Differences in the Levels of the Selected Phytoestrogens and Stable Isotopes in Organic vs. Conventional Hops and Beer
Abstract
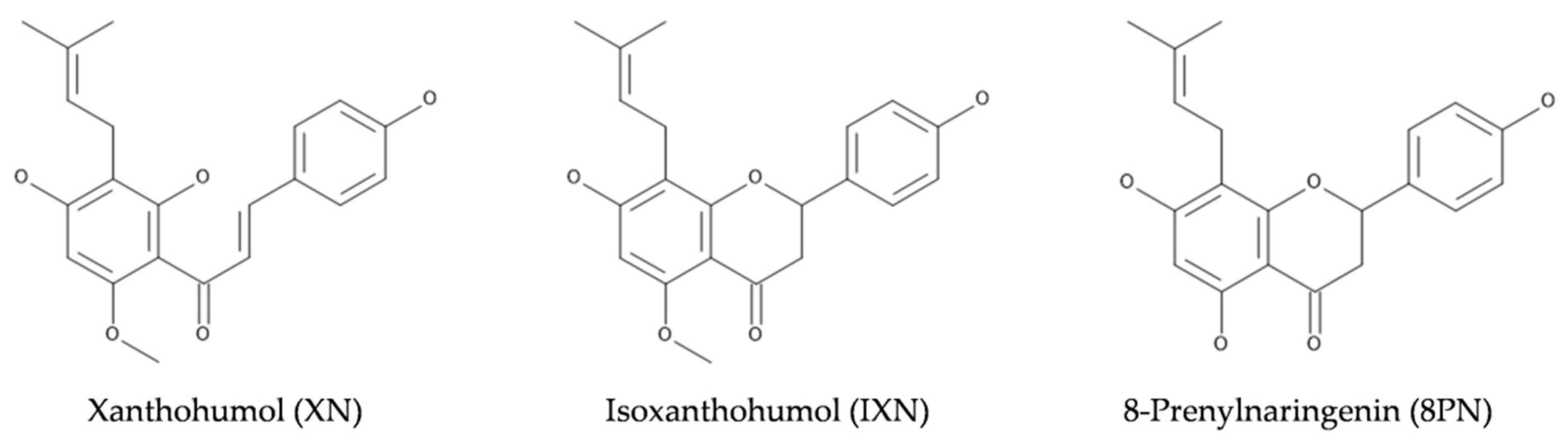
:1. Introduction
1.1. Organic Production
1.2. Stable Isotopes
2. Materials and Methods
2.1. Chemicals, Solvents and Materials
2.2. Sampling
2.3. Sample Preparation
2.4. Solid-Phase Extraction
2.5. LC-MS/MS Method Development and Validation
2.6. Isotope Ratio Mass Spectrometry (IRMS)
2.7. Statistical Evaluation of the Data
3. Results and Discussion
3.1. Method Development and Validation
3.2. Hops and Beer Samples
3.3. Commercial Beer Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yin, H.; Liu, G.; Dong, J.; Qian, Z.; Miao, J. Xanthohumol, a Prenylated Chalcone from Beer Hops, Acts as an α-Glucosidase Inhibitor in Vitro. J. Agric. Food Chem. 2014, 62, 5548–5554. [Google Scholar] [CrossRef]
- Bocquet, L.; Sahpaz, S.; Hilbert, J.L.; Rambaud, C.; Rivière, C. Humulus Lupulus L., a Very Popular Beer Ingredient and Medicinal Plant: Overview of Its Phytochemistry, Its Bioactivity, and Its Biotechnology. Phytochem. Rev. 2018, 17, 1047–1090. [Google Scholar] [CrossRef]
- Sauerwein, H.; Meyer, H.H. D Erfassung Östrogenwirksamer Substanzen in Bier Und in Dessen Rohstoffen. Mon. Brauwiss. 1997, 50, 142–146. [Google Scholar]
- Štulíková, K.; Karabín, M.; Nešpor, J.; Dostálek, P. Therapeutic Perspectives of 8-Prenylnaringenin, a Potent Phytoestrogen from Hops. Molecules 2018, 23, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, J.F.; Page, J.E. Xanthohumol and Related Prenylflavonoids from Hops and Beer: To Your Good Health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Becker, T.; Qian, F.; Ring, J. Beer and Beer Compounds: Physiological Effects on Skin Health. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Barański, M.; Średnicka-Tober, D.; Volakakis, N.; Seal, C.; Sanderson, R.; Stewart, G.B.; Benbrook, C.; Biavati, B.; Markellou, E.; Giotis, C.; et al. Higher Antioxidant and Lower Cadmium Concentrations and Lower Incidence of Pesticide Residues in Organically Grown Crops: A Systematic Literature Review and Meta-Analyses. Br. J. Nutr. 2014, 112, 794–811. [Google Scholar] [CrossRef] [Green Version]
- De Keukeleire, J.; Janssens, I.; Heyerick, A.; Ghekiere, G.; Cambie, J.; Roldán-Ruiz, I.; Van Bockstaele, E.; De Keukeleire, D. Relevance of Organic Farming and Effect of Climatological Conditions on the Formation of α-Acids, β-Acids, Desmethylxanthohumol, and Xanthohumol in Hop (Humulus lupulus L.). J. Agric. Food Chem. 2007, 55, 61–66. [Google Scholar] [CrossRef]
