Changes in Essential Oil Composition, Polyphenolic Compounds and Antioxidant Capacity of Ajowan (Trachyspermum ammi L.) Populations in Response to Water Deficit
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Irrigation Regimes
2.3. Essential Oil Distillation
2.4. GC–MS Analysis
Identification of Essential Oil Constituents
2.5. Methanolic Extract and Total Phenolic Content
2.6. Total Flavonoid Content (TFC)
2.7. Antioxidant Activity
2.7.1. DPPH Assay
2.7.2. Reducing Power
2.8. HPLC Analysis
2.9. Statistical Analysis
3. Results
3.1. Seed Yield
3.2. Essential Oil Yield
3.3. Essential Oil Composition
3.4. Multivariate Analyses
Principle Component Analysis (PCA) of Essential Oils
| Compounds | α-Thujene | α-Pinene | Sabinene | β-Pinene | Myrcene | α-Terpinene | p-Cymene | γ-Terpinene | cis-Sabinenehydrate | β-Thujone | Terpinene-4-ol | Pulegone | Thymol | Carvacrol | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| * RI a/b | 927/929 | 941/937 | 972/974 | 979/979 | 996/991 | 1017/1017 | 1025/1024 | 1057/1060 | 1068/1070 | 1110/1114 | 1181/1177 | 1246/1237 | 1290/1291 | 1301/1299 | ||
| Stress | Populations | |||||||||||||||
| Normal | Arak | 0.36 | 0.15 | 0.18 | 1.01 | 0.35 | 0.38 | 18.74 | 26.89 | 0 | 0.81 | 0.21 | 0 | 50.16 | 0.65 | 99.89 |
| Arakkho | 0.43 | 0.17 | 0.33 | 1.43 | 0.53 | 0.34 | 17.74 | 26.05 | 0 | 0.61 | 0.17 | 0.29 | 50.99 | 0.58 | 99.66 | |
| Ardebil | 0.44 | 0.12 | 0.31 | 0.41 | 0.58 | 0.34 | 20.14 | 26.96 | 0 | 0.73 | 0.27 | 0.36 | 48.84 | 0.5 | 100 | |
| Esfahfo | 0.31 | 0.11 | 0.21 | 0.66 | 0.47 | 0.19 | 20.32 | 23.15 | 0 | 0.74 | 0.29 | 0.71 | 52.2 | 0.63 | 99.99 | |
| Farsfars | 0.2 | 0 | 0.17 | 0.12 | 0.24 | 0.28 | 20.32 | 21.37 | 0 | 0.45 | 0 | 0 | 40.21 | 0 | 83.36 | |
| Farsmar | 0.39 | 0.11 | 0.26 | 0.13 | 0.5 | 0.28 | 18.61 | 22.62 | 0 | 0.77 | 0.2 | 0.38 | 54.98 | 0.64 | 99.87 | |
| Hamdan | 0.37 | 0.11 | 0.32 | 0.56 | 0.57 | 0.25 | 19.46 | 22.11 | 0.21 | 0.74 | 0.26 | 0.43 | 53.91 | 0.7 | 100 | |
| Khorbir | 0.36 | 0.13 | 0.23 | 0.6 | 0.57 | 0.44 | 18.23 | 26.33 | 0 | 0.74 | 0.41 | 0 | 51.4 | 0.5 | 99.94 | |
| Khormo | 0.18 | 0.09 | 0.14 | 0.9 | 0.3 | 0.12 | 15.09 | 19.46 | 0 | 0.57 | 0.19 | 0 | 61.44 | 0.81 | 99.29 | |
| Qazvin | 0.42 | 0.13 | 0.25 | 0.43 | 0.48 | 0.34 | 18.87 | 22.85 | 0 | 0.77 | 0.31 | 0 | 54.29 | 0.66 | 99.8 | |
| Yazd | 0.31 | 0.1 | 0.19 | 0.19 | 0.41 | 0.22 | 16.96 | 20.32 | 0 | 0.47 | 0.28 | 0.62 | 58.92 | 0.72 | 99.71 | |
| Yazshah | 0.4 | 0.12 | 0.3 | 0.37 | 0.55 | 0.19 | 19.08 | 23.02 | 0 | 0.75 | 0.31 | 0.56 | 53.65 | 0.61 | 99.91 | |
| Medium | Arak | 0.68 | 0.21 | 0.35 | 1.2 | 0.48 | 0.54 | 24.64 | 26.19 | 0.17 | 0.71 | 0.17 | 0.1 | 43.91 | 0.44 | 99.79 |
| Arakkho | 0.68 | 0.24 | 0.43 | 1.66 | 0.71 | 0.62 | 21.19 | 29.53 | 0.2 | 0.73 | 0.1 | 0.1 | 42.65 | 0.71 | 99.55 | |
