The Antioxidant and Anti-Inflammatory Effects of Flavonoids from Propolis via Nrf2 and NF-κB Pathways
Abstract
1. Background
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Coupling of HPLC-ESI-QTOF-MS/MS
2.3. Cell Culture and Treatment
2.4. Cell Counting Kit-8 Assay for Cell Viability
2.5. Production of Reactive Oxygen Species (ROS)
2.6. SOD and CAT Assays
2.7. NO and NOS Assays
2.8. Extraction of Whole-Cell Protein, Cytosolic Protein, and Nuclear Protein
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Identification of Chemical Constituents in Propolis Extract
3.2. Effects of H2O2 on Cell Viability and Production of ROS
3.3. Effects of LPSs on Cell Viability and Expression of Pro-Inflammatory Cytokine Proteins
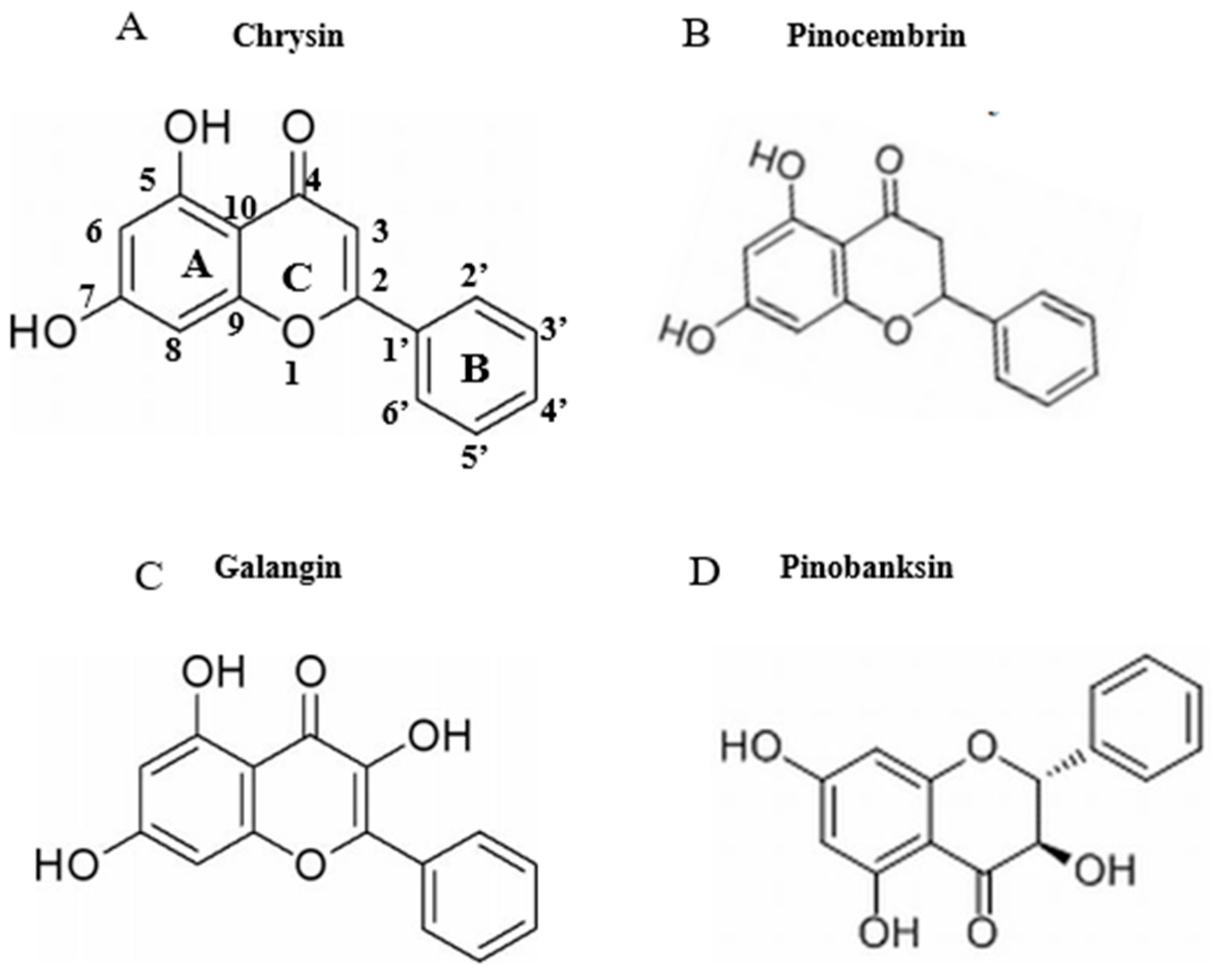
3.4. Effects of Chrysin, Pinocembrin, Galangin, Pinobanksin, and Propolis Extract on Cell Viability
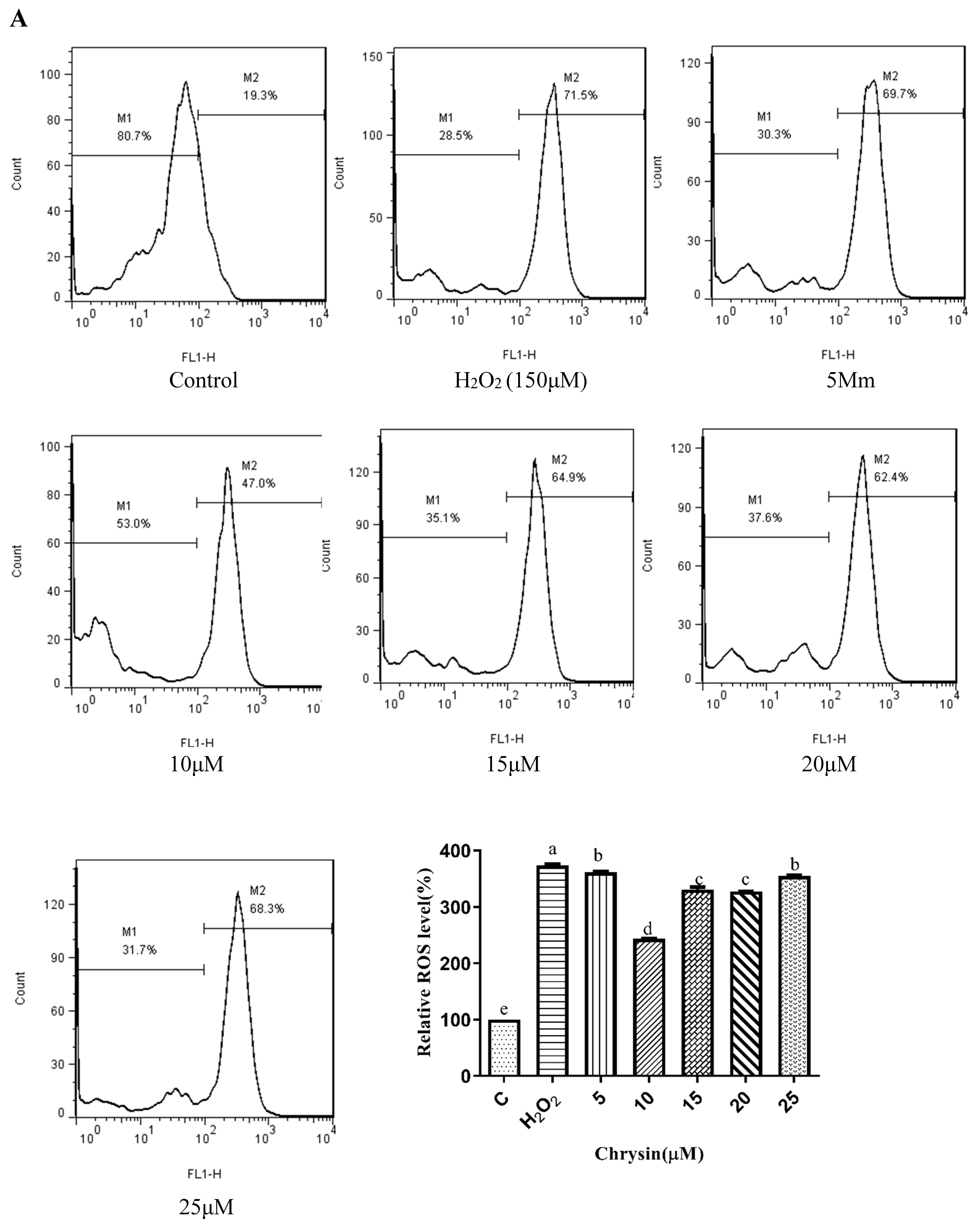
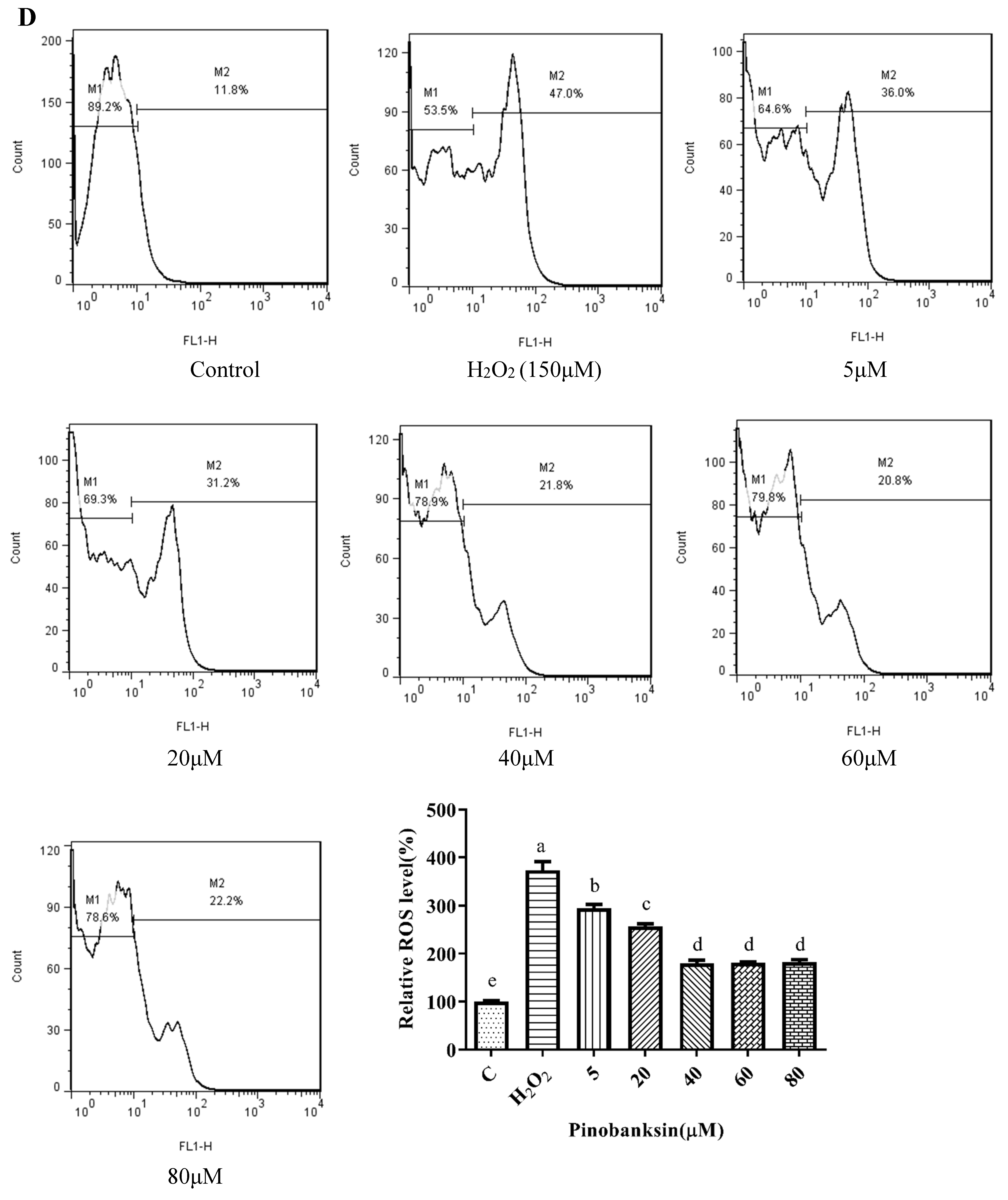
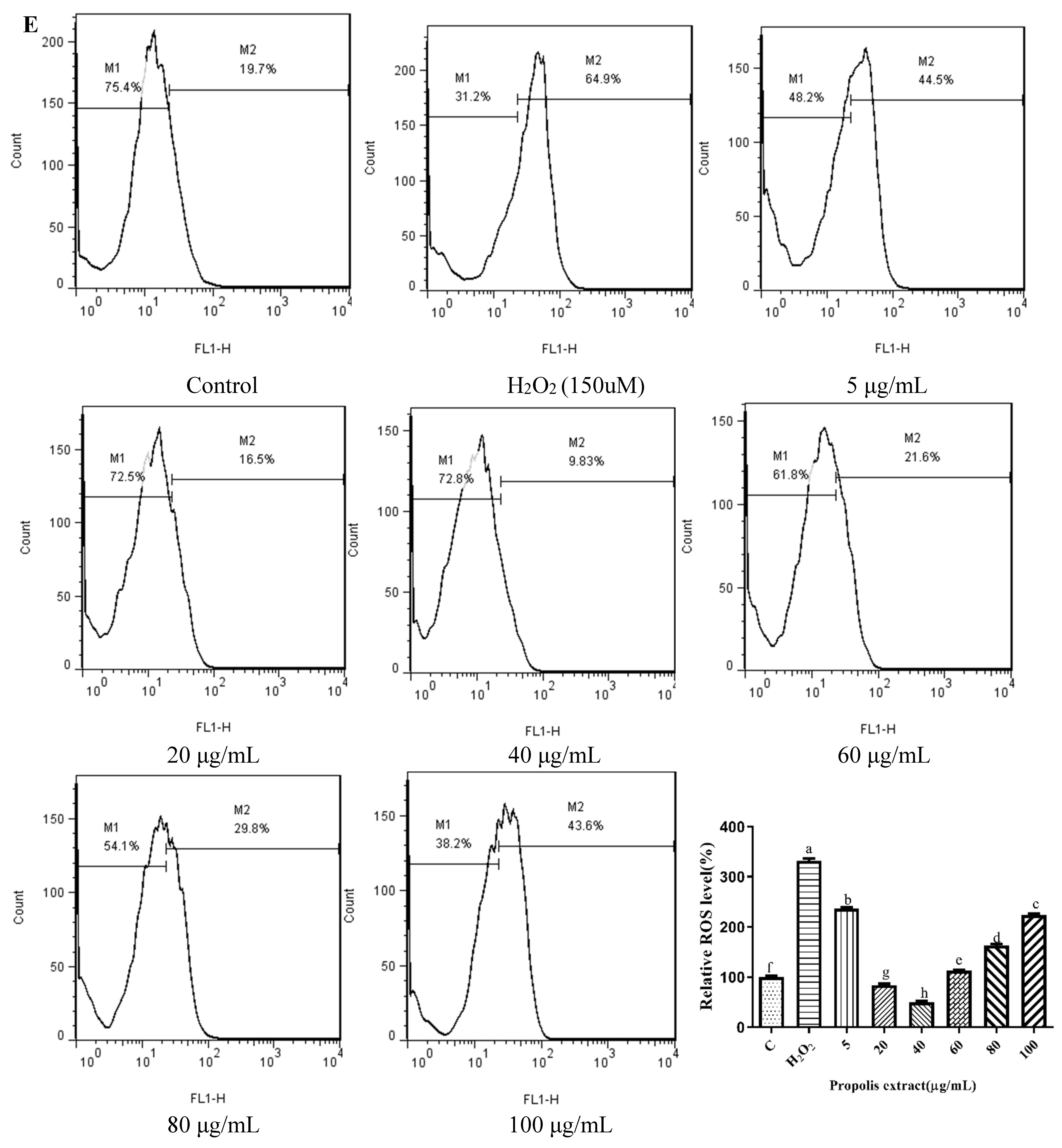
3.5. Effects of Chrysin, Pinocembrin, Galangin, Pinobanksin, and Propolis Extract on Cell Production of ROS
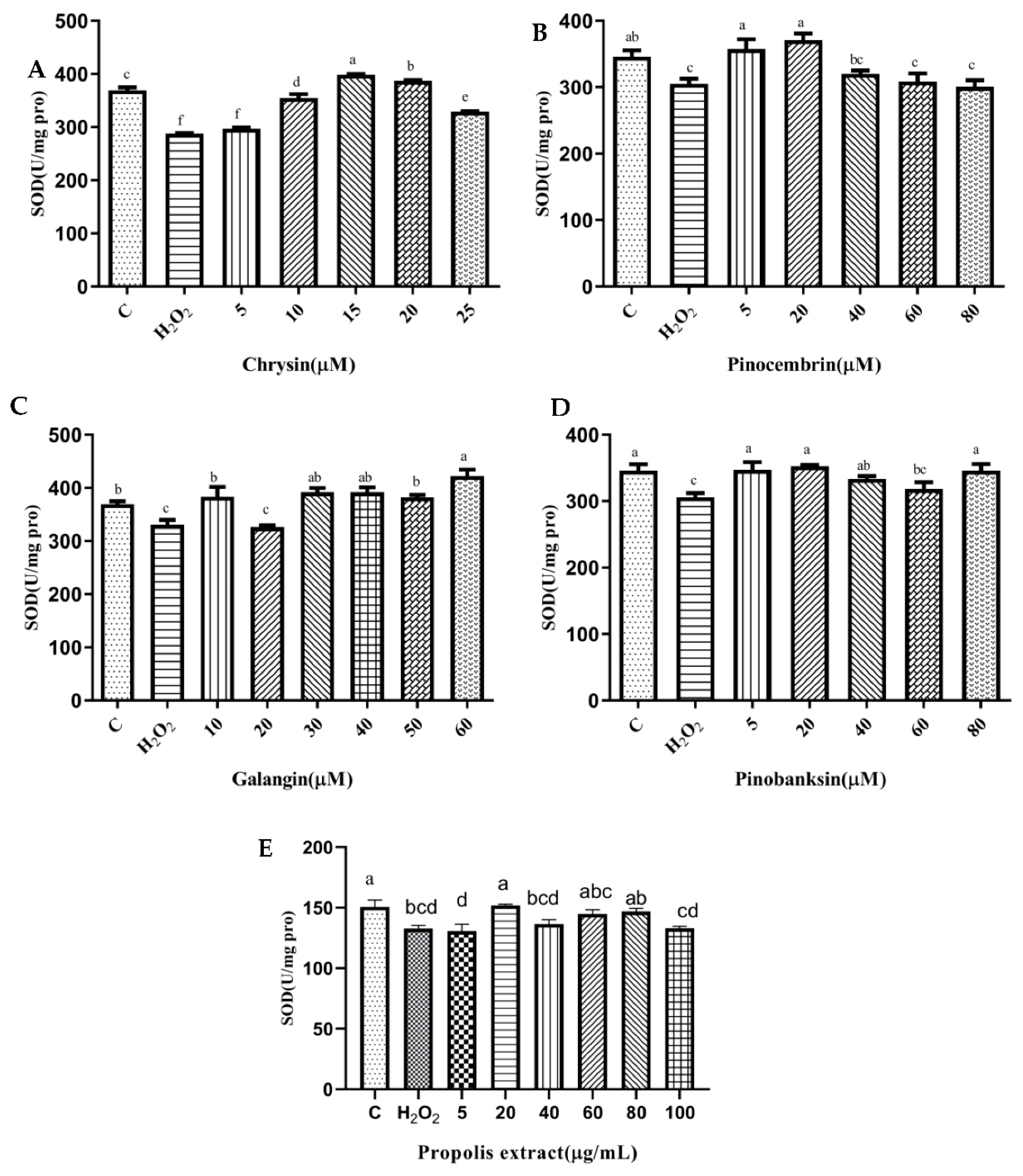
3.6. SOD and CAT Activities
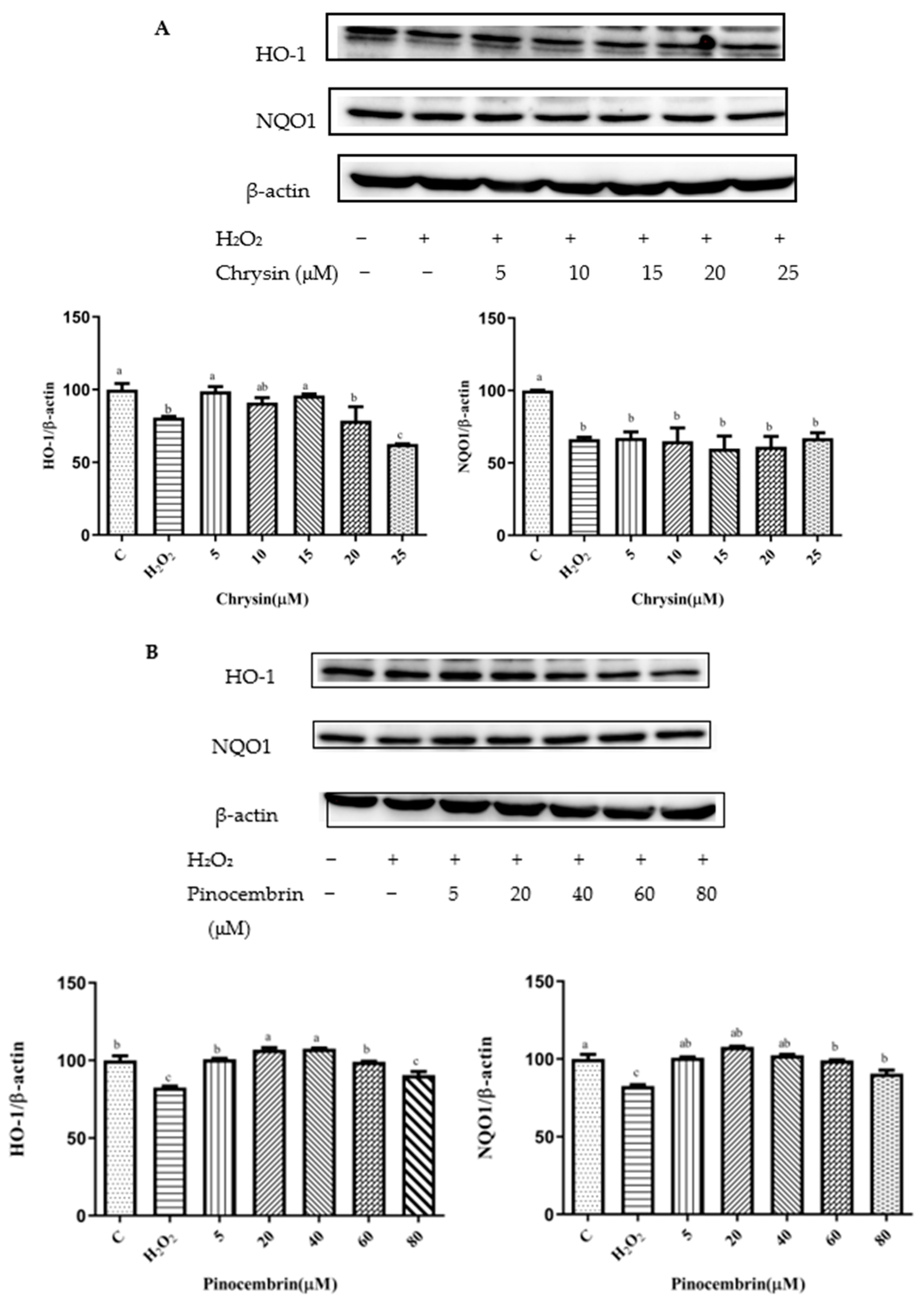
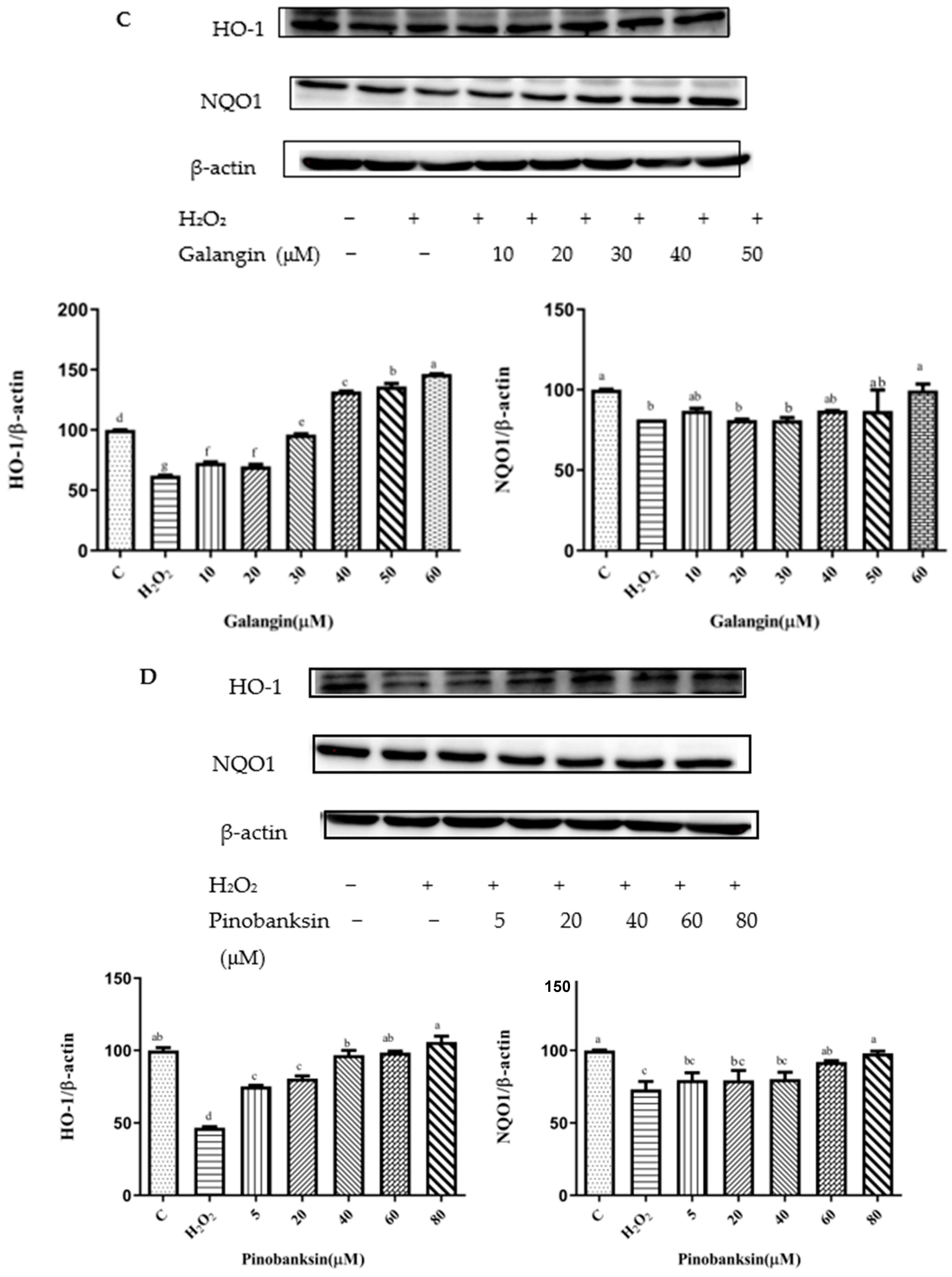
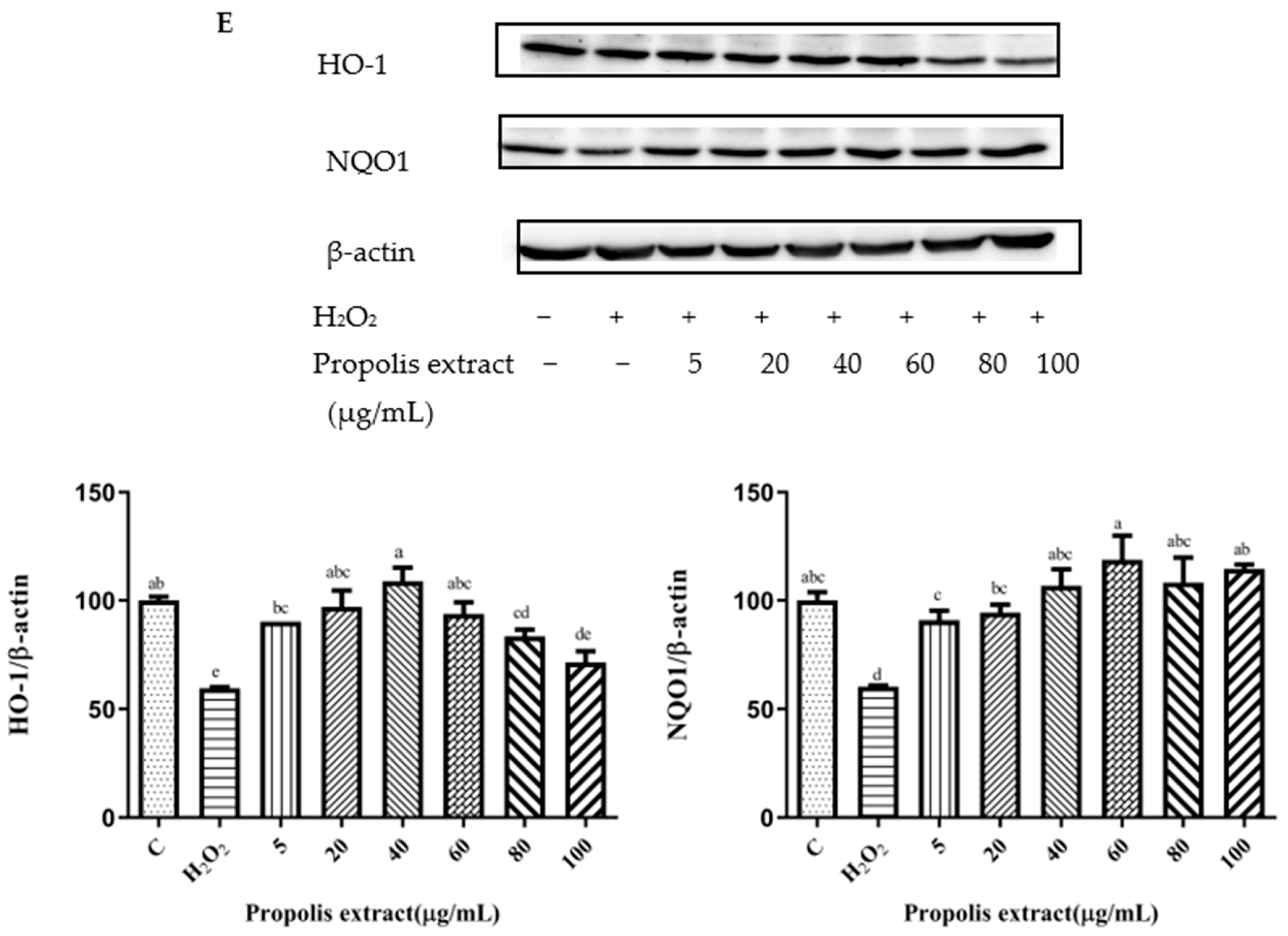
3.7. Effects of Chrysin, Pinocembrin, Galangin, Pinobanksin, and Propolis Extract on the Expression of Proteins Encoded by Antioxidant Genes Downstream of Nrf2
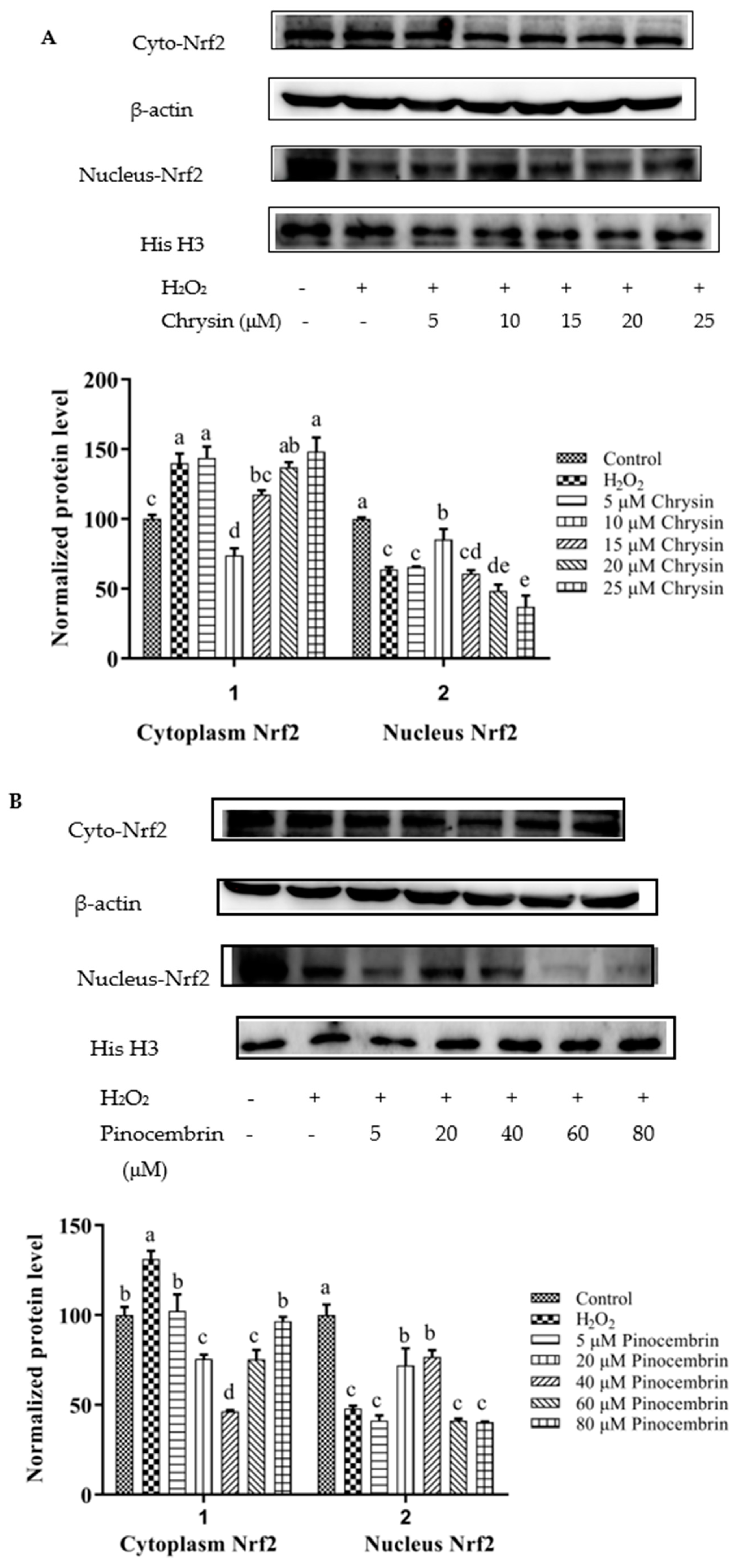
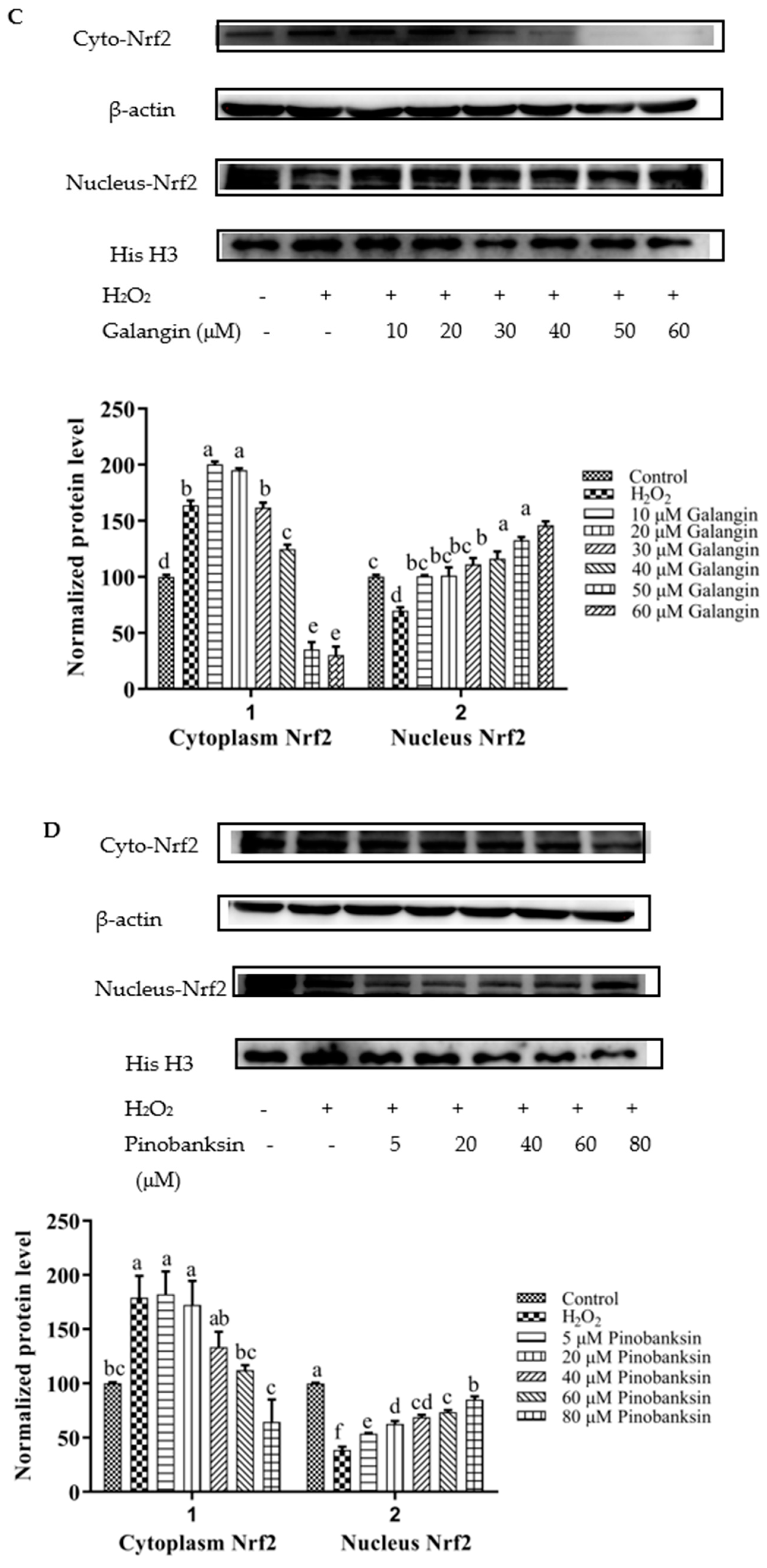
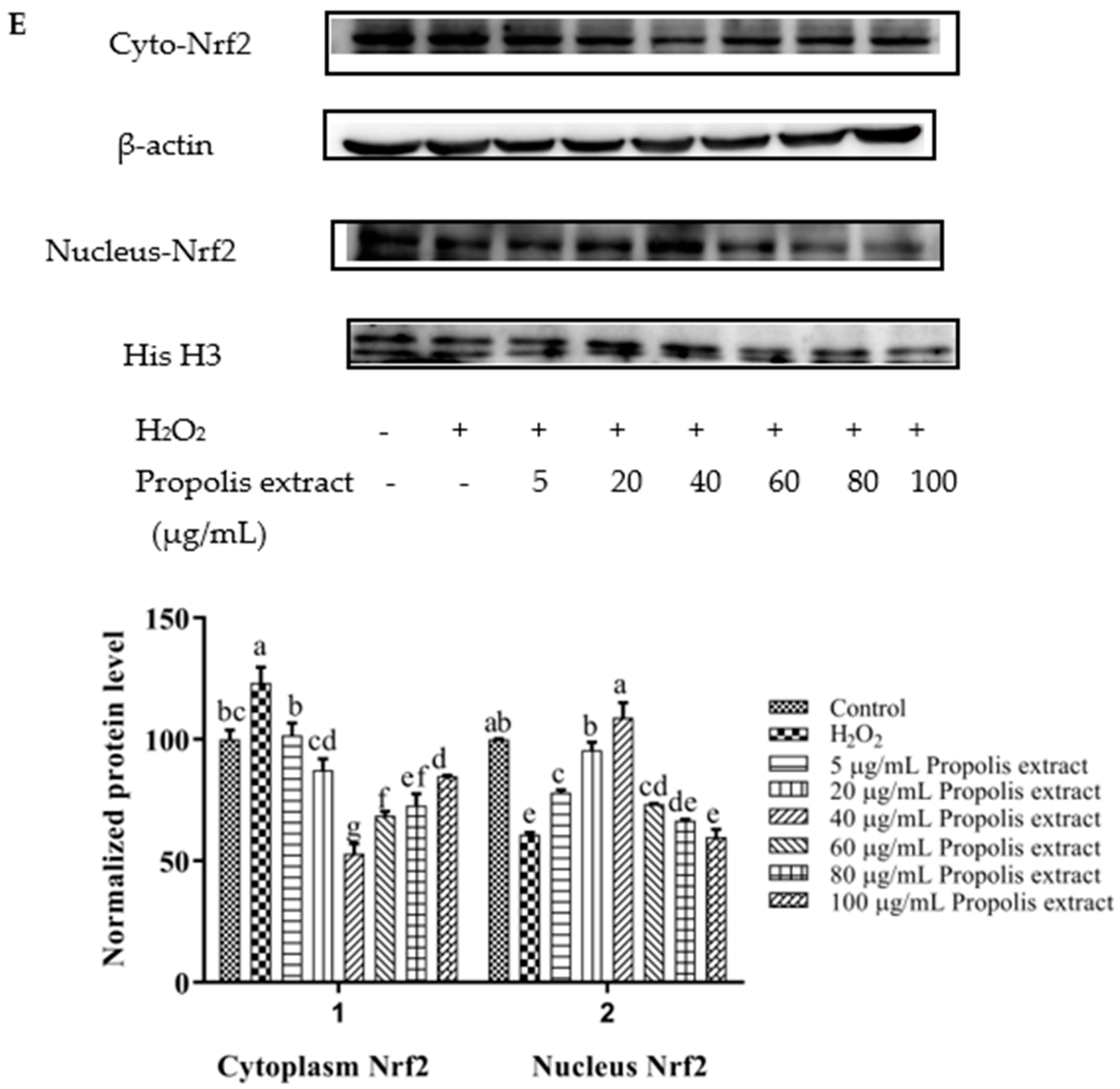
3.8. Activation of Nrf2 by Chrysin, Pinocembrin, Galangin, Pinobanksin, and Propolis Extract
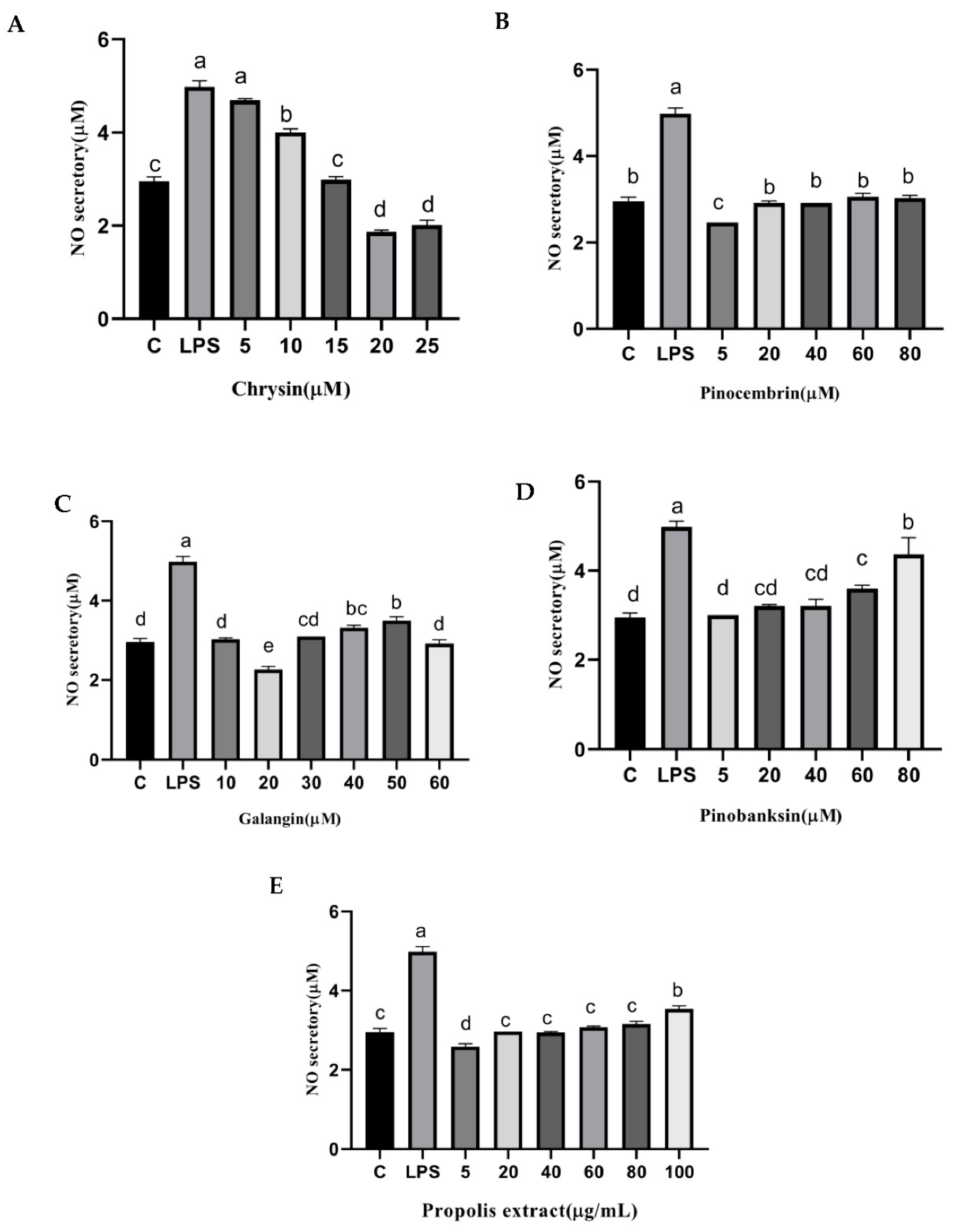
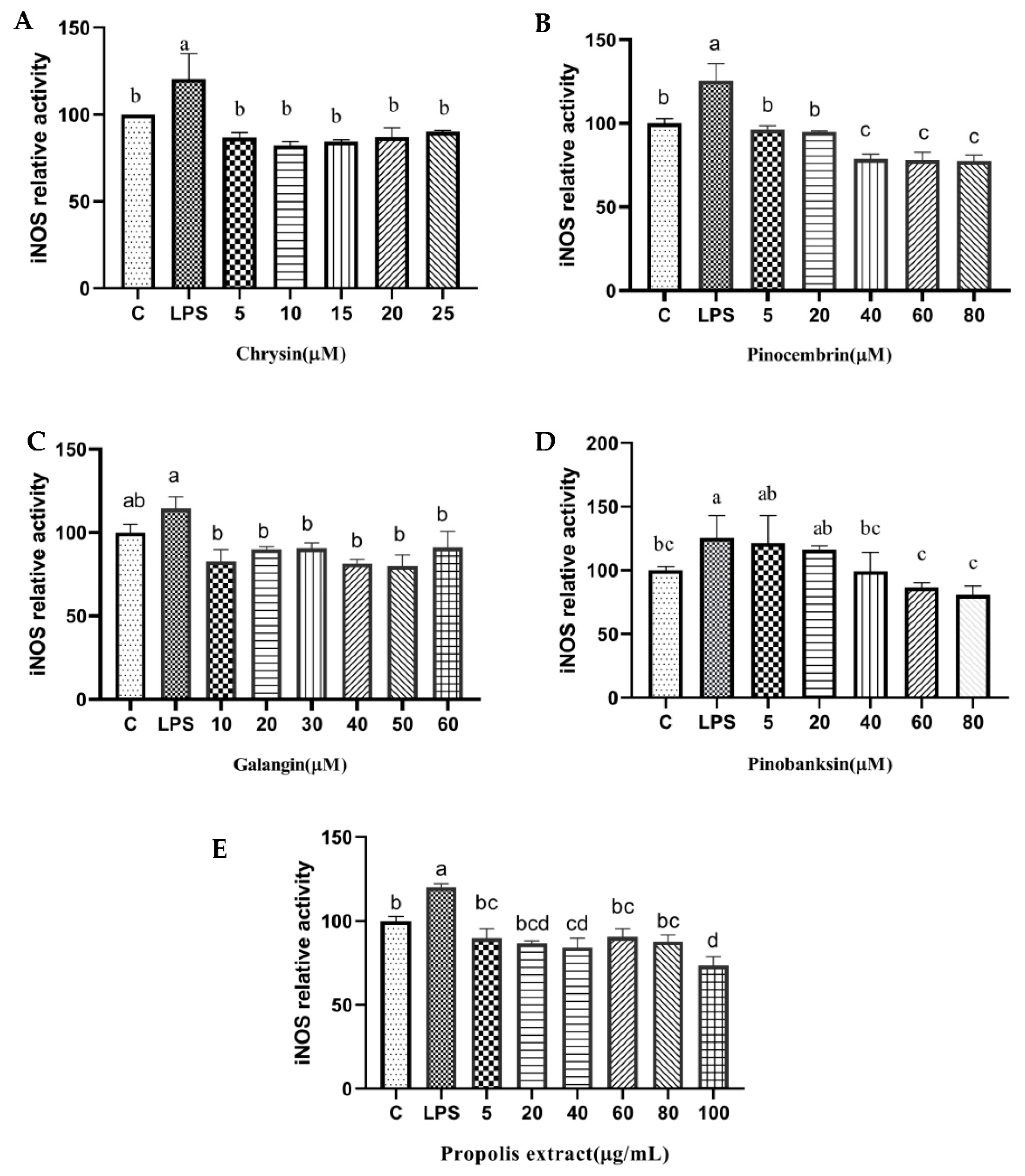
3.9. NO and NOS Levels
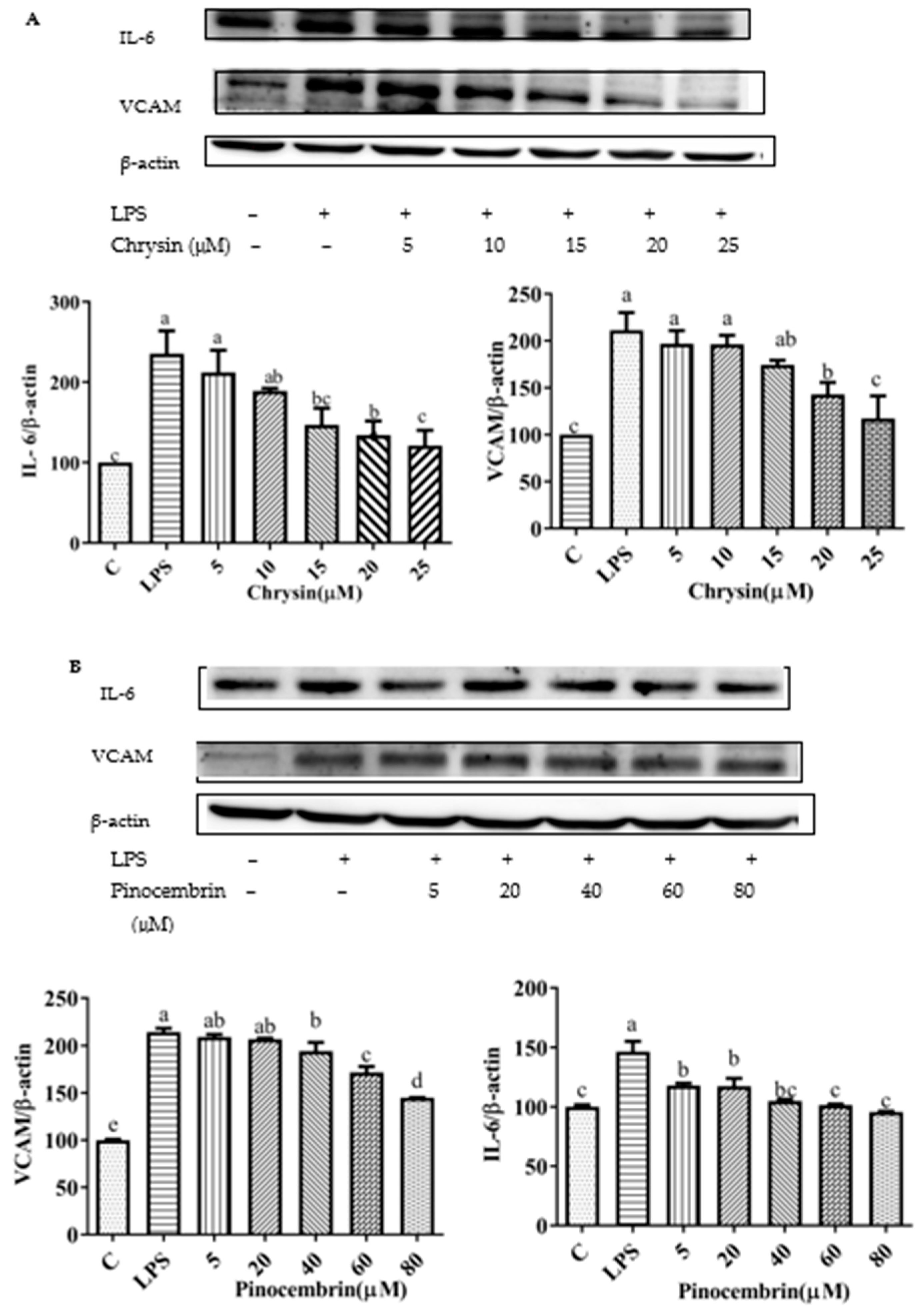
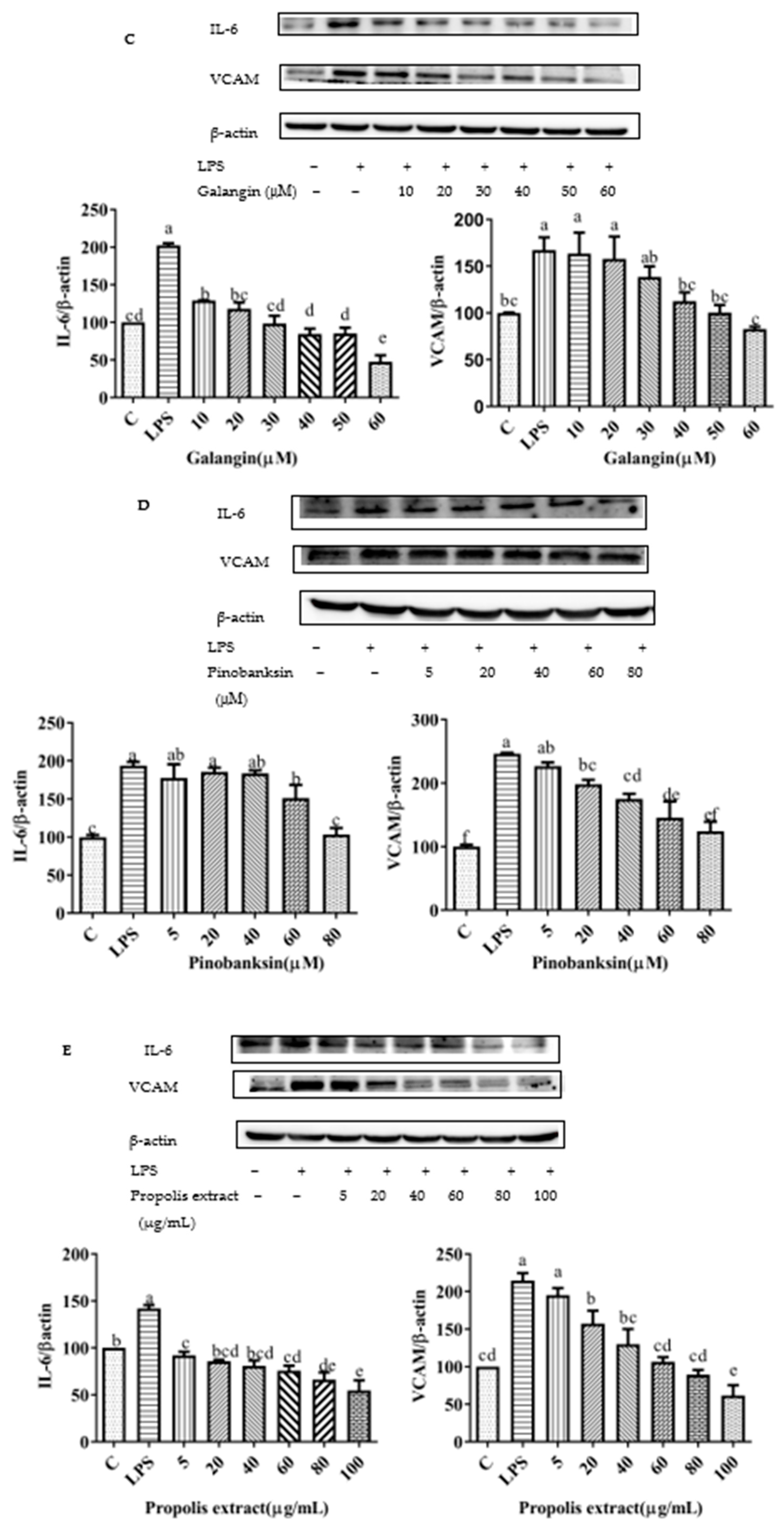
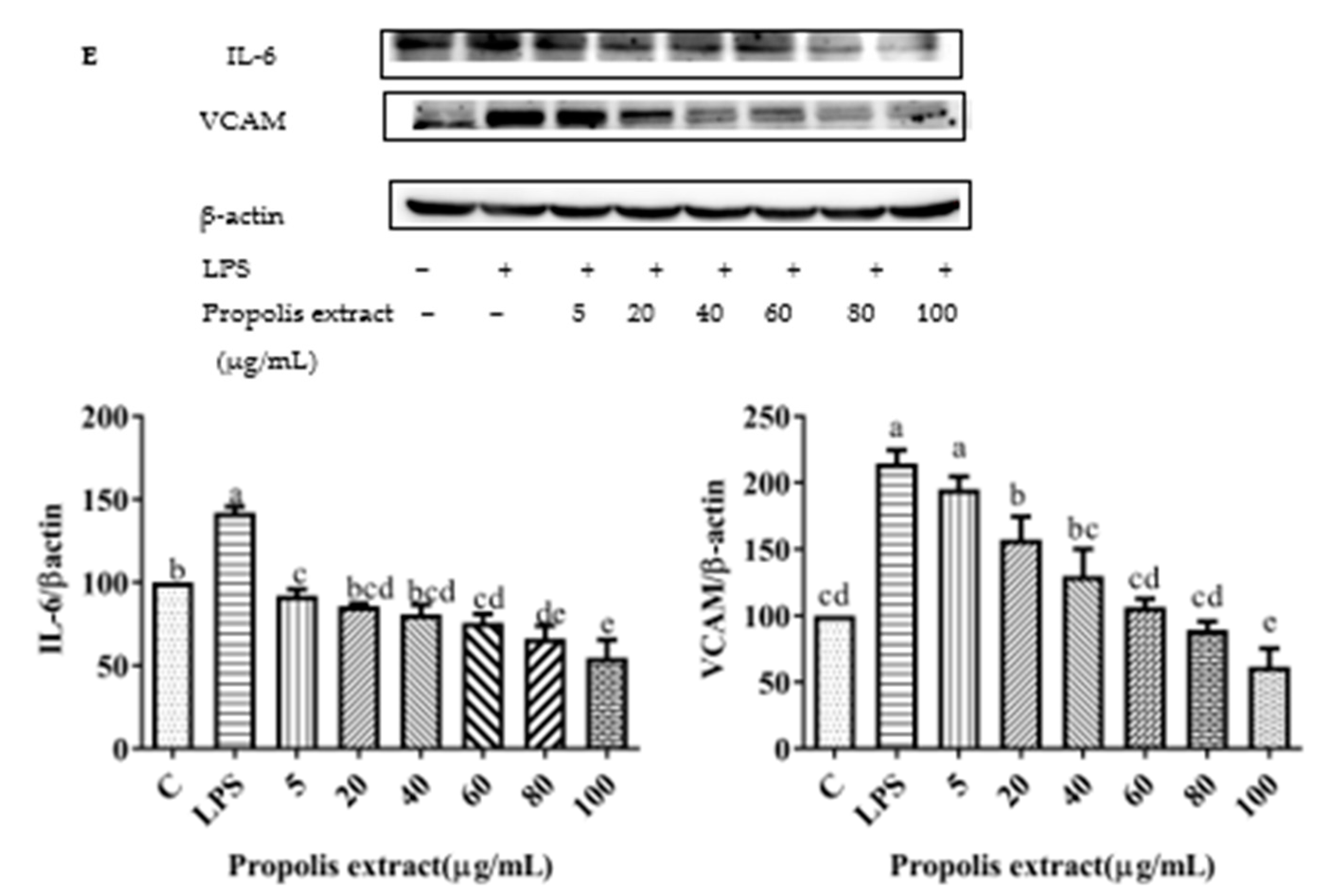
3.10. Chrysin, Pinocembrin, Galangin, Pinobanksin, and Propolis Extract Down-Regulated the Expression of Pro-Inflammatory Cytokines
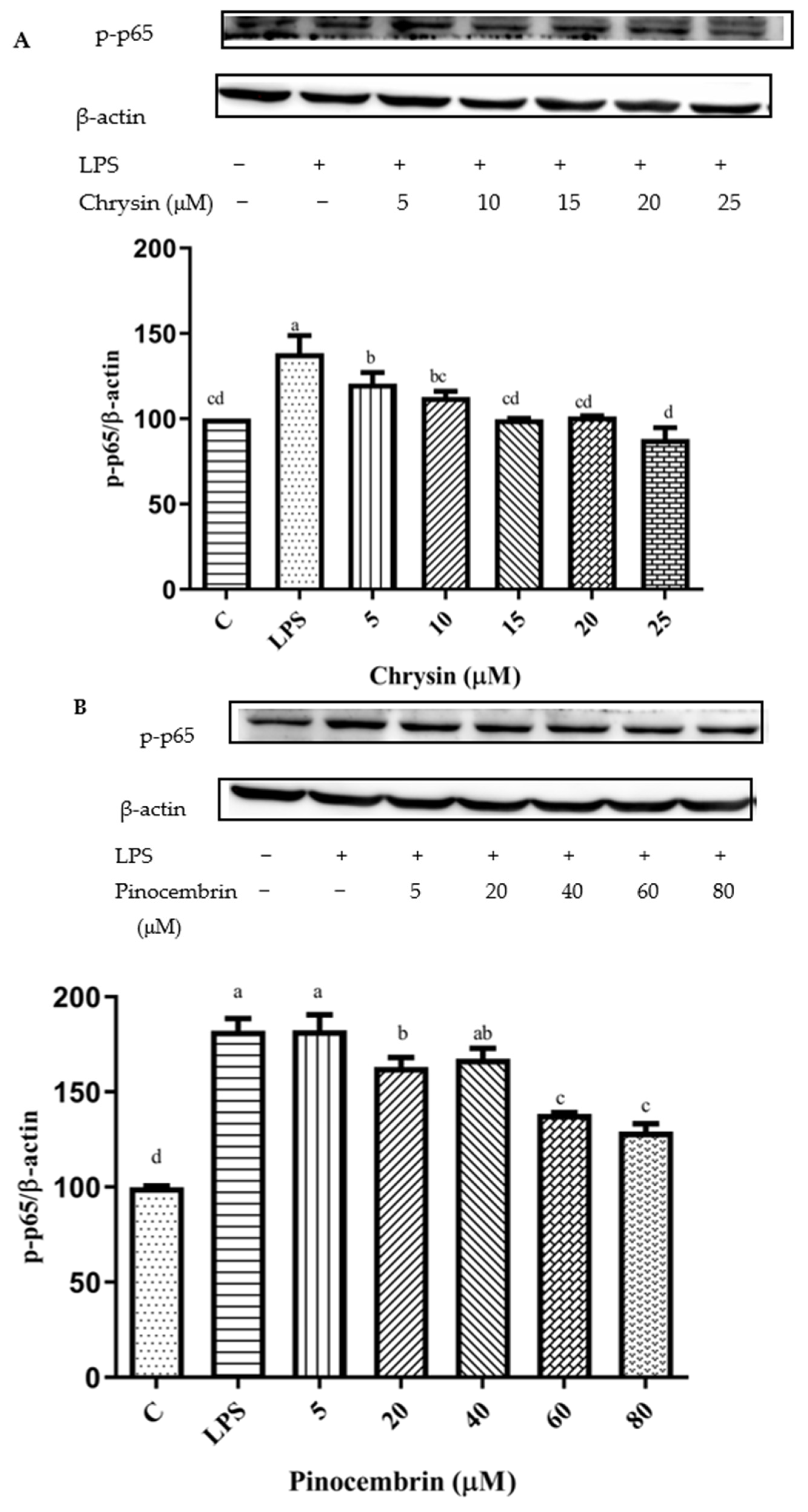
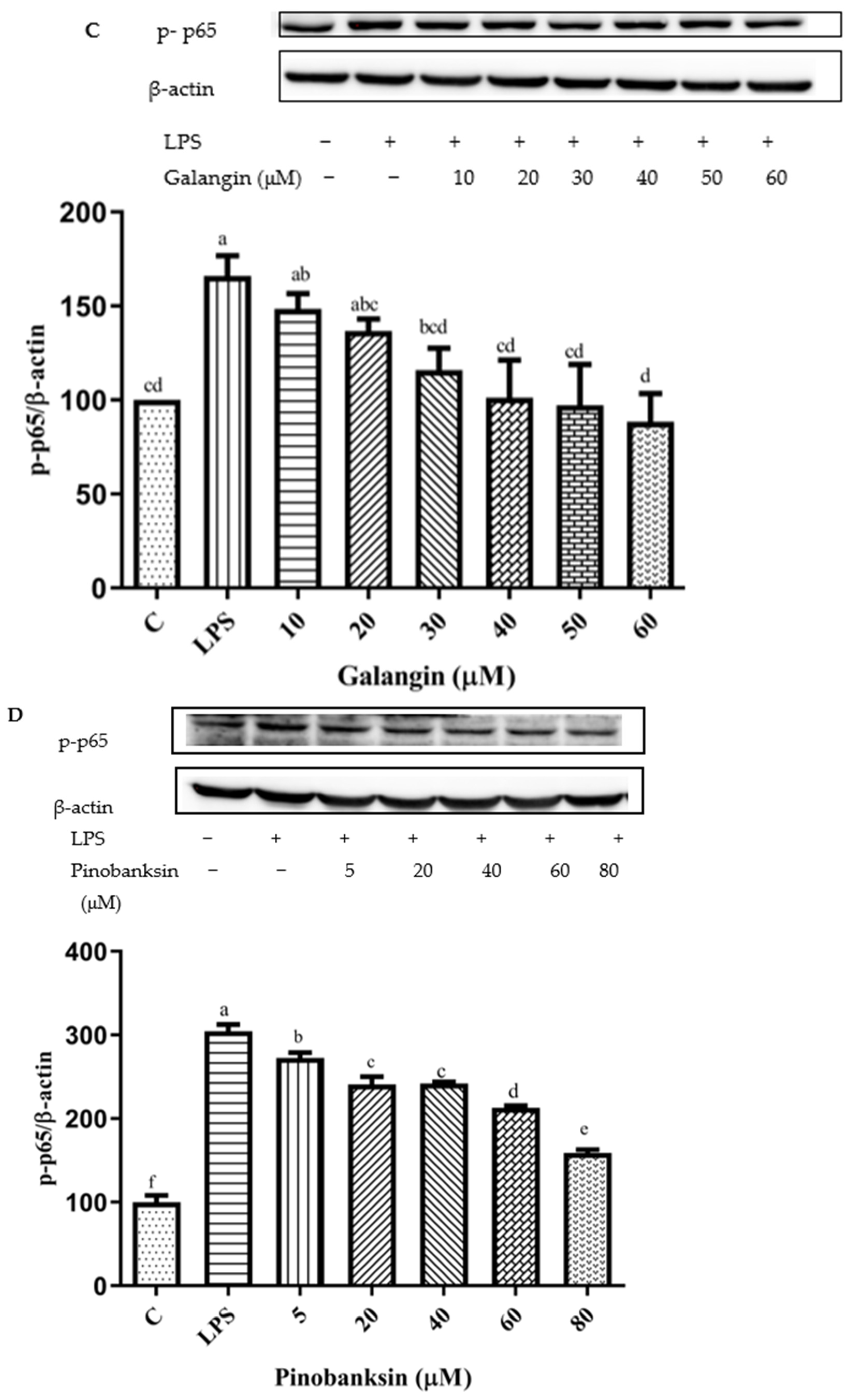
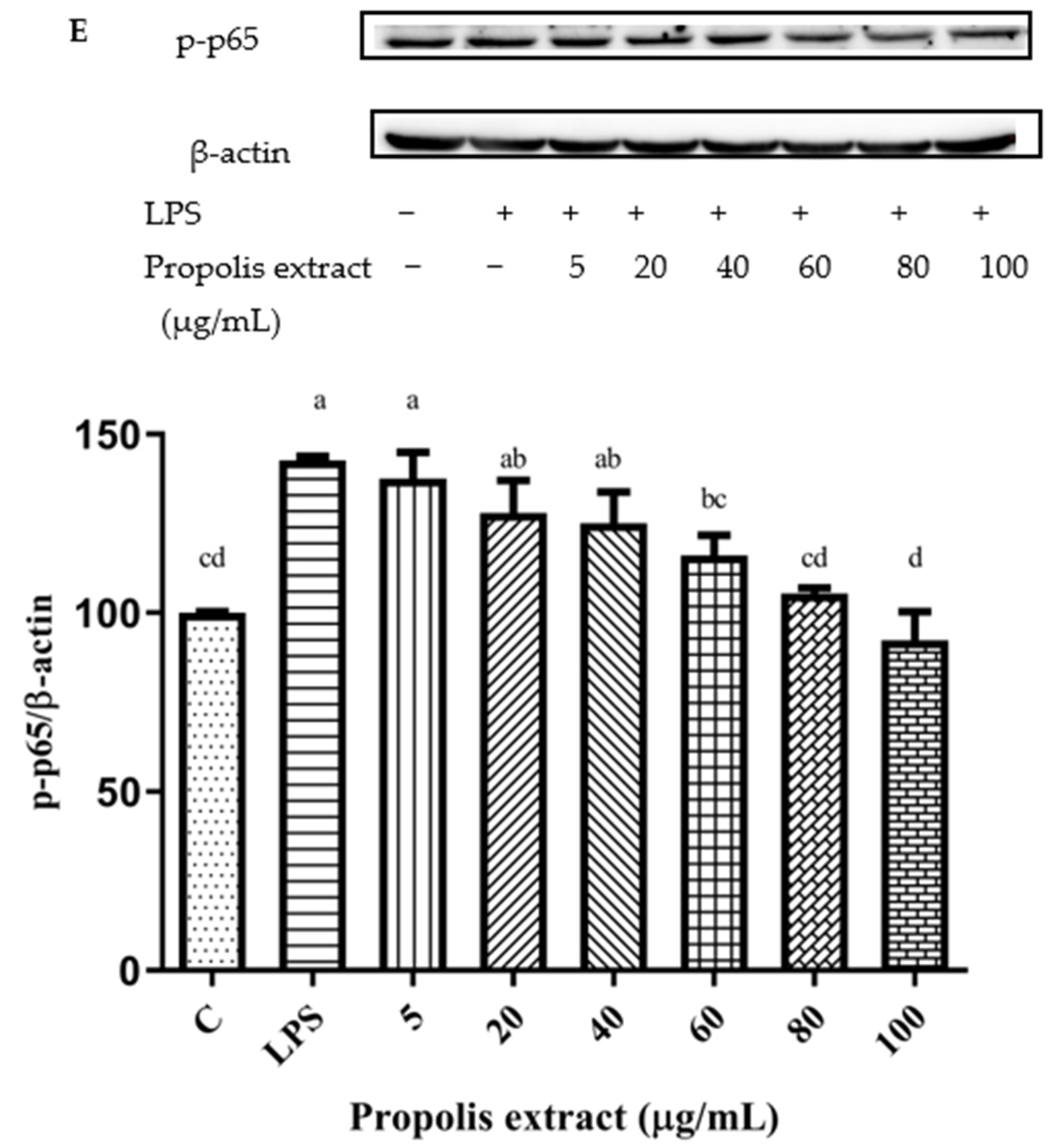
3.11. Inhibition of NF-κB Signaling Pathway
4. Discussion
4.1. Interpretation of Antioxidant and Pro-Oxidant Effects
4.2. Activation of Nrf2 Signaling Pathway and Inhibition of NF-κB Signaling Pathway
4.3. Bioavailability Issue of Chrysin, Pinocembrin, Galangin, Pinobanksin, and Propolis Extract
