The Impact of Childhood Obesity on Adult Dental Caries: A Mendelian Randomization Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Selection of Genetic Instruments
2.3. Statistical Analysis
2.4. Sensitivity and Validation Analysis
3. Results
3.1. Childhood Obesity and Dental Caries
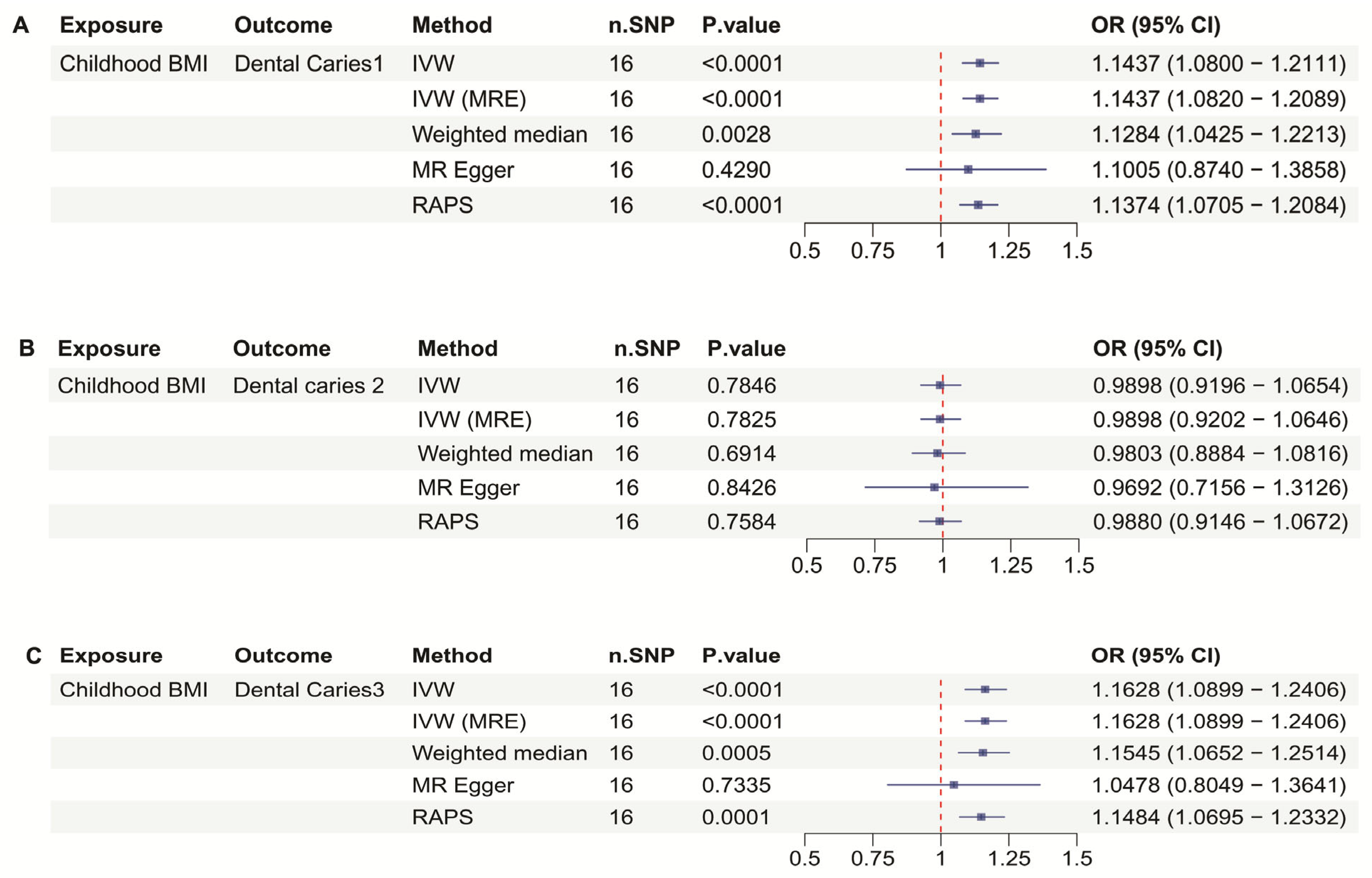
3.2. Childhood BMI and Dental Caries
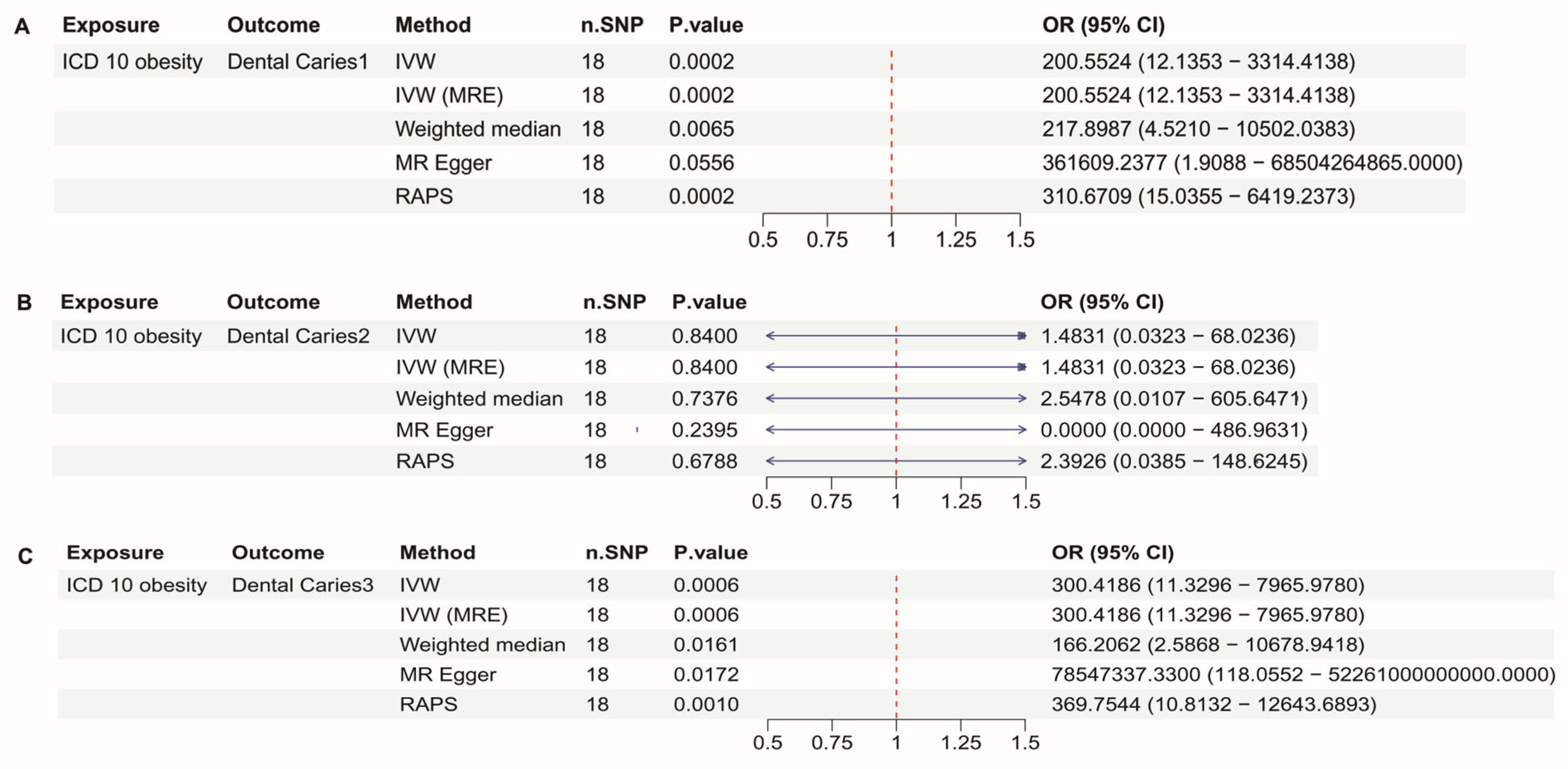
3.3. ICD10 Obesity and Dental Caries
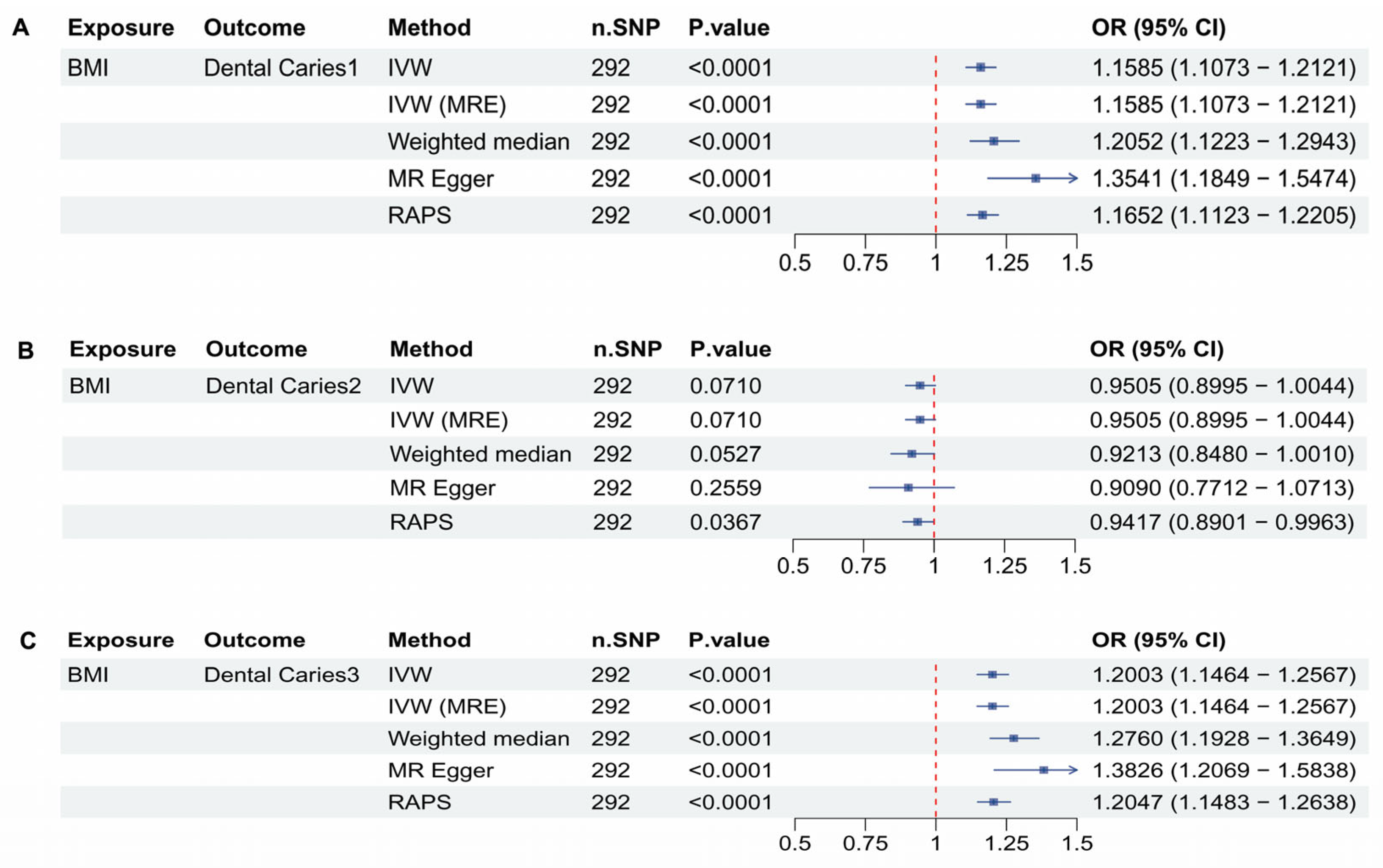
3.4. BMI and Dental Caries
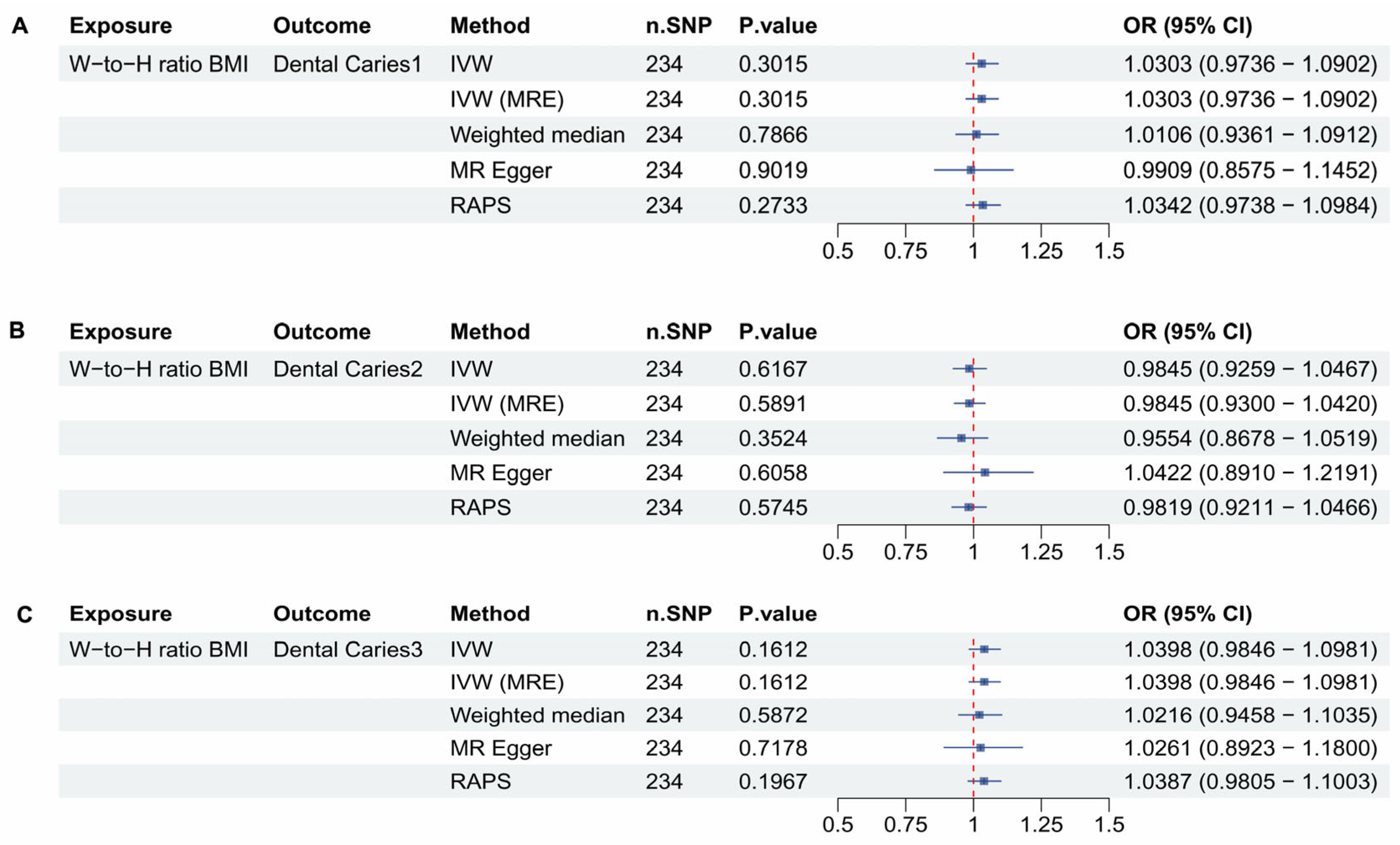
3.5. Waist to Hip Ratio Adjusted to BMI and Dental Caries
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kidd, E.A.; Fejerskov, O. What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms. J. Dent. Res. 2004, 83, C35–C38. [Google Scholar] [CrossRef]
- Kidd, E.A.; Giedrys-Leeper, E.; Simons, D. Take two dentists: A tale of root caries. Dent. Updat. 2000, 27, 222–230. [Google Scholar] [CrossRef]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Anderson, M. Risk assessment and epidemiology of dental caries: Review of the literature. Pediatr. Dent. 2002, 24, 377–385. [Google Scholar]
- Hong, Y.; Ullah, R.; Wang, J.B.; Fu, J.F. Trends of obesity and overweight among children and adolescents in China. World J. Pediatr. 2023, 19, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Muyulema, S.L.; Carpio-Arias, T.V.; Verdezoto, N.; Guanga Lara, V.E.; Manzano, A.S.; Pulgar, H.; Vinueza Veloz, M.F. Worldwide trends in childhood overweight and obesity over the last 20 years. Clin. Nutr. ESPEN 2025, 65, 453–460. [Google Scholar] [CrossRef]
