Abstract
There is a lack of studies evaluating the metabolic contribution of non-Saccharomyces yeasts in early fermentation phases. This study aimed to investigate the volatile aroma profiles produced by various non-Saccharomyces yeasts just before sequential inoculation with Saccharomyces cerevisiae to provide an insight into the particular effects they induce at this stage. The grape must of Malvazija istarska was inoculated with monocultures of Torulaspora delbrueckii, Metschnikowia pulcherrima, Pichia kluyveri, Lachancea thermotolerans, and Schizosaccharomyces pombe, alongside a S. cerevisiae control. Eighty volatile compounds were quantified via headspace solid-phase microextraction and gas chromatography–mass spectrometry, and the data were statistically elaborated. Volatile profiles of non-Saccharomyces yeasts differed significantly from the S. cerevisiae control. Most treatments caused increases in linalool and β-damascenone, decreases in higher alcohols and fatty acids, and improved synthesis of odoriferous esters. Torulaspora delbrueckii and M. pulcherrima produced compounds not commonly found in S. cerevisiae fermented wines. Multivariate statistical analysis linked the investigated yeasts to specific, particularly abundant compounds. Future studies should explore to what degree these contributions persist after sequential inoculation with S. cerevisiae in diverse grape must matrices.
1. Introduction
Winemaking dates back to the beginning of civilization, but the scientific community keeps pursuing new technologies to improve the production and quality of wine. Although the yeasts that are usually selected for winemaking are from the genus Saccharomyces, most often of Saccharomyces cerevisiae species, there is great potential in the use of non-Saccharomyces yeasts. Non-Saccharomyces yeasts play an important role in the pre-fermentative stage of winemaking (e.g., cold soak) and un-inoculated fermentations, characterized by the coexistence and succession of multiple yeast species and strains [1]. Recently, several non-Saccharomyces yeasts with great oenological potential are commercially available, and this is likely to continue in the near future [2].
As most non-Saccharomyces yeasts show limited fermentation aptitudes, sequential or co-inoculation with Saccharomyces yeasts is required for fermentation completion. The sequential fermentation is initiated by a high concentration of a non-Saccharomyces species. For example, after a certain period of time or when the ethanol level reaches the desired level, Saccharomyces yeast is inoculated. In this manner, enough time is provided for the metabolic contribution of non-Saccharomyces yeasts before being inhibited by Saccharomyces yeasts and increasing alcohol levels, whilst avoiding stuck or sluggish fermentation [3].
Fermentation involving non-Saccharomyces yeasts may bring many advantages to the wine production process and the final wine quality, depending on the yeast genus, species and strain. It is a potential solution to reduce ethanol content in wine when mitigating the effects of climate change and the premature ripening of grapes, as well as producing wines with less alcohol to meet specific consumer and stylistic requirements.
It has been shown that Metschnikowia pulcherrima [2] and Lachancea thermotolerans [4], in combination with S. cerevisiae, are good candidates for decreasing wine ethanol content. Schizosaccharomyces pombe is the highest malic acid consumer among yeasts [5] and thus can be used to decrease wine acidity through maloalcoholic fermentation [6]. On the other hand, L. thermotolerans shows the potential to increase total wine acidity through the production of lactic acid [4]. In excess, acetic acid has a negative impact on fermentation and wine aroma. Although S. pombe shows a tendency to produce high levels of acetic acid, an interesting strategy with sequential fermentation of L. thermotolerans and S. pombe, proposed as an alternative to malolactic fermentation, resulted in decreased acetic acid content [7]. High sulphite levels added in various stages of winemaking may cause consumer rejection. Application of non-Saccharomyces yeasts, in particular Torulaspora delbrueckii and M. pulcherrima, in bioprotection by colonizing the environment in pre-fermentative stages and thereby limiting the development of the potentially undesirable microbiota, offers an alternative to SO2 [8]. Moreover, non-Saccharomyces yeasts can positively affect wine protein stability, either by producing more mannose-containing polysaccharides, which act as stabilizers or by acting proteolytically and reducing the levels of pathogenesis-related proteins [9].
One of the most important applications of non-Saccharomyces yeast is their use for obtaining wines with distinct aroma profiles. Non-Saccharomyces yeasts are a great source of different exogenous enzymes that may affect the release of various grape-derived compounds during fermentation, including terpenes, norisoprenoids, and thiols that have a positive impact on wine aroma. Their influence is more direct through the production of odoriferous fermentation aroma compounds, such as higher alcohols, fatty acids, and esters, in quantities and ratios which often differ among the species and from those obtained by S. cerevisiae [7].
Non-Saccharomyces yeasts affect the wine’s volatile composition in a species/strain-specific manner. For example, P. kluyveri and M. pulcherrima [10] decreased, while T. delbrueckii [11,12,13] and L. thermotolerans [13] increased the concentration of 2-phenylethanol in sequential or co-inoculation compared to pure S. cerevisiae fermentation. Although non-Saccharomyces species may have a positive impact on wine profile, there are also studies reporting the opposite, so the results were in many cases contrasting, even for the same yeast species. Pichia kluyveri was shown to be able to both reduce [10,14] and increase [15] the concentration of hexanol in co-fermentation with S. cerevisiae. Similar was observed for T. delbrueckii, causing either a decrease [11,12] or an increase [13,16] in isoamyl acetate concentration. In mixed cultures, non-Saccharomyces yeasts can modulate wine aroma by their own activity but also by changing the genomic expression of S. cerevisiae while coexisting during wine fermentation [17]. Multiple factors, such as the genetic predispositions of a particular non-Saccharomyces yeast, the availability of yeast nutrients, and the general composition of grape must certainly play a significant role in determining the outcomes of such fermentations. Considering such interactions and the fact that most previous studies investigated the influence of co-fermentation of non-Saccharomyces and S. cerevisiae yeasts based on the aroma of the final wine, the actual aromatic potential of non-Saccharomyces yeasts in co-fermentations was often not distinguishable.
On the other hand, studies that investigated volatile profiles of pure culture fermentations of various non-Saccharomyces yeasts were mainly conducted at the end of monoculture fermentations [4,18,19,20]. However, this is not representative of their oenological application, as they are exclusively used in mixed cultures with S. cerevisiae. Indeed, the production of volatile compounds during fermentation fluctuates [21], and the final volatile profiles of non-Saccharomyces monocultures do not necessarily reflect their status at earlier fermentation stages (e.g., when S. cerevisiae is added). To our knowledge, only one study compared early fermentation volatile profiles of inoculated non-Saccharomyces yeasts, highlighting the unique behaviour of each yeast [22], which warrants further research.
The main premise of this study was that in the initial phase of alcoholic fermentation, the species-specific effects of non-Saccharomyces yeasts on the grape must’s volatile profile would be more distinguishable than in the finished wines produced by mixed fermentation with S. cerevisiae. Therefore, the aim was to investigate the production of the volatile aroma compounds by five commercially available non-Saccharomyces yeasts in the early stage of fermentation, before the inoculation of S. cerevisiae. In this manner, their effect would be more distinguishable compared to finished wines produced by mixed fermentations with S. cerevisiae and their monoculture fermentations.