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Toan, S.; Zhou, H. New insights into the role of mitochondria in cardiac microvascular ischemia/reperfusion injury. Angiogenesis 2020, 23, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Taube, A.; Schlich, R.; Sell, H.; Eckardt, K.; Eckel, J. Inflammation and metabolic dysfunction: Links to cardiovascular diseases. Am. J. Physiol.-Heart Circ. Physiol. 2012, 302, H2148–H2165. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Mikhed, Y.; Daiber, A.; Steven, S. Mitochondrial Oxidative Stress, Mitochondrial DNA Damage and Their Role in Age-Related Vascular Dysfunction. Int. J. Mol. Sci. 2015, 16, 15918–15953. [Google Scholar] [CrossRef]
- Oelze, M.; Kroller-Schon, S.; Steven, S.; Lubos, E.; Doppler, C.; Hausding, M.; Tobias, S.; Brochhausen, C.; Li, H.; Torzewski, M.; et al. Glutathione peroxidase-1 deficiency potentiates dysregulation modifications of endothelial nitric oxide synthase and vascular dysfunction in aging. Hypertension 2014, 63, 390–396. [Google Scholar] [CrossRef]
- Wenzel, P.; Kossmann, S.; Munzel, T.; Daiber, A. Redox regulation of cardiovascular inflammation immunomodulatory func-tion of mitochondrial and Nox-derived reactive oxygen and nitrogen species. Free Radic Biol. Med. 2017, 109, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Pittman, K.; Kubes, P. Damage-Associated Molecular Patterns Control Neutrophil Recruitment. J. Innate Immun. 2013, 5, 315–323. [Google Scholar] [CrossRef]
- Ortona, E.; Maselli, A.; Delunardo, F.; Colasanti, T.; Giovannetti, A.; Pierdominici, M. Relationship Between Redox Status and Cell Fate in Immunity and Autoimmunity. Antioxid. Redox Signal. 2014, 21, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Brüne, B.; Dehne, N.; Grossmann, N.; Jung, M.; Namgaladze, D.; Schmid, T.; Von Knethen, A.; Weigert, A. Redox Control of Inflammation in Macrophages. Antioxid. Redox Signal. 2013, 19, 595–637. [Google Scholar] [CrossRef] [PubMed]
- Janko, C.; Filipovic, M.; Munoz, L.E.; Schorn, C.; Schett, G.; Ivanović-Burmazović, I.; Herrmann, M. Redox modulation of HMGB1-related signaling. Antioxid. Redox Signal. 2014, 20, 1075–1085. [Google Scholar] [CrossRef]
