Comparison of Different Drying Methods on the Volatile Components of Ginger (Zingiber officinale Roscoe) by HS-GC-MS Coupled with Fast GC E-Nose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ginger Samples
2.2. Drying Process
2.2.1. Hot Air Drying Process (HAD)
2.2.2. Vacuum Drying Process (VD)
2.2.3. Sun Drying (SD)
2.2.4. Vacuum-Freeze Drying (VFD)
2.3. Preparation of Dried Ginger Powder
2.4. Color Measurements
2.5. Analysis of Volatile Components by HS-GC-MS
2.6. Analysis of Flavor Compounds by Fast GC E-Nose
2.6.1. Sample Incubation
2.6.2. Acquisition of Flavor Components
2.6.3. Qualitative Analysis of Flavor Components
2.7. Statistical Procedures
3. Results and Discussion
3.1. Analysis of the Appearance Characteristics and Color Changes of Dried Gingers
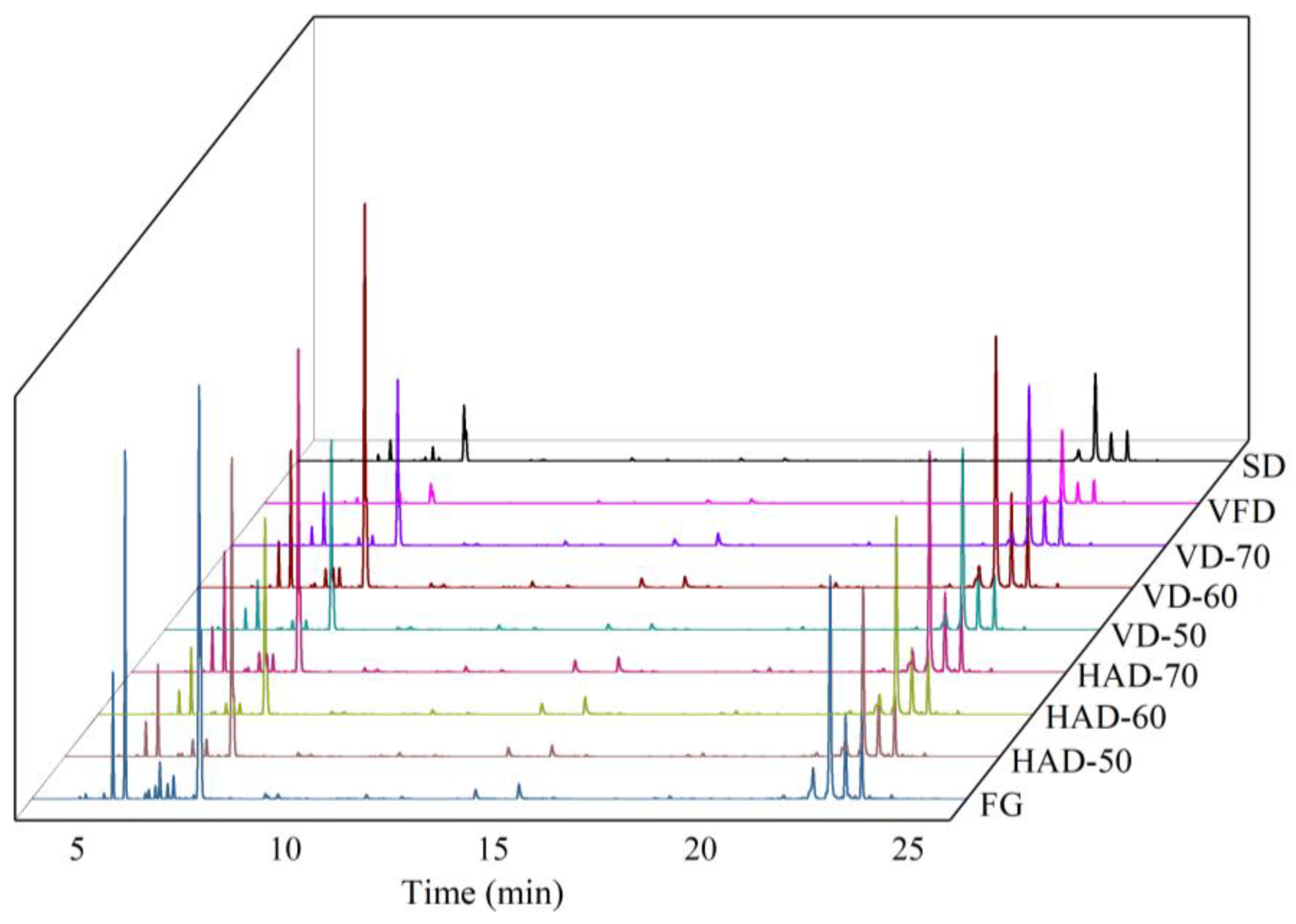
3.2. HS-GC-MS Analysis
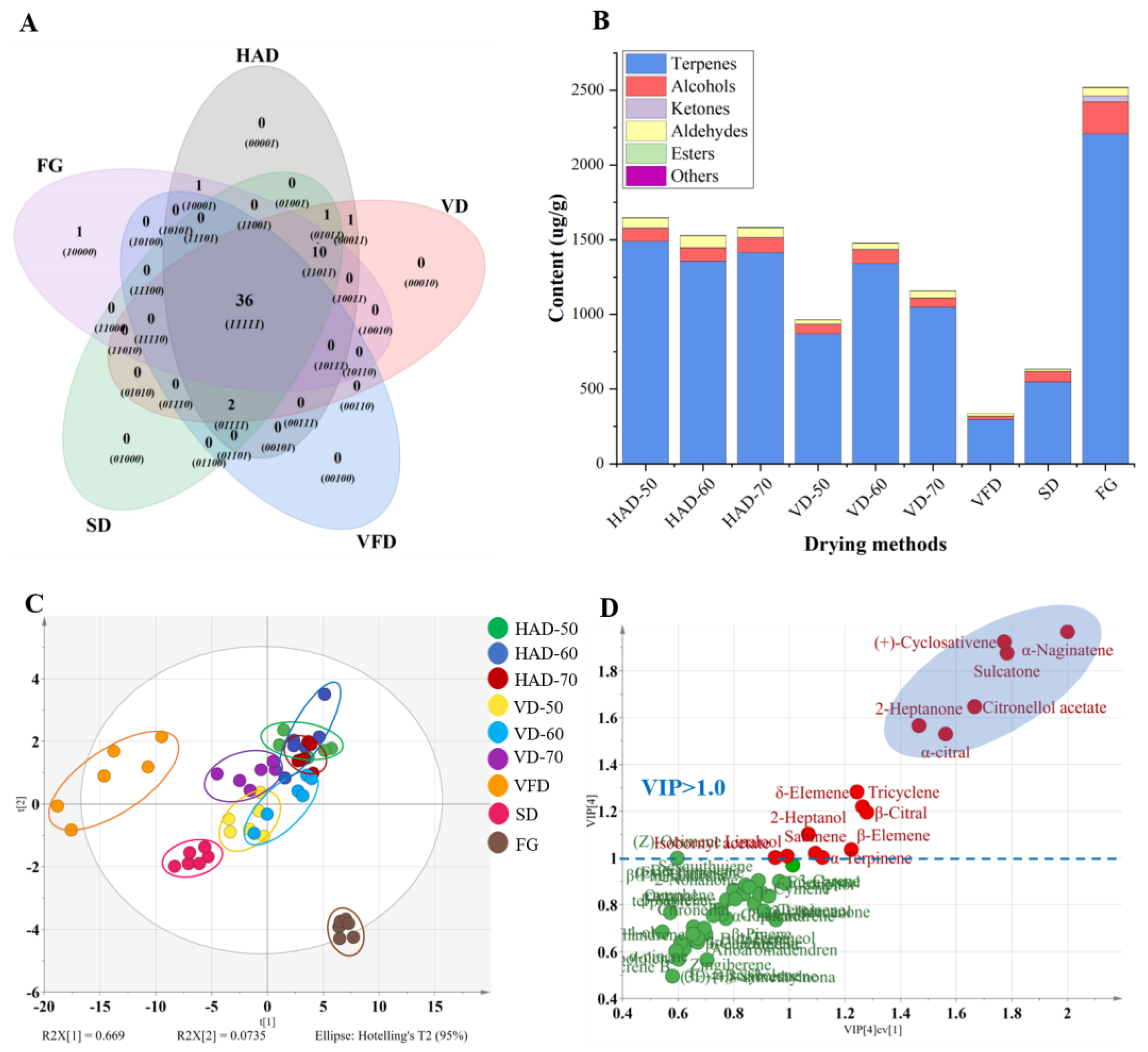
3.2.1. Identification and Analysis of Volatile Components
3.2.2. Discrimination of the Dried Gingers by PCA and PLS-DA Analysis
3.3. Fast GC E-Nose Analysis
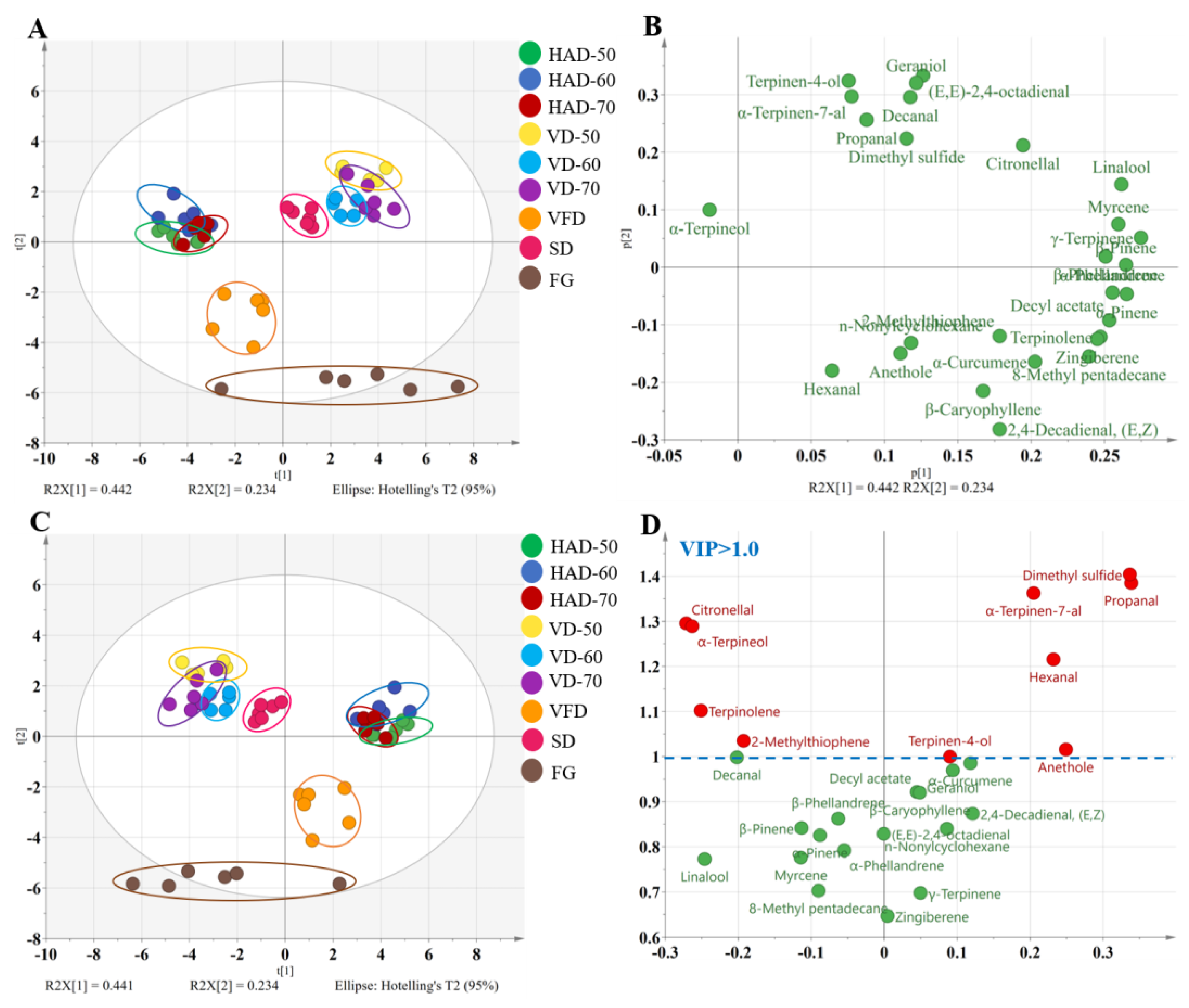
3.3.1. Analysis of Flavor Components
3.3.2. Discrimination of the Dried Gingers by PCA and PLS-DA Analysis
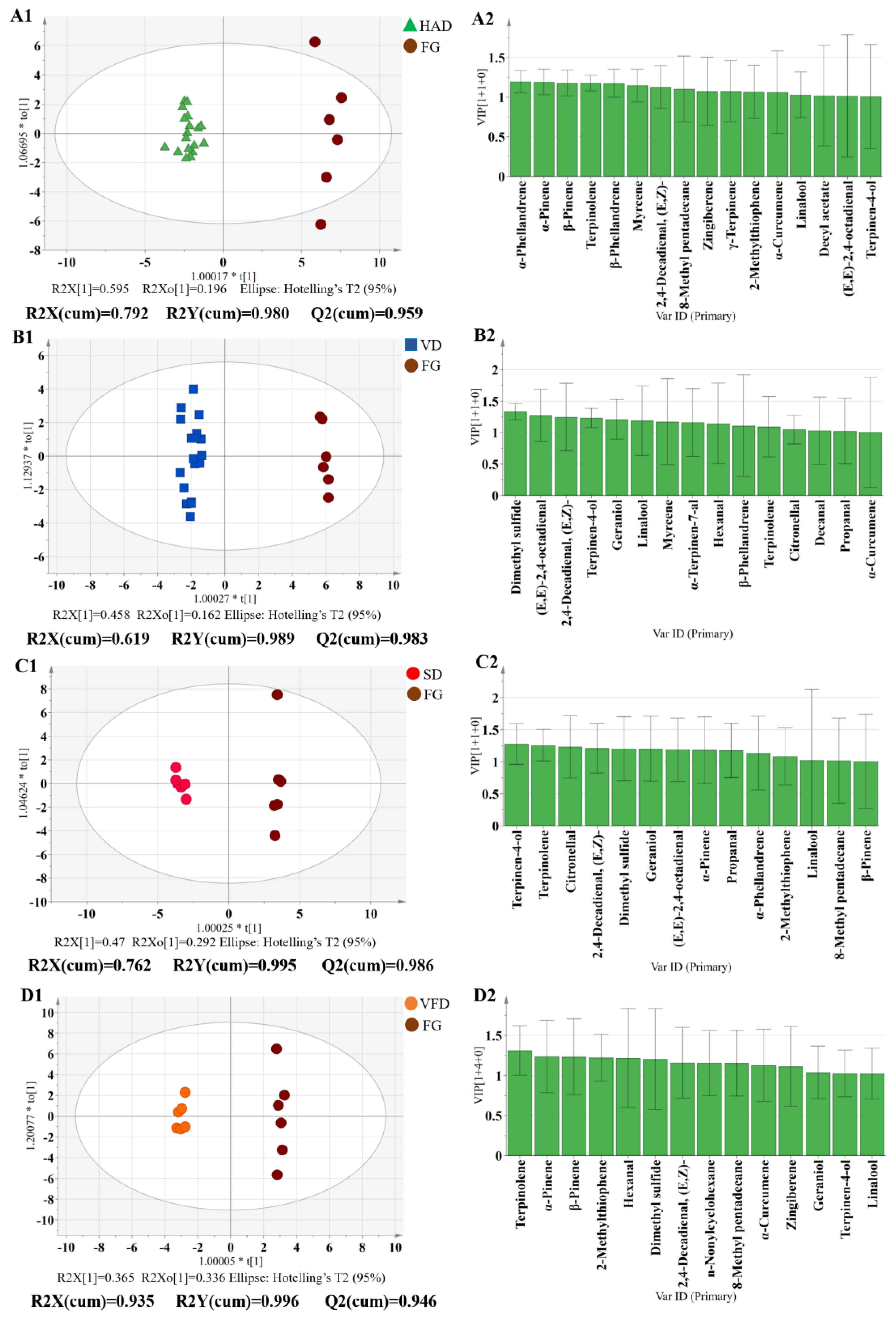
3.3.3. Flavor Comparison between FG and DG by OPLS-DA
3.4. Comparative Analysis of HS-GC-MS and Fast GC E-Nose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, T.; Su, J.; Ding, Z.H.; Zheng, Y.T.; Li, Y.; Leng, Y.; Liu, J.K. Chemical constituents and their bioactivities of “Tongling White Ginger” (Zingiber officinale). J. Agric. Food Chem. 2011, 59, 11690–11695. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. J. Nutr. Biochem. 2020, 86, 108486. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K. Ginger rhizomes (Zingiber officinale): A spice with multiple health beneficial potentials. PharmaNutrition 2017, 5, 18–28. [Google Scholar] [CrossRef]