| Ardebil | 0.51 | 0.13 | 0.27 | 0.53 | 0.45 | 0.41 | 24.36 | 31.63 | 0 | 0.57 | 0 | 0 | 40.88 | 0.26 | 100 | |
| Esfahfo | 0.32 | 0.1 | 0.21 | 0.69 | 0.38 | 0.4 | 17.97 | 30.81 | 0 | 0.46 | 0.12 | 0 | 48.2 | 0.35 | 100 | |
| Farsfars | 0.39 | 0 | 0.24 | 0.16 | 0.36 | 0.36 | 21.44 | 27.77 | 0 | 0.51 | 0 | 0 | 48.78 | 0 | 100 | |
| Farsmar | 0.55 | 0.14 | 0.31 | 0.35 | 0.59 | 0.53 | 21.09 | 26.04 | 0.19 | 0.62 | 0.13 | 0.1 | 48.6 | 0.49 | 99.73 | |
| Hamdan | 0.57 | 0.17 | 0.35 | 0.94 | 0.64 | 0.55 | 20.31 | 28.87 | 0.19 | 0.6 | 0.15 | 0.1 | 45.62 | 0.62 | 99.68 | |
| Khorbir | 0.75 | 0.22 | 0.42 | 1.26 | 0.69 | 0.61 | 23.18 | 27.09 | 0.26 | 0.73 | 0.15 | 0.07 | 43.5 | 0.82 | 99.75 | |
| Khormo | 0.48 | 0.18 | 0.31 | 1.58 | 0.57 | 0.53 | 17.3 | 26.76 | 0.24 | 0.56 | 0.15 | 0.15 | 50.35 | 0.53 | 99.69 | |
| Qazvin | 0.5 | 0.14 | 0.35 | 0.64 | 0.55 | 0.54 | 0.55 | 28.13 | 0.23 | 0.58 | 0.09 | 0 | 46.00 | 1.07 | 79.37 | |
| Yazd | 0.53 | 0.13 | 0.34 | 0.44 | 0.64 | 0.61 | 19.21 | 28.49 | 0.22 | 0.62 | 0.17 | 0.14 | 47.07 | 0.9 | 99.51 | |
| Yazshah | 0.56 | 0.15 | 0.3 | 0.72 | 0.55 | 0.52 | 21.22 | 29.98 | 0.21 | 0.57 | 0.16 | 0 | 45.06 | 0 | 100 | |
| Severe | Arak | 0.63 | 0.17 | 0.42 | 0.53 | 0 | 0.67 | 20.25 | 29.74 | 0.19 | 0.67 | 0.23 | 0.12 | 44.49 | 0.65 | 98.76 |
| Arakkho | 0.57 | 0.22 | 0.26 | 1.81 | 0.54 | 0.74 | 24.98 | 25.65 | 0.09 | 1.29 | 0.17 | 0 | 41.05 | 0.91 | 98.28 | |
| Ardebil | 0.54 | 0.13 | 0.29 | 0.45 | 0.56 | 0.52 | 23.12 | 26.95 | 0.19 | 0.62 | 0.27 | 0 | 45.49 | 0.61 | 99.74 | |
| Esfahfo | 0.51 | 0.13 | 0.26 | 0.5 | 0.52 | 0.46 | 22.93 | 25.73 | 0.14 | 0.6 | 0.14 | 0 | 47.54 | 0.45 | 99.91 | |
| Farsfars | 0.67 | 0.16 | 0.41 | 0.28 | 0.75 | 0.61 | 21.74 | 27.89 | 0.27 | 0.7 | 0.27 | 0.15 | 45.08 | 0.6 | 99.58 | |
| Farsmar | 0 | 0 | 0 | 0 | 0 | 0 | 5.35 | 5.64 | 0 | 0 | 0 | 0 | 89.01 | 0 | 100 | |
| Hamdan | 0.82 | 0.2 | 0.47 | 0.33 | 0.79 | 0.64 | 25.9 | 30.51 | 0.15 | 0.83 | 0.19 | 0.12 | 37.54 | 1.09 | 99.58 | |
| Khorbir | 0.2 | 0.05 | 0.14 | 0.4 | 0.21 | 0.19 | 13.98 | 17.16 | 0.26 | 0.33 | 0.25 | 0.29 | 64.76 | 1.39 | 99.61 | |
| Khormo | 1.11 | 0.65 | 0.24 | 0.87 | 0.82 | 0.51 | 16.48 | 17.52 | 0.18 | 3.93 | 0.2 | 0.1 | 41.42 | 0.6 | 84.63 | |
| Qazvin | 0.68 | 0.17 | 0.37 | 0.67 | 0.68 | 0.51 | 23.51 | 26.08 | 0.19 | 0.67 | 0.4 | 0.16 | 44.92 | 0.68 | 99.69 | |
| Yazd | 0.41 | 0.13 | 0.19 | 0.97 | 0.41 | 0.36 | 20.37 | 22.38 | 0.25 | 0.67 | 0.25 | 0.1 | 51.78 | 1.21 | 99.48 | |
| Yazshah | 0.57 | 0.14 | 0.33 | 0.39 | 0.63 | 0.54 | 23.43 | 30.15 | 0 | 0.64 | 0.18 | 0 | 41.24 | 0.67 | 98.91 |


3.5. Antioxidant Activity
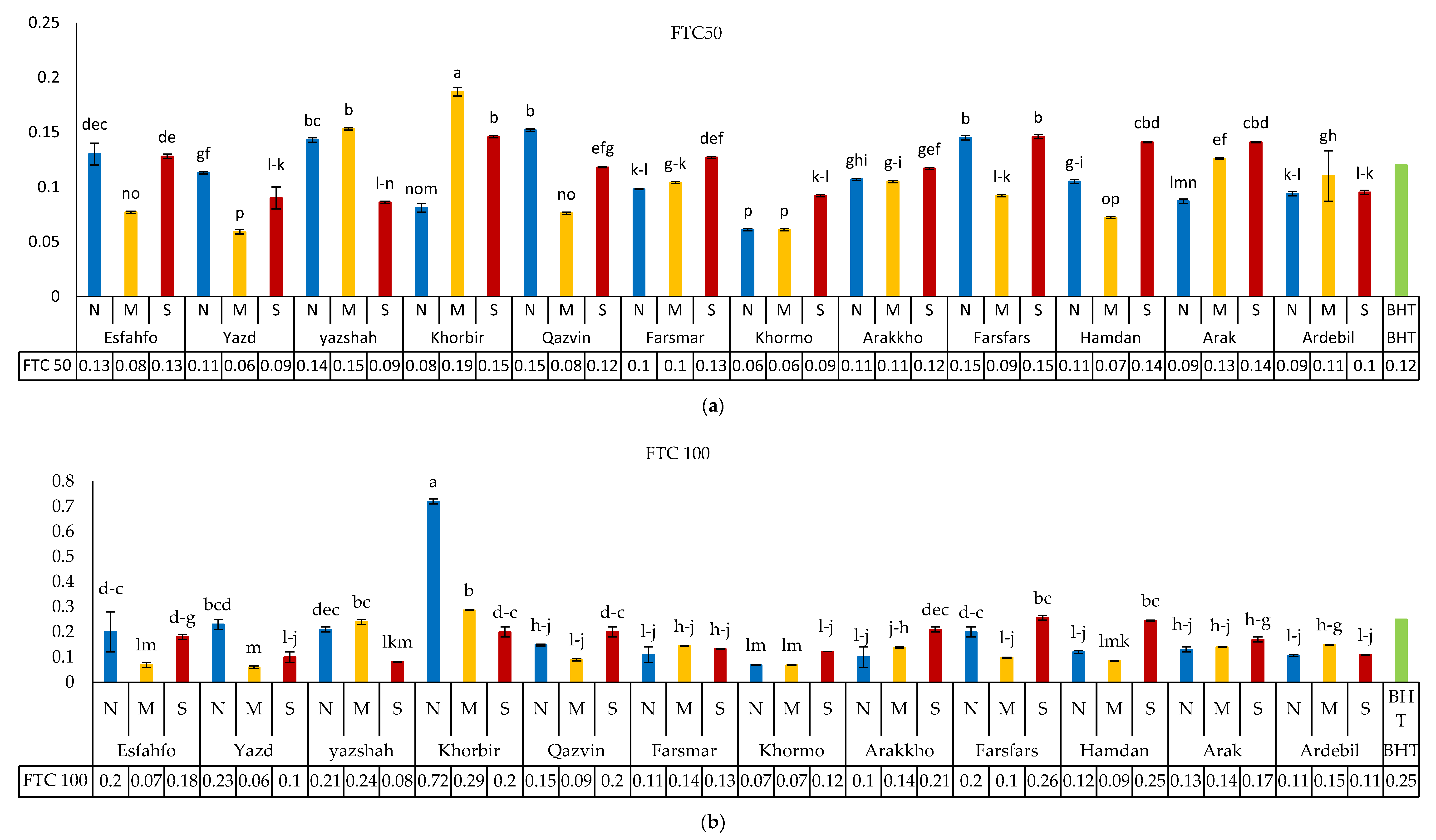
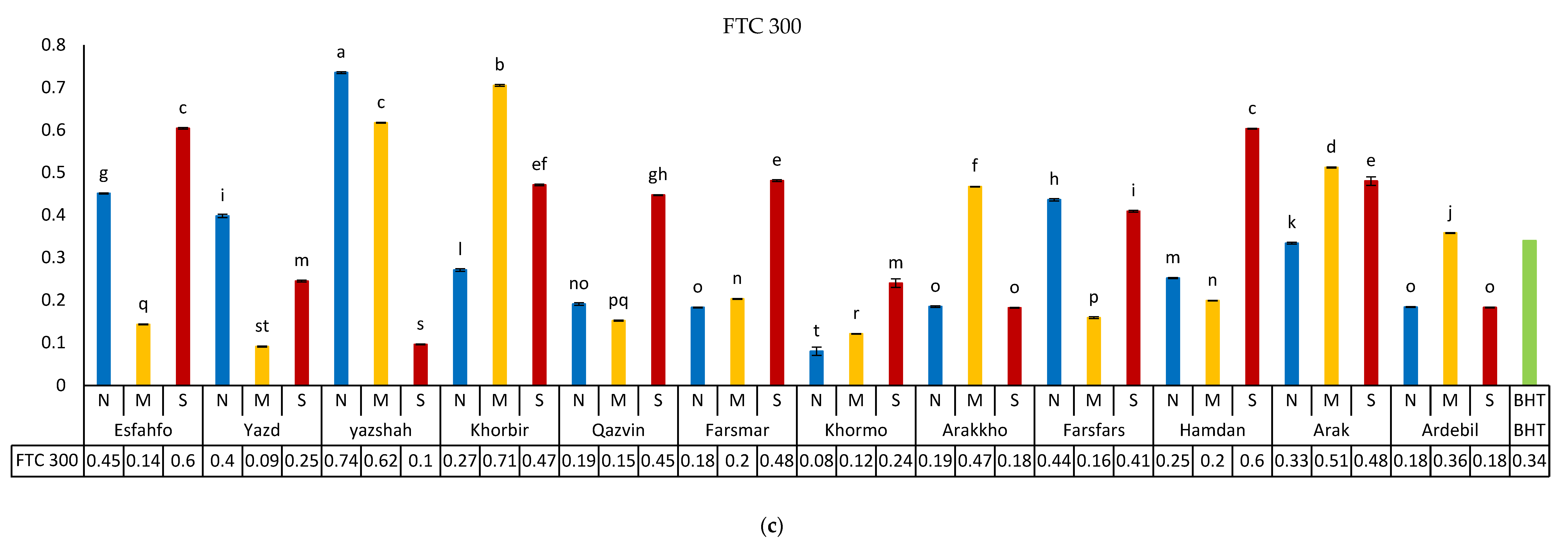
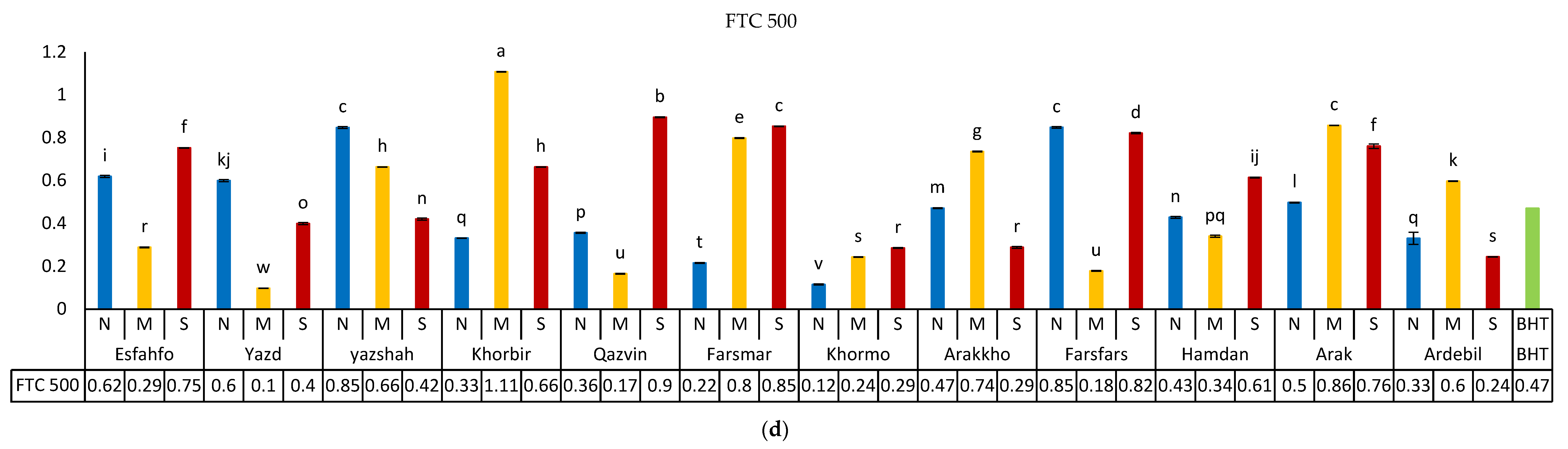
3.5.1. DPPH Method
3.5.2. Fe-Reducing Power
3.6. Total Phenolic (TPC) and Flavonoid Content (TFC)
3.7. HPLC Results
Biplot Analysis of Polyphenolic Compounds
