Synbiotics and Gut Microbiota: New Perspectives in the Treatment of Type 2 Diabetes Mellitus
Abstract
:1. Introduction
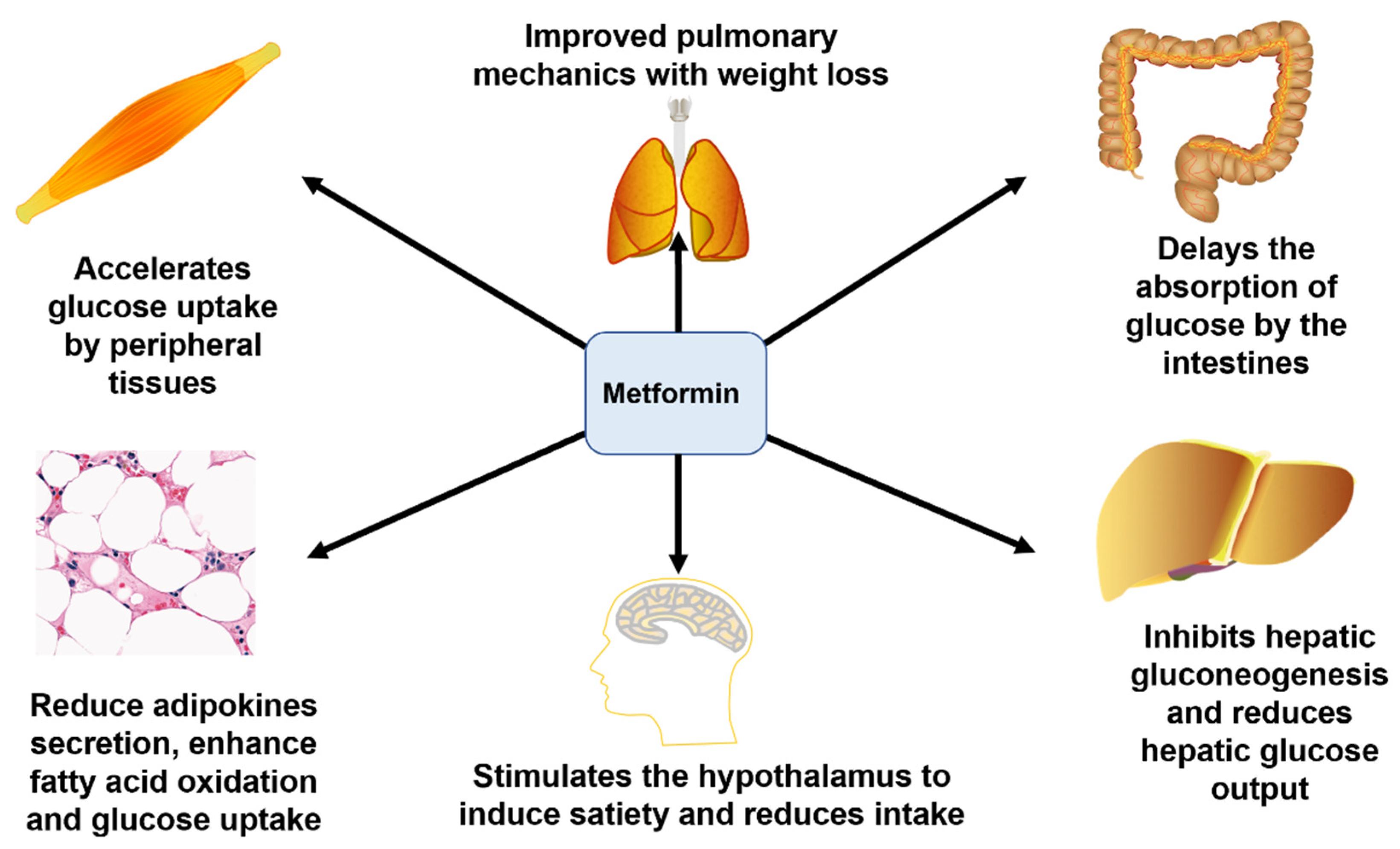
2. The History of T2DM Treatment
3. Definition, Function, and Study of Synbiotics
3.1. Definition
3.2. Synbiotics—Components and Function
3.3. Synbiotics in Disease Treatment
4. Synbiotics in the Treatment of T2DM
4.1. Animal Models
4.2. Clinical Studies
5. Regulation Mechanisms of Gut Microbiota by Synbiotics
- (1)
- The intestinal flora regulates the absorption and utilization of nutrients and energy. The gut is the first gateway for glucose absorption and utilization and plays a crucial role in the regulation of glycemic homeostasis. The gut microbiota can ferment carbohydrates in foods that cannot be digested by the host itself by encoding a large number of glycoside hydrolases to convert them into monosaccharides and broken chain fatty acids (SCFAs), which have been found to alter the composition of the gut microbiota in obese and T2DM patients, affecting the gene expression of broken chain fatty acid receptors and affecting the starvation and repletion cycle of the host [101]. On the other hand, products of intestinal flora (such as methane and SCFAs) can slow intestinal peristalsis, prolong the transit time of intestinal contents, cause enteral nutrition, including glucose, to be more fully absorbed, and directly affect the postprandial blood glucose content. The gut microbiota can also be involved in the pathogenesis of obesity and T2DM by regulating bile acid synthesis and regulating fat and glucose metabolism [102];
- (2)
- Intestinal flora is involved in lipogenesis and storage. Significant pathophysiological features of T2DM are insulin resistance accompanied by an absolute or relative deficiency in insulin secretion due to the fact of a defect in pancreatic beta-cell function, and obesity is strongly associated with insulin resistance. Gut microbiota can affect host lipogenesis and storage by a variety of mechanisms. On the one hand, intestinal flora upregulates the expression of the hepatic carbohydrate response element-binding protein and sterol regulatory element-binding protein-1 mRNA, thereby inducing the production of acetyl-CoA carboxylase and fatty acid synthase, key enzymes of lipogenesis, and promoting hepatic triglyceride synthesis. On the other hand, the intestinal flora downregulates fasting-induced adipocytokine (Fiaf) expression produced by intestinal epithelial cells. Fifa inhibits white adipose and muscle tissue from absorbing fatty acids from triglyceride-rich lipoproteins in the blood by acting on lipoprotein lipase (LPL). It was further found that Fiaf can also resist diet-induced obesity by inducing the expression of peroxisome proliferator-activated receptor costimulators, initiating the fatty acid oxidative metabolic pathway, and increasing the transcriptional activity of fatty acid magnesium oxide to increase fatty acid β-oxidation [103];
- (3)
- Chronic low-grade inflammatory response are caused by intestinal flora disorder. T2DM has varying degrees of chronic low-grade inflammatory responses characterized by metabolic endotoxemia and disorders of the endocannabinoid system [104]. Available evidence suggests that gut microbiota can affect lipid metabolism and induce systemic chronic low-grade inflammatory responses in animals, leading to the development of obesity and insulin resistance, and this pathogenic role may be much greater than the contribution of animal autogenetic defects to pathogenesis [105].
