Analysis of the Chemical Profiles and Anti-S. aureus Activities of Essential Oils Extracted from Different Parts of Three Oregano Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Bacterial Strains, and Standards
2.2. Extraction of OEOs
2.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of OEOs
2.4. Evaluation of Antibacterial Activity
2.4.1. Diameter of the Inhibitory Zone (DIZ) Assays
2.4.2. Minimum Inhibitory Concentration (MIC) Assays
2.5. Statistical Analysis
3. Results and Discussion
3.1. Morphological Observations
3.2. Chemical Composition of OEOs
3.3. Characterization of Common and Unique OEO Components
3.4. Multivariate Statistical Analysis of EO Chemical Profiles
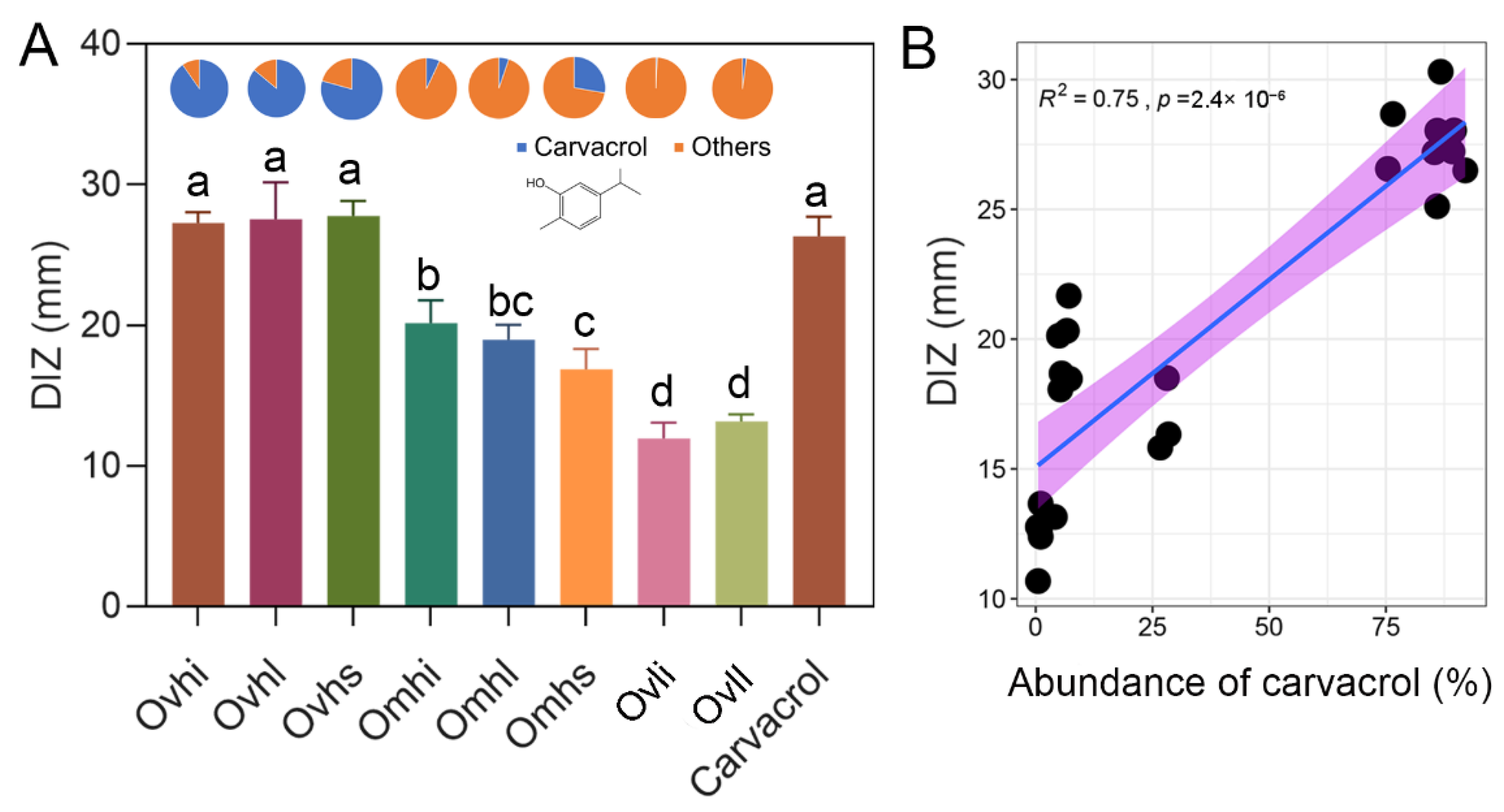
3.5. Antibacterial Activity of OEOs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vokou, D.; Kokkini, S.; Bessiere, J.-M. Geographic Variation of Greek Oregano (Origanum vulgare ssp. hirtum) Essential Oils. Biochem. Syst. Ecol. 1993, 21, 287–295. [Google Scholar] [CrossRef]
- Marrelli, M.; Statti, G.A.; Conforti, F. Origanum spp.: An Update of Their Chemical and Biological Profiles. Phytochem. Rev. 2018, 17, 873–888. [Google Scholar] [CrossRef]
- Haddad, N.; Johnson, N.; Kathariou, S.; Métris, A.; Phister, T.; Pielaat, A.; Tassou, C.; Wells-Bennik, M.H.J.; Zwietering, M.H. Next Generation Microbiological Risk Assessment-Potential of Omics Data for Hazard Characterisation. Int. J. Food Microbiol. 2018, 287, 28–39. [Google Scholar] [CrossRef]
- Kang, J.; Jin, W.; Wang, J.; Sun, Y.; Wu, X.; Liu, L. Antibacterial and Anti-Biofilm Activities of Peppermint Essential Oil against Staphylococcus aureus. LWT 2019, 101, 639–645. [Google Scholar] [CrossRef]
- Begić, M.; Josić, D. Food Borne Bacterial Pathogens and Food Safety—An Outlook. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–13. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial Mechanism of Oregano Essential Oil. Ind. Crops Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Davidson, P.M.; Critzer, F.J.; Taylor, T.M. Naturally Occurring Antimicrobials for Minimally Processed Foods. Annu. Rev. Food Sci. Technol. 