Effects of the Mixture of Xylooligosaccharides and Egg White Protein on the Physicochemical Properties, Conformation, and Gel-Forming Ability of Culter alburnus Myofibrillar Protein during Multiple Freeze–Thaw Cycles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Mixture (XO/EW) of Xylooligosaccharides and Egg White Protein
2.2.2. Preparation of Myofibrillar Proteins
2.2.3. Preparation of Heat-Induced Gel
2.2.4. Protein Solubility
2.2.5. Carbonyl Content
2.2.6. Sulfhydryl Content
2.2.7. Dityrosine Content
2.2.8. Ca2+-ATPase Activity
2.2.9. Surface Hydrophobicity (S0)
2.2.10. Intrinsic Fluorescence Intensity
2.2.11. Secondary Structure
2.2.12. Gel Strength
2.2.13. Water-Holding Capacity
2.2.14. T2 Relaxation Time Analysis
2.2.15. Intermolecular Interaction Force
2.2.16. Statistical Analysis
3. Results and Discussion
3.1. Protein Solubility
3.2. Carbonyl Content
3.3. Sulfhydryl Content
3.4. Dityrosine Content
3.5. Ca2+-ATPase Activity
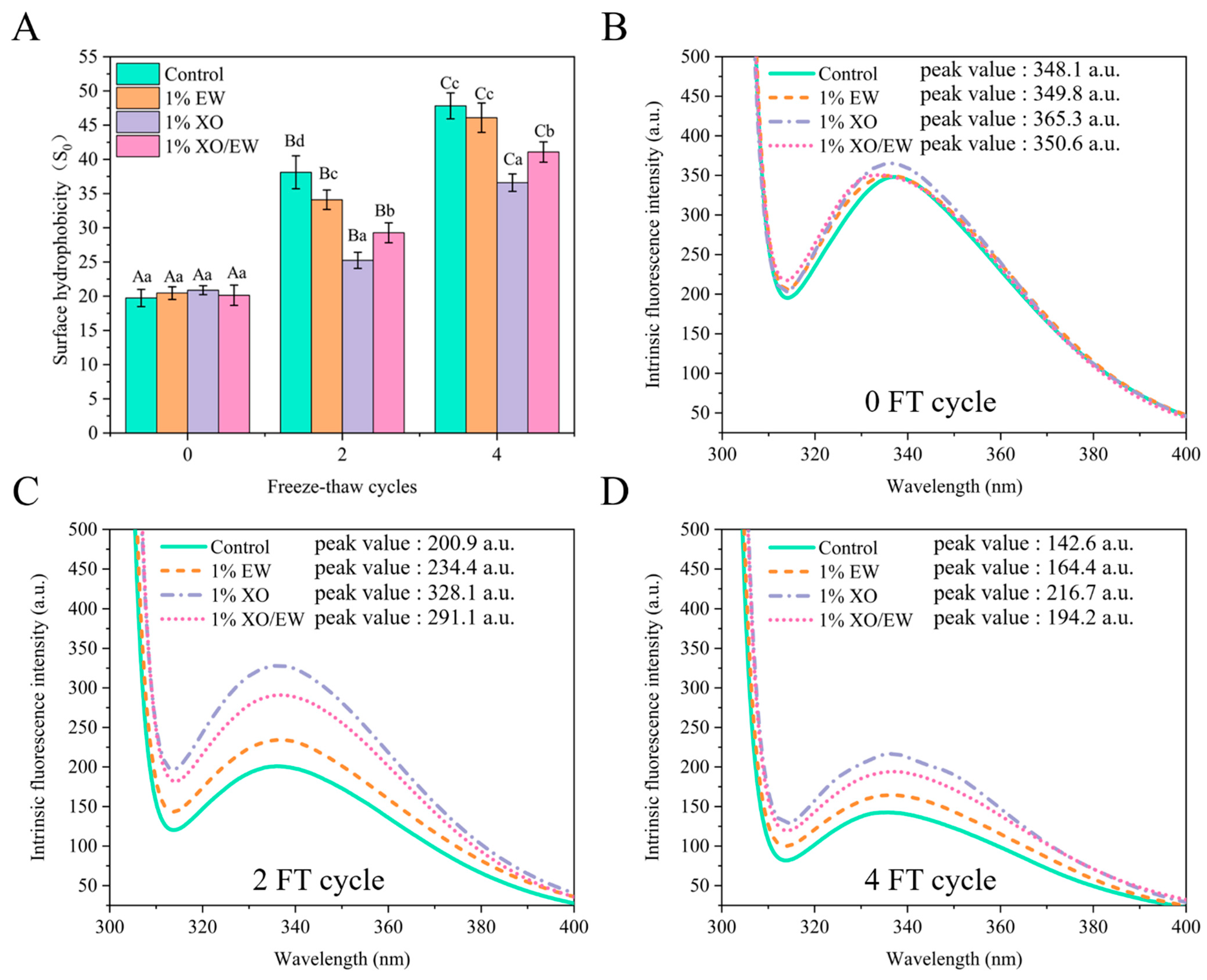
3.6. Surface Hydrophobicity (S0)
3.7. Intrinsic Fluorescence Intensity
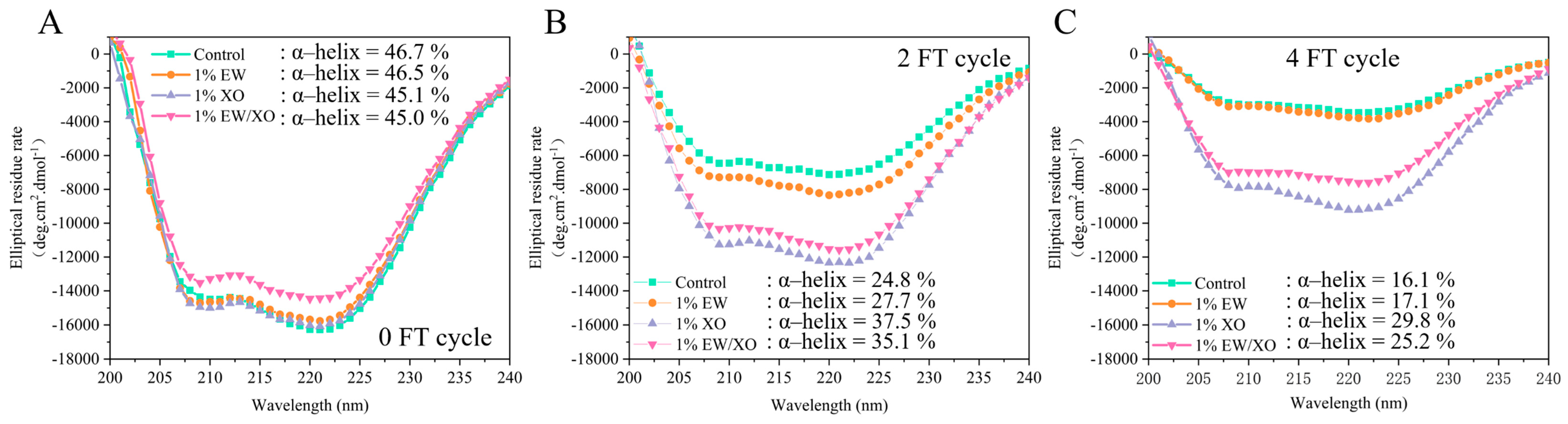
3.8. Circular Dichroism
3.9. Gel Strength
3.10. Water-Holding Capacity
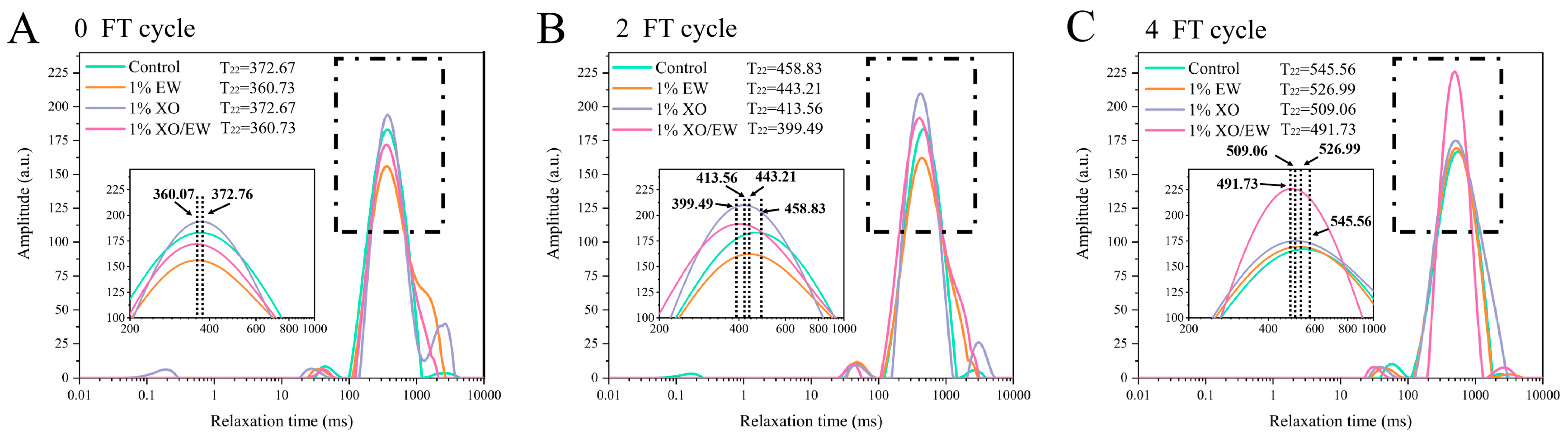
3.11. T2 Relaxation Time Analysis
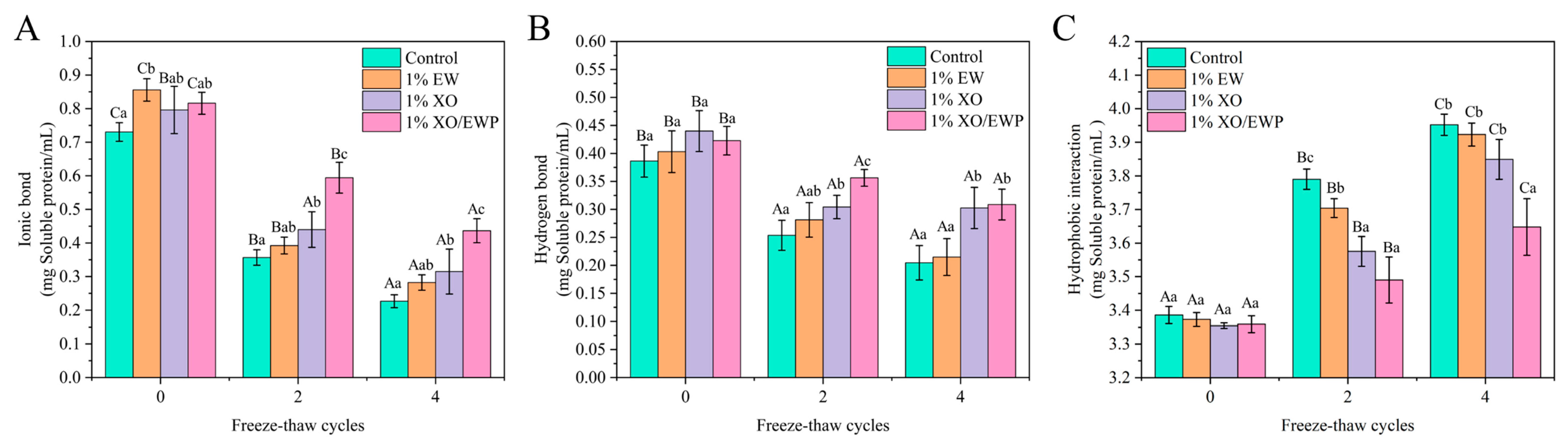
3.12. Intermolecular Interaction Forces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walayat, N.; Xiong, Z.; Xiong, H.; Moreno, H.M.; Niaz, N.; Ahmad, M.N.; Hassan, A.; Nawaz, A.; Ahmad, I.; Wang, P.K. Cryo-protective effect of egg white proteins and xylooligosaccharides mixture on oxidative and structural changes in myofibrillar proteins of Culter alburnus during frozen storage. Int. J. Biol. Macromol. 2020, 158, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Zhao, Y.; Hu, X.; Zhang, X.; Xie, J. Effects of carrageenan oligosaccharide on lipid, protein oxidative changes, and moisture migration of Litopenaeus vannamei during freeze-thaw cycles. J. Food Process. Preserv. 2020, 49, e14675. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, Z.; Lu, S.; Walayat, N.; Hu, C.; Xiong, H. Effects of oxidative modification on the functional, conformational and gelling prop-erties of myofibrillar proteins from Culter alburnus. Int. J. Biol. Macromol. 2020, 162, 1442–1452. [Google Scholar] [CrossRef]
