Abstract
Plant polyphenols have attracted considerable attention because of their key roles in preventing many diseases, including high blood sugar, high cholesterol, and cancer. A variety of functional foods have been designed and developed with plant polyphenols as the main active ingredients. Polyphenols mainly come from vegetables and fruits and can generally be divided according to their structure into flavonoids, astragalus, phenolic acids, and lignans. Polyphenols are a group of plant-derived functional food ingredients with different molecular structures and various biological activities including antioxidant, anti-inflammatory, and anticancer properties. However, many polyphenolic compounds have low oral bioavailability, which limits the application of polyphenols in nutraceuticals. Fortunately, green bio-based nanocarriers are well suited for encapsulating, protecting, and delivering polyphenols, thereby improving their bioavailability. In this paper, the health benefits of plant polyphenols in the prevention of various diseases are summarized, with a review of the research progress into bio-based nanocarriers for the improvement of the oral bioavailability of polyphenols. Polyphenols have great potential for application as key formulations in health and nutrition products. In the future, the development of food-grade delivery carriers for the encapsulation and delivery of polyphenolic compounds could well solve the limitations of poor water solubility and low bioavailability of polyphenols for practical applications.
1. Introduction
Plant-derived functional foods have gradually become an area of increasing research interest because of their health benefits to the human body, and plant polyphenols have gained widespread attention as one of the most abundant and widely distributed chemical components. Plant polyphenols naturally occur in vegetables, fruits, cereals, tea, coffee, and other plants. These compounds take phenolic rings as the basic monomer and have a variety of complex structures, which are generally divided into phenolic acids, flavonoids, anthocyanins, and tannins (Figure 1) [1,2]. Some foods contain a variety of bioactive compounds that can cause a variety of biological effects when interacting with the body; these are known as bioactive substances. Plant-based foods contain bioactive substances, including polyphenols [3,4]. Some can be directly isolated and extracted from natural foods, while others can be obtained by reprocessing natural ingredients. Polyphenols mainly depend on biological use to express their biological properties, and their absorption speed and the limits of their activity in the gut depend on their chemical structure [5,6]. Polyphenols are considered good preventive agents for chronic and degenerative diseases, due to their extensive and special chemical structure that endows them with many biological functions. Food polyphenols are common antioxidants in food that can effectively prevent atherosclerosis and prevent aging [7]. Some studies have also confirmed the beneficial effects of polyphenols in the prevention of cardiovascular diseases, neurological diseases, liver diseases, diabetes, and cancer [8].
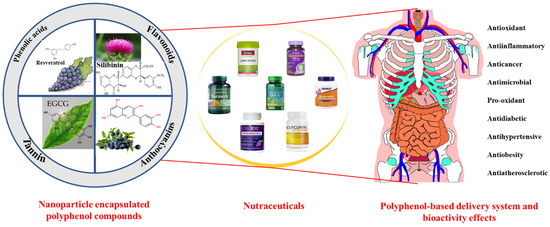
Figure 1.
Beneficial effects of polyphenol-based delivery systems and their potential application in nutraceutical formulations.
Polyphenols are widely found in plant-based foods, and many polyphenols have anti-inflammatory, antioxidant, and anticancer properties [9,10]. Long-term consumption of foods rich in plant polyphenols has been shown to improve conditions as diverse as diabetes, cancer, osteoporosis, neurodegenerative diseases, and cardiovascular disease [11]. Other studies have found that polyphenols have a variety of beneficial effects on intestinal health and are potential prebiotics, able to play a variety of probiotic roles including reducing inflammation and preventing cancer by regulating the intestinal flora [12,13]. Therefore, they are often used as additives in functional dietary foods to achieve beneficial effects through food intake. The market demand for polyphenols is increasing rapidly with social and economic development and the improvement of people’s health awareness. However, many polyphenolic compounds have low oral bioavailability, which limits the application of polyphenols in nutraceuticals.
Fortunately, green bio-based nanocarriers are well suited for encapsulating, protecting, and delivering polyphenols, thus enhancing their bioavailability (Figure 1) [1]. Bio-based polymers (e.g., proteins and polysaccharides) with good biocompatibility, biodegradability, resource sustainability, and good nutritional value are excellent delivery vehicles to address the limitations of low oral utilization of polyphenols when applied as nutritional medicines. Protein is a multifunctional bio-based polymer with rich nutritional value and various functional properties, including emulsification, amphiphilicity, gelation, and foaming properties. Its unique chemical structure and functionality allow it to be engineered into nanoparticles, nanogels, nanoemulsions, nanofilms, and nanofibers that can deliver both hydrophilic and hydrophobic polyphenolic compounds [14]. The unique structure and physiological activity of polysaccharides, as bio-based polymers with a wide range of origins, allow them to be used as raw materials for the preparation of various types of nanocarriers and for the delivery of polyphenolic compounds [1]. In addition, the good biocompatibility and biodegradability of lipid-based nanocarriers make them one of the key technologies for the delivery of polyphenols in the field of food and nutrition. Lipid-based delivery vectors (including liposomes, nano-emulsions, and solid lipid nanoparticles) are good ways to protect fat-soluble polyphenols in the gastrointestinal tract and improve their bioavailability [1]. Some commercialized functionalized polyphenol products have been introduced onto the market, which undoubtedly provides a foundation for the further exploration and development of new polyphenol health nutrition products [15,16].
In this article, we provide an overview of the classification of polyphenolic compounds and an exhaustive summary of the health benefits of polyphenols for human health improvement. We also propose improvements to address the limitations of polyphenolic compounds in their application as health food formulations: namely, the design and preparation of food-grade bio-based nanocarriers to encapsulate, protect, and deliver polyphenolic compounds, thereby improving their bioavailability. Finally, we look forward to the prospective application of polyphenol food-grade bio-based nanocomplexes in nutraceuticals, with the aim of providing valuable insights into the development and utilization of nutraceuticals through the combination of nano-delivery methods and the health benefits of polyphenols.
2. Classification, Properties, and Beneficial Effects of Plant Polyphenols
2.1. Classification and Properties
Plant polyphenols are widely found in various foods and have attracted much research attention because of their antioxidant, anti-inflammatory, and anticancer biological activities. Polyphenols generally refer to chemicals with a benzene ring structure and two or more phenolic hydroxyl groups, which are generally classified into flavonoids and phenolic acids according to their structural characteristics [1].
Generally, flavonoids exist mainly in the form of glycosides in the vesicles of plant cells. Structurally, flavonoids have three cyclic structures of C6–C3–C6 as their basic skeleton. According to the differences in chemical structures, flavonoids are further subdivided into flavonols, flavones, isoflavones, anthocyanins, flavanones, flavanols, and chalcones (Figure 2). Most plants contain flavonoids that play an important role in plant growth, development, flowering, fruiting, antibacterial activity, and disease prevention. Most of these flavonoids have anti-inflammatory, antibacterial, antioxidant, and anticancer physiological activities, and can have beneficial effects on the human body (Table 1).