- Dangour, A.D.; Dodhia, S.K.; Hayter, A.; Allen, E.; Lock, K.; Uauy, R. Nutritional Quality of Organic Foods: A Systematic Review. Am. J. Clin. Nutr. 2009, 90, 680–685. [Google Scholar] [CrossRef] [Green Version]
- Brandt, K.; Leifert, C.; Sanderson, R.; Seal, C.J. Agroecosystem Management and Nutritional Quality of Plant Foods: The Case of Organic Fruits and Vegetables. Crit. Rev. Plant Sci. 2011, 30, 177–197. [Google Scholar] [CrossRef]
- Mulet, J.M. Should We Recommend Organic Crop Foods on the Basis of Health Benefits? Letter to the Editor Regarding the Article by Barański et Al. Br. J. Nutr. 2014, 112, 1745–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mlinarić, A.; Horvat, M.; Smolčić, V.Š. Dealing with the Positive Publication Bias: Why You Should Really Publish Your Negative Results. Biochem. Med. 2017, 27. [Google Scholar] [CrossRef] [Green Version]
- Suñé, P.; Suñé, J.M.; Montoro, J.B. Positive Outcomes Influence the Rate and Time to Publication, but Not the Impact Factor of Publications of Clinical Trial Results. PLoS ONE 2013, 8, e54583. [Google Scholar] [CrossRef] [Green Version]
- Mantha, O.L.; Laxmi Patel, M.; Hankard, R.; De Luca, A. Effect of Organic Food Intake on Nitrogen Stable Isotopes. Nutrients 2020, 12, 2965. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D. On the Nature of Carbon Isotope Discrimination in C4 Species. Funct. Plant Biol. 1983, 10, 205–226. [Google Scholar] [CrossRef]
- Brooks, J.R.; Buchmann, N.; Phillips, S.; Ehleringer, B.; Evans, R.D.; Lott, M.; Martinelli, L.A.; Pockman, W.T.; Sandquist, D.; Sparks, J.P.; et al. Heavy and Light Beer: A Carbon Isotope Approach To Detect C4 Carbon in Beers of Different Origins, Styles, and Prices. J. Agric. Food Chem. 2002, 50, 6413–6418. [Google Scholar] [CrossRef] [PubMed]
- Mardegan, S.F.; Andrade, T.M.B.; de Sousa Neto, E.R.; de Castro Vasconcellos, E.B.; Martins, L.F.B.; Mendonça, T.G.; Martinelli, L.A. Stable Carbon Isotopic Composition of Brazilian Beers—A Comparison between Large- and Small-Scale Breweries. J. Food Compos. Anal. 2013, 29, 52–57. [Google Scholar] [CrossRef]
- Bateman, A.S.; Kelly, S.D. Fertilizer Nitrogen Isotope Signatures. Isot. Environ. Health Stud. 2007, 43, 237–247. [Google Scholar] [CrossRef]