- Sahoo, K.; Sahoo, B.; Choudhury, A.K.; Sofi, N.Y.; Kumar, R.; Bhadoria, A.S. Childhood obesity: Causes and consequences. J. Fam. Med. Prim. Care 2015, 4, 187–192. [Google Scholar] [CrossRef]
- Hayden, C.; Bowler, J.O.; Chambers, S.; Freeman, R.; Humphris, G.; Richards, D.; Cecil, J.E. Obesity and dental caries in children: A systematic review and meta-analysis. Community Dent. Oral Epidemiol. 2013, 41, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.; Mohamed, R.N.; Swamy, H.S.; Ramamurthy, P.H.; Sexena, V. Caries incidence Among Obese Adolescents: A 3-year Prospective Study. Oral Health Prev. Dent. 2017, 15, 65–71. [Google Scholar] [CrossRef]
- Alshihri, A.A.; Rogers, H.J.; Alqahtani, M.A.; Aldossary, M.S. Association between Dental Caries and Obesity in Children and Young People: A Narrative Review. Int. J. Dent. 2019, 2019, 9105759. [Google Scholar] [CrossRef]
- Kabbarah, A.J.; Samman, M.; Alwafi, A.A.; Ashi, H.; Abuljadayel, L.W.; Bahanan, L.O.; Rajeh, M.T.; Farsi, N.J. Association between Obesity and Dental Caries in Adults: An Analysis of WHR, and DMFT Score. Obes. Facts 2025, 18, 39–47. [Google Scholar] [CrossRef]
- Kim, K.; Han, K.; Yang, S. Association between overweight, obesity and incidence of advanced dental caries in South Korean adults: A 10-year nationwide population-based observational study. PLoS ONE 2020, 15, e0229572. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, Y.F.A.; Park, J.S.; Kruger, E.; Tennant, M. Association between body mass index and dental caries in the Kingdom of Saudi Arabia: Systematic review. Saudi Dent. J. 2020, 32, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Alm, A.; Isaksson, H.; Fåhraeus, C.; Koch, G.; Andersson-Gäre, B.; Nilsson, M.; Birkhed, D.; Wendt, L.K. BMI status in Swedish children and young adults in relation to caries prevalence. Swed. Dent. J. 2011, 35, 1–8. [Google Scholar] [PubMed]
- Marshall, T.A.; Eichenberger-Gilmore, J.M.; Broffitt, B.A.; Warren, J.J.; Levy, S.M. Dental caries and childhood obesity: Roles of diet and socioeconomic status. Community Dent. Oral 2007, 35, 449–458. [Google Scholar] [CrossRef]
- Ribeiro, C.C.C.; Da Silva, M.C.B.; Nunes, A.M.M.; Thomaz, E.B.D.F.; Carmo, C.D.S.; Ribeiro, M.R.C.; Da Silva, A.A.M. Overweight, obese, underweight, and frequency of sugar consumption as risk indicators for early childhood caries in Brazilian preschool children. Int. J. Paediatr. Dent. 2017, 27, 532–539. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef]
- Davey Smith, G.; Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Human Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef]
- Fazia, T.; Baldrighi, G.N.; Nova, A.; Bernardinelli, L. A systematic review of Mendelian randomization studies on multiple sclerosis. Eur. J. Neurosci. 2023, 58, 3172–3194. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Kutalik, Z.; Holmes, M.V.; Minelli, C.; et al. Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Timpson, N.J.; Higgins, J.P.T.; Dimou, N.; Langenberg, C.; et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): Explanation and elaboration. BMJ 2021, 375, n2233. [Google Scholar] [CrossRef]