- Sun, Q.; Kong, B.; Liu, S.; Zheng, O.; Zhang, C. Ultrasonic Freezing Reduces Protein Oxidation and Myofibrillar Gel Quality Loss of Common Carp (Cyprinus carpio) during Long-Time Frozen Storage. Foods 2021, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Fang, C.D.; Hao, G.J.; Zhang, Y.Y. Effect of kappa-carrageenan oligosaccharides on myofibrillar protein oxidation in peeled shrimp (Litopenaeus vannamei) during long-term frozen storage. Food Chem. 2018, 245, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Walayat, N.; Xiong, Z.; Xiong, H.; Moreno, H.M.; Nawaz, A.; Niaz, N.; Hu, C.; Taj, M.I.; Mushtaq, B.S.; Khalifa, I. The effect of egg white protein and beta-cyclodextrin mixture on structural and functional properties of silver carp myofibrillar proteins during frozen storage. LWT Food Sci. Technol. 2021, 135, 109975. [Google Scholar] [CrossRef]
- Jia, R.; Katano, T.; Yoshimoto, Y.; Gao, Y.; Watanabe, Y.; Nakazawa, N.; Osako, K.; Okazaki, E. Sweet potato starch with low pasting temperature to improve the gelling quality of surimi gels after freezing. Food Hydrocoll. 2018, 81, 467–473. [Google Scholar] [CrossRef]
- Walayat, N.; Xiong, H.; Xiong, Z.; Moreno, H.M.; Nawaz, A.; Niaz, N.; Randhawa, M.A. Role of Cryoprotectants in Surimi and Factors Affecting Surimi Gel Properties: A Review. Food Rev. Int. 2020, 1–20. Available online: https://www.tandfonline.com/doi/abs/10.1080/87559129.2020.1768403 (accessed on 15 May 2021). [CrossRef]
- Hunt, A.; Park, J.W.; Handa, A. Effect of Various Types of Egg White on Characteristics and Gelation of Fish Myofibrillar Pro-teins. J. Food Sci. 2009, 74, C683–C692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, T.; Lin, H.; Chen, H.; Liu, J.; Lyu, F.; Ding, Y. Physicochemical properties and microstructure of surimi treated with egg white modified by tea polyphenols. Food Hydrocoll. 2019, 90, 82–89. [Google Scholar] [CrossRef]
