New Insights on the Effects of Dietary Omega-3 Fatty Acids on Impaired Skin Healing in Diabetes and Chronic Venous Leg Ulcers
Abstract
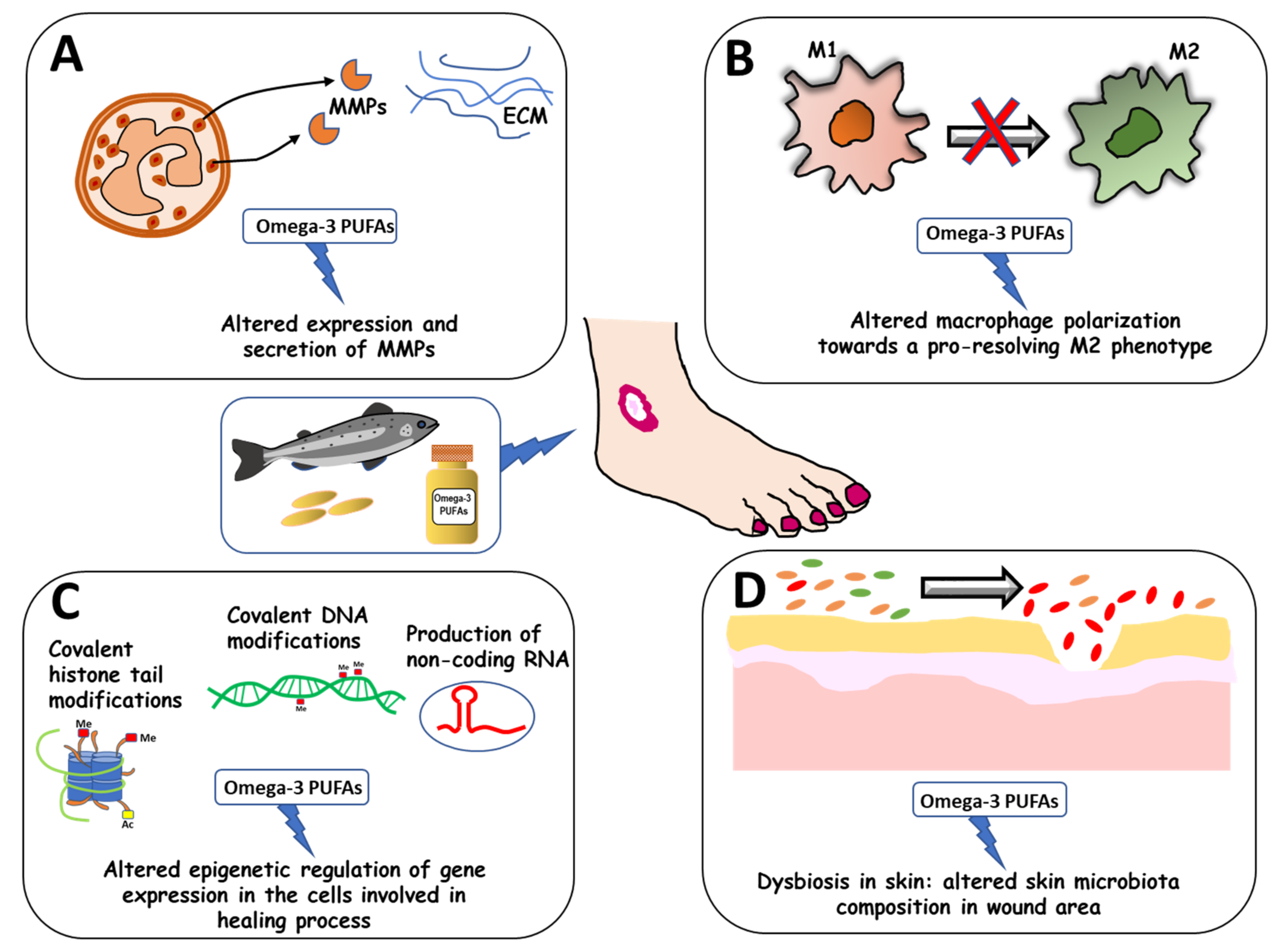
:1. Introduction
2. WH Process in the Skin: Cells and Molecules Involved
2.1. Platelets and Endothelial Cells
2.2. Resident Immune Cells
2.3. Neutrophils and Monocytes/Macrophages
2.4. Keratinocytes, Fibroblasts and Myofibroblasts
3. Impaired Healing in Diabetes and Venous Stasis Ulcers
3.1. Mechanisms of Impaired WH in Diabetes
3.2. Mechanisms of Impaired WH in Patients Affected by Chronic Venous Leg Ulcers
4. Omega-3 PUFA Modulatory Effects on Impaired WH in Diabetes and Vascular Disease
4.1. Omega-3 PUFA Modulatory Effects on Delayed WH in Diabetic Patients
4.2. Omega-3 PUFA Modulatory Effects on Delayed WH in Patients Affected by Chronic Venous Leg Ulcers
5. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Calviello, G.; Su, H.M.; Weylandt, K.H.; Fasano, E.; Serini, S.; Cittadini, A. Experimental evidence of ω-3 polyunsaturated fatty acid modulation of inflammatory cytokines and bioactive lipid mediators: Their potential role in inflammatory, neurodegenerative, and neoplastic diseases. Biomed Res. Int. 2013, 2013, 743171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serini, S.; Ottes Vasconcelos, R.; Fasano, E.; Calviello, G. Epigenetic regulation of gene expression and M2 macrophage polarization as new potential omega-3 polyunsaturated fatty acid targets in colon inflammation and cancer. Expert. Opin. Ther. Targets 2016, 20, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Ottes Vasconcelos, R.; Fasano, E.; Calviello, G. How plausible is the use of dietary n-3 PUFA in the adjuvant therapy of cancer? Nutr. Res. Rev. 2016, 29, 102–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serini, S.; Cassano, R.; Trombino, S.; Calviello, G. Nanomedicine-based formulations containing ω-3 polyunsaturated fatty acids: Potential application in cardiovascular and neoplastic diseases. Int. J. Nanomed. 2019, 14, 2809–2828. [Google Scholar] [CrossRef] [Green Version]
- Chow, O.; Barbul, A. Immunonutrition: Role in Wound Healing and Tissue Regeneration. Adv. Wound Care 2014, 3, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.W.; Supp, D.M. Role of Arginine and Omega-3 Fatty Acids in Wound Healing and Infection. Adv. Wound Care 2014, 3, 682–690. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Glaser, R.; Christian, L.M. Omega-3 fatty acids and stress-induced immune dysregulation: Implications for wound healing. Mil. Med. 2014, 179, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Serini, S.; Calviello, G. Omega-3 PUFA Responders and Non-Responders and the Prevention of Lipid Dysmetabolism and Related Diseases. Nutrients 2020, 12, 1363. [Google Scholar] [CrossRef]
- Mazzocchi, A.; De Cosmi, V.; Risé, P.; Milani, G.P.; Turolo, S.; Syrén, M.L.; Sala, A.; Agostoni, C. Bioactive Compounds in Edible Oils and Their Role in Oxidative Stress and Inflammation. Front. Physiol. 2021, 12, 659551. [Google Scholar] [CrossRef]
- Gilles, S.; Mariani, V.; Bryce, M.; Mueller, M.J.; Ring, J.; Jakob, T.; Pastore, S.; Behrendt, H.; Traidl-Hoffmann, C. Pollen-derived E1-phytoprostanes signal via PPAR-gamma and NF-kappaB-dependent mechanisms. J. Immunol. 2009, 182, 6653–6658. [Google Scholar] [CrossRef] [Green Version]
- Leung, K.S.; Galano, J.M.; Oger, C.; Durand, T.; Lee, J.C. Enrichment of alpha-linolenic acid in rodent diet reduced oxidative stress and inflammation during myocardial infarction. Free Radic. Biol. Med. 2021, 162, 53–64. [Google Scholar] [CrossRef]
- Ohue-Kitano, R.; Yasuoka, Y.; Goto, T.; Kitamura, N.; Park, S.B.; Kishino, S.; Kimura, I.; Kasubuchi, M.; Takahashi, H.; Li, Y.; et al. α-Linolenic acid-derived metabolites from gut lactic acid bacteria induce differentiation of anti-inflammatory M2 macrophages through G protein-coupled receptor 40. FASEB J. 2018, 32, 304–318. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014, 7, a016311. [Google Scholar] [CrossRef] [Green Version]
- Avishai, E.; Yeghiazaryan, K.; Golubnitschaja, O. Impaired wound healing: Facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J. 2017, 8, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Heng, M.C.Y. Wound healing in adult skin: Aiming for perfect regeneration. Int. J. Derm. 2011, 50, 1058–1066. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Red. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Delavary, B.M.; Van der Veer, W.M.; Van Egmond, M.; Niessen, F.B.; Beelen, R.H. Macrophages in skin injury and repair. Immunobiology 2011, 216, 753–762. [Google Scholar] [CrossRef]
- Scully, D.; Sfyri, P.; Wilkinson, H.N.; Acebes-Huerta, A.; Verpoorten, S.; Muñoz-Turrillas, M.C.; Parnell, A.; Patel, K.; Hardman, M.J.; Gutiérrez, L.; et al. Optimising platelet secretomes to deliver robust tissue-specific regeneration. J. Tissue Eng. Regen. Med. 2020, 14, 82–98. [Google Scholar] [CrossRef]