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Federation, I.D. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Wong, J.; Ross, G.P.; Zoungas, S.; Craig, M.E.; Davis, E.A.; Donaghue, K.C.; Maple-Brown, L.J.; McGill, M.J.; Shaw, J.E.; Speight, J.; et al. Management of type 2 diabetes in young adults aged 18–30 years: ADS/ADEA/APEG consensus statement. Med. J. Aust. 2022, 216, 422–429. [Google Scholar] [CrossRef]
- Hermansen, K.; Mortensen, L.S.; Hermansen, M.-L. Combining insulins with oral antidiabetic agents: Effect on hyperglycemic control, markers of cardiovascular risk and disease. Vasc. Health Risk Manag. 2008, 4, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tripathi, P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Zhou, D.-D.; Gan, R.-Y.; Huang, S.-Y.; Zhao, C.-N.; Shang, A.; Xu, X.-Y.; Li, H.-B. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: A narrative review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Benjamin, I.J.; Burke, G.L.; Chait, A.; Eckel, R.H.; Howard, B.V.; Mitch, W.; Smith, S.C., Jr.; Sowers, J.R. Diabetes and cardiovascular disease: A statement for healthcare professionals from the American Heart Association. Circulation 1999, 100, 1134–1146. [Google Scholar] [CrossRef]
- Thomas, M.C.; Cooper, M.E.; Zimmet, P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol. 2016, 12, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Formiguera, X.; Cantón, A. Obesity: Epidemiology and clinical aspects. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 1125–1146. [Google Scholar] [CrossRef]
- Drivsholm, T.; Olivarius, N.D.F.; Nielsen, A.B.S.; Siersma, V. Symptoms, signs and complications in newly diagnosed type 2 diabetic patients, and their relationship to glycaemia, blood pressure and weight. Diabetologia 2005, 48, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Guo, L. Medication treatment of type 2 diabetes. Clinical Medication Journal 2015, 13, 18–22. [Google Scholar]
- Brange, J. Galenics of Insulin: The Physico-Chemical and Pharmaceutical Aspects of Insulin and Insulin Preparations; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Schadewaldt, H. The history of diabetes mellitus. In Diabetes Its Medical and Cultural History; Springer: Berlin/Heidelberg, Germany, 1989; pp. 43–100. [Google Scholar]
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef]
- White, J.R., Jr. A brief history of the development of diabetes medications. Diabetes Spectr. 2014, 27, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Delawter, D.E.; Moss, J.M. Tolbutamide: Orally Effective Drug for Diabetes Mellitus. Am. J. Nurs. 1958, 58, 1106–1108. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Du, L.; Li, L.; Ma, J.; Geng, X.; Yao, X.; Liu, G.; Sun, X. Cancer risk of sulfonylureas in patients with type 2 diabetes mellitus: A systematic review. J. Diabetes 2017, 9, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Shlomai, G.; Neel, B.; Leroith, D.; Gallagher, E.J. Type 2 diabetes mellitus and cancer: The role of pharmacotherapy. J. Clin. Oncol. 2016, 34, 4261–4269. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.B.A.; Gomes, M.B. Metformin: An old but still the best treatment for type 2 diabetes. Diabetol. Metab. Syndr. 2013, 5, 6. [Google Scholar] [CrossRef]
