Chemical Constituents of the Essential Oil Extracted from Elsholtzia densa and Their Insecticidal Activity against Tribolium castaneum and Lasioderma serricorne
Abstract
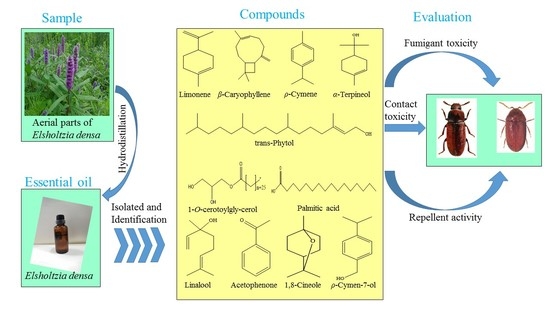
:1. Introduction
2. Materials and Methods
2.1. Plant Material and the Extraction of the EO
2.2. Insects
2.3. GC-MS Analysis
2.4. Isolation and Identification of Pure Compounds
2.5. Bioassay
2.5.1. Contact and Fumigant Activity
2.5.2. Repellant Activity
2.6. Data Analysis
2.7. Chemical Compounds
3. Results and Discussion
3.1. Chemical Composition of the EO for E. densa
3.1.1. Yield of EO
3.1.2. Chemical Composition of the EO
3.2. Structural Analysis of Isolated Compounds
3.3. Bioassay
3.3.1. Contact Activity
3.3.2. Fumigant Activity
3.3.3. Repellent Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Hubert, J.; Stejskal, V.; Athanassiou, C.G.; Throne, J.E. Health hazards associated with arthropod infestation of stored products. Annu. Rev. Entomol. 2018, 63, 553–573. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.P.; Throne, J.E. Biorational approaches to managing stored-product insects. Annu. Rev. Entomol. 2010, 55, 375–397. [Google Scholar] [CrossRef]
- Boyer, S.; Zhang, H.; Lempérière, G. A review of control methods and resistance mechanisms in stored-product insects. Bull. Entomol. Res. 2012, 102, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Suman, C.; Rupinder, K.K.; Alka, S.; David, M.C.; Colin, J.B.; Rakesh, S.; Jagat, R.K. Progress on Azadirachta indica based biopesticidesin replacing synthetic toxic pesticides. Front. Plant Sci. 2017, 8, 610. [Google Scholar] [CrossRef]
- Yadav, I.C.; Devi, N.L.; Syed, J.H.; Cheng, Z.; Li, J.; Zhang, G.; Jones, K.C. Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Sci. Total Environ. 2015, 511, 123–137. [Google Scholar] [CrossRef]
- Baskar, K.; Ignacimuthu, S. Antifeedant, larvicidal and growth inhibitory effects of ononitol monohydrate isolated from Cassia tora L. against Helicoverpa armigera (Hub.) and Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Chemosphere 2012, 88, 384–388. [Google Scholar] [CrossRef]
- Arthur, F.H. Grain protectants: Current status and prospects for the future. J. Stored Prod. Res. 1996, 32, 293–302. [Google Scholar] [CrossRef]
- Duraipandiyan, V.; Ignacimuthu, S.; Paulraj, M.G. Antifeedant and larvicidal activities of Rhein isolated from the flowers of Cassia fistula L. Saudi J. Biol. Sci. 2011, 18, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [Green Version]
- Varma, J.; Dubey, N.K. Efficacy of essential oils of Caesulia axillaris and Mentha arvensis against some storage pests causing biodeterioration of food commodities. Int. J. Food. Microbiol. 2001, 68, 207–210. [Google Scholar] [CrossRef]