- Nicorescu, I.; Vial, C.; Talansier, E.; Lechevalier, V.; Loisel, C.; Della Valle, D.; Riaublanc, A.; Djelveh, G.; Legrand, J. Comparative effect of thermal treatment on the physicochemical properties of whey and egg white protein foams. Food Hydrocoll. 2011, 25, 797–808. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, K.; Ye, T.; Wan, S.; Wang, Y.; Wang, D.; Li, B.; Wang, C. Influence of konjac glucomannan on gelling properties and water state in egg white protein gel. Food Res. Int. 2013, 51, 437–443. [Google Scholar] [CrossRef]
- Wu, Y.-B.; Lin, K.-W. Influences of xylooligosaccharides on the quality of Chinese-style meatball (kung-wan). Meat Sci. 2011, 88, 575–579. [Google Scholar] [CrossRef]
- Okazaki, M.; Fujikawa, S.; Matsumoto, N. Effect of Xylooligosaccharide on the Growth of Bifidobacteria. Bifid-Microflora 1990, 9, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.B.; Lin, K.W. Influences of xylooligosaccharides and saccharides on the properties of meat batter during frozen storage. J. Food Process. Preserv. 2014, 38, 1439–1446. [Google Scholar] [CrossRef]
- Zhang, B.; Hao, G.J.; Cao, H.J.; Tang, H.; Zhang, Y.Y.; Deng, S.G. The cryoprotectant effect of xylooligosaccharides on denatur-ation of peeled shrimp (Litopenaeus vannamei) protein during frozen storage. Food Hydrocoll. 2018, 77, 228–237. [Google Scholar] [CrossRef]
- Walayat, N.; Xiong, Z.; Xiong, H.; Moreno, H.M.; Li, Q.; Nawaz, A.; Zhang, Z.; Wang, P.; Niaz, N. The effectiveness of egg white protein and beta-cyclodextrin during frozen storage: Functional, rheological and structural changes in the myofibrillar pro-teins of Culter alburnus. Food Hydrocoll. 2020, 105, 105842. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, L.; Wu, H.; Xu, X.; Zhou, G.; Zhu, B.; Feng, X. (-)-Epigallocatechin-3-gallate-mediated formation of myofibrillar protein emulsion gels under malondialdehyde-induced oxidative stress. Food Chem. 2019, 285, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Rl, L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar]
