The Nutritional Quality Potential of Microgreens, Baby Leaves, and Adult Lettuce: An Underexploited Nutraceutical Source
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Greenhouse-Field Experiments
2.3. Leaf Sample Preparation
2.4. Nutraceutical Compounds and Antioxidant Capacity
2.4.1. Chlorophyll and Carotenoid Concentration
- (1)
- Chl a = 12.25 × Abs663.6 − 2.79 × Abs646.6 (µg mL−1)
- (2)
- Chl b = 21.3 × Abs646.6 − 5.1 × Abs663.6 (µg mL−1)
- (3)
- Car = [(1000 × Abs470 − 1.82 Chl a) − (85.02 × Chl b)]/198 (µg mL−1)
- (4)
- Chl a + b = 7.15 × Abs663.6 + 18.71 × 646.6
2.4.2. Anthocyanin Concentration
- (1)
- (0.0821 × Abs534 − 0.00687 × Abs643 − 0.002426 × Abs661) × 5 mL g−1 FW
2.4.3. Ascorbic Acid Concentration
2.4.4. Total Phenolic Analysis
2.4.5. Antioxidant Capacity Measurements
2.5. Mineral Determination
2.6. Statistical Analysis
3. Results
3.1. Dry Weight
3.2. Nutraceutical Compounds and Antioxidant Capacity
3.2.1. Total Chlorophyll Concentration
3.2.2. Carotenoids
3.2.3. Anthocyanins
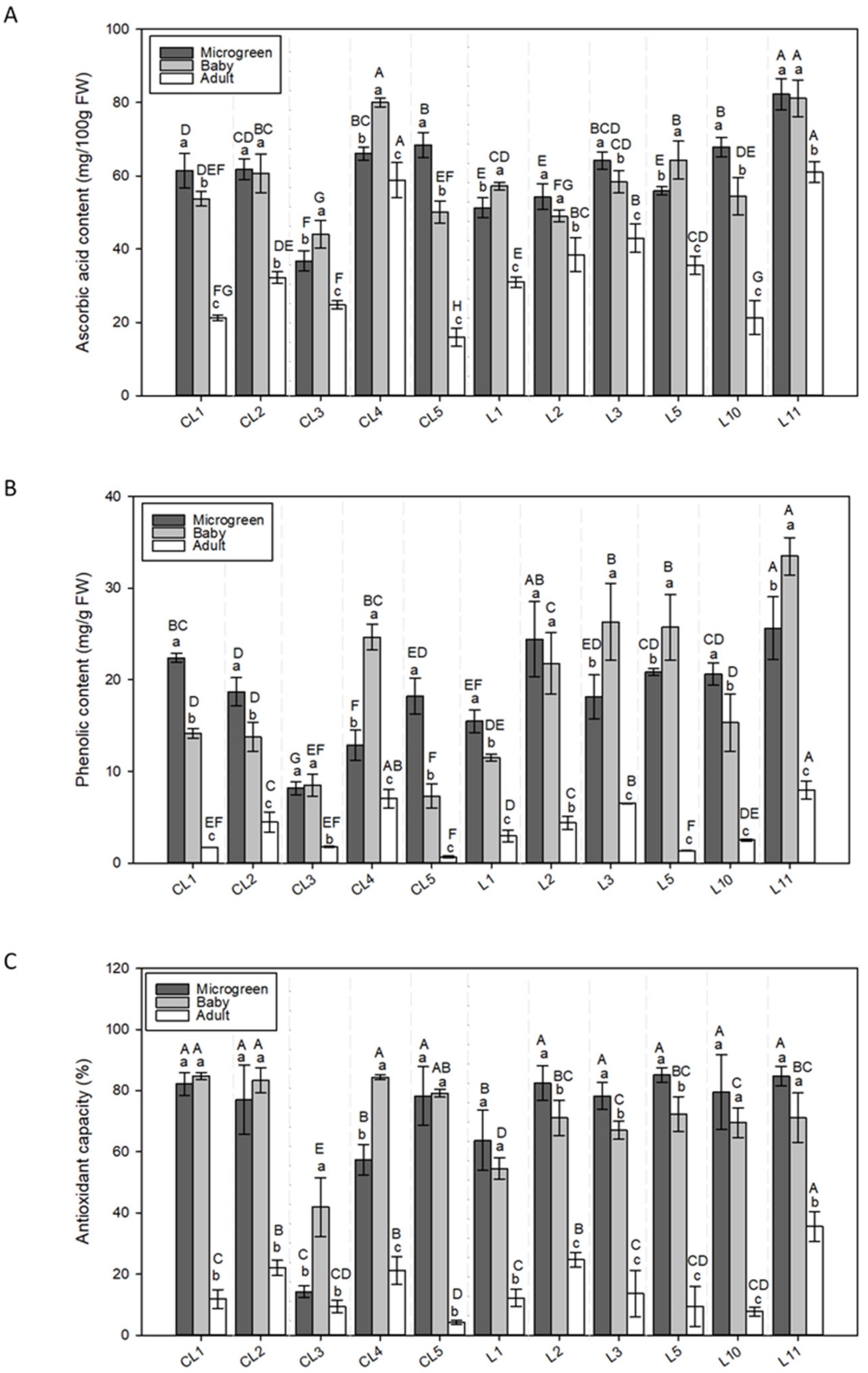
3.2.4. Ascorbic Acid
3.2.5. Phenols
3.2.6. Antioxidant Capacity
3.3. Mineral Concentration
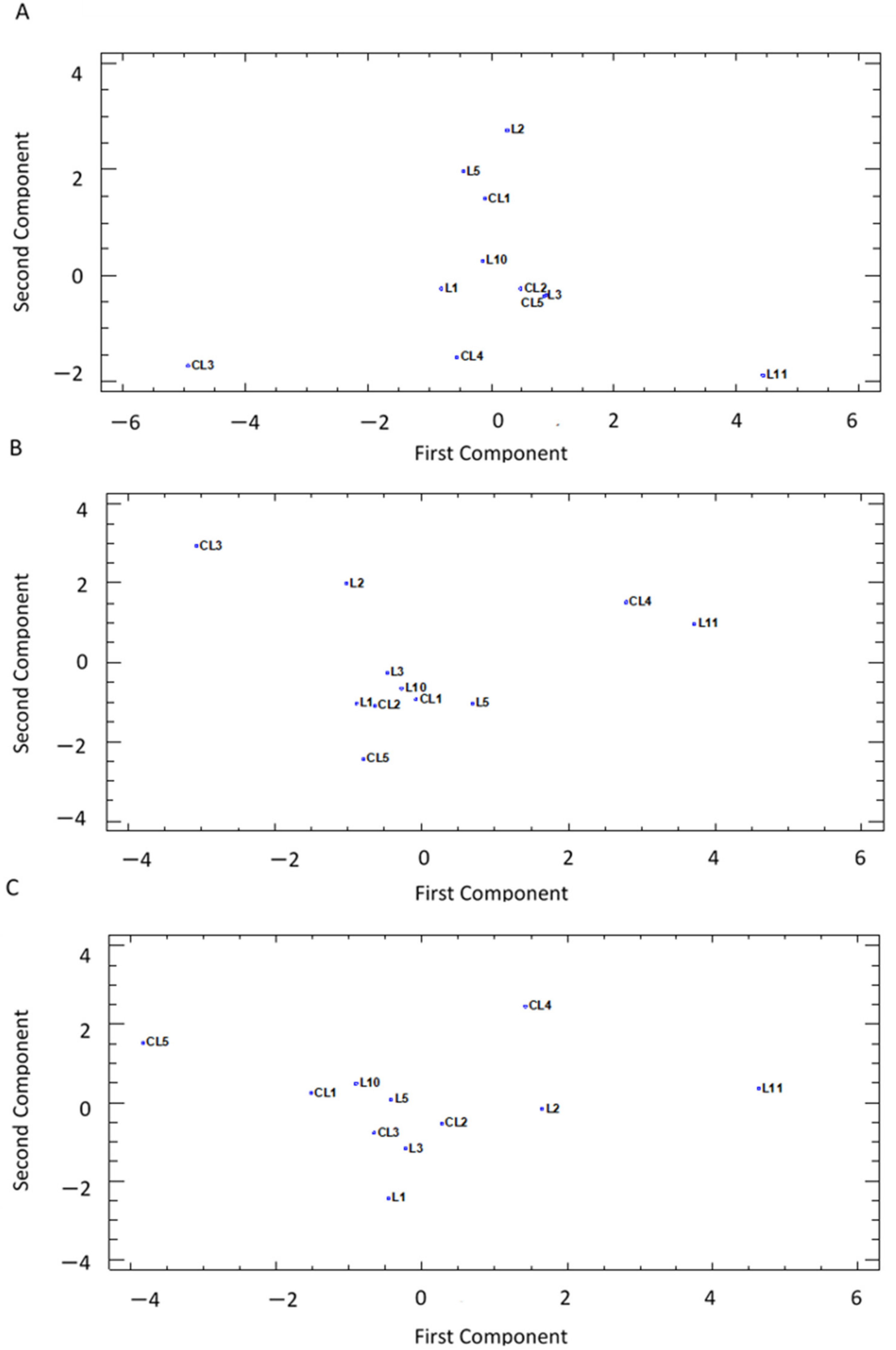
3.4. PCA Analysis
3.5. Correlation between Quality Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenny, O.; O’Beirne, D. The effects of washing treatment on antioxidant retention in ready-to-use iceberg lettuce. Int. J. Food Sci. Technol. 2009, 44, 1146–1156. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants—A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Kopsell, D.A.; Park, S.; Tou, J.C.; Waterland, N.L. Nutritional value of Crisphead ‘Iceberg’ and Romaine lettuces (Lactuca sativa L.). J. Agric. Sci. 2016, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chem. 2016, 199, 702–710. [Google Scholar] [CrossRef]
- Łata, B.; Przeradzka, M. Glutathione and ascorbate contents in broccoli and lettuce cultivars. Folia Hort. 1999, 11, 13–22. [Google Scholar]
- Gazula, A.; Kleinhenz, M.D.; Scheerens, J.C.; Ling, P.P.; Streeter, J.G. Temperature and genotype affect anthocyanin concentrations in lettuce (Lactuca sativa). HortScience 2004, 39, 864A. [Google Scholar] [CrossRef]
- Pinto, E.; Almeida, A.A.; Aguiar, A.A.; Ferreira, I.M. Comparison between the mineral profile and nitrate content of microgreens and mature lettuces. J. Food Compos. Anal. 2015, 37, 38–43. [Google Scholar] [CrossRef]
- Mou, B.; Ryder, E.J. Relationship between the nutritional value and the head structure of lettuce. Acta Hortic. 2004, 637, 361–367. [Google Scholar] [CrossRef]
- Figàs Moreno, M.; Raigón Jiménez, M.; Casanova Calancha, C.; Soler, E.; Pereira Dias, L.; García Martínez, M.; Rosa, E.; Martín, A.; Prohens Tomás, J.; Soler Aleixandre, S. Caracterización de una colección de variedades tradicionales valencianas de lechuga (“Lactuca sativa” L.). Agric. Vergel Frutic. Hortic. Floric. 2017, 401, 157–164. [Google Scholar]
- Kosma, C.; Triantafyllidis, V.; Papasavvas, A.; Salahas, G.; Patakas, A. Yield and nutritional quality of greenhouse lettuce as affected by shading and cultivation season. Emir. J. Food Agric. 2013, 25, 974–979. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M. microgreens: Production, shelf life, and bioactive components. Crit. Rev. Food Sci. Nutr. 2017, 57, 2730–2736. [Google Scholar] [CrossRef]
