Assessing the Gel Quality and Storage Properties of Hypophythalmalmichthys molitrix Surimi Gel Prepared with Epigallocatechin Gallate Subject to Multiple Freeze-Thaw Cycles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Heat-Induced Gel and F-T Cycles
2.3. Texture Profile Analysis (TPA)
2.4. Gel Strength Measurement
2.5. Color Measurement
2.6. Storage Properties Measurement
2.6.1. Aerobic Plate Count (APC) Measurement
2.6.2. Total Volatile Basic Nitrogen (TVB-N) Measurement
2.6.3. pH Value Measurement
2.6.4. TBA Measurement
2.7. Water-Holding Capacity (WHC) Measurement
2.8. Low-Field Nuclear Magnetic Resonance (LF-NMR) Spin-Spin Relaxation (T2) Measurement
2.9. Magnetic Resonance Imaging (MRI) Measurement
2.10. Microstructure Measurement
2.11. Statistical Analysis
3. Results and Discussion
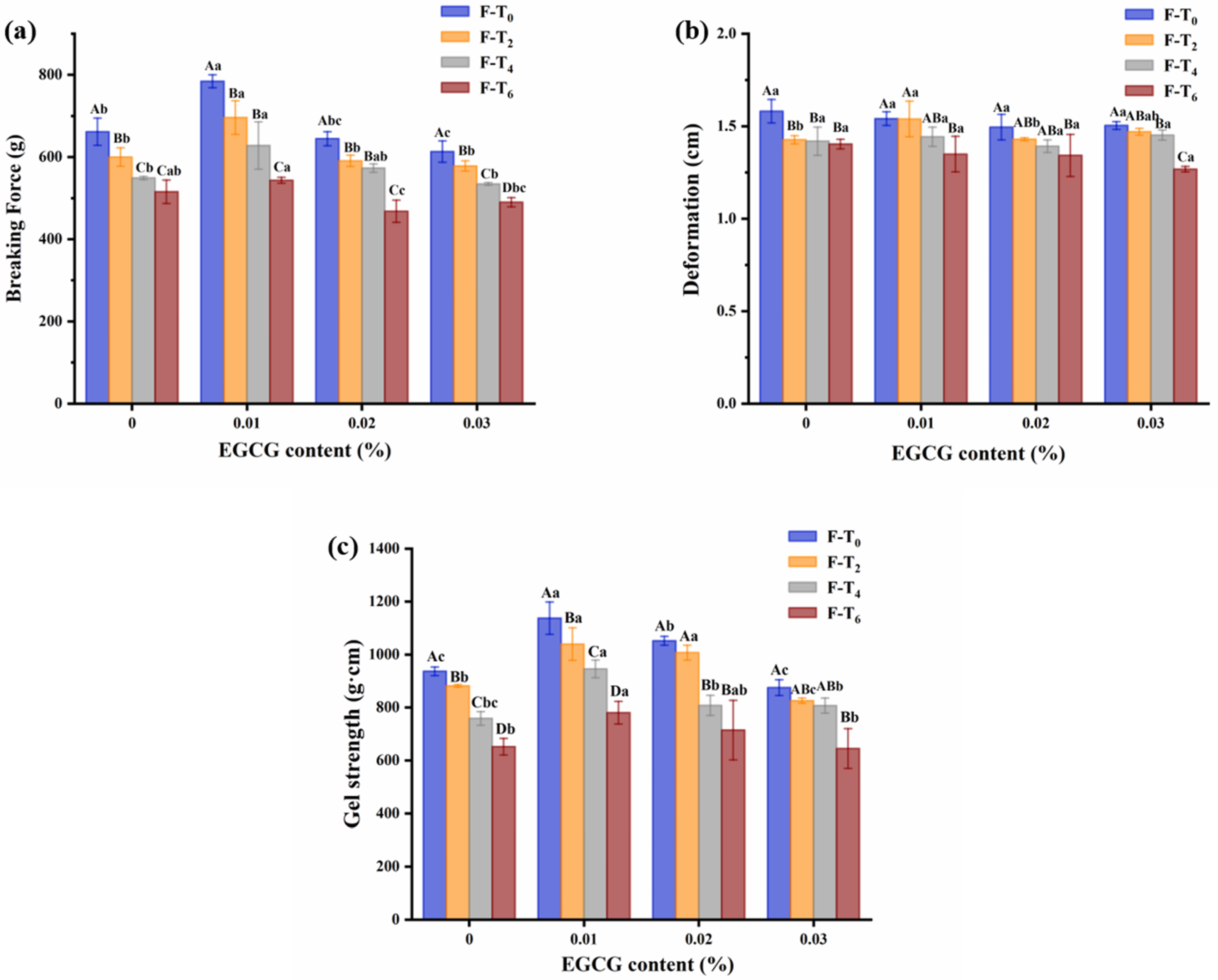
3.1. TPA
3.2. Gel Strength
3.3. Color
3.4. Storage Properties
3.4.1. Aerobic Plate Count (APC)
3.4.2. Total Volatile Basic Nitrogen (TVB-N)
3.4.3. pH Value
3.4.4. TBA
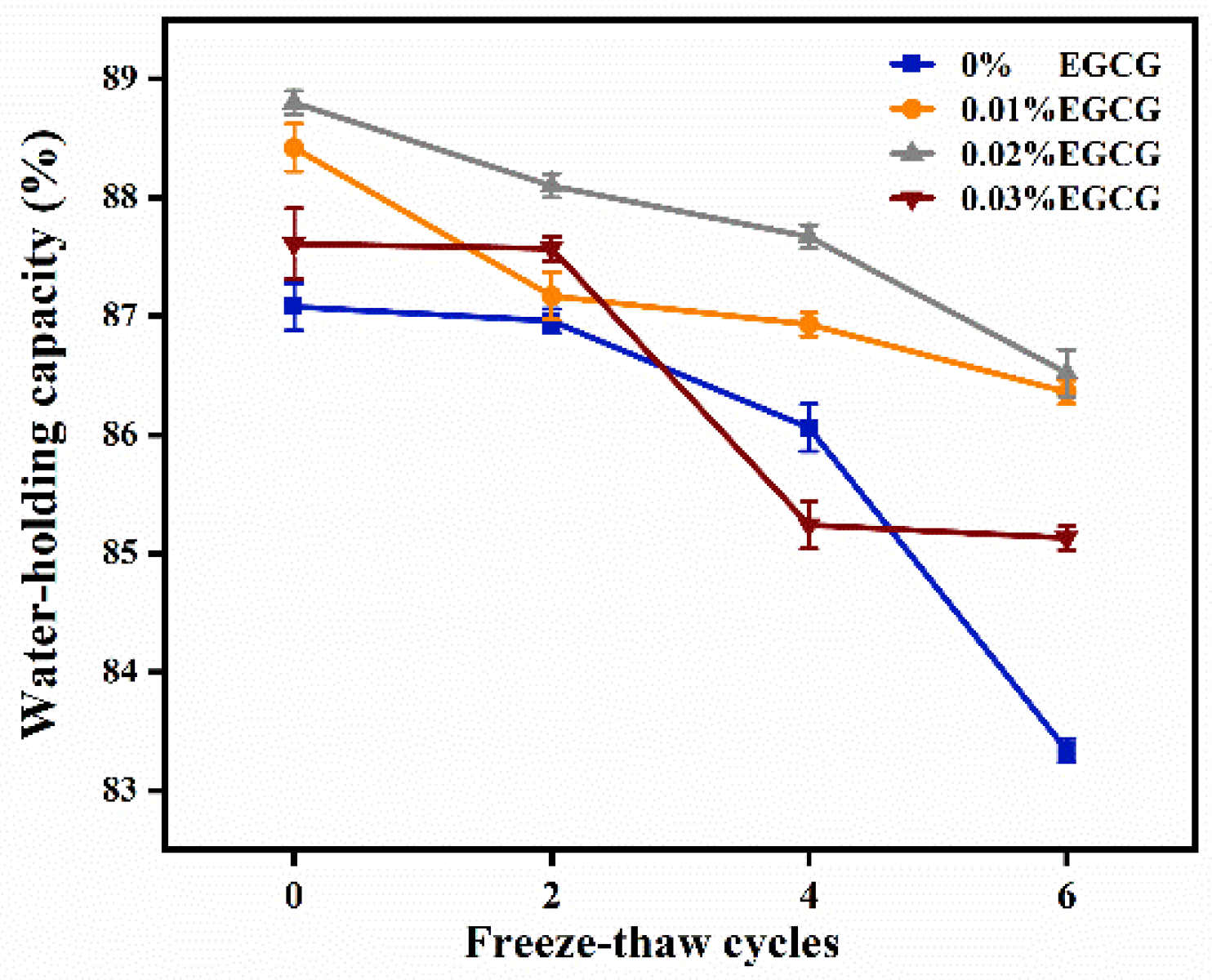
3.5. Water-Holding Capacity (WHC)
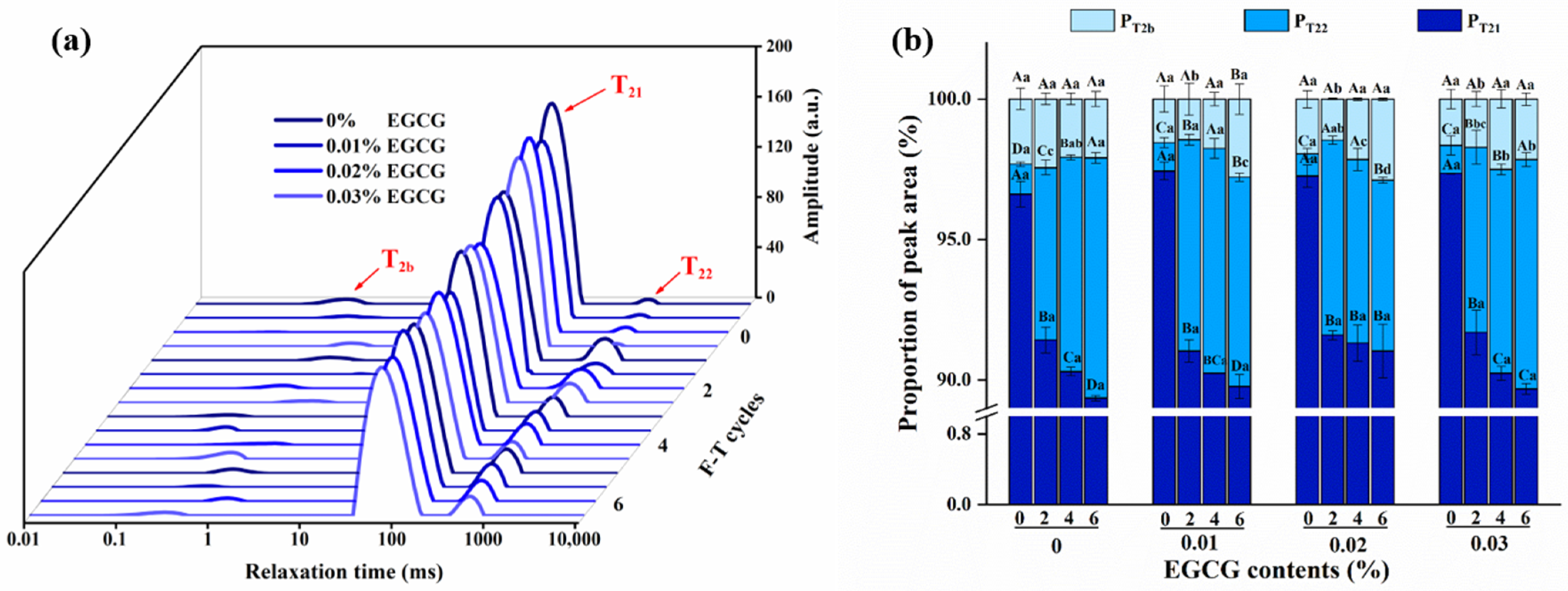
3.6. Moisture Mobility and Distribution Analysis
3.6.1. Moisture Mobility
3.6.2. Moisture Distribution
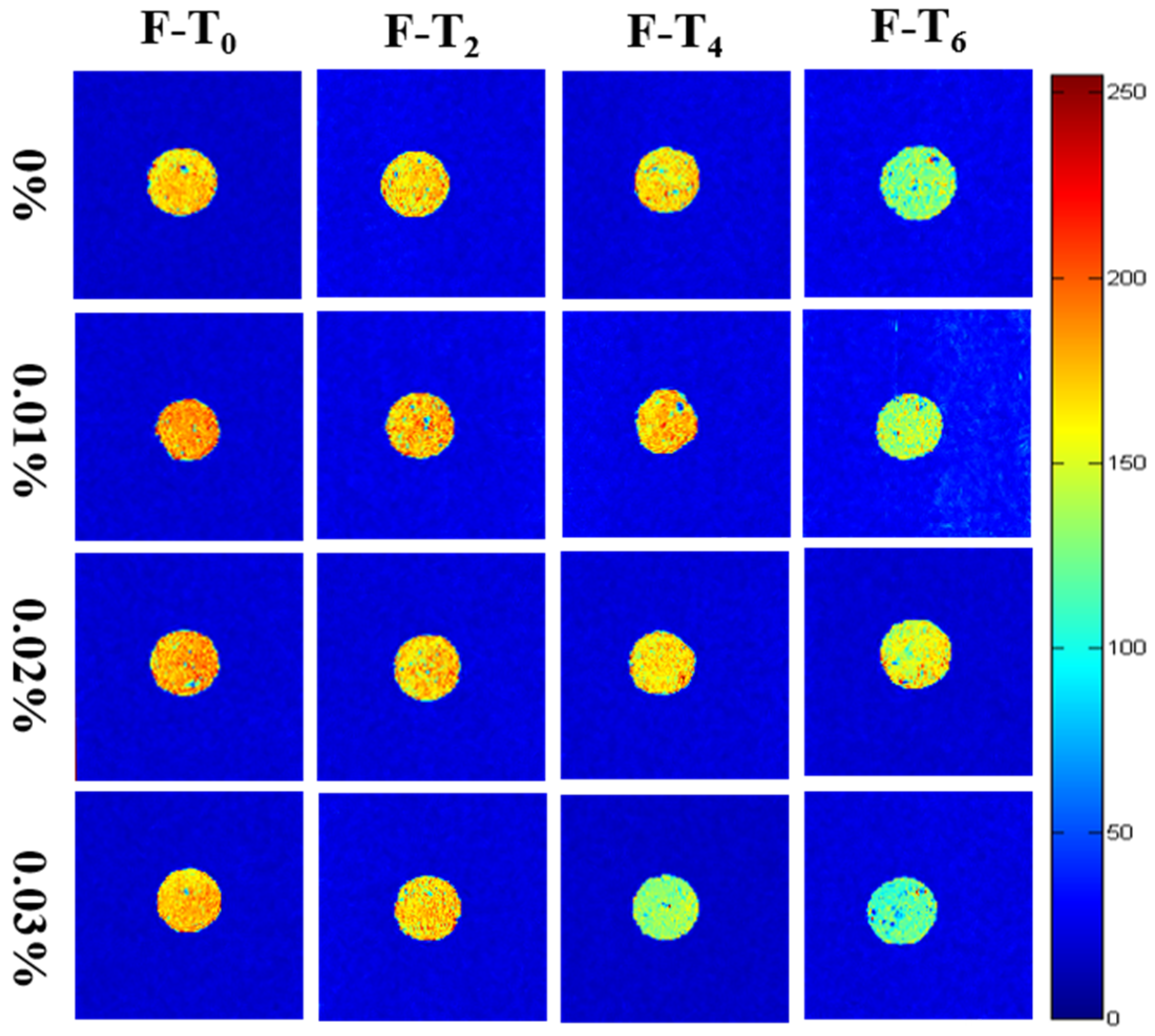
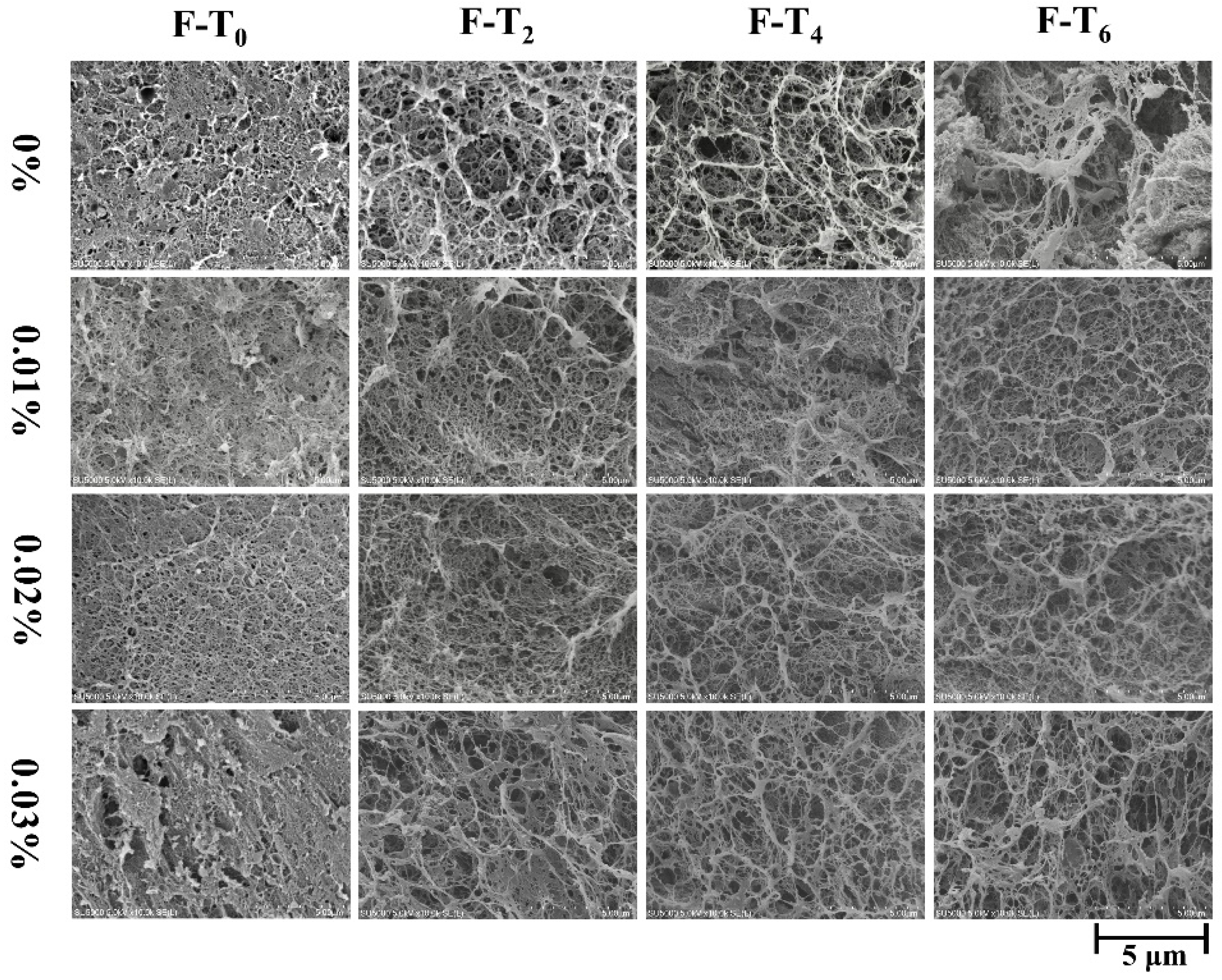
3.7. Microstructure
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Du, X.; Kong, B.; Liu, Q.; Li, F.; Pan, N.; Xia, X.; Zhang, D. Effect of ultrasound thawing, vacuum thawing, and microwave thawing on gelling properties of protein from porcine longissimus dorsi. Ultrason. Sonochem. 