- Nikoo, M.; Benjakul, S.; Xu, X. Antioxidant and cryoprotective effects of Amur sturgeon skin gelatin hydrolysate in unwashed fish mince. Food Chem. 2015, 181, 295–303. [Google Scholar] [CrossRef]
- Chanarat, S.; Benjakul, S. Impact of microbial transglutaminase on gelling properties of Indian mackerel fish protein isolates. Food Chem. 2013, 136, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Fiske, C.H.; Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Hu, Y.M.; Zhang, N.H.; Wang, H.; Yang, Y.F.; Tu, Z.C. Effects of pre-freezing methods and storage temperatures on the quali-ties of crucian carp (Carassius auratus var. pengze) during frozen storage. J. Food Process. Preserv. 2021, 45, e15139. [Google Scholar] [CrossRef]
- Perez-Mateos, M.; Lourenço, H.; Montero, P.; Borderias, A.J. Rheological and Biochemical Characteristics of High-Pressure- and Heat-Induced Gels from Blue Whiting (Micromesistius poutassou) Muscle Proteins. J. Agric. Food Chem. 1997, 45, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Hartree, E.F. Determination of Protein-Modification of lowry method that gives a linear photometric response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef]
- Shui, S.S.; Qi, H.; Shaimaa, H.; Aubourg, S.P.; Zhang, B. Kappa-carrageenan and its oligosaccharides maintain the physico-chemical properties of myofibrillar proteins in shrimp mud (Xia-Hua) during frozen storage. J. Food Sci. 2021, 86, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Lee, D.I.; Lee, C.H.; Ahn, I.S. A dityrosine-based substrate for a protease assay: Application for the selective assessment of papain and chymopapain activity. Anal. Chim. Acta 2012, 723, 101–107. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, M.; Sun, W.; Ren, J.; Cui, C. Effect of oxidation on the emulsifying properties of soy protein isolate. Food Res. Int. 2013, 52, 26–32. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, J.L.; Chen, S.J.; Zhang, X.L.; Wei, W.Y. Influence of trehalose and alginate oligosaccharides on ice crystal growth and recrystallization in whiteleg shrimp (Litopenaeus vannamei) during frozen storage with temperature fluctuations. Int. J. Refrig.-Rev. Int. Du Froid 2019, 99, 176–185. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Q.; Kong, B.; Han, J.; He, X. The influence of superchilling and cryoprotectants on protein oxidation and struc-tural changes in the myofibrillar proteins of common carp (Cyprinus carpio) surimi. LWT Food Sci. Technol. 2014, 57, 603–611. [Google Scholar] [CrossRef]
- Park, D.; Xiong, Y.L.; Alderton, A.L.; Ooizumi, T. Biochemical changes in myofibrillar protein isolates exposed to three oxidiz-ing systems. J. Agric. Food Chem. 2006, 54, 4445–4451. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Gan, X.; Li, H. Effect of peroxyl radicals on the structure and gel properties of isolated rabbit meat myofibril-lar proteins. Int. J. Food Sci. Technol. 2018, 53, 2687–2696. [Google Scholar] [CrossRef]
- Ma, Y.; Xiong, S.; You, J.; Hu, Y.; Huang, Q.; Yin, T. Effects of vacuum chopping on physicochemical and gelation properties of myofibrillar proteins from silver carp (Hypophthalmichthys molitrix). Food Chem. 2018, 245, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, M.; Fang, Z.; Bhandari, B. Gelation properties of myofibrillar protein under malondialdehyde-induced oxidative stress. J. Sci. Food Agric. 2017, 97, 50–57. [Google Scholar] [CrossRef]
- Lina, R.; Yanshun, X.; Qixing, J.; Wenshui, X.; Chunjiang, Q. Investigation on Structural Changes of Myofibrillar Proteins from Silver Carp (Hypophthalmichthys molitrix) during Frozen Storage. Food Sci. Technol. Res. 2013, 19, 1051–1059. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Diao, X.; Kong, B.; Xia, X. Moisture migration, microstructure damage and protein structure changes in por-cine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci. 2017, 133, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, X.L.; Shen, C.L.; Deng, S.G. Understanding the influence of carrageenan oligosaccharides and xylooligosac-charides on ice-crystal growth in peeled shrimp (Litopenaeus vannamei) during frozen storage. Food Funct. 