Improving Soy Sauce Aroma Using High Hydrostatic Pressure and the Preliminary Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Soy Sauce Preparation and HHP Treatment
2.3. Sensory Evaluation
2.4. Volatile Compounds Extraction Using Solid-Phase Micro-Extraction (SPME)
2.5. Analyses of GC-Olfactometry (GC-O) and Flavor Dilution (FD) Factor
2.6. Characterization and Quantification of Volatile Compounds
2.7. Statistical Analysis
3. Results and Discussion
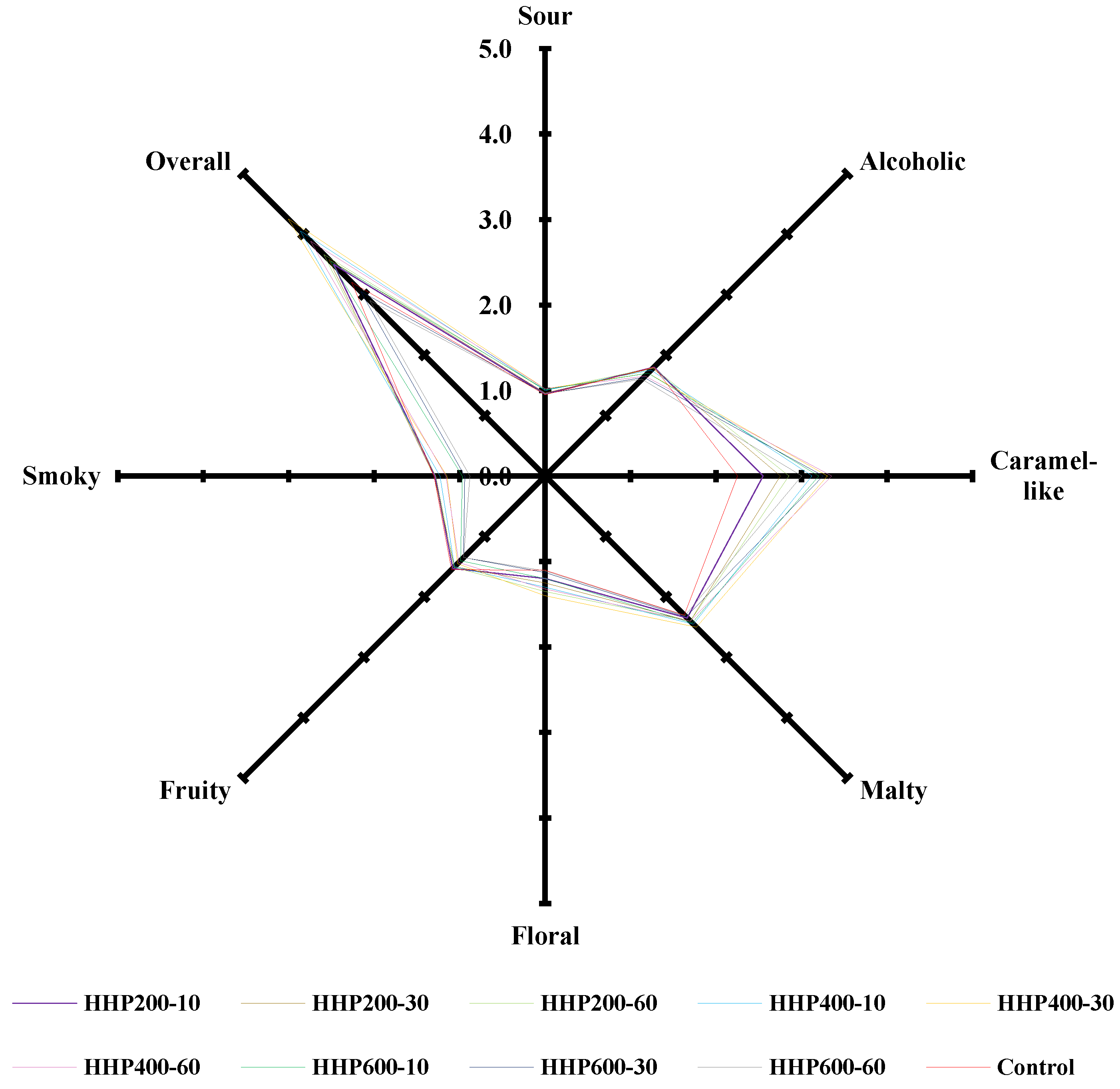
3.1. Sensory Evaluation
3.2. Characterization of Volatile Compounds
3.3. Analyses of GC-O and FD Factor
3.4. OAVs of Aroma-Active Compounds
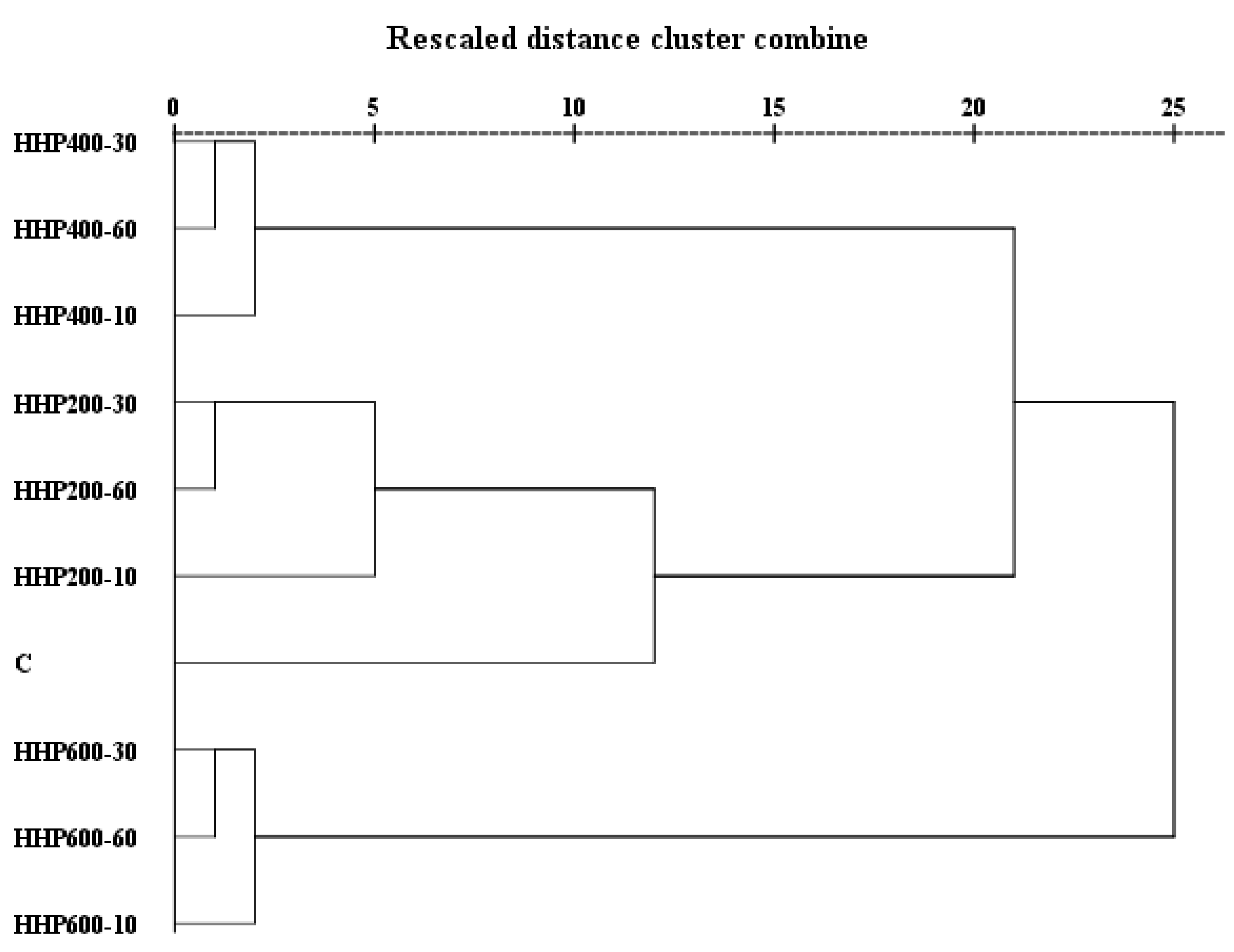
3.5. Hierarchical Cluster Analysis
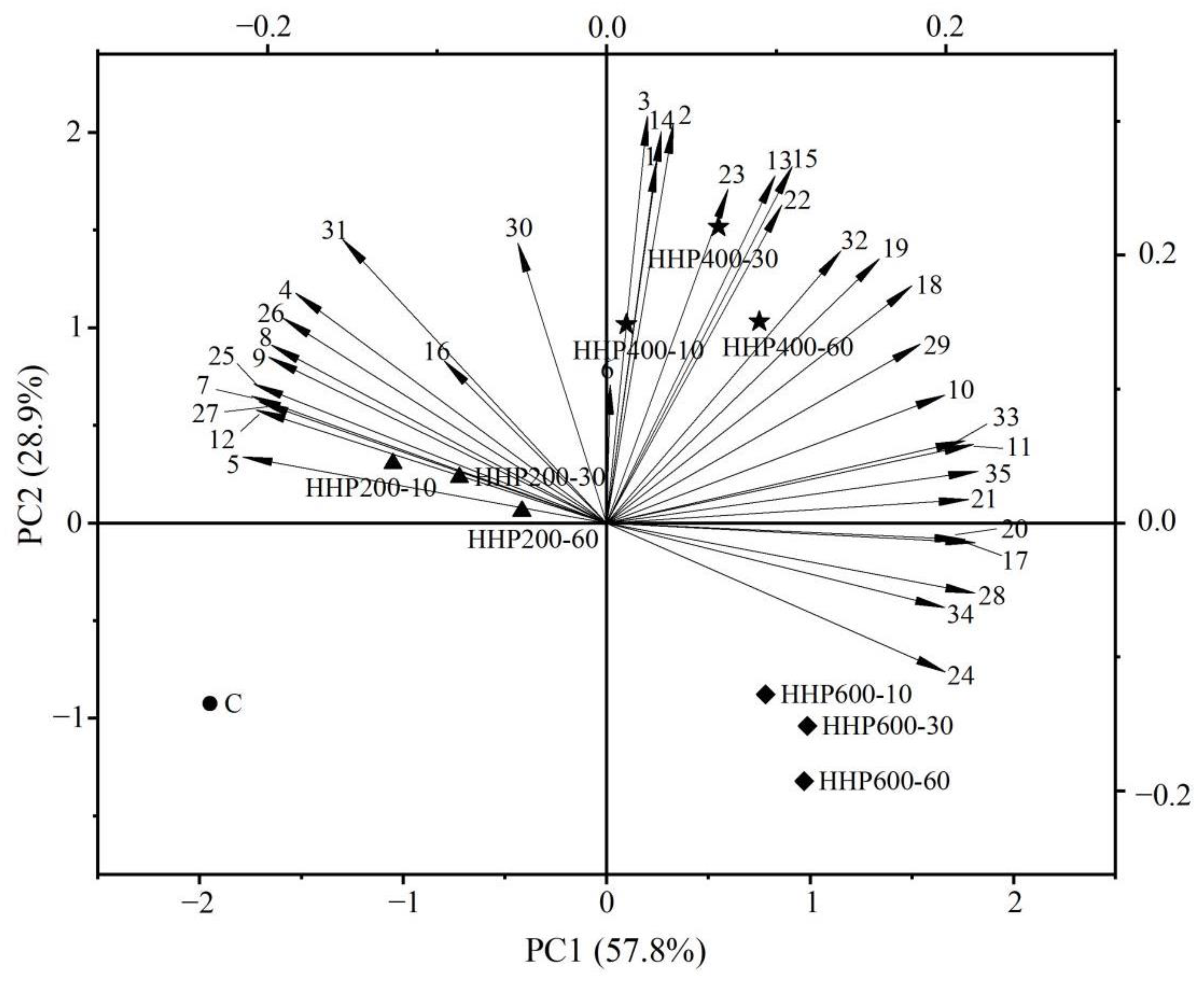
3.6. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, X.L.; Cui, C.; Zhao, H.F.; Zhao, M.M.; Yang, L.; Ren, J.Y. Changes in volatile aroma compounds of traditional Chinese-type soy sauce during moromi fermentation and heat treatment. Food Sci. Biotechnol. 2010, 19, 889–898. [Google Scholar] [CrossRef]
- Gao, X.L.; Zhang, J.K.; Liu, E.M.; Yang, M.Q.; Chen, S.; Hu, F.; Ma, H.L.; Liu, Z.; Yu, X.T. Enhancing the taste of raw soy sauce using low intensity ultrasound treatment during moromi fermentation. Food Chem. 2019, 298, 124928. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.L.; Liu, E.M.; Zhang, J.K.; Yang, L.X.; Huang, Q.R.; Chen, S.; Ma, H.L.; Ho, C.T.; Liao, L. Accelerating aroma maturation of raw soy sauce using low intensity sonication. Food Chem. 2020, 329, 127118. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.L.; Cui, C.; Ren, J.Y.; Zhao, H.F.; Zhao, Q.Z.; Zhao, M.M. Changes in the chemical composition of traditional Chinese-type soy sauce at different stages of manufacture and its relation to taste. Int. J. Food Sci. Technol. 2011, 46, 243–249. [Google Scholar] [CrossRef]
- Feng, Y.Z.; Cai, Y.; Su, G.W.; Zhao, H.F.; Wang, C.X.; Zhao, M.M. Evaluation of aroma differences between high-salt liquid-state fermentation and low-salt solid-state fermentation soy sauces from China. Food Chem. 2014, 145, 126–134. [Google Scholar] [CrossRef]
- Sun, X.Y.; Li, L.; Ma, T.T.; Zhao, F.; Yu, D.; Huang, W.D.; Zhan, J.C. High hydrostatic pressure treatment: An artificial accelerating aging method which did not change the region and variety non-colored phenolic characteristic of red wine. Innov. Food Sci. Emerg. Technol. 2016, 33, 123–134. [Google Scholar] [CrossRef]
- Umego, E.C.; He, R.H.; Huang, G.P.; Dai, C.H.; Ma, H.L. Ultrasound-assisted fermentation: Mechanisms, technologies, and challenges. J. Food Process Pres. 2021, 45, 15559. [Google Scholar] [CrossRef]
- Yu, Z.; Su, Y.; Zhang, Y.L.; Zhu, P.Y.; Mei, Z.L.; Zhou, X.N.; Yu, H. Potential use of ultrasound to promote fermentation, maturation, and properties of fermented foods: A review. Food Chem. 2021, 357, 129805. [Google Scholar] [CrossRef]
- Al Daccache, M.; Koubaa, M.; Salameh, D.; Maroun, R.G.; Louka, N.; Vorobiev, E. Ultrasound-assisted fermentation for cider production from Lebanese apples. Ultrason Sonochem. 2020, 63, 104952. [Google Scholar] [CrossRef]
- Wang, Z.; Li, T.; Liu, F.; Zhang, C.; Ma, H.; Wang, L.; Zhao, S. Effects of ultrasonic treatment on the maturation of Zhenjiang vinegar. Ultrason. Sonochem. 2017, 39, 272–280. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Liu, Y.; Xu, M.; Zhang, T.H.; Ren, H.; Liu, W.; Li, M.Y. Accelerated aging of grape pomace vinegar by using additives combined with physical methods. J. Food Process Eng. 2020, 43, 13398. [Google Scholar] [CrossRef]
- Aganovic, K.; Hertel, C.; Vogel, R.F.; Johne, R.; Schlüter, O.; Schwarzenbolz, U.; Jager, H.; Holzhauser, T.; Bergmair, J.; Roth, A.; et al. Aspects of high hydrostatic pressure food processing: Perspectives on technology and food safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3225–3266. [Google Scholar] [CrossRef] [PubMed]