2020, 64, 104860. [Google Scholar] [CrossRef] [PubMed]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kingwascharapong, P.; Benjakul, S. Effect of phosphate and bicarbonate replacers on quality changes of raw and cooked Pacific white shrimp as influenced by the repeated freeze–thawing. Int. J. Refrig. 2016, 67, 345–354. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2019, 11, 39. [Google Scholar] [CrossRef] [Green Version]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R.; Thabrew, I.; de Silva, E.D. Protective Effects of Six Selected Dietary Compounds against Leptin-Induced Proliferation of Oestrogen Receptor Positive (MCF-7) Breast Cancer Cells. Medicines 2017, 4, 56. [Google Scholar] [CrossRef]
- Li, J.; Munir, S.; Yu, X.; Yin, T.; You, J.; Liu, R.; Xiong, S.; Hu, Y. Double-crosslinked effect of TGase and EGCG on myofibrillar proteins gel based on physicochemical properties and molecular docking. Food Chem. 2021, 345, 128655. [Google Scholar] [CrossRef]
- Lv, Y.; Feng, X.; Wang, Y.; Guan, Q.; Qian, S.; Xu, X.; Zhou, G.; Ullah, N.; Chen, L. The gelation properties of myofibrillar proteins prepared with malondialdehyde and (-)-epigallocatechin-3-gallate. Food Chem. 2021, 340, 10. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Zhou, P.; Zhang, B.; Deng, S. Effect of squid pen chitooligosaccharide and epigallocatechin gallate on discoloration and shelf-life of yellowfin tuna slices during refrigerated storage. Food Chem. 2021, 351, 129296. [Google Scholar] [CrossRef]
- Pei, J.; Mei, J.; Yu, H.; Qiu, W.; Xie, J. Effect of Gum Tragacanth-Sodium Alginate Active Coatings Incorporated With Epigallocatechin Gallate and Lysozyme on the Quality of Large Yellow Croaker at Superchilling Condition. Front. Nutr. 2022, 8. [Google Scholar] [CrossRef]