- Miresmailli, S.; Isman, M.B. Botanical insecticides inspired by plant-herbivore chemical interactions. Trends Plant Sci. 2014, 19, 29–35. [Google Scholar] [CrossRef]
- Isman, M.B.; Grieneisen, M.L. Botanical insecticide research: Many publications, limited useful data. Trends Plant Sci. 2014, 19, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y. Common types and advantages and disadvantages of plant-derived pesticides. Mod. Gard. 2007, 6, 22–23. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.T.; Feng, Y.X.; Zhang, D.; Du, S.S. Comparative evaluation of the chemical composition and bioactivities of essential oils from four spice plants (Lauraceae) against stored-product insects. Ind. Crops Prod. 2019, 140, 111640. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, S.S.; Zhang, W.J.; Geng, Z.F.; Liang, J.Y.; Du, S.S.; Wang, C.F.; Deng, Z.W. Essential oil and polyacetylenes from Artemisia ordosica and their bioactivities against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Ind. Crops Prod. 2017, 100, 132–137. [Google Scholar] [CrossRef]
- Feng, Y.X.; Wang, Y.; Geng, Z.F.; Zhang, D.; Almaz, B.; Du, S.S. Contact toxicity and repellent efficacy of Valerianaceae spp. to three stored-product insects and synergistic interactions between two major compounds camphene and bornyl acetate. Ecotoxicol. Environ. Saf. 2020, 190, 106–110. [Google Scholar] [CrossRef]
- You, C.X.; Guo, S.S.; Zhang, W.J.; Geng, Z.F.; Liang, J.Y.; Ning, L.; Du, S.S.; Deng, Z.W. Chemical Constituents of Murraya tetramera Huang and Their Repellent Activity against Tribolium castaneum. Molecules 2017, 22, 1379. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.Y.; Guo, S.S.; Cao, J.Q.; Pang, X.; Geng, Z.F.; Wang, Y.; Zhang, Z.; Du, S.S. Insecticidal and repellent activity of essential oil from Amomum villosum Lour., its main compounds against two stored-product insects. Int. J. Food Prop. 2018, 21, 2265–2275. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.X.; Wang, Y.; Chen, Z.Y.; Guo, S.S.; Du, S.S. Efficacy of bornyl acetate and camphene from Valeriana officinalis essential oil against two storage insects. Environ. Sci. Poll. Res. 2019, 26, 16157–16165. [Google Scholar] [CrossRef]
- Fu, G.L. Chinese Higher Plants; Qingdao Publishing House: Qingdao, China, 2004; pp. 544–554. [Google Scholar]
- Ren, Q.R.; Wang, Y.N.; Wang, Y.; Chen, H.; Xin, W.Y.; Ma, D.W. In vitro antioxidant activity and anti-tumor activity of total flavonoids from Elshltzia densa Benth. Nat. Prod. Res. Dev. 2017, 29, 14–21. [Google Scholar] [CrossRef]
- Shao, Y.Z.; Hou, Q.Z.; Xie, Z.Y.; Yang, Y.Y.; He, C.Y.; Zhou, F.; Zhang, J.; Liang, J.Y. Bioactivities and chemical constituents of essential oils extracted from Caryopteris mongholica Bunge against two stored product insects. J. Essen. Oil Bear. Plants 2021, 24, 22–30. [Google Scholar] [CrossRef]