- Treadwell, D.; Hochmuth, R.; Landrum, L.; Laughlin, W. Microgreens: A New Specialty Crop; University of Florida IFAS Extension HS1164: Gainesville, FL, USA, 2010. [Google Scholar]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.F. Nutrient content of cabbage and lettuce microgreens grown on vermicompost and hydroponic growing pads. J. Hortic. 2016, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Variation in macronutrient content, phytochemical constitution and in vitro antioxidant capacity of green and red Butterhead lettuce dictated by different developmental stages of harvest maturity. Antioxidants 2020, 9, 300. [Google Scholar] [CrossRef] [Green Version]
- Ebert, A.W. Potential of underutilized traditional vegetables and legume crops to contribute to food and nutritional security, income and more sustainable production systems. Sustainability 2014, 6, 319. [Google Scholar] [CrossRef] [Green Version]
- Penella, C.; Nebauer, S.G.; Bautista, A.S.; López-Galarza, S.; Calatayud, Á. Rootstock alleviates PEG-induced water stress in grafted pepper seedlings: Physiological responses. J. Plant Physiol. 2014, 171, 842–851. [Google Scholar] [CrossRef]
- Maroto, J.V. Horticultura Herbácea Especial, 5th ed.; Mundi-Prensa: Madrid, Spain, 2002. [Google Scholar]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. BBA-Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Szepesi, Á.; Csiszár, J.; Gallé, Á.; Gémes, K.; Poór, P.; Tari, I. Effects of long-term salicylic acid pre-treatment on tomato (Lycopersicon esculentum Mill. L.) salt stress tolerance: Changes in glutathione S-transferase activities and anthocyanin contents. Acta Agron. Hung. 2008, 56, 129–138. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Xianzhong, W.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mampholo, B.M.; Maboko, M.M.; Soundy, P.; Sivakumar, D. Phytochemicals and overall quality of leafy lettuce (Lactuca sativa L.) varieties grown in closed hydroponic system. J. Food Qual. 2016, 39, 805–815. [Google Scholar] [CrossRef]
- Mou, B. Nutritional quality of lettuce. Curr. Nutr. Food Sci. 2012, 8, 177–187. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Palladino, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Phenolic constitution, phytochemical and macronutrient content in three species of microgreens as modulated by natural fiber and synthetic substrates. Antioxidants 2020, 9, 252. [Google Scholar] [CrossRef] [Green Version]
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. Microgreens as novel functional foods: Variation of nutritional and phytochemical profiles and their in vitro bioactive properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef]
- Choe, U.; Yu, L.L.; Wang, T.T. The science behind microgreens as an exciting new food for the 21st century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef]
- Lenzi, A.; Orlandini, A.; Bulgari, R.; Ferrante, A.; Bruschi, P. Antioxidant and mineral composition of three wild leafy species: A comparison between microgreens and baby greens. Foods 2019, 8, 487. [Google Scholar] [CrossRef] [Green Version]