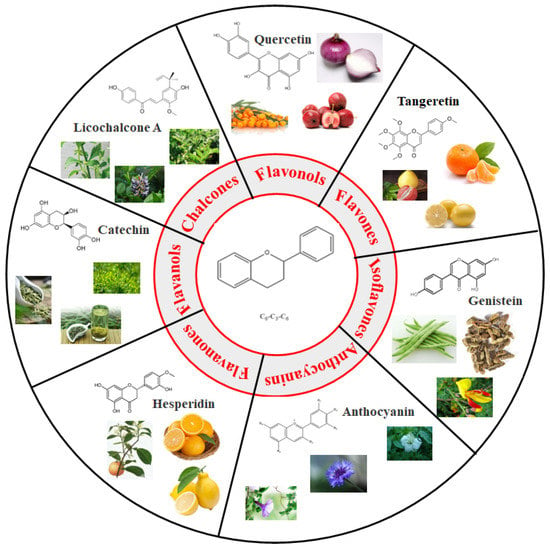
Figure 2.
Classification of flavonoids and examples.

Table 1.
Chemical structure, physiological activity, and food sources of flavonoid compounds.
Phenolic acids are widely found in fruits, vegetables, and beverages. Low molecular weight phenolic acids exhibit water-soluble properties during further processing, as well as during human digestion, and when phenolic acids undergo condensation reactions with glucose and quinic acid, they assume a water-insoluble state [17]. Phenolic acids are generally classified into monohydroxybenzoic acid, dihydroxybenzoic acid, and trihydroxybenzoic acid, according to their hydroxyl content. Phenolic acids are widely used in various functional foods because of their good antioxidant, anti-inflammatory, antiallergic, and anticancer properties, as well as cardioprotective and other health-functional activities [18].
2.2. Beneficial Effects
Plant polyphenols have a variety of beneficial effects on human health. Their excellent biological activities such as antioxidant and antibacterial effects, as well as their natural availability and biocompatibility, make it possible for them to be added to foods and endowed with unique functional properties to exert their beneficial effects on human health. Some of the beneficial effects of plant polyphenols on human health are presented in Table 2, showing the promising application of polyphenols in functional food formulations. The potential role of functional foods containing polyphenolic compounds is extremely important in the prevention of many chronic diseases including diabetes, hypertension, and cancer.

Table 2.
Biological activity and health benefits of polyphenols.
2.2.1. Antioxidant Effect
Plant polyphenolic compounds generally have good antioxidant activity due to their specific structural characteristics. Fruits and vegetables, as well as some cereals, are rich in polyphenolic compounds, which play an important role in human health. Polyphenols as antioxidants can prevent several diseases by scavenging free radicals and thus protecting DNA from oxidative damage. Jakubczyk et al. [38] conducted a search of PubMed and Embase for randomized clinical trials of >20 patients treated with curcumin supplements from the start of the database until 27 September 2019. A total of 308 participants were enrolled in the study on the antioxidant potential of curcumin. A total of 40% of the respondents were men. The mean age of the participants was 27.60 ± 3.79 years. The average duration of supplementation was 67 days, and the average dose of curcumin administered was 645 mg/24 h. The results showed that curcumin had good antioxidant capacity and tended to reduce malondialdehyde concentrations. In a study by Grzesik et al. [39], the antioxidant capacities of catechins, glutathione, and ascorbic acid were compared. It was found that catechins were the most effective for scavenging ABTS radicals, had the highest reduction equivalents for trivalent iron ions, and were effective in protecting against the oxidation of dihydrorhodamine. The excellent antioxidant properties of polyphenolic compounds such as catechins make them ideal candidates for antioxidant prophylaxis and therapy.
2.2.2. Anti-Inflammatory Effect
Plant polyphenols have good inhibitory and killing effects on some inflammatory cells, either by affecting cytokines and their receptors or by influencing their secretion processes. It was reported that hydrogels containing rutin exhibited good anti-inflammatory activity comparable to that of standard drugs, as demonstrated in the study of Singhai et al. [46]. Hesperidin was evaluated by Ding et al. [47]. Hesperidin was tested for anti-inflammatory activity using RAW264.7 cells and a CCl4-induced acute liver injury model, and it was found that hesperidin was effective in reducing nitric oxide (NO), interleukin (IL-6), and tumor necrosis factor (TNF-α), both in vivo and in vitro, exhibiting good anti-inflammatory activity.
2.2.3. Anticancer Effect
Polyphenols have good protective effects against some types of cancer. They can inhibit tumor proliferation and have toxic effects on cells, inducing apoptosis. Lee et al. [49] reported that resveratrol can affect the course of cancer and other related diseases through its inhibitory effect on cell proliferation, its apoptotic effect, and its good antioxidant properties. Their report also indicated that quercetin has been widely used in the prevention and treatment of esophageal cancer. The anticancer activity of polyphenolic compounds has also been confirmed in several other studies. For example, silymarin has been observed to induce apoptosis in liver cancer cells and has shown good preventive and therapeutic effects against liver diseases, and epigallocatechin gallate (EGCG) and curcumin have good anticancer effects on breast cancer [51,52].
2.2.4. Antimicrobial Effect
Polyphenols have good antibacterial effects on a variety of microorganisms. This is especially the case for flavonoids, which have significantly higher antibacterial activity than some other polyphenolic compounds. Some reports have shown that polyphenolic compounds can synergize with antibiotics and exhibit excellent antibacterial properties. Liu et al. [55] reported that chitosan-film-loaded curcumin showed good antibacterial activity against Staphylococcus aureus and Rhizopus solani. In addition, the antibacterial activities of tea polyphenols, silymarin, and rutin have been widely reported and confirmed [41,46,56].
2.2.5. Pro-Oxidant Effect
Polyphenolic compounds have excellent antioxidant effects. However, some polyphenolic compounds can cause DNA damage at high doses, even to the point of causing apoptosis [75]. Canedo-Santos et al. [57] showed that the pro-oxidant properties of resveratrol accelerated the aging process and its pro-oxidant activity shortened the chronological life span of brewer’s yeast. In contrast, gallic acid, while inhibiting lipid oxidation and protein carbonyl formation, tends to promote the oxidative loss of thiol and amine groups, thereby affecting the structural properties and biological activity of proteins [58]. The addition of high doses of soybean into the feed of meat pigs promoted oxidative changes in pork fat, liver, and plasma, and their addition for 64 days increased superoxide dismutase activity and total antioxidant capacity, showing strong pro-oxidant activity [59]. In view of this, the dosage of polyphenolic compounds used in applications is an important point of attention.