2018, 9, 4394–4403. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Cheng, J.-H.; Sun, D.-W. Effects of atmospheric pressure plasma jet on the conformation and physicochemical properties of myofibrillar proteins from king prawn (Litopenaeus vannamei). Food Chem. 2019, 276, 147–156. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Bhandari, B.; Gao, Z. Effects of malondialdehyde-induced protein modification on water functionality and physicochemical state of fish myofibrillar protein gel. Food Res. Int. 2016, 86, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Wang, Y.; Li, J.; Li, F.; Teng, C.; Li, X. Effects of Water-Extractable Arabinoxylan on the Physicochemical Properties and Structure of Wheat Gluten by Thermal Treatment. J. Agric. Food Chem. 2017, 65, 4728–4735. [Google Scholar] [CrossRef] [PubMed]
- Boonchuay, P.; Wongpoomchai, R.; Jaturasitha, S.; Mahatheeranont, S.; Watanabe, M.; Chaiyaso, T. Prebiotic properties, antioxidant activity, and acute oral toxicity of xylooligosaccharides derived enzymatically from corncob. Food Biosci. 2021, 40, 100895. [Google Scholar] [CrossRef]
- Shen, H.; Elmore, J.S.; Zhao, M.; Sun, W. Effect of oxidation on the gel properties of porcine myofibrillar proteins and their binding abilities with selected flavour compounds. Food Chem. 2020, 329, 127032. [Google Scholar] [CrossRef]
- McDonnell, C.; Allen, P.; Duggan, E.; Arimi, J.; Casey, E.; Duane, G.; Lyng, J. The effect of salt and fibre direction on water dynamics, distribution and mobility in pork muscle: A low field NMR study. Meat Sci. 2013, 95, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ni, N.; Wang, Z.; He, F.; Wang, L.; Pan, H.; Li, X.; Wang, Q.; Zhang, D. Gel properties and molecular forces of lamb myofibrillar protein during heat induction at different pH values. Process. Biochem. 2014, 49, 631–636. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Zheng, B.; Guo, Z. Effects of high pressure processing on gelation properties and molecular forces of myosin containing deacetylated konjac glucomannan. Food Chem. 2019, 291, 117–125. [Google Scholar] [CrossRef] [PubMed]