- Le, T.N.; Chiu, C.; Hsieh, P. Microgreens: An updated overview from a nutraceutical perspective. Plants 2020, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional quality in novel food sources: Genotypic variation in the nutritive and phytochemical composition of thirteen microgreens species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Choe, U.; Li, Y.; Gao, B.; Yu, L.; Wang, T.T.Y.; Sun, J.; Chen, P.; Liu, J.; Yu, L. Chemical compositions of cold-pressed broccoli, carrot, and cucumber seed flours and their in vitro gut microbiota modulatory, anti-inflammatory, and free radical scavenging properties. J. Agric. Food Chem. 2018, 66, 9309–9317. [Google Scholar] [CrossRef] [PubMed]
- Castagnino, A.; Marina, J.; Benvenuti, s.; Castro, M. Microgreens and sprouts, two innovative functional foods for a healthy diet in Km 0. Hortic. Argent. 2020, 39, 55–95. [Google Scholar]
- Xiao, Z.; Lester, G.E.; Park, E.; Saftner, R.A.; Luo, Y.; Wang, Q. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: Microgreens. Postharvest Biol. Technol. 2015, 110, 140–148. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J. Food Compos. Anal. 2020, 91, 103495. [Google Scholar] [CrossRef]
- Liu, X.; Ardo, S.; Bunning, M.; Parry, J.; Zhou, K.; Stushnoff, C.; Stoniker, F.; Yu, L.; Kendall, P. Total phenolic content and DPPH radical scavenging activity of lettuce (Lactuca sativa L.) grown in Colorado. LWT-Food Sci. Technol. 2007, 40, 552–557. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, X.; Xiao, Z.; Yu, L.; Pham, Q.; Sun, J.; Chen, P.; Yokoyama, W.; Yu, L.L.; Luo, Y.S.; et al. Red cabbage microgreens lower circulating low-density lipoprotein (LDL), liver cholesterol, and inflammatory cytokines in mice fed a high-fat diet. J. Agric. Food Chem. 2016, 64, 9161–9171. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- Ribarova, F.; Atanassova, M.; Marinova, D.; Ribarova, F.; Atanassova, M.J. Total phenolics and flavonoids in Bulgarian fruits and vegetables. Chem. Technol. 2005, 40, 255–260. [Google Scholar]
- Lin, J.Y.; Tang, C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Jabłonska-Rys, E.; Zalewska-Korona, M.; Kalbarczyk, J. Antioxidant capacity, ascorbic acid and phenolics content in wild edible fruits. J. Fruit Ornam. Plant Res. 2009, 17, 115–120. [Google Scholar]
- Senevirathne, G.I.; Gama-Arachchige, N.S.; Karunaratne, A.M. Germination, harvesting stage, antioxidant activity and consumer acceptance of ten microgreens. Ceylon J. Sci. 2019, 48, 91. [Google Scholar] [CrossRef]
- Viacava, G.E.; Gonzalez-Aguilar, G.; Roura, S.I. Determination of phytochemicals and antioxidant activity in butterhead lettuce related to leaf age and position. J. Food Biochem. 2014, 38, 352–362. [Google Scholar] [CrossRef]
- Medina-Lozano, I.; Bertolín, J.R.; Díaz, A. Nutritional value of commercial and traditional lettuce (Lactuca sativa L.) and wild relatives: Vitamin C and anthocyanin content. Food Chem. 2021, 359. [Google Scholar] [CrossRef]
- Favell, D.J. A comparison of the vitamin C content of fresh and frozen vegetables. Food Chem. 1998, 62, 59–64. [Google Scholar] [CrossRef]
- Szeto, Y.T.; Tomlinson, B.; Benzie, I.F.F. Total antioxidant and ascorbic acid content of fresh fruits and vegetables: Implications for dietary planning and food preservation. Br. J. Nutr. 2002, 87, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Kapur, A.A.; Hasković, A.; Čopra-Janićijević; Klepo, L. Spectrophotometric analysis of total ascorbic acid contetnt in various fruits and vegetables. Glas. Hem. Tehnol. Bosne Herceg. 2012, 38, 39–42. [Google Scholar]