2. Materials and Methods
2.1. Vinification
For this experiment, the grapes of Malvazija istarska (Vitis vinifera L.), the most spread and important native white grape cultivar in Croatia, were handpicked from the experimental vineyard of the Institute for Agriculture and Tourism in Poreč situated in the region of Istria, Croatia. Before grape processing, the equipment was cleaned with caustic soda solution and washed off, and then sanitized with an aqueous solution of potassium metabisulfite and citric acid and washed off again. The tanks were additionally washed with 70% ethanol. All the equipment was carefully and thoroughly washed off with hot water before use. The grapes were destemmed, crushed, and pressed immediately after harvest using a closed-type pneumatic press of 500 L capacity with the pressures of 2 × 0.5 bar and 1 × 0.8 bar (Letina Inox d.o.o., Čakovec, Croatia). The obtained juice was sulfited and cold-settled with the aid of Endozym Rapid pectolytic enzymes at 2 g/hL (AEB s.p.a. Brescia, Italy) for 48 h at 10 °C. The grape must had total acidity of 4.7 g/L, pH of 3.41 and 22.1 Brix˚. The total acidity was adjusted by adding 1.3 g/L of tartaric acid to obtain the concentration of 6 g/L; after the addition, the pH was set to 3.27. The must was distributed in 80 L stainless steel tanks and inoculated with yeast to start the fermentation. All fermentations were performed at 17 °C in triplicates. Diammonium phosphate (Corimpex Service Srl, Romans d’Isonzo, Italy) was added at 30 g/hL 36 h after inoculation. The concentration of sugars was monitored daily by a portable density meter DMA 35 (Anton Paar, Graz, Austria). After measuring the sugar concentration, the alcohol content was estimated based on the conversion table by Ribéreau-Gayon et al. [23]. When the alcohol level reached approximately 1.5–2%, fermented samples were collected for analysis.
2.2. Preparation of the Yeasts
Five non-Saccharomyces yeasts were used in this experiment: T. delbrueckii (BIODIVA®), M. pulcherrima (FLAVIA®), and L. thermotolerans (LAKTIA®) were purchased from Lallemand Inc. (Montreal, Canada), P. kluyveri (Frootzen®) was purchased from CHR Hansen (Hoersholm, Denmark) and S. pombe (Atecrem 12H®) was purchased from BioEnologia 2.0 (Oderzo, Italy). Saccharomyces cerevisiae var. bayanus (Lalvin EC1118®) purchased from Lallemand Inc. was used as a control.
Torulaspora delbrueckii, M. pulcherrima, L. thermotolerans, and S. cerevisiae were rehydrated according to the manufacturers’ protocols, while S. pombe in cream form and P. kluyveri frozen at −45 °C were added directly to the must. Torulaspora delbrueckii, M. pulcherrima, L. thermotolerans, S. pombe, and S. cerevisiae yeasts were added in the amounts recommended by the producers, which corresponded to the cell density of approximately 4–5 × 106 cells/mL. The cell density of P. kluyveri recommended by the producer is much lower, 1 × 105 cells/mL, but in this work, approximately 1 × 106 cells/mL were inoculated to keep a similar order of magnitude as for other yeasts.
2.3. Analysis of Volatile Aroma Compounds by Headspace Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry
Volatile aroma compounds were extracted from grape must by headspace solid-phase microextraction (HS-SPME) by the modified method proposed by Bubola et al. [24]. Prior to analysis, 7 µL of 5% sulfurous acid (Agrolit, Litija, Slovenia) and 50 µL of sodium azide (VWR BDH Prolabo, Radnor, SAD) were added to inhibit oxidation and microbial activity, respectively, and samples were centrifuged at 4000 rpm at 4 °C for 5 min using a laboratory centrifuge Universal 320 R (Hettich, Westphalia, Germany). Half a milliliter of the supernatant was placed in a 10 mL glass vial containing 3.45 mL of deionized water. A gram of ammonium sulphate and 50 μL of internal standards solution (2-octanol at 0.84 mg/L, 1-nonanol at 0.82 mg/L, and heptanoic acid at 2.57 mg/L) were added. The samples were incubated for 15 min under stirring at 800 rpm, and the extraction using a divinylbenzene/Carboxen/polydimethylsiloxane (DVB/CAR/PDMS; StableFlex, 50/30 μm, 1 cm; Supelco, Bellafonte, PA, USA) fiber took place for 40 min at 40 °C. When the extraction finished, the fiber was inserted into the GC/MS injector port at 248 °C for 10 min, with the first 3 min in splitless mode.
For the identification and quantification of volatile aroma compounds a Varian 3900 gas chromatograph (GC) coupled to a Varian Saturn 2100T ion trap mass spectrometer (MS) (Varian Inc., Harbour City, CA, USA) was used. The GC-MS was equipped with an Rtx-WAX capillary column of the following dimensions: 60 m × 0.25 mm i.d. × 0.25 μm d.f. (Restek, Belafonte, PA, USA). The initial column temperature was 40 °C, then it was increased at 2 °C/min to 240 °C, and it remained at this temperature for the next 10 min. The carrier gas was helium with a 1.2 mL/min flow rate. Electron ionization mode (EI, 70 eV) in the range of 20–350 m/z was used to acquire mass spectra.
A comparison of retention times and mass spectra with those of the pure standards and with those available in the NIST05 library was used for identification. Spectra reverse match numbers RM > 800 were considered satisfactory. In the cases of RM < 800, the identification was based on the similarity of the intensities of a quantifier ion and other major ions in the spectra to those in the reference spectra. A solution containing C10 to C28 n-alkanes was injected under the same chromatographic conditions, the linear retention indices were calculated, and the identity of volatile compounds was additionally confirmed by comparison with the retention indices reported in the literature. Standard solutions were also injected, and the calibration curves were constructed with r2 > 0.99 in all cases. Internal standards were used for normalization before quantification by using calibration curves. The compounds present in high concentrations were quantified based on total ion current peak area, while quantifier ions were used to quantify others. Method validation results were previously published in the study of Bubola et al. [24]. Compounds for which the authentic standards were not available were semi-quantified as equivalents of the corresponding internal standards. A response factor equal to one was used.
2.4. Statistical Data Elaboration
One-way analysis of variance (ANOVA) and the Least Significant Difference (LSD) test (p < 0.05) were used to determine statistically significant differences between the treatments. After normalization, forward stepwise linear discriminant analysis (SLDA) and hierarchical clustering analysis (HCA) were applied to 40 volatile compounds with the highest Fisher ratio values (F-ratios) obtained by ANOVA. Wilk’s lambda was used as a selection criterion in SLDA, with F-value to enter = 1 and F-value to remove = 0.5. Statistica v. 13.2 software (StatSoft Inc., Tulsa, OK, USA) was used for ANOVA and SLDA. MetaboAnalyst v. 5.0 [25] was used for generating box plots and performing HCA using the Ward algorithm and Euclidean distance analysis.