2013, 4, 163–190. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of Plant Volatiles in Defence against Microbial Pathogens and Microbial Exploitation of Volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef] [Green Version]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential Oils: A Promising Eco-Friendly Food Preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Dadalioglu, I.; Evrendilek, G.A. Chemical Compositions and Antibacterial Effects of Essential Oils of Turkish Oregano (Origanum Minutiflorum), Bay Laurel (Laurus nobilis), Spanish Lavender (Lavandula stoechas L.), and Fennel (Foeniculum vulgare) on Common Foodborne Pathogens. J. Agric. Food Chem. 2004, 52, 8255–8260. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- Marques, J.d.L.; Volcão, L.M.; Funck, G.D.; Kroning, I.S.; da Silva, W.P.; Fiorentini, Â.M.; Ribeiro, G.A. Antimicrobial Activity of Essential Oils of Origanum vulgare L. and Origanum majorana L. against Staphylococcus aureus Isolated from Poultry Meat. Ind. Crops Prod. 2015, 77, 444–450. [Google Scholar] [CrossRef]
- Hao, Y.; Kang, J.; Guo, X.; Yang, R.; Chen, Y.; Li, J.; Shi, L. Comparison of Nutritional Compositions and Essential Oil Profiles of Different Parts of a Dill and Two Fennel Cultivars. Foods 2021, 10, 1784. [Google Scholar] [CrossRef]
- Chalvin, C.; Drevensek, S.; Dron, M.; Bendahmane, A.; Boualem, A. Genetic Control of Glandular Trichome Development. Trends Plant Sci. 2020, 25, 477–487. [Google Scholar] [CrossRef]
- Gavalas, N.; Bosabalidis, A.M.; Kokkini, S. Comparative Study of Leaf Anatomy and Essential Oils of the Hybrid Mentha x Villoso-Nervata and Its Parental Species M. longifolia and M. spicata. Isr. J. Plant Sci. 1998, 46, 27–33. [Google Scholar] [CrossRef]