- Igarashi, K.; Kimura, Y.; Takenaka, A. Preventive Effects of dietary cabbage acylated anthocyanins on paraquat-induced oxidative stress in rats. Biosci. Biotechnol. Biochem. 2000, 64, 1600–1607. [Google Scholar] [CrossRef]
- Simko, I.; Hayes, R.J.; Furbank, R.T. Non-destructive phenotyping of lettuce plants in early stages of development with optical sensors. Front. Plant Sci. 2016, 7, 1985. [Google Scholar] [CrossRef] [Green Version]
- Sytar, O.; Zivcak, M.; Bruckova, K.; Brestic, M.; Hemmerich, I.; Rauh, C.; Simko, I. Shift in accumulation of flavonoids and phenolic acids in lettuce attributable to changes in ultraviolet radiation and temperature. Sci. Hortic. 2018, 239, 193–204. [Google Scholar] [CrossRef]
- Baslam, M.; Morales, F.; Garmendia, I.; Goicoechea, N. Nutritional quality of outer and inner leaves of green and red pigmented lettuces (Lactuca sativa L.) consumed as salads. Sci. Hortic. 2013, 151, 103–111. [Google Scholar] [CrossRef]
- Brücková, K.; Sytar, O.; Ẑivĉák, M.; Brestič, M.; Lebeda, A. Vplyv podmienok pestovania na akumuláciu flavonolov a antokyánov v zelenom a červenom šaláte. J. Cent. Eur. Agric. 2016, 17, 986–997. [Google Scholar] [CrossRef]
- Delian, E.; Chira, A.; Bădulescu, L.; Chira, L. Insights into microgreens physiology. Sci. Pap. Ser. B Hortic. 2015, 59, 447–454. [Google Scholar]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Influence of light on health-promoting phytochemicals of broccoli sprouts. J. Sci. Food Agric. 2008, 88, 904–910. [Google Scholar] [CrossRef]
- Meas, S.; Luengwilai, K.; Thongket, T. Enhancing growth and phytochemicals of two amaranth microgreens by LEDs light irradiation. Sci. Hortic. 2020, 265, 109204. [Google Scholar] [CrossRef]
- Mou, B. Nutrient content of lettuce and its improvement. Curr. Nutr. Food Sci. 2009, 5, 242–248. [Google Scholar] [CrossRef]
- Sytar, O.; Brücková, K.; Kovár, M.; Živčák, M.; Hemmerich, I.; Brestič, M. Nondestructive detection and biochemical quantification of buckwheat leaves using visible (VIS) and near-infrared (NIR) hyperspectral reflectance imaging Nedeštrukčná detekcia a biochemická kvantifikácia listov pohánky s využitím hyperspektrálneho zobrazovania s reflektanciou vo viditeľnej (VIS) a blízkej infračervenej (NIR) oblasti. J. Cent. Eur. Agric. 2017, 18, 864–878. [Google Scholar] [CrossRef] [Green Version]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Proc. Bioch. Biop. Acta. 2005, 1740, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Frégeau-Reid, J. Breeding line selection based on multiple traits. Crop Sci. 2008, 48, 417–423. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Trokowski, K.; Karlovits, G.; Szłyk, E. Effect of refining processes on antioxidant capacity, total contents of phenolics and carotenoids in palm oils. Food Chem. 2011, 129, 1187–1192. [Google Scholar] [CrossRef]
- Gisbert-Mullor, R.; Ceccanti, C.; Padilla, Y.G.; López-Galarza, S.; Calatayud, Á.; Conte, G.; Guidi, L. Effect of grafting on the production, physico-chemical characteristics and nutritional quality of fruit from pepper landraces. Antioxidants 2020, 9, 501. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ispizua, E.; Martínez-Cuenca, M.-R.; Marsal, J.I.; Díez, M.J.; Soler, S.; Valcárcel, J.V.; Calatayud, Á. Bioactive compounds and antioxidant capacity of valencian pepper landraces. Molecules 2021, 26, 1031. [Google Scholar] [CrossRef] [PubMed]