- Hundal, R.S.; Krssak, M.; Dufour, S.; Laurent, D.; Lebon, V.; Chandramouli, V.; Inzucchi, S.E.; Schumann, W.C.; Petersen, K.F.; Landau, B.R.; et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000, 49, 2063–2069. [Google Scholar] [CrossRef]
- Lang, J.E. Obesity and asthma in children: Current and future therapeutic options. Paediatr. Drugs 2014, 16, 179–188. [Google Scholar] [CrossRef]
- Lee, C.; Chae, S.; Jo, S.; Jerng, U.; Bae, S. The relationship between the gut microbiome and metformin as a key for treating type 2 diabetes mellitus. Int. J. Mol. Sci. 2021, 22, 3566. [Google Scholar] [CrossRef] [PubMed]
- Mardinoglu, A.; Boren, J.; Smith, U. Confounding effects of metformin on the human gut microbiome in type 2 diabetes. Cell Metab. 2016, 23, 10–12. [Google Scholar] [CrossRef]
- Lalau, J.-D. Lactic acidosis induced by metformin. Drug Saf. 2010, 33, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Raptis, S.A.; Dimitriadis, G.D. Oral hypoglycemic agents: Insulin secretagogues, α-glucosidase inhibitors and insulin sensitizers. Exp. Clin. Endocrinol. Diabetes 2001, 109 (Suppl. S2), S265–S287. [Google Scholar] [CrossRef] [PubMed]
- Juan, L.N.T. Clinical understanding of glinides secretagogues. Chin. J. Pract. Intern. Med. 2014, 34, 959–961. [Google Scholar]
- Nissen, S.; Wolski, K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. J. Vasc. Surg. 2007, 356, 2457–2471. [Google Scholar]
- Mahboobi, S.; Rahimi, F.; Jafarnejad, S. Effects of prebiotic and synbiotic supplementation on glycaemia and lipid profile in type 2 diabetes: A meta-analysis of randomized controlled trials. Adv. Pharm. Bull. 2018, 8, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Mirmiranpour, H.; Huseini, H.F.; Derakhshanian, H.; Khodaii, Z.; Tavakoli-Far, B. Effects of probiotic, cinnamon, and synbiotic supplementation on glycemic control and antioxidant status in people with type 2 diabetes; a randomized, double-blind, placebo-controlled study. J. Diabetes Metab. Disord. 2020, 19, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Kolida, S.; Gibson, G.R. Synbiotics in health and disease. Annu. Rev. Food Sci. Technol. 2011, 2, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Ooi, L.-G.; Liong, M.-T. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. Int. J. Mol. Sci. 2010, 11, 2499–2522. [Google Scholar] [CrossRef]
- Lye, H.-S.; Kuan, C.-Y.; Ewe, J.-A.; Fung, W.-Y.; Liong, M.-T. The improvement of hypertension by probiotics: Effects on cholesterol, diabetes, renin, and phytoestrogens. Int. J. Mol. Sci. 2009, 10, 3755–3775. [Google Scholar] [CrossRef] [PubMed]
- Cunningham-Rundles, S.; Ahrné, S.; Bengmark, S.; Johann-Liang, R.; Marshall, F.; Metakis, L.; Califano, C.; Dunn, A.-M.; Grassey, C.; Hinds, G.; et al. Probiotics and immune response. Am. J. Gastroenterol. 2000, 95, S22–S25. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.; Kachrimanidou, V.; Bosnea, L.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Sarao, L.K.; Arora, M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V. Prebiotics and probiotics: New concepts in nutrition and health. Heinz Infant Nutr. Inst. 2002, 19, 1–4. [Google Scholar]
- Maftei, N.-M. Probiotic, prebiotic and synbiotic products in human health. In Frontiers and New Trends in the Science of Fermented Food and Beverages; IntechOpen: London, UK, 2019. [Google Scholar]
- Vyas, U.; Ranganathan, N. Probiotics, prebiotics, and synbiotics: Gut and beyond. Gastroenterol. Res. Pract. 2012, 2012, 872716. [Google Scholar] [CrossRef]