| Standards | Gallic Acid | Chlorogenic Acid | Caffeic Acid | p-Coumaric Acid | Rutin | Ferulic Acid | Rosmarinic Acid | Apigenin | |
|---|---|---|---|---|---|---|---|---|---|
| RT * | 4.692 | 12.315 | 13.13 | 26.548 | 28.927 | 29.375 | 36.257 | 56.356 | |
| Stress | Populations | ||||||||
| Normal | Arak | 12.850 ± 0.2 j | 47.355 ± 1.6 a | 14.446 ± 0.8 qr | 18.722 ± 0.6 i | 14.949 ± 0.4 k | 20.481 ± 0.4 n | 2.996 ± 0.2 hij | 8.775 ± 0.2 ghi |
| Arakkho | 8.544 ± 0.4 t | 8.499 ± 0.4 x | 13.246 ± 0.4 w | 16.816 ± 0.4 mn | 13.477 ± 0.3 mn | 16.525 ± 0.4 t | 3.721 ± 0.3 de | 8.412 ± 0.3 klm | |
| Ardebil | 16.103 ± 0.4 d | 12.623 ± 0.4 q | 18.877 ± 0.5 i | 16.047 ± 0.4 p | 12.724 ± 0.4 r | 13.434 ± 0.5 y | 2.534 ± 0.2 lm | 8.637 ± 0.3 ij | |
| Esfahfo | 13.945 ± 0.3 h | 8.085 ± 0.3 y | 15.288 ± 0.6 no | 16.422 ± 0.2 o | 13.598 ± 0.2 m | 17.043 ± 0.7 s | 2.820 ± 0.2 jk | 8.377 ± 0.2 m | |
| Farsfars | 8.855 ± 0.6 rs | 22.495 ± 0.4 h | 13.635 ± 0.2 uv | 17.958 ± 0.6 jk | 13.120 ± 0.6 op | 11.254 ± 0.6 z | 2.883 ± 0.1 ijk | 8.741 ± 0.6 hi | |
| Farsmar | 6.152 ± 0.4 w | 3.750 ± 0.4 b | 13.977 ± 0.4 t | 15.575 ± 0.5 r | 19.282 ± 0.5 b | 14.602 ± 0.5 x | 2.445 ± 0.2 mno | 9.033 ± 0.3 ef | |
| Hamdan | 11.694 ± 0.5 l | 7.127 ± 0.5 z | 13.569 ± 0.4 v | 15.990 ± 0.5 pq | 11.783 ± 0.8 w | 32.799 ± 0.7 c | 2.224 ± 0.3 pqrs | 8.477 ± 0.4 jklm | |
| Khorbir | 9.876 ± 0.4 p | 9.445 ± 0.3 w | 14.060 ± 0.6 st | 18.197 ± 0.6 j | 16.669 ± 0.6 g | 21.156 ± 0.7 m | 5.134 ± 0.2 b | 8.626 ± 0.3 ijk | |
| Khormo | 9.398 ± 0.5 q | 11.634 ± 0.5 s | 18.419 ± 0.4 j | 14.605 ± 0.5 t | 12.229 ± 0.6 t | 22.381 ± 0.5 k | 2.291 ± 0.2 nopqr | 8.763 ± 0.3 ghi | |
| Qazvin | 14.380 ± 0.3 g | 10.791 ± 0.4 u | 23.947 ± 0.8 d | 17.269 ± 0.7 l | 19.424 ± 0.4 b | 15.769 ± 0.5 u | 3.313 ± 0.3 f | 9.184 ± 0.3 de | |
| Yazd | 11.657 ± 0.4 l | 6.984 ± 0.4 z | 21.173 ± 0.7 f | 17.400 ± 0.3 l | 13.306 ± 0.3 no | 18.333 ± 0.4 qr | 2.196 ± 0.1 qrs | 8.439 ± 0.4 ijklm | |
| Yazshah | 15.417 ± 0.7 e | 36.003 ± 1.4 d | 14.285 ± 0.4 rs | 21.725 ± 0.3 c | 15.939 ± 0.6 i | 26.630 ± 0.9 g | 2.291 ± 0.2 nopqr | 8.383 ± 0.5 lm | |
| Medium | Arak | 8.969 ± 0.3 r | 15.718 ± 0.7 m | 17.183 ± 0.6 l | 14.736 ± 0.7 t | 14.897 ± 0.6 k | 15.044 ± 0.5 w | 3.175 ± 0.2 fgh | 9.289 ± 0.5 cd |
| Arakkho | 11.773 ± 0.7 l | 16.842 ± 0.5 kl | 19.545 ± 0.4 h | 17.811 ± 0.8 k | 12.478 ± 0.3 s | 22.754 ± 0.7 j | 4.539 ± 0.4 c | 8.958 ± 0.5 fgh | |
| Ardebil | 10.366 ± 0.5 o | 11.617 ± 0.5 s | 22.992 ± 0.7 m | 16.710 ± 0.6 n | 15.343 ± 0.5 j | 15.782 ± 0.5 u | 3.242 ± 0.3 fg | 8.424 ± 0.3 jklm | |
| Esfahfo | 12.927 ± 0.4 j | 11.102 ± 0.7 t | 18.260 ± 0.3 j | 16.975 ± 0.6 m | 15.469 ± 0.6 j | 25.144 ± 0.7 h | 3.907 ± 0.3 d | 8.487 ± 0.3 jklm | |
| Farsfars | 13.936 ± 0.5 h | 11.681 ± 0.4 s | 21.276 ± 0.6 f | 15.775 ± 0.5 qr | 18.029 ± 0.6 d | 16.884 ± 0.5 s | 3.094 ± 0.2 ghi | 9.927 ± 0.4 a | |
