Ozone Therapy in Medicine and Dentistry: A Review of the Literature
Abstract
1. Introduction
1.1. Mode of Action of Ozone
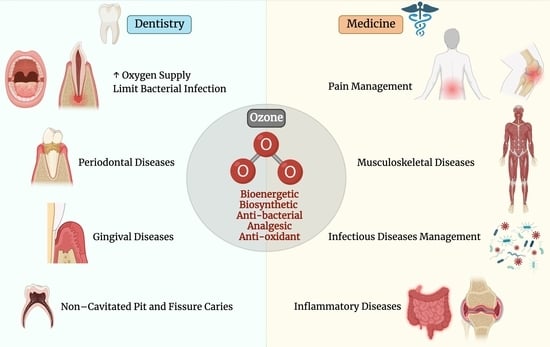
1.2. Applications of Ozone in Medicine
1.3. Applications of Ozone in Dentistry
2. Methods
3. Results
4. The Uses of Ozone in Medicine
4.1. Effect of Ozone on Pain Management
4.2. Effect of Ozone on Musculoskeletal Diseases
4.3. Effect of Ozone on Infectious Disease Management
5. The Uses of Ozone in Dentistry
5.1. Effect of Ozone on Soft Tissues
5.2. Effect of Ozone on Dental Hard Tissues
5.3. Effect of Ozone on Physical Properties of Enamel and Dentin
5.4. Ozone in the Management of Non-Cavitated Pit and Fissure Caries
5.5. Ozone in the Management of Cavitated Occlusal Carious Lesions
5.6. Role of Ozone in Oral Medicine and Periodontology
5.7. Ozone and Dental Unit Water Lines
6. Safety, Toxicity, and Contraindications of Ozone
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barbe, A.; Mikhailenko, S.; Starikova, E.; Tyuterev, V. High Resolution Infrared Spectroscopy in Support of Ozone Atmospheric Monitoring and Validation of the Potential Energy Function. Molecules 2022, 27, 911. [Google Scholar] [CrossRef] [PubMed]
- Baysan, A.; Lynch, E. The use of ozone in dentistry and medicine. Prim. Dent. Care 2005, 12, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.G.; de Oliveira, L.D.; Koga-Ito, C.Y.; Jorge, A.O. Effectiveness of ozonated water on Candida albicans, Enterococcus faecalis, and endotoxins in root canals. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2008, 105, e85–e91. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.; Zanardi, I.; Travagli, V. Oxygen/ozone as a medical gas mixture. A critical evaluation of the various methods clarifies positive and negative aspects. Med. Gas Res. 2011, 1, 6. [Google Scholar] [CrossRef]
- Re, K.; Gandhi, J.; Liang, R.; Patel, S.; Joshi, G.; Smith, N.L.; Reid, I.; Khan, S.A. Clinical utility of ozone therapy and hyperbaric oxygen therapy in degenerative disc disease. Med. Gas Res. 2023, 13, 1–6. [Google Scholar] [CrossRef]
- Bocci, V. Is it true that ozone is always toxic? The end of a dogma. Toxicol. Appl. Pharmacol. 2006, 216, 493–504. [Google Scholar] [CrossRef]
- Hidalgo-Tallón, F.J.; Torres-Morera, L.M.; Baeza-Noci, J.; Carrillo-Izquierdo, M.D.; Pinto-Bonilla, R. Updated Review on Ozone Therapy in Pain Medicine. Front. Physiol. 2022, 13, 840623. [Google Scholar] [CrossRef]
- Alfaro, M.F.; Walby, W.F.; Adams, W.C.; Schelegle, E.S. Breath condensate levels of 8-isoprostane and leukotriene B4 after ozone inhalation are greater in sensitive versus nonsensitive subjects. Exp. Lung Res. 2007, 33, 115–133. [Google Scholar] [CrossRef]
- Martínez-Sánchez, G.; Al-Dalain, S.M.; Menéndez, S.; Re, L.; Giuliani, A.; Candelario-Jalil, E.; Alvarez, H.; Fernández-Montequín, J.I.; León, O.S. Therapeutic efficacy of ozone in patients with diabetic foot. Eur. J. Pharmacol. 2005, 523, 151–161. [Google Scholar] [CrossRef]