- Bradfield, J.P.; Taal, H.R.; Timpson, N.J.; Scherag, A.; Lecoeur, C.; Warrington, N.M.; Hypponen, E.; Holst, C.; Valcarcel, B.; Thiering, E.; et al. A genome-wide association meta-analysis identifies new childhood obesity loci. Nat. Genet. 2012, 44, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipila, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Vogelezang, S.; Bradfield, J.P.; Ahluwalia, T.S.; Curtin, J.A.; Lakka, T.A.; Grarup, N.; Scholz, M.; van der Most, P.J.; Monnereau, C.; Stergiakouli, E.; et al. Novel loci for childhood body mass index and shared heritability with adult cardiometabolic traits. PLoS Genet. 2020, 16, e1008718. [Google Scholar] [CrossRef]
- Loh, P.R.; Kichaev, G.; Gazal, S.; Schoech, A.P.; Price, A.L. Mixed-model association for biobank-scale datasets. Nat. Genet. 2018, 50, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Wightman, D.P.; Savage, J.E.; de Leeuw, C.A.; Jansen, I.E.; Posthuma, D. Rare variant aggregation in 148,508 exomes identifies genes associated with proxy dementia. Sci. Rep. 2023, 13, 2179. [Google Scholar] [CrossRef]
- Barton, A.R.; Sherman, M.A.; Mukamel, R.E.; Loh, P.R. Whole-exome imputation within UK Biobank powers rare coding variant association and fine-mapping analyses. Nat. Genet. 2021, 53, 1260–1269. [Google Scholar] [CrossRef]
- Wang, X.; Gao, H.; Zeng, Y.; Chen, J. A Mendelian analysis of the relationships between immune cells and breast cancer. Front. Oncol. 2024, 14, 1341292. [Google Scholar] [CrossRef]