- Huang, W.; Xu, L.; Zhou, X.Q.; Gao, C.L.; Yang, M.J.; Chen, G.; Zhu, J.H.; Jiang, L.; Gan, H.K.; Gou, F.; et al. High glucose induces activation of NF-κB inflammatory signaling through IκBα sumoylation in rat mesangial cells. Biochem. Biophys. Res. Commun. 2013, 438, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Búfalo, M.C.; Ferreira, I.; Costa, G.; Francisco, V.; Liberal, J.; Cruz, M.T.; Lopes, M.C.; Batista, M.T.; Sforcin, J.M. Propolis and its constituent caffeic acid suppress LPS-stimulated pro-inflammatory response by blocking NF-κB and MAPK activation in macrophages. J. Ethnopharmacol. 2013, 149, 84–92. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cha, Y.-N.; Surh, Y.-J. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2010, 690, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Daleprane, J.; Abdalla, D.S. Emerging Roles of Propolis: Antioxidant, Cardioprotective, and Antiangiogenic Actions. Evid.-Based Complement. Altern. Med. 2013, 2013, 175135. [Google Scholar] [CrossRef]
- Cuevas, A.; Saavedra, N.; Cavalcante, M.F.; Salazar, L.A.; Abdalla, D.S.P. Identification of microRNAs involved in the modulationof pro-angiogenic factors in atherosclerosis by a polyphenol-rich extract from propolis. Arch. Biochem. Biophys. 2014, 557, 28–35. [Google Scholar] [CrossRef]
- Fukuda, T.; Fukui, M.; Tanaka, M.; Senmaru, T.; Iwase, H.; Yamazaki, M.; Aoi, W.; Inui, T.; Nakamura, N.; Marunaka, Y. Effect of Brazilian green propolis in patients with type 2 diabetes: A double-blind randomized placebo-controlled study. Biomed. Rep. 2015, 3, 355–360. [Google Scholar] [CrossRef]
- Gao, W.; Pu, L.; Wei, J.; Yao, Z.; Wang, Y.; Shi, T.; Zhao, L.; Jiao, C.; Guo, C. Serum Antioxidant Parameters are Significantly Increased in Patients with Type 2 Diabetes Mellitus after Consumption of Chinese Propolis: A Randomized Controlled Trial Based on Fasting Serum Glucose Level. Diabetes Ther. 2018, 9, 101–111. [Google Scholar] [CrossRef]
- Bhadauria, M. Propolis Prevents Hepatorenal Injury Induced by Chronic Exposure to Carbon Tetrachloride. Evid.-Based Complement. Altern. Med. 2012, 2012, 235358. [Google Scholar] [CrossRef]
- Ulusoy, H.B.; Öztürk, I.; Sönmez, M.F. Protective effect of propolis on methotrexate-induced kidney injury in the rat. Ren. Fail. 2016, 38, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Nanaware, S.; Shelar, M.; Sinnathambi, A.; Mahadik, K.; Lohidasan, S. Neuroprotective effect of Indian propolis in β-amyloid induced memory deficit: Impact on behavioral and biochemical parameters in rats. Biomed. Pharmacother. 2017, 93, 543–553. [Google Scholar] [CrossRef]