| Farsmar | 13.284 ± 0.6 i | 37.070 ± 1.4 c | 14.622 ± 0.3 pq | 19.167 ± 0.6 h | 12.149 ± 0.4 tu | 18.164 ± 0.5 r | 2.838 ± 0.1 jk | 9.566 ± 0.5 b | |
| Hamdan | 14.122 ± 0.3 h | 14.883 ± 0.6 n | 15.280 ± 0.6 no | 17.734 ± 0.7 k | 12.505 ± 0.3 s | 22.477 ± 0.7 k | 2.430 ± 0.2 mnop | 8.599 ± 0.5 ijkl | |
| Khorbir | 12.364 ± 0.6 k | 13.275 ± 0.5 p | 14.720 ± 0.7 p | 19.059 ± 0.7 h | 11.931 ± 0.3 uvw | 22.548 ± 0.7 jk | 5.658 ± 0.2 a | 8.461 ± 0.4 jklm | |
| Khormo | 5.366 ± 0.5 x | 6.488 ± 0.4 a | 15.139 ± 0.7 no | 15.179 ± 0.5 s | 12.831 ± 0.4 qr | 15.299 ± 0.4 v | 2.477 ± 0.2 mn | 8.968 ± 0.4 efg | |
| Qazvin | 14.000 ± 0.6 h | 16.705 ± 0.6 l | 30.524 ± 1.4 b | 17.967 ± 0.6 jk | 13.901 ± 0.4 l | 18.444 ± 0.5 q | 5.330 ± 0.3 b | 9.439 ± 0.3 bc | |
| Yazd | 10.629 ± 0.7 n | 12.205 ± 0.4 r | 27.637 ± 0.6 c | 16.822 ± 0.7 mn | 14.948 ± 0.6 k | 21.178 ± 0.7 m | 3.289 ± 0.2 fg | 8.418 ± 0.3 jklm | |
| Yazshah | 19.172 ± 0.5 b | 9.532 ± 0.4 vw | 18.210 ± 0.4 j | 21.332 ± 0.7 d | 16.264 ± 0.4 h | 23.578 ± 0.9 i | 2.383 ± 0.2 mnopq | 8.511 ± 0.5 jklm | |
| Severe | Arak | 16.256 ± 0.5 d | 24.053 ± 1.3 g | 16.829 ± 0.7 m | 19.573 ± 0.7 g | 11.859 ± 0.6 vw | 21.430 ± 0.9 l | 2.136 ± 0.2 rst | 8.610 ± 0.4 ijk |
| Arakkho | 14.068 ± 0.7 h | 11.486 ± 0.6 sh | 13.869 ± 0.3 tu | 27.151 ± 1.5 a | 19.986 ± 0.8 a | 38.352 ± 1.7 b | 3.591 ± 0.3 e | 9.524 ± 0.5 b | |
| Ardebil | 8.658 ± 0.3 st | 9.698 ± 0.8 sh | 17.578 ± 0.5 k | 16.167 ± 0.5 op | 18.397 ± 0.3 c | 14.807 ± 0.3 x | 3.235 ± 0.2 fg | 8.628 ± 0.3 ijk | |
| Esfahfo | 11.681 ± 0.6 l | 20.288 ± 1.2 i | 16.800 ± 0.5 m | 20.664 ± 0.5 e | 13.024 ± 0.4 pq | 30.924 ± 1.4 d | 2.529 ± 0.3 lm | 8.607 ± 0.3 ijk | |
| Farsfars | 8.694 ± 0.4 st | 35.553 ± 2.3 e | 13.974 ± 0.5 t | 17.786 ± 0.6 k | 15.434 ± 0.5 j | 26.638 ± 0.5 g | 2.739 ± 0.2 kl | 9.062 ± 0.4 ef | |
| Farsmar | 7.657 ± 0.4 v | 13.902 ± 0.6 o | 14.024 ± 0.4 st | 19.506 ± 0.9 g | 12.062 ± 0.2 tuv | 19.469 ± 0.4 o | 2.243 ± 0.3 opqrs | 8.463 ± 0.3 jklm | |
| Hamdan | 17.354 ± 0.5 c | 13.950 ± 0.8 o | 15.039 ± 0.8 o | 19.143 ± 0.7 h | 17.772 ± 0.7 e | 40.098 ± 1.2 a | 1.939 ± 0.2 tu | 8.476 ± 0.5 jklm | |
| Khorbir | 11.316 ± 0.6 m | 15.065 ± 0.9 n | 13.683 ± 0.6 uv | 21.146 ± 0.8 d | 12.202 ± 0.7 t | 18.894 ± 0.3 p | 3.351 ± 0.3 f | 8.557 ± 0.5 ijklm | |
| Khormo | 37.695 ± 2.4 a | 16.944 ± 0.8 k | 13.225 ± 0.6 w | 23.483 ± 0.9 b | 11.741 ± 0.7 w | 29.615 ± 0.6 e | 2.099 ± 0.2 u | 8.409 ± 0.4 klm | |
| Qazvin | 8.291 ± 0.7 u | 27.190 ± 1.4 f | 20.361 ± 1.2 g | 17.880 ± 0.6 k | 18.128 ± 0.7 d | 20.474 ± 0.4 n | 2.209 ± 0.3 qrs | 9.506 ± 0.1 bc | |
| Yazd | 14.615 ± 0.6 f | 43.357 ± 2.3 b | 40.099 ± 1.6 a | 20.042 ± 0.9 f | 17.316 ± 0.6 f | 28.522 ± 0.4 f | 2.041 ± 0.4 st | 8.582 ± 0.5 ijklm | |