- Tatyana, P.; Pamela Guerra, B.; Arizbeth Pérez, M.; Isaac Chairez, O.; Clara, L.S.C. Ozone Dosage is the Key Factor of Its Effect in Biological Systems. In Ozone in Nature and Practice; Ján, D., Marian, K., Eds.; IntechOpen: Rijeka, Croatia, 2018; Chapter 3. [Google Scholar]
- Ajamieh, H.H.; Berlanga, J.; Merino, N.; Sánchez, G.M.; Carmona, A.M.; Cepero, S.M.; Giuliani, A.; Re, L.; León, O.S. Role of protein synthesis in the protection conferred by ozone-oxidative-preconditioning in hepatic ischaemia/reperfusion. Transpl. Int. 2005, 18, 604–612. [Google Scholar] [CrossRef]
- Bocci, V.A. Why orthodox medicine has not yet taken advantage of ozone therapy. Arch. Med. Res. 2008, 39, 259–260. [Google Scholar] [CrossRef]
- Mawsouf, M.N.; Tanbouli, T.T.; Viebahn-Hänsler, R. Ozone Therapy in Patients with Viral Hepatitis C: Ten Years’ Experience. Ozone Sci. Eng. 2012, 34, 451–458. [Google Scholar] [CrossRef]
- Shah, M.A.; Anande, L.K.; Powar, A.; Captain, J.; Mk Nair, P. The Role of Medical Ozone in Improving Antioxidant Status in Multiple Drug-Resistant Tuberculosis Patients: A Quasi-experimental Study. Middle East J. Rehabil. Health Stud. 2019, 6, e97125. [Google Scholar] [CrossRef]
- Dobkin, V.G.; Sadovnikova, S.S.; Kuz’min, G.P.; Bondarev, G.B. [Local ozone therapy in the complex surgical treatment of pulmonary and pleural tuberculosis patients]. Probl. Tuberk. 2001, 7, 18–20. [Google Scholar]
- Galluccio, F. Rapid and Sustained Effect of Ozone Major Autohemotherapy for Raynaud and Hand Edema in Systemic Sclerosis Patient: A Case Report. Cureus 2022, 14, e31831. [Google Scholar] [CrossRef]
- Hidalgo-Tallón, J.; Menéndez-Cepero, S.; Vilchez, J.S.; Rodríguez-López, C.M.; Calandre, E.P. Ozone therapy as add-on treatment in fibromyalgia management by rectal insufflation: An open-label pilot study. J. Altern. Complement. Med. 2013, 19, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Cespedes-Suarez, J.; Martin-Serrano, Y.; Carballosa-Peña, M.R.; Dager-Carballosa, D.R. The immune response behavior in HIV-AIDS patients treated with ozone therapy for two years. J. Ozone Ther. 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Takahashi, H.; Fujimoto, C.; Matsui, H.; Igarashi, T.; Shiwa, T.; Ohara, K.; Sugita, T. Anterior chamber irrigation with an ozonated solution as prophylaxis against infectious endophthalmitis. J. Cataract. Refract. Surg. 2004, 30, 1773–1780. [Google Scholar] [CrossRef]
- Bocci, V. The Actual Six Therapeutic Modalities. In OZONE: A New Medical Drug; Bocci, V., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 35–74. [Google Scholar]
- Bocci, V. Physical-Chemical Properties of Ozone—Natural Production of Ozone: The Toxicology of Ozone. In OZONE: A New Medical Drug; Bocci, V., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 1–4. [Google Scholar]
- Lipatov, K.V.; Sopromadze, M.A.; Shekhter, A.B.; Rudenko, T.G.; Emel’ianov, A. [Ozone-ultrasonic therapy in the treatment of purulent wounds]. Khirurgiia 2002, 1, 36–39. [Google Scholar]