- Xu, Y.; Li, L.; Xia, W.; Zang, J.; Gao, P. The role of microbes in free fatty acids release and oxidation in fermented fish paste. LWT 2019, 101, 323–330. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Shi, W. Effect of different drying time on physicochemical properties of black carp (Mylopharyngodon piceus) by hot air. J. Food Process. Preserv. 2021, 46, e16217. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, Q.; Xiao, N.; Du, Y.; Feng, Q.; Shi, W. Changes in Gel Structure and Chemical Interactions of Hypophthalmichthys molitrix Surimi Gels: Effect of Setting Process and Different Starch Addition. Foods 2021, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.F.; Zhang, M.; Adhikari, B.; Islam, M.N. Effect of Power Ultrasound and Pulsed Vacuum Treatments on the Dehydration Kinetics, Distribution, and Status of Water in Osmotically Dehydrated Strawberry: A Combined NMR and DSC Study. Food Bioprocess Technol. 2014, 7, 2782–2792. [Google Scholar] [CrossRef]
- Alakhrash, F.; Anyanwu, U.; Tahergorabi, R. Physicochemical properties of Alaska pollock (Theragra chalcograma) surimi gels with oat bran. LWT Food Sci. Technol. 2016, 66, 41–47. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Chen, S.; Xu, C.; Yu, Y.; Wang, X.; Shi, W. Determination of the effects of different high-temperature treatments on texture and aroma characteristics in Alaska pollock surimi. Food Sci. Nutr. 2018, 6, 2079–2091. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.H.; Hamid, N.; Liu, T.; Sarojini, V. Effect of Antifreeze Peptide Pretreatment on Ice Crystal Size, Drip Loss, Texture, and Volatile Compounds of Frozen Carrots. J Agric Food Chem. 2016, 64, 4327–4335. [Google Scholar] [CrossRef]