| Yazshah | 8.183 ± 0.4 u | 20.017 ± 1.3 j | 15.344 ± 0.3 n | 19.991 ± 0.9 f | 20.123 ± 0.8 a | 26.509 ± 0.5 g | 2.165 ± 0.1 rs | 8.585 ± 0.4 ijklm |
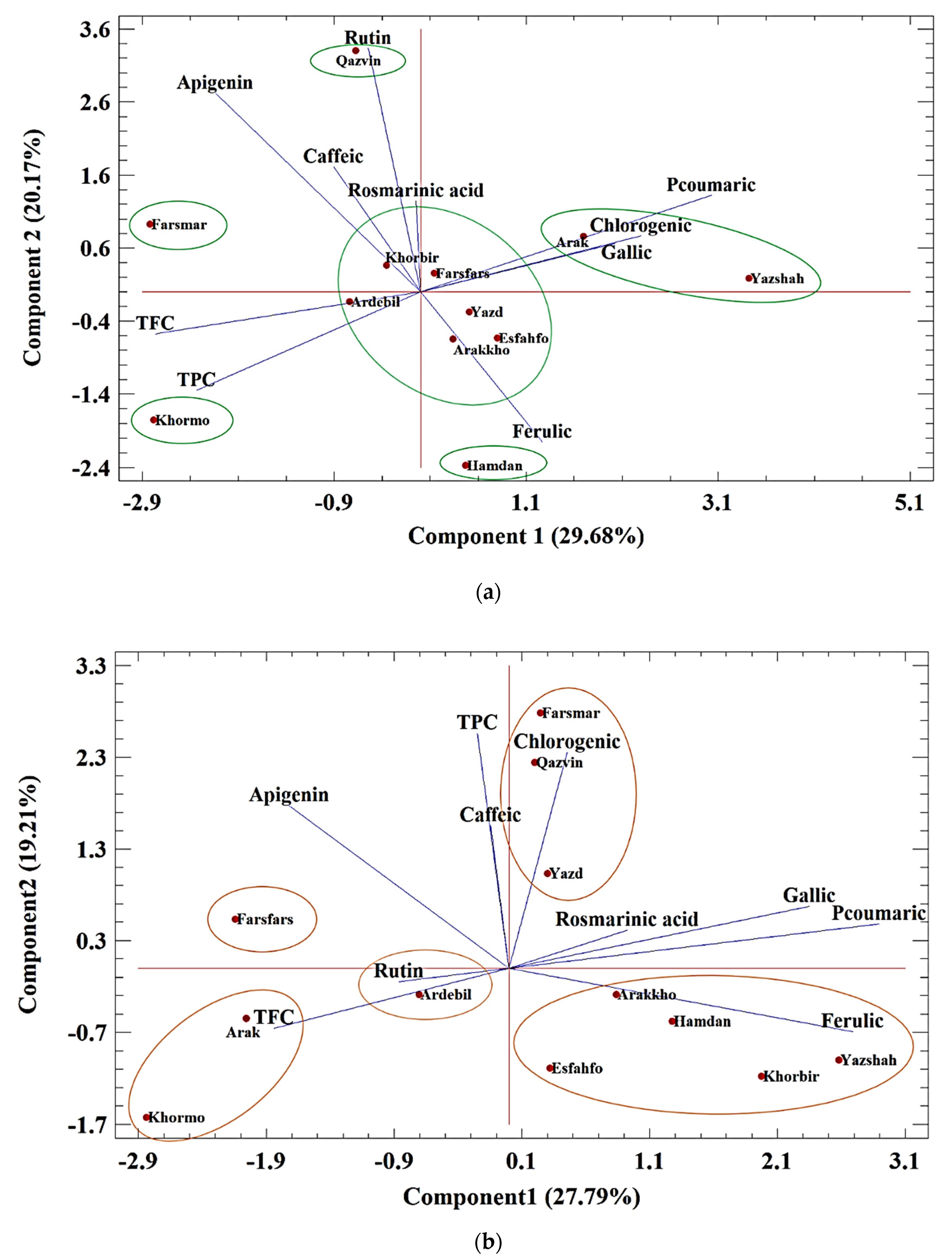

3.8. Cluster Analysis of All Compounds
4. Discussion

5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gholami-Ahangaran, M.; Ahmadi-Dastgerdi, A.; Azizi, S.; Basiratpour, A.; Zokaei, M.; Derakhshan, M. Thymol and carvacrol supplementation in poultry health and performance. Vet. Med. Sci. 2022, 8, 267–288. [Google Scholar] [CrossRef]
- Bonetti, A.; Tugnoli, B.; Piva, A.; Grilli, E. Thymol as an Adjuvant to Restore Antibiotic Efficacy and Reduce Antimicrobial Resistance and Virulence Gene Expression in Enterotoxigenic Escherichia coli Strains. Antibiotics 2022, 11, 1073. [Google Scholar] [CrossRef] [PubMed]
- Davazdahemami, S.; Allahdadi, M. Essential oil yield and composition of four annual plants (ajowan, dill, Moldavian balm and black cumin) under saline irrigation. Food Ther. Health Care 2022, 4, 5. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Ghassemi, S.; Salmasi, S.Z. Changes in essential oil-content and composition of ajowan (Carum copticum L.) seeds in response to growth regulators under water stress. Sci. Hortic. 2018, 231, 219–226. [Google Scholar] [CrossRef]
- Gaba, B.; Khan, T.; Haider, M.F.; Alam, T.; Baboota, S.; Parvez, S.; Ali, J. Vitamin E loaded naringenin nanoemulsion via intranasal delivery for the management of oxidative stress in a 6-OHDA Parkinson’s disease model. Biomed Res. Int. 2019, 2019, 2382563. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Elnesr, S.S. A review on the beneficial effect of thymol on health and production of fish. Rev. Aquac. 2021, 13, 632–641. [Google Scholar] [CrossRef]
- Krist, S. Ajowan Oil. In Vegetable Fats and Oils; Springer: Cham, Switzerland, 2020; pp. 27–31. [Google Scholar]
- Heidari, E.F.; Rahimmalek, M.; Mohammadi, S.; Ehtemam, M.H. Genetic structure and diversity of ajowan (Trachyspermum ammi) populations based on molecular, morphological markers, and volatile oil content. Ind. Crops Prod. 2016, 92, 186–196. [Google Scholar] [CrossRef]
- Soltani Howyzeh, M.; Sadat Noori, S.A.; Shariati, J.V.; Niazian, M. Essential oil chemotype of Iranian Ajowan (Trachyspermum ammi L.). J. Essent. Oil Bear. Plants 2018, 21, 273–276. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Heidari, E.F.; Ehtemam, M.H.; Mohammadi, S. Essential oil variation in Iranian ajowan (Trachyspermum ammi L.) Sprague populations collected from different geographical regions in relation to climatic factors. Ind. Crops Prod. 2017, 95, 591–598. [Google Scholar] [CrossRef]
- Singh, H.; Meghwal, M. Ajwain a potential source of phytochemical for better health. Pharma Innov. 2019, 8, 599–603. [Google Scholar]
