Chemical-Based Methodologies to Extend the Shelf Life of Fresh Fish—A Review
Abstract
:1. Introduction
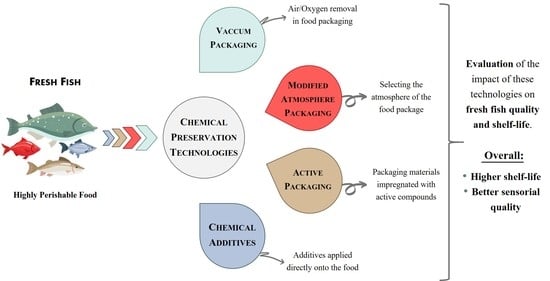
2. Chemical Methodologies for Shelf-Life Extension
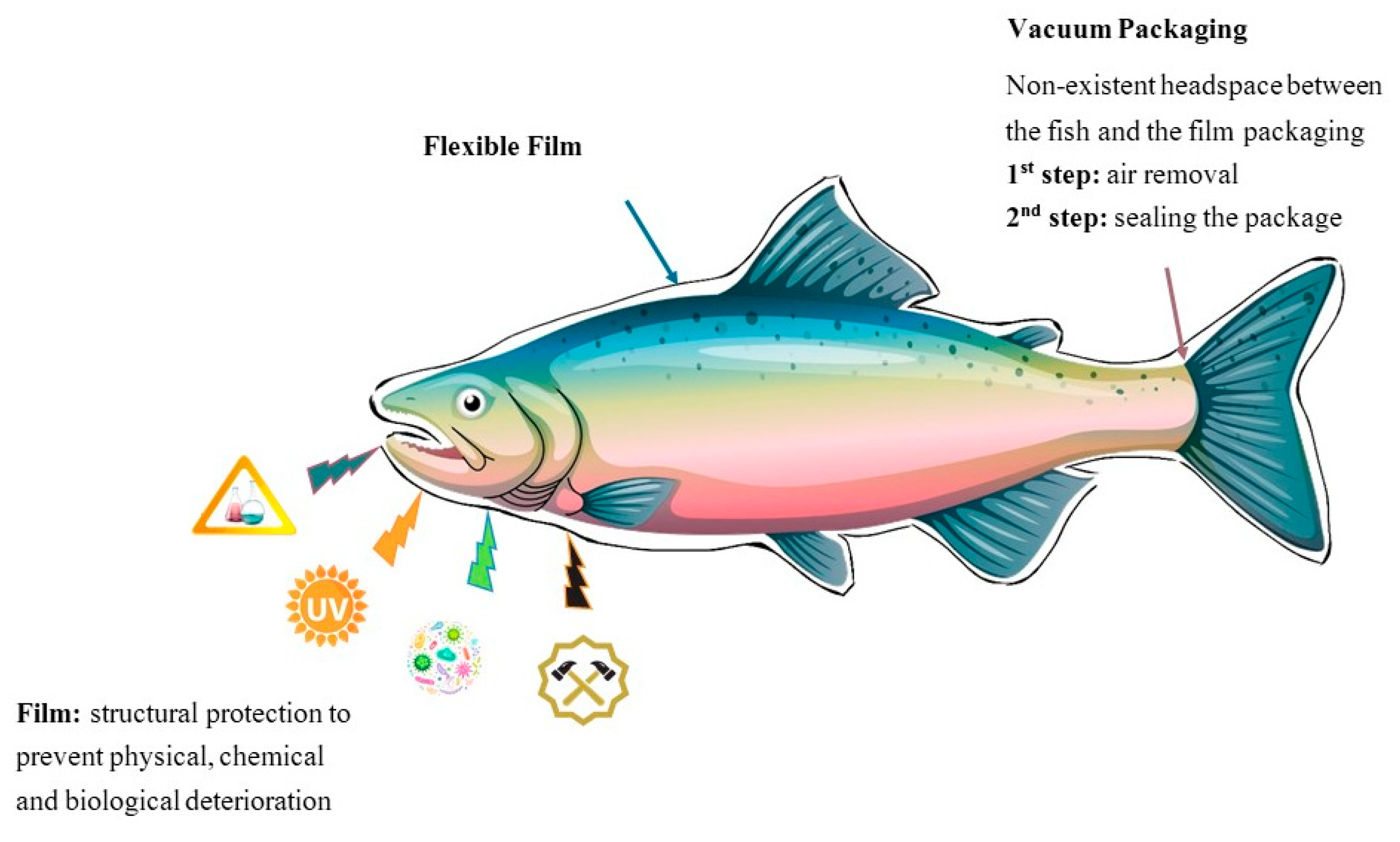
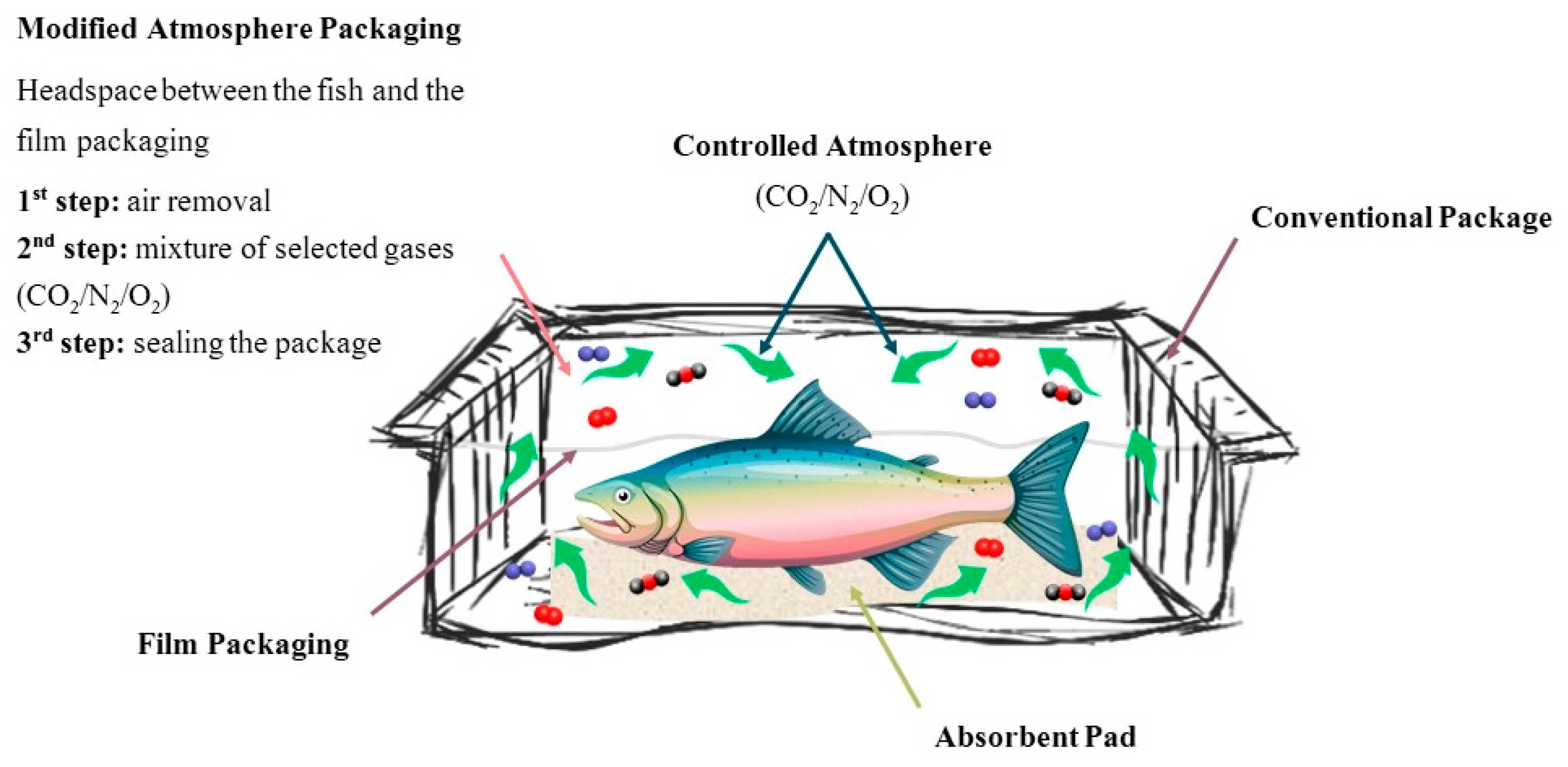
2.1. Conventional Packaging: Vacuum-Packaging and Modified Atmosphere Packaging
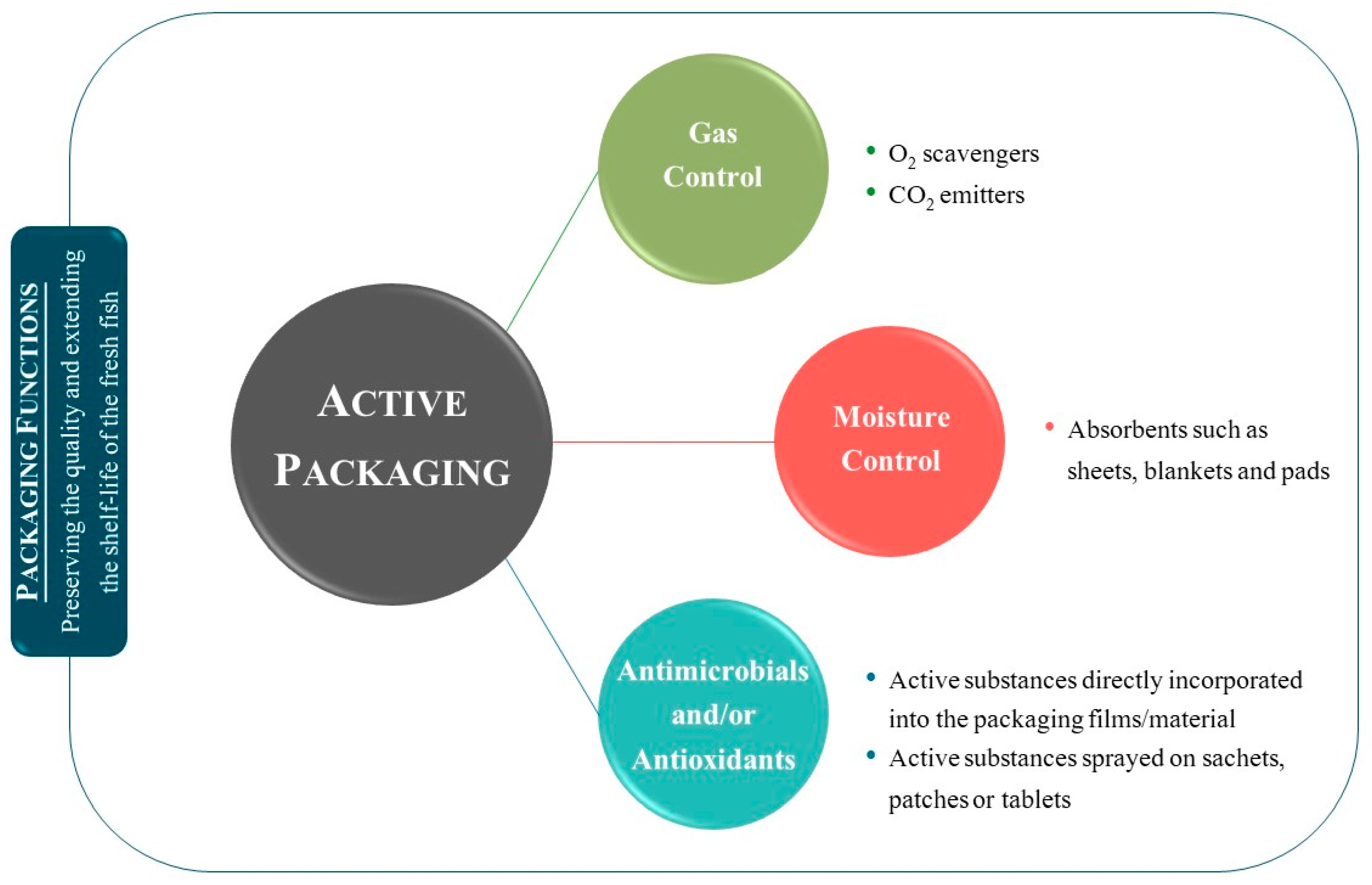
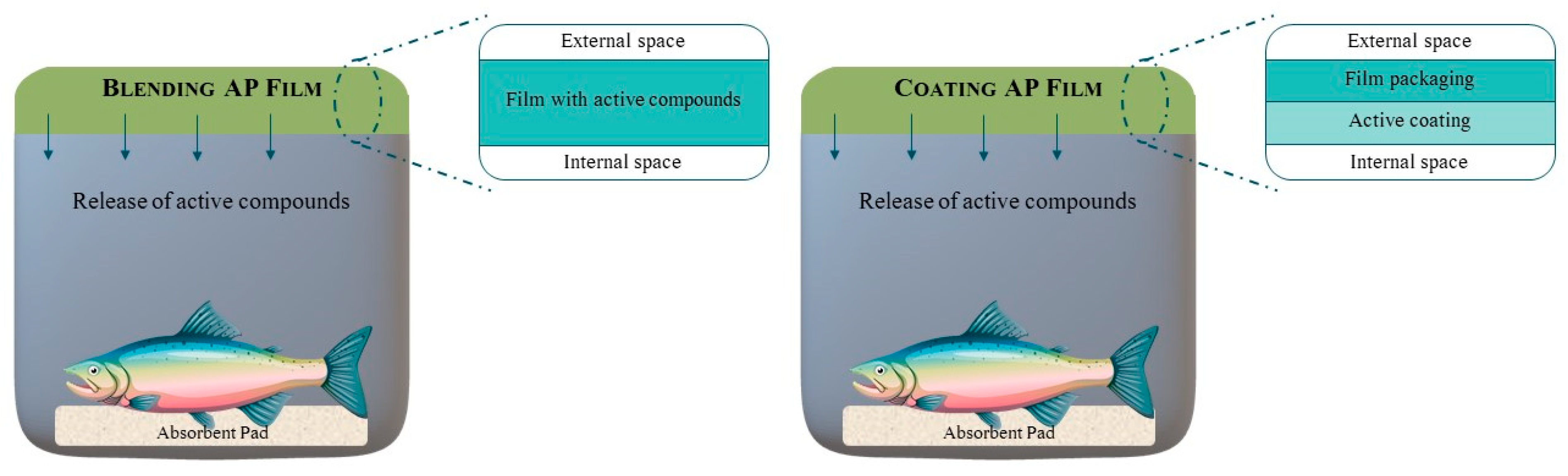
2.2. Active Packaging
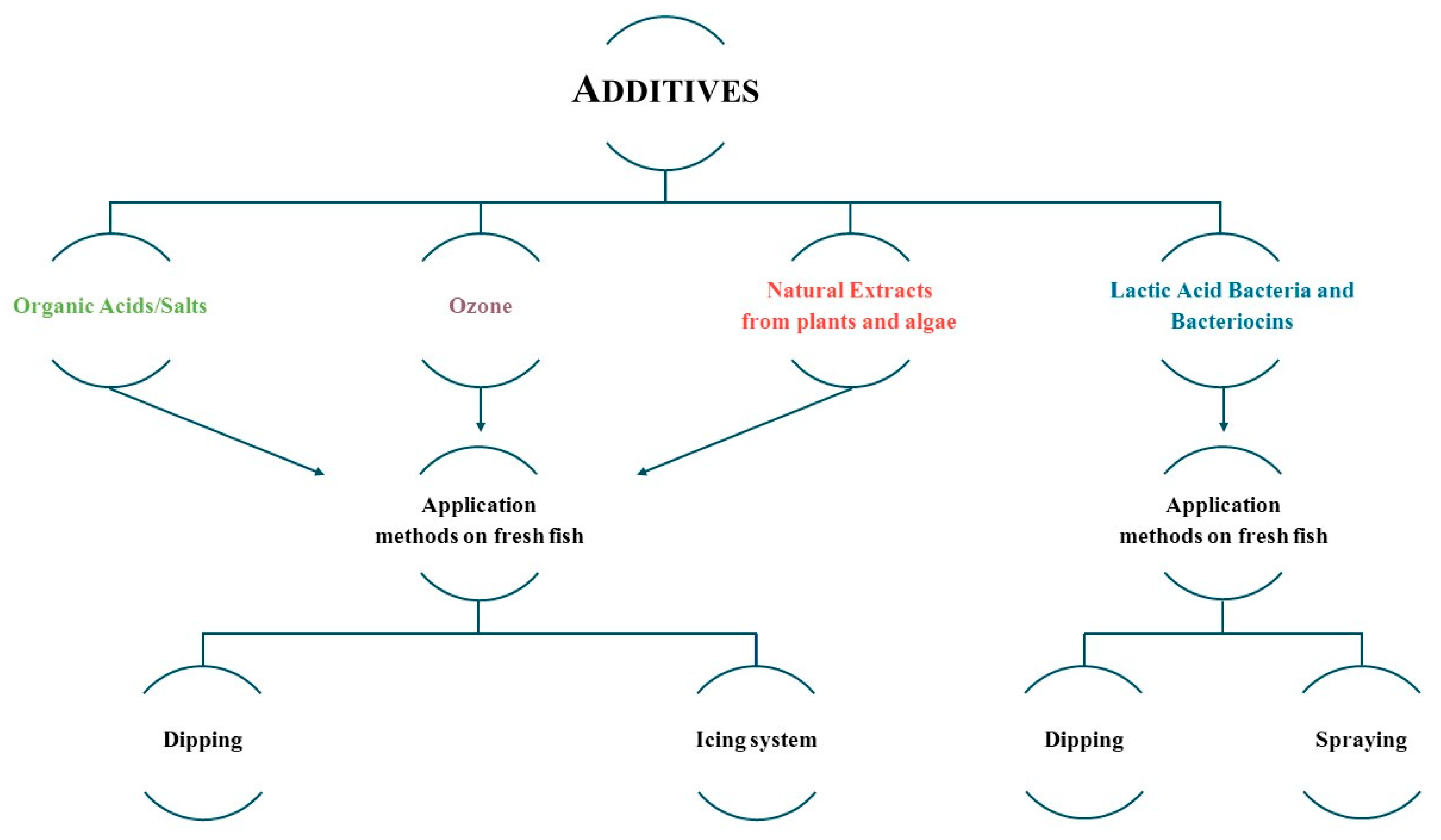
2.3. Chemical Additives
2.3.1. Organic Acids
Fresh Fish Dipping
Fresh Fish Organic Acid-Icing
2.3.2. Ozone
Ozonized Water Dipping
Ozonized Icing-Systems
2.4. Natural Extracts
2.4.1. Extracts from Plants
2.4.2. Extracts from Algae
2.4.3. Latic Acid Bacteria and Bacteriocins
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prabhakar, P.K.; Vatsa, S.; Srivastav, P.P.; Pathak, S.S. A Comprehensive Review on Freshness of Fish and Assessment: Analytical Methods and Recent Innovations. Food Res. Int. 2020, 133, 109157. [Google Scholar] [CrossRef]
- Mei, J.; Ma, X.; Xie, J. Review on Natural Preservatives for Extending Fish Shelf Life. Foods 2019, 8, 490. [Google Scholar] [CrossRef] [Green Version]
- FAO. Contributing to Food Security and Nutrition for All; The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2016; ISBN 978-92-5-109185-2. [Google Scholar]
- Yu, D.; Wu, L.; Regenstein, J.M.; Jiang, Q.; Yang, F.; Xu, Y.; Xia, W. Recent Advances in Quality Retention of Non-Frozen Fish and Fishery Products: A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Wang, L. The Microbial Safety of Fish and Fish Products: Recent Advances in Understanding Its Significance, Contamination Sources, and Control Strategies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 738–786. [Google Scholar] [CrossRef] [PubMed]
- Silbande, A.; Adenet, S.; Chopin, C.; Cornet, J.; Smith-Ravin, J.; Rochefort, K.; Leroi, F. Effect of Vacuum and Modified Atmosphere Packaging on the Microbiological, Chemical and Sensory Properties of Tropical Red Drum (Sciaenops ocellatus) Fillets Stored at 4 °C. Int. J. Food Microbiol. 2018, 266, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Biji, K.B.; Ravishankar, C.N.; Venkateswarlu, R.; Mohan, C.O.; Gopal, T.K.S. Biogenic Amines in Seafood: A Review. J. Food Sci. Technol. 2016, 53, 2210–2218. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Zhu, J.; Wang, Y.; Fu, L.; Xuan, W. Postmortem Changes in Yellow Grouper (Epinephelus awoara) Fillets Stored under Vacuum Packaging at 0 °C. Food Chem. 2011, 126, 896–901. [Google Scholar] [CrossRef]
- Kostaki, M.; Giatrakou, V.; Savvaidis, I.N.; Kontominas, M.G. Combined Effect of MAP and Thyme Essential Oil on the Microbiological, Chemical and Sensory Attributes of Organically Aquacultured Sea Bass (Dicentrarchus labrax) Fillets. Food Microbiol. 2009, 26, 475–482. [Google Scholar] [CrossRef]
- Kykkidou, S.; Giatrakou, V.; Papavergou, A.; Kontominas, M.G.; Savvaidis, I.N. Effect of Thyme Essential Oil and Packaging Treatments on Fresh Mediterranean Swordfish Fillets during Storage at 4 °C. Food Chem. 2009, 115, 169–175. [Google Scholar] [CrossRef]
- Genç, I.Y.; Esteves, E.; Aníbal, J.; Diler, A. Effects of Chilled Storage on Quality of Vacuum Packed Meagre Fillets. J. Food Eng. 2013, 115, 486–494. [Google Scholar] [CrossRef]
- Ježek, F.; Buchtová, H. Physical and Chemical Changes in Fresh Chilled Muscle Tissue of Common Carp (Cyprinus carpio L.) Packed in a Modified Atmosphere. Acta Vet. Brno 2007, 76, S83–S92. [Google Scholar] [CrossRef] [Green Version]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An Overview of the Intelligent Packaging Technologies in the Food Sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and Intelligent Biodegradable Packaging Films Using Food and Food Waste-Derived Bioactive Compounds: A Review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Brodowska, A.J.; Nowak, A.; Śmigielski, K. Ozone in the Food Industry: Principles of Ozone Treatment, Mechanisms of Action, and Applications: An Overview. Crit. Rev. Food Sci. Nutr. 2018, 58, 2176–2201. [Google Scholar] [CrossRef] [PubMed]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Srinivasa Gopal, T.K. Smart Packaging Systems for Food Applications: A Review. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Tsironi, T.N.; Taoukis, P.S. Current Practice and Innovations in Fish Packaging. J. Aquat. Food Prod. Technol. 2018, 27, 1024–1047. [Google Scholar] [CrossRef]
- Sethi, S.; Nayak, S.L.; Joshi, A.; Sharma, R.R. 5—Sanitizers for fresh-cut fruits and vegetables. In Fresh-Cut Fruits and Vegetables; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 99–119. ISBN 978-0-12-816184-5. [Google Scholar]