- Samrat, N.H.; Johnson, J.B.; White, S.; Naiker, M.; Brown, P. A rapid non-destructive hyperspectral imaging data model for the prediction of pungent constituents in dried ginger. Foods 2022, 11, 649. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H. Bin bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, J.; Zhang, Y. Research progress on chemical constituents of Zingiber officinale Roscoe. Biomed Res. Int. 2019, 2019, 5370823. [Google Scholar] [CrossRef] [Green Version]
- Krüger, S.; Bergin, A.; Morlock, G.E. Effect-directed analysis of ginger (Zingiber officinale) and its food products, and quantification of bioactive compounds via high-performance thin-layer chromatography and mass spectrometry. Food Chem. 2018, 243, 258–268. [Google Scholar] [CrossRef]
- Si, W.; Chen, Y.P.; Zhang, J.; Chen, Z.Y.; Chung, H.Y. Antioxidant activities of ginger extract and its constituents toward Lipids. Food Chem. 2018, 239, 1117–1125. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Ezzat, M.I.; Okba, M.M.; Menze, E.T.; Abdel-Naim, A.B. The hidden mechanism beyond ginger (Zingiber officinale Rosc.) potent in vivo and in vitro anti-inflammatory activity. J. Ethnopharmacol. 2018, 214, 113–123. [Google Scholar] [CrossRef]
- Dalsasso, R.R.; Valencia, G.A.; Monteiro, A.R. Impact of drying and extractions processes on the recovery of gingerols and shogaols, the main bioactive compounds of ginger. Food Res. Int. 2022, 154, 111043. [Google Scholar] [CrossRef]
- Bhaskar, A.; Kumari, A.; Singh, M.; Kumar, S.; Kumar, S.; Dabla, A.; Chaturvedi, S.; Yadav, V.; Chattopadhyay, D.; Prakash Dwivedi, V. [6]-Gingerol exhibits potent anti-mycobacterial and immunomodulatory activity against Tuberculosis. Int. Immunopharmacol. 2020, 87, 106809. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Guo, Z.; Fang, S.; Gu, J.; Liang, X. Effect of drying methods on volatile compounds of burdock (Arctium lappa L.) root tea as revealed by gas chromatography mass spectrometry-based metabolomics. Foods 2021, 70, 868. [Google Scholar] [CrossRef] [PubMed]