3. Results and Discussion
3.1. Fermentation Dynamics
The dynamics of the production of ethanol in the early fermentation phase after inoculation with the six investigated yeast species are shown in Figure 1. Sacharomyces cerevisiae was the first to reach and even exceed the target ethanol level in three days. Metschnikowia pulcherrima, P. kluyveri, L. thermotolerans, and S. pombe exhibited similar patterns with a relatively slow start during the first three days, followed by accelerated fermentation/ethanol production on day four, when they were sampled. Interestingly, T. delbrueckii followed a different course, starting more intensively than the other non-Saccharomyces yeasts, but keeping approximately the same pace until the fourth day. Since it was practically impossible to sample the ferments at exactly the same point of fermentation, the ethanol levels produced slightly differed among the investigated yeasts, which possibly had a small impact on the concentrations of volatile aroma compounds released and produced in this phase.
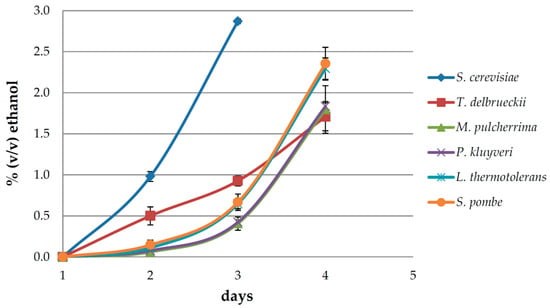
Figure 1.
Ethanol production over time in early fermentation of Malvazija istarska (Vitis vinifera L.) white grape must inoculated with Saccharomyces cerevisiae and five non-Saccharomyces yeasts: Torulaspora delbrueckii, Metschnikowia pulcherrima, Pichia kluyveri, Lachancea thermotolerans, and Schizosaccharomyces pombe.
3.2. Volatile Aroma Compounds
The concentrations of volatile compounds produced in the early phase of fermentation Malvazija istarska grape must by various non-Saccharomyces yeasts, and a S. cerevisiae monoculture control are reported in Table 1. A total of 80 compounds were identified, including 11 terpenes, five C13-norisoprenoids, nine alcohols, six acids, 40 esters, and nine miscellaneous compounds. Statistically significant differences between various yeasts were found for the majority of compounds.

Table 1.
Concentrations (μg/L)* of volatile aroma compounds identified in the early phase of fermentation of the Malvazija istarska grape must inoculated by Saccharomyces cerevisiae and five non-Saccharomyces yeasts obtained by headspace solid-phase microextraction and gas chromatography-mass spectrometry.
3.2.1. Terpenes
Terpenes originate from grapes and are considered among the leading carriers of varietal aroma, especially in wines containing higher concentrations, where they play a significant role in determining varietal typicity. Monoterpenols, such as geraniol, citronellol, nerol, ho-trienol, and especially linalool, are the most relevant terpenes in wine in sensory terms, imparting positive floral and fruity notes [26]. Several previous reports emphasized a significant contribution of linalool to Malvazija istarska varietal aroma, with concentrations repeatedly higher than its odor detection threshold [27]. Although it is generally considered that terpenes are less influenced by fermentation parameters, the enzymatic activity of yeast can influence their behaviour during fermentation and their composition in the final wine, principally by affecting the release of their free volatile forms from the corresponding glycosides [28]. Moreover, terpenes undergo numerous interconversions during fermentation, which can also be affected by the yeast species [29].
Linalool was the only major monoterpenol identified in this study. The highest concentration was found in the must inoculated with P. kluyveri yeast (Table 1, Figure S1). Several studies reported limited or no effects of P. kluyveri in sequential fermentation on the content of total terpenes when compared to pure S. cerevisiae inoculation [10,15], presumably because of its limited β-glucosidase activity [30]. However, the concentrations of particular monoterpenes, such as ho-trienol, were found to be positively affected [10].
The lowest concentration of linalool, although not significantly different from that found in some other treatments, was found in the control S. cerevisiae must, which was in line with the relatively low β-glucosidase activity of this commercial S. cerevisiae (ex-bayanus) strain, as shown earlier [31]. Other yeast species investigated in this study were previously shown to increase the content of certain terpenes in sequential or co-inoculations compared to S. cerevisiae monoculture.
Azzolini et al. [11] recorded an increase in α-terpineol¸ ho-diendiol I, and endiol concentrations after sequential fermentation with T. delbrueckii in red wine, while Čuš and Jenko [32] observed an increase in linalool and a decrease in citronellol and geraniol concentration after a similar experiment with the same yeast. Linalool concentration increased after T. delbrueckii monoculture inoculation in the early phase of fermentation (2–3% v/v ethanol), as reported by Beckner Whitener et al. [22]. In this work, early fermentation T. delbrueckii must contained the lowest concentration of geranyl acetate among all the treatments, as well as a lower concentration of β-pinene and a higher concentration of eucalyptol in relation to S. cerevisiae must (Table 1). Torulaspora delbrueckii was previously highlighted as yeast capable of inducing interconversion reactions between monoterpenes [29], so it is possible that this phenomenon also had an effect in this study.
In previous studies, L. thermotolerans inoculation exhibited a positive effect on the concentrations of nerol and 4-terpineol in monoculture [22], as well as of geraniol, farnesol, and citronellyl acetate in sequential fermentation with S. cerevisiae [33], implying a significant β-glucosidase activity. In this study, L. thermotolerans did not have an effect on terpene concentrations when compared to S. cerevisiae control. Similar was observed for M. pulcherrima (Table 1), contrary to a previous investigation where it positively affected the content of linalool in early fermentation [22].
3.2.2. C13-Norisoprenoids
Like terpenes, C13-norisoprenoids are not products of fermentation. They are formed by degradation of carotenoid precursors whose amounts are mainly predetermined by pedoclimatic and grape growing conditions in a vineyard, as well as pre-fermentation grape processing steps and parameters, such as harvest, transport, crushing, pressing, etc. [34]. However, the degree of liberation of free volatile C13-norisoprenoids during fermentation can also be conditioned by yeast [35]. The most important C13-norisoprenoids in grapes and wine are β-damascenone, due to its extremely low odor detection threshold [26], and, to a lesser degree, β-ionone. Both are considered positive contributors due to pleasant odors they produce, which are reminiscent of stewed apple and violet flowers, respectively.
The highest concentration of β-damascenone was found in P. kluyveri must, although not significantly different than that found in L. thermotolerans and S. pombe inoculated musts (Table 1, Figure S1). Torulaspora delbrueckii and M. pulcherrima early must ferments also contained concentrations higher than that found in S. cerevisiae control, which was in line with previous findings [22]. The lowest concentration of β-damascenone in S. cerevisiae must corroborated the relatively low enzymatic activity of the yeast strain used [31]. S. pombe must contained higher concentrations of other C13-norisoprenoids compared to T. delbrueckii must, with all of them being β-ionone derivatives (Table 1).