- Sun, X.D.; Holley, R.A. Factors Influencing Gel Formation by Myofibrillar Proteins in Muscle Foods. Compr. Rev. Food Sci. Food Saf. 2011, 10, 33–51. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kumazawa, Y.; Toiguchi, S.; Seguro, K.; Soeda, T.; Motoki, M. Gel strength enhancement by addition of microbial transglutaminase during onshore surimi manufacture. J. Food Sci. 1995, 60, 300–304. [Google Scholar] [CrossRef]
- Balange, A.; Benjakul, S. Enhancement of gel strength of bigeye snapper (Priacanthus tayenus) surimi using oxidised phenolic compounds. Food Chem. 2009, 113, 61–70. [Google Scholar] [CrossRef]
- Sochaya, C.; Soottawat, B. Effect of formaldehyde on protein cross-linking and gel forming ability of surimi from lizardfish induced by microbial transglutaminase. Food Hydrocoll. 2013, 30, 704–711. [Google Scholar] [CrossRef]
- Medic, H.; Djurkin Kusec, I.; Pleadin, J.; Kozacinski, L.; Njari, B.; Hengl, B.; Kusec, G. The impact of frozen storage duration on physical, chemical and microbiological properties of pork. Meat Sci. 2018, 140, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Thanonkaew, A.; Benjakul, S.; Visessanguan, W.; Decker, E.A. The effect of metal ions on lipid oxidation, colour and physicochemical properties of cuttlefish (Sepia pharaonis) subjected to multiple freeze–thaw cycles. Food Chem. 2006, 95, 591–599. [Google Scholar] [CrossRef]
- Aguirrezabal, M.M.; Mateo, J.; Dominguez, M.C.; Zumalacarregui, J.M. The effect of paprika, garlic and salt on rancidity in dry sausages. Meat Sci. 2000, 54, 77–81. [Google Scholar] [CrossRef]