- Schaller, T.; Schieberle, P. Comparison of the key aroma compounds in fresh, raw ginger (Zingiber officinale Roscoe) from China and roasted ginger by application of aroma extract dilution analysis. J. Agric. Food Chem. 2020, 68, 15292–15300. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.G.; Kim, M.; Choe, J.S.; Choi, A.J. Effects of high-pressure, hydrothermal, and enzyme-assisted treatment on the taste and flavor profile of water-soluble ginger (Zingiber officinale) extract. Foods 2022, 11, 508. [Google Scholar] [CrossRef]
- Ren, Z.; Yu, X.; Yagoub, A.E.G.A.; Fakayode, O.A.; Ma, H.; Sun, Y.; Zhou, C. Combinative effect of cutting orientation and drying techniques (hot air, vacuum, freeze and catalytic infrared drying) on the physicochemical properties of ginger (Zingiber officinale Roscoe). LWT-Food Sci. Technol. 2021, 144, 111238. [Google Scholar] [CrossRef]
- Liu, H.; Hui, T.; Fang, F.; Ma, Q.; Li, S.; Zhang, D.; Wang, Z. Characterization and discrimination of key aroma compounds in pre- and postrigor roasted mutton by GC-O-MS, GC E-Nose and aroma recombination experiments. Foods 2021, 10, 2387. [Google Scholar] [CrossRef]
- Virgiliou, C.; Zisi, C.; Kontogiannopoulos, K.N.; Nakas, A.; Iakovakis, A.; Varsamis, V.; Gika, H.G.; Assimopoulou, A.N. Headspace gas chromatography-mass spectrometry in the analysis of Lavender’s essential oil: Optimization by response surface methodology. J. Chromatogr. B 2021, 1179, 122852. [Google Scholar] [CrossRef]
- Brendel, R.; Schwolow, S.; Rohn, S.; Weller, P. Volatilomic profiling of citrus juices by dual-detection HS-GC-MS-IMS and machine learning-an alternative authentication approach. J. Agric. Food Chem. 2021, 69, 1727–1738. [Google Scholar] [CrossRef]