- Mukazayire, M.J.; Tomani, J.C.; Chalchat, J.C.; Stévigny, C.; Duez, P. Chemical composition, antimicrobial and antioxidant activities of the essential oil of Guizotia scabra and Microglossa pyrifolia from Rwanda. Planta Med. 2009, 75, 9. [Google Scholar] [CrossRef]
- Liu, Z.L.; Ho, S.H. Bioactivity of the essential oil extracted from Evodia rutaecarpa Hook f. et Thomas against the grain storage insects, Sitophilus zeamais Motsch., Tribolium castaneum (Herbst). J. Stored Prod. Res. 1999, 35, 317–328. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhao, N.N.; Liu, Q.Z. Repellent constituents of essential oil of Cymbopogon distans aerial parts against two stored-product insects. J. Agric. Food Chem. 2011, 59, 9910–9915. [Google Scholar] [CrossRef]
- Liang, J.Y.; Guo, S.S.; You, C.X.; Zhang, W.L.; Wang, C.F.; Geng, Z.F.; Deng, Z.W.; Du, S.S.; Zhang, J. Chemical constituents and insecticidal activities of Ajania fruticulosa essential oil. Chem. Biodiver. 2016, 13, 1053–1057. [Google Scholar] [CrossRef]
- Sakuma, M. Probit analysis of preference data. Appl. Entomol. Zool. 1998, 33, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Si, J.Y.; Cao, L.; Jia, X.G.; Li, X.J. Chemical composition, Antimicrobial and antiviral activities of the essential oil of Elsholtzia densa Benth. Nat. Prod. Res. Dev. 2012, 24, 1070–1074. [Google Scholar] [CrossRef]
- Talzi, V.P. A 13C and 1H NMR analysis of perfumes. Russ. J. Appl. Chem. 2006, 79, 107–116. [Google Scholar] [CrossRef]
- Kazutoshi, S.; Jun-ichiro, H.; Mariko, K. Purification and structural analysis of volatile sesquiterpenes produced by Escherichia coli carrying unidentified terpene synthase genes from edible plants of the family Araliaceae. Biosci. Biotechnol. Biochem. 2018, 82, 978–985. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.H.; Chen, L.G. Isolation and identification of the main chemical components in perilla oil. Flavour Fragr. Cosmet. 1998, 1, 19–20. [Google Scholar]
- Chu, S.S.; Feng, H.J.; Liu, Z.L. Composition of essential oil of Chinese Chenopodium ambrosioides and insecticidal activity against maize weevil, Sitophilus zeamais. Pest. Manag. Sci. 2011, 67, 714–718. [Google Scholar] [CrossRef]
- Dirk, U.; Josef, Z.; Hans, B. Biosynthesis of the irregular monoterpene artemisia ketone, the sesquiterpene germacrene D and other isoprenoids in Tanacetum vulgare L. (Asteraceae). Phytochemistry 2004, 65, 2463–2470. [Google Scholar] [CrossRef]
- Lou, H.X.; Li, X.; Zhu, Y.R. New monoterpene components in North China white fore. J. Pharm. Sci. 1992, 10, 752–757. [Google Scholar]