- Fletcher, G.C. Advances in vacuum and modified atmosphere packaging of fish and crustaceans. In Advances in Meat, Poultry and Seafood Packaging; Woodhead Publishing: Swaston, UK, 2012; pp. 261–297. ISBN 9781845697518. [Google Scholar]
- Kachele, R.; Zhang, M.; Gao, Z.; Adhikari, B. Effect of Vacuum Packaging on the Shelf-Life of Silver Carp (Hypophthalmichthys molitrix) Fillets Stored at 4 °C. LWT-Food Sci. Technol. 2017, 80, 163–168. [Google Scholar] [CrossRef]
- Rodrigues, B.L.; da Silveira Alvares, T.; Sampaio, G.S.L.; Cabral, C.C.; Araujo, J.V.A.; Franco, R.M.; Mano, S.B.; Conte Junior, C.A. Influence of Vacuum and Modified Atmosphere Packaging in Combination with UV-C Radiation on the Shelf Life of Rainbow Trout (Oncorhynchus mykiss) Fillets. Food Control 2016, 60, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Bakar, A. Effect of Modified Atmosphere Packaging on Microbial Flora Changes in Fishery Products. Int. Food Res. J. 2013, 20, 17–26. [Google Scholar]
- Sáez, M.I.; Martínez, T.F.; Cárdenas, S.; Suárez, M.D. Effects of Different Preservation Strategies on Microbiological Counts, Lipid Oxidation and Color of Cultured Meagre (Argyrosomus regius, L.) Fillets. J. Food Process. Preserv. 2015, 39, 768–775. [Google Scholar] [CrossRef]
- Lerfall, J.; Bjørge Thomassen, G.M.; Jakobsen, A.N. Quality of Fresh Saithe (Pollachius virens) in Modified Atmosphere Packages as Affected by the Gas Composition. Food Packag. Shelf Life 2018, 18, 147–156. [Google Scholar] [CrossRef]
- Noseda, B.; Islam, M.T.; Eriksson, M.; Heyndrickx, M.; De Reu, K.; Van Langenhove, H.; Devlieghere, F. Microbiological Spoilage of Vacuum and Modified Atmosphere Packaged Vietnamese Pangasius Hypophthalmus Fillets. Food Microbiol. 2012, 30, 408–419. [Google Scholar] [CrossRef]
- Hansen, A.Å.; Moen, B.; Rødbotten, M.; Berget, I.; Pettersen, M.K. Effect of Vacuum or Modified Atmosphere Packaging (MAP) in Combination with a CO2 Emitter on Quality Parameters of Cod Loins (Gadus morhua). Food Packag. Shelf Life 2016, 9, 29–37. [Google Scholar] [CrossRef]
- Babic, J.; Milijasevic, M.; Vranic, D.; Veskovic-Moracanin, S.; Djinovic-Stojanovic, J. Effect of Modified Atmosphere Pakaging on the Shelf-Life of Common Carp (Cyprinus carpio) Steaks. Procedia Food Sci. 2015, 5, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Stamatis, N.; Arkoudelos, J. Quality Assessment of Scomber Colias Japonicus under Modified Atmosphere and Vacuum Packaging. Food Control 2007, 18, 292–300. [Google Scholar] [CrossRef]
- Hansen, A.Å.; Mørkøre, T.; Rudi, K.; Rødbotten, M.; Bjerke, F.; Eie, T. Quality Changes of Prerigor Filleted Atlantic Salmon (Salmo salar L.) Packaged in Modified Atmosphere Using CO2 Emitter, Traditional MAP, and Vacuum. J. Food Sci. 2009, 74, M242–M249. [Google Scholar] [CrossRef]
- Mbarki, R.; Ben Miloud, N.; Selmi, S.; Dhib, S.; Sadok, S. Effect of Vacuum Packaging and Low-Dose Irradiation on the Microbial, Chemical and Sensory Characteristics of Chub Mackerel (Scomber japonicus). Food Microbiol. 2009, 26, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Milijasevic, M.; Babic, J.; Veskovic-Moracanin, S. Effect of Vacuum and Modified Atmosphere on Enterobacteriaceae Count Determined in Rainbow Trout (Oncorhynchus mykiss) and Carp (Cyprinus carpio) Steaks. Procedia Food Sci. 2015, 5, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, Y.; Liu, X.; Lei, Y.; Regenstein, J.M.; Luo, Y. Characterization of the Microbial Composition and Quality of Lightly Salted Grass Carp (Ctenopharyngodon idellus) Fillets with Vacuum or Modified Atmosphere Packaging. Int. J. Food Microbiol. 2019, 293, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Stamatis, N.; Arkoudelos, J.S. Effect of Modified Atmosphere and Vacuum Packaging on Microbial, Chemical and Sensory Quality Indicators of Fresh, FilletedSardina Pilchardus at 3 °C. J. Sci. Food Agric. 2007, 87, 1164–1171. [Google Scholar] [CrossRef]
- Silbande, A.; Adenet, S.; Smith-Ravin, J.; Joffraud, J.J.; Rochefort, K.; Leroi, F. Quality Assessment of Ice-Stored Tropical Yellowfin Tuna (Thunnus albacares) and Influence of Vacuum and Modified Atmosphere Packaging. Food Microbiol. 2016, 60, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, A.; Abraha, B.; Samuel, M.; Abraham, W.; Mahmud, E. Fish Preservation: A Multi-Dimensional Approach. MOJ Food Process. Technol. 2018, 6, 303–310. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No. 450/2009 of 29 May 2009 on Active and Intelligent Materials and Articles Intended to Come into Contact with Food. Off. J. Eur. Union 2009, L135, 3–11. [Google Scholar]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and Active Films and Coatings as Carriers of Natural Antioxidants for Lipid Food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in Antioxidant Active Food Packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Lee, K.T. Quality and Safety Aspects of Meat Products as Affected by Various Physical Manipulations of Packaging Materials. Meat Sci. 2010, 86, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Remya, S.; Mohan, C.O.; Venkateshwarlu, G.; Sivaraman, G.K.; Ravishankar, C.N. Combined Effect of O2 Scavenger and Antimicrobial Film on Shelf Life of Fresh Cobia (Rachycentron canadum) Fish Steaks Stored at 2 °C. Food Control 2017, 71, 71–78. [Google Scholar] [CrossRef]