- Choi, W.-J.; Ro, H.-M.; Hobbie, E.A. Patterns of Natural N-15 in Soils and Plants from Chemically and Organically Fertilized Uplands. Soil Biol. Biochem. 2003, 35, 1493–1500. [Google Scholar] [CrossRef]
- Rogers, K.M. Nitrogen Isotopes as a Screening Tool To Determine the Growing Regimen of Some Organic and Nonorganic Supermarket Produce from New Zealand. J. Agric. Food Chem. 2008, 56, 4078–4083. [Google Scholar] [CrossRef] [PubMed]
- Inácio, C.T.; Chalk, P.M.; Magalhães, A.M.T. Principles and Limitations of Stable Isotopes in Differentiating Organic and Conventional Foodstuffs: 1. Plant Products. Crit. Rev. Food Sci. Nutr. 2015, 55, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Voica, C.; Magdas, D.-A.; Feher, I. Metal Content and Stable Isotope Determination in Some Commercial Beers from Romanian Markets. J. Chem. 2015, 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Ocvirk, M.; Ogrinc, N.; Košir, I.J. Determination of the Geographical and Botanical Origin of Hops (Humulus Lupulus L.) Using Stable Isotopes of C, N, and S. J. Agric. Food Chem. 2018, 66, 2021–2026. [Google Scholar] [CrossRef]
- Stevens, J.F.; Taylor, A.W.; Deinzer, M.L. Quantitative Analysis of Xanthohumol and Related Prenylflavonoids in Hops and Beer by Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 1999, 832, 97–107. [Google Scholar] [CrossRef]
- Trass, M.; Koerner, P.; Layne, J. Rapid Analysis of Hop Acids in Beer Using StrataTM-X SPE and Kinetex® 2.6 Μm Core-Shell Technology; HPLC Column. Application note ©Phenomenex: Torrance, CA, USA, 2011. [Google Scholar]
- Bernal, J.; García-Mauriño, C.M.; Reglero, G.; Marin, F.R.; Ibáñez, E. Fast Screening Method to Determine Hop’s Phytoestrogens in Beer. Food Anal. Methods 2011, 4, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, P.J.; Guido, L.F.; Cruz, J.M.; Barros, A.A. Analysis of Xanthohumol and Isoxanthohumol in Different Hop Products by Liquid Chromatography-Diode Array Detection-Electrospray Ionization Tandem Mass Spectrometry. J. Chromatogr. A 2007, 1150, 295–301. [Google Scholar] [CrossRef] [PubMed]
- AOAC. AOAC Guidelines for Single-Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals, Official Methods of Analysis, 19th ed.; AOAC: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Magnusson, B.; Örnemark, U. (Eds.) Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed. 2014. Available online: Www.Eurachem.Org (accessed on 14 May 2019).