- Guerrini, A.; Rossi, D.; Paganetto, G. Chemical characterization (GC/MS and NMR Fingerprinting) and bioactivities of South-African Pelargonium capitatum (L.) L’ Her. (Geraniaceae) essential oil. Chem. Biodiver. 2011, 8, 624–642. [Google Scholar] [CrossRef]
- Toshikazu, S.; Nobuaki, F.; Fumio, I. Structure and synthesis of a new monoterpenoidal carboxamide from the seeds of the Thai medicinal plant Acacia concinna. Chem. Pharm. Bull. 1997, 45, 148–151. [Google Scholar] [CrossRef]
- Senthil, K.R.; Karthikeyan, K.; Paramasivan, T.P. An Efficient Procedure for the TEMPO-Catalyzed Oxidation of Alcohols to Aldehydes and Ketones Using Ferric Chloride Hexahydrate as Terminal Oxidant; NRC Research Press: Ottawa, ON, Canada, 2008; Volume 86, pp. 720–725. [Google Scholar] [CrossRef]
- Xu, S.Q.; Dun, S.Q.; Yang, X.L.; Pan, Y. Isolation and determination of α-pinene and 1, 8-cineole from Oleum Vitics Negundo. Mod. Chin. Med. 2008, 10, 11–18. [Google Scholar] [CrossRef]
- Nanjing University of Science and Technology. A Method for the Synthesis of Primary Alcohols. China Patent 201710683922.0, 26 February 2019. [Google Scholar]
- Dong, Z.Y.; Wang, H.; Ma, Y.X.; Lan, X.Z.; Chen, M. Chemical constituents from Herpetospermum caudigerum. Chin. Trad. Patent Med. 2019, 41, 341–344. [Google Scholar] [CrossRef]
- Ren, B.R.; Xia, B.; Li, W.L.; Wu, J.L.; Zhang, H.Q. Chemical constituents of Stenoloma chusanum. Chin. Tradit. Herb. Drugs 2007, 38, 20–23. [Google Scholar] [CrossRef]
- Liang, J.Y.; You, C.X.; Guo, S.S.; Zhang, W.J.; Li, Y.; Geng, Z.F.; Wang, C.F.; Du, S.S.; Deng, Z.W.; Zhang, J. Chemical constituents of the essential oil extracted from Rhododendron thymifolium and their insecticidal activities against Liposcelis bostrychophila or Tribolium castaneum. Ind. Crops Prod. 2016, 79, 267–273. [Google Scholar] [CrossRef]
- Jiang, C.H.; Liu, Q.Z.; Du, S.S.; Deng, Z.W.; Liu, Z.L. Essential oil composition and insecticidal activity of Evodia lepta (Spreng) Merr. root barks from China against two grain storage insects. J. Med. Plants Res. 2012, 6, 3464–3469. [Google Scholar] [CrossRef]
- Chen, H.; Yang, K.; You, C.X. Chemical constituents and biological activities against Tribolium castaneum (Herbst) of the essential oil from Citrus wilsonii leave. J. Ser. Chem. Soc. 2014, 79, 1213–1222. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, S.S.; Huang, D.Y.; Wang, C.F.; Wei, J.Y.; Li, Z.H.; Sun, J.S.; Bai, J.F.; Tian, Z.F.; Wang, P.J.; et al. Contact and repellent activities of Zingober zerumbet (L.) Smith against Lasioderma serricorne. J. Oleo Sci. 2017, 66, 399–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavela, R.; Sedlák, P. Post-application temperature as a factor influencing the insecticidal activity of essential oil from Thymus vulgaris. Ind. Crops Prod. 2018, 113, 46–49. [Google Scholar] [CrossRef]