- Lomelí-Martín, A.; Martínez, L.M.; Welti-Chanes, J.; Escobedo-Avellaneda, Z. Induced changes in aroma compounds of foods treated with high hydrostatic pressure: A review. Foods 2021, 10, 878. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; House, J.D.; Pouliot, Y.; Doyen, A. Effects of high hydrostatic pressure processing on hen egg compounds and egg products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 707–720. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K. Food processing by high hydrostatic pressure. Biosci. Biotechnol. Biochem. 2017, 81, 672–679. [Google Scholar] [CrossRef] [Green Version]
- Valdés, M.E.; Ramírez, R.; Martínez-Cañas, M.A.; Frutos-Puerto, S.; Moreno, D. Accelerating aging of white and red wines by the application of hydrostatic high pressure and maceration with holm oak (Quercus ilex) chips: Influence on physicochemical and sensory characteristics. Foods 2021, 10, 889. [Google Scholar] [CrossRef]
- Huang, H.W.; Wu, S.J.; Lu, J.K.; Shyu, Y.T.; Wang, C.Y. Current status and future trends of high-pressure processing in food industry. Food Control 2017, 72, 1–8. [Google Scholar] [CrossRef]
- Tian, Y.T.; Huang, J.M.; Xie, T.T.; Huang, L.Q.; Zhuang, W.J.; Zheng, Y.F.; Zheng, B.D. Oenological characteristics, amino acids and volatile profiles of Hongqu rice wines during pottery storage: Effects of high hydrostatic pressure processing. Food Chem. 2016, 203, 456–464. [Google Scholar] [CrossRef]
- Martinez-Monteagudo, S.I.; Saldana, M.D.A. Chemical reactions in food systems at high hydrostatic pressure. Food Eng. Rev. 2014, 6, 105–127. [Google Scholar] [CrossRef]
- Guclu, G.; Sevindik, O.; Kelebek, H.; Selli, S. Determination of Volatiles by Odor Activity Value and Phenolics of cv. Ayvalik Early-Harvest Olive Oil. Foods 2016, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.C.; Nunes, C.; Rocha, M.A.M.; Rodrigues, A.; Rocha, S.M.; Saraiva, J.A.; Coimbra, M.A. High pressure treatments accelerate changes in volatile composition of sulphur dioxide-free wine during bottle storage. Food Chem. 2015, 188, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, M.; Zieliński, H. How Maillard reaction influences sensorial properties (color, flavor and texture) of food products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Xiao, Z.B.; Chen, L.N.; Niu, Y.W.; Zhu, J.C.; Zhang, J.; Deng, J.M. Evaluation of the interaction between esters and sulfur compounds in pineapple using feller’s additive model, oav, and odor activity coefficient. Food Anal. Methods 2021, 14, 1714–1729. [Google Scholar] [CrossRef]
- Moreira, N.; De Pinho, P.G.; Santos, C.; Vasconcelos, I. Volatile sulphur compounds composition of monovarietal white wines. Food Chem. 2010, 123, 1198–1203. [Google Scholar] [CrossRef]
- Xia, Q.; Li, Y.F. Ultra-high pressure effects on color, volatile organic compounds and antioxidants of wholegrain brown rice (Oryza Sativa L.) during Storage: A comparative study with high-intensity ultrasound and germination pretreatments. Innov. Food Sci. Emerg. Technol. 2018, 45, 390–400. [Google Scholar] [CrossRef]
- Chen, X.H.; Qin, W.D.; Ma, L.H.; Xu, F.; Jin, P.; Zheng, Y.H. Effect of high pressure processing and thermal treatment on physicochemical parameters, antioxidant activity and volatile compounds of green asparagus juice. LWT Food Sci. Technol. 2015, 62, 927–933. [Google Scholar] [CrossRef]
- González-Cebrino, F.; García-Parra, J.; Ramírez, R. Aroma profile of a red plum purée processed by high hydrostatic pressure and analysed by SPME-GC/MS. Innov. Food Sci. Emerg. Technol. 2016, 33, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Pei, L.Y.; Hou, S.H.; Wang, L.L.; Chen, J.L. Effects of high hydrostatic pressure, dense phase carbon dioxide, and thermal processing on the quality of hami melon juice. J. Food Process Eng. 2018, 41, e12828. [Google Scholar] [CrossRef]