- Melucci, D.; Bendini, A.; Tesini, F.; Barbieri, S.; Zappi, A.; Vichi, S.; Conte, L.; Gallina Toschi, T. Rapid direct analysis to discriminate geographic origin of extra virgin olive oils by flash gas chromatography electronic nose and chemometrics. Food Chem. 2016, 204, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Fei, C.; Mao, C.; Ji, D.; Gong, J.; Qin, Y.; Qu, L.; Zhang, W.; Bian, Z.; Su, L.; et al. Physicochemical parameters combined flash GC E-Nose and artificial neural network for quality and volatile characterization of vinegar with different brewing techniques. Food Chem. 2022, 374, 131658. [Google Scholar] [CrossRef]
- Song, J.; Chen, Q.; Bi, J.; Meng, X.; Wu, X.; Qiao, Y.; Lyu, Y. GC/MS coupled with MOS e-nose and flash GC e-nose for volatile characterization of Chinese jujubes as affected by different drying methods. Food Chem. 2020, 331, 127201. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, J.; Jiang, Y.; Qian, M.C.; Deng, Y.; Xie, J.; Li, J.; Wang, J.; Dong, C.; Yuan, H. Aroma dynamic characteristics during the drying process of green tea by gas phase electronic nose and gas chromatography-ion mobility spectrometry. LWT 2022, 154, 112691. [Google Scholar] [CrossRef]
- Rocchi, R.; Mascini, M.; Faberi, A.; Sergi, M.; Compagnone, D.; Di Martino, V.; Carradori, S.; Pittia, P. Comparison of IRMS, GC-MS and E-Nose data for the discrimination of saffron samples with different origin, process and age. Food Control 2019, 106, 106736. [Google Scholar] [CrossRef]
- He, X.; Yangming, H.; Górska-Horczyczak, E.; Wierzbicka, A.; Jeleń, H.H. Rapid analysis of Baijiu volatile compounds fingerprint for their aroma and regional origin authenticity assessment. Food Chem. 2021, 337, 128002. [Google Scholar] [CrossRef]
- Singha, P.; Muthukumarappan, K. Quality changes and freezing time prediction during freezing and thawing of ginger. Food Sci. Nutr. 2016, 4, 521–533. [Google Scholar] [CrossRef]
- Ding, S.H.; An, K.J.; Zhao, C.P.; Li, Y.; Guo, Y.H.; Wang, Z.F. Effect of drying methods on volatiles of Chinese ginger (Zingiber officinale Roscoe). Food Bioprod. Process. 2012, 90, 515–524. [Google Scholar] [CrossRef]