- Saini, R.K.; Song, M.H.; Yu, J.W.; Shang, X.; Keum, Y.S. Phytosterol Profiling of Apiaceae Family Seeds Spices Using GC-MS. Foods 2021, 10, 2378. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Vilkickyte, G.; Raudone, L. Phenological and Geographical Effects on Phenolic and Triterpenoid Content in Vaccinium vitis-idaea L. Leaves. Plants 2021, 10, 1986. [Google Scholar] [CrossRef]
- Choudhary, S.; Zehra, A.; Mukarram, M.; Naeem, M.; Khan, M.; Hakeem, K.R.; Aftab, T. An insight into the role of plant growth regulators in stimulating abiotic stress tolerance in some medicinally important plants. In Plant Growth Regul; Springer: Cham, Switzerland, 2021; pp. 75–100. [Google Scholar]
- Suleman, M.; Khan, A.; Baqi, A.; Kakar, M.S.; Ayub, M. 2. Antioxidants, its role in preventing free radicals and infectious diseases in human body. Pure Appl. Biol. PAB 2019, 8, 380–388. [Google Scholar] [CrossRef]
- Razavi, S.M.; Ghorbanian, A.; Abadi, A. Impact of drought stress on growth–yield parameters, volatile constituents and physio-biochemical traits of three Foeniculum vulgare genotypes. Agric. Res. 2021, 1–17. [Google Scholar] [CrossRef]
- Afshari, M.; Pazoki, A.; Sadeghipour, O. Foliar-applied Silicon and its Nanoparticles Stimulate Physio-chemical Changes to Improve Growth, Yield and Active Constituents of Coriander (Coriandrum Sativum L.) Essential Oil Under Different Irrigation Regimes. Silicon 2021, 13, 4177–4188. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Guerra, I.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Figueirinha, A.; Salgueiro, L. Chemical composition of Crithmum maritimum L. essential oil and hydrodistillation residual water by GC-MS and HPLC-DAD-MS/MS, and their biological activities. Ind. Crops Prod. 2020, 149, 112329. [Google Scholar] [CrossRef]
- Kikowska, M.; Thiem, B. In vitro systems of selected Eryngium species (E. planum, E. campestre, E. maritimum, and E. alpinum) for studying production of desired secondary metabolites (phenolic acids, flavonoids, triterpenoid saponins, and essential oil). In Plant Cell and Tissue Differentiation and Secondary Metabolites. Fundamentals and Applications; Reference Series in Phytochemistry; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Springer: Cham, Switzerland; Heidelberg, Germany, 2020; pp. 1–33. [Google Scholar]
- Demir, S.; Korukluoglu, M. A comparative study about antioxidant activity and phenolic composition of cumin (Cuminum cyminum L.) and coriander (Coriandrum sativum L.). Indian J. Tradit. Knowl. IJTK 2020, 19, 383–393. [Google Scholar]
- Goudarzi, G.R.; Saharkhiz, M.J.; Sattari, M.; Zomorodian, K. Antibacterial activity and chemical composition of Ajowan (Carum copticum Benth. & Hook) essential oil. J. Agric. Sci. Technol. 2011, 13, 203–208. [Google Scholar]
- Rechinger, K.H. Flora Iranica; Akademische Druck- und Verlagsanstalt & Naturhistorisches Museum Wien, Graz & Wien: Wien, Austria, 1963–2015.