- Han, F.; Ma, G.Q.; Yang, M.; Yan, L.; Xiong, W.; Shu, J.C.; Zhao, Z.D.; Xu, H.L. Chemical Composition and Antioxidant Activities of Essential Oils from Different Parts of the Oregano. J. Zhejiang Univ. Sci. B 2017, 18, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Lukas, B.; Schmiderer, C.; Novak, J. Essential Oil Diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. [Google Scholar] [CrossRef]
- Emrahi, R.; Morshedloo, M.R.; Ahmadi, H.; Javanmard, A.; Maggi, F. Intraspecific Divergence in Phytochemical Characteristics and Drought Tolerance of Two Carvacrol-Rich Origanum vulgare subspecies: Subsp. hirtum and subsp. gracile. Ind. Crops Prod. 2021, 168, 113557. [Google Scholar] [CrossRef]
- Fang, C.; Fernie, A.R.; Luo, J. Exploring the Diversity of Plant Metabolism. Trends Plant Sci. 2019, 24, 83–98. [Google Scholar] [CrossRef]
- Hassiotis, C.N.; Ntana, F.; Lazari, D.M.; Poulios, S.; Vlachonasios, K.E. Environmental and Developmental Factors Affect Essential Oil Production and Quality of Lavandula angustifolia during Flowering Period. Ind. Crops Prod. 2014, 62, 359–366. [Google Scholar] [CrossRef]
- El-Serafy, R.S.; El-Sheshtawy, A.A. Effect of Nitrogen Fixing Bacteria and Moringa Leaf Extract on Fruit Yield, Estragole Content and Total Phenols of Organic Fennel. Sci. Hortic. 2020, 265, 109209. [Google Scholar] [CrossRef]
- Giannoulis, K.D.; Kamvoukou, C.-A.; Gougoulias, N.; Wogiatzi, E. Irrigation and Nitrogen Application Affect Greek Oregano (Origanum vulgare ssp. hirtum) Dry Biomass, Essential Oil Yield and Composition. Ind. Crops Prod. 2020, 150, 112392. [Google Scholar] [CrossRef]
- Król, B.; Sęczyk, Ł.; Kołodziej, B.; Paszko, T. Biomass Production, Active Substance Content, and Bioaccessibility of Greek Oregano (Origanum vulgare ssp. hirtum (Link) Ietswaart) Following the Application of Nitrogen. Ind. Crops Prod. 2020, 148, 112271. [Google Scholar] [CrossRef]
- Lukas, B.; Schmiderer, C.; Novak, J. Phytochemical Diversity of Origanum vulgare L. subsp. vulgare (Lamiaceae) from Austria. Biochem. Syst. Ecol. 2013, 50, 106–113. [Google Scholar] [CrossRef]