- Etxabide, A.; Kilmartin, P.A.; Maté, J.I. Color stability and PH-indicator ability of curcumin, anthocyanin and betanin containing colorants under different storage conditions for intelligent packaging development. Food Control 2021, 121, 107645. [Google Scholar] [CrossRef]
- Biancolillo, A.; Aloia, R.; Rossi, L.; D’Archivio, A.A. Organosulfur volatile profiles in italian red garlic (Allium sativum L.) varieties investigated by HS-SPME/GC-MS and chemometrics. Food Control 2022, 131, 108477. [Google Scholar] [CrossRef]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016, 197, 1292–1300. [Google Scholar] [CrossRef]
- Qin, H.W.; Yang, T.M.; Yang, S.B.; Yang, M.Q.; Wang, Y.Z.; Zhang, J.Y. Effects of different pre-drying and drying methods on volatile compounds in the pericarp and kernel of amomum Tsao-Ko. Front. Plant Sci. 2022, 13, 803776. [Google Scholar] [CrossRef]
- Pang, X.; Cao, J.; Wang, D.; Qiu, J.; Kong, F. Identification of ginger (Zingiber officinale Roscoe) volatiles and localization of aroma-active constituents by GC-olfactometry. J. Agric. Food Chem. 2017, 65, 4140–4145. [Google Scholar] [CrossRef] [PubMed]
- Maoz, I.; Kaplunov, T.; Raban, E.; Dynkin, I.; Degani, O.; Lewinsohn, E.; Lichter, A. Insights into the chemosensory basis of flavor in table grapes. J. Sci. Food Agric. 2020, 100, 1405–1417. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Drying Methods | FG | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HAD-50 | HAD-60 | HAD-70 | VD-50 | VD-60 | VD-70 | VFD | SD | ||
| L* | 79.50 bc | 79.02 c | 76.96 e | 78.11 d | 79.20 c | 77.46 de | 86.60 a | 80.04 b | 77.01 e |
| a* | 0.39 e | 1.05 c | 1.99 a | 1.01 cd | 0.60 e | 1.50 b | −1.88 g | 0.67 e | −1.19 f |
| b* | 32.18 de | 32.44 de | 33.18 bcd | 34.59 a | 34.94 a | 34.36 ab | 33.40 bc | 26.18 f | 31.42 e |
| Chroma | 32.18 ef | 32.46 def | 33.24 cde | 34.60 ab | 34.94 a | 34.40 abc | 33.46 bcd | 26.19 g | 31.45 f |
| Hue angle | 89.32 c | 88.15 ef | 86.58 g | 88.33 de | 89.01 cd | 87.50 f | 93.22 a | 88.54 de | 92.24 b |