- Qudah, J.; El-Qudah, J.M. Identification and quantification of major carotenoids in some vegetables evaluation of hospital diets view project identification and quantification of major carotenoids in some vegetables. Am. J. Appl. Sci. 2009, 6, 492–497. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briat, J.-F.; Vert, G. Acquisition et gestion du fer par les plantes. Cah. Agric. 2004, 13, 183–201. [Google Scholar]
- Msilini, N.; Amdouni, T.; Chebbi, M.; Lachaâl, M.; Ouerghi, Z. Antagonistic effects of iron and/or magnesium deficiencies on enzyme activities in lettuce (Lactuca sativa L.) plants. J. Hortic. Sci. Biotechnol. 2014, 89, 361–366. [Google Scholar] [CrossRef]
- Kumar Tewari, R.; Kumar, P.; Nand Sharma, P. Magnesium deficiency induced oxidative stress and antioxidant responses in mulberry plants. Sci. Hortic. 2006, 108, 7–14. [Google Scholar] [CrossRef]
- Ciccarese, A.; Stellacci, A.M.; Gentilesco, G.; Rubino, P. Effectiveness of pre- and post-veraison calcium applications to control decay and maintain table grape fruit quality during storage. Postharvest Biol. Technol. 2013, 75, 135–141. [Google Scholar] [CrossRef]
- Holb, I.J.; Balla, B.; Vámos, A.; Gáll, J.M. Influence of preharvest calcium applications, fruit injury, and storage atmospheres on postharvest brown rot of apple. Postharvest Biol. Technol. 2012, 67, 29–36. [Google Scholar] [CrossRef]
- Rincón, A.; Martínez, E. Funciones del calcio en la calidad poscosecha de frutas y hortalizas. Aliment. Hoy Rev. Asoc. Colomb. y Tecnol. Aliment. 2015, 24, 13–25. [Google Scholar]
- Gómez-zeledón, J.; Sc, B.; Jiménez, V.M.; Ph, D. In vitro production of anthocyanins—A literature review. Acta Biol. Colomb. 2011, 16, 3–20. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: Cambridge, MA, USA, 1955. [Google Scholar]
- Battistelli, A.; Fallovo, C.; Rouphael, Y.; Cardarelli, M.; Rea, E.; Colla, G.; Rastilantie, M. Yield and quality of leafy lettuce in response to nutrient solution composition and growing season Yield and quality of leafy lettuce in response to nutrient solution composition and growing season WFL Publisher Science and Technology. J. Food, Agric. Environ. 2009, 7, 456–462. [Google Scholar]
- Hoque, M.M.; Ajwa, H.; Othman, M.; Smith, R.; Cahn, M. Yield and postharvest quality of lettuce in response to nitrogen, phosphorus, and potassium fertilizers. HortScience 2010, 45, 1539–1544. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Maruo, T. Plant growth and photosynthesis response to low potassium conditions in three lettuce (Lactuca sativa) types. Hortic. J. 2017, 86, 229–237. [Google Scholar] [CrossRef] [Green Version]
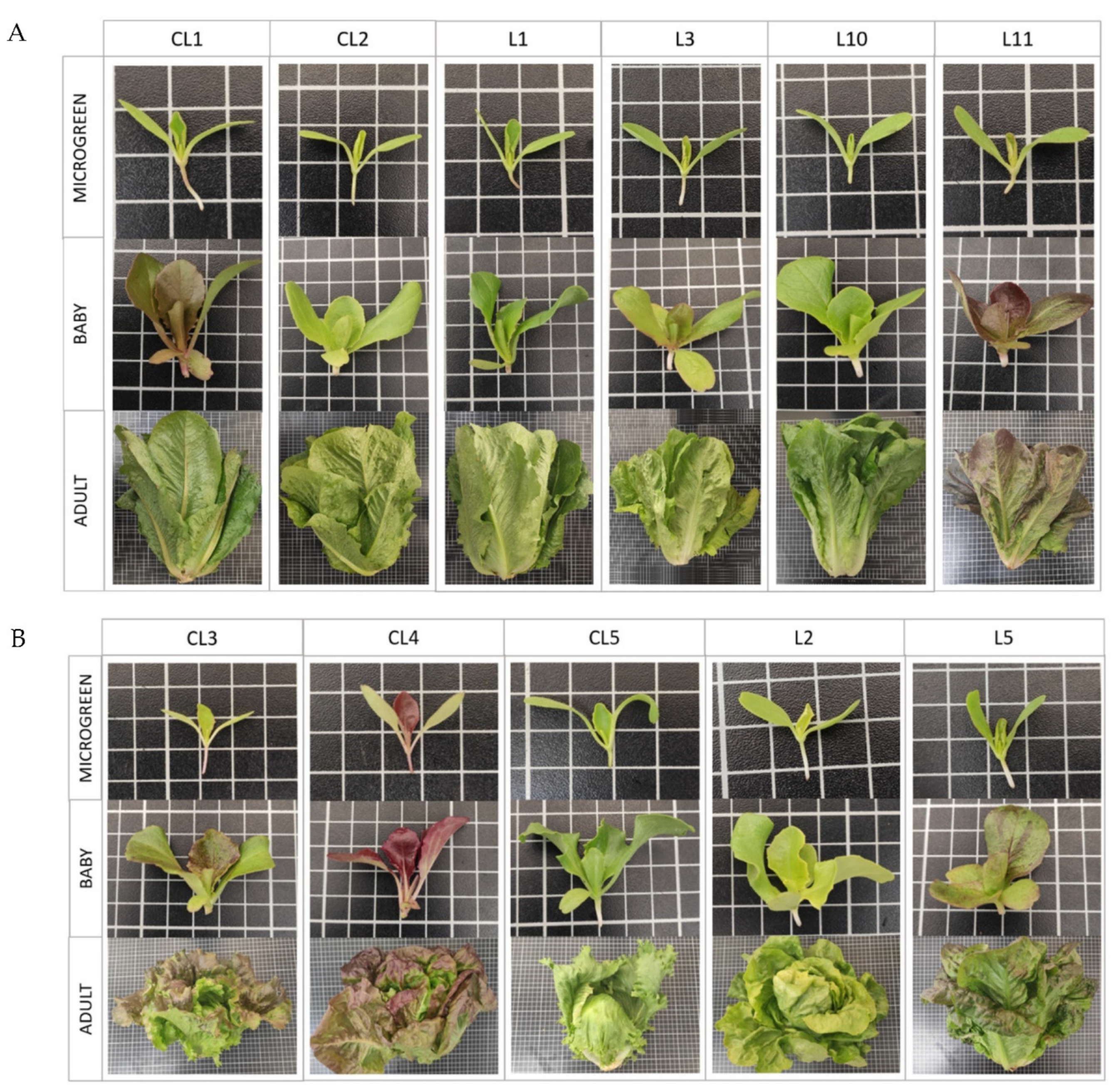


| Abbreviation Code | Origin | Identification | Plant Description |
|---|---|---|---|
| CL1 | Commercial | Romaine lettuce long mule ear (Battle) a | Dark green. Elongated shape. Compact and narrow head, barely prominent. |
| CL2 | Commercial | Romaine lettuce from the gardeners (Vilmorín) a | Green-yellowish. Elongated shape. Compact and narrow head, barely prominent. |
| CL3 | Commercial | Wonder summer (Battle) a | Green with reddish shades. Remarkable width in relation to height. Compact, rounded and quite prominent head. |
| CL4 | Commercial | Marvel of Four Seasons Butterhead (Battle) a | Dark green with reddish shades; the red is prominent at the edges. Round shape. Quite rounded shape. Full-sized head. |