- Gao, X.L.; Shan, P.; Feng, T.; Zhang, L.J.; He, P.; Ran, J.L.; Fu, J.Y.; Zhou, C.S. Enhancing selenium and key flavor compounds contents in soy sauce using selenium-enriched soybean. J. Food Compos. Anal. 2022, 106, 104299. [Google Scholar] [CrossRef]
- Xia, Q.; Mei, J.; Yu, W.J.; Li, Y.F. High hydrostatic pressure treatments enhance volatile components of pre-germinated brown rice revealed by aromatic fingerprinting based on HS-SPME/GC–MS and chemometric methods. Food Res. Int. 2017, 91, 103–114. [Google Scholar] [CrossRef]
- Wang, F.; Du, B.L.; Cui, Z.W.; Xu, L.P.; Li, C.Y. Effects of high hydrostatic pressure and thermal processing on bioactive compounds, antioxidant activity, and volatile profile of mulberry juice. Food Sci. Technol. Int. 2016, 23, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Feng, X.P.; Ren, H.; Yang, H.K.; Liu, Y.; Gao, Z.P. Changes in physicochemical properties and volatiles of kiwifruit pulp beverage treated with high hydrostatic pressure. Foods 2020, 9, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duong, T.; Balaban, M.; Perera, C. Effects of Combined High Hydrostatic Pressure and Dense Phase Carbon Dioxide on the Activity, Structure and Size of Polyphenoloxidase. J. Food Sci. 2015, 80, e2486–e2494. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Sun, D.W.; Górecki, A.; Błaszczak, W.; Lamparski, G.; Amarowicz, R.; Fornal, J.; Jelinski, T. Effects of high hydrostatic pressure processing on the physicochemical and sensorial properties of a red wine. Innov. Food Sci. Emerg. Technol. 2012, 16, 409–416. [Google Scholar] [CrossRef]
- Gao, X.L.; Zhang, J.K.; Regenstein, J.M.; Yin, Y.Y.; Zhou, C.S. Characterization of taste and aroma compounds in Tianyou, a traditional fermented wheat flour condiment. Food Res. Int. 2018, 106, 156–163. [Google Scholar] [CrossRef]
| Compounds | RI (DB-Wax) | Aroma Description | FD | Identification Method | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | HHP200-10 | HHP200-30 | HHP200-60 | HHP400-10 | HHP400-30 | HHP400-60 | HHP600-10 | HHP600-30 | HHP600-60 | ||||
| Acids | |||||||||||||
| Acetic acid | 1452 | Sour 1,2,3 | 2 | 2 | 2 | 2 | 4 | 4 | 4 | 8 | 8 | 8 | ABC |
| 2-Methylpropanoic acid | 1543 | Sour, rancid 1,2 | 1 | 2 | 2 | 2 | 4 | 4 | 4 | 4 | 4 | 4 | ABC |
| Butanoic acid | 1622 | Sour, sweaty 1,2,3 | nd | 1 | 1 | – | 1 | 1 | – | – | – | – | ABC |
| 2-Methylbutanoic acid | 1667 | Sour, smelly 1,2,3 | 32 | 32 | 32 | 32 | 32 | 32 | 16 | 8 | 8 | 8 | ABC |
| 3-Methylbutanoic acid | 1657 | Sour, smelly 1,2,3 | 8 | 16 | 16 | 16 | 32 | 32 | 16 | 32 | 32 | 32 | ABC |
| 4-Methylpentanoic acid | 1803 | Sour, smelly 2 | nd | – | – | – | – | – | – | – | – | – | A |
| Hexanoic acid | 1825 | Sweat, pungent 2 | nd | – | – | – | – | – | – | – | – | – | AB |
| 2-Ethylhexanoic acid | 1908 | un | – | – | – | nd | – | – | – | – | – | – | A |
| 2-Methyl-2-butenoic acid | 1869 | Pungent 3 | nd | – | – | – | – | – | – | – | – | – | A |
| Nonanoic acid | 2202 | Green, fat 3 | – | nd | – | – | – | – | – | nd | – | – | A |
| Decanoic acid | 2351 | Rancid, fat 3 | nd | – | – | – | – | – | – | – | – | – | A |
| Benzoic acid | 2410 | Flower, fruity 1,2,3 | 4 | 4 | 8 | 8 | 16 | 16 | 16 | 8 | 8 | 8 | AB |
| Phenylacetic acid | 2546 | Sour, honey 1,2,3 | 2 | 4 | 4 | 4 | 8 | 8 | 8 | 8 | 8 | 8 | ABC |
| Alcohols | |||||||||||||
| Ethanol | 928 | Alcoholic 1,2,3 | 128 | 128 | 128 | 128 | 64 | 64 | 64 | 32 | 32 | 32 | ABC |
| 1-Propanol | 1042 | Alcoholic, pungent 3 | – | – | – | – | – | – | – | – | – | – | A |
| 2-Methylpropanol | 1095 | Wine, solvent, bitter 3 | – | – | – | – | – | – | – | – | – | – | A |