- Choudhari, M.K.; Haghniaz, R.; Rajwade, J.M.; Paknikar, K.M. Anticancer activity of Indian stingless bee propolis: An in vitro study. Evid.-Based Complement. Altern. Med. 2013, 2013, 928280. [Google Scholar] [CrossRef] [PubMed]
- Mantawy, E.M.; El-Bakly, W.M.; Esmat, A.; Badr, A.M.; El-Demerdash, E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur. J. Pharmacol. 2014, 728, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Khezri, S.; Sabzalipour, T.; Jahedsani, A.; Azizian, S.; Atashbar, S.; Salimi, A. Chrysin ameliorates aluminum phosphide-induced oxidative stress and mitochondrial damages in rat cardiomyocytes and isolated mitochondria. Environ. Toxicol. 2020, 35, 1114–1124. [Google Scholar] [CrossRef]
- Kim, M.E.; Park, P.R.; Na, J.Y.; Jung, I.; Cho, J.H.; Lee, J.S. Anti-neuroinflammatory effects of galangin in LPS-stimulated BV2 microglia through regulation of IL-1β production and the NF-κB signaling pathways. Mol. Cell Biol. 2019, 451, 145–153. [Google Scholar]
- Aladaileh, S.H.; Abukhalil, M.H.; Saghir, S.A.; Hanieh, H.; Alfwuaires, M.; Almaiman, A.A.; Bin-Jumah, M.; Mahmoud, A.M. Galangin activates Nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclo-phosphamide-induced hepatotoxicity. Biomolecules 2019, 9, 346. [Google Scholar] [CrossRef]
- Jiang, X.; Cai, S.; Jin, Y.; Wu, F.; He, J.; Wu, X.; Tan, Y.; Wang, Y. Irisin Attenuates Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis in the H9C2 Cellular Model of Septic Cardiomyopathy through Augmenting Fundc1-Dependent Mitophagy. Oxidative Med. Cell. Longev. 2021, 2021, 2989974. [Google Scholar] [CrossRef]
- Hao, R.; Su, G.; Sun, X.; Kong, X.; Zhu, C.; Su, G. Adiponectin attenuates lipopolysaccharide-induced cell injury of H9c2 cells by regulating AMPK pathway. Acta Biochim. Biophys. Sin. 2019, 51, 168–177. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Long, K.; Qiu, W.; Wang, Y.; Hu, Z.; Liu, C.; Luo, Y.; Jiang, A.; Jin, L.; et al. Overexpression of Exosomal Cardioprotective miRNAs Mitigates Hypoxia-Induced H9c2 Cells Apoptosis. Int. J. Mol. Sci. 2017, 18, 711. [Google Scholar] [CrossRef]
- Lama, S.; Monda, V.; Rizzo, M.R.; Dacrema, M.; Maisto, M.; Annunziata, G.; Tenore, G.C.; Novellino, E.; Stiuso, P. Cardio-protective Effects of Taurisolo® in Cardiomyoblast H9c2 Cells under High-Glucose and Trimethylamine N-Oxide Treatment via De Novo Sphingolipid Synthesis. Oxid. Med. Cell Longev. 2020, 2020, 2961406. [Google Scholar] [CrossRef] [PubMed]