- Wang, Y.; You, C.X.; Yang, K. Bioactivity of essential oil of Zingiber pur pureum Rhizomes and its main compounds against two stored product insects. J. Econo. Entomol. 2015, 8, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, C.F.; You, C.X.; Geng, Z.F.; Sun, R.Q.; Guo, S.S.; Du, S.S.; Liu, Z.L.; Deng, Z.W. Bioactivity of essential oil of Litsea cubeba from China and its main compounds against two stroed product insects. J. Asia Pac. Entomol. 2014, 17, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhang, W.J.; Huang, D.Y.; Wang, Y.; Wei, J.Y.; Li, Z.H.; Sun, J.S.; Bai, J.F.; Tian, Z.F.; Wang, P.J.; et al. Chemical compositions and insecticidal activities of Alpinia kwangsiensis essential oil against Lasioderma serricorne. Molecules 2015, 20, 21939–21945. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; You, C.X.; Wang, C.F.; Yang, K.; Chen, R.; Zhang, W.J.; Du, S.S.; Geng, Z.F.; Deng, Z.W. Chemical constituents and insecticida activities of the essential oil from Amomum tsaoko against two stored-productinsects. J. Oleo Sci. 2014, 63, 1019–1026. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.S.; You, C.X.; Liang, J.Y.; Zhang, W.J.; Yang, K.; Geng, Z.F.; Wang, C.F.; Du, S.S.; Lei, N. Essential oil of Amomum maximum Roxb, and its bioaxtivities against two stored-product insects. J. Oleo Sci. 2015, 64, 1307–1314. [Google Scholar] [CrossRef] [Green Version]
- Trongtokit, Y.; Rongsriyam, Y.; Komalamisra, N.; Apiwathnasorn, C. Comparative repellency of 38 essential oils against mosquito bites. Phytother. Res. 2005, 19, 303–309. [Google Scholar] [CrossRef]



| Grade | 0 | I | II | III | IV | V |
|---|---|---|---|---|---|---|
| PR (%) | 0.01~0.1 | 0.1~20 | 20.1~40 | 40.1~60 | 60.1~80 | 80.1~100 |
| Name | Sample Size (kg) | Volume (mL) | Yield (v/w, %) | Relative Density (g/mL) |
|---|---|---|---|---|
| E. densa | 24.80 | 84.30 | 0.34 | 0.88 |
| Peak NO. | RT (min) | Compound | RI * | Relative Content (%) | Formula |
|---|---|---|---|---|---|
| 1 | 3.539 | 3-Thujene | 925 | 0.10 | C10H16 |
| 2 | 3.653 | 2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene | 939 | 0.41 | C10H16 |
| 3 | 4.306 | β-Pinene | 978 | 0.86 | C10H16 |
| 4 | 4.357 | 1-Octen-3-ol | 986 | 0.67 | C8H16O |
| 5 | 4.414 | 3-Octanol | 994 | 0.16 | C8H18O |
| 6 | 4.683 | β-Phellandrene | 1028 | 0.39 | C10H16 |
| 7 | 5.170 | o-Cymene | 1029 | 0.38 | C10H14 |
| 8 | 5.267 | Limonene | 1035 | 22.05 | C10H16 |
| 9 | 5.370 | β-Ocimene | 1037 | 0.33 | C10H16 |
| 10 | 5.553 | 3,7-Dimethyl-1,3,6-octatriene | 1044 | 5.79 | C10H16 |
| 11 | 5.719 | γ-Terpinene | 1061 | 0.81 | C10H16 |
| 12 | 5.919 | Acetophenone | 1065 | 0.52 | C8H8O |
| 13 | 6.354 | 3-Oxabicyclo[4.3.0]non-8-en-2-one | 1084 | 0.38 | C9H12O |