| CL5 | Commercial | Batavia, iceberg type (Battle) a | Not very intense green. Rounded shape. Full-sized head. |
| L1 | Local landrace | BGV5721 b,1 | Dark green. Pink shades near the principal stem. Elongated shape. Compact and narrow head, barely prominent. |
| L2 | Local landrace | BGV5722 b,1 | Green-yellowish. Round shape. Full-sized head. |
| L3 | Local landrace | BGV5723 b,1 | Green-yellowish. Remarkable width in relation to height. Head not appreciated. |
| L5 | Local landrace | BGV5736 b,1 | Dark green with reddish shades. Elongated shape. Compact and narrow head, quite prominent. |
| L10 | Local landrace | L-10 b,2 | Dark green. Elongated shape. Compact and narrow head, barely prominent. |
| L11 | Local landrace | L-11 b,2 | Dark red, almost purple. Remarkable width in relation to height. Head not appreciated. |
| Unit/Scale | Mean | Range | CV (%) | F-Ratio | |
|---|---|---|---|---|---|
| Microgreen | |||||
| DW | % | 7.53 ± 0.59 | 5.81–8.84 *** | 7.84 | 4.81 |
| Chl | µg g−1 FW | 393.77 ± 92.38 | 200.89–647.87 *** | 23.46 | 29.45 |
| Car | µg g−1 FW | 3.70 ± 9.62 | 0–43.48 *** | 259.62 | 70.10 |
| Ant | µmol 100 g−1 FW | 50.67 ± 10.29 | 36.12–72.34 *** | 20.31 | 20.55 |
| AsA | mg 100 g−1 FW | 61.05 ± 11.61 | 34.26–87.01 *** | 19.02 | 54.38 |
| Phe | mg g−1 DW | 18.69 ± 5.23 | 7.43–28.78 *** | 28.01 | 24.3 |
| DPPH | % | 71.52 ± 21.17 | 12.65–88.46 *** | 29.61 | 32.08 |
| Ca | mg g−1 DW | 9.76 ± 1.75 | 6.61–12.81 *** | 17.96 | 22.48 |
| K | mg g−1 DW | 48.23 ± 3.93 | 39.54–60.08 ** | 8.15 | 2.97 |
| Fe | µg g−1 DW | 225.08 ± 60.74 | 68.1–362.18 *** | 26.99 | 132.5 |
| Baby | |||||
| DW | % | 10.14 ± 1.14 | 7.65–12.74 *** | 11.26 | 13.03 |
| Chl | µg g−1 FW | 502.85 ± 65.60 | 339.05–606.61 *** | 13.05 | 14.63 |
| Car | µg g−1 FW | 36.76 ± 28.89 | 1.87–126.98 *** | 78.6 | 36.93 |
| Ant | µmol 100 g−1 FW | 56.16 ± 29.88 | 26.46–141.96 *** | 56.19 | 296.27 |
| AsA | mg 100 g−1 FW | 58.35 ± 11.19 | 39.25–86.49 *** | 19.18 | 34.83 |
| Phe | mg g−1 DW | 18.43 ± 8.49 | 5.58–36.11 *** | 46.06 | 45.24 |
| DPPH | % | 71.07 ± 13.46 | 32.61–87.02 *** | 18.93 | 23.79 |
| Ca | mg g−1 DW | 7.23 ± 1.28 | 5.39–10.07 *** | 17.65 | 7.39 |
| K | mg g−1 DW | 28.18 ± 3.60 | 22.21–36.82 * | 12.78 | 2.53 |
| Fe | µg g−1 DW | 104.64 ± 18.07 | 71.24–146.55 *** | 17.27 | 4.28 |
| Adult | |||||
| DW | % | 6.48 ± 1.46 | 3.83–13.25 *** | 22.5 | 6.84 |
| Chl | µg g−1 FW | 434.36 ± 46.61 | 359.85–564.58 *** | 10.73 | 7.88 |
| Car | µg g−1 FW | 11.11 ± 11.01 | 0–39.73 *** | 99.10 | 35.03 |
| Ant | µmol 100 g−1 FW | 47.63 ± 10.50 | 29.15–71.76 *** | 22.08 | 12.65 |
| AsA | mg 100 g−1 FW | 34.45 ± 14.33 | 13.44–64.2 *** | 41.58 | 88.68 |
| Phe | mg g−1 DW | 3.91 ± 2.50 | 0.56–9.38 *** | 63.89 | 49.97 |
| DPPH | % | 15.88 ± 9.55 | 3.58–39.41 *** | 60.14 | 19.21 |
| Ca | mg g−1 DW | 8.87 ± 1.79 | 6.13–13.79 *** | 20.18 | 23.04 |
| K | mg g−1 DW | 59.89 ± 7.66 | 44.36–75.96 *** | 12.80 | 11.55 |
| Fe | µg g−1 DW | 171.44 ± 114.32 | 74.51–514.25 *** | 66.68 | 131.98 |
| Variety | State | Ca (mg g−1 DW) | K (mg g−1 DW) | Fe (µg g−1 DW) | |||
|---|---|---|---|---|---|---|---|
| CL1 | Microgreen | 8.76 ± 0.87 | DEFa | 48.06 ± 1.26 | Bb | 230.48 ± 20.05 | CDa |
| Baby | 5.64 ± 0.16 | Cc | 26.47 ± 3.03 | CDc | 119.34 ± 16.77 | Ab | |
| Adult | 7.21 ± 0.03 | Eb | 58.13 ± 2.63 | Ca | 108.80 ± 7.19 | DEb | |
| CL2 | Microgreen | 8.66 ± 0.54 | DEFa | 45.85 ± 4.56 | BCb | 230.51 ± 2.06 | CDa |