- Sengoopta, C. Rejuvenation and the prolongation of life: Science or quackery? Perspect. Biol. Med. 1993, 37, 55–66. [Google Scholar] [CrossRef]
- Gordon, S. Elie Metchnikoff, the man and the myth. J. Innate Immun. 2016, 8, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E. Probiotics in 2015: Their scope and use. J. Clin. Gastroenterol. 2015, 49, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.L.; Rajkumar, C.; Cooke, J.; Bulpitt, C.J. Probiotics in prevention of antibiotic associated diarrhoea: Meta-analysis. BMJ 2002, 324, 1361. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, G.; Rao, S.; Patole, S.; Bulsara, M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 2010, 125, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zuo, Z.; Mao, A.-P. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn’s disease, and pouchitis: Meta-analysis of randomized controlled trials. Inflamm. Bowel Dis. 2014, 20, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Sun, J.; He, J.; Chen, F.; Chen, R.; Chen, H. Effect of probiotics on glycemic control: A systematic review and meta-analysis of randomized, controlled trials. PLoS ONE 2015, 10, e0132121. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, Y.; Wen, Y.; Liu, G.; Wan, C. Efficacy of probiotics in non-alcoholic fatty liver disease in adult and children: A meta-analysis of randomized controlled trials. Hepatol. Res. 2016, 46, 1226–1233. [Google Scholar] [CrossRef]
- Sun, J.; Buys, N. Glucose-and glycaemic factor-lowering effects of probiotics on diabetes: A meta-analysis of randomised placebo-controlled trials. Br. J. Nutr. 2016, 115, 1167–1177. [Google Scholar] [CrossRef]
- Jonkers, D.M. Microbial perturbations and modulation in conditions associated with malnutrition and malabsorption. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 161–172. [Google Scholar] [CrossRef]
- Krumbeck, J.A.; Maldonado-Gomez, M.X.; Ramer-Tait, A.E.; Hutkins, R.W. Prebiotics and synbiotics: Dietary strategies for improving gut health. Curr. Opin. Gastroenterol. 2016, 32, 110–119. [Google Scholar] [CrossRef]
- Verspreet, J.; Damen, B.; Broekaert, W.F.; Verbeke, K.; Delcour, J.A.; Courtin, C.M. A critical look at prebiotics within the dietary fiber concept. Annu. Rev. Food Sci. Technol. 2016, 7, 167–190. [Google Scholar] [CrossRef]
- Preidis, G.; Versalovic, J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: Gastroenterology enters the metagenomics era. Gastroenterology 2009, 136, 2015–2031. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Takayama, H.; Morotomi, M.; Kuroshima, T.; Ueyama, S.; Matsumoto, K.; Kuroda, A.; Mutai, M. Effects of administration of TOS and Bifidobacterium breve 4006 on the human fecal flora. Bifidobact. Microflora 1983, 2, 17–24. [Google Scholar] [CrossRef]
- Catford, J.A.; Jansson, R.; Nilsson, C. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers. Distrib. 2009, 15, 22–40. [Google Scholar] [CrossRef]
- Costello, E.K.; Stagaman, K.; Dethlefsen, L.; Bohannan, B.J.M.; Relman, D.A. The application of ecological theory toward an understanding of the human microbiome. Science 2012, 336, 1255–1262. [Google Scholar] [CrossRef]
- Walter, J.; Maldonado-Gómez, M.X.; Martínez, I. To engraft or not to engraft: An ecological framework for gut microbiome modulation with live microbes. Curr. Opin. Biotechnol. 2018, 49, 129–139. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Trivedi, M.K.; Jha, A.; Lin, Y.-F.; Dimaano, L.; García-Romero, M.T. Synbiotics for prevention and treatment of atopic dermatitis: A meta-analysis of randomized clinical trials. JAMA Pediatr. 2016, 170, 236–242. [Google Scholar] [CrossRef]