- Boufadi, Y.M.; Van Antwerpen, P.; Alard, I.C.; Nève, J.; Djennas, N.; Riazi, A.; Soubhye, J. Antioxidant effects and bioavailability evaluation of propolis extract and its content of pure polyphenols. J. Food Biochem. 2018, 42, e12434. [Google Scholar] [CrossRef]
- Ferreira, J.M.; Fernandes-Silva, C.C.; Salatino, A.; Negri, G.; Message, D. New propolis type from north-east Brazil: Chemical composition, antioxidant activity and botanical origin. J. Sci. Food Agric. 2017, 97, 3552–3558. [Google Scholar] [CrossRef] [PubMed]
- Midorikawa, K.; Banskota, A.H.; Tezuka, Y.; Nagaoka, T.; Matsushige, K.; Message, D.; Huertas, A.A.G.; Kadota, S. Liquid chromatography-mass spectrometry analysis of propolis. Phytochem. Anal. 2001, 12, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Falcão, S.I.; Vale, N.; Gomes, P.; Domingues, M.R.; Freire, C.; Cardoso, S.M.; Vilas-Boas, M. Phenolic Profiling of Portuguese Propolis by LC-MS Spectrometry: Uncommon Propolis Rich in Flavonoid Glycosides. Phytochem. Anal. 2013, 24, 309–318. [Google Scholar] [CrossRef]
- Pellati, F.; Orlandini, G.; Pinetti, D.; Benvenuti, S. HPLC-DAD and HPLC-ESI-MS/MS methods for metabolite profiling of propolis extracts. J. Pharm. Biomed. Anal. 2011, 55, 934–948. [Google Scholar] [CrossRef]
- Castro, C.; Mura, F.; Valenzuela, G.; Figueroa, C.; Salinas, R.; Zuñiga, M.C.; Torres, J.L.; Fuguet, E.; Delporte, C. Identification of phenolic compounds by HPLC-ESI-MS/MS and antioxidant activity from Chilean propolis. Food Res. Int. 2014, 64, 873–879. [Google Scholar] [CrossRef]
- Yuan, Y.; Zheng, S.L.; Zeng, L.H.; Deng, Z.Y.; Zhang, B.; Li, H.Y. The phenolic compounds, metabolites, and antioxidant activity of propolis extracted by ultrasound-assisted method. J. Food Sci. 2019, 84, 3850–3865. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Manda, G.; Nechifor, M.; Neagu, M. Reactive oxygen species, cancer and anti-cancer therapies. Curr. Chem. Biol. 2009, 3, 22–46. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef] [PubMed]
- Hanus, J.; Kolkin, A.; Chimienti, J.; Botsay, S.; Wang, S. 4-Acetoxyphenol Prevents RPE Oxidative Stress–Induced Necrosis by Functioning as an NRF2 Stabilizer. Investig. Opthalmol. Vis. Sci. 2015, 56, 5048–5059. [Google Scholar] [CrossRef][Green Version]
- Bai, X.; Chen, Y.; Hou, X.; Huang, M.; Jin, J. Emerging role of NRF2 in chemoresistance by regulating drug-metabolizing enzymes and efflux transporters. Drug Metab. Rev. 2016, 48, 541–567. [Google Scholar] [CrossRef]