| 1-Butanol | 1146 | Medicine, fruit 3 | – | nd | – | – | – | – | – | – | – | – | AB |
| 2-Butanol | 1027 | Wine 3 | nd | – | – | – | – | – | – | – | – | – | AB |
| 2-Methyl-1-butanol | 1212 | Wine, onion 1,2,3 | 32 | 16 | 16 | 16 | 16 | 16 | 8 | 8 | 8 | 8 | ABC |
| 3-Methyl-1-butanol | 1206 | Whiskey, malty, burnt 1,2,3 | 128 | 128 | 128 | 64 | 128 | 128 | 64 | 128 | 64 | 64 | ABC |
| 2,3-Butanediol | 1581 | Fruit, onion 2,3 | – | – | – | – | – | – | – | – | – | – | A |
| 2-Pentanol | 1121 | Green 3 | – | – | – | – | – | – | – | – | – | – | A |
| Prenol | 1129 | Herb 3 | nd | – | – | – | – | – | – | – | – | – | A |
| 4-Methyl-2-pentanol | 1147 | un | – | – | – | – | nd | – | – | – | – | – | A |
| 2,3-Dimethylpentanol | 1187 | un | nd | – | – | – | – | – | – | – | – | – | A |
| 1-Hexanol | 1364 | Resin, flower, green 3 | – | – | – | – | – | – | – | – | – | nd | A |
| 4-Methyl-1-hexanol | 1263 | Sweat 3 | nd | nd | – | – | – | – | – | – | – | – | A |
| 2-Ethyl-1-hexanol | 1482 | Rose 2 | – | – | – | – | – | – | – | – | – | – | A |
| 2-Heptanol | 1277 | Mushroom 3 | – | – | – | – | – | – | – | nd | – | – | A |
| 6-Methyl-2-heptanol | 1312 | un | nd | – | – | – | – | – | – | – | – | – | A |
| 3-Octanol | 1343 | Nut, mushroom 3 | – | – | – | – | – | – | – | – | – | – | AB |
| 1-Octen-3-ol | 1468 | Mushroom 1,2,3 | 8 | 16 | 16 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | ABC |
| 1-Nonanol | 1506 | Fat, green 3 | – | – | – | – | – | – | – | – | – | – | A |
| 2-Nonanol | 1537 | Cucumber 3 | nd | – | – | – | – | – | – | – | – | – | A |
| Benzyl alcohol | 1867 | Sweet, flower 3 | – | – | – | – | – | – | – | – | – | – | AB |
| Methylbenzyl alcohol | 1912 | Flower 3 | nd | – | – | – | – | – | – | – | – | – | A |
| Phenylethanol | 1894 | Flower, honey 1,2,3 | 128 | 64 | 64 | 64 | 64 | 64 | 64 | 32 | 32 | 32 | ABC |
| α-Ethyl phenylethanol | 2013 | un | – | – | – | – | – | – | – | – | – | – | A |
| 1-(2-Butoxyethoxy)-ethanol | 1799 | un | nd | – | – | – | – | – | – | – | – | – | A |
| 4-Phenyl-3-buten-2-ol | nd | un | nd | – | – | – | – | – | – | – | – | nd | A |
| Aldehydes | |||||||||||||
| Acetaldehyde | 718 | Pungent, ether 3 | – | – | – | – | – | – | – | – | – | – | AB |
| 2-Methylpropanal | 826 | Malty 1,2,3 | – | 32 | – | – | 32 | 32 | 32 | – | – | nd | ABC |
| 2-Methylbutanal | 934 | Malty 1,2,3 | 64 | 64 | 64 | 64 | 64 | 128 | 128 | 64 | 64 | 64 | ABC |
| 3-Methylbutanal | 925 | Malty 1,2,3 | 64 | 128 | 128 | 128 | 256 | 256 | 128 | 128 | 128 | 128 | ABC |
| Benzaldehyde | 1500 | Almond, caramel-like 2,3 | – | – | – | – | – | – | – | – | – | – | AB |
| Benzeneacetaldehyde | 1625 | Flower, sweet 1,2,3 | 32 | 32 | 64 | 64 | 64 | 64 | 64 | 64 | 32 | 32 | ABC |
| 3-Furaldehyde | 1453 | Bread, sweet, almond 3 | – | 1 | 2 | – | 2 | 4 | 4 | 4 | 4 | 2 | ABC |
| Methyl cinnamaldehyde | 1946 | Cinnamon, sweet 3 | nd | – | – | – | – | – | – | – | – | nd | A |
| Ketones | |||||||||||||
| Acetone | 869 | Mild 3 | nd | – | – | – | – | – | – | – | – | – | AB |
| 2-Butanone | 943 | Cheese 2,3 | – | – | – | – | 1 | 1 | 1 | 1 | 1 | – | A |
| Acetoin | 1288 | Butter, creamy 3 | – | – | – | – | – | – | – | – | – | – | A |
| Methyl isobutyl ketone | 1007 | Fruity 3 | nd | – | – | – | – | – | – | – | – | – | A |
| 2,3-Pentanedione | 1058 | Butter, creamy 3 | – | – | – | – | – | – | – | – | – | – | A |
| 3-Penten-2-one | 1099 | un | nd | – | – | – | – | – | – | – | – | – | A |
| 5-Methyl-2-hexanone | 1138 | un | – | – | – | – | – | – | – | – | – | – | A |
| 6-Methyl-2-heptanone | nd | un | nd | – | – | – | – | – | – | – | – | – | A |
| 3-Octanone | 1248 | Herb, butter, resin 2,3 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | – | 1 | nd | AB |
| Isophorone | 1603 | Camphor 2,3 | nd | – | – | – | – | – | – | – | – | – | A |
| 1-Phenyl-1-propanone | 1723 | Mild 3 | – | – | – | – | – | – | – | – | – | – | A |