- Pärtty, A.; Rautava, S.; Kalliomäki, M. Probiotics on pediatric functional gastrointestinal disorders. Nutrients 2018, 10, 1836. [Google Scholar] [CrossRef]
- Shukla, S.; Shukla, A.; Mehboob, S.; Guha, S. Meta-analysis: The effects of gut flora modulation using prebiotics, probiotics and synbiotics on minimal hepatic encephalopathy. Aliment. Pharmacol. Ther. 2011, 33, 662–671. [Google Scholar] [CrossRef]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef]
- Núñez-Sánchez, M.A.; Herisson, F.M.; Cluzel, G.L.; Caplice, N.M. Metabolic syndrome and synbiotic targeting of the gut microbiome. Curr. Opin. Food Sci. 2021, 41, 60–69. [Google Scholar] [CrossRef]
- Han, J.-L.; Lin, H.-L. Intestinal microbiota and type 2 diabetes: From mechanism insights to therapeutic perspective. World J. Gastroenterol.: WJG 2014, 20, 17737. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Hulston, C.J.; Churnside, A.A.; Venables, M. Probiotic supplementation prevents high-fat, overfeeding-induced insulin resistance in human subjects. Br. J. Nutr. 2015, 113, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, Q.; Guang, C.; Zhang, W.; Mu, W. An overview on biological production of functional lactose derivatives. Appl. Microbiol. Biotechnol. 2019, 103, 3683–3691. [Google Scholar] [CrossRef]
- Aït-Aissa, A.; Aïder, M. Lactulose: Production and use in functional food, medical and pharmaceutical applications. Practical and critical review. Int. J. Food Sci. Technol. 2014, 49, 1245–1253. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kumari, S. Lactulose: Production, purification and potential applications. Biotechnol. Adv. 2011, 29, 940–948. [Google Scholar] [CrossRef]
- Ballongue, J.; Schumann, C.; Quignon, P. Effects of lactulose and lactitol on colonic microflora and enzymatic activity. Scand. J. Gastroenterol. 1997, 32 (Suppl. S222), 41–44. [Google Scholar] [CrossRef]
- Hu, B. Production and utilization of L-arabinose in China. World J. Eng. Technol. 2018, 6, 24–36. [Google Scholar] [CrossRef]
- Seri, K.; Sanai, K.; Matsuo, N.; Kawakubo, K.; Xue, C.; Inoue, S. L-arabinose selectively inhibits intestinal sucrase in an uncompetitive manner and suppresses glycemic response after sucrose ingestion in animals. Metabolism 1996, 45, 1368–1374. [Google Scholar] [CrossRef]
- Osaki, S.; Kimura, T.; Sugimoto, T.; Hizukuri, S.; Iritani, N. L-arabinose feeding prevents increases due to dietary sucrose in lipogenic enzymes and triacylglycerol levels in rats. J. Nutr. 2001, 131, 796–799. [Google Scholar] [CrossRef]
- Murosaki, S.; Yamamoto, Y.; Ito, K.; Inokuchi, T.; Kusaka, H.; Ikeda, H.; Yoshikai, Y. Heat-killed Lactobacillus plantarum L-137 suppresses naturally fed antigen–specific IgE production by stimulation of IL-12 production in mice. J. Allergy Clin. Immunol. 1998, 102, 57–64. [Google Scholar] [CrossRef]
- de Vries, M.C.; Vaughan, E.E.; Kleerebezem, M.; de Vos, W.M. Lactobacillus plantarum—Survival, functional and potential probiotic properties in the human intestinal tract. Int. Dairy J. 2006, 16, 1018–1028. [Google Scholar] [CrossRef]
- Sáez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: A review of human clinical trials. Int. J. Mol. Sci. 2016, 17, 928. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, Y.; Sato, M.; Ogawa, A.; Miyoshi, M.; Uenishi, H.; Ogawa, H.; Ikuyama, K.; Kagoshima, M.; Tsuchida, T. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br. J. Nutr. 2013, 110, 1696–1703. [Google Scholar] [CrossRef]