- Rickard, G.D.; Richardson, R.; Johnson, T.; McColl, D.; Hooper, L. Ozone therapy for the treatment of dental caries. Cochrane Database Syst. Rev. 2004, 3, CD004153. [Google Scholar] [CrossRef]
- Dukic, W.; Juric, H.; Andrasevic, A.T.; Kovacevic, V.; Dukic, O.L.; Delija, B. The efficacy of gaseous ozone on some cariogenic bacteria. Coll. Antropol. 2013, 37, 109–113. [Google Scholar]
- Kollmuss, M.; Kist, S.; Obermeier, K.; Pelka, A.K.; Hickel, R.; Huth, K.C. Antimicrobial effect of gaseous and aqueous ozone on caries pathogen microorganisms grown in biofilms. Am. J. Dent. 2014, 27, 134–138. [Google Scholar]
- Rangel, K.; Cabral, F.O.; Lechuga, G.C.; Carvalho, J.P.R.S.; Villas-Bôas, M.H.S.; Midlej, V.; De-Simone, S.G. Detrimental Effect of Ozone on Pathogenic Bacteria. Microorganisms 2022, 10, 40. [Google Scholar] [CrossRef]
- AbuNaba’a, L.; Al Shorman, H.; Holmes, J.; Peterson, L.; Tagami, J.; Lynch, E. Evidence-based research into ozone treatment in dentistry: An overview. In Ozone: The Revolution in Dentistry; Quintessence Publishing Co.: London, UK, 2004; pp. 73–115. [Google Scholar]
- Grootveld, M.; Baysan, A.; Sidiiqui, N.; Sim, J.; Silwood, C.; Lynch, E. History of the clinical applications of ozone. In Ozone: The Revolution in Dentistry; Quintessence Publishing Co.: London, UK, 2004; pp. 23–30. [Google Scholar]
- Han, S.G.; Andrews, R.; Gairola, C.G.; Bhalla, D.K. Acute pulmonary effects of combined exposure to carbon nanotubes and ozone in mice. Inhal. Toxicol. 2008, 20, 391–398. [Google Scholar] [CrossRef]
- Elvis, A.M.; Ekta, J.S. Ozone therapy: A clinical review. J. Nat. Sci. Biol. Med. 2011, 2, 66–70. [Google Scholar] [CrossRef]
- De Sire, A.; Marotta, N.; Ferrillo, M.; Agostini, F.; Sconza, C.; Lippi, L.; Respizzi, S.; Giudice, A.; Invernizzi, M.; Ammendolia, A. Oxygen-Ozone Therapy for Reducing Pro-Inflammatory Cytokines Serum Levels in Musculoskeletal and Temporomandibular Disorders: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 2528. [Google Scholar] [CrossRef] [PubMed]
- Clavo, B.; Pérez, J.L.; López, L.; Suárez, G.; Lloret, M.; Rodríguez, V.; Macías, D.; Santana, M.; Morera, J.; Fiuza, D.; et al. Effect of ozone therapy on muscle oxygenation. J. Altern. Complement. Med. 2003, 9, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J. Clinical reversal of root caries using ozone, double-blind, randomised, controlled 18-month trial. Gerodontology 2003, 20, 106–114. [Google Scholar] [CrossRef]
- Wentworth, P.; Nieva, J.; Takeuchi, C.; Galve, R.; Wentworth, A.D.; Dilley, R.B.; DeLaria, G.A.; Saven, A.; Babior, B.M.; Janda, K.D.; et al. Evidence for Ozone Formation in Human Atherosclerotic Arteries. Science 2003, 302, 1053–1056. [Google Scholar] [CrossRef] [PubMed]