- Noseda, B.; Vermeulen, A.; Ragaert, P.; Devlieghere, F. Packaging of Fish and Fishery Products. In Seafood Processing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 237–261. ISBN 978-1-118-34617-4. [Google Scholar]
- Barbosa-Pereira, L.; Cruz, J.M.; Sendón, R.; Rodríguez Bernaldo de Quirós, A.; Ares, A.; Castro-López, M.; Abad, M.J.; Maroto, J.; Paseiro-Losada, P. Development of Antioxidant Active Films Containing Tocopherols to Extend the Shelf Life of Fish. Food Control 2013, 31, 236–243. [Google Scholar] [CrossRef]
- da Rocha, M.; Alemán, A.; Romani, V.P.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P.; Prentice, C. Effects of Agar Films Incorporated with Fish Protein Hydrolysate or Clove Essential Oil on Flounder (Paralichthys orbignyanus) Fillets Shelf-Life. Food Hydrocoll. 2018, 81, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Kakaei, S.; Shahbazi, Y. Effect of Chitosan-Gelatin Film Incorporated with Ethanolic Red Grape Seed Extract and Ziziphora Clinopodioides Essential Oil on Survival of Listeria Monocytogenes and Chemical, Microbial and Sensory Properties of Minced Trout Fillet. LWT-Food Sci. Technol. 2016, 72, 432–438. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Pereira Santos, J.C.; Camilloto, G.P.; Miranda, A.L.; Druzian, J.I.; Guimarães, A.G. Development of Active Films Poly (Butylene Adipate Co-Terephthalate)—PBAT Incorporated with Oregano Essential Oil and Application in Fish Fillet Preservation. Ind. Crop. Prod. 2017, 108, 388–397. [Google Scholar] [CrossRef]
- López de Lacey, A.M.; López-Caballero, M.E.; Montero, P. Agar Films Containing Green Tea Extract and Probiotic Bacteria for Extending Fish Shelf-Life. LWT-Food Sci. Technol. 2014, 55, 559–564. [Google Scholar] [CrossRef]
- Carissimi, M.; Flôres, S.H.; Rech, R. Effect of Microalgae Addition on Active Biodegradable Starch Film. Algal Res. 2018, 32, 201–209. [Google Scholar] [CrossRef]
- Vázquez, J.A.; González, M.P.; Murado, M.A. Effects of Lactic Acid Bacteria Cultures on Pathogenic Microbiota from Fish. Aquaculture 2005, 245, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Bockisch, M. (Ed.) Chapter 8—Fat as or in Food. In Fats and Oils Handbook; AOCS Press: Urbana, IL, USA, 1998; pp. 719–802. ISBN 978-0-9818936-0-0. [Google Scholar]
- Bushell, F.M.L.; Tonner, P.D.; Jabbari, S.; Schmid, A.K.; Lund, P.A. Synergistic Impacts of Organic Acids and PH on Growth of Pseudomonas Aeruginosa: A Comparison of Parametric and Bayesian Non-Parametric Methods to Model Growth. Front. Microbiol. 2019, 9, 3196. [Google Scholar] [CrossRef]
- Nazer, A.I.; Kobilinsky, A.; Tholozan, J.-L.; Dubois-Brissonnet, F. Combinations of Food Antimicrobials at Low Levels to Inhibit the Growth of Salmonella Sv. Typhimurium: A Synergistic Effect? Food Microbiol. 2005, 22, 391–398. [Google Scholar] [CrossRef]
- Brul, S.; Coote, P. Preservative Agents in Foods: Mode of Action and Microbial Resistance Mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17. [Google Scholar] [CrossRef]
- Pandey, R.; Vischer, N.O.E.; Smelt, J.P.P.M.; van Beilen, J.W.A.; Ter Beek, A.; De Vos, W.H.; Brul, S.; Manders, E.M.M. Intracellular PH Response to Weak Acid Stress in Individual Vegetative Bacillus Subtilis Cells. Appl. Environ. Microbiol. 2016, 82, 6463–6471. [Google Scholar] [CrossRef] [Green Version]
- Hazan, R.; Levine, A.; Abeliovich, H. Benzoic Acid, a Weak Organic Acid Food Preservative, Exerts Specific Effects on Intracellular Membrane Trafficking Pathways in Saccharomyces Cerevisiae. Appl. Environ. Microbiol. 2004, 70, 4449–4457. [Google Scholar] [CrossRef] [Green Version]
- Martinez, A.; Gilderb, A. Autolytic Degradation of Belly Tissue in Anchovy (Engraulis encrasicholus). Int. J. Food Sci. Technol. 2007, 23, 185–194. [Google Scholar] [CrossRef]
- Kim, C.R.; Hearnsberger, J.O.; Eun, J.B. Gram-Negative Bacteria in Refrigerated Catfish Fillets Treated with Lactic Culture and Lactic Acid. J. Food Prot. 1995, 58, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Manju, S.; Mohan, C.O.; Mallick, A.K.; Ravishankar, C.N.; Gopal, T.K.S. Influence of Vacuum Packaging and Organic Acid Treatment on the Chilled Shelf Life of Pearl Spot (Etroplus suratensis, Bloch 1790). J. Food Qual. 2008, 31, 347–365. [Google Scholar] [CrossRef]