- Klancnik, A.; Mozina, S.S.; Zhang, Q. Anti-Campylobacter activities and resistance mechanisms of natural phenolic compounds in Campylobacter. PLoS ONE 2012, 7, e51800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef] [Green Version]
- Klancnik, A.; Piskernik, S.; Bucar, F.; Vuckovic, D.; Mozina, S.S.; Jersek, B. Reduction of microbiological risk in minced meat by a combination of natural antimicrobials. J. Sci. Food Agric. 2014, 94, 2758–2765. [Google Scholar] [CrossRef]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef]
- Goulas, A.E.; Kontominas, M.G. Combined effect of light salting, modified atmosphere packaging and oregano essential oil on the shelf-life of sea bream (Sparus aurata): Biochemical and sensory attributes. Food Chem. 2007, 100, 287–296. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H. Effects of calcium ion on gel properties and gelation of tilapia (Oreochromis niloticus) protein isolates processed with pH shift method. Food Chem. 2019, 277, 327–335. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Thongkaew, C.; Tanaka, M. Comparative study on physicochemical changes of muscle proteins from some tropical fish during frozen storage. Food Res. Int. 2003, 36, 787–795. [Google Scholar] [CrossRef]
- Suvanich, V.; Jahncke, M.L.; Marshall, D.L. Changes in selected chemical quality characteristics of channel catfish frame mince during chill and frozen storage. J. Food Sci. 2000, 65, 24–29. [Google Scholar] [CrossRef]
- Ozpolat, E.; Patir, B. Determination of Shelf Life for Sausages Produced From Some Freshwater Fish Using Two Different Smoking Methods. J. Food Saf. 2016, 36, 69–76. [Google Scholar] [CrossRef]
- Li, F.; Zhong, Q.; Kong, B.; Wang, B.; Pan, N.; Xia, X. Deterioration in quality of quick-frozen pork patties induced by changes in protein structure and lipid and protein oxidation during frozen storage. Food Res. Int. 2020, 133, 109142. [Google Scholar] [CrossRef] [PubMed]