- Kingsley, K.; Huff, J.; Rust, W.; Carroll, K.; Martinez, A.; Fitchmun, M.; Plopper, G.E. ERK1/2 mediates PDGF-BB stimulated vascular smooth muscle cell proliferation and migration on laminin-5. Biochem. Biophys. Res. Commun. 2002, 293, 1000–1006. [Google Scholar] [CrossRef]
- Strbo, N.; Yin, N.; Stojadinovic, O. Innate and Adaptive Immune Responses in Wound Epithelialization. Adv. Wound Care (New Rochelle) 2014, 3, 492–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; DiPietro, L.A. Toll-like receptor function in acute wounds. Adv. Wound Care 2017, 6, 344–355. [Google Scholar] [CrossRef] [Green Version]
- Martin, P.; Leibovich, S.J. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Segel, G.B.; Halterman, M.W.; Lichtman, M.A. The paradox of the neutrophil’s role in tissue injury. J. Leukoc. Biol. 2011, 89, 359–372. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Kim, M.H.; Liu, W.; Borjesson, D.L.; Curry, F.R.E.; Miller, L.S.; Cheung, A.L.; Liu, F.T.; Isseroff, R.R.; Simon, S.I. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J. Investig. Dermatol. 2008, 128, 1812–1820. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Sica, A.; Locati, M. Macrophage polarization comes of age. Immunity 2005, 23, 344–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginhoux, F.; Schultze, J.L.; Murray, P.J.; Ochando, J.; Biswas, S.K. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat. Immunol. 2016, 17, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.J.; Lantis, J.; Kirsner, R.S.; Shah, V.; Molyneaux, M.; Carter, M.J. Macrophages: A review of their role in wound healing and their therapeutic use. Wound Repair Regen. 2016, 24, 613–629. [Google Scholar] [CrossRef]
- Khallou-Laschet, J.; Varthaman, A.; Fornasa, G.; Compain, C.; Gaston, A.T.; Clement, M.; Dussiot, M.; Levillain, O.; Graff-Dubois, S.; Nicoletti, A.; et al. Macrophage plasticity in experimental atherosclerosis. PLoS ONE 2010, 5, e8852. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am. J. Pathol. 2015, 185, 2596–2606. [Google Scholar] [CrossRef] [Green Version]
- Nelson, S.M.; Lei, X.; Prabhu, K.S. Selenium levels affect the IL-4–induced expression of alternative activation markers in murine macrophages. J. Nutr. 2011, 141, 1754–1761. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-W.; Lee, B.; Liu, P.-S.; Wei, L.-N. Receptor interacting protein 140 orchestrates the dynamics of macrophage M1/M2 polarization. J. Innate Immun. 2016, 8, 97–107. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Campbell, L.; Saville, C.R.; Murray, P.J.; Cruickshank, S.M.; Hardman, M.J. Local arginase 1 activity is required for cutaneous wound healing. J. Investig. Dermatol. 2013, 133, 2461–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurevich, D.B.; Severn, C.E.; Twomey, C.; Greenhough, A.; Cash, J.; Toye, A.M.; Mellor, H.; Martin, P. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J. 2018, 37, e97786. [Google Scholar] [CrossRef] [PubMed]
- Wager, L.J.; Leavesley, D.I. MicroRNA regulation of epithelial-to-mesenchymal transition during re-epithelialisation: Assessing an open wound. Wound Pract. Res. 2015, 23, 132–142. [Google Scholar]
- Santoro, M.M.; Gaudino, G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp. Cell Res. 2005, 304, 274–286. [Google Scholar] [CrossRef]
- Rousselle, P.; Braye, F.; Dayan, G. Reepithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef]
- Baum, C.L.; Arpey, C.J. Normal cutaneous wound healing clinical correlation with cellular and molecular events. Dermatol. Surg. 2005, 31, 674–686. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Beyer, S.; Koch, M.; Lee, Y.H.; Jung, F.; Blocki, A. An In Vitro Model of Angiogenesis during Wound Healing Provides Insights into the Complex Role of Cells and Factors in the Inflammatory and Proliferation Phase. Int. J. Mol. Sci. 2018, 19, 2913. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-P.; Wu, M.-S.; Shun, C.-T.; Wang, H.-P.; Hsieh, C.-Y.; Kuo, M.-L.; Lin, J.-T. Cyclooxygenase-2 increases hypoxia-inducible factor-1 and vascular endothelial growth factor to promote angiogenesis in gastric carcinoma. J. Biomed. Sci. 2005, 12, 229–241. [Google Scholar] [CrossRef]
- Honnegowda, T.M.; Kumar, P.; Udupa, E.G.; Kumar, S.; Kumar, U.; Rao, P. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast. Aesthet. Res. 2015, 2, 24324–24329. [Google Scholar]