2.2.6. Antidiabetic Effect
A diet rich in polyphenols may reduce the risk of diabetes. Some studies have shown that polyphenols can regulate the insulin pathway and enhance insulin sensitivity in the periphery of tissues [76]. Many polyphenolic compounds demonstrate strong inhibition of alpha amylase and alpha glucosidase, which regulate the intestinal absorption of glucose and maintain blood sugar balance [77]. Some foods, such as tea, are rich in a variety of polyphenolic compounds including catechins, which have excellent antioxidant and antidiabetic activities. The therapeutic potential of quercetin as an antidiabetic bioactive substance was reported in detail in a review by Eid et al. [64]. A comprehensive and systematic summary and description of the mechanism of action, the targets, and the effects of quercetin were reported, showing that quercetin has, in vitro and in vivo, been found to have good preventive and therapeutic potential against diabetes [64]. Thus, polyphenolic compounds have great potential for application in the prevention and control of diabetogenesis.
2.2.7. Antihypertensive Effect
Some polyphenolic compounds, such as cocoa that is rich in flavanol compounds including catechins and proanthocyanidins, can effectively improve endothelial function, effectively reduce the oxidative sensitivity of low-density lipoproteins, and increase vasodilation. This effect has been recognized by the European Food Safety Authority [78]. A report by Huang et al. [68] described in detail the beneficial effects of flavonoids, flavanols, anthocyanins, phenolic acids, tannins, resveratrol, and other polyphenolic compounds on vasodilation and other aspects of blood pressure control. In another study, researchers evaluated the vasodilatory effects of curcumin, amlodipine, and curcumin and amlodipine in combination on isolated rat aortic rings, and found that hypertensive patients taking amlodipine could consume curcumin or turmeric for food or other medical purposes without inhibiting the antihypertensive effects of amlodipine [65]. This provides a valuable basis for the use of curcumin and other polyphenolic compounds with antihypertensive effects as food ingredients for the prevention and treatment of hypertension.
2.2.8. Antiobesity Effect
Polyphenols can affect obesity through various effects, such as inhibiting adipocyte proliferation, stimulating adipocyte apoptosis, promoting lipolysis, and fat oxidation. Green tea is a beverage with a variety of health benefits and is a rich source of gallocatechin gallate and catechin, as well as a variety of polyphenolic compounds such as catechins, and can play an important role in obesity control [79]. Evidence suggests that soybean is able to control obesity by inhibiting the activity of pancreatic lipase and pancreatic protein lipase, thereby inhibiting adipocyte differentiation, and by activating hormone-sensitive lipase to stimulate lipolysis [69]. Similarly, in a study by Ting et al. [70], quercetin supplementation was found to have a significant inhibitory effect on the accumulation of adipose tissue in obese rats, with potential anti-obesity effects.
2.2.9. Antiatherosclerotic Effect
A variety of plant polyphenolic compounds can protect the cardiovascular system through different mechanisms, such as increasing high-density lipoprotein, decreasing low-density lipoprotein, and preventing low-density lipoprotein oxidation [80]. For example, ellagic acid and resveratrol can play an anti-atherosclerotic role by improving endothelial barrier function [72,74,81]. In addition, according to the research of Tanaka et al. [73], EGCG has also been found to exhibit certain anti-atherosclerotic effects, and this mechanism of action was elaborated and demonstrated in detail in the report. Polyphenolic compounds have shown good effects in the prevention and treatment of atherosclerosis, and have attracted attention in the development and application of functional foods.
3. Common Bio-Based Polymer Nano-Delivery of Polyphenolic Compounds
Biopolymers have many excellent properties, such as being biodegradable and bio-compatible, and are good choices as delivery carriers for polyphenolic compounds. Among several types of biopolymers, the most frequent and extensive studies have been of protein-based, polysaccharide-based, and lipid-based carriers (Table 3). To enhance the trend of including polyphenolic compounds in nutraceuticals, some limitations in their application have been weakened or overcome. The effective design and preparation of nanocarriers using a variety of biomolecules such as proteins, polysaccharides, and lipids for the encapsulation, protection, and delivery of polyphenolic compounds has been widely studied (Figure 3) [1].

Table 3.
Common bio-based nano-delivery systems for polyphenols.
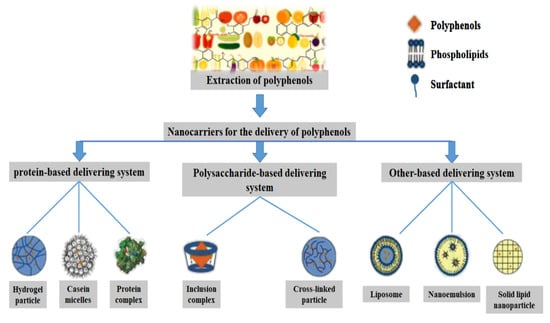
Figure 3.
Different types of bio-based nanocarriers for delivery of polyphenols.
It is encouraging that some commercialized functionalized products containing polyphenols have been introduced onto the market (Table 4), which undoubtedly provides a point of reference for the further exploration and development of new polyphenol health nutrition products [98]. WackerChemie AG has developed CAVACURCUMIN@ for enhanced absorption in combination with γ-cyclodextrin [15]. New formulations containing curcumin nanoparticles, such as Theracurcumin®, a brand name of curcumin nanoparticles, have also shown improved pharmacokinetic properties in clinical trials with human volunteers [16]. Preparation of polyphenol nanocomposites using nanotechnology has significantly improved the physicochemical properties of the compounds and provided unique characteristics in terms of size, surface, drug delivery and targeting potential. Polyphenol-supported biological nanocomposites have broad application prospects for health care products, functional food and cosmetics. This phenomenon provides continuous support for the development of food-grade bio-based nanocarriers.

Table 4.
Commercialized nano-polyphenols [98]. Reprinted from Rambaran T.F. (2022), copyright (2022), with permission from Elsevier.
Different types of bio-based nanocarriers can be used as embedding agents and protective barriers for plant polyphenols, which can be divided into nanoparticles, nanomicelles, nanogels, nanoemulsions, and liposomes. A range of nanocarriers, including zein, soy protein, albumin, starch, cellulose, and lipids have been shown to efficiently deliver polyphenolic compounds and improve their bioavailability [1]. Different packaging materials have different effects on the physical and chemical properties of the carrier. Some studies have found that protein and chitosan are more popular as materials for carrier preparation, and extensive studies have reported their favorable effects on increasing intestinal absorption and the bioavailability of plant polyphenols [14]. The encapsulation mechanisms of polyphenols by nanocarriers are diverse, including ionic gel, eutectic, condensation, encapsulation, emulsification, freeze-drying, and yeast encapsulation. These encapsulation mechanisms can improve the water dispersion, gastrointestinal environmental stability, targeting, and sustained release of polyphenols [1]. For example, between curcumin and zein there exists hydrogen bonding, along with electrostatic interaction, and hydrophobicity, which are the main driving forces of nanoencapsulation [82].