- Alakomi, H.-L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic Acid Permeabilizes Gram-Negative Bacteria by Disrupting the Outer Membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, G.W. Methods for Preservation and Extension of Shelf Life. Int. J. Food Microbiol. 1996, 33, 51–64. [Google Scholar] [CrossRef]
- Al-Hajj, N.Q.M.; Alzoreky, N.; Al-Zaimy, A.; Wang, H.; Asamet, S.; Noor, Y.; Thabit, R. Destruction of Some Food Poisoning Bacteria and Shelf-Life Extension of Seafood. JAIR 2014, 3, 10–15. [Google Scholar]
- Rossi, B.; Esteban, M.A.; García-Beltran, J.M.; Giovagnoni, G.; Cuesta, A.; Piva, A.; Grilli, E. Antimicrobial Power of Organic Acids and Nature-Identical Compounds against Two Vibrio Spp.: An In Vitro Study. Microorganisms 2021, 9, 966. [Google Scholar] [CrossRef]
- Sallam, K.I. Antimicrobial and Antioxidant Effects of Sodium Acetate, Sodium Lactate, and Sodium Citrate in Refrigerated Sliced Salmon. Food Control 2007, 18, 566–575. [Google Scholar] [CrossRef] [Green Version]
- El-Shemy, M.G.Y.; Hanafi, E.K.N.; Gadallah, M.G.E.; Yasin, N.M.N. Effect of Organic Acids Pretreatments on Physicochemical Properties and Shelf Life of Refrigerated Bolti Fish: Tilapia Nilotica. J. Agric. Veterninary Sci. 2015, 8, 57–68. [Google Scholar] [CrossRef]
- El-Shemy, M.G.Y.; Yasin, N.M.N.; Gadallah, M.G.E. Microbiological Quality and Enzymes Activity of Refrigerated Bolti Fish (Tilapia nilotica) Pretreated with Organic Acids. J. Agric. Veterninary Sci. 2016, 9, 55–70. [Google Scholar] [CrossRef]
- Elnimr, T. Evaluation of Some Heavy Metals in Pangasius Hypothalmus and Tilapia Nilotica and the Role of Acetic Acid in Lowering Their Levels. Int. J. Fish. Aquac. 2011, 3, 151–157. [Google Scholar]
- Dhimmer, H.; Vala, S.R.; Lende, S.R.; Jora, K.; Vagh, S.N.; Mevada, J.; Fofandi, D.C.; Dhimmer, S. Quality Attributes and Shelf Life Assessment of Black Pomfret (Formio niger) Steaks Treated with Salts of Organic Acids. J. Entomol. Zool. Stud. 2020, 8, 69–72. [Google Scholar] [CrossRef]
- Ingham, S.C. Lactic Acid Dipping for Inhibiting Microbial Spoilage of Refrigerated Catfish Fillet Pieces. J. Food Qual. 1989, 12, 433–443. [Google Scholar] [CrossRef]
- Bal’a, M.F.A.; Marshall, D.L. Organic Acid Dipping of Catfish Fillets: Effect on Color, Microbial Load, and Listeria Monocytogenes. J. Food Prot. 1998, 61, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
- Metin, S.; Erkan, N.; Varlik, C.; Aran, N. Extension of Shelf-Life of Chub Mackerel (Scomber japonicus Houttuyn 1780) Treated with Lactic Acid. Eur. Food Res. Technol. 2001, 213, 174–177. [Google Scholar] [CrossRef]
- Gerges, T.; Selim, A.; Osman, M. Improvement the Shelf Life of Tilapia Fillets Stored at Chilling Condition. Benha Vet. Med J. 2016, 31, 45–55. [Google Scholar] [CrossRef]
- Sallam, K.I. Chemical, Sensory and Shelf Life Evaluation of Sliced Salmon Treated with Salts of Organic Acids. Food Chem. 2007, 101, 2–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gogus, U.; Bozoglu, F.; Yurdugul, S. Comparative Effects of Lactic Acid, Nisin, Coating Combined and Alone Applications on Some Postmortem Quality Criteria of Refrigerated Sardina Pilchardus. J. Food Qual. 2006, 29, 658–671. [Google Scholar] [CrossRef]
- Mohan, C.O.; Ravishankar, C.N.; Ashok Kumar, K.; Srinivasa Gopal, T.K. Quality and Shelf Life of Sodium-Acetate-Treated Seer Fish (Scomberomorus commerson) Steaks Packed in EVOH Pouches During Chilled Storage. J. Packag. Technol. Res. 2019, 3, 109–116. [Google Scholar] [CrossRef]
- Monirul, I.; Yang, F.; Niaz, M.; Qixing, J.; Wenshui, X. Effectiveness of Combined Acetic Acid and Ascorbic Acid Spray on Fresh Silver Carp (Hypophthalmichthys molitrix) Fish to Increase Shelf-Life at Refrigerated Temperature. Curr. Res. Nutr. Food Sci. J. 2019, 7, 415–426. [Google Scholar] [CrossRef] [Green Version]
- Rey, M.S.; García-Soto, B.; Fuertes-Gamundi, J.R.; Aubourg, S.; Barros-Velázquez, J. Effect of a Natural Organic Acid-Icing System on the Microbiological Quality of Commercially Relevant Chilled Fish Species. LWT-Food Sci. Technol. 2012, 46, 217–223. [Google Scholar] [CrossRef]
- García-Soto, B.; Sanjuás, M.; Barros-Velázquez, J.; Fuertes-Gamundi, J.R.; Aubourg, S.P. Preservative Effect of an Organic Acid-Icing System on Chilled Fish Lipids. Eur. J. Lipid Sci. Technol. 2011, 113, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Sanjuás-Rey, M.; Gallardo, J.M.; Barros-Velázquez, J.; Aubourg, S.P. Microbial Activity Inhibition in Chilled Mackerel (Scomber scombrus) by Employment of an Organic Acid-Icing System. J. Food Sci. 2012, 77, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, A.A. Ozone as a Safe and Environmentally Friendly Tool for the Seafood Industry. J. Aquat. Food Prod. Technol. 2016, 25, 210–229. [Google Scholar] [CrossRef]