- Mirowsky, J.E.; Carraway, M.S.; Dhingra, R.; Tong, H.; Neas, L.; Diaz-Sanchez, D.; Cascio, W.; Case, M.; Crooks, J.; Hauser, E.R.; et al. Ozone exposure is associated with acute changes in inflammation, fibrinolysis, and endothelial cell function in coronary artery disease patients. Environ. Health 2017, 16, 126. [Google Scholar] [CrossRef] [PubMed]
- Seyam, O.; Smith, N.L.; Reid, I.; Gandhi, J.; Jiang, W.; Khan, S.A. Clinical utility of ozone therapy for musculoskeletal disorders. Med. Gas Res. 2018, 8, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Re, L.; Sanchez, G.M.; Mawsouf, N. Clinical evidence of ozone interaction with pain mediators. Saudi Med. J. 2010, 31, 1363–1367. [Google Scholar] [PubMed]
- Fuccio, C.; Luongo, C.; Capodanno, P.; Giordano, C.; Scafuro, M.A.; Siniscalco, D.; Lettieri, B.; Rossi, F.; Maione, S.; Berrino, L. A single subcutaneous injection of ozone prevents allodynia and decreases the over-expression of pro-inflammatory caspases in the orbito-frontal cortex of neuropathic mice. Eur. J. Pharmacol. 2009, 603, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, M.; Lin, X.; Li, Y.; Fu, Z. Low-Concentration Oxygen/Ozone Treatment Attenuated Radiculitis and Mechanical Allodynia via PDE2A-cAMP/cGMP-NF-κB/p65 Signaling in Chronic Radiculitis Rats. Pain Res. Manag. 2018, 2018, 5192814. [Google Scholar] [CrossRef]
- Gjonovich, A.; Marchetto, R.; Montemarà, E.; Girotto, T. Refractory tendinopathies of the knee: Use of oxygen-ozone therapy. Riv. Ital. Ossigeno-Ozonoterapia 2003, 2, 187–192. [Google Scholar]
- Gurger, M.; Once, G.; Yilmaz, E.; Demir, S.; Calik, I.; Say, Y.; Kavakli, A.; Key, S.; Gurbuz, M.U.; Bingollu, O. The effect of the platelet-rich plasma and ozone therapy on tendon-to-bone healing in the rabbit rotator cuff repair model. J. Orthop. Surg. Res. 2021, 16, 202. [Google Scholar] [CrossRef]
- Manzi, R.; Raimondi, D. The role of oxygen-ozone therapy in patellofemoral chondromalacia. Riv. Ital. Ossigeno-Ozonoterapia 2002, 1, 31–35. [Google Scholar]
- Zhang, J.; Wei, Y.; Fang, Z. Ozone Pollution: A Major Health Hazard Worldwide. Front. Immunol. 2019, 10, 2518. [Google Scholar] [CrossRef]
- Trenti, G.F.; Gheza, G. Efficacy of oxygen-ozone pain therapy associated with shock waves to treat calcifying tendinitis of the shoulder. Preliminary findings. Riv. Ital. Ossigeno-Ozonoterapia 2002, 1, 45–50. [Google Scholar]
- Ikonomidis, S.T.; Iliakis, E.M.; Charalambus Dvakirtzian, L. Nonoperative treatment of shoulder impingement syndrome with topical injections of medical oxygen-ozone mixture. A double blind clinical trial. Riv. Ital. Ossigeno-Ozonoterapia 2002, 1, 41–44. [Google Scholar]
- De Sire, A.; Agostini, F.; Lippi, L.; Mangone, M.; Marchese, S.; Cisari, C.; Bernetti, A.; Invernizzi, M. Oxygen–Ozone Therapy in the Rehabilitation Field:State of the Art on Mechanisms of Action, Safety andEffectiveness in Patients with Musculoskeletal Disorders. Biomolecules 2021, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Moretti, B.; Lanzisera, R.; Pesce, V.; Moretti, L.; Patella, S.; Patella, V.S.C. O2-O3 vs. anti-inflammatory drugs in the treatment of neck pain. Riv. Ital. Ossigeno-Ozonoterapia 2004, 3, 131–137. [Google Scholar]