- Kamat, M.A.; Blackshaw, J.A.; Young, R.; Surendran, P.; Burgess, S.; Danesh, J.; Butterworth, A.S.; Staley, J.R. PhenoScanner V2: An expanded tool for searching human genotype-phenotype associations. Bioinformatics 2019, 35, 4851–4853. [Google Scholar] [CrossRef]
- Burgess, S.; Dudbridge, F.; Thompson, S.G. Combining information on multiple instrumental variables in Mendelian randomization: Comparison of allele score and summarized data methods. Stat. Med. 2016, 35, 1880–1906. [Google Scholar] [CrossRef]
- Burgess, S.; Scott, R.A.; Timpson, N.J.; Davey Smith, G.; Thompson, S.G.; Consortium, E.-I. Using published data in Mendelian randomization: A blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol. 2015, 30, 543–552. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Davey Smith, G.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Boehm, F.J.; Zhou, X. Statistical methods for Mendelian randomization in genome-wide association studies: A review. Comput. Struct. Biotechnol. J. 2022, 20, 2338–2351. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Adams, R.J.; Berry, J.D.; Brown, T.M.; Carnethon, M.R.; Dai, S.; de Simone, G.; Ford, E.S.; et al. Heart disease and stroke statistics—2011 update: A report from the American Heart Association. Circulation 2011, 123, e18–e209. [Google Scholar] [CrossRef] [PubMed]
- Writing Group, M.; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef]
- Logan, W.H.; Kronfeld, R. Development of the Human Jaws and Surrounding Structures from Birth to the Age of Fifteen Years. J. Am. Dent. Assoc. 1933, 20, 379–428. [Google Scholar]