Proteins are commonly used for the encapsulation and delivery of polyphenolic compounds, due to their many excellent properties such as high nutritional value, low toxicity, biocompatibility, and biodegradability. They are designed in various forms (nanoparticles, thin films, hydrogel fibers, etc.) and can also be modified or compounded to improve their surface functionality, thus achieving increased bioavailability of polyphenolic compounds [99]. Lozano-Pérez et al. [100] conducted encapsulation, adsorption, and release studies on quercetin using nanoparticles prepared from silk proteins, and the encapsulated quercetin maintained good free radical scavenging ability and was able to achieve a slow-release effect in simulated intestinal fluids. Similarly, whey protein nanoparticles are a suitable carrier for the encapsulation, delivery, and slow gastrointestinal release of polyphenolic compounds such as curcumin, and the system can maintain good stability at pH 7, which provides a good theoretical basis for its application in functional beverages [101]. Other proteins, including bovine serum albumin [102] and zein [99], have often been used as materials for the preparation of nanocarriers to encapsulate polyphenol compounds and improve their bioavailability.
Polysaccharides have likewise received much attention as raw materials for the preparation of bio-based nanoparticles. Various technological approaches allow the modification of polysaccharide-based nanomaterials suitable for the encapsulation and delivery of bioactive molecules in various fields, especially for the protection of polyphenolic compounds [103,104]. Geetha et al. [105] designed and prepared curcumin-loaded starch nanoparticles from cassava starch, and the successful loading of curcumin was confirmed by fluorescence spectroscopy and Fourier infrared transform. Chitosan, a natural polysaccharide with good inter-solubility and biodegradability, has been widely studied for the delivery of polyphenolic compounds. Chitosan nanoparticles can effectively achieve the slow release, controlled release, and improved bioavailability of polyphenolic compounds such as tea polyphenols [106].
As well as proteins and polysaccharides, lipids are another class of bio-based nanocarrier that are commonly used to encapsulate polyphenolic compounds, and are equally biocompatible and biodegradable. Bioactive systems based on solid lipid nanoparticles have been shown to be effective in delivering hydrophobic nutraceuticals. For example, Qin et al. [95] used solid lipid nanoparticles to encapsulate and deliver resveratrol, and found that resveratrol-loaded solid lipid nanoparticles were effective in enhancing antioxidant defense and conferring anti-fatigue capacity after extensive exercise in mice, which provides a valuable reference for resveratrol delivery systems within the field of novel anti-fatigue sports nutrition. Similarly, the bioavailability of other polyphenolic compounds such as quercetin was enhanced to varying degrees by the solid lipid nanocarriers [107]. In addition, nanoemulsions and liposomes are also effective delivery systems for lipophilic polyphenolic compounds, and provide excellent support for the enhancement of plant polyphenol bioavailability. In the study by Li et al. [96], nanoemulsions were extensively studied for the encapsulation, protection, and delivery of lipophilic functional components including polyphenols, pigments, and flavors. Polyphenolic compounds delivered via nanoemulsions have the ability to improve the solubility of hydrophobic compounds, providing better kinetic and biological effects. This gives a key reference for the use of nanoemulsions in the application of plant polyphenols as nutritional formulations in the food industry. Liposomes have also recently been studied for the delivery of different functional compounds in food systems; their biodegradability, non-toxicity, small size, and unique amphiphilic nature were found to confer excellent delivery [97]. Nowadays, the application of lipid delivery systems for the bioavailability enhancement of polyphenolic compounds has been extensively studied, laying the theoretical foundation for the application of polyphenolic compounds in nutraceuticals.
In addition, some protein-based nanoparticles encapsulated with polysaccharides can better improve the stability of delivery systems, such as the preparation of polysaccharide–protein composite nanoparticles using pectin [108], carrageenan [109], chitosan [110], etc., in complex with zein. This can improve the limitation of single zein nanoparticles that tend to aggregate, and then enhance the delivery capacity and protection effect of composite particles to polyphenol compounds. Dai et al. [111] prepared zein-rhamnoid composite nanoparticles by combining zein with rhamnolipid. The study found that the composite nanoparticles had a good encapsulation and protection effect on curcumin, providing an alternative food-grade nano-carrier for the delivery of hydrophobic health care products in functional food and beverages.
In conclusion, biomolecule-based nanoparticles have many advantages, such as environmental friendliness, biocompatibility, and abundant obtainability, which make them excellent materials for the application of polyphenol compound delivery carriers in dietary foods. The continuous development and gradual maturation of nanotechnology has greatly reduced the limitations associated with the low bioavailability of polyphenolic compounds, and the design and preparation of food-grade polyphenol-bio-based nanocomposite systems have become important areas in the development of functional foods.
4. Conclusions
Polyphenolic compounds are receiving increased attention due to their wide distribution in plants and their good biological activities. As the application of nanotechnology in the delivery of bioactive ingredients has gradually matured, the development and design of food-grade nanocarriers has gradually advanced, opening up broad application prospects for polyphenols as functional food ingredients. With the increasing pursuit of food diversification and functionalization, some functional foods and special dietary foods designed with plant polyphenols as their main active ingredients have received relevant safety certifications and are widely appreciated. However, certain other polyphenolic compounds, which also have excellent biological activities and functional properties that have been confirmed by researchers, have not yet been approved for food addition. This continues to pose limitations for the development of functional foods. This paper has reviewed the types of polyphenolic compounds and health benefits, including antioxidant, anticancer, antibacterial, antiviral, antiatherosclerotic, and anti-obesity effects. It has discussed the promising development and application of plant polyphenols encapsulated, protected, and delivered through nanocarriers as food ingredients in sustainable and healthy functional foods. This paper also looks forward to the broad prospects of plant polyphenols as food ingredients as part of the development and application of healthy, environmentally friendly functional foods, hoping to provide new insights and research ideas for the development of polyphenolic compounds in food applications. In addition, the potential nanotoxicity of polyphenol nanocomposites is also a concern. The development of commercial polyphenol nanomaterials must be subject to adequate safety assessment. Regulators should take steps to establish standardized safety assessment guidelines so that their entry into the market can be appropriately monitored.