| 2,6,6-Trimethyl-2,4-cycloheptadien-1-one | 1691 | un | nd | – | – | – | – | – | – | – | – | – | A |
| 3-Hydroxy-3-phenylbutan-2-one | 1789 | un | – | – | – | – | – | – | – | – | – | – | A |
| (E)-β-damascenone | 1815 | Rose, honey, apple 1,2,3 | – | 16 | 16 | 16 | 64 | 64 | 32 | 16 | 16 | 16 | ABC |
| Esters | |||||||||||||
| Ethyl acetate | 905 | Pineapple 1,2,3 | 64 | 32 | 32 | 32 | 32 | 32 | 32 | 16 | 16 | 16 | ABC |
| Ethyl propanoate | 979 | Fruity 1,2,3 | 16 | 8 | 8 | 8 | 8 | 8 | 8 | 4 | 4 | 4 | ABC |
| Ethyl lactate | 1355 | Fruity 2,3 | – | – | – | – | – | – | – | – | – | – | A |
| Ethyl butanoate | 1055 | Fruity 2,3 | – | – | – | – | – | – | – | – | – | – | AB |
| Ethyl isobutyrate | 966 | Apple 2,3 | – | – | – | – | – | – | – | – | – | – | A |
| Ethyl 2-methylbutyrate | 1055 | Pineapple 3 | – | – | – | – | – | – | – | – | – | – | A |
| Ethyl isovalerate | 1061 | Sweet, fruity 1,2,3 | 128 | 128 | 128 | 128 | 128 | 128 | 128 | 64 | 64 | 64 | ABC |
| Ethyl hexanoate | 1233 | Wine, fruity 2,3 | – | – | – | – | – | – | – | – | – | – | AB |
| Ethyl isohexanoate | 1203 | Fruity 3 | – | – | – | nd | – | – | – | – | – | – | A |
| Ethyl 5-methylhexanoate | 1285 | un | – | – | – | – | – | nd | – | – | – | – | A |
| Ethyl 2-ethylhexanoate | 1309 | un | – | – | – | – | – | – | – | – | – | – | A |
| Ethyl heptanoate | 1455 | Fruity 2,3 | – | – | – | nd | – | nd | nd | nd | nd | nd | A |
| Ethyl caprylate | 1443 | Fruity, fat 2,3 | – | – | – | – | nd | nd | nd | – | nd | nd | AB |
| Ethyl nonanoate | 1468 | Grape 2,3 | – | – | nd | nd | – | – | nd | nd | nd | nd | A |
| Ethyl palmitate | 2265 | Wax 3 | – | – | – | – | – | – | – | – | – | – | A |
| Ethyl 9-hexadecenoate | 2274 | un | – | – | – | – | – | – | – | – | – | – | A |
| Ethyl oleate | 2403 | Flower 3 | – | – | – | nd | nd | nd | nd | nd | – | nd | A |
| (E)-9-Octadecenoic acid ethyl ester | 2391 | Flower, fruity, fat 3 | – | – | nd | nd | – | nd | nd | – | nd | nd | A |
| Ethyl tiglate | 1241 | Mushroom 3 | – | – | – | nd | nd | nd | nd | nd | nd | nd | A |
| Ethyl benzoate | 1649 | Fruity, flower 2,3 | 8 | 8 | 4 | 4 | 4 | 4 | 4 | 4 | 2 | 2 | AB |
| Ethyl phenylacetate | 1771 | Fruity, sweet 2,3 | – | – | – | – | – | – | – | – | – | – | A |
| 2-Phenylethyl acetate | 1827 | Rose, honey, tobacco 1,2,3 | 1 | 1 | – | – | 1 | 1 | 1 | 1 | – | – | ABC |
| Ethyl 3-phenylpropionate | 1908 | Flower 3 | – | – | – | nd | – | nd | nd | nd | – | – | A |
| Butanedioic acid diethyl ester | 1632 | Wine, fruity 3 | – | – | nd | nd | – | nd | nd | nd | nd | nd | A |
| Dimethyl phthalate | 2316 | Mild 3 | – | – | – | – | – | – | – | – | – | – | A |
| Methyl benzoate | 1609 | Flower, fruity 1,2,3 | nd | 2 | 2 | – | 4 | 8 | 8 | – | – | – | ABC |
| Methyl phenylacetate | 1712 | Honey 3 | nd | – | – | – | – | – | – | – | – | – | A |
| Isobutyl acetate | 1019 | Fruity, banana, apple 2,3 | – | – | – | – | – | – | – | – | – | – | A |
| Isoamyl acetate | 1127 | Fruity, banana 2,3 | – | – | – | – | – | – | – | – | – | – | A |
| Isoamyl lactate | 1499 | Sweet 3 | nd | – | – | – | – | – | – | – | – | – | A |
| Formic acid heptyl ester | 1483 | Flower, fruity, fat 3 | nd | – | – | – | – | – | – | – | – | – | A |
| Butyrolactone | 1521 | Caramel-like, sweet 2,3 | – | – | – | – | – | – | – | – | – | – | AB |
| Furan(one)s | |||||||||||||
| Furan | 1456 | Sweet, bread 2,3 | nd | – | – | – | – | – | – | – | – | – | AB |
| 1-(2-Furanyl)-ethanone | 1487 | Smoky 2,3 | nd | – | – | – | – | – | – | – | – | – | A |
| 1-(2-Furanyl)-1-propanone | 1511 | un | – | – | – | – | – | – | – | – | – | – | A |
| 2,2-Dimethyl-5-isopropyl -furan | 1569 | un | – | – | – | – | – | – | – | – | – | – | A |
| Benzofuran | nd | Mild 3 | – | – | – | – | – | – | – | – | – | – | A |
| 3-Phenylfuran | 1839 | Green bean 2 | – | – | – | – | 1 | 2 | 2 | 2 | 2 | 2 | A |