- Du Cheyne, C.; Tay, H.; De Spiegelaere, W. The complex TIE between macrophages and angiogenesis. Anat. Histol. Embryol. 2020, 49, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Poché, R.A.; Hsu, C.-W.; McElwee, M.L.; Burns, A.R.; Dickinson, M.E. Macrophages engulf endothelial cell membrane particles preceding pupillary membrane capillary regression. Dev. Biol. 2015, 403, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefater, J.A., 3rd; Lewkowich, I.; Rao, S.; Mariggi, G.; Carpenter, A.C.; Burr, A.R.; Fan, J.; Ajima, R.; Molkentin, J.D.; Williams, B.O.; et al. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature 2011, 474, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Jung, N.; Yu, J.; Um, J.; Dubon, M.J.; Park, K.-S. Substance P modulates properties of normal and diabetic dermal fibroblasts. Tissue Eng. Regen. Med. 2016, 13, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Um, J.; Jung, N.; Chin, S.; Cho, Y.; Choi, S.; Park, K.S. Substance P enhances EPC mobilization for accelerated wound healing. Wound Repair Regen. 2016, 24, 402–410. [Google Scholar] [CrossRef]
- Leal, E.C.; Carvalho, E.; Tellechea, A.; Kafanas, A.; Tecilazich, F.; Kearney, C.; Kuchibhotla, S.; Auster, M.E.; Kokkotou, E.; Mooney, D.J.; et al. Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am. J. Pathol. 2015, 185, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Um, J.; Yu, J.; Park, K.S. Substance P accelerates wound healing in type 2 diabetic mice through endothelial progenitor cell mobilization and Yes-associated protein activation. Mol. Med. Rep. 2017, 15, 3035–3040. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.-B.; Fang, X.-J.; Liu, D.-W.; Shao, Y.; Zhang, H.-Y.; Peng, Y.; Zhong, Q.; Li, Y.; De-Ming, L. Substance P combined with epidermal stem cells promotes wound healing and nerve regeneration in diabetes mellitus. Neural Regen. Res. 2016, 11, 493–501. [Google Scholar]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar]
- Witte, M.B.; Barbul, A. General principles of wound healing. Surg. Clin. N. Am. 1997, 77, 509–528. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2011, 29, 475–479. [Google Scholar] [CrossRef]
- Almine, J.F.; Wise, S.G.; Weiss, A.S. Elastin signaling in wound repair. Birth Defects Res. C Embryo Today 2012, 96, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Amadeu, T.P.; Braune, A.S.; Porto, L.C.; Desmoulière, A.; Costa, A.M. Fibrillin-1 and elastin are differentially expressed in hypertrophic scars and keloids. Wound Repair Regen. 2004, 12, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Mastrangelo, D.; Iselin, C.E.; Chaponnier, C.; Gabbiani, G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am. J. Pathol. 2001, 159, 1009–1020. [Google Scholar] [CrossRef] [Green Version]
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg. 1998, 176, 26S–38S. [Google Scholar] [CrossRef]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune regulation of skin wound healing: Mechanisms and novel therapeutic targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Blakytny, R.; Jude, E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet. Med. 2006, 23, 594–608. [Google Scholar] [CrossRef]
- Kahle, B.; Hermanns, H.J.; Gallenkemper, G. Evidence-based treatment of chronic leg ulcers. Dtsch Arztebl. Int. 2011, 108, 231–237. [Google Scholar] [CrossRef]
- Guest, J.F.; Staines, K.; Murphy, N. Cost-effectiveness of using intermittent pneumatic compression to manage hard-to-heal venous leg ulcers in the UK. J. Wound Care 2021, 30, 544–552. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Walsh, J.W.; Hoffstad, O.J.; Sullivan, M.O.; Margolis, D.J. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet. Med. 2016, 33, 1493–1498. [Google Scholar] [CrossRef]
- Bachar-Wikstrom, E.; Manchanda, M.; Bansal, R.; Karlsson, M.; Kelly-Pettersson, P.; Sköldenberg, O.; Wikstrom, J.D. Endoplasmic reticulum stress in human chronic wound healing: Rescue by 4-phenylbutyrate. Int. Wound J. 2021, 18, 49–61. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, T.F., Jr.; Passman, M.A.; Marston, W.A.; Ennis, W.J.; Dalsing, M.; Kistner, R.L.; Lurie, F.; Henke, P.K.; Gloviczki, M.L.; Eklöf, B.G.; et al. Management of venous leg ulcers: Clinical practice guidelines of the Society for Vascular Surgery® and the American Venous Forum. J. Vasc. Surg. 2014, 60, 3S–59S. [Google Scholar] [CrossRef] [Green Version]
- McDaniel, J.C.; Rausch, J.; Tan, A. Impact of omega-3 fatty acid oral therapy on healing of chronic venous leg ulcers in older adults: Study protocol for a randomized controlled single-center trial. Trials 2020, 21, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agale, S.V. Chronic Leg Ulcers: Epidemiology, Aetiopathogenesis, and Management. Ulcers 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chi, Y.W.; Raffetto, J.D. Venous leg ulceration pathophysiology and evidence-based treatment. Vasc. Med. 2015, 20, 168–181. [Google Scholar] [CrossRef]
- Geng, K.; Ma, X.; Jiang, Z.; Huang, W.; Gao, C.; Pu, Y.; Luo, L.; Xu, Y.; Xu, Y. Innate Immunity in Diabetic Wound Healing: Focus on the Mastermind Hidden in Chronic Inflammatory. Front. Pharmacol. 2021, 12, 653940. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Ligi, D.; Maniscalco, R.; Khalil, R.A.; Mannello, F. Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J. Clin. Med. 2020, 10, 29. [Google Scholar] [CrossRef]