- Anu, V.; Brindha, J.R.; Carol, P.T.; Diana, P.C.; Elsy, J.D.; Garima, S. Does Body Mass Index affect Tooth Eruption Sequence? A Study among 6-7 Years Old Schoolchildren in Chennai, India. Int. J. Clin. Pediatr. Dent. 2020, 13, 261–263. [Google Scholar] [CrossRef]
- Farges, J.C.; Alliot-Licht, B.; Renard, E.; Ducret, M.; Gaudin, A.; Smith, A.J.; Cooper, P.R. Dental Pulp Defence and Repair Mechanisms in Dental Caries. Mediat. Inflamm. 2015, 2015, 230251. [Google Scholar] [CrossRef]
- Couve, E.; Osorio, R.; Schmachtenberg, O. Reactionary Dentinogenesis and Neuroimmune Response in Dental Caries. J. Dent. Res. 2014, 93, 788–793. [Google Scholar] [CrossRef]
- Maffeis, C.; Moghetti, P.; Grezzani, A.; Clementi, M.; Gaudino, R.; Tato, L. Insulin resistance and the persistence of obesity from childhood into adulthood. J. Clin. Endocrinol. Metab. 2002, 87, 71–76. [Google Scholar] [CrossRef] [PubMed]
- McNay, E.C.; Recknagel, A.K. Brain insulin signaling: A key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol. Learn. Mem. 2011, 96, 432–442. [Google Scholar] [CrossRef]
- Amir, M.; Jeevithan, L.; Barkat, M.; Fatima, S.H.; Khan, M.; Israr, S.; Naseer, F.; Fayyaz, S.; Elango, J.; Wu, W.; et al. Advances in Regenerative Dentistry: A Systematic Review of Harnessing Wnt/beta-Catenin in Dentin-Pulp Regeneration. Cells 2024, 13, 1153. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Majeed, F.; Penumetcha, R.; Flack, J.M.; Brook, R.D. Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both: A 2 × 2 factorial Mendelian randomization study. J. Am. Coll. Cardiol. 2015, 65, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.L.; Wang, S.; Wang, L.Y.; Xiao, L.X.; Yao, T.X.; Zeng, Y.; Zhang, L. Childhood Obesity and Risk of Stroke: A Mendelian Randomisation Analysis. Front. Genet. 2021, 12, 727475. [Google Scholar] [CrossRef]
- Pereira Ciochetti, N.; Lugli-Moraes, B.; Santos da Silva, B.; Rovaris, D.L. Genome-wide association studies: Utility and limitations for research in physiology. J. Physiol. 2023, 601, 2771–2799. [Google Scholar] [CrossRef]
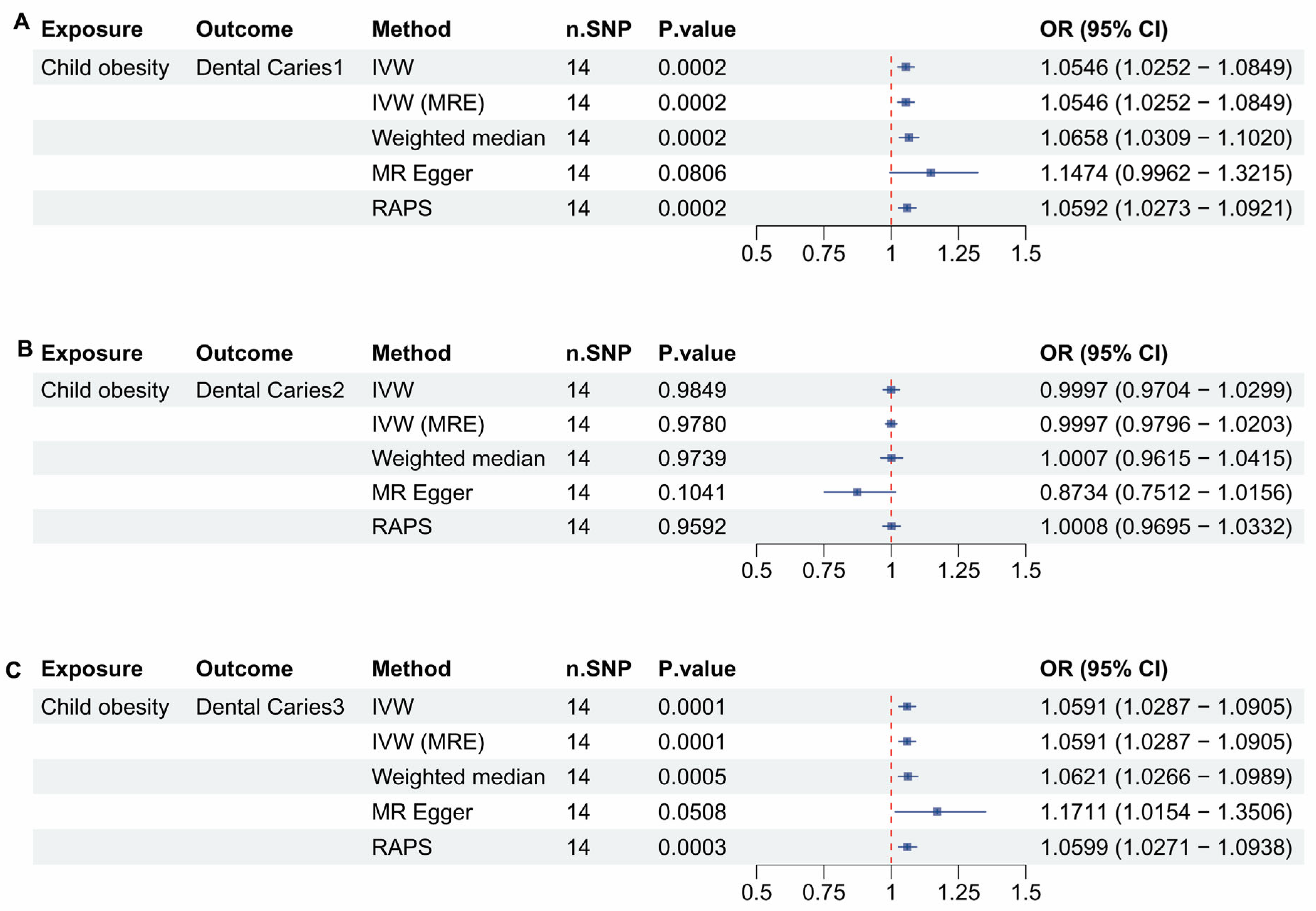
| Outcome in Childhood Obesity | IVW OR 95%Cl | IVW p-Value | MRE OR 95%Cl | MRE p-Value | WM OR 95%Cl | WM p-Value | MR Egger OR 95%Cl | MR Egger p-Value | RAPS OR 95%Cl | RAPS p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Caries 1 | 1.0546 (1.0252–1.0849) | 0.0002 | 1.0546 (1.0252–1.0849) | 0.0002 | 1.0658 (1.0309–1.1020) | 0.0002 | 1.1474 (0.9962–1.3215) | 0.0806 | 1.0592 (1.0273–1.0921) | 0.0002 |
| Caries 2 | Not valid | 0.9849 | Not valid | 0.9780 | Not valid | 0.9739 | Not valid | 0.1041 | Not valid | 0.9592 |
| Caries 3 | 1.0591 (1.0287–1.0905) | 0.0001 | 1.0591 (1.0287–1.0905) | 0.0001 | 1.0621 (1.0621–1.0989) | 0.0005 | Not valid | 0.0508 | 1.0599 (1.0271–1.0938) | 0.0003 |
| Outcome in Childhood BMI | IVW OR 95%Cl | IVW p-Value | MRE OR 95%Cl | MRE p-Value | WM OR 95%Cl | WM p-Value | MR Egger OR 95%Cl | MR egger p-Value | RAPS OR 95%Cl | RAPS p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Caries 1 | 1.1437 (1.0800–1.2111) | <0.0001 | 1.1437 (1.0820–1.2089) | <0.0001 | 1.1284 (1.0425–1.2213) | 0.0028 | 1.1005 (0.8740–1.3858) | 0.4290 | 1.1374 (1.0705–1.2084) | <0.0001 |