| ∆E | 3.14 e | 3.27 e | 3.82 d | 4.20 d | 4.73 c | 4.19 d | 9.87 a | 6.15 b | ------ |
| NO. | RT (min) | Compounds | Type | Formula | CAS | R.Match | RI-m | RI-r |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.66 | 2-Heptanone | ketones | C7H14O | 110-43-0 | 749 | 890.3 | 891 |
| 2 | 4.80 | 2-Heptanol | alcohols | C7H16O | 543-49-7 | 859 | 899.6 | 901 |
| 3 | 5.24 | Tricyclene | monoterpenes | C10H16 | 508-32-7 | 826 | 922.1 | 925 |
| 4 | 5.32 | α-Thujene | monoterpenes | C10H16 | 2867-05-2 | 743 | 926.1 | 929 |
| 5 | 5.45 | α-Pinene | monoterpenes | C10H16 | 80-56-8 | 883 | 933.0 | 937 |
| 6 | 5.74 | Camphene | monoterpenes | C10H16 | 79-92-5 | 877 | 947.8 | 952 |
| 7 | 6.24 | Sabinene | monoterpenes | C10H16 | 3387-41-5 | 872 | 973.0 | 974 |
| 8 | 6.32 | β-Pinene | monoterpenes | C10H16 | 127-91-3 | 846 | 977.3 | 979 |
| 9 | 6.48 | Sulcatone | ketones | C8H14O | 110-93-0 | 763 | 985.5 | 986 |
| 10 | 6.58 | β-Myrcene | monoterpenes | C10H16 | 123-35-3 | 874 | 990.6 | 991 |
| 11 | 6.83 | Octanal | aldehydes | C8H16O | 124-13-0 | 890 | 1002.6 | 1003 |
| 12 | 6.91 | α-Phellandrene | monoterpenes | C10H16 | 99-83-2 | 885 | 1005.5 | 1005 |
| 13 | 7.05 | 3-Carene | monoterpenes | C10H16 | 13466-78-9 | 878 | 1010.9 | 1011 |
| 14 | 7.20 | α-Terpinene | monoterpenes | C10H16 | 99-86-5 | 808 | 1016.4 | 1017 |
| 15 | 7.40 | p-Cymene | monoterpenes | C10H14 | 99-87-6 | 824 | 1023.8 | 1025 |
| 16 | 7.52 | β-Phellandrene | monoterpenes | C10H16 | 555-10-2 | 864 | 1028.4 | 1031 |
| 17 | 7.56 | Eucalyptol | alcohols | C10H18O | 470-82-6 | 873 | 1030.1 | 1032 |
| 18 | 7.79 | (Z)-Ocimene | monoterpenes | C10H16 | 3338-55-4 | 662 | 1038.5 | 1038 |
| 19 | 8.28 | γ-Terpinene | monoterpenes | C10H16 | 99-85-4 | 824 | 1057.0 | 1060 |
| 20 | 9.12 | Terpinolene | monoterpenes | C10H16 | 586-62-9 | 882 | 1088.7 | 1088 |
| 21 | 9.19 | 2-Nonanone | ketones | C9H18O | 821-55-6 | 813 | 1091.3 | 1092 |
| 22 | 9.34 | α-Naginatene | alkenes | C10H14O | 15186-51-3 | 703 | 1097.1 | 1093 |