- Nikoo, M.; Regenstein, J.M.; Ahmadi Gavlighi, H. Antioxidant and Antimicrobial Activities of (-)-Epigallocatechin-3-gallate (EGCG) and its Potential to Preserve the Quality and Safety of Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 732–753. [Google Scholar] [CrossRef] [Green Version]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, X. Assessing the gelling properties of the silver carp surimi gel prepared with large yellow croaker processing by-product in freeze-thaw cycles. J. Food Process. Preserv. 2021, 45, e15479. [Google Scholar] [CrossRef]
- Jiang, Q.; Nakazawa, N.; Hu, Y.; Osako, K.; Okazaki, E. Changes in quality properties and tissue histology of lightly salted tuna meat subjected to multiple freeze-thaw cycles. Food Chem. 2019, 293, 178–186. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Regenstein, J.M.; Zhou, P.; Yang, Y.L. Effects of high intensity ultrasound modification on physicochemical property and water in myofibrillar protein gel. Ultrason. Sonochem. 2017, 34, 960–967. [Google Scholar] [CrossRef]
- Provesi, J.G.; Valentim Neto, P.A.; Arisi, A.C.M.; Amante, E.R. Extraction of antifreeze proteins from cold acclimated leaves of Drimys angustifolia and their application to star fruit (Averrhoa carambola) freezing. Food Chem. 2019, 289, 65–73. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Diao, X.; Kong, B.; Xia, X. Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci. 2017, 133, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, F.; Pan, N.; Kong, B.; Xia, X. Effect of ice structuring protein on the quality of quick-frozen patties subjected to multiple freeze-thaw cycles. Meat Sci. 2021, 172, 108335. [Google Scholar] [CrossRef] [PubMed]