3.2.3. C6-Alcohols
C6-alcohols are formed by degradation of lipids, i.e., long-chain fatty acids, in a series of enzymatic reactions during harvest and pre-fermentation grape-processing steps. A fraction is present in grapes and transfers to grape must and wine in both free or glycosidically bound form, where the latter can be cleaved by the action of (yeast) enzymes to release free volatile molecules. Another small portion of a major C6-alcohol 1-hexanol is formed in fermentation. Although these compounds are often mentioned among the possible negative contributors to wine aroma by their herbaceous odors, they rarely have an impact due to relatively high odor perception thresholds [26].
Since, in this study, the same homogenized must was used, the differences in C6-alcohol concentrations were a result of differential yeast activity. The differences observed were marginal but statistically significant for all the identified C6-alcohols. Hexanol was found in higher concentration in T. delbrueckii, M. pulcherrima, and L. thermotolerans than in S. pombe and control S. cerevisiae musts. Similar was observed for cis-3-hexen-1-ol, while T. delbrueckii must was the most abundant in trans-3-hexen-1-ol. The highest concentration of cis-2-hexen-1-ol was found in S. pombe. In previous studies, P. kluyveri was shown to be able to both reduce [10,14] and increase the concentration of hexanol in sequential fermentation compared to S. cerevisiae alone [15].
3.2.4. Higher Alcohols
Wine major higher alcohols are formed in fermentation by yeast either from sugars or from amino acids by the Ehrlich mechanism [17]. They form a basis of wine aroma and, at a low concentration, contribute to its complexity and character, while at total levels above 350 mg/L can have a direct negative impact and also mask other, positive aromas. The importance of higher alcohols is not solely in their contribution to wine aroma as they are also precursors to particular odoriferous volatile esters.
The highest concentration of the major wine higher alcohol, i.e., isoamyl alcohol was detected in the control S. cerevisiae must, although not significantly different compared to the levels found in P. kluyveri and L. thermotolerans inoculated musts (Table 1, Figure S2). It was shown in previous studies that particular non-Saccharomyces yeast, such as P. kluyveri [10] and M. pulcherrima [10,36], produce lower concentrations of higher alcohols in sequential fermentation compared to pure S. cerevisiae inoculation, although opposite results were also published for co-inoculation with M. pulcherrima in red wine [37]. In this work, a lower concentration of isobutanol was recorded in M. pulcherrima and the lowest in T. delbrueckii must in relation to the other musts (Table 1). It was shown previously that the application of different commercial preparations, and therefore different strains of the same non-Saccharomyces yeast, e.g., T. delbrueckii, in sequential fermentation can lead to contrasting outcomes regarding the content and composition of higher alcohols in relation to pure S. cerevisiae inoculation [11,38]. Besides the strain, the availability of yeast nutrients and suppressors in a given grape must matrix, as well as the genetically predetermined regulation system for the selection of nitrogen from various sources, certainly have a large effect [18].
In contrast to other major higher alcohols in wine, 2-phenylethanol is a carrier of a pleasant odor reminiscent of roses. It is mainly a product of alcoholic fermentation, although a smaller part derives from grapes in both free and glycosidically bound form. The concentration found in the control must inoculated with S. cerevisiae almost doubled those found in the musts of non-Saccharomyces starters (Table 1, Figure S2). As in the case of the abovementioned other major fermentation alcohols, previous results regarding the effect of non-Saccharomyces yeasts in sequential or co-inoculation with S. cerevisiae on 2-phenylethanol concentration were also contrasting. While certain authors observed a decrease after the use of P. kluyveri and M. pulcherrima [10], others noted an increase after inoculation with T. delbrueckii [11,12,13] and L. thermotolerans [13].
3.2.5. Volatile Acids
Volatile acids are known to impart mostly undesirable odors, described as vinegary in the case of acetic acid and fatty, cheesy, and rancid in the case of short- and middle-chain volatile fatty acids. The level of the major volatile acid in wine, acetic acid, was higher in P. kluyveri than in T. delbrueckii inoculated must. S. pombe is generally known for generating high levels of acetic acid [39], which was not the case at the early fermentation stage in this study (Table 1). On the other hand, one of the advantages of using T. delbrueckii as a co-starter is the reduction of volatile acidity compared to standard S. cerevisiae monoculture fermentations [12,40]. The results of this investigation partially corresponded to this hypothesis, since the concentrations of the majority of volatile acids, including acetic acid (although without a statistically significant difference), as well as total fatty acids, were lower in T. delbrueckii than in S. cerevisiae inoculated must.
Saccharomyces cerevisiae produced the highest and T. delbrueckii the lowest concentrations of the majority of middle-chain fatty acids. The exception was hexanoic acid, which was the highest in S. pombe must (Table 1, Figure S2). Pichia kluyveri was previously shown not to affect [10] or even reduce the concentrations of volatile fatty acids, especially decanoic acid [15].
3.2.6. Esters
Esters are among the most important contributors to wine aroma with their positive fruity and floral odors. They are mainly formed during alcoholic fermentation by yeast but can also be synthesized by bacteria during malolactic fermentation and via chemically induced esterification during wine aging. Several yeast enzymes are involved in the biosynthesis of esters, and their activity is mainly determined by the expression of corresponding genes [18]. The variability in ester-related enzymatic activities among Saccharomyces yeasts was already described [41] and suggested for non-Saccharomyces yeasts. The final concentration of esters in the wine after alcoholic fermentation is determined by the competing activity of ester-synthesizing enzymes and esterases from yeasts that can synthesize esters but are mostly responsible for their cleavage [42]. It is known that extracellular esterases are present in S. cerevisiae [43], while non-Saccharomyces yeasts still need to be investigated more from this aspect [30]. Another factor that strongly influences the content and composition of esters in wine is the composition of the grape juice matrix, especially the availability of substrates generated in carbon, nitrogen, and fatty acid metabolism [18].
Two main classes of esters are formed in wine: the ethyl esters, which are esters of ethanol and fatty acids, and the acetate esters, which are esters of higher alcohols and acetic acid. The concentration of the main wine ester, ethyl acetate, was higher in S. cerevisiae and T. delbrueckii than in P. kluyveri, S. pombe, and especially M. pulcherrima must, which contained the lowest concentration (Table 1, Figure S3).
The concentration of ethyl propanoate differentiated well the early fermentation profiles of the investigated yeasts. The highest concentration was found in T. delbrueckii, followed by S. cerevisiae, L. thermotolerans, and P. kluyveri, with the lowest concentrations found in M. pulcherrima and S. pombe musts which did not differ among each other (Table 1, Figure S3). Torulaspora delbrueckii was also relatively abundant in ethyl isobutyrate when compared to the other musts. Such results are in accordance with previous studies that observed an increase in these esters in mixed T. delbrueckii/S. cerevisiae fermentations [13,16]. Odd-chain and branched-chain fatty acids, which served as precursors to the mentioned ethyl esters, are not formed from acetyl-CoA through the fatty acid synthase (FAS) complex but from the degradation of threonine and valine, respectively, via the Erlich pathway [21], so the results obtained implied particular differences in amino acid metabolism between the investigated yeast.