| FT Cycles | Group | Protein Solubility (%) | Carbonyl Content (nmol/mg) | Sulfhydryl Content (nmol/mg) | Dityrosine Content (a.u.) | Ca2+-ATPase Activity (μmol/mg/min) |
|---|---|---|---|---|---|---|
| 0 | Control | 91.26 ± 3.44 Da | 1.01 ± 0.33 Aa | 21.60 ± 0.80 Da | 1.52 ± 0.24 Aa | 0.1543 ± 0.0078 Da |
| 1% EW | 90.97 ± 2.08 Da | 0.98 ± 0.19 Aa | 21.33 ± 1.01 Da | 1.50 ± 0.21 Aa | 0.1678 ± 0.0075 Ea | |
| 1% XO | 91.9 ± 2.46 Da | 0.97 ± 0.25 Aa | 20.93 ± 0.74 Da | 1.54 ± 0.24 Aa | 0.1637 ± 0.0054 Ea | |
| 1% XO/EW | 91.07 ± 3.09 Da | 1.01 ± 0.24 Aa | 21.74 ± 1.01 Da | 1.53 ± 0.26 Aa | 0.1620 ± 0.0060 Ea | |
| 1 | Control | 60.67 ± 3.35 Ca | 4.26 ± 0.56 Bc | 14.68 ± 0.54 Ca | 3.59 ± 0.32 Bb | 0.0918 ± 0.0094 Ca |
| 1% EW | 69.24 ± 3.08 Cb | 3.01 ± 0.31 Bb | 16.30 ± 0.81 Ca | 3.16 ± 0.29 Bb | 0.1076 ± 0.0063 Db | |
| 1% XO | 83.59 ± 4.40 Cc | 1.85 ± 0.62 Aba | 19.65 ± 0.71 Dd | 2.11 ± 0.27 Ba | 0.1404 ± 0.0076 Dd | |
| 1% XO/EW | 77.99 ± 3.23 Cc | 2.33 ± 0.48 Bab | 17.94 ± 0.66 Dc | 2.51 ± 0.25 Ba | 0.1257 ± 0.0072 Dc | |
| 2 | Control | 45.11 ± 3.86 Ba | 6.19 ± 1.12 Cc | 10.09 ± 0.97 Ba | 5.34 ± 0.34 Cd | 0.0712 ± 0.0071 Ba |
| 1% EW | 54.45 ± 3.52 Bb | 5.14 ± 0.89 Cbc | 11.60 ± 0.84 Ba | 4.76 ± 0.30 Cc | 0.0834 ± 0.0083 Ca | |
| 1% XO | 77.49 ± 3.27 Cd | 2.67 ± 0.63 Ba | 17.89 ± 0.88 Cc | 2.55 ± 0.31 Ba | 0.1235 ± 0.0082 Cc | |
| 1% XO/EW | 65.91 ± 3.54 Bc | 3.68 ± 0.38 Cab | 14.72 ± 0.58 Cb | 3.59 ± 0.29 Cb | 0.0996 ± 0.0077 Cb | |
| 3 | Control | 38.53 ± 4.12 Aa | 7.56 ± 0.50 Dd | 7.07 ± 0.63 Aa | 6.73 ± 0.35 Dd | 0.0527 ± 0.0069 Aa |
| 1% EW | 43.72 ± 4.65 Aab | 6.44 ± 0.33 Dc | 8.13 ± 0.44 Aa | 6.19 ± 0.28 Dc | 0.0673 ± 0.0087 Bab | |
| 1% XO | 67.73 ± 3.11 Bc | 3.63 ± 0.43 Ca | 15.14 ± 0.80 Bc | 3.30 ± 0.33 Ca | 0.0905 ± 0.0094 Bc | |