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements-FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998; Volume 300, p. D05109. [Google Scholar]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IT, USA, 2007; Volume 456. [Google Scholar]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Yao, X.H.; Duan, M.H.; Wei, F.Y.; Wu, G.H.; Li, L. Variation of essential oil content and antioxidant activity of Lonicera species in different sites of China. Ind. Crops Prod. 2015, 77, 772–779. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Talebi, M.; Matkowski, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Alaghemand, A.; Khaghani, S.; Bihamta, M.R.; Gomarian, M.; Ghorbanpour, M. Physiological responses of Nigella Sativa ecotypes to drought stress condition. Plant Physiol. 2019, 9, 2695–2702. [Google Scholar]
- García-Caparrós, P.; Romero, M.J.; Llanderal, A.; Cermeño, P.; Lao, M.T.; Segura, M.L. Effects of Drought Stress on Biomass, Essential Oil Content, Nutritional Parameters, and Costs of Production in Six Lamiaceae Species. Water 2019, 11, 573. [Google Scholar] [CrossRef]
- Nassiri, H.; Seghatoleslami, M.; Mousavi, G.; Ebrahimi, A. Effect of irrigation and planting date on yield and water use efficiency of ajowan (Carum copticum). Annu. Res. Rev. Biol. 2014, 4, 1968. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Siddique, K.H.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and heat-stress effects on seed filling in food crops: Impacts on functional biochemistry, seed yields, and nutritional quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Amirikia, F.; Ghorbanpour, M.; Fatehi, F.; Hashempour, H. Salicylic acid induced changes in physiological traits and essential oil constituents in different ecotypes of Thymus kotschyanus and Thymus vulgaris under well-watered and water stress conditions. Ind. Crops Prod. 2019, 129, 561–574. [Google Scholar] [CrossRef]
- Baher, Z.F.; Mirza, M.; Ghorbanli, M.; Bagher Rezaii, M. The influence of water stress on plant height, herbal and essential oil yield and composition in Satureja hortensis L. Flavour Fragr. J. 2002, 17, 275–277. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Daferera, D.; Polissiou, M.G.; Passam, H.C. The effect of water deficit stress on the growth, yield and composition of essential oils of parsley. Sci. Hortic. 2008, 115, 393–397. [Google Scholar] [CrossRef]
- Zhu, Z.; Liang, Z.; Han, R. Saikosaponin accumulation and antioxidative protection in drought-stressed Bupleurum chinense DC. plants. Environ. Exp. Bot. 2009, 66, 326–333. [Google Scholar] [CrossRef]
- Rebey, I.B.; Jabri-Karoui, I.; Hamrouni-Sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Effect of drought on the biochemical composition and antioxidant activities of cumin (Cuminum cyminum L.) seeds. Ind. Crops Prod. 2012, 36, 238–245. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chang, Y.H.; Shao, Y.Y. Effects of genotype and treatment on the antioxidant activity of sweet potato in Taiwan. Food Chem. 2006, 98, 529–538. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef]
- Neffati, M.; Sriti, J.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Salinity impact on fruit yield, essential oil composition and antioxidant activities of Coriandrum sativum fruit extracts. Food Chem. 2011, 124, 221–225. [Google Scholar] [CrossRef]
- Terzi, R.; Sağlam, A.; Kutlu, N.; Nar, H.; Kadioğlu, A. Impact of soil drought stress on photochemical efficiency of photosystem II and antioxidant enzyme activities of Phaseolus vulgaris cultivars. Turk. J. Bot. 2010, 34, 1–10. [Google Scholar] [CrossRef]
- De Abreu, I.N.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Sellami, I.H.; Wannes, W.A.; Bettaieb, I.; Berrima, S.; Chahed, T.; Marzouk, B.; Limam, F. Qualitative and quantitative changes in the essential oil of Laurus nobilis L. leaves as affected by different drying methods. Food Chem. 2011, 126, 691–697. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Goli, S.A.H. Evaluation of six drying treatments with respect to essential oil yield, composition and color characteristics of Thymys daenensis subsp. daenensis. Celak leaves. Ind. Crops Prod. 2013, 42, 613–619. [Google Scholar] [CrossRef]
- Wormuth, D.; Heiber, I.; Shaikali, J.; Kandlbinder, A.; Baier, M.; Dietz, K.J. Redox regulation and antioxidative defence in Arabidopsis leaves viewed from a systems biology perspective. J. Biotechnol. 2007, 129, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Selmar, D.; Kleinwächter, M. Stress enhances the synthesis of secondary plant products: The impact of stress-related over-reduction on the accumulation of natural products. Plant Cell Environ. 2013, 54, 817–826. [Google Scholar] [CrossRef]
- Llusià, J.; Pen~uelas, J. Seasonal patterns of terpene content and emission from seven Mediterranean woody species in field conditions. Am. J. Bot. 2000, 87, 133–140. [Google Scholar] [CrossRef]
- Gershenzon, J.; Croteau, R. “Terpenoids.”. In Herbivores: Their Interactions with Secondary Plant Metabolites, 2nd ed.; The Chemical Participants; Academic Press: Cambridge, MA, USA, 1992; Volume 1, pp. 165–219. [Google Scholar]
- Hartmann, T.; Toppel, G. Senecionine N-oxide, the primary product of pyrrolizidine alkaloid biosynthesis in root cultures of Senecio vulgaris. Phytochemistry 1987, 26, 1639–1643. [Google Scholar] [CrossRef]
- Baldwin, I.T. Inducible nicotine production in native Nicotiana as an example of adaptive phenotypic plasticity. J. Chem. Ecol. 1999, 25, 3–30. [Google Scholar] [CrossRef]
- Harborne, A.J. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Opitz, S.; Schneider, B. Organ-specific analysis of phenylphenalenone-related compounds in Xiphidium caeruleum. Phytochemistry 2002, 61, 819–825. [Google Scholar] [CrossRef]