- Warschawski, D.E.; Arnold, A.A.; Marcotte, I. A New Method of Assessing Lipid Mixtures by P-31 Magic-Angle Spinning NMR. Biophys. J. 2018, 114, 1368–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunez-Flores, R.; Cando, D.; Javier Borderias, A.; Moreno, H.M. Importance of salt and temperature in myosin polymerization during surimi gelation. Food Chem. 2018, 239, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hua, Y.; Qiu, A. Soybean protein aggregation induced by lipoxygenase catalyzed linoleic acid oxidation. Food Res. Int. 2006, 39, 240–249. [Google Scholar] [CrossRef]


| Color | EGCG Content (%) | F-T Cycles | |||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | ||
| L* | 0 | 72.80 ± 0.40 Aa | 71.30 ± 0.13 Ba | 70.90 ± 0.07 Ba | 70.2 ± 0.29 Ca |
| 2 | 71.00 ± 0.59 Ab | 70.10 ± 0.11 Bb | 69.40 ± 0.47 BCb | 69.0 ± 0.12 Cb | |
| 4 | 70.80 ± 0.11 Ab | 69.20 ± 0.36 Bc | 68.90 ± 0.14 Bc | 68.1 ± 0.25 Cbc | |
| 6 | 70.40 ± 0.38 Ab | 69.20 ± 0.40 Bc | 68.60 ± 0.47 BCc | 68.0 ± 0.43 Cc | |
| a* | 0 | −1.60 ± 0.08 Ac | −1.70 ± 0.03 Ac | −1.60 ± 0.01 Ad | −1.70 ± 0.10 Ac |
| 2 | −0.50 ± 0.05 Ab | −0.70 ± 0.04 Bb | −0.80 ± 0.05 Bc | −0.60 ± 0.10 ABb | |
| 4 | −0.30 ± 0.04 Aa | −0.60 ± 0.02 Bab | −0.20 ± 0.06 Ab | −0.30 ± 0.08 Aa | |
| 6 | −0.30 ± 0.08 Ba | −0.50 ± 0.09 Ca | 0.10 ± 0.03 Aa | −0.20 ± 0.04 Ba | |
| b* | 0 | 4.60 ± 0.09 Aa | 3.50 ± 0.16 Ca | 3.60 ± 0.21 Ca | 4.00 ± 0.26 Ba |
| 2 | 2.40 ± 0.01 Ab | 2.30 ± 0.13 ABb | 2.30 ± 0.46 ABb | 2.20 ± 0.25 Bb | |
| 4 | 2.50 ± 0.08 Ab | 2.10 ± 0.03 Bc | 2.00 ± 0.31 BCbc | 1.70 ± 0.18 Cc | |