Linear even-chain fatty acid ethyl esters are some of the most important positive contributors to wine aroma due to their low perception thresholds and relatively high concentrations. The highest concentration of ethyl butyrate was found in S. pombe, S. cerevisiae, and L. thermotolerans musts, while T. delbrueckii must was the least abundant in this, as well as in middle-chain ethyl esters formed through the FAS complex, such as ethyl hexanoate, ethyl octanoate, and ethyl decanoate (Table 1, Figure S3). At the investigated stage of fermentation, L. thermotolerans and S. pombe starters produced the highest amounts of ethyl hexanoate and together with P. kluyveri were the most abundant in ethyl octanoate, the two among the most important fruity esters [26]. The concentration of ethyl 9-decenoate, another carrier of fruity notes, was higher in L. thermotolerans and S. pombe than in most other musts. Lachancea thermotolerans was previously found to enhance the concentration of ethyl esters in sequential fermentation [10]. It is known that during fermentation the production of linear ethyl esters and other volatile compounds fluctuates, often peaking at the end of the growth phase and decreasing during the stationary phase, sometimes with the second peak corresponding to the start of the decline phase and release of intracellular volatiles after yeast cell autolysis [21]. Various non-Saccharomyces yeasts exhibit diverse performance and dynamics of the production of volatile compounds during fermentation, which is among the probable reasons for the differences observed in this study at the monitored point of fermentation.
Compared to ethyl esters, the concentrations of acetates formed during alcoholic fermentation depend more on yeast enzymatic activity, especially that of acetyltransferases, and less on substrate availability [21]. The highest concentrations of propyl and isobutyl acetate, esters exerting fruity notes, were found in the control treatment inoculated with S. cerevisiae, while M. pulcherrima must contained the lowest amounts (Table 1, Figure S4). In general, the must inoculated with P. kluyveri contained elevated levels of isoamyl and hexyl acetate, two major acetate esters responsible for fruity and flowery notes [26], respectively. Pichia kluyveri must was the most abundant in both 3-hexen-1-yl acetate isomers and also the sole treatment with the ratio of trans to cis form higher than one (Table 1, Figure S4), suggesting a possible activity of particular invertases. Metschnikowia pulcherrima and especially T. delbrueckii starters produced the lowest quantities of the abovementioned fruity acetates. Despite its positive influence on the concentration of particular esters, M. pulcherrima was previously shown to be able to reduce the content of acetates [10]. The results obtained for T. delbrueckii were partly in agreement with previous studies that observed both a decrease [11,12] and an increase [13,16] in isoamyl acetate concentration after inoculation with this yeast in co-fermentation.
The largest difference among the acetates was observed for 2-phenethyl acetate, an ester imparting pleasant floral notes to wine. It was produced in the highest concentration by P. kluyveri, with a two- to three-fold increase relative to the other musts. Such an outcome at this stage corresponded significantly to previous findings showing an increase in the concentration of this ester in finished wines after sequential or mixed inoculation with this non-Saccharomyces yeast [10,15,16], suggesting this effect remains significant throughout fermentation. Schizosaccharomyces pombe fermented must contained the highest concentration of isobornyl acetate.
A number of esters of methanol and higher alcohols with various fatty acids were identified (Table 1, Figure S5). While the odor of methyl hexanoate is generally described as fruity, methyl esters of higher molecular weights are perceived as fatty and waxy. Saccharomyces cerevisiae and especially T. delbrueckii musts contained the lowest concentration of the majority of these esters at this stage of fermentation. Interestingly, T. delbrueckii must, previously found to contain an elevated level of ethyl propanoate, was characterized by the highest concentrations of other esters of propanoic acid, such as isoamyl propanoate (fruity) and 2-phenethyl propanoate (floral), the latter not commonly synthesized by S. cerevisiae (Table 1, Figure S5). The observed highest concentration of 2-phenethyl propanoate was in accordance with a previous investigation reporting high concentration in T. delbrueckii inoculated must [22]. Beckner Whitener et al. [22] found this ester in grape musts fermented by some other non-Saccharomyces yeasts as well, such as M. pulcherrima, L. thermotolerans, and especially Kazachstania gamospora. In this study, M. pulcherrima also produced a notable concentration of 2-phenethyl propanoate, although lower than that found in T. delbrueckii inoculated must, while traces of this ester (m/z 104) were detected in musts of the remaining non-Saccharomyces treatments (Table 1). Both this and the cited study [22] tentatively identified 2-phenethyl propanoate only in the initial stage of fermentation, so it is yet to be confirmed if it remains detectable in the wine after fermentation completion. No such data were found in the literature.
Esters of isoamyl alcohol and isobutanol with middle-chain fatty acids were generally lower in T. delbrueckii and S. cerevisiae treatments (Table 1). As in the case of the majority of minor esters identified, their odor perception thresholds are still unknown, which makes it impossible to assess their potential impact on wine aroma.
3.2.7. Miscellaneous Compounds
The most abundant aldehyde identified was hexanal, a precursor to hexanol and other C6-alcohols imparting ´green´ notes. The highest level was observed in M. pulcherrima, followed by S. cerevisiae must. Metschnikowia pulcherrima and P. kluyveri contained the highest, and T. delbrueckii the lowest level of 3-methylbutanal. Saccharomyces cerevisiae produced the highest benzaldehyde level. Among other miscellaneous compounds, the concentration of dihydro-2-methyl-3(2H)-thiophenone differentiated well the majority of the treatments. It was the highest in T. delbrueckii, followed by L. thermotolerans, then the M. pulcherrima and P. kluyveri musts, which did not differ from each other; even lower concentrations were recorded in S. pombe, while the lowest amount was produced by S. cerevisiae yeast (Table 1, Figure S6). The potential of the majority of compounds from this group to affect wine aroma is still unknown, mostly due to a lack of information about their odor perception thresholds.
3.2.8. Multivariate Statistical Analysis
To better visualize the diversity in volatile aroma compound composition produced by the investigated non-Saccharomyces yeasts in the early phase of grape must fermentation, and to extract the most useful variables for their differentiation, SLDA was applied on mean-centred data of a reduced dataset including 40 variables (volatile compound concentrations) with the highest F-ratios. This SLDA model correctly classified all the grape must samples according to yeast species and extracted the nine most useful variables for their differentiation (Figure 2), with rather high squared Mahalanobis distances from group centroids (numerical data not shown). All the samples (100%) were classified correctly after including only two variables already, ethyl propanoate and 2-phenethyl acetate, while the SLDA model further extracted butyric acid, dihydro-2-methyl-3(2H)-thiophenone, 3-methylbutanal, 3-buten-2-ol, decanoic acid, hexanoic acid, and isoamyl alcohol.