- DiPietro, L.A.; Wilgus, T.A.; Koh, T.J. Macrophages in Healing Wounds: Paradoxes and Paradigms. Int. J. Mol. Sci. 2021, 22, 950. [Google Scholar] [CrossRef]
- Bannon, P.; Wood, S.; Restivo, T.; Campbell, L.; Hardman, M.J.; Mace, K.A. Diabetes induces stable intrinsic changes to myeloid cells that contribute to chronic inflammation during wound healing in mice. Dis. Model. Mech. 2013, 6, 1434–1447. [Google Scholar] [CrossRef] [Green Version]
- Barman, P.K.; Pang, J.; Urao, N.; Koh, T.J. Skin wounding-induced monocyte expansion in mice is not abrogated by IL-1 receptor 1 deficiency. J. Immunol. 2019, 202, 2720–2727. [Google Scholar] [CrossRef]
- Barman, P.K.; Koh, T.J. Macrophage Dysregulation and Impaired Skin Wound Healing in Diabetes. Front. Cell Dev. Biol. 2020, 8, 528. [Google Scholar] [CrossRef]
- Gallagher, K.A.; Joshi, A.; Carson, W.F.; Schaller, M.; Allen, R.; Mukerjee, S.; Kittan, N.; Feldman, E.L.; Henke, P.K.; Hogaboam, C.; et al. Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes. Diabetes 2015, 64, 1420–1430. [Google Scholar] [CrossRef] [Green Version]
- Kimball, A.; Schaller, M.; Joshi, A.; Davis, F.M.; DenDekker, A.; Boniakowski, A.; Bermick, J.; Obi, A.; Moore, B.; Henke, P.K.; et al. Ly6CHi Blood Monocyte/Macrophage Drive Chronic Inflammation and Impair Wound Healing in Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1102–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastar, I.; Marjanovic, J.; Stone, R.C.; Chen, V.; Burgess, J.L.; Mervis, J.S.; Tomic-Canic, M. Epigenetic regulation of cellular functions in wound healing. Exp. Dermatol. 2021, 30, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Kittan, N.A.; Allen, R.M.; Dhaliwal, A.; Cavassani, K.A.; Schaller, M.; Gallagher, K.A.; Carson, W.F., 4th; Mukherjee, S.; Grembecka, J.; Cierpicki, T.; et al. Cytokine induced phenotypic and epigenetic signatures are key to establishing specific macrophage phenotypes. PLoS ONE 2013, 8, e78045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamam, H.J.; Khan, M.A.; Palaniyar, N. Histone Acetylation Promotes Neutrophil Extracellular Trap Formation. Biomolecules 2019, 9, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabanel, M.; Da Costa, T.P.; El-Cheikh, M.C.; Carneiro, K. The epigenome as a putative target for skin repair: The HDAC inhibitor Trichostatin A modulates myeloid progenitor plasticity and behavior and improves wound healing. J. Transl. Med. 2019, 17, 247. [Google Scholar] [CrossRef] [Green Version]
- Teena, R.; Dhamodharan, U.; Ali, D.; Rajesh, K.; Ramkumar, K.M. Gene expression profiling of multiple histone deacetylases (HDAC) and Its correlation with NRF2-mediated redox regulation in the pathogenesis of diabetic foot ulcers. Biomolecules 2020, 10, 1466. [Google Scholar] [CrossRef]
- Kimball, A.S.; Joshi, A.; Carson, W.F., 4th; Boniakowski, A.E.; Schaller, M.; Allen, R.; Bermick, J.; Davis, F.M.; Henke, P.K.; Burant, C.F.; et al. The Histone Methyltransferase MLL1 Directs Macrophage-Mediated Inflammation in Wound Healing and Is Altered in a Murine Model of Obesity and Type 2 Diabetes. Diabetes 2017, 66, 2459–2471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawaya, A.P.; Pastar, I.; Stojadinovic, O.; Lazovic, S.; Davis, S.C.; Gil, J.; Kirsner, R.S.; Tomic-Canic, M. Topical mevastatin promotes wound healing by inhibiting the transcription factor c-Myc via the glucocorticoid receptor and the long non-coding RNA Gas5. J. Biol. Chem. 2018, 293, 1439–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Zhang, L.; Liechty, C.; Zgheib, C.; Hodges, M.M.; Liechty, K.W.; Xu, J. Long Noncoding RNA GAS5 Regulates Macrophage Polarization and Diabetic Wound Healing. J. Investig. Dermatol. 2020, 140, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Luqman, A.; Götz, F. The Ambivalent Role of Skin Microbiota and Adrenaline in Wound Healing and the Interplay between Them. Int. J. Mol. Sci. 2021, 22, 4996. [Google Scholar] [CrossRef]
- Flowers, L.; Grice, E.A. The Skin Microbiota: Balancing Risk and Reward. Cell Host Microbe 2020, 28, 190–200. [Google Scholar] [CrossRef]
- Grice, E.A.; Snitkin, E.S.; Yockey, L.J.; Bermudez, D.M.; NISC Comparative Sequencing Program; Liechty, K.W.; Segre, J.A. Longitudinal shift in diabetic wound microbiota correlates with prolonged skin defense response. Proc. Natl. Acad. Sci. USA 2010, 107, 14799–14804. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Ruegger, P.R.; Lebig, E.G.; Van Schalkwyk, S.; Jeske, D.R.; Hsiao, A.; Borneman, J.; Martins-Green, M. High Levels of Oxidative Stress Create a Microenvironment That Significantly Decreases the Diversity of the Microbiota in Diabetic Chronic Wounds and Promotes Biofilm Formation. Front. Cell Infect. Microbiol. 2020, 10, 259. [Google Scholar] [CrossRef]