| 2-Furanmethanol | 1125 | Caramel-like 1,2,3 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ABC |
| Dihydro-5-pentyl-2(3H) -furanone | 1971 | Coconut, peach 3 | nd | – | – | – | – | – | – | – | – | – | A |
| HDMF | 2032 | Caramel-like, sweet 1,2,3 | 16 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 16 | 16 | ABC |
| HEMF | 2089 | Caramel-like, sweet 1,2,3 | 32 | 32 | 32 | 32 | 64 | 64 | 64 | 32 | 32 | 32 | ABC |
| Phenols | |||||||||||||
| Phenol | 1485 | Phenol 3 | – | – | – | – | – | – | – | – | – | – | AB |
| 4-Ethylphenol | 2158 | Smoky 2,3 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | A |
| 3-Tert-butyl-phenol | 2243 | Phenol 3 | – | – | – | – | – | – | – | – | – | – | A |
| 2,4-Di-tert-butylphenol | 2289 | Phenol 3 | – | – | – | – | – | – | – | – | – | – | A |
| 4-Ethylguaiacol | 2016 | Smoky 1,2,3 | 128 | 128 | 128 | 128 | 64 | 64 | 64 | 64 | 32 | 32 | ABC |
| 4-Vinylguaiacol | 2177 | Pungent, smoky 1,2,3 | 64 | 64 | 64 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | ABC |
| 5-Methylguaiacol | 1932 | Sweet 3 | nd | – | – | – | – | – | – | – | – | – | A |
| Pyrazines | |||||||||||||
| Pyrazine | 1205 | Rancid 3 | nd | – | – | – | – | – | – | – | – | – | A |
| 2-Methyl pyrazine | 1266 | Popcorn 2,3 | nd | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | AB |
| 2,3-Dimethyl pyrazine | 1259 | Roasted nut, cocoa 3 | nd | – | – | – | – | – | – | – | – | – | A |
| 2,5-Dimethyl pyrazine | 1255 | Roasted nut, cocoa 3 | – | – | – | – | – | – | – | – | – | – | A |
| 2,6-Dimethyl pyrazine | 1329 | Roasted nut, cocoa 2,3 | – | – | – | 8 | 16 | 16 | 16 | 16 | 16 | 16 | AB |
| 2-Isopropyl pyrazine | 1353 | un | nd | – | – | – | – | – | – | – | – | – | A |
| Trimethyl pyrazine | 1388 | Roast, potato 2,3 | 1 | 2 | 2 | 2 | 4 | 4 | 4 | 4 | 4 | 4 | A |
| 2-Ethyl-6-methyl pyrazine | 1345 | Fruity, sweet 3 | – | – | – | – | 1 | 2 | 2 | 2 | 4 | 2 | A |
| 2-Ethyl-3,5-dimethyl pyrazine | 1453 | Potato 3 | – | – | – | – | – | – | – | – | – | – | A |
| Sulfur-containing compounds | |||||||||||||
| Disulfide dimethyl | 1068 | Onion, cabbage, putrid 2,3 | 1 | 1 | 2 | 2 | 2 | 4 | 4 | 4 | 4 | 4 | AB |
| Dimethyl trisulfide | 1372 | Sulfur, fish, cabbage 1,2,3 | 2 | – | 4 | 4 | 4 | 8 | 8 | 8 | 8 | 8 | ABC |
| 3-Methylthio-1-propanol | 1718 | Sweet, potato 1,2,3 | 2 | 4 | 4 | 4 | 8 | 8 | 8 | 8 | 4 | 4 | ABC |
| 3-(Methylthio)propanal | 1423 | Cooked potato 1,2,3 | 128 | 256 | 256 | 256 | 256 | 512 | 512 | 256 | 256 | 256 | ABC |
| 2-Hydroxyethyl isobutyl sulfide | 1583 | un | – | – | – | – | – | – | – | – | – | – | A |
| Ethyl 3-(methylthio) propionate | 1417 | Onion, garlic, pineapple 2 | 8 | 8 | 4 | 4 | 4 | 4 | 4 | 4 | 2 | 2 | A |
| 3-(Methylthio) propyl acetate | 1583 | Mushroom, onion, garlic 2 | – | – | – | – | – | – | – | – | – | – | A |
| Others | |||||||||||||
| 2-Acetylpyrroline | 1396 | Nut, roasted, bread 1,2,3 | 4 | 4 | 4 | 4 | 8 | 8 | 8 | 8 | 16 | 16 | AB |
| 3-Methyl-2-pyrrolidinone | nd | un | – | – | – | – | – | – | – | – | – | – | A |
| Maltol | 1952 | Caramel-like, sweet 2,3 | – | – | – | – | – | – | – | – | – | nd | AB |
| Naphthalene | 1725 | Mothball-like 2,3 | – | – | – | – | – | – | – | – | – | – | A |
| Dodecane | 1198 | Alkane-like 3 | – | – | – | – | – | – | – | – | – | – | A |
| Hexadecane | 1599 | Alkane-like 3 | – | – | – | – | – | – | – | – | – | – | A |
| Aroma-Active Compound | Odor Threshold (μg/L) | Odor Activity Value (OAV) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | HHP200-10 | HHP200-30 | HHP200-60 | HHP400-10 | HHP400-30 | HHP400-60 | HHP600-10 | HHP600-30 | HHP600-60 | ||
| 2-Methylpropanal | 1.5 A | – | 67.1 ± 3.1 b | – | – | 82.5 ± 2.9 b | 84.1 ± 3.0 b | 87.7 ± 3.6 a | – | – | – |