- European Commission Commission Regulation (EC). No 401/2006, Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs; Official Journal of the European Communities: Brussels, Belgium, 2006. [Google Scholar]
- European Commission. Directorate General for Health and Food Safety Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed SANTE/11813/2017; European Commission: Brussels, Belgium, 2017. [Google Scholar]




| Label | Type | δ15N (‰) | δ13C (‰) | Water Content (%) | Hops | Beer | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IXN (μg/g) | 8PN (μg/g) | XN (μg/g) | Weighed hops (g) | IXN (ng/mL) | 8PN (ng/mL) | XN (μg/g) | |||||
| H1 | organic | 7.9 | −27.6 | 8.1 | 2.2 | 9.6 | 378 | 16.5 | 1030 | 19.8 | 263 |
| H2 | conventional | 6.0 | −27.1 | 7.0 | 1.5 | 11.2 | 345 | 24 | 1410 | 23.0 | 538 |
| H3 | organic | 7.7 | −27.1 | 8.0 | 1.1 | 4.7 | 173 | 27 | 553 | 17.2 | 296 |
| H4 | conventional | 9.5 | −27.1 | 9.0 | 1.2 | 4.9 | 188 | 35 | 671 | 18.4 | 363 |
| H5 | organic | 6.6 | −27.2 | 8.1 | 1.3 | 6.1 | 287 | 15 | 718 | 14.9 | 198 |
| H6 | conventional | 7.5 | −27.2 | 8.0 | 1.0 | 5.9 | 232 | 19 | 811 | 19.5 | 344 |
| H7 | organic | 6.6 | −27.7 | 8.4 | 1.9 | 13.5 | 511 | 5.8 | 1850 | 25.8 | 188 |
| H8 | conventional | 6.2 | −27.5 | 8.0 | 1.6 | 15.9 | 491 | 8.5 | 1120 | 19.5 | 283 |
| H9 | organic | 5.3 | −26.6 | 6.5 | 0.6 | 10.9 | 407 | 5.6 | 517 | 3.8 | 102 |
| H10 | conventional | 8.1 | −26.1 | 7.5 | 0.6 | 9.6 | 445 | 4.3 | 621 | 6.6 | 71.2 |
| H11 | organic | 10.3 | −26.9 | 8.7 | 1.1 | 7.1 | 199 | 27 | 644 | 8.2 | 79.2 |
| H12 | conventional | 6.3 | −26.6 | 8.1 | 1.2 | 7.9 | 264 | 21 | 1040 | 13.2 | 81.2 |
| H13 | organic | 7.2 | −25.5 | 8.0 | 1.3 | 11.4 | 376 | 18 | 1470 | 21.5 | 97.4 |
| H14 | conventional | 4.8 | −27.1 | 7.5 | 1.6 | 13.4 | 463 | 7.8 | 1130 | 16.9 | 66.9 |
| H15 | organic | 7.5 | −26.2 | 5.9 | 1.5 | 18.6 | 555 | 4.9 | 1290 | 14.2 | 38.6 |
| H16 | conventional | 5.9 | −26.8 | 5.9 | 1.0 | 9.4 | 384 | 8.5 | 1170 | 16.5 | 84.8 |
| H19 | organic | 8.6 | −26.2 | 9.9 | 1.3 | 8.1 | 242 | 11 | 570 | 11.5 | 44.4 |
| H20 | conventional | 5.5 | −26.4 | 8.0 | 1.2 | 10.3 | 314 | 9.6 | 617 | 13.4 | 65.6 |
| H21 | organic | 10.9 | −28.1 | 9.5 | 1.3 | 11.0 | 341 | 12 | 858 | 13.7 | 91.1 |
| H22 | conventional | 5.9 | −27.3 | 8.5 | 1.3 | 13.2 | 366 | 12 | 987 | 16.0 | 129 |
| H23 | organic | 7.9 | −26.9 | 8.5 | 1.5 | 11.9 | 327.3 | 7.7 | 896 | 17.5 | 67.1 |
| H24 | conventional | 6.4 | −27.0 | 8.0 | 1.9 | 19.6 | 480 | 8.2 | 1114.1 | 19.2 | 64.4 |
| H25 | organic | 6.5 | −25.8 | 11.4 | 1.1 | 8.3 | 460 | 11 | 595.6 | 6.5 | 54.5 |
| H26 | conventional | 5.7 | −25.6 | 7.0 | 1.5 | 24.8 | 301 | 5.2 | 196.1 | 5.0 | 76.0 |
| H27 | organic | 5.4 | −25.6 | 21.6 | 0.8 | 4.2 | 87.4 | 71 | 984.5 | 16.7 | 32.5 |
| H28 | conventional | 5.4 | −25.1 | 8.5 | 0.9 | 2.5 | 230 | 16 | 964.9 | 16.3 | 29.8 |