| 23 | 9.42 | Linalool | alcohols | C10H18O | 78-70-6 | 788 | 1100.1 | 1099 |
| 24 | 9.94 | (3E)-4,8-Dimethylnona-1,3,7-triene | alkenes | C11H18 | 19945-61-0 | 755 | 1115.9 | 1116 |
| 25 | 10.12 | (+)-Sylvestrene | monoterpenes | C10H16 | 1461-27-4 | 790 | 1121.1 | 1027 |
| 26 | 10.85 | (-)-Camphor | monoterpenes | C10H16O | 464-48-2 | 840 | 1143.4 | 1142 |
| 27 | 10.87 | Camphor | monoterpenes | C10H16O | 76-22-2 | 799 | 1143.9 | 1145 |
| 28 | 11.11 | Citronellal | aldehydes | C10H18O | 106-23-0 | 884 | 1151.1 | 1153 |
| 29 | 11.55 | (-)-Borneol | monoterpenes | C10H18O | 464-45-9 | 869 | 1164.6 | 1166 |
| 30 | 11.95 | Terpinen-4-ol | monoterpenes | C10H18O | 562-74-3 | 697 | 1176.7 | 1177 |
| 31 | 12.41 | α-Terpineol | monoterpenes | C10H18O | 98-55-5 | 876 | 1190.7 | 1189 |
| 32 | 14.18 | β-Citral | aldehydes | C10H16O | 106-26-3 | 819 | 1241.1 | 1240 |
| 33 | 15.23 | α-Citral | aldehydes | C10H16O | 141-27-5 | 885 | 1270.6 | 1270 |
| 34 | 15.77 | Isobornyl acetate | esters | C12H20O2 | 125-12-2 | 793 | 1285.9 | 1286 |
| 35 | 16.05 | 2-Undecanone | ketones | C11H22O | 112-12-9 | 818 | 1293.8 | 1294 |
| 36 | 17.56 | δ-EIemene | sesquiterpenes | C15H24 | 20307-84-0 | 795 | 1338.1 | 1338 |
| 37 | 18.11 | Citronellol acetate | esters | C12H22O2 | 150-84-5 | 741 | 1354.2 | 1354 |
| 38 | 18.56 | (+)-Cyclosativene | sesquiterpenes | C15H24 | 22469-52-9 | 805 | 1367.7 | 1368 |
| 39 | 18.87 | Copaene | sesquiterpenes | C15H24 | 3856-25-5 | 867 | 1376.6 | 1376 |
| 40 | 19.40 | β-Elemene | sesquiterpenes | C15H24 | 515-13-9 | 744 | 1392.4 | 1391 |
| 41 | 19.84 | Sesquithujene | sesquiterpenes | C15H24 | 58319-06-5 | 879 | 1405.7 | 1402 |
| 42 | 20.80 | α-Bergamotene | sesquiterpenes | C15H24 | 17699-05-7 | 811 | 1435.8 | 1435 |
| 43 | 21.47 | (E)-β-Famesene | sesquiterpenes | C15H24 | 18794-84-8 | 810 | 1456.9 | 1457 |
| 44 | 21.59 | Alloaromadendren | sesquiterpenes | C15H24 | 25246-27-9 | 834 | 1460.6 | 1461 |