- Jan, S.; Mir, J.I.; Shafi, W.; Faktoo, S.Z.; Singh, D.B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Divergence in Tissue-Specific Expression Patterns of Genes Associated with the Terpeniod Biosynthesis in Two Oregano Species Origanum vulgare L., and Origanum majorana. Ind. Crops Prod. 2018, 123, 546–555. [Google Scholar] [CrossRef]
- Farrés, M.; Platikanov, S.; Tsakovski, S.; Tauler, R. Comparison of the Variable Importance in Projection (VIP) and of the Selectivity Ratio (SR) Methods for Variable Selection and Interpretation. J. Chemom. 2015, 29, 528–536. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Al-Reza, S.M.; Choi, U.K.; Lee, J.H.; Kang, S.C. Chemical Composition, Antibacterial and Antioxidant Activities of Leaf Essential Oil and Extracts of Metasequioa glyptostroboides Miki ex Hu. Food Chem. Toxicol. 2009, 47, 1876–1883. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The Antibacterial Properties of Phenolic Isomers, Carvacrol and Thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- AlSheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem Jan, A.; Haq, Q.M.R. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef]
- Khan, I.; Bahuguna, A.; Kumar, P.; Bajpai, V.K.; Kang, S.C. Antimicrobial Potential of Carvacrol against Uropathogenic Escherichia coli via Membrane Disruption, Depolarization, and Reactive Oxygen Species Generation. Front. Microbiol. 2017, 8, 2421. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.; Kim, H.; Beuchat, L.R.; Ryu, J.H. Synergistic Activities of Gaseous Oregano and Thyme Thymol Essential Oils against Listeria monocytogenes on Surfaces of a Laboratory Medium and Radish Sprouts. Food Microbiol. 2020, 86, 103357. [Google Scholar] [CrossRef]
- Cava-Roda, R.; Taboada-Rodríguez, A.; López-Gómez, A.; Martínez-Hernández, G.B.; Marín-Iniesta, F. Synergistic Antimicrobial Activities of Combinations of Vanillin and Essential Oils of Cinnamon Bark, Cinnamon Leaves, and Cloves. Foods 2021, 10, 1406. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Baek, K.H. Antibacterial Mode of Action of Cudrania tricuspidata Fruit Essential Oil, Affecting Membrane Permeability and Surface Characteristics of Food-borne Pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