Author Contributions
Conceptualization, S.S. and L.C.; software, Z.Z.; validation, Z.Z., X.L. and C.Q.; formal analysis, J.L.; investigation, Z.Z.; resources, C.Q.; writing—original draft preparation, Z.Z.; writing—review and editing, D.J.M. and C.Q.; visualization, A.J.; supervision, Z.J.; project administration, C.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [Natural Science Foundation of Jiangsu Province] grant number [BK20210458], [Special Support for Post-doc Creative Funding in Shandong Province] grant number [2020003072]. Thanks to the medical staff who worked on the front line during the COVID-19 pandemic and the editors and reviewers who reviewed our work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Z.; Qiu, C.; Li, X.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z. Advances in research on interactions between polyphenols and biology-based nano-delivery systems and their applications in improving the bioavailability of polyphenols. Trends Food Sci. Technol. 2021, 116, 492–500. [Google Scholar] [CrossRef]
- Guo, Q.; Xiao, X.; Lu, L.; Ai, L.; Xu, M.; Liu, Y.; Goff, H. Polyphenol-Polysaccharide Complex: Preparation, Characterization, and Potential Utilization in Food and Health. Annu. Rev. Food Sci. Technol. 2022, 13, 59–87. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Rezaei, M.; McClements, D.J. Bioactive functional ingredients from aquatic origin: A review of recent progress in marine-derived nutraceuticals. Crit. Rev. Food Sci. 2022, 62, 1242–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Wang, J.; Jin, Z.; Qiu, C. A review of nanostructured delivery systems for the encapsulation, protection, and delivery of silymarin: An emerging nutraceutical. Food Res. Int. 2022, 156, 111314. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Chen, Y. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis (Review). J. Funct. Foods 2020, 66, 103829. [Google Scholar] [CrossRef]
- Ashwin, K.; Pattanaik, A.K.; Howarth, G.S. Polyphenolic bioactives as an emerging group of nutraceuticals for promotion of gut health: A review. Food Biosci. 2021, 44, 101376. [Google Scholar] [CrossRef]
- Fan, G.; Beta, T. Discrimination of geographical origin of Napirira bean (Phaseolus vulgaris L.) based on phenolic profiles and antioxidant activity. J. Food Compos. Anal. 2017, 62, 217–222. [Google Scholar] [CrossRef]
- Mehmood, A.; Usman, M.; Patil, P.; Zhao, L.; Wang, C. A review on management of cardiovascular diseases by olive polyphenols. Food Sci. Nutr. 2020, 8, 4639–4655. [Google Scholar] [CrossRef]
- Delmas, D.; Hichami, A.; Rebe, C.; Ghiringhelli, F. Immunomodulation and Anti-inflammatory Roles of Polyphenols as Anticancer Agents. Anti-Cancer Agent Med. 2012, 12, 852–873. [Google Scholar] [CrossRef]
- Qiu, C.; Hu, Y.; Jin, Z.; McClements, D.J.; Qin, Y.; Xu, X.; Wang, J. A review of green techniques for the synthesis of size-controlled starch-based nanoparticles and their applications as nanodelivery systems (Review). Trends Food Sci. Technol. 2019, 92, 138–151. [Google Scholar] [CrossRef]
- García-Conesa, M.; Larrosa, M. Polyphenol-rich foods for human health and disease (Editorial). Nutrients 2020, 12, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, G.; Wang, X.; Wu, C.; Pan, H.; Yang, J.; Li, T.; Cao, F. Effect of heating on the content and composition of ginkgolic acids in ginkgo seeds. Qual. Assur. Saf. Crop. 2017, 9, 195–199. [Google Scholar] [CrossRef]
- Thilakarathna, W.W.; Langille, M.G.; Rupasinghe, H.V. Polyphenol-based prebiotics and synbiotics: Potential for cancer chemoprevention (Review). Curr. Opin. Food Sci. 2018, 20, 51–57. [Google Scholar] [CrossRef]
- Guan, T.; Zhang, Z.; Li, X.; Cui, S.; McClements, D.J.; Wu, X.; Chen, L.; Long, J.; Jiao, A.; Qiu, C.; et al. Preparation, Characteristics, and Advantages of Plant Protein-Based Bioactive Molecule Delivery Systems. Foods 2022, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Hundshammer, C.; Schön, C.; Kimura, M.; Furune, T.; Terao, K.; Elgeti, D.; Mohr, R. Enhanced metabolic bioavailability of tetrahydrocurcumin after oral supplementation of a γ-cyclodextrin curcumin complex. J. Funct. Foods 2021, 79, 104410. [Google Scholar] [CrossRef]
- Naksuriya, O.; Okonogi, S.; Schiffelers, R.M.; Hennink, W.E. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 2014, 35, 3365–3383. [Google Scholar] [CrossRef]
- Bennourab, N.; Mighria, H.; Eljania, H.; Zammouria, T.; Akrouta, A. Effect of solvent evaporation method on phenolic compounds and the antioxidant activity of Moringa oleifera cultivated in Southern Tunisia. S. Afr. J. Bot. 2020, 129, 181–190. [Google Scholar] [CrossRef]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Petrillo, A.D.; Orru, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef]
- Semwal, R.; Joshi, S.K.; Semwal, R.B.; Semwal, D.K. Health benefits and limitations of rutin-A natural flavonoid with high nutraceutical value. Phytochem. Lett. 2021, 46, 119–128. [Google Scholar] [CrossRef]
- Ibrahim, A.; Nasr, M.; El-Sherbiny, I.M. Baicalin as an emerging magical nutraceutical molecule: Emphasis on pharmacological properties and advances in pharmaceutical delivery. J. Drug Deliv. Sci. Technol. 2022, 70, 103269. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Farsad, N.A.; Mohammad, A. Antioxidant properties of the flavonoid fisetin: An updated review of in vivo and in vitro studies. Trends Food Sci. Technol. 2017, 70, 34–44. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kim, H.; Lee, Y.; Lee, Y.I. Phytochemical and Pharmacological role of liquiritigenin and isoliquiritigenin from radix glycyrrhizae in human health and disease models. Front. Aging Neurosci. 2018, 10, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Wang, F.; Zhou, R.; Song, X.; Xie, M. Apigenin: A current review on its beneficial biological activities. J. Food Biochem. 2017, 41, e12376. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.; Rahaman, A.; Muhammad, A.R.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef]