- Sanchez, M.; Darimont, C.; Drapeau, V.; Emady-Azar, S.; Lepage, M.; Rezzonico, E.; Ngom-Bru, C.; Berger, B.; Philippe, L.; Ammon-Zuffrey, C.; et al. Effect of Lactobacillus rhamnosus CGMCC1. 3724 supplementation on weight loss and maintenance in obese men and women. Br. J. Nutr. 2014, 111, 1507–1519. [Google Scholar] [CrossRef]
- Moroti, C.; Souza Magri, L.F.S.; de Rezende Costa, M.D.R.; Cavallini, D.C.; Sivieri, K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.-S.; Esmaillzadeh, A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann. Nutr. Metab. 2013, 63, 1–9. [Google Scholar] [CrossRef]
- Tajadadi-Ebrahimi, M.; Bahmani, F.; Shakeri, H.; Hadaegh, H.; Hijijafari, M.; Abedi, F.; Asemi, Z. Effects of daily consumption of synbiotic bread on insulin metabolism and serum high-sensitivity C-reactive protein among diabetic patients: A double-blind, randomized, controlled clinical trial. Ann. Nutr. Metab. 2014, 65, 34–41. [Google Scholar] [CrossRef]
- Shakeri, H.; Hadaegh, H.; Abedi, F.; Tajabadi-Ebrahimi, M.; Mazroii, N.; Ghandi, Y.; Asemi, Z. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids 2014, 49, 695–701. [Google Scholar] [CrossRef]
- Kassaian, N.; Aminorroaya, A.; Feizi, A.; Jafari, P.; Amini, M. The effects of probiotic and synbiotic supplementation on metabolic syndrome indices in adults at risk of type 2 diabetes: Study protocol for a randomized controlled trial. Trials 2017, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, A.; Motamedzadeh, A.; Zarrati Mojarrad, M.; Bahmani, F.; Amirani, E.; Ostadmohammadi, V.; Tajabadi-Ebrahimi, M.; Asemi, Z. The effects of synbiotic supplementation on metabolic status in diabetic patients undergoing hemodialysis: A randomized, double-blinded, placebo-controlled trial. Probiotics Antimicrob. Proteins 2019, 11, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Velayati, A.; Kareem, I.; Sedaghat, M.; Sohrab, G.; Nikpayam, O.; Hedayati, M.; Abhari, K.; Hejazi, E. Does symbiotic supplementation which contains Bacillus Coagulans Lactobacillus rhamnosus, Lactobacillus acidophilus and fructooligosaccharide has favourite effects in patients with type-2 diabetes? A randomised, double-blind, placebo-controlled trial. Arch. Physiol. Biochem. 2021, 2, 1–8. [Google Scholar] [CrossRef]
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Erratum: Enterotypes of the human gut microbiome (Nature (2011) 473 (174-180)). Nature 2011, 474, 666. [Google Scholar] [CrossRef]
- Siezen, R.J.; Kleerebezem, M. The human gut microbiome: Are we our enterotypes? Microb. Biotechnol. 2011, 4, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Hehemann, J.-H.; Correc, G.; Barbeyron, T.; Helbert, W.; Czjzek, M.; Michel, G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 2010, 464, 908–912. [Google Scholar] [CrossRef]
- Xu, X.; Xu, P.; Ma, C.; Tang, J.; Zhang, X. Gut microbiota, host health, and polysaccharides. Biotechnol. Adv. 2013, 31, 318–337. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef]