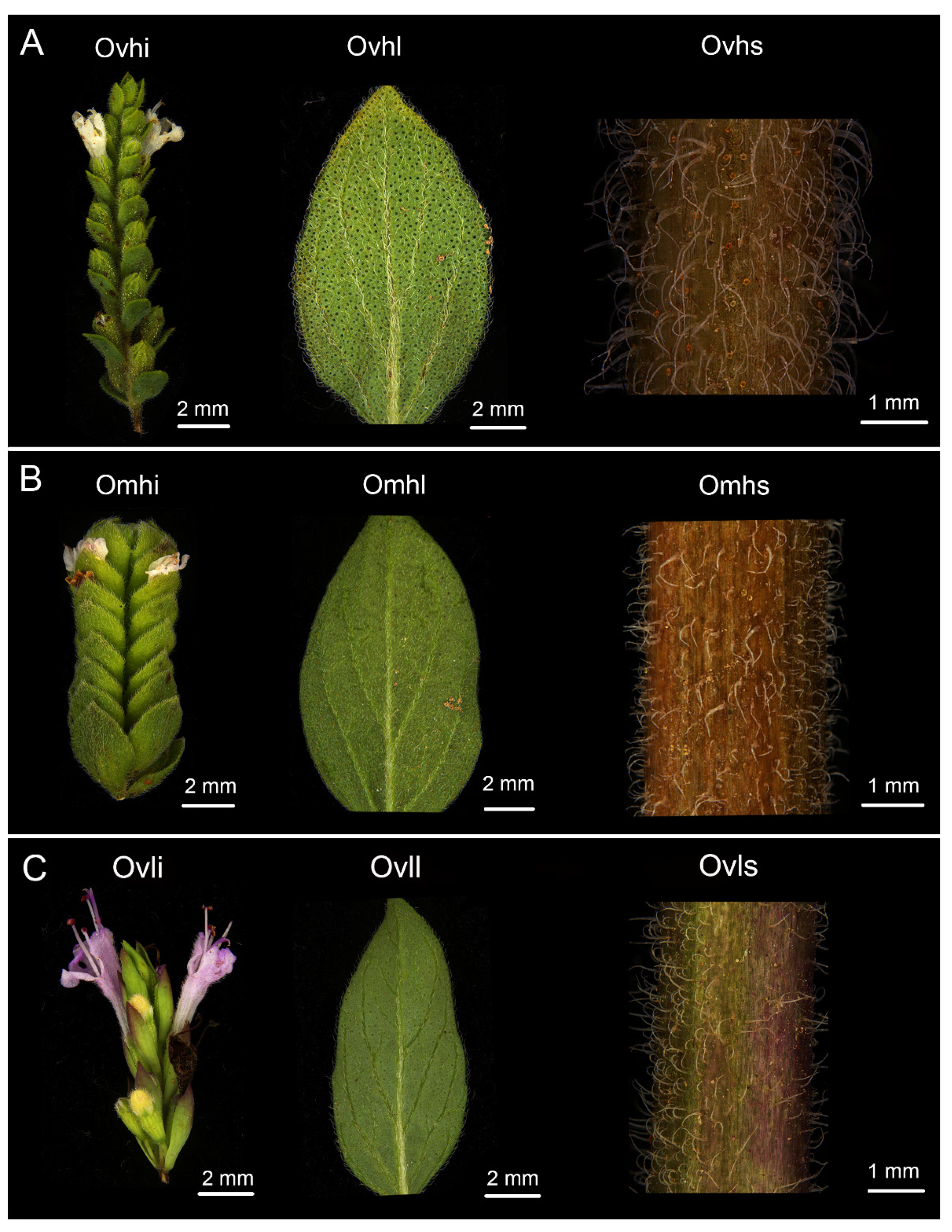
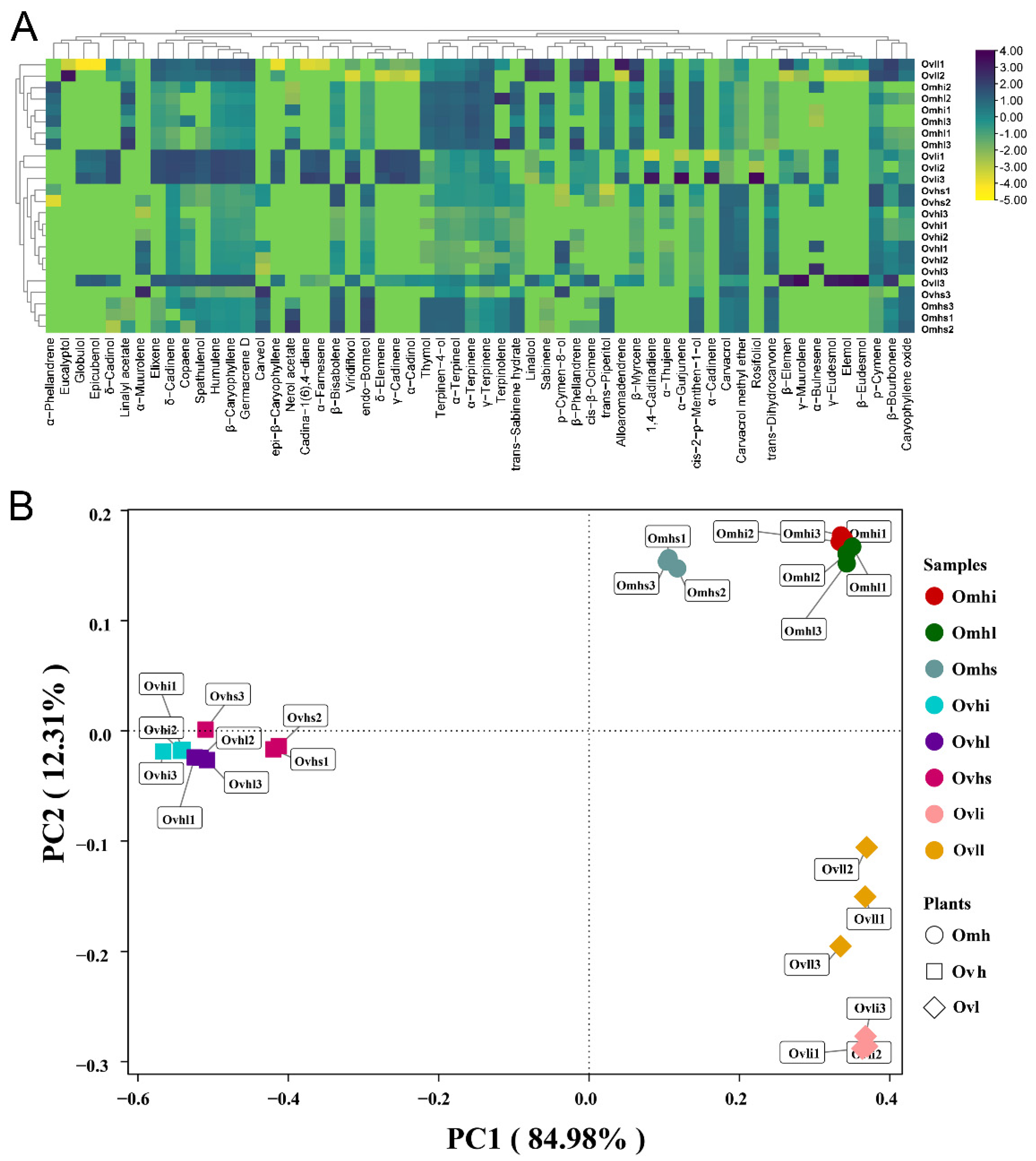
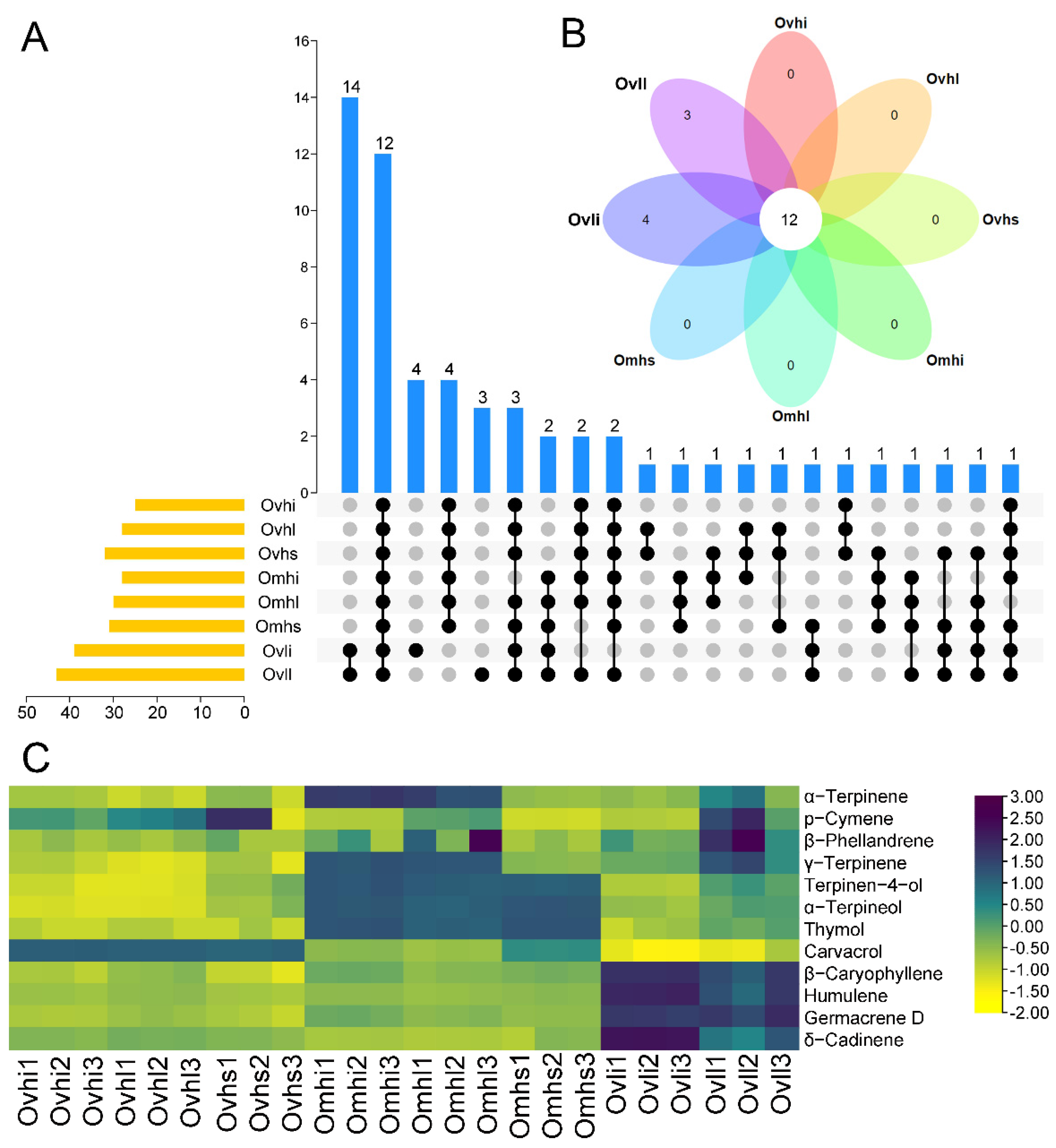
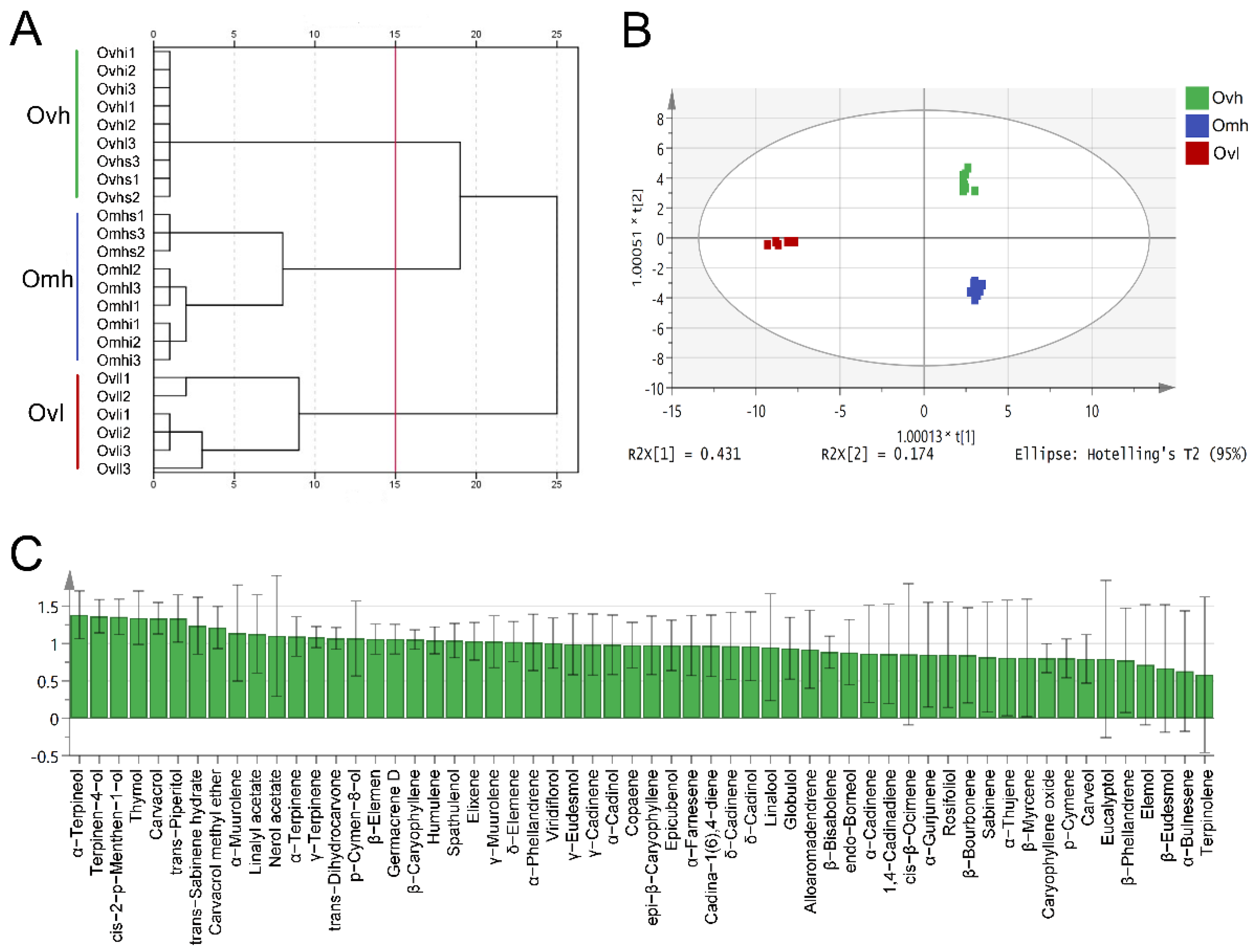
| Compounds | RI | Ovhi | Ovhl | Ovhs | Omhi | Omhl | Omhs | Ovli | Ovll |
|---|---|---|---|---|---|---|---|---|---|
| α-Thujene | 932 | 0.06 ± 0.06 | 0.08 ± 0.03 | 0.19 ± 0.26 | 1.01 ± 0.4 | 0.97 ± 0.97 | - | - | 0.33 ± 0.23 |
| Sabinene | 973 | - | - | - | 0.29 ± 0.26 | 0.88 ± 0.6 | 0.05 ± 0.01 | 0.15 ± 0.14 | 1.65 ± 1.35 |
| β-Myrcene | 991 | 0.08 ± 0.01 | 0.09 ± 0.04 | 0.13 ± 0.11 | 0.24 ± 0.04 | 0.48 ± 0.04 | - | - | 0.87 ± 0.65 |
| α-Phellandrene | 1006 | - | - | 0.07 ± 0.08 | 0.49 ± 0.1 | 0.37 ± 0.15 | - | - | - |
| α-Terpinene | 1018 | 0.39 ± 0.05 | 0.07 ± 0.13 | 0.5 ± 0.43 | 11.27 ± 0.78 | 8.74 ± 1.54 | 0.6 ± 0.07 | 0.68 ± 0.13 | 2.92 ± 1.99 |
| p-Cymene | 1026 | 2.16 ± 0.3 | 3.37 ± 0.51 | 5.65 ± 4.58 | 0.79 ± 0.01 | 2.02 ± 0.15 | 0.51 ± 0.02 | 0.78 ± 0.04 | 5.72 ± 3.54 |
| β-Phellandrene | 1030 | 0.04 ± 0.06 | 0.06 ± 0.06 | 0.1 ± 0.17 | 0.25 ± 0.25 | 1.3 ± 1.26 | 0.04 ± 0.07 | 0.3 ± 0.15 | 1.67 ± 1.08 |
| Eucalyptol | - | - | - | - | - | - | - | 0.18 ± 0.16 | |
| cis-β-Ocimene | 1038 | - | - | 0.04 ± 0.04 | - | - | 0.04 ± 0.04 | 0.14 ± 0 | 3.47 ± 2.16 |
| γ-Terpinene | 1060 | 0.77 ± 0.2 | 0.23 ± 0.06 | 0.79 ± 0.56 | 13.79 ± 0.99 | 13.3 ± 0.16 | 1.69 ± 0.11 | 2.62 ± 0.12 | 12.28 ± 6.59 |
| trans-Sabinene hydrate | 1069 | 0.3 ± 0.12 | 0.4 ± 0.08 | 1.02 ± 0.1 | 6.54 ± 3.76 | 14.6 ± 0.5 | 9.36 ± 0.14 | - | - |
| Terpinolene | 1091 | 0.12 ± 0.03 | 0.26 ± 0.15 | 0.19 ± 0.17 | - | 1.8 ± 1.57 | 0.41 ± 0.02 | 0.15 ± 0.13 | 0.23 ± 0.21 |
| Linalool | - | - | - | - | - | - | 0.21 ± 0.04 | 0.98 ± 0.28 | |
| cis-2-p-Menthen-1-ol | 1124 | 0.09 ± 0.03 | 0.08 ± 0.02 | 0.23 ± 0.07 | 2.28 ± 0.16 | 2.58 ± 0.18 | 2.46 ± 0.12 | - | 0.27 ± 0.22 |