- Thakur, K.; Zhu, Y.; Feng, J.; Zhang, J.; Hu, F.; Prasad, C.; Wei, Z. Morin as an imminent functional food ingredient: An update on its enhanced efficacy in the treatment and prevention of metabolic syndromes. Food Funct. 2020, 11, 8424–8443. [Google Scholar] [CrossRef]
- Mayo, B.; Vzquez, L.; Flrez, A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, S.; Wang, S.; Gao, P.; Dai, L. A comprehensive review on Pueraria: Insights on its chemistry and medicinal value. Biomed. Pharmacother. 2020, 131, 110734. [Google Scholar] [CrossRef]
- Leis, K.; Kulczyńska, A.; Racinowski, M.; Kaczor, P.; Golebiewski, J.; Januszko-Giergielewicz, B. Genistein-a supplement improving efficiency of the human body: A review. Sci. Sport 2021, 36, 359–367. [Google Scholar] [CrossRef]
- Raheja, S.; Girdhar, A.; Lather, V.; Pandita, D. Biochanin A: A phytoestrogen with therapeutic potential (Review). Trends Food Sci. Technol. 2018, 79, 55–66. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, R.; Shi, W.; Li, L.; Liu, H.; Liu, Z.; Wu, L. The Multifunctional Benefits of Naturally Occurring Delphinidin and Its Glycosides. J. Agric. Food Chem. 2019, 67, 11288–11306. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Chen, L.; Qian, L.; Lin, X.; Fan, X.; Teng, H.; Cao, H. Fabrication of caseins nanoparticles to improve the stability of cyanidin 3-O-glucoside. Food Chem. 2020, 317, 126418. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. 2017, 57, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.H.; Augustin, M.A. Nano- and micro-particles for delivery of catechins: Physical and biological performance. Crit. Rev. Food Sci. 2019, 59, 1563–1579. [Google Scholar] [CrossRef]
- Xiao, Y.; Lee, I. Effects of Microbial Transformation on the Biological Activities of Prenylated Chalcones from Angelica keiskei. Foods 2022, 11, 543. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Druzga, A.; Katarzyna, J.; Skonieczna-Zydecka, K. Antioxidant Potential of Curcumin-A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, e1092. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparlo, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Song, H.; Wang, Q.; He, A.; Li, S.; Guan, X.; Hu, Y.; Feng, S. Antioxidant activity, storage stability and in vitro release of epigallocatechin-3-gallate (EGCG) encapsulated in hordein nanoparticles. Food Chem. 2022, 388, 132903. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Wu, S. Health Benefits of Silybum marianum: Phytochemistry, Pharmacology, and Applications. J. Agric. Food Chem. 2020, 68, 11644–11664. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.; Zhang, N.; Zhou, Q.; Fan, D.; Wang, M. Lipophilized apigenin derivatives produced during the frying process as novel antioxidants. Food Chem. 2022, 379, 132178. [Google Scholar] [CrossRef] [PubMed]
- Sneharani, A.H. Curcumin-sunflower protein nanoparticles—A potential antiinflammatory agent. J. Food Biochem. 2019, 43, e12909. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; McClements, D.J.; Jin, Z.; Qin, Y.; Hu, Y.; Xu, X.; Wang, J. Resveratrol-loaded core-shell nanostructured delivery systems: Cyclodextrin-based metal-organic nanocapsules prepared by ionic gelation. Food Chem. 2020, 317, 126328. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Huang, S.; Chen, Y.; Wang, Q.; Luo, S.; Li, Y.; Wang, X.; Chen, J.; Luo, X.; Zhou, L. Synergistic effect of combined treatment with baicalin and emodin on DSS-induced colitis in mouse. Phytother. Res. 2021, 35, 5708–5719. [Google Scholar] [CrossRef]
- Singhai, A.K.; Malik, J.; Sont, H. Antimicrobial and Antiinflammatory Activity of the Hydrogels Containing Rutin Delivery. Asian J. Chem. 2013, 25, 8371–8373. [Google Scholar] [CrossRef]
- Ding, H.; Huang, A.; Zhang, Y.; Li, B.; Huang, C.; Ma, T.; Meng, X.; Li, J. Design, synthesis and biological evaluation of hesperetin derivatives as potent anti-inflammatory agent. Fitoterapia 2017, 121, 212–222. [Google Scholar] [CrossRef]
- Stanca, E.; Serviddio, G.; Bellanti, F.; Vendemiale, G.; Siculella, L.; Giudetti, A.M. Down-regulation of LPCAT expression increases platelet-activating factor level in cirrhotic rat liver: Potential antiinflammatory effect of silybin. BBA-Mol. Basis Dis. 2019, 1832, 2019–2026. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Lee, Y.J. Synergistic anticancer activity of resveratrol in combination with docetaxel in prostate carcinoma cells. Nutr. Res. Pract. 2021, 15, 12–25. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Shabani, V.M.; Clark, C.C.T.; Jafarnejad, S. Quercetin as an anticancer agent: Focus on esophageal cancer. J. Food Biochem. 2020, 44, e1337. [Google Scholar] [CrossRef]
- Liu, H.T.; Ho, Y.S. Anticancer effect of curcumin on breast cancer and stem cells. Food Sci. Hum. Well. 2018, 7, 134–137. [Google Scholar] [CrossRef]
- Bimonte, S.; Cascella, M.; Barbieri, A.; Arra, C.; Cuomo, A. Current shreds of evidence on the anticancer role of EGCG in triple negative breast cancer: An update of the current state of knowledge. Infect. Agents Cancer 2020, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Verebova, V.; Benes, J.; Stanicova, J. Biophysical Characterization and Anticancer Activities of Photosensitive Phytoanthraquinones Represented by Hypericin and Its Model Compounds. Molecules 2020, 25, E5666. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Bhattacharjee, M.; Bhattacharya, S.; Ahir, M.; Adhikary, A.; Patra, P. Silymarin-Loaded, Lactobionic Acid-Conjugated Porous PLGA Nanoparticles Induce Apoptosis in Liver Cancer Cells. ACS Appl. Bio Mater. 2020, 3, 7178–7192. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Jiang, X.; Wu, J.; Le, X. Molecular interactions, characterization and antimicrobial activity of curcumin-chitosan blend films (Article). Food Hydrocolloid. 2016, 52, 564–572. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, T. Antimicrobial Activities of Tea Polyphenol on Phytopathogens: A Review. Molecules 2019, 24, 816. [Google Scholar] [CrossRef] [Green Version]
- Canedo-Santos, J.C.; Carrillo-Garmendia, A.; Mora-Martinez, I.; Gutierrez-Garcia, I.K.; Ramirez-Romero, M.G.; Regalado-Gonzalez, C.; Nava, G.M.; Madrigal-Perez, L.A. Resveratrol shortens the chronological lifespan of Saccharomyces cerevisiae by a pro-oxidant mechanism. Yeast 2022, 39, 193–207. [Google Scholar] [CrossRef]