| Baby | 6.65 ± 0.82 | BCb | 25.37 ± 3.35 | Dc | 92.90 ± 9.12 | Cb | |
| Adult | 7.19 ± 0.17 | Eb | 68.73 ± 4.84 | Aa | 84.30 ± 10.40 | Eb | |
| CL3 | Microgreen | 10.91 ± 1.35 | Ba | 53.59 ± 4.36 | Ab | 77.74 ± 10.88 | Gb |
| Baby | 7.93 ± 0.82 | Ab | 32.53 ± 2.61 | Ac | 114.69 ± 8.51 | ABb | |
| Adult | 8.76 ± 0.65 | CDb | 66.02 ± 4.46 | ABa | 218.88 ± 38.54 | Ba | |
| CL4 | Microgreen | 9.66 ± 1.29 | Ca | 47.14 ± 2.89 | Bb | 155.56 ± 4.73 | Fb |
| Baby | 7.76 ± 0.47 | Ab | 31.86 ± 1.87 | ABc | 118.73 ± 6.45 | Ab | |
| Adult | 11.07 ± 0.70 | Ba | 57.91 ± 6.47 | Ca | 193.38 ± 12.79 | Ba | |
| CL5 | Microgreen | 8.21 ± 0.23 | EFa | 48.92 ± 3.79 | ABa | 255.54 ± 12.62 | Ba |
| Baby | 5.88 ± 0.46 | Cc | 26.46 ± 2.02 | CDb | 94.77 ± 16.44 | Cb | |
| Adult | 6.82 ± 0.61 | Eb | 46.21 ± 1.68 | Da | 110.06 ± 22.83 | DEb | |
| L1 | Microgreen | 7.72 ± 0.58 | Fa | 48.73 ± 4.69 | Bb | 216.87 ± 8.87 | DEa |
| Baby | 6.23 ± 0.55 | Cb | 27.89 ± 1.65 | BCDc | 91.43 ± 13.50 | Cb | |
| Adult | 7.51 ± 0.83 | Ea | 66.53 ± 5.29 | ABa | 123.30 ± 10.28 | CDab | |
| L2 | Microgreen | 12.37 ± 0.15 | Aa | 47.69 ± 0.86 | Ba | 344.91 ± 13.64 | Aa |
| Baby | 8.89 ± 1.02 | Ab | 30.25 ± 5.41 | ABCb | 119.75 ± 20.78 | Aa | |
| Adult | 9.47 ± 0.71 | CDb | 50.63 ± 1.14 | Da | 119.34 ± 3.73 | Db | |
| L3 | Microgreen | 7.77 ± 0.79 | EFab | 49.18 ± 3.43 | ABb | 244.98 ± 3.66 | BCDa |
| Baby | 6.50 ± 1.03 | Cb | 28.21 ± 5.60 | ABCDc | 98.21 ± 5.05 | BCb | |
| Adult | 8.78 ± 0.73 | CDa | 66.10 ± 4.57 | ABa | 100.87 ± 13.03 | DEb | |
| L5 | Microgreen | 12.38 ± 0.23 | Aa | 49.73 ± 2.88 | ABb | 222.59 ± 9.10 | DEa |
| Baby | 8.58 ± 1.18 | Ab | 25.48 ± 0.46 | Dc | 89.74 ± 13.70 | Cb | |
| Adult | 9.70 ± 1.01 | Cb | 59.69 ± 4.42 | Ca | 153.94 ± 27.85 | Cab | |
| L10 | Microgreen | 10.66 ± 0.15 | BCa | 49.57 ± 1.33 | ABb | 208.97 ± 2.41 | Eab |
| Baby | 7.77 ± 0.68 | ABb | 26.68 ± 1.41 | CDc | 88.93 ± 12.13 | Cb | |
| Adult | 8.65 ± 0.92 | Db | 56.93 ± 4.46 | Ca | 152.15 ± 19.59 | Cb | |
| L11 | Microgreen | 10.28 ± 0.12 | BCb | 42.13 ± 2.81 | Cb | 236.34 ± 9.73 | BCDb |
| Baby | 8.07 ± 1.09 | Ac | 28.77 ± 2.17 | ABCDc | 122.49 ± 17.73 | Ab | |
| Adult | 12.41 ± 0.92 | Aa | 61.91 ± 1.62 | BCa | 493.47 ± 23.01 | Aa | |
| First PC | Second PC | Third PC | |
|---|---|---|---|
| Microgreen | |||
| DW | 0.355 | −0.230 | |
| Chl a + b | 0.203 | −0.557 | |
| Car | 0.334 | −0.294 | 0.213 |
| Ant | −0.665 | ||
| AsA | 0.419 | ||
| Phe | 0.366 | 0.352 | |
| DPPH | 0.373 | 0.346 | |
| Ca | 0.270 | 0.653 | |
| K | −0.409 | −0.182 | |
| Fe | 0.295 | 0.435 | |
| Eigenvalue | 4.70 | 2.28 | 1.35 |
| Variance explained (%) | 46.99 | 22.80 | 13.49 |
| Cumulative variance explained (%) | 46.99 | 69.79 | 83.28 |
| Baby | |||
| DW | 0.201 | −0.471 | |
| Chl a + b | 0.292 | 0.509 | |
| Car | 0.459 | 0.221 | |
| Ant | 0.335 | −0.545 | |
| AsA | 0.510 | ||
| Phe | 0.413 | 0.167 | 0.180 |
| DPPH | 0.284 | −0.269 | −0.466 |
| Ca | 0.403 | 0.292 | |
| K | 0.553 | −0.153 | |
| Fe | 0.169 | 0.436 | −0.179 |
| Eigenvalue | 3.46 | 2.64 | 1.39 |
| Variance explained (%) | 34.60 | 26.43 | 13.90 |
| Cumulative variance explained (%) | 34.60 | 61.02 | 74.93 |
| Adult | |||
| DW | 0.328 | −0.326 | |
| Chl a + b | −0.360 | ||
| Car | 0.593 | −0.415 | |
| Ant | 0.331 | −0.270 | 0.242 |
| AsA | 0.396 | 0.157 | |
| Phe | 0.369 | 0.334 | |
| DPPH | 0.406 | ||
| Ca | 0.248 | −0.428 | −0.218 |