- Jones, S.G.; Edwards, R.; Thomas, D.W. Inflammation and Wound Healing: The Role of Bacteria in the Immuno-Regulation of Wound Healing. Int. J. Low Extrem. Wounds 2004, 3, 201–208. [Google Scholar] [CrossRef]
- Romana-Souza, B.; Otranto, M.; Almeida, T.F.; Porto, L.C.; Monte-Alto-Costa, A. Stress-induced epinephrine levels compromise murine dermal fibroblast activity through beta-adrenoceptors. Exp. Dermatol. 2011, 20, 413–419. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, A.P.; Fox, J.M.; Pullar, C.E. Beta-Adrenoceptor Activation Reduces Both Dermal Microvascular Endothelial Cell Migration via a cAMP-Dependent Mechanism and Wound Angiogenesis. J. Cell Physiol. 2015, 230, 356–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romana-Souza, B.; Porto, L.C.; Monte-Alto-Costa, A. Cutaneous wound healing of chronically stressed mice is improved through catecholamines blockade. Exp. Derm. 2010, 19, 821–829. [Google Scholar] [CrossRef]
- Kim, M.H.; Gorouhi, F.; Ramirez, S.; Granick, J.L.; Byrne, B.A.; Soulika, A.M.; Simon, S.I.; Isseroff, R.R. Catecholamine stress alters neutrophil trafficking and impairs wound healing by beta2-adrenergic receptor-mediated upregulation of IL-6. J. Investig. Dermatol. 2014, 134, 809–817. [Google Scholar] [CrossRef] [Green Version]
- Rhoads, D.D.; Cox, S.B.; Rees, E.J.; Sun, Y.; Wolcott, R.D. Clinical identification of bacteria in human chronic wound infections: Culturing vs. 16S ribosomal DNA sequencing. BMC Infect. Dis. 2012, 12, 321. [Google Scholar] [CrossRef] [Green Version]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.J.; Moser, C.; Jensen, P.O.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Williams, H.; Campbell, L.; Crompton, R.A.; Singh, G.; McHugh, B.J.; Davidson, D.J.; McBain, A.J.; Cruickshank, S.M.; Hardman, M.J. Microbial Host Interactions and Impaired Wound Healing in Mice and Humans: Defining a Role for BD14 and NOD2. J. Investig. Dermatol. 2018, 138, 2264–2274. [Google Scholar] [CrossRef] [Green Version]
- Cambronel, M.; Nilly, F.; Mesguida, O.; Boukerb, A.M.; Racine, P.-J.; Baccouri, O.; Borrel, V.; Martel, J.; Fécamp, F.; Knowlton, R.; et al. Influence of Catecholamines (Epinephrine/Norepinephrine) on Biofilm Formation and Adhesion in Pathogenic and Probiotic Strains of Enterococcus faecalis. Front. Microbiol. 2020, 11, 1501. [Google Scholar] [CrossRef] [PubMed]
- Danilova, N.D.; Solovyeva, T.V.; Mart’Yanov, S.V.; Zhurina, M.V.; Gannesen, A.V. Stimulatory Effect of Epinephrine on Biofilms of Micrococcus luteus C01. Microbiology 2020, 89, 493–497. [Google Scholar] [CrossRef]
- Martinon, F.; Agostini, L.; Meylan, E.; Tschopp, J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr. Biol. 2004, 14, 1929–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meissner, M.H.; Eklof, B.; Smith, P.C.; Dalsing, M.C.; De Palma, R.G.; Gloviczki, P.; Moneta, G.; Neglén, P.; O’Donnell, T.; Partsch, H.; et al. Secondary chronic venous disorders. J. Vasc. Surg. 2007, 46, 68S–83S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alavi, A.; Sibbald, R.G.; Phillips, T.J.; Miller, O.F.; Margolis, D.J.; Marston, W.; Woo, K.; Romanelli, M.; Kirsner, R.S. What’s new: Management of venous leg ulcers: Treating venous leg ulcers. J. Am. Acad. Dermatol. 2016, 74, 643–664. [Google Scholar] [CrossRef]
- Gordon, P.; Widener, J.M.; Heffline, M. Venous leg ulcers: Impact and dysfunction of the venous system. J. Vasc. Nurs. 2015, 33, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Saharay, M.; Shields, D.A.; Porter, J.B.; Scurr, J.H.; Coleridge Smith, P.D. Leukocyte activity in the microcirculation of the leg in patients with chronic venous disease. J. Vasc. Surg. 1997, 26, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Nicolaides, A.N. Chronic venous disease and the leukocyte-endothelium interaction: From symptoms to ulceration. Angiology 2005, 56, S11–S19. [Google Scholar] [CrossRef]
- Moor, A.N.; Vachon, D.J.; Gould, L.J. Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Repair Regen. 2009, 17, 832–839. [Google Scholar] [CrossRef]
- Gill, S.E.; Parks, W.C. Metalloproteinases and their inhibitors: Regulators of wound healing. Int. J. Biochem. Cell Biol. 2008, 40, 1334–1347. [Google Scholar] [CrossRef] [Green Version]
- Amato, B.; Coretti, G.; Compagna, R.; Amato, M.; Buffone, G.; Gigliotti, D.; Grande, R.; Serra, R.; De Franciscis, S. Role of matrix metalloproteinases in non-healing venous ulcers. Int. Wound J. 2015, 12, 641–645. [Google Scholar] [CrossRef]
- Bergant Suhodolčan, A.; Luzar, B.; Kecelj Leskovec, N. Matrix metalloproteinase (MMP)-1 and MMP-2, but not COX-2 serve as additional predictors for chronic venous ulcer healing. Wound Repair Regen. 2021, 29, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Grande, R.; Buffone, G.; Molinari, V.; Perri, P.; Perri, A.; Amato, B.; Colosimo, M.; De Franciscis, S. Extracellular matrix assessment of infected chronic venous leg ulcers: Role of metalloproteinases and inflammatory cytokines. Int. Wound J. 2016, 13, 53–58. [Google Scholar] [CrossRef]