- de Mendonça Silva, A.M.; Gonçalves, A.A. Effect of Aqueous Ozone on Microbial and Physicochemical Quality of Nile Tilapia Processing. J. Food Process. Preserv. 2017, 41, e13298. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; de Alba, M.; Sun, D.-W.; Tiwari, B. Principles and Recent Applications of Novel Non-Thermal Processing Technologies for the Fish Industry—A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Ben-Gigirey, B.; Barros-Velázquez, J.; Price, R.J.; An, H. Histamine and Biogenic Amine Production by Morganella Morganii Isolated from Temperature-Abused Albacore. J. Food Prot. 2000, 63, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Aponte, M.; Anastasio, A.; Marrone, R.; Mercogliano, R.; Peruzy, M.F.; Murru, N. Impact of Gaseous Ozone Coupled to Passive Refrigeration System to Maximize Shelf-Life and Quality of Four Different Fresh Fish Products. LWT 2018, 93, 412–419. [Google Scholar] [CrossRef]
- Nerantzaki, A.; Tsiotsias, A.; Paleologos, E.K.; Savvaidis, I.N.; Bezirtzoglou, E.; Kontominas, M.G. Effects of Ozonation on Microbiological, Chemical and Sensory Attributes of Vacuum-Packaged Rainbow Trout Stored at 4 ± 0.5 °C. Eur. Food Res. Technol. 2005, 221, 675–683. [Google Scholar] [CrossRef]
- Bono, G.; Badalucco, C. Combining Ozone and Modified Atmosphere Packaging (MAP) to Maximize Shelf-Life and Quality of Striped Red Mullet (Mullus surmuletus). LWT 2012, 47, 500–504. [Google Scholar] [CrossRef]
- Crowe, K.M.; Skonberg, D.; Bushway, A.; Baxter, S. Application of Ozone Sprays as a Strategy to Improve the Microbial Safety and Quality of Salmon Fillets. Food Control 2012, 25, 464–468. [Google Scholar] [CrossRef]
- Gelman, A.; Sachs, O.; Khanin, Y.; Drabkin, V.; Glatman, L. Effect of Ozone Pretreatment on Fish Storage Life at Low Temperatures. J. Food Prot. 2005, 68, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, F.R.; Nickerson, J.T.R. Studies on Salting and Drying Fish. I. Equilibrium Considerations in Salting. J. Food Sci. 1967, 32, 173–179. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, Y.; Li, Z.; Wang, Y.; Yang, W.; Xue, C. Effects of Ozone on the Removal of Geosmin and the Physicochemical Properties of Fish Meat from Bighead Carp (Hypophthalmichthys nobilis). Innov. Food Sci. Emerg. Technol. 2016, 34, 16–23. [Google Scholar] [CrossRef]
- Campos, C.A.; Losada, V.; Rodríguez, Ó.; Aubourg, S.P.; Barros-Velázquez, J. Evaluation of an Ozone-Slurry Ice Combined Refrigeration System for the Storage of Farmed Turbot (Psetta maxima). Food Chem. 2006, 97, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Aubourg, S.P.; Losada, V.; Gallardo, J.M.; Miranda, J.M.; Barros-Velázquez, J. On-Board Quality Preservation of Megrim (Lepidorhombus whiffiagonis) by a Novel Ozonised-Slurry Ice System. Eur. Food Res. Technol. 2006, 223, 232–237. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Huang, J.; Deng, S.; Huang, Y. Combining Ozone and Slurry Ice to Maximize Shelf-Life and Quality of Bighead Croaker (Collichthys niveatus). J. Food Sci. Technol. 2016, 53, 3651–3660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gyawali, R.; Ibrahim, S.A. Natural Products as Antimicrobial Agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural Food Additives: Quo Vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef] [Green Version]
- Wright, M.H.; Shalom, J.; Matthews, B.; Greene, A.C.; Cock, I.E. Terminalia Ferdinandiana Exell: Extracts Inhibit Shewanella Spp. Growth and Prevent Fish Spoilage. Food Microbiol. 2019, 78, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Li, J.; Hu, W.; Zhang, X.; Li, X.; Zhao, J. Shelf-Life Extension of Crucian Carp (Carassius auratus) Using Natural Preservatives during Chilled Storage. Food Chem. 2012, 135, 140–145. [Google Scholar] [CrossRef]
- Jadhav, R.; Anal, A.K. Experimental Investigation on Biochemical, Microbial and Sensory Properties of Nile Tilapia (Oreochromis niloticus) Treated with Moringa (Moringa oleifera) Leaves Powder. J. Food Sci. Technol. 2018, 55, 3647–3656. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Feng, L.; Jiang, T.; Zhu, J.; Fu, L.; Yuan, D.; Li, J. The Use of Rosemary Extract in Combination with Nisin to Extend the Shelf Life of Pompano (Trachinotus ovatus) Fillet during Chilled Storage. Food Control 2014, 37, 1–8. [Google Scholar] [CrossRef]
- Raeisi, S.; Quek, S.Y.; Ojagh, S.M.; Alishahi, A.R. Effects of Cumin (C Uminum cyminum L.) Seed and Wild Mint (M Entha longifolia L.) Leaf Extracts on the Shelf Life and Quality of Rainbow Trout (O Ncorhynchus mykiss) Fillets Stored at 4C ± 1. J. Food Saf. 2016, 36, 271–281. [Google Scholar] [CrossRef]
- Anon Effect of Some Natural Extracts On Shelf Life of Chilled Lucioperca Lucioperca Fillets. In Proceedings of the 5th International Conference on Food, Agricultural and Biological Sciences, Bangkok, Thailand, 25–26 December 2016.