| K | 0.388 | 0.718 | |
| Fe | 0.313 | −0.289 | 0.283 |
| Eigenvalue | 5.09 | 1.44 | 1.07 |
| Variance explained (%) | 50.88 | 14.40 | 10.73 |
| Cumulative variance explained (%) | 50.88 | 65.27 | 76.00 |
| Microgreen | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chl a + b | Car | Ant | AsA | Phe | DPPH | Ca | K | Fe | |
| Chl a + b | 0.4143 * | −0.0364 | 0.3928 * | 0.0747 | 0.0346 | −0.117 | −0.119 | −0.0901 | |
| Car | 0.0732 | 0.5045 ** | 0.1708 | 0.2554 | −0.1941 | −0.4941 ** | 0.2132 | ||
| Ant | 0.0124 | 0.2169 | 0.1641 | −0.4458 ** | −0.1202 | 0.2338 | |||
| AsA | 0.3244 * | 0.4498 ** | −0.1398 | −0.3722 * | 0.1853 | ||||
| Phe | 0.7212 *** | 0.262 | −0.2496 | 0.2812 | |||||
| DPPH | 0.1729 | −0.1802 | 0.1598 | ||||||
| Ca | 0.0285 | 0.0943 | |||||||
| K | −0.604 *** | ||||||||
| Fe | |||||||||
| Baby | |||||||||
| Chla+b | Car | Ant | AsA | Phe | DPPH | Ca | K | Fe | |
| Chl a + b | 0.5116 ** | 0.2391 | 0.3653 * | 0.27 | 0.0557 | 0.0786 | 0.0099 | 0.0448 | |
| Car | 0.271 | 0.6154 *** | 0.4764 ** | 0.2258 | 0.1565 | −0.0187 | 0.4546 ** | ||
| Ant | 0.6243 *** | 0.2022 | 0.4963 *** | −0.0116 | 0.1843 | 0.116 | |||
| AsA | 0.6974 *** | 0.4557 ** | 0.1692 | −0.0295 | 0.3639 * | ||||
| Phe | 0.184 | 0.3394 * | 0.0149 | 0.3192 * | |||||
| DPPH | −0.1262 | −0.2486 | 0.0874 | ||||||
| Ca | 0.4761 ** | 0.1332 | |||||||
| K | 0.2664 | ||||||||
| Fe | |||||||||
| Adult | |||||||||
| Chla+b | Car | Ant | AsA | Phe | DPPH | Ca | K | Fe | |
| Chl a + b | −0.3158 | 0.3714 * | 0.2159 | 0.1143 | 0.2218 | 0.0939 | 0.419 ** | −0.0149 | |
| Car | 0.15 | 0.0572 | 0.2014 | 0.0254 | 0.147 | −0.0818 | 0.0858 | ||
| Ant | 0.5291 *** | 0.4224 ** | 0.5695 *** | 0.5614 *** | 0.2667 | 0.584 *** | |||
| AsA | 0.8672 *** | 0.7029 *** | 0.7997 *** | 0.2131 | 0.5939 *** | ||||
| Phe | 0.7243 *** | 0.6467 *** | 0.1911 | 0.5194 *** | |||||
| DPPH | 0.5732 *** | 0.0936 | 0.5226 *** | ||||||
| Ca | 0.0815 | 0.7591 *** | |||||||
| K | 0.0814 | ||||||||
| Fe |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Cannata, C.; Basile, F.; Abdelkhalik, A.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.-R. The Nutritional Quality Potential of Microgreens, Baby Leaves, and Adult Lettuce: An Underexploited Nutraceutical Source. Foods 2022, 11, 423. https://doi.org/10.3390/foods11030423
Martínez-Ispizua E, Calatayud Á, Marsal JI, Cannata C, Basile F, Abdelkhalik A, Soler S, Valcárcel JV, Martínez-Cuenca M-R. The Nutritional Quality Potential of Microgreens, Baby Leaves, and Adult Lettuce: An Underexploited Nutraceutical Source. Foods. 2022; 11(3):423. https://doi.org/10.3390/foods11030423
Chicago/Turabian StyleMartínez-Ispizua, Eva, Ángeles Calatayud, José Ignacio Marsal, Claudio Cannata, Federico Basile, Abdelsattar Abdelkhalik, Salvador Soler, José Vicente Valcárcel, and Mary-Rus Martínez-Cuenca. 2022. "The Nutritional Quality Potential of Microgreens, Baby Leaves, and Adult Lettuce: An Underexploited Nutraceutical Source" Foods 11, no. 3: 423. https://doi.org/10.3390/foods11030423
APA StyleMartínez-Ispizua, E., Calatayud, Á., Marsal, J. I., Cannata, C., Basile, F., Abdelkhalik, A., Soler, S., Valcárcel, J. V., & Martínez-Cuenca, M.-R. (2022). The Nutritional Quality Potential of Microgreens, Baby Leaves, and Adult Lettuce: An Underexploited Nutraceutical Source. Foods, 11(3), 423. https://doi.org/10.3390/foods11030423