| Label | Type | δ15N (‰) | δ13C (‰) | IXN (μg/L) | 8PN (μg/L) | XN (μg/L) |
|---|---|---|---|---|---|---|
| UO1 | organic | 3.4 | −27.2 | 1.834 | 0.010 | 0.027 |
| UO2 | organic | 3.6 | −27.0 | 1.549 | 0.012 | 0.032 |
| UO3 | organic | 4.0 | −27.4 | 0.727 | 0.020 | 0.020 |
| UO4 | organic | 5.1 | −28.3 | 1.096 | 0.012 | 0.018 |
| UO5 | organic | 5.2 | −25.9 | 0.676 | 0.022 | 0.014 |
| UO6 | organic | 4.9 | −28.4 | 0.856 | 0.011 | 0.017 |
| UO7 | organic | 4.2 | −27.4 | 0.894 | 0.016 | 0.036 |
| UO8 | organic | 5.1 | −27.2 | 0.662 | 0.013 | 0.026 |
| UO9 | organic | 4.0 | −27.9 | 0.907 | 0.016 | 0.013 |
| UO10 | organic | 4.7 | −27.9 | 0.754 | 0.012 | 0.012 |
| UO11 | organic | 7.4 | −26.7 | 1.35 | 0.022 | 0.019 |
| UO12 | organic | 3.3 | −27.9 | 0.705 | 0.015 | 0.023 |
| UO13 | organic | 4.9 | −28.4 | 1.11 | 0.023 | 0.017 |
| UO14 | organic | 4.3 | −27.9 | 1.64 | 0.022 | 0.042 |
| UO15 | organic | 3.9 | −27.9 | 1.20 | 0.012 | 0.015 |
| UK3 | conventional | 4.1 | −27.2 | 0.716 | 0.019 | 0.060 |
| UK7 | conventional | 2.9 | −28.3 | 0.492 | 0.006 | 0.012 |
| UK8 | conventional | 4.6 | −28.4 | 3.11 | 0.014 | 0.025 |
| UK9 | conventional | 3.6 | −27.4 | 0.740 | 0.017 | 0.029 |
| UK10 | conventional | 3.3 | −27.0 | 0.632 | 0.018 | 0.031 |
| UK11 | conventional | 2.4 | −27.4 | 0.221 | 0.006 | 0.006 |
| UK12 | conventional | 3.3 | −26.9 | 1.31 | 0.023 | 0.037 |
| UK13 | conventional | 3.4 | −27.3 | 0.155 | 0.003 | 0.007 |
| UK14 | conventional | 3.2 | −27.2 | 0.205 | 0.007 | 0.009 |
| UK15 | conventional | 4.4 | −27.2 | 0.713 | 0.013 | 0.018 |
| UK16 | conventional | 4.9 | −22.6 | 0.486 | 0.022 | 0.050 |
| UK17 | conventional | 4.3 | −27.5 | 1.24 | 0.032 | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubović, J.B.; Heath, E.; Košir, I.J.; Ogrinc, N.; Potočnik, D.; Strojnik, L.; Heath, D. Differences in the Levels of the Selected Phytoestrogens and Stable Isotopes in Organic vs. Conventional Hops and Beer. Foods 2021, 10, 1839. https://doi.org/10.3390/foods10081839
Golubović JB, Heath E, Košir IJ, Ogrinc N, Potočnik D, Strojnik L, Heath D. Differences in the Levels of the Selected Phytoestrogens and Stable Isotopes in Organic vs. Conventional Hops and Beer. Foods. 2021; 10(8):1839. https://doi.org/10.3390/foods10081839
Chicago/Turabian StyleGolubović, Jelena B., Ester Heath, Iztok Jože Košir, Nives Ogrinc, Doris Potočnik, Lidija Strojnik, and David Heath. 2021. "Differences in the Levels of the Selected Phytoestrogens and Stable Isotopes in Organic vs. Conventional Hops and Beer" Foods 10, no. 8: 1839. https://doi.org/10.3390/foods10081839
APA StyleGolubović, J. B., Heath, E., Košir, I. J., Ogrinc, N., Potočnik, D., Strojnik, L., & Heath, D. (2021). Differences in the Levels of the Selected Phytoestrogens and Stable Isotopes in Organic vs. Conventional Hops and Beer. Foods, 10(8), 1839. https://doi.org/10.3390/foods10081839