| endo-Borneol | 1170 | 0.17 ± 0.01 | 0.25 ± 0.02 | 0.53 ± 0.12 | 0.18 ± 0 | 0.19 ± 0.01 | 0.88 ± 0.04 | - | - |
| Terpinen-4-ol | 1181 | 1.36 ± 0.32 | 1.08 ± 0.11 | 3.64 ± 0.91 | 25.05 ± 1.55 | 22.13 ± 0.98 | 21.42 ± 0.76 | 2.17 ± 0.1 | 7.21 ± 1.32 |
| p-Cymen-8-ol | 1188 | - | 0.17 ± 0.01 | 0.14 ± 0.04 | - | - | - | - | - |
| α-Terpineol | 1194 | 0.19 ± 0.04 | 0.17 ± 0.02 | 0.78 ± 0.24 | 4.71 ± 0.08 | 4.15 ± 0.14 | 4.77 ± 0.08 | 0.75 ± 0.09 | 1.58 ± 0.21 |
| trans-Dihydrocarvone | 1201 | 0.24 ± 0.05 | 0.31 ± 0.04 | 0.33 ± 0.06 | 0.1 ± 0 | 0.09 ± 0.02 | 0.32 ± 0.01 | - | - |
| trans-Piperitol | 1211 | - | - | 0.12 ± 0.06 | 0.72 ± 0.04 | 0.78 ± 0.04 | 0.97 ± 0.01 | - | - |
| Carveol | 1223 | - | 0.06 ± 0.02 | 0.12 ± 0.05 | - | - | 0.13 ± 0.01 | - | - |
| Carvacrol methyl ether | 1248 | 0.63 ± 0.1 | 0.99 ± 0.18 | 0.87 ± 0.07 | 0.05 ± 0 | 0.05 ± 0.01 | 0.6 ± 0.03 | - | 0.17 ± 0.03 |
| Linalyl acetate | 1259 | - | - | - | 0.5 ± 0.18 | 1.14 ± 0.14 | 0.2 ± 0.09 | - | 0.17 ± 0.15 |
| Thymol | 1294 | 0.1 ± 0.17 | 0.23 ± 0.2 | 0.21 ± 0.37 | 18.74 ± 0.62 | 14.31 ± 0.75 | 18.72 ± 0.08 | 0.45 ± 0.4 | 2.49 ± 0.51 |
| Carvacrol | 1304 | 90.28 ± 1.5 | 86.03 ± 0.66 | 79.31 ± 5.83 | 7.01 ± 0.32 | 5.25 ± 0.28 | 27.73 ± 0.94 | 0.67 ± 0.33 | 2 ± 1.81 |
| δ-EIemene | - | - | - | - | - | - | 0.83 ± 0.04 | 0.43 ± 0.11 | |
| Nerol acetate | 1368 | - | - | - | 0.09 ± 0.1 | 0.15 ± 0.09 | 0.26 ± 0.13 | - | - |
| Copaene | 1382 | 0.05 ± 0.04 | 0.07 ± 0.01 | 0.05 ± 0.01 | 0.11 ± 0.1 | - | 0.12 ± 0.1 | 0.63 ± 0.06 | 0.33 ± 0.13 |
| β-Bourbonene | 1393 | 0.03 ± 0.02 | 0.15 ± 0.02 | 0.05 ± 0.04 | - | 0.05 ± 0.01 | 0.15 ± 0.02 | 0.1 ± 0.08 | 0.85 ± 0.19 |
| β-Elemen | - | - | - | - | - | - | 0.44 ± 0.01 | 0.48 ± 0.1 | |
| α-Gurjunene | - | - | - | - | - | - | 0.18 ± 0.04 | - | |
| β-Caryophyllene | 1428 | 1.36 ± 0.17 | 1.95 ± 0.18 | 0.93 ± 0.23 | 2.69 ± 0.05 | 2.03 ± 0.13 | 2.42 ± 0.1 | 13.99 ± 0.18 | 10.51 ± 2.41 |
| Alloaromadendrene | - | - | - | - | - | - | 0.3 ± 0.09 | 0.35 ± 0.28 | |
| Humulene | 1462 | 0.16 ± 0.02 | 0.23 ± 0.02 | 0.11 ± 0.02 | 0.24 ± 0 | 0.19 ± 0.02 | 0.26 ± 0.02 | 3 ± 0.09 | 1.95 ± 0.53 |