- Andreula, C.F.; Simonetti, L.; De Santis, F.; Agati, R.; Ricci, R.; Leonardi, M. Minimally invasive oxygen-ozone therapy for lumbar disk herniation. AJNR Am. J. Neuroradiol. 2003, 24, 996–1000. [Google Scholar] [PubMed]
- Bonetti, M.; Fontana, A.; Cotticelli, B.; Volta, G.D.; Guindani, M.; Leonardi, M. Intraforaminal O2-O3 versus periradicular steroidal infiltrations in lower back pain: Randomized controlled study. AJNR Am. J. Neuroradiol. 2005, 26, 996–1000. [Google Scholar]
- Somay, H.; Emon, S.T.; Uslu, S.; Orakdogen, M.; Meric, Z.C.; Ince, U.; Hakan, T. The histological effects of ozone therapy on sciatic nerve crush injury in rats. World Neurosurg. 2017, 105, 702–708. [Google Scholar] [CrossRef]
- Biazzo, A.; Corriero, A.S.; Confalonieri, N. Intramuscular oxygen-ozone therapy in the treatment of low back pain. Acta Bio-Med. Atenei Parm. 2018, 89, 41–46. [Google Scholar] [CrossRef]
- Clavo, B.; Robaina, F.; Urrutia, G.; Bisshopp, S.; Ramallo, Y.; Szolna, A.; Caramés, M.A.; Fiuza, M.D.; Linertová, R. Ozone therapy versus surgery for lumbar disc herniation: A randomized double-blind controlled trial. Complement. Ther. Med. 2021, 59, 102724. [Google Scholar] [CrossRef]
- Chen, H.; Yu, B.; Lu, C.; Lin, Q. The effect of intra-articular injection of different concentrations of ozone on the level of TNF-α, TNF-R1, and TNF-R2 in rats with rheumatoid arthritis. Rheumatol. Int. 2013, 33, 1223–1227. [Google Scholar] [CrossRef]
- Bozbas, G.T.; Sendur, O.F. New Therapeutıc Aproach in Rheumatoıd Arthrıtıs: Ozone. Int. J. Physiatry 2016, 2, 7. [Google Scholar] [CrossRef]
- León Fernández, O.S.; Viebahn-Haensler, R.; Cabreja, G.L.; Espinosa, I.S.; Matos, Y.H.; Roche, L.D.; Santos, B.T.; Oru, G.T.; Polo Vega, J.C. Medical ozone increases methotrexate clinical response and improves cellular redox balance in patients with rheumatoid arthritis. Eur. J. Pharmacol. 2016, 789, 313–318. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, Z.; Jiang, Y.; Ma, N.; Zhang, Y.; Feng, L.; Wang, K. IL-22+ CD4+ T cells in patients with rheumatoid arthritis. Int. J. Rheum. Dis. 2013, 16, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Wang, M. Mechanism of medical ozone and its clinical application in HIV/AIDS patients. J. Chin. Physician 2021, 12, 1588–1591. [Google Scholar]
- Fitzpatrick, E.; Holland, O.J.; Vanderlelie, J.J. Ozone therapy for the treatment of chronic wounds: A systematic review. Int. Wound J. 2018, 15, 633–644. [Google Scholar] [CrossRef]
- Andrade, R.R.; Oliveira-Neto, O.B.; Barbosa, L.T.; Santos, I.O.; Sousa-Rodrigues, C.F.; Barbosa, F.T. [Effectiveness of ozone therapy compared to other therapies for low back pain: A systematic review with meta-analysis of randomized clinical trials]. Braz. J. Anesthesiol. 2019, 69, 493–501. [Google Scholar] [CrossRef]