- Velikonja, A.; Lipoglavšek, L.; Zorec, M.; Orel, R.; Avguštin, G. Alterations in gut microbiota composition and metabolic parameters after dietary intervention with barley beta glucans in patients with high risk for metabolic syndrome development. Anaerobe 2019, 55, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.-C.D. Diet and gut microbiota in health and disease. Intest. Microbiome: Funct. Asp. Health Dis. 2017, 88, 117–126. [Google Scholar]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The long-term stability of the human gut microbiota. Science 2013, 341, 6141. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, F.; Liang, B.; Liang, Y.; Chen, S.; Mo, X.; Ju, Y.; Zhao, H.; Jia, H.; Spector, T.D.; et al. A metagenome-wide association study of gut microbiota in asthma in UK adults. BMC Microbiol. 2018, 18, 114. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Remely, M.; Aumueller, E.; Merold, C.; Dworzak, S.; Hippe, B.; Zanner, J.; Pointner, A.; Brath, H.; Haslberger, A.G. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene 2014, 537, 85–92. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Tang, W.; Li, D.Y.; Hazen, S.L. Dietary metabolism, the gut microbiome, and heart failure. Nat. Rev. Cardiol. 2019, 16, 137–154. [Google Scholar] [CrossRef]
- Bessac, A.; Cani, P.D.; Meunier, E.; Dietrich, G.; Knauf, C. Inflammation and gut-brain axis during type 2 diabetes: Focus on the crosstalk between intestinal immune cells and enteric nervous system. Front. Neurosci. 2018, 12, 725. [Google Scholar] [CrossRef]
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Frittitta, L. Type 2 diabetes mellitus and Alzheimer’s disease: Role of insulin signalling and therapeutic implications. Int. J. Mol. Sci. 2018, 19, 3306. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Everard, A. Talking microbes: When gut bacteria interact with diet and host organs. Mol. Nutr. Food Res. 2016, 60, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Ni, Q.; Gu, Y.; Xie, Y.; Yin, Q.; Zhang, H.; Nie, A.; Li, W.; Wang, Y.; Ning, G.; Wang, W.; et al. Raptor regulates functional maturation of murine beta cells. Nat. Commun. 2017, 8, 15755. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.J.; Guo, J.; Jia, Q.; Huang, Y.S.; Huang, W.-J.; Zhang, W.; Zhang, F.; Liu, W.J.; Wang, Y. The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 142, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Moosazadeh, M.; Lankarani, K.B.; Akbari, M.; Heydari, S.T.; Kolahdooz, F.; Asemi, Z. The effects of synbiotic supplementation on glucose metabolism and lipid profiles in patients with diabetes: A systematic review and meta-analysis of randomized controlled trials. Probiotics Antimicrob. Proteins 2018, 10, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Ostadmohammadi, V.; Lankarani, K.B.; Akbari, M.; Akbari, H.; Vakili, S.; Shokrpour, M.; Kolahdooz, F.; Rouhi, V.; Asemi, Z. The effects of probiotic and synbiotic supplementation on inflammatory markers among patients with diabetes: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Pharmacol. 2019, 852, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Roshan, H.; Ghaedi, E.; Rahmani, J.; Barati, M.; Najafi, M.; Karimzedeh, M.; Nikpayam, O. Effects of probiotics and synbiotic supplementation on antioxidant status: A meta-analysis of randomized clinical trials. Clin. Nutr. ESPEN 2019, 30, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Leylabadlo, H.E.; Sanaie, S.; Heravi, F.S.; Ahmadian, Z.; Ghotaslou, R. From role of gut microbiota to microbial-based therapies in type 2-diabetes. Infect. Genet. Evol. 2020, 81, 104268. [Google Scholar] [CrossRef]