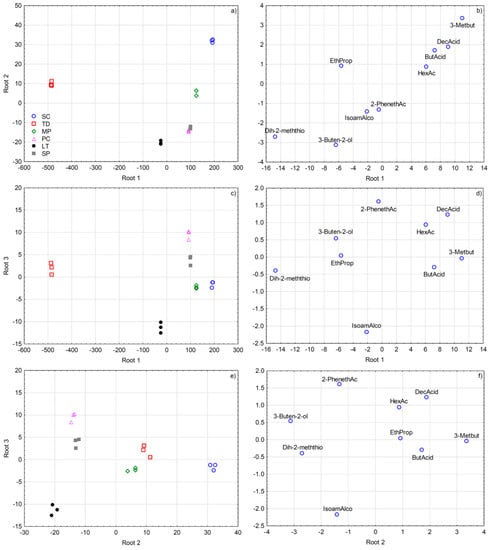
Figure 2.
Separation of Malvazija istarska grape musts in early fermentation according to inoculated yeast species defined by the first three discriminant functions (roots) obtained by forward stepwise discriminant analysis on the basis of volatile aroma compound composition. Projection of grape must samples is shown in sub-figures (a,c,e), while standardized coefficients of the variables (volatile aroma compounds) are shown in sub-figures (b,d,f). Abbreviations: SC—Saccharomyces cerevisiae; TD—Torulaspora delbrueckii; MP—Metschnikowia pulcherrima; PC—Pichia kluyveri; LT—Lachancea thermotolerans; SP—Schizosaccharomyces pombe; Dih-2-meththio—dihydro-2-methyl-3(2H)-thiophenone; EthProp—ethyl propanoate; IsoamAlco—isoamyl alcohol; 2-PhenethAc—2-phenethyl acetate; HexAc—hexyl acetate; ButAcid—butanoic acid; DecAcid—decanoic acid; 3-Metbut—3-methylbutanal.
Hierarchical clustering analysis performed on a reduced dataset including 40 variables (volatile compound concentrations) with the highest F-ratios, confirmed that each yeast species studied produced a distinct volatile profile in the early phase of fermentation (Figure 3). Saccharomyces cerevisiae early ferment was separated from the musts inoculated by non-Saccharomyces yeasts mostly by higher concentrations of propyl acetate, isobutyl acetate, butyric acid, decanoic acid, 2-phenylethanol, and benzaldehyde, and the lowest concentrations of compounds such as β-damascenone and dihydro-2-methyl-3(2H)-thiophenone. Saccharomyces cerevisiae grape must was also characterized by lower concentrations of particular esters compared to the musts inoculated with non-Saccharomyces yeasts except T. delbrueckii, such as methyl octanoate, ethyl dodecanoate, isobutyl octanoate, isoamyl hexanoate, and ethyl 9-decenoate. Torulaspora delbrueckii must contain the lowest concentrations of a large array of compounds and, at the same time, was distinguished by the highest concentrations of ethyl propanoate, 2-phenethyl propanoate, dihydro-2-methyl-3(2H)-thiophenone, and trans-3-hexen-1-ol, which resulted in the largest distance from the other species. Pichia kluyveri stood out with increased levels of linalool, 3-buten-2-ol, β-damascenone and a whole range of acetate esters. The highest levels of β-pinene, hexanoic acid, and ethyl hexanoate were characteristic of the S. pombe early ferment. Although evidently different and distant from each other, grape musts inoculated with S. cerevisiae and S. pombe were distinguished from the others by high octanoic acid and ethyl butyrate, and low trans-2-hexen-1-ol, 1-hexanol, and dihydro-2-methyl-3(2H)-thiophenone concentrations.
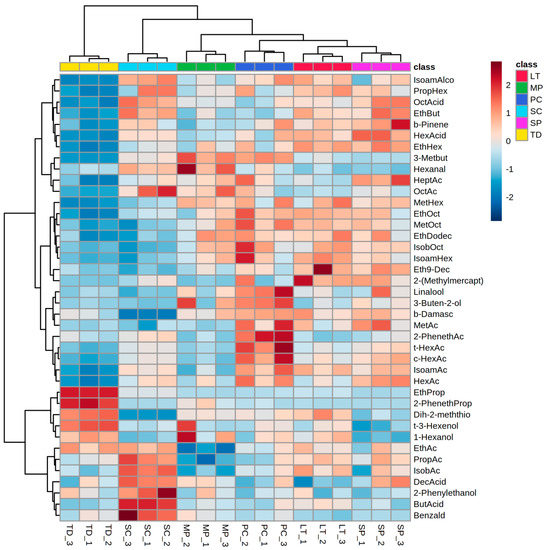
Figure 3.
Hierarchical clustering analysis performed using volatile aroma compound composition of Malvazija istarska grape must in early fermentation by Saccharomyces cerevisiae and various non-Saccharomyces yeasts. The rows in the heatmap represent compounds, and the columns indicate samples. Colours of heatmap cells indicate low (dark blue), medium (white), and high (dark red) abundance of a particular compound. Abbreviations: IsoamAlco—isoamyl alcohol; PropHex—propyl hexanoate; OctAcid—octanoic acid; EthBut—ethyl butyrate; b-Pinene—β-pinene; HexAcid—hexanoic acid; EthHex—ethyl hexanoate; 3-Metbut—3-methylbutanal; HeptAc—heptyl acetate; OctAc—octyl acetate; MetHex—methyl hexanoate; EthOct—ethyl octanoate; MetOct—methyl octanoate; EthDodec—Ethyl dodecanoate; IsobOct—Isobutyl octanoate; IsoamHex—isoamyl hexanoate; Eth9-Dec—ethyl 9-decenoate; 2-(Methylmercapt)—2-(methylmercapto)benzothiazole; b-Damasc—β-damascenone; MetAc—methyl acetate; 2-PhenethAc—2-phenethyl acetate; t-HexAc—trans-hexen-1-yl acetate; c-HexAc—cis-hexen-1-yl acetate; IsoamAc—isoamyl acetate; HexAc—hexyl acetate; EthProp—ethyl propanoate; 2-PhenethProp—2-phenethyl propanoate; Dih-2-meththio—dihydro-2-methyl-3(2H)-thiophenone; t-3-Hexenol—trans-3-hexen-1-ol; EthAc—ethyl acetate; PropAc—propyl acetate; LT—Lachancea thermotolerans; MP—Metschnikowia pulcherrima; PC—Pichia kluyveri; SC—Saccharomyces cerevisiae; SP—Schizosaccharomyces pombe; TD—Torulaspora delbrueckii.
4. Conclusions
The results of this study showed that the studied non-Saccharomyces yeasts produce diverse volatile aroma profiles in the early phase of monoculture fermentation, significantly different from each other and from that produced by S. cerevisiae. Many of the investigated non-Saccharomyces yeasts exhibited undoubtedly positive characteristics in this phase of fermentation in quantitative terms, such as increases in linalool and β-damascenone concentrations, lower production of higher alcohols, and improved synthesis of many major and minor odoriferous esters.