- Baig, M.S.; Zaichick, S.V.; Mao, M.; de Abreu, A.L.; Bakhshi, F.R.; Hart, P.C.; Saqib, U.; Deng, J.; Chatterjee, S.; Block, M.L.; et al. NOS-derived nitric oxide promotes NF-κB transcriptional activity throughinhibition of suppressor of cytokine signaling-1. J. Exp. Med. 2015, 212, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.; Korbut, R.; Adamek-Guzik, T. Nitricoxide and superoxide ininflammation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar] [PubMed]
- Xie, Q.; Kashiwabara, Y.; Nathan, C. Role of transcription factor NF-κB/Relin induction of nitric oxide synthase. J. Biol. Chem. 1994, 269, 4705–4708. [Google Scholar] [CrossRef]
- Han, L.; Yu, J.; Chen, Y.Y.; Cheng, D.; Wang, X.; Wang, C.L. Immunomodulatory Activity of docosahexenoic acid on RAW264.7 cells activation through GPR120-mediated signaling pathway. J. Agric. Food Chem. 2018, 66, 926–934. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and pro-oxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D.; Tsai, H.-L.; Huang, S.-L. Pro-oxidative Properties of Flavonoids in Human Lymphocytes. Biosci. Biotechnol. Biochem. 2003, 67, 1215–1222. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Wang, Y.-H.; Liou, C.-C.; Lin, Y.-C.; Huang, H.; Liu, Y.-C. Induction of Oxidative DNA Damage by Flavonoids of Propolis: Its Mechanism and Implication about Antioxidant Capacity. Chem. Res. Toxicol. 2012, 25, 191–196. [Google Scholar] [CrossRef]
- Hodnick, W.F.; Milosavljević, E.B.; Nelson, J.H.; Pardini, R.S. Electrochemistry of flavonoids: Relationships between redox potentials, inhibition of mitochondrial respiration, and production of oxygen radicals by flavonoids. Biochem. Pharmacol. 1988, 37, 2607–2611. [Google Scholar] [CrossRef]
- Fiorucci, S.; Golebiowski, J.; Cabrol-Bass, D.; Antonczak, S. DFT Study of quercetin activated forms involved in antiradical, antioxidant, and pro-oxidant biological processes. J. Agric. Food Chem. 2007, 55, 903–911. [Google Scholar] [CrossRef]
- Chen, W.; Lin, Y.C.; Ma, X.Y.; Jiang, Z.Y.; Lan, S.P. High concentrations of genistein exhibit pro-oxidant effects in primary muscle cells through mechanisms involving 5-lipoxygenase-mediated production of reactive oxygen species. Food Chem. Toxicol. 2014, 67, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; Lin, A.; Sultan, A.M.; O’Brien, P.J. Cellular and in vivo hepatotoxicity caused by green teaphenolic acids and catechins. Free Radic Biol. Med. 2006, 40, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defense pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef]