- Onida, S.; Tan, M.K.H.; Kafeza, M.; Bergner, R.T.; Shalhoub, J.; Holmes, E.; Davies, A.H. Metabolic Phenotyping in Venous Disease: The Need for Standardization. J. Proteome Res. 2019, 18, 3809–3820. [Google Scholar] [CrossRef]
- Babaei, S.; Ansarihadipour, H.; Nakhaei, M.; Darabi, M.; Bayat, P.; Sakhaei, M.; Baazm, M.; Mohammadhoseiny, A. Effect of Omegaven on mast cell concentration in diabetic wound healing. J. Tissue Viability 2017, 26, 125–130. [Google Scholar] [CrossRef]
- Jia, Y.C.; Qiu, S.; Xu, J.; Kang, Q.L.; Chai, Y.M. Docosahexaenoic Acid Improves Diabetic Wound Healing in a Rat Model by Restoring Impaired Plasticity of Macrophage Progenitor Cells. Plast. Reconstr. Surg. 2020, 145, 942e–950e. [Google Scholar] [CrossRef]
- Tian, H.; Lu, Y.; Shah, S.P.; Hong, S. 14S,21R-dihydroxydocosahexaenoic acid remedies impaired healing and mesenchymal stem cell functions in diabetic wounds. J. Biol. Chem. 2011, 286, 4443–4453. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Zhang, M.J.; Hellmann, J.; Kosuri, M.; Bhatnagar, A.; Spite, M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes 2013, 62, 618–627. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Tian, H.; Lu, Y.; Laborde, J.M.; Muhale, F.A.; Wang, Q.; Alapure, B.V.; Serhan, C.N.; Bazan, N.G. Neuroprotectin/protectin D1: Endogenous biosynthesis and actions on diabetic macrophages in promoting wound healing and innervation impaired by diabetes. Am. J. Physiol. Cell Physiol. 2014, 307, C1058–C1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gercek, A.; Yildirim, O.; Konya, D.; Bozkurt, S.; Ozgen, S.; Kilic, T.; Sav, A.; Pamir, N. Effects of parenteral fish-oil emulsion (Omegaven) on cutaneous wound healing in rats treated with dexamethasone. JPEN J. Parenter. Enter. Nutr. 2007, 31, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ruthig, D.J.; Meckling-Gill, K.A. Both (n-3) and (n-6) fatty acids stimulate wound healing in the rat intestinal epithelial cell line, IEC-6. J. Nutr. 1999, 129, 1791–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shingel, K.I.; Faure, M.P.; Azoulay, L.; Roberge, C.; Deckelbaum, R.J. Solid emulsion gel as a vehicle for delivery of polyunsaturated fatty acids: Implications for tissue repair, dermal angiogenesis and wound healing. J. Tissue Eng. Regen. Med. 2008, 2, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.R.; Souza, M.A.; Ferro, E.A.; Favoreto, S., Jr.; Pena, J.D. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen. 2004, 12, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.H.; Yan, S.; Wang, J.; Liang, Q.Y. Oral administration of docosahexaenoic acid activates the GDNF-MAPK-CERB pathway in hippocampus of natural aged rat. Pharm. Biol. 2013, 51, 1188–1195. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Zhong, W.; Shao, C.; Liu, P.; Wang, C.; Wang, Z.; Jiang, M.; Lu, Y.; Yan, J. Docosahexaenoic acid inhibits monocrotaline-induced pulmonary hypertension via attenuating endoplasmic reticulum stress and inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L243–L255. [Google Scholar] [CrossRef]
- Fasano, E.; Serini, S.; Cittadini, A.; Calviello, G. Long-chain n-3 PUFA against breast and prostate cancer: Which are the appropriate doses for intervention studies in animals and humans? Crit. Rev. Food Sci. Nutr. 2017, 57, 2245–2262. [Google Scholar] [CrossRef] [PubMed]
- Bougnoux, P.; Hajjaji, N.; Ferrasson, M.N.; Giraudeau, B.; Couet, C.; Le Floch, O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: A phase II trial. Br. J. Cancer 2009, 101, 1978–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleimani, Z.; Hashemdokht, F.; Bahmani, F.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. Clinical and metabolic response to flaxseed oil omega-3 fatty acids supplementation in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. J. Diabetes Complicat. 2017, 31, 1394–1400. [Google Scholar] [CrossRef]
- Brenna, J.T. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Lullove, E.J.; Liden, B.; Winters, C.; McEneaney, P.; Raphael, A.; Lantis Li, J.C. A Multicenter, Blinded, Randomized Controlled Clinical Trial Evaluating the Effect of Omega-3-Rich Fish Skin in the Treatment of Chronic, Nonresponsive Diabetic Foot Ulcers. Wounds 2021, 33, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, T.; Chant, T.; Chant, H. Treatment of diabetic foot wounds with acellular fish skin graft rich in omega-3: A prospective evaluation. J. Wound Care 2019, 28, 76–80. [Google Scholar] [CrossRef]