| 1% XO/EW | 52.81 ± 3.94 Ab | 4.92 ± 0.28 Db | 11.45 ± 0.63 Bb | 4.72 ± 0.02 Dd | 0.0812 ± 0.0085 Bbc | |
| 4 | Control | 37.14 ± 4.27 Aa | 8.15 ± 0.50 Dc | 6.01 ± 0.74 Aa | 7.86 ± 0.25 Ed | 0.0421 ± 0.0074 Aa |
| 1% EW | 40.43 ± 5.03 Aab | 7.60 ± 0.41 Ec | 6.69 ± 0.80 Aa | 7.34 ± 0.25 Ec | 0.0471 ± 0.0076 Aab | |
| 1% XO | 58.41 ± 5.61 Ac | 5.31 ± 0.58 Da | 9.29 ± 0.71 Ab | 5.15 ± 0.22 Da | 0.0694 ± 0.0074 Ac | |
| 1% XO/EW | 47.87 ± 3.97 Ab | 6.29 ± 0.37 Eb | 8.77 ± 0.55 Ab | 6.13 ± 0.02 Eb | 0.0607 ± 0.0073 Abc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Xiong, Z.; Walayat, N.; Lorenzo, J.M.; Nawaz, A.; Xiong, H. Effects of the Mixture of Xylooligosaccharides and Egg White Protein on the Physicochemical Properties, Conformation, and Gel-Forming Ability of Culter alburnus Myofibrillar Protein during Multiple Freeze–Thaw Cycles. Foods 2021, 10, 2007. https://doi.org/10.3390/foods10092007
Zhang Z, Xiong Z, Walayat N, Lorenzo JM, Nawaz A, Xiong H. Effects of the Mixture of Xylooligosaccharides and Egg White Protein on the Physicochemical Properties, Conformation, and Gel-Forming Ability of Culter alburnus Myofibrillar Protein during Multiple Freeze–Thaw Cycles. Foods. 2021; 10(9):2007. https://doi.org/10.3390/foods10092007
Chicago/Turabian StyleZhang, Zhongli, Zhouyi Xiong, Noman Walayat, Jose M. Lorenzo, Asad Nawaz, and Hanguo Xiong. 2021. "Effects of the Mixture of Xylooligosaccharides and Egg White Protein on the Physicochemical Properties, Conformation, and Gel-Forming Ability of Culter alburnus Myofibrillar Protein during Multiple Freeze–Thaw Cycles" Foods 10, no. 9: 2007. https://doi.org/10.3390/foods10092007
APA StyleZhang, Z., Xiong, Z., Walayat, N., Lorenzo, J. M., Nawaz, A., & Xiong, H. (2021). Effects of the Mixture of Xylooligosaccharides and Egg White Protein on the Physicochemical Properties, Conformation, and Gel-Forming Ability of Culter alburnus Myofibrillar Protein during Multiple Freeze–Thaw Cycles. Foods, 10(9), 2007. https://doi.org/10.3390/foods10092007