- Yazgan, H.; Burgut, A.; Durmus, M.; Kosker, A.R. The Impacts of Water and Ethanolic Extracts of Propolis on Vacuum Packaged Sardine Fillets Inoculated with Morganella Psychrotolerans during Chilly Storage. J. Food Saf. 2020, 40, e12767. [Google Scholar] [CrossRef]
- Cottica, S.M.; Sawaya, A.C.H.F.; Eberlin, M.N.; Franco, S.L.; Zeoula, L.M.; Visentainer, J.V. Antioxidant Activity and Composition of Propolis Obtained by Different Methods of Extraction. J. Braz. Chem. Soc. 2011, 22, 929–935. [Google Scholar] [CrossRef]
- Houicher, A.; Kuley, E.; Özogul, F.; Bendeddouche, B. Effect of Natural Extracts (Mentha spicata L. and Artemisia campestris) on Biogenic Amine Formation of Sardine Vacuum-Packed and Refrigerated (Sardina pilchardus) Fillets. J. Food Process. Preserv. 2015, 39, 2393–2403. [Google Scholar] [CrossRef]
- Miranda, J.M.; Carrera, M.; Pastén, A.; Vega-Gálvez, A.; Barros-Velázquez, J.; Aubourg, S.P. The Impact of Quinoa (Chenopodium quinoa Willd.) Ethanolic Extracts in the Icing Medium on Quality Loss of Atlantic Chub Mackerel (Scomber colias) under Chilling Storage. Eur. J. Lipid Sci. Technol. 2018, 120, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Speranza, B.; Bevilacqua, A.; Conte, A.; Del Nobile, M.A.; Sinigaglia, M.; Corbo, M.R. Use of Desirability Approach to Predict the Inhibition of Pseudomonas Fluorescens, Shewanella Putrefaciens and Photobacterium Phosphoreum in Fish Fillets Through Natural Antimicrobials and Modified Atmosphere Packaging. Food Bioprocess Technol. 2013, 6, 2319–2330. [Google Scholar] [CrossRef]
- Ozogul, Y.; Ayas, D.; Yazgan, H.; Ozogul, F.; Boga, E.K.; Ozyurt, G. The Capability of Rosemary Extract in Preventing Oxidation of Fish Lipid. Int. J. Food Sci. Technol. 2010, 45, 1717–1723. [Google Scholar] [CrossRef]
- Sun Pan, B. Analysis of EPA and DHA and Distinction between Fish Oils and Concentrates. Lipid Technol. 2012, 24, 178–180. [Google Scholar] [CrossRef]
- Messina, C.M.; Bono, G.; Renda, G.; La Barbera, L.; Santulli, A. Effect of Natural Antioxidants and Modified Atmosphere Packaging in Preventing Lipid Oxidation and Increasing the Shelf-Life of Common Dolphinfish (Coryphaena hippurus) Fillets. LWT-Food Sci. Technol. 2015, 62, 271–277. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Retardation of Quality Changes of Pacific White Shrimp by Green Tea Extract Treatment and Modified Atmosphere Packaging during Refrigerated Storage. Int. J. Food Microbiol. 2011, 149, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Shinde, P.A.; Patange, S.B. Quality of Indian Mackerel as Affected by Pomegranate Peel and Tea Leaf Extracts during Ice Storage. SAARC J. Agric. 2015, 13, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, Z.; Bach, S.J.; McAllister, T.A. Sensitivity of Escherichia Coli to Seaweed (Ascophyllum nodosum) Phlorotannins and Terrestrial Tannins. Asian-Australas. J. Anim. Sci. 2009, 22, 238–245. [Google Scholar] [CrossRef]
- Amorim, R.D.N.D.S.; Rodrigues, J.A.G.; Holanda, M.L.; Quinderé, A.L.G.; de Paula, R.C.M.; Melo, V.M.M.; Benevides, N.M.B. Antimicrobial Effect of a Crude Sulfated Polysaccharide from the Red Seaweed Gracilaria Ornata. Braz. Arch. Biol. Technol. 2012, 55, 171–181. [Google Scholar] [CrossRef]
- Oucif, H.; Miranda, J.M.; Mehidi, S.A.; Abi-Ayad, S.M.E.A.; Barros-Velázquez, J.; Aubourg, S.P. Effectiveness of a Combined Ethanol–Aqueous Extract of Alga Cystoseira Compressa for the Quality Enhancement of a Chilled Fatty Fish Species. Eur. Food Res. Technol. 2018, 244, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, I.S.; Shirahigue, L.D.; Ferraz De Arruda Sucasas, L.; Anbe, L.; Da Cruz, P.G.; Gallo, C.R.; Carpes, S.T.; Marques, M.J.; Oetterer, M. Shelf Life and Quality Study of Minced Tilapia with Nori and Hijiki Seaweeds as Natural Additives. Sci. World J. 2014, 2014, 485287. [Google Scholar] [CrossRef] [Green Version]
- Yarnpakdee, S.; Benjakul, S.; Senphan, T. Antioxidant Activity of the Extracts from Freshwater Macroalgae (Cladophora glomerata) Grown in Northern Thailand and Its Preventive Effect against Lipid Oxidation of Refrigerated Eastern Little Tuna Slice. Turk. J. Fish. Aquat. Sci. 2019, 19, 209–219. [Google Scholar] [CrossRef]
- Kirkholt, E.M.; Dikiy, A.; Shumilina, E. Changes in the Composition of Atlantic Salmon upon the Brown Seaweed (Saccharina latissima) Treatment. Foods 2019, 8, 625. [Google Scholar] [CrossRef] [Green Version]
- Huss, H.H.; Jeppesen, V.F.; Johansen, C.; Gram, L. Biopreservation of Fish Products—A Review of Recent Approaches and Results. J. Aquat. Food Prod. Technol. 1995, 4, 5–26. [Google Scholar] [CrossRef]
- Johnson, E.M.; Jung, D.Y.G.; Jin, D.Y.Y.; Jayabalan, D.R.; Yang, D.S.H.; Suh, J.W. Bacteriocins as Food Preservatives: Challenges and Emerging Horizons. Crit. Rev. Food Sci. Nutr. 2018, 58, 2743–2767. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Liu, Q.; Chen, S.; Yang, X.; Li, L. Application of Lactic Acid Bacteria (LAB) in Freshness Keeping of Tilapia Fillets as Sashimi. J. Ocean Univ. China 2015, 14, 675–680. [Google Scholar] [CrossRef]
- Anacarso, I.; Messi, P.; Condò, C.; Iseppi, R.; Bondi, M.; Sabia, C.; de Niederhäusern, S. A Bacteriocin-like Substance Produced from Lactobacillus Pentosus 39 Is a Natural Antagonist for the Control of Aeromonas Hydrophila and Listeria Monocytogenes in Fresh Salmon Fillets. LWT-Food Sci. Technol. 2014, 55, 604–611. [Google Scholar] [CrossRef]
- Sarika, A.R.; Lipton, A.P.; Aishwarya, M.S.; Dhivya, R.S. Efficacy of Bacteriocin of Enterococcus Faecalis CD1 as a Biopreservative for High Value Marine Fish Reef Cod (Epinephelus diacanthus) under Different Storage Conditions. J. Microbiol. Biotechnol. Res. 2011, 1, 18–24. [Google Scholar]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a New Generation of Antimicrobials: Toxicity Aspects and Regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef] [PubMed]
- Laulund, S.; Wind, A.; Derkx, P.M.F.; Zuliani, V. Regulatory and Safety Requirements for Food Cultures. Microorganisms 2017, 5, 28. [Google Scholar] [CrossRef] [Green Version]
| Species (Scientific Name) | Storage Conditions (Temperature, Duration) | Major Results | Reference |
|---|---|---|---|
| Atlantic Salmon (Salmo salar) | 1.2 °C, 25 days Packaging: MAP (60% CO2 and 40% N2) and VP | MAP (15/18 days) extended the shelf-life 7 days compared to VP samples (8/11 days). Negative odors and liquid losses were detected earlier for VP. Lower firmness and higher color intensity in MAP samples. | [29] |
| Chub mackerel (Scomber colias japonicus) | 3 °C and 6 °C, 15 days Packaging: MAP (50% CO2 and 50% N2), air and VP | Lower pH values on MAP and VP samples. Longer shelf-life in MAP (12–10 days), followed by VP (10–8 days) and air (8–7 days) at 3 and 6 °C, respectively; Faster growth and higher microbial load for air samples. | [28] |
| Chub mackerel (Scomber japonicus) | 1 °C, 14 days Packaging: air and VP | VP reduced TMA content but was ineffective to reduce biogenic amine contents. | [30] |
| Cod (Gadus morhua) | 2 °C, 15 days Packaging: MAP (60% CO2 and 40% N2) and VP | Shelf-life of 7 days for VP and 9 days for MAP samples. | [26] |
| Common carp (Cyprinus carpio) | 3 °C, 15 days Packaging: MAP (1: 40% CO2 and 60% N2 2: 100% CO2) | MAP2 slowed total viable microorganisms’ growth, presented significant lower counts of Enterobacteriaceae, lower pH value and no sensory changes were detected throughout the storage period. Higher values of TVB-N were observed for MAP1. | [27] |
| Common carp (Cyprinus carpio) and rainbow trout (Oncorhynchus mykiss) | 3 °C, 14 days Packaging: MAP (1: 60% CO2 and 40% N2; 2: 40% CO2 and 60% N2) and VP | Higher counts of Enterobacteriaceae for VP samples, followed by MAP2 and finally MAP1 in both fish species. | [31] |
| Grass Carp (Ctenopharyngodon idellus) | 4 °C, 8 days (air), 16 days (VP) or 24 days (MAP) Packaging: MAP (75% CO2 and 25% N2), air and VP | Doubled and tripled sensorial shelf-life for VP (16 days) and MAP (24 days), respectively. Lower pH and improved sensorial parameters for VP and MAP. Significantly higher TVB-N for air samples in the first 8 days. Higher levels of tyramine for VP and MAP, and putrescine and cadaverine for air samples. | [32] |
| Meagre (Argyrosomus regius) | 4 °C, 8 days (air) or 13 days (VP) Packaging: air and VP | Increased shelf life by approx. 4 days for VP (~10 days). Lightness and hardness increased over time regardless of the type of packaging. Significantly less microbial growth on VP samples. | [11] |
| 4 °C, 15 days Packaging: MAP (40% CO2, 30% N2 and 30% O2), air and VP | Microbial loads were significantly lower under VP. Color was not affected by any packaging method. | [23] | |
| Rainbow trout (Oncorhynchus mykiss) | 4 °C, 22 days Packaging: MAP (80% CO2 and 20% N2), air and VP | MAP reduced total production of ammonia, TVB-N and cadaverine. MAP enhanced the shelf-life at least twice since total mesophilic count and psychotropic microorganisms reached the upper limit of 7 log CFU g−1 on the 11th day (air—5th day; VP—7th day). | [21] |