Regarding particular volatile compounds, T. delbrueckii produced the highest levels of trans-3-hexen-1-ol, ethyl propanoate, ethyl isobutyrate, isoamyl propanoate, 2-phenethyl propanoate, and dihydro-2-methyl-3(2H)-thiophenone, P. kluyveri excelled in the production of acetates, particularly cis-3-hexen-1-yl acetate, trans-3-hexen-1-yl acetate, and 2-phenethyl acetate, while the highest levels of cis-2-hexen-1-ol and hexanoic acid were found in must inoculated with S. pombe. The Saccharomyces cerevisiae control must contained the highest concentrations of 2-phenylethanol, butyric acid, propyl acetate, and isobutyl acetate. Particular yeasts, such as T. delbrueckii and M. pulcherrima synthesized esters not commonly found in S. cerevisiae fermented wines, such as 2-phenethyl propanoate. However, it is yet to be established if and to what degree these contributions persist after sequential inoculation with S. cerevisiae, which certainly varies depending on the biocompatibility of the co-fermenting yeasts in diverse grape must matrices. Our group is currently working on this topic.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods11193088/s1, Figure S1: Concentrations of selected monoterpenes and C13–norisoprenoids with high differentiating ability among yeasts (high F-ratios obtained by one-way ANOVA) identified in the early phase of fermentation of Malvazija istarska grape must inoculated by Saccharomyces cerevisiae and five non-Saccharomyces yeasts. Abbreviations: SC—Saccharomyces cerevisiae; TD—Torulaspora delbrueckii; MP—Metschnikowia pulcherrima; PC—Pichia kluyveri; LT—Lachancea thermotolerans; SP—Schizosaccharomyces pombe; Figure S2: Concentrations of selected alcohols and acids with high differentiating ability among yeasts (high F-ratios obtained by one-way ANOVA) identified in the early phase of fermentation of Malvazija istarska grape must inoculated by Saccharomyces cerevisiae and five non-Saccharomyces yeasts. Abbreviations: SC—Saccharomyces cerevisiae; TD—Torulaspora delbrueckii; MP—Metschnikowia pulcherrima; PC—Pichia kluyveri; LT—Lachancea thermotolerans; SP—Schizosaccharomyces pombe; Figure S3: Concentrations of ethyl esters with high differentiating ability among yeasts (high F-ratios obtained by one-way ANOVA) identified in the early phase of fermentation of Malvazija istarska grape must inoculated by Saccharomyces cerevisiae and five non-Saccharomyces yeasts. Abbreviations: SC—Saccharomyces cerevisiae; TD—Torulaspora delbrueckii; MP—Metschnikowia pulcherrima; PC—Pichia kluyveri; LT—Lachancea thermotolerans; SP—Schizosaccharomyces pombe; Figure S4: Concentrations of acetate esters with high differentiating ability among yeasts (high F-ratios obtained by one-way ANOVA) identified in the early phase of fermentation of Malvazija istarska grape must inoculated by Saccharomyces cerevisiae and five non-Saccharomyces yeasts. Abbreviations: SC—Saccharomyces cerevisiae; TD—Torulaspora delbrueckii; MP—Metschnikowia pulcherrima; PC—Pichia kluyveri; LT—Lachancea thermotolerans; SP—Schizosaccharomyces pombe; Figure S5: Concentrations of other esters with high differentiating ability among yeasts (high F-ratios obtained by one-way ANOVA) identified in the early phase of fermentation of Malvazija istarska grape must inoculated by Saccharomyces cerevisiae and five non-Saccharomyces yeasts. Abbreviations: SC—Saccharomyces cerevisiae; TD—Torulaspora delbrueckii; MP—Metschnikowia pulcherrima; PC—Pichia kluyveri; LT—Lachancea thermotolerans; SP—Schizosaccharomyces pombe; Figure S6: Concentrations of miscellaneous volatile compounds with high differentiating ability among yeasts (high F-ratios) identified in the early phase of fermentation of Malvazija istarska grape must inoculated by Saccharomyces cerevisiae and five non-Saccharomyces yeasts. Abbreviations: SC—Saccharomyces cerevisiae; TD—Torulaspora delbrueckii; MP—Metschnikowia pulcherrima; PC—Pichia kluyveri; LT—Lachancea thermotolerans; SP—Schizosaccharomyces pombe.
Author Contributions
D.D.S.: formal analysis, investigation, visualization, writing—original draft. I.H.: formal analysis, investigation. A.H.: methodology, writing—review and editing. T.P.: investigation. S.R.: investigation. I.P.: data curation. I.L.: conceptualization, methodology, validation, data curation, visualization, resources, writing—review and editing, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Croatian Science Foundation under the projects IP-2020-02-4551 and DOK-2021-02-5500.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zott, K.; Miot-Sertier, C.; Claisse, O.; Lonvaud-Funel, A.; Masneuf-Pomarede, I. Dynamics and diversity of non-Saccharomyces yeasts during the early stages in winemaking. Int. J. Food Microbiol. 2008, 125, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Contribution of Non-Saccharomyces Yeasts to Wine Freshness. Biomolecules 2020, 10, 34. [Google Scholar] [CrossRef]
- Lleixà, J.; Manzano, M.; Mas, A.; Portillo, M.D. Saccharomyces and non-Saccharomyces competition during microvinification under different sugar and nitrogen conditions. Front. Microbiol. 2016, 7, 1959. [Google Scholar] [CrossRef]
- Blanco, P.; Rabuñal, E.; Neira, N.; Castrillo, D. Dynamic of Lachancea thermotolerans population in monoculture and mixed fermentations: Impact on wine characteristics. Beverages 2020, 6, 36. [Google Scholar] [CrossRef]
- Benito, S. The impacts of Schizosaccharomyces on winemaking. Appl. Microbiol. Biotechnol. 2019, 103, 4291–4312. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Palomero, F.; González, C.; Suárez-Lepe, J.A. Schizosaccharomyces pombe: A Promising Biotechnology for Modulating Wine Composition. Fermentation 2018, 4, 70. [Google Scholar] [CrossRef]
- Vejarano, R.; Gil-Calderón, A. Commercially available non-Saccharomyces yeasts for winemaking: Current Market, Advantages over Saccharomyces, Biocompatibility and safety. Fermentation 2021, 7, 171. [Google Scholar] [CrossRef]
- Windholtz, S.; Redon, P.; Lacampagne, S.; Farris, L.; Lytra, G.; Cameleyre, M.; Barbe, J.C.; Coulon, J.; Thibon, J.; Masneuf-Pomarède, I. Non-Saccharomyces yeasts as bioprotection in the composition of red wine and in the reduction of sulfur dioxide. LWT—Food Sci. Technol. 2021, 149, 111781. [Google Scholar] [CrossRef]