- Su, C.; Lei, L.; Duan, Y.; Zhang, K.-Q.; Yang, J. Culture-independent methods for studying environmental microorganisms: Methods, application, and perspective. Appl. Microbiol. Biotechnol. 2012, 93, 993–1003. [Google Scholar] [CrossRef] [PubMed]



| Experimental Group Assignment | Symbiotic Components | ||
|---|---|---|---|
| Experimental treatment groups | 30% Lactulose | 30% Arabinose | 40% Lactobacillus plantarum CGMCC 8198 |
| Control Treated Group 1 | 60% Lactulose | \ | 40% Lactobacillus plantarum CGMCC 8198 |
| Control Treatment Group 2 | \ | 60% arabinose | 40% Lactobacillus plantarum CGMCC 8198 |
| Control Treatment Group 3 | 30% Lactulose | 30% Arabinose | 40% Lactobacillus plantarum CGMCC 1258 |
| Reference | Sample | Strain/Dose | Time | Results |
|---|---|---|---|---|
| Moroti et al., 2012 [81] | 20 patients with T2DM | B. bifidum 108 CFU, L. acidophilus 108 CFU, and 2 g oligofructose | 2 weeks | Increased HDL-C and reduced fasting glycemia. |
| Asemi et al., 2013 [82] | 54 patients with T2DM | L. acidophilus, L. casei, L.rhamnosus, L.bulgaricus, B. breve, B. longum, S. thermophilus, 109 CFU and 100 mg FOS | 8 weeks | TGL and HOMA-IR plasma levels increased; serum CRP decreased. |
| Tajadadi-Ebrahimi et al., 2014 [83] | 81 patients with T2DM | L. sporogenes, 108 CFU and 0.07 g inulin per 1 g | 8 weeks | Reduce serum insulin levels; conducive to insulin metabolism. |
| Shakeri et al., 2014 [84] | 78 patients with T2DM | L. sporogenes, 108 CFU and 0.07 g inulin per 1 g | 8 weeks | The serum HDL-C level significantly increased; the blood lipid profile decreased (TAG, TC/HDL-C). |
| Nazila Kassaian et al., 2016 [85] | 120 adults with impaired glucose tolerance | Lactobacillus acidophilus, Bifidobacter bifidum, Bifidobacter lactis, and Bifidobacter longum (109 CFU) with maltodextrin as filler and 6 g inulin | 6 months | Elevated HDL-C, and improved (LDL)/HDL. |
| Hossein et al., 2019 [30] | 136 patients with T2DM | Lactobacillus acidophilus 108 CFU and 0.5 g of powdered cinnamon | 3 months | Improved antioxidant enzyme activity modestly. |
| Soleimani et al., 2019 [86] | 60 patients with diabetes mellitus complicated with hemodialysis | Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum (2 × 109 CFU) and 0.8 g inulin | 12 weeks | Reduced blood glucose, insulin levels, and insulin resistance; improved insulin sensitivity. |
| Aynaz Velayati et al., 2021 [87] | 50 patients with T2DM | Bacillus Coagulans, Lactobacillus rhamnosus, Lactobacillus acidophilus and fructooligosaccharide | 12 weeks | Reduced insulin level, HOMA-IR CRP, and HOMA-β levels. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Cai, M.; Shen, B.; Wang, Q.; Zhang, T.; Zhou, X. Synbiotics and Gut Microbiota: New Perspectives in the Treatment of Type 2 Diabetes Mellitus. Foods 2022, 11, 2438. https://doi.org/10.3390/foods11162438
Jiang H, Cai M, Shen B, Wang Q, Zhang T, Zhou X. Synbiotics and Gut Microbiota: New Perspectives in the Treatment of Type 2 Diabetes Mellitus. Foods. 2022; 11(16):2438. https://doi.org/10.3390/foods11162438
Chicago/Turabian StyleJiang, Haoran, Miaomiao Cai, Boyuan Shen, Qiong Wang, Tongcun Zhang, and Xiang Zhou. 2022. "Synbiotics and Gut Microbiota: New Perspectives in the Treatment of Type 2 Diabetes Mellitus" Foods 11, no. 16: 2438. https://doi.org/10.3390/foods11162438
APA StyleJiang, H., Cai, M., Shen, B., Wang, Q., Zhang, T., & Zhou, X. (2022). Synbiotics and Gut Microbiota: New Perspectives in the Treatment of Type 2 Diabetes Mellitus. Foods, 11(16), 2438. https://doi.org/10.3390/foods11162438