| 2-Methylbutanal | 4.4 A | 237 ± 13 c | 270 ± 10 b | 275 ± 12 b | 280 ± 12 a,b | 287 ± 11 a,b | 295 ± 10 a | 298 ± 10 a | 249 ± 8 c | 248 ± 10 c | 249 ± 11 c |
| 3-Methylbutanal | 1.2 A | 314 ± 15 d | 347 ± 18 b,c | 358 ± 17 a,b | 352 ± 17 a,b,c | 361 ± 15 a,b | 376 ± 13 a | 366 ± 16 a,b | 327 ± 13 c,d | 325 ± 14 c,d | 318 ± 11 d |
| OAV of compounds with malty aroma | 551 | 684 | 633 | 632 | 731 | 755 | 752 | 576 | 573 | 567 | |
| Ethanol | 40,000 A | 238 ± 12 a,b | 243 ± 10 a | 232 ± 11 a,b,c | 216 ± 10 c,d | 227 ± 10 a,b,c,d | 221 ± 10 b,c,d | 212 ± 9 d | 192 ± 9 e | 184 ± 8 e | 177 ± 9 e |
| 2-Methyl-1-butanol | 16 A | 43.3 ± 2.1 a | 40.9 ± 1.8 a,b | 41.2 ± 1.9 a,b | 39.2 ± 1.7 b | 35.5 ± 1.5 c | 32.6 ± 1.2 d | 32.0 ± 1.3 d,e | 29.4 ± 1.2 e,f | 29.0 ± 1.3 f | 29.7 ± 1.2 e,f |
| 3-Methyl-1-butanol | 4 A | 253 ± 13 a | 242 ± 12 a | 236 ± 12 a | 253 ± 13 a | 251 ± 14 a | 254 ± 12 a | 254 ± 11 a | 249 ± 11 a | 253 ± 10 a | 232 ± 11 a |
| OAV of compounds with alcoholic aroma | 534 | 526 | 509 | 508 | 514 | 507 | 498 | 470 | 466 | 439 | |
| Ethyl acetate | 5000 A | 105 ± 6 a | 101 ± 6 a,b | 100 ± 5 a,b | 97.3 ± 6.1 a,b,c | 93.5 ± 5.2 b,c | 89.1 ± 4.2 c,d,e | 89.6 ± 5.0 c,d | 83.7 ± 4.1 d,e,f | 80.3 ± 4.2 e,f | 78.5 ± 3.9 f |
| Ethyl propanoate | 10 A | 16.4 ± 0.8 a | 15.8 ± 0.7 a | 14.2 ± 0.7 b | 13.2 ± 0.7 b,c | 12.5 ± 0.6 c | 13.6 ± 0.5 b | 10.6 ± 0.6 d | 8.62 ± 0.43 e | 8.51 ± 0.40 e | 7.26 ± 0.37 f |
| Ethyl isovalerate | 1.5 A | 217 ± 12 a | 210 ± 11 a | 211 ± 10 a | 206 ± 10 a | 185 ± 9 b | 188 ± 9 b | 175 ± 8 b,c | 159 ± 8 c,d | 147 ± 6 d | 145 ± 7 d |
| Benzoic acid | 3000 A | 16.6 ± 0.8 d | 17.9 ± 0.8 c,d | 17.9 ± 0.8 c,d | 18.5 ± 0.9 c | 19.1 ± 1.0 a,b,c | 20.3 ± 1.0 a,b | 20.6 ± 1.0 a | 19.4 ± 0.9 a,b,c | 20.5 ± 0.9 a,b | 18.9 ± 0.9 b,c |
| Ethyl 3-(methylthio) propionate | 80 C | – | 1.25 ± 0.06 d | 1.29 ± 0.05 d | 1.47 ± 0.08 c | 2.01 ± 0.09 b | 2.52 ± 0.12 a | 2.55 ± 0.12 a | 2.37 ± 0.10 a | 2.40 ± 0.11 a | 2.50 ± 0.11 a |
| OAV of compounds with fruity aroma | 354 | 346 | 344 | 336 | 312 | 313 | 298 | 272 | 259 | 252 | |
| Phenylethanol | 564 A | 218 ± 11 a | 196 ± 9 b | 187 ± 9 b | 185 ± 8 b | 182 ± 7 b,c | 187 ± 7 b | 170 ± 9 c,d | 162 ± 6 d,e | 157 ± 6 d,e | 155 ± 8 e |
| Benzeneacetaldehyde | 10 A | 42.0 ± 2.1 d | 46.3 ± 2.3 d | 52.8 ± 3.0 c | 54.5 ± 3.5 c | 69.0 ± 3.2 a,b | 71.2 ± 3.5 a | 65.1 ± 2.9 b | 52.8 ± 2.4 c | 53.5 ± 3.0 c | 44.6 ± 2.1 d |
| Methyl benzoate | 125 A | – | 4.32 ± 0.19 c | 4.94 ± 0.23 c | – | 7.62 ± 0.35 b | 10.2 ± 0.5 a | 9.74 ± 0.45 a | – | – | – |
| (E)-β-damascenone | 0.002 A | – | 42.8 ± 1.9 e | 47.5 ± 2.1 d,e | 48.6 ± 2.5 d | 59.4 ± 2.9 c | 88.2 ± 4.7 a | 79.4 ± 4.2 b | 32.3 ± 1.3 f | 37.2 ± 1.5 f | 35.0 ± 1.6 f |
| 2-Phenylethyl acetate | 250 A | 2.01 ± 0.12 a | 1.72 ± 0.08 b | – | – | 1.41 ± 0.07 c | 1.40 ± 0.06 c | 1.35 ± 0.06 c | 0.92 ± 0.04 d | – | – |
| OAV of compounds with floral aroma | 262 | 291 | 293 | 288 | 319 | 358 | 326 | 248 | 248 | 235 | |
| 2-Furanmethanol | 2000 A | 1.81 ± 0.08 e | 2.02 ± 0.09 d | 2.11 ± 0.09 d | 2.07 ± 0.08 d | 2.31 ± 0.11 c | 2.57 ± 0.11 a,b | 2.54 ± 0.10 b | 2.63 ± 0.10 a,b | 2.62 ± 0.11 a,b | 2.73 ± 0.12 a |
| 3-Methylthio-1-propanol | 856.1 A | 0.60 ± 0.04 g | 2.05 ± 0.10 f | 1.93 ± 0.09 f | 1.91 ± 0.08 f | 3.08 ± 0.13 c | 3.65 ± 0.16 a | 3.43 ± 0.15 b | 2.43 ± 0.14 e | 2.72 ± 0.15 d | 2.60 ± 0.12 d,e |
| 3-(Methylthio)propanal | 0.5 A | 378 ± 21 f | 438 ± 20 e | 471 ± 24 d,e | 457 ± 23 e | 519 ± 22 b,c | 554 ± 25 a,b | 562 ± 26 a | 448 ± 23 e | 509 ± 27 c,d | 478 ± 24 c,d,e |
| 2,6-Dimethyl pyrazine | 0.4 C | – | – | – | 16.4 ± 0.9 e | 26.2 ± 1.3 d | 32.1 ± 1.5 c | 33.5 ± 1.4 b,c | 35.1 ± 1.6 a,b | 36.1 ± 1.5 a,b | 36.5 ± 1.7 a |
| Trimethyl pyrazine | 0.8 C | 2.76 ± 0.13 f | 4.69 ± 0.21 d | 3.86 ± 0.19 e | 4.84 ± 0.23 d | 5.64 ± 0.27 c | 6.66 ± 0.31 a,b | 6.21 ± 0.30 b | 6.79 ± 0.35 a | 6.36 ± 0.32 a,b | 6.78 ± 0.33 a |