| Red Drum (Sciaenops ocellatus) | 4 °C, 29 days Packaging: MAP (50% CO2 and 50% N2) and VP | Putrescine and cadaverine were the prevalent amines and had higher counts on VP. At the end of the storage VP samples retained a slightly better appearance than MAP samples, with a firmer texture but had a stronger odor. | [6] |
| Saithe (Pollachius virens) | 4 °C, 13 days Packaging: MAP ● high CO2/low N2: 67.2 ± 0.2% CO2, 32.8 ± 0.2% N2 and 0.0 ± 0.0% O2 ● low CO2/high N2: 31.8 ± 0.2% CO2, 68.2 ± 0.3% N2 and 0.1 ± 0.1% O2 ● high CO2/low O2: 66.4 ± 0.4% CO2, 32.2 ± 0.0% O2 and 1.3 ± 0.3% N2 ● low CO2/high O2: 31.3 ± 0.2% CO2, 66.0 ± 0.1% O2 and 2.7 ± 0.3% N2 ● and VP | All MAP conditions had the same shelf-life of 13 days, 3 days longer than VP samples (10 days). Lower muscle pH was observed in packages balanced with O2 compared to those balanced with N2. Differences were found between off-odors produced in MAP with mix of CO2 and O2 (butter-like) and a mix with CO2 and N2 (ammonium-like). Drip loss was higher in “high CO2/low N2” MAP. Higher cadaverine formation in packages balanced with N2. | [24] |
| Sardine (Sardina pilchardus) | 3 °C, 15 days Packaging: MAP (50% CO2 and 50% N2) and air | Longer shelf-life for MAP (9 days), followed by VP (7 days) and air (5 days). Higher concentration of ammonia and a significant increase of pH in air samples. | [33] |
| Sea bass (Dicentrarchus labrax) | 4 °C, 21 days Packaging: MAP (1: 40% CO2, 50% N2 and 10% O2 2: 60% CO2, 30% N2 and 10% O2) and air | MAP1 extended shelf-life by 3 days (8/9 days) while MAP2 extended it by 7/8 days (13 days) based on sensory analysis. Lower TVB-N and TMA-N values for MAP2 samples. | [9] |
| Silver carp (Hypophthalmichthys molitrix) | 4 °C, 14 days Packaging: air and VP (30-50 kPa) | Significant decrease of microbial growth with VP; Lower pH and TVB-N for VP samples; Better sensory quality and increased shelf-life (by 3 days) for 30 kPa VP samples. | [20] |
| Sutchi catfish (Pangasius hypophthalmus) | 4 °C, 21 days Packaging: MAP (1: 50% CO2 and 50% N2; 2: 50% CO2 and 50% O2), air and VP | MAP with O2 significantly extended the lag phase compared to the MAP without O2. Shelf-life was extended by 3, 5, and 7 days with VP (10 days), MAP 1 (12 days) and MAP2 (14 days), respectively, in comparison to air samples. | [25] |
| Swordfisfh (Xiphias gladius) | 4 °C, 18 days Packaging: MAP (50% CO2, 45% N2 and 5% O2) and air | Microbial and sensorial shelf-life extension by 5/6 days on MAP samples (12/13 days) compared to air samples (6/8 days). Lower values of TMA-N for MAP samples. | [10] |
| Tropical yellowfin tuna (Thunnus albacares) | 0 °C for air, 4 °C in the first week then 8 °C for VP and MAP, 13 days Packaging: MAP (70% CO2 and 30% O2), air and VP | No extension of shelf-life was provided by VP and MAP (13 days). Similar bacterial evolution. Very low levels of TVB-N and no differences between treatments. TMA-N increased for MAP and VP samples but not for air samples. VP and MAP presented a slight discoloration and MAP samples were less firm. | [34] |
| Yellow grouper (Epinephelus awoara) | 0 °C, 15 days Packaging: VP | TVB-N and TMA-N significantly increased over time. Significant variations for hardness, gumminess, and chewiness values with storage time. Evolution of color to grey-blue tones and reduction in color intensity and purity. | [8] |
| Fish Specie (Scientific Name) | Polymers | Active Compounds | Main Results | Reference |
|---|---|---|---|---|
| Fish * | Poly (butylene adipate co-terephthalate)—PBAT | Oregano (Origanum vulgare) essential oil (OEO) | A high antioxidant action and antimicrobial effect due to the lower counts of coliforms, Staphylococcus aureus and psychrotrophic microorganisms. | [45] |
| Flounder (Paralichthys orbignyanus) | Agar | Fish protein hydrolysate (PH) or clove essential oil (CEO) | Improvement of the biochemical (TVB-N and pH values, etc.) and microbiological (H2S-producing bacteria, etc.) parameters of chilled flounder fillets and, thus, increasing the shelf-life. | [43] |
| Hake (Merluccius capensis) | Agar | Green tea extract (Camellia sinensis L.) and probiotic bacteria (Lactobacillus paracasei L26 and Bifidobacterium lactis B94) | Green tea films effectively reduced microbial growth and some spoilage indicators such as TVB-N, TMA-N, and pH value, in hake. Probiotic films have been able to extend the shelf-life of hake and transmit some probiotic bacteria to fish. | [46] |
| Rainbow trout (Oncorhynchus mykiss) | Chitosan-gelatin | Ethanolic red grape seed extract (GSE) and Ziziphora clinopodioides essential oil (ZEO) | Reduction of lipid oxidation and bacterial growth, increasing the shelf life of rainbow trout at refrigerated storage. | [44] |
| Salmon (Salmo Solar) | Low Density Polyethylene (LDPE) | Natural tocopherols (commercial names: NUTRABIOL®® T90 and TOCOBIOL®®—PV) | Antioxidant effectiveness, through the reduction/inhibition of the lipid oxidation of salmon during storage period, by up to 40%. | [42] |
| Cassava starch | Extract of microalgae Heterochlorella luteoviridis | A reduction in lipid oxidation and moisture loss. | [47] |
| Specie (Scientific Name) | Organic Acid or Salt (Concentration, Dipping Time) | Storage Donditions | Results | Reference |
|---|---|---|---|---|
| Bigeye trevally (Caranx sexfasciatus) | Lactic, acetic, and citric acids (all at 2%, 30 min) | 5 °C, vacuum packaging, for 7 days | Elimination of pathogenic Escherichia coli and Listeria monocytogenes. Total aerobic mesophiles development slowed down. Minor color changes because of the dipping process. | [60] |
| Black pomfret (Formio niger) | Sodium acetate (2.5%, 5 min) | 4 °C, air packed, for 7 days | Improved moisture retention, tenderness, and higher water holding capacity, lower drip loss, and total TMA-N and TVB-N values in dipped fish samples compared to undipped fish. | [66] |
| Bolti fish (Oreochromis niloticus) | Acetic and citric acid (1 and 3%, respectively, 5 min) and a mixture of both | 4 °C, air packed, for 12 days | Slower microbial proliferation, along with catalase and protease activity decrease in dipped fish, especially when the mixture of both acids was used. | [64] |
| Catifish fillets (*) | Lactic acid (1.70 and 2.55%, 10 min) | 2 and 7 °C, air packed, for 3 and 6 days, respectively | Shelf-life extension up to 6 days for 2.55% dipped samples stored at both temperatures. Sensorial panel did not consistently distinguish dipped and undipped samples. | [67] |
| Lactic acid (2%, 5 min) (3%, 1 and 5 min) | 4 and 10 °C, air packed, for 9 days | Dipped samples presented less than 2 log units of Gram-negative bacteria by the 9th day of storage. Higher concentrations and dipping times slowed down microbial development. | [56] | |
| Catifish fillets (Ictalurus punctatus) | Acetic, citric, hydrochloric, lactic, malic, and tartaric acids (2%, 10 min) | 4 °C, air packed, for 8 days | Reduced microbial proliferation (total aerobic mesophiles, total coliforms, and L. monocytogenes) on acid treated samples. Significant color changes in catfish fillets after dipping (malic acid had the smallest impact on lightness while yellowness was less impacted by hydrochloric acid). | [68] |
| Chub mackerel (Scomber japonicus) | Lactic acid (0, 2 and 4%, 30 min) | 4 °C, vacuum packed, for 12 days | Shelf-life extension of lactic acid dipped fillets. Improved control of TMA-N and TVB-N production. | [69] |
| Nile tilapia (Oreochromis niloticus) | Acetic and citric acid (1 and 3%, respectively, 5 min) and a mixture of both | 4 °C, air packed, for 12 days | Reduction of thiobarbituric acid-reactive substances (TBARS) and TVB-N concentration. Improved WHC and lipid content for fish dipped in citric and acetic acid compared to undipped fish. | [63] |
| Acetic acid (1%, 2 min) | 2 °C, air and modified atmosphere (80% CO2 and 20% N2) packaging, for 21 days | Microbiological shelf-life extension for modified atmosphere packaged dipped fish samples, improvement of TVB-N and TBARS values, and good overall acceptability after 21 days of storage. | [70] | |
| Pearl spot (Etroplus suratensis) | Sodium acetate and potassium sorbate (both at 2%, 30 min) | 1–2 °C, air and vacuum packed, for 18 days | Microbiological and sensorial shelf-life extension of vacuum packaged pearl spot when combined with salts (16 days, compared to 7 days for control samples), with improved sensorial properties and reduced TMA-N and TVB-N compared to untreated samples (without salts). | [57] |
| Salmon (Salmo salar) | Sodium acetate, sodium citrate and sodium lactate (2.5%, 10 min) | 1 °C, air packed, for 15 days | Microbial development inhibited by dipping treatment, with sodium citrate showing the best results. Both lipid oxidation and TBARS values were delayed. | [62] |
| Shelf-life extension of 12 days for sodium lactate and sodium citrate, and 15 days for sodium acetate. Reduction of k-value, hypoxanthine, TVB-N, TMA-N values, and improved sensorial attributes in dipped salmon fillets. | [71] | |||