| 14 | 7.064 | 4-Pyridinol | 1154 | 0.15 | C5H5NO |
| 15 | 7.521 | 2-Methyl-3-methylene-1-cyclopentanecarboxylic acid methyl ester | 1169 | 20.00 | C9H14O2 |
| 16 | 7.624 | Bornyl chloride | 1203 | 0.57 | C10H17Cl |
| 17 | 7.848 | 1,3-Dimethylcyclohexene | 1264 | 0.57 | C8H14 |
| 18 | 8.248 | ρ-Cymen-7-ol | 1291 | 7.50 | C10H14O |
| 19 | 9.925 | 1-Methylene-2-methyl-3-isopropenylcyclopentane | 1306 | 0.17 | C10H16 |
| 20 | 10.457 | α-Copaene | 1376 | 0.16 | C15H24 |
| 21 | 10.588 | β-Bourbonene | 1384 | 0.56 | C15H24 |
| 22 | 10.663 | β-Elemene | 1394 | 0.56 | C15H24 |
| 23 | 11.058 | β-Caryophyllene | 1399 | 4.41 | C15H24 |
| 24 | 11.332 | 1,1,7-Trimethyl-4-methylenedecahydro-Alloaromadendrene | 1433 | 0.10 | C15H24 |
| 25 | 11.492 | 2,6,6,9-Tetramethyl-1,4,8-cycloundecatriene | 1438 | 3.68 | C15H24 |
| 26 | 11.607 | 1,2,3,5,6,7,8,8a-Octahydro-1-methyl-6-methylene-4-(1-methylethyl)-naphthalene | 1453 | 0.14 | C15H24 |
| 27 | 11.773 | γ-Muurolene | 1474 | 0.18 | C15H24 |
| 28 | 11.859 | 7-Methyl-3-methylidene-4-(propan-2-yl)octahydro-1H-cyclopenta[1,3]cyclopropa[1,2]benzene | 1486 | 11.17 | C15H24 |
| 29 | 11.933 | α-Farnesene | 1524 | 1.19 | C15H24 |
| 30 | 12.030 | Bicyclogermacrene | 1532 | 2.09 | C15H24 |
| 31 | 12.231 | 1-Isopropyl-7-methyl-4-methylene-1,2,3,4,4a,5,6,8a-octahydronaphthalene | 1558 | 0.27 | C15H24 |
| 32 | 12.344 | Cadinene | 1572 | 0.76 | C15H24 |
| 33 | 12.986 | 1-Hydroxy-1,7-dimethyl-4-isopropyl-2,7-cyclodecadiene | 1573 | 0.33 | C15H26O |
| 34 | 13.020 | Spathulenol | 1576 | 0.19 | C15H24O |
| 35 | 13.095 | 1-Ethenyl-1-methyl-2,4-bis(1-methylethenyl)-cyclohexane | 1585 | 0.27 | C15H24 |
| 36 | 13.398 | Spiro[4.4]nonan-2-one | 1608 | 0.15 | C9H14O |
| 37 | 13.741 | T-Cadinol | 1649 | 0.50 | C15H26O |
| 38 | 13.896 | α-Cadinol | 1663 | 0.60 | C15H26O |
| 39 | 14.462 | Decahydro-4a-methyl-1-methylene-7-(1-methylethenyl)-naphthalene | 1731 | 0.28 | C15H24 |
| 40 | 18.090 | Phytol | 2111 | 0.27 | C20H40O |
| 41 | 19.572 | Bis(2-ethylhexyl) adipate | 2398 | 0.13 | C22H42O4 |
| 42 | 23.263 | Didecan-2-yl phthalate | 2956 | 0.45 | C28H46O4 |
| Total | 90.25 |
| Target | Compounds | LD50 (μg/Adult) | 95% FL | Slope ± SE | Chi Square (χ2) | p-Value |
|---|---|---|---|---|---|---|
| T. castaneum | EO | 29.70 | 14.00–40.40 | 0.12 ± 0.03 | 1.02 | 0.99 |
| Limonene | 46.61 | 39.19–53.38 | 0.12 ± 0.02 | 6.92 | 1.00 | |
| β-Caryophyllene | 80.70 | 67.93–96.59 | 0.07 ± 0.01 | 10.14 | 0.93 | |
| ρ-Cymene | 33.84 | 31.00–36.64 | 0.35 ± 0.05 | 1.71 | 1.00 | |
| Phytol | 124.26 | 112.01–136.55 | 0.07 ± 0.01 | 6.25 | 1.00 | |
| α-Terpineol | 86.17 | 76.38–96.69 | 0.09 ± 0.01 | 7.89 | 0.99 | |
| Linalool | 69.12 | 63.67–75.15 | 0.16 ± 0.02 | 4.45 | 1.00 | |