- Kumar, S.; Moniruzzaman, M.; Chakraborty, A.; Sarbajna, A.; Chakraborty, S.B. Crosstalk between heat shock proteins, Nrf2, NF-κB and different endogenous antioxidants during lead-induced hepatotoxicity in Puntius ticto. Aquat. Toxicol. 2021, 233, 105771. [Google Scholar] [CrossRef]
- Robaszkiewicz, A.; Balcerczyk, A.; Bartosz, G. Antioxidative and pro-oxidative effects of quercetin on A549 cells. Cell Biol. Int. 2007, 31, 1245–1250. [Google Scholar] [CrossRef]
- Falcão, S.I.; Vale, N.; Cos, P.; Gomes, P.; Freire, C.; Maes, L.; Vilas-Boas, M. In Vitro Evaluation of Portuguese Propolis and Floral Sources for Antiprotozoal, Antibacterial and Antifungal Activity. Phytother. Res. 2014, 28, 437–443. [Google Scholar] [CrossRef]
- Ren, N.; Kim, E.; Li, B.; Pan, H.; Tong, T.; Yang, C.S.; Tu, Y. Flavonoids Alleviating Insulin Resistance through Inhibition of Inflammatory Signaling. J. Agric. Food Chem. 2019, 67, 5361–5373. [Google Scholar] [CrossRef]
- Zhou, L.T.; Wang, K.J.; Li, L.; Li, H.; Geng, M. Pinocembrin inhibits lipopolysaccharide-induced inflammatory mediators production in BV2 microglial cells through suppression of PI3K/Akt/NF-κB pathway. Eur. J. Pharmacol. 2015, 761, 211–216. [Google Scholar] [CrossRef]
- Surh, Y.-J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Curti, V.; Zaccaria, V.; Sokeng, A.J.; Dacrema, M.; Masiello, I.; Mascaro, A.; D’Antona, G.; Daglia, M. Bioa-vailability and In Vivo Antioxidant Activity ofa Standardized Polyphenol Mixture Extracted from Brown Propolis. Int. J. Mol. Sci. 2019, 20, 1250. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.L.; Zhao, F.; Yin, J.T.; Liang, C.J.; Niu, X.L.; Qiu, Z.H.; Zhang, L.T. Two Approaches for Evaluating the Effects of Galangin on the Activities and mRNA Expression of Seven CYP450. Molecules 2019, 24, 1171. [Google Scholar] [CrossRef]
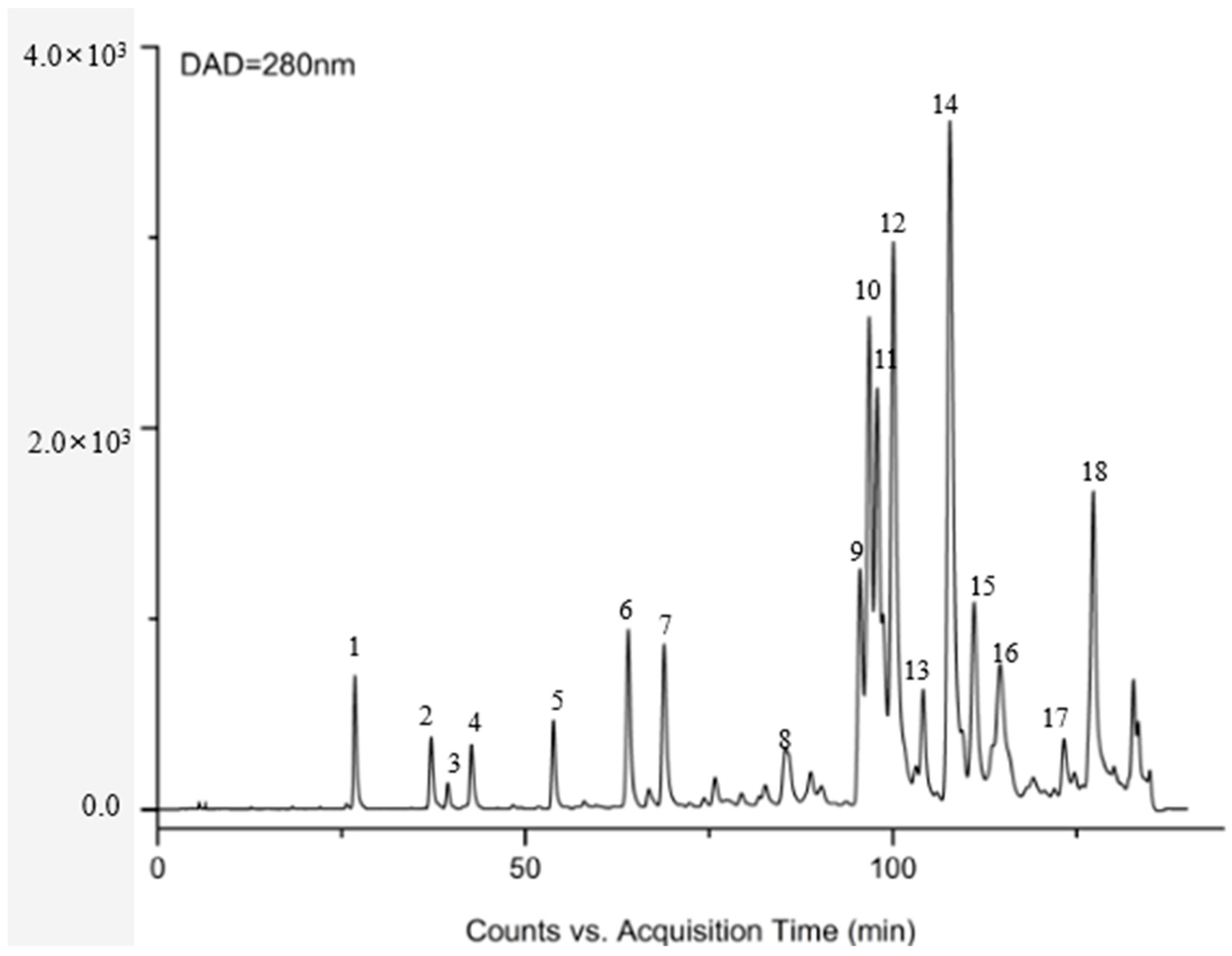



| No. | Identification | RT (min) | Formula | [M-H]/(m/z) | Major Fragment Ions (m.z) | |
|---|---|---|---|---|---|---|
| Measured | Calculated | |||||
| 5 | Pinobanksin-5-methylether | 63.98 | C16H14O5 | 285.0689 | 285.0692 | [M-H-H2O]− = 267.0559 [M-H-H2O-CH3]− = 252.0333 [M-H-H2O-CH3-CO]− = 224.0397 [1,3A]− = 195.0382 [1,4A]− =138.0270 |