| epi-β-Caryophyllene | - | - | - | - | - | - | 1.48 ± 0.08 | 0.55 ± 0.46 | |
| Cadina-1(6),4-diene | - | - | - | - | - | - | 0.33 ± 0.03 | 0.09 ± 0.09 | |
| Germacrene D | 1484 | 0.41 ± 0.03 | 0.66 ± 0.07 | 0.32 ± 0.17 | 1.34 ± 0.11 | 0.92 ± 0.09 | 0.76 ± 0.03 | 11.13 ± 0.65 | 11.26 ± 2.56 |
| γ-Muurolene | - | - | - | - | - | - | 0.85 ± 0.17 | 1.01 ± 0.41 | |
| α-Bulnesene | 1502 | - | 0.11 ± 0.1 | 0.06 ± 0.05 | 0.06 ± 0.04 | - | - | - | - |
| Elixene | 1505 | - | - | - | 0.79 ± 0.04 | 0.56 ± 0.03 | 0.34 ± 0.12 | 25.27 ± 0.93 | 13.69 ± 2.67 |
| α-Muurolene | 1507 | 0.04 ± 0.04 | 0.1 ± 0 | 0.07 ± 0.07 | - | - | - | - | - |
| α-Farnesene | - | - | - | - | - | - | 3.39 ± 0.13 | 0.98 ± 0.91 | |
| β-Bisabolene | 1514 | 0.19 ± 0.01 | 0.34 ± 0.02 | 0.72 ± 0.08 | 0.32 ± 0.01 | 0.29 ± 0.03 | 0.72 ± 0.05 | - | - |
| γ-Cadinene | - | - | - | - | - | - | 2.33 ± 0.1 | 0.77 ± 0.29 | |
| δ-Cadinene | 1530 | 0.38 ± 0.04 | 0.51 ± 0.02 | 0.36 ± 0.06 | 0.11 ± 0.01 | 0.05 ± 0.01 | 0.24 ± 0.21 | 7.75 ± 0.11 | 2.35 ± 0.88 |
| 1,4-Cadinadiene | - | - | - | - | - | - | 0.21 ± 0.02 | - | |
| α-Cadinene | - | - | - | - | - | - | 0.45 ± 0.01 | - | |
| Elemol | - | - | - | - | - | - | - | 0.43 ± 0.28 | |
| Globulol | - | - | - | - | - | - | 1.88 ± 0.36 | 0.74 ± 1.19 | |
| Spathulenol | 1586 | - | - | 0.08 ± 0 | - | 0.06 ± 0.05 | 0.73 ± 0.19 | 4.6 ± 0.28 | 3.47 ± 1.62 |
| Caryophyllene oxide | 1592 | 0.17 ± 0.04 | 0.91 ± 0.11 | 1.22 ± 0.12 | - | 0.09 ± 0.01 | 1.15 ± 0.21 | 0.16 ± 0.01 | 0.64 ± 0.61 |
| Viridiflorol | - | - | - | - | - | - | 1.05 ± 0.22 | 0.51 ± 0.3 | |
| Rosifoliol | - | - | - | - | - | - | 0.33 ± 0.08 | - | |
| Epicubenol | - | - | - | - | - | - | 0.25 ± 0.01 | 0.12 ± 0.14 | |
| γ-Eudesmol | - | - | - | - | - | - | 0.3 ± 0.02 | 0.29 ± 0.21 | |
| δ-Cadinol | 1648 | - | - | - | - | - | 0.2 ± 0.12 | 4.25 ± 0.16 | 1.09 ± 0.61 |
| β-Eudesmol | - | - | - | - | - | - | - | 0.4 ± 0.32 | |
| α-Cadinol | - | - | - | - | - | - | 4.43 ± 0.01 | 1.42 ± 0.86 | |