- He, J.; Bazan, H.E. Mapping the nerve architecture of diabetic human corneas. Ophthalmology 2012, 119, 956–964. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Hu, X.; Qi, X.; Di, G.; Zhang, Y.; Wang, Q.; Zhou, Q. Resolvin D1 promotes corneal epithelial wound healing and restoration of mechanical sensation in diabetic mice. Mol. Vis. 2018, 24, 274–285. [Google Scholar]
- Lee, H.N.; Surh, Y.J. Resolvin D1-mediated NOX2 inactivation rescues macrophages undertaking efferocytosis from oxidative stress-induced apoptosis. Biochem. Pharmacol. 2013, 86, 759–769. [Google Scholar] [CrossRef]
- David, J.A.; Rifkin, W.J.; Rabbani, P.S.; Ceradini, D.J. The Nrf2/ Keap1/ARE Pathway and Oxidative Stress as a Therapeutic Target in Type II Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 4826724. [Google Scholar] [CrossRef]
- Norling, L.V.; Headland, S.E.; Dalli, J.; Arnardottir, H.H.; Haworth, O.; Jones, H.R.; Irimia, D.; Serhan, C.N.; Perretti, M. Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI Insight 2016, 1, e85922. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Pham, T.L.; Kakazu, A.; Bazan, H.E.P. Recovery of Corneal Sensitivity and Increase in Nerve Density and Wound Healing in Diabetic Mice After PEDF Plus DHA Treatment. Diabetes 2017, 66, 2511–2520. [Google Scholar] [CrossRef] [Green Version]
- Manalac, J.; Mukherjee, P.K.; Bazan, N.G. Pigment epithelial derived factor (PEDF) and docosahexaenoic acid (DHA) induce antioxidant responsive element (ARE) upregulation in retinal pigment (RPE-19) cells. Invest. Ophthalmol. Vis. Sci. 2012, 53, 4780. [Google Scholar]
- McDaniel, J.C.; Szalacha, L.; Sales, M.; Roy, S.; Chafee, S.; Parinandi, N. EPA + DHA supplementation reduces PMN activation in microenvironment of chronic venous leg ulcers: A randomized, double-blind, controlled study. Wound Repair Regen. 2017, 25, 680–690. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, J.C.; Kemmner, K.G.; Rusnak, S. Nutritional profile of older adults with chronic venous leg ulcers: A pilot study. Geriatr. Nurs. 2015, 36, 381–386. [Google Scholar] [CrossRef] [Green Version]
- Grenon, S.M.; Owens, C.D.; Alley, H.; Chong, K.; Yen, P.K.; Harris, W.; Hughes-Fulford, M.; Conte, M.S. n-3 polyunsaturated fatty acids supplementation in peripheral artery disease: The OMEGA-PAD trial. Vasc. Med. 2013, 18, 263–274. [Google Scholar] [CrossRef]
- Calviello, G.; Palozza, P.; Franceschelli, P.; Bartoli, G.M. Low-dose eicosapentaenoic or docosahexaenoic acid administration modifies fatty acid composition and does not affect susceptibility to oxidative stress in rat erythrocytes and tissues. Lipids 1997, 32, 1075–1083. [Google Scholar] [CrossRef]
- McDaniel, J.C.; Belury, M.; Ahijevych, K.; Blakely, W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008, 16, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Beidler, S.K.; Douillet, C.D.; Berndt, D.F.; Keagy, B.A.; Rich, P.B.; Marston, W.A. Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression therapy. Wound Repair Regen. 2008, 16, 642–648. [Google Scholar] [CrossRef]
- Serena, T.E.; Cullen, B.M.; Bayliff, S.W.; Gibson, M.C.; Carter, M.J.; Chen, L.; Yaakov, R.A.; Samies, J.; Sabo, M.; DeMarco, D.; et al. Defining a new diagnostic assessment parameter for wound care: Elevated protease activity, an indicator of nonhealing, for targeted protease-modulating treatment. Wound Repair Regen. 2016, 24, 589–595. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. PUFA, inflammatory processes and rheumatoid arthritis. Proc. Nutr. Soc. 2008, 67, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinohara, M.; Mirakaj, V.; Serhan, C.N. Functional Metabolomics Reveals Novel Active Products in the DHA Metabolome. Front. Immunol. 2012, 3, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaydakov, M.E.; Ting, W.; Sadek, M.; Aziz, F.; Diaz, J.A.; Raffetto, J.D.; Marston, W.A.; Lal, B.K.; Welch, H.J.; American Venous Forum Research Committee. Review of the Current Evidence for Topical Treatment for Venous Leg Ulcers. J. Vasc. Surg. Venous Lymphat. Disord. 2021. [Google Scholar] [CrossRef] [PubMed]
- Calviello, G.; Resci, F.; Serini, S.; Piccioni, E.; Toesca, A.; Boninsegna, A.; Monego, G.; Ranelletti, F.O.; Palozza, P. Docosahexaenoic acid induces proteasome-dependent degradation of beta-catenin, down-regulation of survivin and apoptosis in human colorectal cancer cells not expressing COX-2. Carcinogenesis 2007, 28, 1202–1209. [Google Scholar] [CrossRef] [Green Version]
- Serini, S.; Zinzi, A.; Ottes Vasconcelos, R.; Fasano, E.; Riillo, M.G.; Celleno, L.; Trombino, S.; Cassano, R.; Calviello, G. Role of β-catenin signaling in the anti-invasive effect of the omega-3 fatty acid DHA in human melanoma cells. J. Dermatol. Sci. 2016, 84, 149–159. [Google Scholar] [CrossRef]