| 6 | apigenin-7-O-glucoside | 67.33 | C21H20O10 | 431.0854 | 431.0849 | [M-H-C6H11O5]− = 268.0295 |
| 7 | pinobanksin | 68.98 | C15H12O5 | 271.0612 | 271.0552 | [M-H-H2O]− = 253.0428 [M-H-H2O-CO]− = 225.0489 [M-H-H2O-2CO]− =197.0547 [1,3A]− = 150.9997 [1,4A]− = 125.0218 |
| 8 | methoxyl-cyanidenon | 88.81 | C16H12O6 | 299.0464 | 299.0464 | [M-H-CO2]− = 255.0218 [M-H-CH3]− = 284.0228 [M-H-CO2-CO]− = 227.0276 |
| 11 | Pinocembrin | 97.01 | C15H12O4 | 255.0663 | 255.0582 | [M-H-C2H2O]− = 213.0472 [M-H-C3O2]− = 187.0691 [1,3A]− = 150.9974 [1,3A-CO2]− = 107.0091 |
| 12 | Pinobanksin 3-O-acetate | 100.02 | C17H14O6 | 313.0612 | 313.0609 | [M-acetate]− = 271.0509 [M-acetate-H2O]− = 253.0419 [M-acetate-H2O-CO2]− = 209.0529 [M-acetate-H2O-C3O2-C2H2O]− = 143.0457 |
| 14 | chrysin | 107.44 | C15H10O4 | 253.0419 | 253.0424 | [M-H-CO2]− = 209.0517 [M-H-CO2-CO]− = 181.0579 [1,3A]− = 143.0441 [1,4A]− = 107.0000 |
| 15 | galangin | 110.87 | C15H10O5 | 269.0418 | 269.0423 | [M-H-2CO]− = 213.0504 [M-H-2CO-CO2]− = 169.0611 |
| 16 | galangin- 5-methylether | 114.38 | C16H12O5 | 283.052 | 283.0519 | [M-H-CH3]− = 268.0273 [M-H-CO2]− = 239.0262 [M-H-CO2-CO]− = 211.0319 |
| 17 | pinocembrin-7-methylether | 123.22 | C16H14O4 | 269.073 | 269.0729 | [M-H-CH3]− =254.0485 [M-H-CH3-CO]− = 226.0548 [M-H-C8H8]− = 165.0132 [M-H-C8H8-CO2]− = 121.996 |
| 18 | Pinobanksin-3-O-butyric | 126.72 | C19H18O6 | 341.0917 | 341.092 | [M-butyrate-H2O]− = 253.0401 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Lu, H.; Yuan, Y.; Deng, Z.; Zheng, L.; Li, H. The Antioxidant and Anti-Inflammatory Effects of Flavonoids from Propolis via Nrf2 and NF-κB Pathways. Foods 2022, 11, 2439. https://doi.org/10.3390/foods11162439
Xu W, Lu H, Yuan Y, Deng Z, Zheng L, Li H. The Antioxidant and Anti-Inflammatory Effects of Flavonoids from Propolis via Nrf2 and NF-κB Pathways. Foods. 2022; 11(16):2439. https://doi.org/10.3390/foods11162439
Chicago/Turabian StyleXu, Wenzhen, Han Lu, Yuan Yuan, Zeyuan Deng, Liufeng Zheng, and Hongyan Li. 2022. "The Antioxidant and Anti-Inflammatory Effects of Flavonoids from Propolis via Nrf2 and NF-κB Pathways" Foods 11, no. 16: 2439. https://doi.org/10.3390/foods11162439
APA StyleXu, W., Lu, H., Yuan, Y., Deng, Z., Zheng, L., & Li, H. (2022). The Antioxidant and Anti-Inflammatory Effects of Flavonoids from Propolis via Nrf2 and NF-κB Pathways. Foods, 11(16), 2439. https://doi.org/10.3390/foods11162439