| Caries 2 | Not Valid | 0.7846 | Not valid | 0.7825 | Not valid | 0.6914 | Not valid | 0.8426 | Not valid | 0.7584 |
| Caries 3 | 1.1628 (1.0899–1.2406) | <0.0001 | 1.1628 (1.0899–1.2406) | <0.0001 | 1.1545 (1.0652–1.2514) | 0.0005 | Not valid | 0.7335 | 1.1484 (1.0695–1.2332) | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.W.; Wu, J.; Lim, S.W.; Lim, S.; Choi, S.-J.; Lee, T.-K.; Choi, S.C.; Kim, D.W. The Impact of Childhood Obesity on Adult Dental Caries: A Mendelian Randomization Study. Dent. J. 2025, 13, 573. https://doi.org/10.3390/dj13120573
Kim YW, Wu J, Lim SW, Lim S, Choi S-J, Lee T-K, Choi SC, Kim DW. The Impact of Childhood Obesity on Adult Dental Caries: A Mendelian Randomization Study. Dentistry Journal. 2025; 13(12):573. https://doi.org/10.3390/dj13120573
Chicago/Turabian StyleKim, Yeon Woo, Junhua Wu, Sun Woo Lim, Seongjin Lim, Su-Jeong Choi, Tae-Kyeong Lee, Sung Chul Choi, and Dong Woon Kim. 2025. "The Impact of Childhood Obesity on Adult Dental Caries: A Mendelian Randomization Study" Dentistry Journal 13, no. 12: 573. https://doi.org/10.3390/dj13120573
APA StyleKim, Y. W., Wu, J., Lim, S. W., Lim, S., Choi, S.-J., Lee, T.-K., Choi, S. C., & Kim, D. W. (2025). The Impact of Childhood Obesity on Adult Dental Caries: A Mendelian Randomization Study. Dentistry Journal, 13(12), 573. https://doi.org/10.3390/dj13120573