| Total | 99.77 ± 0.13 | 98.98 ± 0.24 | 98.93 ± 0.31 | 99.75 ± 0.16 | 99.54 ± 0.55 | 98.25 ± 0.54 | 98.96 ± 0.32 | 98.95 ± 0.7 | |
| EO yields | 5.83 ± 0.15 | 2.23 ± 0.06 | 0.2 ± 0 | 2.3 ± 0.1 | 1.03 ± 0.12 | 0.1 ± 0 | 0.27 ± 0.06 | 0.1 ± 0 |
| Bacteria | Concentrations of OEO (mg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.0625 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | |
| Ovhi | + | - | - | - | - | - | - | - | - | - | - | - |
| Ovhl | + | - | - | - | - | - | - | - | - | - | - | - |
| Ovhs | + | + | - | - | - | - | - | - | - | - | - | - |
| Omhi | + | + | + | + | + | - | - | - | - | - | - | - |
| Omhl | + | + | + | + | + | - | - | - | - | - | - | - |
| Ovli | + | + | + | + | + | + | + | + | - | - | - | - |
| Ovll | + | + | + | + | + | + | + | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Li, J.; Zhang, W.; Sun, M.; Li, H.; Xia, F.; Cui, H.; Bai, H.; Shi, L. Analysis of the Chemical Profiles and Anti-S. aureus Activities of Essential Oils Extracted from Different Parts of Three Oregano Cultivars. Foods 2021, 10, 2328. https://doi.org/10.3390/foods10102328
Hao Y, Li J, Zhang W, Sun M, Li H, Xia F, Cui H, Bai H, Shi L. Analysis of the Chemical Profiles and Anti-S. aureus Activities of Essential Oils Extracted from Different Parts of Three Oregano Cultivars. Foods. 2021; 10(10):2328. https://doi.org/10.3390/foods10102328
Chicago/Turabian StyleHao, Yuanpeng, Jingyi Li, Wenying Zhang, Meiyu Sun, Hui Li, Fei Xia, Hongxia Cui, Hongtong Bai, and Lei Shi. 2021. "Analysis of the Chemical Profiles and Anti-S. aureus Activities of Essential Oils Extracted from Different Parts of Three Oregano Cultivars" Foods 10, no. 10: 2328. https://doi.org/10.3390/foods10102328
APA StyleHao, Y., Li, J., Zhang, W., Sun, M., Li, H., Xia, F., Cui, H., Bai, H., & Shi, L. (2021). Analysis of the Chemical Profiles and Anti-S. aureus Activities of Essential Oils Extracted from Different Parts of Three Oregano Cultivars. Foods, 10(10), 2328. https://doi.org/10.3390/foods10102328