| Sardine (Sardina pilchardus) | Lactic acid (5%, 2 min) | 4 °C, air packed, for 7 days | Lower total aerobic mesophiles and Pseudomonas spp. counts for lactic-acid dipped fish samples. Improved odor, appearance, and aroma compared to undipped samples. | [72] |
| Seer fish (Scomberomorus commerson) | Sodium acetate (2%, 10 min) | 1–2 °C, packed in air permeable ethylene vinyl alcohol, for 24 days | Shelf-life extension of dipped fish samples (21 days) compared to undipped samples (12 days). Extended lag phase of microbial development in dipped samples. Reduced lipid oxidation and nucleotide breakdown inhibition in dipped fish. | [73] |
| Silver carp (Hypophthalmichthys molitrix) | Acetic and ascorbic acid (1 and 2%, respectively, sprayed) and a mixture of both | 4 °C, air packed, for 9 days | Lower microbial loads, pH, and peroxide values in fish fillets sprayed with the combination of both organic acids, along with improved sensorial characteristics. | [74] |
| Specie (Scientific Name) | Concentration, Dipping Time, Water Temperature | Storage Conditions | Results | Reference |
|---|---|---|---|---|
| Catfish (Ictalurus punctatus) | 5 and 10 mg/L, 10 min, 20 °C | 4 °C, air packed, for 12 days | Total psychrophiles and coliform loads reduction after fish dipping, minor impact on microbial evolution during storage. TBARS values remained unchanged for 12 days. | [81] |
| Cod (Merluccius merluccius) | 3.5 mg/L, 3 cycles of 5 min and 4.7 mg/L, 4 cycles of 10 min, ** | 2 °C (passive refrigeration), air packed, for 12 days | Microbial development inhibition. Lower TVB-N and TMA-N values, along with higher lipid hydrolysis and TBARS values compared with undipped samples. | [82] |
| Nile tilapia (Oreochromis niloticus) | 4.0 mg/L, 30 min, ** | Ice storage (replaced every 24 h), air packed, for 18 days | Microbiological shelf-life extension for ozonated samples, along with lower TVB-N and higher TBARS values. No sensorial differences between unozonized and ozonized samples. | [80] |
| Rainbow trout (Onchorynchous mykiss) | 0.6 and 0.4 mg/mL, for 60 and 90 min respectively), 5 °C | 4 °C, vacuum packed, for 15 days | Microbial development retarded by ozone dipping, although with minor differences between ozone concentrations and dipping time. TVB-N values for dipped samples were considerably lower. | [83] |
| Red mullet (Mullus surmuletus) | 0.3 mg/L, 10 min, 5 °C | 1 °C, MAP (50% CO2 and 50% N2), for 24 days | Lower microbial loads, TVB-N and TMA-N levels compared to unozonized samples under MAP. Similar peroxide values and sensorial acceptability. | [84] |
| Salmon (Salmo salar) | 1 and 1.5 mg/L, 1–3 spray nozzles, ** | 4 °C, air packed, for 10 days | Inhibitory effect against Listeria innocua for 6 days. Lower TBARS and propanal values for ozone-spayed fish, especially at 1.5 mg/L. Number of spray nozzles showed minor impacts on the aforementioned parameters. | [85] |
| Scaldfish (Arnoglossus laterna) | 8 mg/L, 6 cycles of 5 min, ** | 2 °C, air packed, for 12 days | Shelf-life extension of 1 week by microbial development inhibition in ozonated samples. Lower TVB-N lipid hydrolysis, along with higher TMA-N and TBARS values compared to undipped fish. | [82] |
| Tilapia (Oreochromis niloticus x Oreochromis aureus) | 0.1 mg/L, 1 h, 20 °C | 0–5 °C, air packed, for 30 days | Microbiological and sensorial shelf-life extension of ozonized samples compared to control, improved freshness, especially for those kept at 0 °C. Lower TVB-N, TBA, and values for ozonized tilapia muscles along with lower scores for odor and taste. No effects on texture. | [86] |
| Trout * | 0.1 mg/L, 2 h, ** | 5 °C, air packed, for 9 days | Shelf-life extension of ozonized trout from 4 to 6 days compared to control samples, along with lower values of TVB-N and peroxides’ index. No major differences in protein content for ozonized samples compared to controls. | [87] |
| Species (Scientific Name) | Plant or Extract, Concentration, Dipping Time, Temperature | Storage Conditions | Results | Reference |
|---|---|---|---|---|
| Black bream (Acanthopagrus butcheri) | Kakadu plum, 0.05–0.2%, 6 h, ** | 4 °C, air packed, 15 days | Both leaf and fruit extracts slowed down microbial development, especially at higher concentration. | [94] |
| Crucian carp (Carassius auratus) | Rosemary, 0.2%, 2 min, 4 °C | 4 °C, air packed, 20 days | Sensorial shelf-life extension (2-fold) of fish samples dipped in rosemary extract, validated by microbiology results, along with pH, TVB-N, and k-value increase. | [95] |
| Nile tilapia (Oreochromis niloticus) | Moringa, 1–4%, ice incorporation | 5 °C, ***, 12 days | Microbial growth delayed with increasing moringa extract concentration. Lower peroxides, TBARS, and TVB-N values compared to control samples (with no extracts). | [96] |
| Pompano (Trachinotus ovatus) | Rosemary, 0.2%, 30 min, 4 °C | 4 °C, air packed, 15 days | Lower microbial loads and k-values during storage, along with lower TMA-N and TVB-N values compared to control. No effects on TBARS values and peroxide index. | [97] |
| Rainbow trout (Oncorhynchus mykiss) | Cumin and wild mint, 3–6%, 30 min, ** | 4 °C, air packed, 18 days | Wild mint extract provided the best antimicrobial and antioxidant activities, resulting in lower peroxide, TBARS, TVB-N, and TMA-N values, along with the best sensorial scores. | [98] |
| Silver carp (Hypophthalmichthys molitrix) | Few-flowered garlic, 2.0–4-0%, 30 min, ** | 4 °C, air packed, 15 days | Shelf-life extension of fish treated with garlic extracts of up to 15 days. Lipid oxidation rates slowed down. Improved sensorial attributes. | [98] |
| Zander (Lucioperca lucioperca) | Green tea, 1%, 10 min, ** | 4 °C, air packed, 15 days | Lower microbial counts, TVB-N and TBARS values, improved organoleptic scores. Lower content in histamine, cadaverine, and putrescine. | [99] |
| Species (Scientific Name) | Plant Extract, Concentration, Dipping Time, Temperature | Storage Conditions | Results | Reference |
|---|---|---|---|---|
| Sardine (Sardine aurita) | Propolis, 0.4–0.8%, 4 min, * | 3 °C, vacuum packed, 15 days | Higher doses of water and ethanolic extracts improved sardine shelf-life and resulted in lower TVB-N and TBARS. Ethanolic extracts provided the best sensorial scores. | [100] |
| Sardine (Sardinella pilchardus) | Rosemary, 1–2%, 2 min, * | 4 °C, vacuum packed, 20 days | Sensorial analyses scores were better for samples treated with 1% of rosemary extracts, despite the lower TVB-N, peroxide index, and TBARS values for 2% concentration. | [105] |
| Yellow corvina (Larimichthys polyactis) | Bayberry leaf, 0.2%, 1 h, 4 °C | 4 °C, air and vacuum packed, 16 days | Microbial growth slow down. Lower values of TVB-N and TBARS values. Improved sensorial scores. No impact on fish color. | [106] |
| Sea bream (Sparus auratus) | Grapefruit seed extract (GFSE) and tymol and chitosan, 1500–6000 ppm(for GFSE and tymol) (1–4% chitosan), 60 s, * | 4 °C, air and MAP (30:40:30 O2/CO2/N2 and 5:95 O2/CO2), 10 days | Inhibition of Pseudomonas fluorescens required a mininum active solution containing 2% of chitosan and 6000 ppm of GFSE and tymol. The use of MAP consisting of 5:95 O2/CO2, maintained the microbiological quality for up to 10 days, as opposed to undipped samples that spoiled in 3–4 days. | [104] |
| Common dolphin fish fillets (Coryphaena hippurus) | H. strobilaceum, 1%, 2 min, ** | −1 °C and MAP (45% CO2, 50% N2, 5% O2), up to 18 days | Lipid oxidation was retarded and lower peroxide values and malondialdehyde content compared to control groups (placed in trays and not sealed). The use of H. strobilaceum allowed maintainance of the content of n-3 PUFAs | [107] |
| Pacific white shrimps (Litopenaeus vannamei) | Green tea, 0.1%, 15 min, 4 °C | 4 °C and MAP (50% CO2, 5% O2, 45%, N2), up to 10 days | The combination of extracts and MAP allowed for better microbial growth control and lower melanosis formation compared to MAP alone. | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, R.A.; Pinto, C.A.; Lima, V.; Tavares, J.; Martins, A.P.; Fidalgo, L.G.; Silva, A.M.; Gil, M.M.; Teixeira, P.; Barbosa, J.; et al. Chemical-Based Methodologies to Extend the Shelf Life of Fresh Fish—A Review. Foods 2021, 10, 2300. https://doi.org/10.3390/foods10102300
Amaral RA, Pinto CA, Lima V, Tavares J, Martins AP, Fidalgo LG, Silva AM, Gil MM, Teixeira P, Barbosa J, et al. Chemical-Based Methodologies to Extend the Shelf Life of Fresh Fish—A Review. Foods. 2021; 10(10):2300. https://doi.org/10.3390/foods10102300
Chicago/Turabian StyleAmaral, Renata A., Carlos A. Pinto, Vasco Lima, Jéssica Tavares, Ana P. Martins, Liliana G. Fidalgo, Ana M. Silva, Maria M. Gil, Paula Teixeira, Joana Barbosa, and et al. 2021. "Chemical-Based Methodologies to Extend the Shelf Life of Fresh Fish—A Review" Foods 10, no. 10: 2300. https://doi.org/10.3390/foods10102300
APA StyleAmaral, R. A., Pinto, C. A., Lima, V., Tavares, J., Martins, A. P., Fidalgo, L. G., Silva, A. M., Gil, M. M., Teixeira, P., Barbosa, J., Barba, F. J., & Saraiva, J. A. (2021). Chemical-Based Methodologies to Extend the Shelf Life of Fresh Fish—A Review. Foods, 10(10), 2300. https://doi.org/10.3390/foods10102300