- Sconza, C.; Respizzi, S.; Virelli, L.; Vandenbulcke, F.; Iacono, F.; Kon, E.; Di Matteo, B. Oxygen-Ozone Therapy for the Treatment of Knee Osteoarthritis: A Systematic Review of Randomized Controlled Trials. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 277–286. [Google Scholar] [CrossRef]
- Sconza, C.; Leonardi, G.; Kon, E.; Respizzi, S.; Massazza, G.; Marcacci, M.; Di Matteo, B. Oxygen-ozone therapy for the treatment of low back pain: A systematic review of randomized controlled trials. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6034–6046. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.E.G.; Baeza-Noci, J.; Mendes Abdala, C.V.; Luvisotto, M.M.; Bertol, C.D.; Anzolin, A.P. The role of ozone treatment as integrative medicine. An evidence and gap map. Front. Public Health 2023, 10, 1112296. [Google Scholar] [CrossRef] [PubMed]
- Setyo Budi, D.; Fahmi Rofananda, I.; Reza Pratama, N.; Sutanto, H.; Sukma Hariftyani, A.; Ratna Desita, S.; Zinedinita Rahmasari, A.; Pudy Asmarawati, T.; Agung Waskito, L.; Dyah Kencono Wungu, C. Ozone as an adjuvant therapy for COVID-19: A systematic review and meta-analysis. Int. Immunopharmacol. 2022, 110, 109014. [Google Scholar] [CrossRef] [PubMed]
- Domb, W.C. Ozone therapy in dentistry. A brief review for physicians. Interv. Neuroradiol. 2014, 20, 632–636. [Google Scholar] [CrossRef]
- Sen, S.; Sen, S. Ozone therapy a new vista in dentistry: Integrated review. Med. Gas Res. 2020, 10, 189–192. [Google Scholar] [CrossRef]
- Gupta, G.; Mansi, B. Ozone therapy in periodontics. J. Med. Life 2012, 5, 59–67. [Google Scholar] [PubMed]
- Badhe, H.; Kalaskar, R.; Balasubramanian, S.; Kamki, H.; Kalaskar, A. Antimicrobial Effect of Ozone Therapy in Deep Dentinal Carious Lesion: A Systematic Review. Int. J. Clin. Pediatr. Dent. 2022, 15, S252–S260. [Google Scholar] [CrossRef] [PubMed]
- Baysan, A.; Lynch, E. The use of ozone in dentistry and medicine. Part 2. Ozone and root caries. Prim. Dent. Care 2006, 13, 37–41. [Google Scholar] [CrossRef]
- Burke, F.J. Ozone and caries: A review of the literature. Dent. Update 2012, 39, 271–272, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Almaz, M.E.; Sonmez, I.S. Ozone therapy in the management and prevention of caries. J. Formos. Med. Assoc. 2015, 114, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Unal, M.; Oztas, N. Remineralization Capacity of Three Fissure Sealants with and without Gaseous Ozone on Non-Cavitated Incipient Pit and Fissure Caries. J. Clin. Pediatr. Dent. 2015, 39, 364–370. [Google Scholar] [CrossRef]
- Safwat, O.; Elkateb, M.; Dowidar, K.; Salam, H.A.; El Meligy, O. Microbiological Evaluation of Ozone on Dentinal Lesions in Young Permanent Molars using the Stepwise Excavation. J. Clin. Pediatr. Dent. 2018, 42, 11–20. [Google Scholar] [CrossRef]
- Sancakli, H.S.; Siso, S.H.; Yildiz, S.O.; Gokce, Y.B. Antibacterial Effect of Surface Pretreatment Techniques against Streptococcus Mutans. Niger. J. Clin. Pract. 2018, 21, 170–175. [Google Scholar]