| 6 | 2.50 ± 0.09 Ab | 1.80 ± 0.06 Bd | 1.80 ± 0.08 Bc | 1.70 ± 0.13 Bc | |
| W | 0 | 72.40 ± 0.40 Aa | 71.00 ± 0.04 Ba | 70.70 ± 0.09 Ba | 69.9 ± 0.30 Ca |
| 2 | 70.90 ± 0.60 Ab | 70.00 ± 0.11 Bb | 69.30 ± 0.50 BCb | 69.0 ± 0.11 Cab | |
| 4 | 70.30 ± 0.40 Ab | 69.10 ± 0.36 Bc | 68.90 ± 0.10 Bc | 68.0 ± 0.23 Cbc | |
| 6 | 70.70 ± 0.10 Ab | 69.20 ± 0.40 Bc | 68.50 ± 0.50 BCc | 68.0 ± 0.43 Cc | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Jiang, X.; Xiao, N.; Zhang, Q.; Shi, W.; Guo, Q. Assessing the Gel Quality and Storage Properties of Hypophythalmalmichthys molitrix Surimi Gel Prepared with Epigallocatechin Gallate Subject to Multiple Freeze-Thaw Cycles. Foods 2022, 11, 1612. https://doi.org/10.3390/foods11111612
Tian Z, Jiang X, Xiao N, Zhang Q, Shi W, Guo Q. Assessing the Gel Quality and Storage Properties of Hypophythalmalmichthys molitrix Surimi Gel Prepared with Epigallocatechin Gallate Subject to Multiple Freeze-Thaw Cycles. Foods. 2022; 11(11):1612. https://doi.org/10.3390/foods11111612
Chicago/Turabian StyleTian, Zhihang, Xin Jiang, Naiyong Xiao, Qiang Zhang, Wenzheng Shi, and Quanyou Guo. 2022. "Assessing the Gel Quality and Storage Properties of Hypophythalmalmichthys molitrix Surimi Gel Prepared with Epigallocatechin Gallate Subject to Multiple Freeze-Thaw Cycles" Foods 11, no. 11: 1612. https://doi.org/10.3390/foods11111612
APA StyleTian, Z., Jiang, X., Xiao, N., Zhang, Q., Shi, W., & Guo, Q. (2022). Assessing the Gel Quality and Storage Properties of Hypophythalmalmichthys molitrix Surimi Gel Prepared with Epigallocatechin Gallate Subject to Multiple Freeze-Thaw Cycles. Foods, 11(11), 1612. https://doi.org/10.3390/foods11111612