| Acetophenone | 55.80 | 49.04–63.47 | 0.12 ± 0.02 | 23.14 | 4.45 | |
| 1,8-Cineole | 64.21 | 59.11–67.94 | 0.19 ± 0.02 | 5.31 | 1.00 | |
| ρ-Cymen-7-ol | 13.30 | 11.44–15.15 | 0.05 ± 0.07 | 5.22 | 1.00 | |
| Furfural | 58.58 | 51.47–65.44 | 0.12 ± 0.01 | 5.84 | 1.00 | |
| Dibutyl phthalate | 105.10 | 94.50–119.42 | 0.08 ± 0.01 | 7.44 | 0.99 | |
| Dioctyl phthalate | 116.90 | 104.82–135.09 | 0.08 ± 0.01 | 12.23 | 0.97 | |
| Geraniol | 64.08 | 54.11–75.52 | 0.08 ± 0.01 | 11.73 | 0.98 | |
| 1-Octen-3-ol | 13.52 | 10.25–16.54 | 0.30 ± 0.04 | 11.73 | 0.97 | |
| 3-Octanol | 17.45 | 14.19–20.42 | 0.26 ± 0.03 | 7.99 | 0.99 | |
| 2-Ethyl-1H-imidazole | >300 | |||||
| 2,4-Ditert-butylphenol | 53.49 | 45.67–62.34 | 0.09 ± 0.01 | 4.72 | 1.00 | |
| Pyrethrins | 0.10 | 0.08–0.13 | 2.40 ± 0.39 | 8.23 | 0.79 | |
| L. serricorne | EO | 24.29 | 20.94–27.61 | 0.55 ± 0.11 | 1.06 | 0.99 |
| Limonene | 59.78 | 55.02–64.97 | 5.43 ± 0.57 | 11.26 | 0.98 | |
| β-Caryophyllene | 45.86 | 42.59–49.20 | 7.30 ± 0.85 | 4.25 | 1.00 | |
| ρ-Cymene | 47.56 | 44.18–51.04 | 7.18 ± 0.82 | 5.72 | 1.00 | |
| Phytol | 46.88 | 42.54–51.97 | 4.56 ± 0.51 | 7.25 | 0.99 | |
| α-Terpineol | 11.64 | 10.15–13.22 | 4.01 ± 0.44 | 5.34 | 1.00 | |
| Linalool | 16.69 | 14.75–18.97 | 3.30 ± 0.32 | 18.90 | 0.90 | |
| Acetophenone | 7.07 | 6.72–7.43 | 10.34 ± 1.21 | 5.10 | 1.00 | |
| 1,8-Cineole | 11.01 | 9.88–12.25 | 4.22 ± 0.45 | 9.93 | 0.99 | |
| ρ-Cymen-7-ol | 8.42 | 6.98–9.81 | 3.34 ± 0.42 | 6.36 | 1.00 | |
| Furfural | 10.21 | 8.08–12.47 | 3.19 ± 0.52 | 2.24 | 1.00 | |
| Dibutyl phthalate | 30.87 | 23.69–41.78 | 2.06 ± 0.30 | 6.68 | 0.92 | |
| Dioctyl phthalate | 24.04 | 18.88–31.05 | 2.36 ± 0.33 | 4.07 | 0.99 | |
| Geraniol | 3.14 | 2.29–4.14 | 2.12 ± 0.30 | 5.68 | 0.96 | |
| 1-Octen-3-ol | 3.39 | 2.57–4.38 | 2.42 ± 0.33 | 5.37 | 0.94 | |
| 3-Octanol | 7.75 | 6.45–9.30 | 4.29 ± 0.62 | 3.08 | 0.99 | |
| 2-Ethyl-1H-imidazole | 31.38 | 25.05–40.17 | 2.64 ± 0.36 | 3.77 | 0.99 | |
| 2,4-Di-tert-butylphenol | 32.19 | 26.26–40.26 | 4.23 ± 0.35 | 3.16 | 0.99 | |
| Pyrethrins | 0.09 | 0.07–0.11 | 3.07 ± 0.36 | 6.19 | 0.99 |
| Target | Compounds | LC50 (mg/L Air) | 95% FL | Slope ± SE | Chi Square (χ2) | p-Value |
|---|---|---|---|---|---|---|
| T. castaneum | EO | 18.45 | 14.89–21.62 | 0.42 ± 0.10 | 0.82 | 0.99 |
| Limonene | 25.94 | 23.35–28.31 | 0.32 ± 0.05 | 2.08 | 1 | |
| β-Caryophyllene | >200 | |||||
| ρ-Cymene | 10.91 | 9.45–12.39 | 0.56 ± 0.07 | 4.12 | 1 | |
| Phytol | >200 | |||||
| α-Terpineol | >200 | |||||
| Linalool | 16.46 | 14.18–18.48 | 0.37 ± 0.05 | 5.05 | 1 | |
| Acetophenone | 37.55 | 28.39–49.52 | 0.16 ± 0.02 | 83.45 | 0 | |
| 1,8-Cineole | >200 | |||||
| ρ-Cymen-7-ol | 10.47 | 8.92–11.99 | 0.53 ± 0.07 | 3.82 | 1 | |