- Cao, Y.; True, A.D.; Chen, J.; Xiong, Y. Dual Role (Anti- and Pro-oxidant) of Gallic Acid in Mediating Myofibrillar Protein Gelation and Gel in vitro Digestion. J. Agric. Food Chem. 2016, 64, 3054–3061. [Google Scholar] [CrossRef]
- Chen, W.; Ma, X.; Lin, Y.; Xiong, Y.; Zheng, C.; Hu, Y.; Yu, D.; Jiang, Z. Dietary supplementation with a high dose of daidzein enhances the antioxidant capacity in swine muscle but experts pro-oxidant function in liver and fat tissues. J. Anim. Sci. Biotechnol. 2016, 7, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Lucas, M.; Freitas, M.; Xavier, J.A.; Moura, F.A.; Goulart, M.O.F.; Ribeiro, D.; Fernandes, E. The scavenging effect of curcumin, piperine and their combination against physiological relevant reactive pro-oxidant species using in vitro non-cellular and cellular models. Chem. Pap. 2021, 75, 5269–5277. [Google Scholar] [CrossRef]
- Natarajan, S.B.; Chandran, S.P.; Khan, S.H.; Natarajan, P.; Rengarajan, K. Versatile Health Benefits of Catechin from Green Tea (Camellia sinensis). Curr. Res. Nutr. Food Sci. 2019, 15, 3–10. [Google Scholar] [CrossRef]
- Liu, B.; Kang, Z.; Yan, W. Synthesis, Stability, and Antidiabetic Activity Evaluation of (-)-Epigallocatechin Gallate (EGCG) Palmitate Derived from Natural Tea Polyphenols. Molecules 2021, 26, E393. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Orhan, I.E.; Bawazeer, S. Health perspectives of a bioactive compound curcumin: A review. Trends Food Sci. Technol. 2018, 74, 33–45. [Google Scholar] [CrossRef]
- Eid, H.M.; Haddad, P.S. The antidiabetic potential of quercetin: Underlying mechanisms. Curr. Med. Chem. 2017, 24, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jo, C.; Choi, H.; Lee, K. Effect of Co-Administration of Curcumin with Amlodipine in Hypertension. Nutrients 2021, 13, 2797. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and Its Consumption: Benefits and Risks. Crit. Rev. Food Sci. 2015, 55, 939–954. [Google Scholar] [CrossRef]
- Muguerza, B.; Quinones, M.; Suarez, M.; Aleixandre, A.; Pons, Z.; Arola, L.; Guerrero, L. Low-molecular procyanidin rich grape seed extract exerts antihypertensive effect in males spontaneously hypertensive rats. Food Res. Int. 2013, 51, 587–595. [Google Scholar] [CrossRef]
- Huang, W.Y.; Davidge, S.T.; Wu, J.P. Bioactive Natural Constituents from Food Sources-Potential Use in Hypertension Prevention and Treatment. Crit. Rev. Food Sci. 2013, 53, 615–630. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, G.; Su, X.; Yang, H.; Zhang, J. Antiobesity action of a daidzein derivative on male obese mice induced by a high-fat diet. Nutr. Res. 2009, 29, 656–663. [Google Scholar] [CrossRef]
- Ting, Y.; Chang, W.; Shiau, D.K.; Chou, P.; Wu, M.; Hsu, C.L. Antiobesity Efficacy of Quercetin-Rich Supplement on Diet-Induced Obese Rats: Effects on Body Composition, Serum Lipid Profile, and Gene Expression. J. Agric. Food Chem. 2018, 66, 70–80. [Google Scholar] [CrossRef]
- Rains, T.M.; Agarwal, S.; Maki, K.C. Antiobesity effects of green tea catechins: A mechanistic review. J. Nutr. Biochem. 2011, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, I.; Piemontese, A.; Bernini, F.; Rio, D.D. Evaluation of antiatherosclerotic effects of ellagic acid metabolites in cultured macrophages. Atherosclerosis 2014, 235, e113–e114. [Google Scholar] [CrossRef]
- Tanaka, M.; Zhao, J.; Suyama, A.; Matsui, T. Epigallocatechin Gallate Promotes the Vasorelaxation Power of the Antiatherosclerotic Dipeptide Trp-His in Contracted Rat Aorta. J. Agric. Food Chem. 2012, 60, 9048–9054. [Google Scholar] [CrossRef]
- Zheng, S.; Feng, Q.; Cheng, J.; Zheng, J. Maternal resveratrol consumption and its programming effects on metabolic health in offspring mechanisms and potential implications. Biosci. Rep. 2018, 38, BSR20171741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunes-Bayir, A.; Toprak, A.; Kiziltan, H.S.; Kocyigit, A.; Karatas, E.; Guler, E.M. Effects of natural phenolic compound carvacrol on the human gastric adenocarcinoma (AGS) cells in vitro. Anti-Cancer Drug 2017, 28, 522–530. [Google Scholar] [CrossRef]
- Domínguez, A.J.A.; Rodrigo, G.J.; González, A.G.A.; Rosa, L.A. The Antidiabetic Mechanisms of Polyphenols Related to Increased Glucagon-Like Peptide-1 (GLP1) and Insulin Signaling (Review). Molecules 2017, 22, 903. [Google Scholar] [CrossRef] [Green Version]
- Vayalil, P.K. Date Fruits (Phoenix dactylifera Linn): An Emerging Medicinal Food. Crit. Rev. Food Sci. 2012, 52, 249. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Sung, H.; Hong, C.; Suh, Y.S. Role of (-)-epigallocatechin-3-gallate in cell viability, lipogenesis, and retinol-binding protein 4 expression in adipocytes. N-S Arch. Pharmacol. 2010, 382, 303–310. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Ebrahimi, F.; Farzaei, M.H.; Baratpourmoghaddam, A.; Ahmadi, P.; Rostamiasrabadi, P.; Rasouli, A.; Amir, H.; Rahimi, R. Dietary polyphenols for atherosclerosis: A comprehensive review and future perspectives. Crit. Rev. Food Sci. 2019, 59, 114–132. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, J.; Zhang, H.; Qin, Y.; Xu, X.; Jin, Z. Novel Approach with Controlled Nucleation and Growth for Green Synthesis of Size-Controlled Cyclodextrin-Based Metal-Organic Frameworks Based on Short-Chain Starch Nanoparticles. J. Agric. Food Chem. 2018, 66, 9785–9793. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. Zein and zein -based nano-materials for food and nutrition applications: A review (Review). Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Tang, C.H. Nanostructured soy proteins: Fabrication and applications as delivery systems for bioactives (a review). Food Hydrocolloid. 2019, 91, 92–116. [Google Scholar] [CrossRef]
- Kelemen, V.; Pichler, A.; Ivić, I.; Buljeta, I.; Šimunović, J.; Kopjar, M. Brown rice proteins as delivery system of phenolic and volatile compounds of raspberry juice. Int. J. Food Sci. Technol. 2022, 57, 1866–1874. [Google Scholar] [CrossRef]