- Kim, H.H.; Shin, C.M.; Park, C.H.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Eicosapentaenoic acid inhibits UV-induced MMP-1 expression in human dermal fibroblasts. J. Lipid Res. 2005, 46, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.H.; Cho, S.; Lee, S.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Photoprotective and anti-skin-aging effects of eicosapentaenoic acid in human skin in vivo. J. Lipid Res. 2006, 47, 921–930. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.E.; Nho, Y.H.; Yun, S.K.; Park, S.M.; Kang, S.; Yeo, H. Caviar Extract and Its Constituent DHA Inhibits UVB-Irradiated Skin Aging by Inducing Adiponectin Production. Int. J. Mol. Sci. 2020, 21, 3383. [Google Scholar] [CrossRef]
- Artiach, G.; Carracedo, M.; Plunde, O.; Wheelock, C.E.; Thul, S.; Sjövall, P.; Franco-Cereceda, A.; Laguna-Fernandez, A.; Arnardottir, H.; Bäck, M. Omega-3 Polyunsaturated Fatty Acids Decrease Aortic Valve Disease Through the Resolvin E1 and ChemR23 Axis. Circulation 2020, 142, 776–789. [Google Scholar] [CrossRef]
- Li, D.; Wang, M.; Ye, J.; Zhang, J.; Xu, Y.; Wang, Z.; Zhao, M.; Ye, D.; Wan, J. Maresin 1 alleviates the inflammatory response, reduces oxidative stress and protects against cardiac injury in LPS-induced mice. Life Sci. 2021, 277, 119467. [Google Scholar] [CrossRef]
- Qiao, N.; Lin, Y.; Wang, Z.; Chen, J.Y.; Ge, Y.Y.; Yao, S.L.; Gong, J. Maresin1 Promotes M2 Macrophage Polarization Through Peroxisome Proliferator-Activated Receptor-γ Activation to Expedite Resolution of Acute Lung Injury. J. Surg. Res. 2020, 256, 584–594. [Google Scholar] [CrossRef]
- Serini, S.; Calviello, G. Reduction of Oxidative/Nitrosative Stress in Brain and its Involvement in the Neuroprotective Effect of n-3 PUFA in Alzheimer’s Disease. Curr. Alzheimer Res. 2016, 13, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Fiala, M.; Kooij, G.; Wagner, K.; Hammock, B.; Pellegrini, M. Modulation of innate immunity of patients with Alzheimer’s disease by omega-3 fatty acids. FASEB J. 2017, 31, 3229–3239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huoman, J.; Martínez-Enguita, D.; Olsson, E.; Ernerudh, J.; Nilsson, L.; Duchén, K.; Gustafsson, M.; Jenmalm, M.C. Combined prenatal Lactobacillus reuteri and ω-3 supplementation synergistically modulates DNA methylation in neonatal T helper cells. Clin. Epigenetics 2021, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, S.; Zamorano, M.J.; Xu, H.; Fernández-Palacios, H.; Robaina, L.; Kaushik, S.; Izquierdo, M. Parental LC-PUFA biosynthesis capacity and nutritional intervention with alpha-linolenic acid affect performance of Sparus aurata progeny. J. Exp. Biol. 2020, 223, jeb214999. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of omega-3 fatty acids on the gut microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menni, C.; Zierer, J.; Pallister, T.; Jackson, M.A.; Long, T.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Omega-3 Fatty Acids Correlate with Gut Microbiome Diversity and Production of N-Carbamylglutamate in Middle Aged and ElderlyWomen. Sci. Rep. 2017, 7, 11079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellenger, J.; Bellenger, S.; Escoula, Q.; Bidu, C.; Narce, M. N-3 polyunsaturated fatty acids: An innovative strategy against obesity and related metabolic disorders, intestinal alteration and gut microbiota dysbiosis. Biochimie 2019, 159, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kaliannan, K.; Wang, B.; Li, X.Y.; Bhan, A.K.; Kang, J.X. Omega-3 fatty acids prevent early-life antibiotic exposure-induced gut microbiota dysbiosis and later-life obesity. Int. J. Obes. 2016, 40, 1039–1042. [Google Scholar] [CrossRef]
- Gui, L.; Chen, S.; Wang, H.; Ruan, M.; Liu, Y.; Li, N.; Zhang, H.; Liu, Z. ϖ-3 PUFAs alleviate high-fat diet-induced circadian intestinal microbes dysbiosis. Mol. Nutr. Food Res. 2019, 63, e1900492. [Google Scholar] [CrossRef]
- Hantsoo, L.; Zemel, B.S. Stress Gets into the Belly: Early Life Stress and the Gut Microbiome. Behav. Brain Res. 2021, 414, 113474. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serini, S.; Calviello, G. New Insights on the Effects of Dietary Omega-3 Fatty Acids on Impaired Skin Healing in Diabetes and Chronic Venous Leg Ulcers. Foods 2021, 10, 2306. https://doi.org/10.3390/foods10102306
Serini S, Calviello G. New Insights on the Effects of Dietary Omega-3 Fatty Acids on Impaired Skin Healing in Diabetes and Chronic Venous Leg Ulcers. Foods. 2021; 10(10):2306. https://doi.org/10.3390/foods10102306
Chicago/Turabian StyleSerini, Simona, and Gabriella Calviello. 2021. "New Insights on the Effects of Dietary Omega-3 Fatty Acids on Impaired Skin Healing in Diabetes and Chronic Venous Leg Ulcers" Foods 10, no. 10: 2306. https://doi.org/10.3390/foods10102306
APA StyleSerini, S., & Calviello, G. (2021). New Insights on the Effects of Dietary Omega-3 Fatty Acids on Impaired Skin Healing in Diabetes and Chronic Venous Leg Ulcers. Foods, 10(10), 2306. https://doi.org/10.3390/foods10102306