- Balkhyour, M.A.; Tammar, A.M.; Summan, A.S.; Hassan, A.H. Enhancing biomass and productivity of coumarins and essential oil in ajwain (Trachyspermum ammi) sprouts via laser light treatment. Ind. Crops Prod. 2021, 170, 113837. [Google Scholar] [CrossRef]
- Pandey, A.; Niranjan, A.; Misra, P.; Lehri, A.; Tewari, S.K.; Trivedi, P.K. Simultaneous separation and quantification of targeted group of compounds in Psoralea corylifolia L. using HPLC-PDA-MS-MS. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 2567–2583. [Google Scholar] [CrossRef]
- Mahmood, S.; Mahmood, T.; Hussian, I.; Javed, S.; Afzal, B.; Ghaffar, F.; Iqbal, M.; Akram, M.; Shah, S.M.A. Efficacy of differently applied tyrosine and tryptophan for modulation of phenolic metabolism in Trachyspermum ammi (L.) sprague seedlings. Pak. J. Pharm. Sci. 2016, 29, 1847–1851. [Google Scholar] [PubMed]
- Chiyaneh, S.F.; Rezaei-Chiyaneh, E.; Amirnia, R.; Afshar, R.K.; Siddique, K.H. Changes in the essential oil, fixed oil constituents, and phenolic compounds of ajowan and fenugreek in intercropping with pea affected by fertilizer sources. Ind. Crops Prod. 2022, 178, 114587. [Google Scholar] [CrossRef]
- Nouraei, S.; Rahimmalek, M.; Saeidi, G. Variation in polyphenolic composition, antioxidants and physiological characteristics of globe artichoke (Cynara cardunculus var. scolymus Hayek L.) as affected by drought stress. Sci. Hortic. 2018, 233, 378–385. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Kadioglu, A.S.İ.M.; Turgut, R.A.B.İ.Y.E. Water stress effects on the content of low molecular weight carbohydrates and phenolic acids in Ctenanthe setosa (Rosc.) Eichler. Can. J. Plant Sci. 2000, 80, 373–378. [Google Scholar] [CrossRef]
- Mumivand, H.; Ebrahimi, A.; Shayganfar, A.; Khoshro, H.H. Screening of tarragon accessions based on physiological and phytochemical responses under water deficit. Sci. Rep. 2021, 11, 17839. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]

| Stress | Populations | TFC* (mg QE g−1 DW) | TPC** (mg TAE g−1 DW) | Essential Oil Yield (%) |
|---|---|---|---|---|
| Normal | Arak | 2.8 ± 0.06 mn | 33.15 ± 0.32 hi | 5.15 ± 0.06 a |
| Arakkho | 1.2 ± 0.05 pq | 28.65 ± 0.41 ij | 5.22 ± 0.06 a | |
| Ardebil | 9.7 ± 0.63 c | 45.07 ± 3.46 f | 3.85 ± 0.05 e | |
| Esfahfo | 0.7 ± 0.05 q | 33.32 ± 0.54 hi | 5.37 ± 0.1 a | |
| Farsfars | 4.00 ± 0.08 ghi | 48.09 ± 0.99 jkl | 2.1 ± 0.15 mno | |
| Farsmar | 9.5 ± 0.49 c | 68.69 ± 0.72 e | 4.33 ± 0.10 c | |
| Hamdan | 4.6 ± 0.24 hij | 33.84 ± 0.53 hi | 2.79 ± 0.08 ghi | |
| Khorbir | 3.1 ± 0.15 mn | 119.30 ± 10.11 b | 3.61 ± 0.15 e | |
| Khormo | 10.6 ± 0.18 a | 171.36 ± 2.13 a | 4.08 ± 0.03 cd | |
| Qazvin | 3.3 ± 0.24 mn | 12.04 ± 0.87 op | 4.73 ± 0.15 b | |
| Yazd | 1.3 ± 0.14 pq | 25.84 ± 0.57 jk | 5.55 ± 0.2 a | |
| Yazshah | 1.9 ± 0.08 op | 12.70 ± 0.63 op | 1.68 ± 0.16 lmn | |
| Medium | Arak | 3.9 ± 0.08 ijk | 13.14 ± 0.34 nop | 1.27 ± 0.01 p |
| Arakkho | 4.1 ± 0.05 hij | 21.33 ± 0.12 klm | 2.9 ± 0.11 g | |
| Ardebil | 3 ± 0.18 lmn | 45.07 ± 3.46 lm | 3.85 ± 0.05 mno | |
| Esfahfo | 8.5 ± 0.23 c | 48.71 ± 0.21 f | 0.7 ± 0.03 r | |
| Farsfars | 7.6 ± 0.07 d | 48.09 ± 0.99 f | 2.1 ± 0.15 jk | |
| Farsmar | 4.7 ± 0.08 fg | 91.66 ± 0.93 c | 2.8 ± 0.05 gh | |
| Hamdan | 1.7 ± 0.08 p | 38.71 ± 0.60 g | 3.34 ± 0.06 f | |
| Khorbir | 2.6 ± 0.29 no | 9.29 ± 0.13 p | 2.22 ± 0.21 ij | |
| Khormo | 8.4 ± 0.43 cd | 33.61 ± 0.81 hi | 2.21 ± 0.16 jk | |
| Qazvin | 3.7 ± 0.11 ijkl | 76.38 ± 0.69 d | 2.51 ± 0.14 hij | |
| Yazd | 1.2 ± 0.05 pq | 100.55 ± 0.61 b | 1.8 ± 0.03 lm | |
| Yazshah | 3.4 ± 0.05 klm | 17.69 ± 0.03 mn | 2.6 ± 0.05 ghi | |
| Severe | Arak | 5.2 ± 0.14 f | 11.97 ± 0.41 nop | 1.67 ± 0.04 mno |
| Arakkho | 4.7 ± 0.14 fgh | 49.21 ± 1.18 f | 1.5 ± 0.06 mnop | |
| Ardebil | 1.3 ± 0.05 p | 47.32 ± 1.24 f | 0.7 ± 0.05 r | |
| Esfahfo | 1.7 ± 0.03 p | 13.56 ± 0.65 nop | 2 ± 0.05 kl | |
| Farsfars | 6.3 ± 0.11 e | 12.90 ± 0.64 nop | 3.87 ± 0.12 de | |
| Farsmar | 3.5 ± 0.20 jklm | 17.39 ± 1.40 mn | 1.4 ± 0.14 op | |
| Hamdan | 4.2 ± 0.26 ghi | 7.00 ± 1.67 p | 1.72 ± 0.014 lmn | |
| Khorbir | 4.8 ± 0.17 fgh | 17.36 ± 0.22 mno | 0.91 ± 0.04 qr | |
| Khormo | 9.6 ± 0.14 b | 45.54 ± 2.57 f | 0.84 ± 0.03 r | |
| Qazvin | 6.6 ± 0.63 e | 16.95 ± 2.88 mno | 1.44 ± 0.09 nop | |
| Yazd | 4.4 ± 0.43 ghi | 36.87 ± 0.57 gh | 1.57 ± 0.22 mno | |
| Yazshah | 2.4 ± 0.02 no | 32.95 ± 0.61 hi | 1.21 ± 0.06 pq |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirniyam, G.; Rahimmalek, M.; Arzani, A.; Matkowski, A.; Gharibi, S.; Szumny, A. Changes in Essential Oil Composition, Polyphenolic Compounds and Antioxidant Capacity of Ajowan (Trachyspermum ammi L.) Populations in Response to Water Deficit. Foods 2022, 11, 3084. https://doi.org/10.3390/foods11193084
Mirniyam G, Rahimmalek M, Arzani A, Matkowski A, Gharibi S, Szumny A. Changes in Essential Oil Composition, Polyphenolic Compounds and Antioxidant Capacity of Ajowan (Trachyspermum ammi L.) Populations in Response to Water Deficit. Foods. 2022; 11(19):3084. https://doi.org/10.3390/foods11193084
Chicago/Turabian StyleMirniyam, Gita, Mehdi Rahimmalek, Ahmad Arzani, Adam Matkowski, Shima Gharibi, and Antoni Szumny. 2022. "Changes in Essential Oil Composition, Polyphenolic Compounds and Antioxidant Capacity of Ajowan (Trachyspermum ammi L.) Populations in Response to Water Deficit" Foods 11, no. 19: 3084. https://doi.org/10.3390/foods11193084
APA StyleMirniyam, G., Rahimmalek, M., Arzani, A., Matkowski, A., Gharibi, S., & Szumny, A. (2022). Changes in Essential Oil Composition, Polyphenolic Compounds and Antioxidant Capacity of Ajowan (Trachyspermum ammi L.) Populations in Response to Water Deficit. Foods, 11(19), 3084. https://doi.org/10.3390/foods11193084