- Zang, J.C.; Chen, H.; Zhao, G.H.; Wang, F.D.; Ren, F.Z. Ferritin cage for encapsulation and delivery of bioactive nutrients: From structure, property to applications. Crit. Rev. Food Sci. 2017, 57, 3673–3683. [Google Scholar] [CrossRef] [PubMed]
- Visentini, F.F.; Perez, A.A.; Santiago, L.G. Bioactive compounds: Application of albumin nanocarriers as delivery systems. Crit. Rev. Food Sci. 2022, 63, 1–31. [Google Scholar] [CrossRef]
- Silvia, V.; Massimo, F.; Donato, C. Gliadins as versatile biomaterials for drug delivery applications. J. Control. Release 2021, 329, 385–400. [Google Scholar] [CrossRef]
- Sadiq, U.; Gill, H.; Chandrapala, J. Casein Micelles as an Emerging Delivery System for Bioactive Food Components. Foods 2021, 10, 1965. [Google Scholar] [CrossRef]
- Ha, H.K.; Rankin, S.A.; Lee, M.R.; Lee, W.J.; Luo, Y.C. Development and Characterization of Whey Protein-Based Nano-Delivery Systems: A Review. Molecules 2019, 24, 3254. [Google Scholar] [CrossRef] [Green Version]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Starch-based nanocarriers as cutting-edge natural cargos for nutraceutical delivery (Review). Trends Food Sci. Technol. 2019, 88, 397–415. [Google Scholar] [CrossRef]
- Mu, R.J.; Hong, X.; Ni, Y.S.; Li, Y.Z.; Pang, J.; Wang, Q.; Xiao, J.B.; Zheng, Y.F. Recent trends and applications of cellulose nanocrystals in food industry. Trends Food Sci. Technol. 2019, 93, 136–144. [Google Scholar] [CrossRef]
- Christian, J.W.; Suryadi, I.; Setiyo, G. A Review of Lignocellulosic-Derived Nanoparticles for Drug Delivery Applications: Lignin Nanoparticles, Xylan Nanoparticles, and Cellulose Nanocrystals. Molecules 2021, 26, 676. [Google Scholar] [CrossRef]
- Tie, S.; Tan, M. Current Advances in Multifunctional Nanocarriers Based on Marine Polysaccharides for Colon Delivery of Food Polyphenols. J. Agric. Food Chem. 2022, 70, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; Vadivelu, J.; Saravanan, S.; Al-Maleki, A.R.; Ramesh, M. A review of natural polysaccharides for drug delivery applications: Special focus on cellulose, starch and glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Lu, T.; Qin, Y.; He, Y.; Cui, N.; Du, A.; Sun, J. In Vivo Effect of Resveratrol-Loaded Solid Lipid Nanoparticles to Relieve Physical Fatigue for Sports Nutrition Supplements. Molecules 2020, 25, E5302. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Z.; Liu, H.; Hu, L. Nanoemulsion-based delivery approaches for nutraceuticals: Fabrication, application, characterization, biological fate, potential toxicity and future trends. Food Funct. 2021, 12, 1933–1953. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Azadmard-Damirchi, S.; Peighambardoust, S.H.; Valizadeh, H.; Hesari, J. Liposomes as carrier vehicles for functional compounds in food sector (Article). J. Exp. Nanosci. 2016, 11, 737–759. [Google Scholar] [CrossRef]
- Rambaran, T.F. A patent review of polyphenol nano-formulations and their commercialization. Trends Food Sci. Technol. 2022, 120, 111–122. [Google Scholar] [CrossRef]
- Ba, C.; Fu, Y.; Niu, F.; Wang, M.; Jin, B.; Li, Z.; Chen, G.; Zhang, H.; Li, X. Effects of environmental stresses on physiochemical stability of β-carotene in zein-carboxymethyl chitosan-tea polyphenols ternary delivery system. Food Chem. 2020, 311, 125878. [Google Scholar] [CrossRef]
- Lozano-Pérez, A.A.; Rivero, H.C.; Pérez, H.; María, C.; Pagán, A.; Montalbán, M.G.; Víllora, G.; Cénis, J.L. Silk fibroin nanoparticles: Efficient vehicles for the natural antioxidant quercetin. Int. J. Pharmaceut. 2017, 518, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Solghi, S.; Emam-Djomeh, Z.; Fathi, M.; Farahani, F. The encapsulation of curcumin by whey protein: Assessment of the stability and bioactivity. J. Food Process. Eng. 2020, 43, 1–10. [Google Scholar] [CrossRef]
- Marziyeh, S.; Hamed, N.; Elham, J.; Faezeh, A.; Hamidreza, K.M.; Soodabeh, D.; Hossein, D. Production of biological nanoparticles from bovine serum albumin as controlled release carrier for curcumin delivery. Int. J. Biol. Macromol. 2018, 115, 83–89. [Google Scholar] [CrossRef]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohyd. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xue, L.; Hu, Y.; Qiu, C.; Jin, Z.; Xu, X.; Wang, J. Green fabrication and characterization of debranched starch nanoparticles via ultrasonication combined with recrystallization. Ultrason. Sonochem. 2020, 66, 105074. [Google Scholar] [CrossRef] [PubMed]
- Geetha, K.A.; Alummoottil, N.J. Cassava starch-poly (vinyl alcohol) nanocomposites for the controlled delivery of curcumin in cancer prevention and treatment. Starch-Starke 2015, 67, 549–558. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Talarico, L.; Consumi, M.; Leone, G.; Tamasi, G.; Magnani, A. Solid Lipid Nanoparticles Produced via a Coacervation Method as Promising Carriers for Controlled Release of Quercetin. Molecules 2021, 26, 2694. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, D.; Li, F.; Li, D.; Huang, Q. Cinnamon essential oil Pickering emulsion stabilized by zein-pectin composite nanoparticles: Characterization, antimicrobial effect and advantages in storage application. Int. J. Biol. Macromol. 2020, 148, 1280–1289. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; McClements, D.J.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Co-delivery of curcumin and piperine in zein-carrageenan core-shell nanoparticles: Formation, structure, stability and in vitro gastrointestinal digestion. Food Hydrocoll. 2020, 99, 105334. [Google Scholar] [CrossRef]
- Pauluk, D.; Padilha, A.K.; Khalil, N.M.; Mainardes, R.M. Chitosan-coated zein nanoparticles for oral delivery of resveratrol: Formation, characterization, stability, mucoadhesive properties and antioxidant activity (Article). Food Hydrocoll. 2019, 94, 411–417. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, H.; Wei, Y.; Gao, Y.; McClements, D.J. Curcumin encapsulation in zein-rhamnolipid composite nanoparticles using a pH-driven method. Food Hydrocoll. 2019, 93, 342–350. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).