- Jolly, N.; Pretorius, I.S.; Varela, C. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2013, 14, 215–237. [Google Scholar] [CrossRef]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Azzolini, M.; Fedrizzi, B.; Tosi, E.; Finato, F.; Vagnoli, P.; Scrinzi, C.; Zapparoli, G. Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae mixed cultures on fermentation and aroma of Amarone wine. Eur. Food Res. Technol. 2012, 235, 303–313. [Google Scholar] [CrossRef]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2015, 31, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Hranilovic, A.; Li, S.; Boss, P.K.; Bindon, K.; Ristic, R.; Grbin, P.R.; Van der Westhuizen, T.; Jiranek, V. Chemical and sensory profiling of Shiraz wines co-fermented with commercial non-Saccharomyces inocula. Aust. J. Grape Wine Res. 2018, 24, 166–180. [Google Scholar] [CrossRef]
- Beckner Whitener, M.E.; Stanstrup, J.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Effect of non-Saccharomyces yeasts on the volatile chemical profile of Shiraz wine. Aust. J. Grape Wine Res. 2017, 23, 179–192. [Google Scholar] [CrossRef]
- Dutraive, O.; Benito, S.; Fritsch, S.; Beisert, B.; Patz, C.-D.; Rauhut, D. Effect of Sequential inoculation with non-Saccharomyces and Saccharomyces yeasts on Riesling wine chemical composition. Fermentation 2019, 5, 79. [Google Scholar] [CrossRef]
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.C.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48. [Google Scholar] [CrossRef]
- Barbosa, C.; Mendes-Faia, A.; Lage, P.; Mira, N.P.; Mendes-Ferreira, A. Genomic expression program of Saccharomyces cerevisiae along a mixed-culture wine fermentation with Hanseniaspora guilliermondii. Microb. Cell Factories 2015, 14, 124. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial Contribution to Wine Aroma and Its Intended Use for Wine Quality Improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulospora delbrueckii in the brewing process: A new approach to enhance bioflavour and to reduce ethanol content. Food Microbiol. 2015, 56, 45–51. [Google Scholar] [CrossRef]
- Cioch-Skoneczny, M.; Grabowski, M.; Satora, P.; Skoneczny, S.; Klimczak, K. The use of yeast mixed cultures for deacidification and improvement of the composition of cold climate grape wines. Molecules 2021, 26, 2628. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Beckner Whitener, M.E.; Carlin, S.; Jacobson, D.; Weighill, D.; Divol, B.; Conterno, L.; Du Toit, M.; Vrhovsek, U. Early fermentation volatile metabolite profile of non-Saccharomyces yeasts in red and white grape must: A targeted approach. LWT—Food Sci. Technol. 2015, 64, 412–422. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology: The Microbiology of Wine and Vinifications; John Wiley and Sons, Ltd.: Bordeaux, France, 2016. [Google Scholar]
- Bubola, M.; Lukić, I.; Radeka, S.; Sivilotti, P.; Grozic, K.; Vanzo, A.; Bavčar, D.; Lisjak, K. Enhancement of Istrian Malvasia wine aroma and hydroxycinnamate composition by hand and mechanical leaf removal. J. Sci. Food Agric. 2019, 99, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sinelnikov, I.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Lukić, I.; Carlin, S.; Vrhovsek, U. Comprehensive 2D Gas Chromatography with TOF-MS Detection Confirms the Matchless Discriminatory Power of Monoterpenes and Provides In-Depth Volatile Profile Information for Highly Efficient White Wine Varietal Differentiation. Foods 2020, 9, 1787. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, M.; Di Stefano, R.; Briones, A. Hydrolysis and transformation of terpene glycosides from muscat must by different yeast species. Food Microbiol. 2003, 20, 35–41. [Google Scholar] [CrossRef]
- King, A.; Dickinson, J.R. Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. Yeast 2020, 16, 499–506. [Google Scholar] [CrossRef]
- Escribano, R.; González-Arenzana, L.; Garijo, P.; Berlanas, C.; López-Alfaro, I.; López, R.; Gutiérrez, A.R.; Santamaría, P. Screening of enzymatic activities within different enological non-Saccharomyces yeasts. J. Food Sci. Technol. 2017, 54, 1555–1564. [Google Scholar] [CrossRef]
- Bisotto, A.; Julien, A.; Rigou, P.; Schneider, R.; Salmon, J.M. Evaluation of the inherent capacity of commercial yeast strains to release glycosidic aroma precursors from Muscat grape must. Aust. J. Grape Wine Res. 2015, 21, 194–199. [Google Scholar] [CrossRef]
- Čuš, F.; Jenko, M. The influence of yeast strains on the composition and sensory quality of Gewürztraminer wine. Food Technol. Biotechnol. 2013, 51, 547–553. [Google Scholar]
- Beckner Whitener, M.E.; Stanstrup, J.; Panzeri, V.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Untangling the wine metabolome by combining untargeted SPME-GCxGC-TOF-MS and sensory analysis to profile Sauvignon blanc co-fermented with seven different yeasts. Metabolomics 2016, 12, 53. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M. Carotenoid breakdown products—The norisoprenoids—In wine aroma. Arch. Biochem. Biophys. 2009, 483, 236–245. [Google Scholar] [CrossRef]
- Lloyd, N.D.R.; Capone, D.L.; Ugliano, M.; Taylor, D.K.; Skouroumounis, G.K.; Sefton, M.A.; Elsey, G.M. Formation of Damascenone under both Commercial and Model Fermentation Conditions. J. Agric. Food Chem. 2011, 59, 1338–1343. [Google Scholar] [CrossRef]
- Ruiz, J.; Belda, I.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Santos, A.; Benito, S. Analytical impact of Metschnikowia pulcherrima in the volatile profile of Verdejo white wines. Appl. Microbiol. Biotechnol. 2018, 102, 8501–8509. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Barker, A.; Tran, T.; Borneman, A.; Curtin, C. Sensory profile and volatile aroma composition of reduced alcohol Merlot wines fermented with Metschnikowia pulcherrima and Saccharomyces uvarum. Int. Food Microbiol. 2017, 252, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic analysis of physiological properties of Torulospora delbrueckii in wine fermentation and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar] [CrossRef]
- Mylona, A.E.; Del Fresno, J.M.; Palomero, F.; Loira, I.; Bañuelos, M.A.; Morata, A.; Calderón, F.; Benito, S.; Suárez-Lepe, J.A. Use of Schizosaccharomyces strains for wine fermentation-Effect on the wine composition and food safety. Int. Int. J. Food Microbiol. 2016, 232, 63–72. [Google Scholar] [CrossRef] [PubMed]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.M.; Zamora, F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2015, 240, 999–1012. [Google Scholar] [CrossRef]
- Stribny, J.; Querol, A.; Pérez-Torrado, R. Differences in enzymatic properties of the Saccharomyces kudriavzevii and Saccharomyces uvarum alcohol acetyltransferases and their impact on aroma-active compounds production. Front. Microbiol. 2016, 7, 897. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar] [CrossRef]
- Iranzo, J.U.; Perez, A.B.; Canas, P.I. Study of the oenological characteristics and enzymatic activities of wine yeasts. Food Microbiol. 1998, 15, 399–406. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).