| Furfural | 23.16 | 21.26–25.13 | 0.40 ± 0.05 | 10.38 | 0.99 | |
| Dibutyl phthalate | >200 | |||||
| Dioctyl phthalate | >200 | |||||
| Geraniol | >200 | |||||
| 1-Octen-3-ol | 19.17 | 16.77–21.38 | 0.03 ± 0.04 | 7.61 | 0.99 | |
| 3-Octanol | 19.85 | 17.09–22.34 | 0.27 ± 0.03 | 2.47 | 1 | |
| 2-Ethyl-1H-imidazole | >200 | |||||
| 2,4-Ditert-butylphenol | >200 | |||||
| MeBr | 0.18 | 0.17–0.20 | 5.71 ± 0.21 | 5.63 | 0.96 | |
| L. serricorne | EO | 14.49 | 12.44–17.12 | 0.61 ± 0.12 | 3.34 | 0.97 |
| Limonene | 5.86 | 18.34–23.47 | 4.03 ± 0.46 | 9.22 | 0.99 | |
| β-Caryophyllene | 153.09 | 101.82–514.78 | 1.79 ± 0.51 | 1.95 | 1 | |
| ρ-Cymene | 29.06 | 27.03–31.22 | 7.26 ± 0.85 | 6.45 | 1 | |
| Phytol | >200 | |||||
| α-Terpineol | 6.90 | 5.69–8.07 | 3.18 ± 0.38 | 8.19 | 0.99 | |
| Linalool | 10.85 | 9.68–12.08 | 5.47 ± 0.63 | 5.36 | 0.99 | |
| Acetophenone | 5.47 | 5.20–5.92 | 10.33 ± 1.26 | 5.56 | 1 | |
| 1,8-Cineole | 9.57 | 7.99–11.20 | 2.820 ± 0.32 | 3.86 | 1 | |
| ρ-Cymen-7-ol | >200 | |||||
| Furfural | 7.02 | 5.60–8.75 | 2.64 ± 0.36 | 3.94 | 0.99 | |
| Dibutyl phthalate | >200 | |||||
| Dioctyl phthalate | >200 | |||||
| Geraniol | >200 | |||||
| 1-Octen-3-ol | 6.71 | 5.61–8.04 | 3.81 ± 0.53 | 7.29 | 0.88 | |
| 3-Octanol | 5.05 | 4.38–6.61 | 3.09 ± 0.44 | 6.11 | 0.94 | |
| 2-Ethyl-1H-imidazole | 95.85 | 80.12–131.75 | 3.55 ± 0.73 | 2.77 | 0.99 | |
| 2,4-Ditert-butylphenol | 50.41 | 37.82–68.15 | 1.84 ± 0.42 | 2.12 | 1 | |
| Phosphine * | 9.23 × 10−3 | (7.13~11.37) × 10−3 | 2.12 ± 0.27 | 11.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Shao, Y.; Wu, H.; An, Y.; Wang, J.; Zhang, J.; Kong, W. Chemical Constituents of the Essential Oil Extracted from Elsholtzia densa and Their Insecticidal Activity against Tribolium castaneum and Lasioderma serricorne. Foods 2021, 10, 2304. https://doi.org/10.3390/foods10102304
Liang J, Shao Y, Wu H, An Y, Wang J, Zhang J, Kong W. Chemical Constituents of the Essential Oil Extracted from Elsholtzia densa and Their Insecticidal Activity against Tribolium castaneum and Lasioderma serricorne. Foods. 2021; 10(10):2304. https://doi.org/10.3390/foods10102304
Chicago/Turabian StyleLiang, Junyu, Yazhou Shao, Haoshu Wu, Yue An, Junlong Wang, Ji Zhang, and Weibao Kong. 2021. "Chemical Constituents of the Essential Oil Extracted from Elsholtzia densa and Their Insecticidal Activity against Tribolium castaneum and Lasioderma serricorne" Foods 10, no. 10: 2304. https://doi.org/10.3390/foods10102304
APA StyleLiang, J., Shao, Y., Wu, H., An, Y., Wang, J., Zhang, J., & Kong, W. (2021). Chemical Constituents of the Essential Oil Extracted from Elsholtzia densa and Their Insecticidal Activity against Tribolium castaneum and Lasioderma serricorne. Foods, 10(10), 2304. https://doi.org/10.3390/foods10102304