| 45 | 22.06 | β-Chamigrene | sesquiterpenes | C15H24 | 18431-82-8 | 814 | 1475.4 | 1476 |
| 46 | 22.30 | α-Curcumene | sesquiterpenes | C15H22 | 644-30-4 | 864 | 1483.0 | 1483 |
| 47 | 22.71 | Zingiberene | sesquiterpenes | C15H24 | 495-60-3 | 903 | 1495.8 | 1495 |
| 48 | 22.98 | α-Bulnesene | sesquiterpenes | C15H24 | 3691-11-0 | 769 | 1506.6 | 1505 |
| 49 | 23.08 | β-Bisabololene | sesquiterpenes | C15H24 | 495-61-4 | 816 | 1511.2 | 1509 |
| 50 | 23.47 | β-Sesquiphellandrene | sesquiterpenes | C15H24 | 20307-83-9 | 908 | 1529.9 | 1524 |
| 51 | 23.67 | (E)-γ-Bisabolene | sesquiterpenes | C15H24 | 53585-13-0 | 876 | 1539.2 | 1533 |
| 52 | 24.19 | Germacrene B | sesquiterpenes | C15H24 | 15423-57-1 | 898 | 1564.3 | 1557 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.-X.; Guo, S.; Wang, J.-M.; Yan, H.; Zhang, Z.-Y.; Yang, J.; Duan, J.-A. Comparison of Different Drying Methods on the Volatile Components of Ginger (Zingiber officinale Roscoe) by HS-GC-MS Coupled with Fast GC E-Nose. Foods 2022, 11, 1611. https://doi.org/10.3390/foods11111611
Yu D-X, Guo S, Wang J-M, Yan H, Zhang Z-Y, Yang J, Duan J-A. Comparison of Different Drying Methods on the Volatile Components of Ginger (Zingiber officinale Roscoe) by HS-GC-MS Coupled with Fast GC E-Nose. Foods. 2022; 11(11):1611. https://doi.org/10.3390/foods11111611
Chicago/Turabian StyleYu, Dai-Xin, Sheng Guo, Jie-Mei Wang, Hui Yan, Zhen-Yu Zhang, Jian Yang, and Jin-Ao Duan. 2022. "Comparison of Different Drying Methods on the Volatile Components of Ginger (Zingiber officinale Roscoe) by HS-GC-MS Coupled with Fast GC E-Nose" Foods 11, no. 11: 1611. https://doi.org/10.3390/foods11111611
APA StyleYu, D.-X., Guo, S., Wang, J.-M., Yan, H., Zhang, Z.-Y., Yang, J., & Duan, J.-A. (2022). Comparison of Different Drying Methods on the Volatile Components of Ginger (Zingiber officinale Roscoe) by HS-GC-MS Coupled with Fast GC E-Nose. Foods, 11(11), 1611. https://doi.org/10.3390/foods11111611