| HDMF | 25 A | 49.8 ± 2.4 e | 63.1 ± 3.2 b,c | 65.6 ± 3.0 a,b | 65.2 ± 3.1 a,b,c | 69.0 ± 3.2 a | 70.9 ± 3.5 a | 69.4 ± 3.2 a | 67.1 ± 3.4 a,b | 57.3 ± 2.9 d | 59.9 ± 3.0 c,d |
| HEMF | 20 A | 93.3 ± 4.5 d | 90.4 ± 3.7 d | 93.1 ± 3.6 d | 98.5 ± 4.8 c,d | 105 ± 6 b,c | 116 ± 5a | 109 ± 5 a,b | 90.6 ± 4.2 d | 92.1 ± 4.6 d | 95.3 ± 4.9 d |
| 2-Acetylpyrroline | 0.1 A | 8.29 ± 0.41 e | 8.96 ± 0.40 e | 9.04 ± 0.44 e | 9.16 ± 0.45 d,e | 10.0 ± 0.4 d | 11.9 ± 0.5 c | 11.4 ± 0.5 c | 12.9 ± 0.8 b | 14.4 ± 0.7 a | 14.0 ± 0.7 a |
| OAV of compounds with caramel-like aroma | 535 | 609 | 646 | 655 | 741 | 798 | 798 | 666 | 720 | 696 | |
| 4-Ethylphenol | 140 B | 2.79 ± 0.15 a | 2.58 ± 0.13 b | 2.43 ± 0.13 b,c | 2.31 ± 0.12 c | 2.04 ± 0.10 d | 1.88 ± 0.09 d | 1.53 ± 0.06 e | 1.25 ± 0.04 f | 1.11 ± 0.05 f | 0.86 ± 0.04 g |
| 4-Ethylguaiacol | 10 A | 307 ± 18 a | 287 ± 16 a,b | 262 ± 13 c,d | 268 ± 12 b,c | 269 ± 13 b,c | 259 ± 12 c,d | 242 ± 12 d | 202 ± 11 e | 182 ± 9 e,f | 179 ± 9 f |
| 4-Vinylguaiacol | 12 A | 90.1 ± 4.7 a | 78.8 ± 4.1 b | 76.1 ± 3.9 b | 68.3 ± 3.5 c,d | 66.9 ± 2.7 d,e | 72.9 ± 3.2 b,c | 61.3 ± 3.0 e,f | 56.8 ± 2.3 f,g | 53.9 ± 2.8 g,h | 50.3 ± 2.1 h |
| OAV of compounds with smoky aroma | 400 | 368 | 340 | 339 | 338 | 334 | 305 | 260 | 238 | 231 | |
| Acetic acid | 10000 A | 10.5 ± 0.6 e | 14.6 ± 0.9 d | 14.7 ± 0.8 d | 15.5 ± 0.8 d | 18.5 ± 1.0 c | 20.1 ± 1.2 b,c | 21.8 ± 1.0 b | 23.7 ± 1.5 a | 24.0 ± 1.3 a | 24.5 ± 1.3 a |
| 2-Methylpropanoic acid | 3500 A | 9.61 ± 0.45 d | 11.8 ± 0.7 c | 12.2 ± 0.4 b,c | 12.9 ± 0.6 a,b,c | 13.1 ± 0.6 a,b | 13.9 ± 0.5 a | 13.7 ± 0.8 a | 13.1 ± 0.5 a,b | 13.8 ± 0.5 a | 12.2 ± 0.6 b,c |
| Butanoic acid | 173 A | – | 7.25 ± 0.32 a | 7.53 ± 0.27 a | – | 7.31 ± 0.51 a | 7.64 ± 0.35 a | – | – | – | – |
| 2-Methylbutanoic acid | 225 A | 150 ± 8 a | 146 ± 8 a,b | 149 ± 7 a,b | 147 ± 8 a,b | 141 ± 6 a,b | 137 ± 7 b | 138 ± 7 a,b | 57.4 ± 3.4 c | 52.5 ± 3.1 c | 60.4 ± 3.7 c |
| 3-Methylbutanoic acid | 540 A | 15.8 ± 0.9 d | 34.6 ± 2.0 c | 39.0 ± 1.5 b | 39.7 ± 1.7 b | 43.8 ± 2.4 a | 44.0 ± 2.2 a | 45.2 ± 1.9 a | 40.0 ± 2.1 b | 32.2 ± 1.7 c | 35.4 ± 1.8 c |
| Phenylacetic acid | 1000 A | 10.2 ± 0.5 b | 11.2 ± 0.5 a | 11.6 ± 0.7 a | 11.6 ± 0.6 a | 11.9 ± 0.5 a | 12.1 ± 0.4 a | 12.2 ± 0.7 a | 12.1 ± 0.5 a | 12.1 ± 0.6 a | 12.2 ± 0.4 a |
| OAV of compounds with sour aroma | 196 | 225 | 234 | 227 | 235 | 235 | 231 | 146 | 135 | 145 | |
| Dimethyl trisulfide | 0.01 A | 4.13 ± 0.25 f | – | 7.16 ± 0.40 e | 6.39 ± 0.35 e | 8.77 ± 0.49 d | 10.8 ± 0.5 c | 12.1 ± 0.7 b | 13.9 ± 0.5 a | 13.3 ± 0.6 a | 14.0 ± 0.8 a |
| 1-Octen-3-ol | 1.5 A | 21.2 ± 1.7 d | 30.4 ± 1.4 c | 32.9 ± 2.5 c | 37.7 ± 1.5 b | 40.2 ± 1.7 b | 44.4 ± 2.3 a | 45.2 ± 2.0 a | 45.0 ± 2.0 a | 45.2 ± 2.1 a | 44.6 ± 2.3 a |
| OAV of compoundswith other aroma | 25.3 | 30.4 | 40.1 | 44.1 | 48.9 | 55.1 | 57.3 | 58.9 | 58.5 | 58.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, Z.-H.; He, R.; Xu, R.; Zhang, L.; Gao, X. Improving Soy Sauce Aroma Using High Hydrostatic Pressure and the Preliminary Mechanism. Foods 2022, 11, 2190. https://doi.org/10.3390/foods11152190
Zhang Y, Zhang Z-H, He R, Xu R, Zhang L, Gao X. Improving Soy Sauce Aroma Using High Hydrostatic Pressure and the Preliminary Mechanism. Foods. 2022; 11(15):2190. https://doi.org/10.3390/foods11152190
Chicago/Turabian StyleZhang, Yaqiong, Zhi-Hong Zhang, Ronghai He, Riyi Xu, Lei Zhang, and Xianli Gao. 2022. "Improving Soy Sauce Aroma Using High Hydrostatic Pressure and the Preliminary Mechanism" Foods 11, no. 15: 2190. https://doi.org/10.3390/foods11152190
APA StyleZhang, Y., Zhang, Z.-H., He, R., Xu, R., Zhang, L., & Gao, X. (2022). Improving Soy Sauce Aroma Using High Hydrostatic Pressure and the Preliminary Mechanism. Foods, 11(15), 2190. https://doi.org/10.3390/foods11152190