- Kaptan, F.; Guven, E.P.; Topcuoglu, N.; Yazici, M.; Kulekci, G. In vitro assessment of the recurrent doses of topical gaseous ozone in the removal of Enterococcus faecalis biofilms in root canals. Niger. J. Clin. Pract. 2014, 17, 573–578. [Google Scholar] [CrossRef]
- Kuska-Kielbratowska, A.; Wiench, R.; Mertas, A.; Bobela, E.; Kielbratowski, M.; Lukomska-Szymanska, M.; Tanasiewicz, M.; Skaba, D. Evaluation of the Sensitivity of Selected Candida Strains to Ozonated Water-An In Vitro Study. Medicina 2022, 58, 1731. [Google Scholar] [CrossRef]
- Shetty, N.; Mathew, T.; Shetty, A.; Hegde, M.N.; Attavar, S. Ozonated water as an irrigant in disinfecting root canal systems—A systematic review. Evid.-Based Dent. 2022. [Google Scholar] [CrossRef]
- Lena, K.; Marianne, K. Ozone Treatment on Dentin Hypersensitivity Surfaces—A Pilot Study. Open Dent. J. 2017, 11, 65–70. [Google Scholar] [CrossRef]
- Talukdar, A.; Langthasa, M.; Barman, I. Ozone therapy: Boon to dentistry and medicine. Int. J. Prev. Clin. Dent. Res. 2015, 2, 59–66. [Google Scholar]
- Makkar, S.; Makkar, M. Ozone-Treating Dental Infections. Indian J. Stomatol. 2011, 2, 256–259. [Google Scholar]
- Marchesi, G.; Petris, L.C.; Navarra, C.O.; Locatelli, R.; Di Lenarda, R.; Breschi, L.; Cadenaro, M. Effect of ozone application on the immediate shear bond strength and microleakage of dental sealants. Pediatr. Dent. 2012, 34, 284–288. [Google Scholar] [PubMed]
- Pires, P.T.; Ferreira, J.C.; Oliveira, S.A.; Silva, M.J.; Melo, P.R. Effect of ozone gas on the shear bond strength to enamel. J. Appl. Oral Sci. Rev. FOB 2013, 21, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Floare, A.D.; Focht, D.; Hajdu, A.I.; Niculescu Talpoş, I.C.; Bălean, O.I.; Muntean, C.V.; Sebeşan, D.; Jumanca, D.E.; Găluşcan, A. Ozone and microstructural morphological changes of tooth enamel. Rom. J. Morphol. Embryol. 2022, 63, 539–544. [Google Scholar] [CrossRef]
- Meligy, O.; Almushayt, A. One Year Follow up Study for Ozone and Fissure Sealant on Non-Cavitated Carious Lesions. J. King Abdulaziz Univ. 2013, 20, 79–101. [Google Scholar] [CrossRef]
- Johansson, E.; van Dijken, J.W.; Karlsson, L.; Andersson-Wenckert, I. Treatment effect of ozone and fluoride varnish application on occlusal caries in primary molars: A 12-month study. Clin. Oral Investig. 2014, 18, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Rickard, G.D.; Richardson, R.J.; Johnson, T.M.; McColl, D.C.; Hooper, L. WITHDRAWN: Ozone therapy for the treatment of dental caries. Cochrane Database Syst. Rev. 2019, 2, Cd004153. [Google Scholar] [CrossRef] [PubMed]
- Safwat, O.; Elkateb, M.; Dowidar, K.; El Meligy, O. Clinical Evaluation of Ozone on Dentinal Lesions in Young Permanent Molars using the Stepwise Excavation. J. Clin. Pediatr. Dent. 2017, 41, 429–441. [Google Scholar] [CrossRef]
- Düzyol, E.; Gürbüz, T.; Bariş, Ö. Antimicrobial Efficacy of Ozone Therapy on Cariogenic Bacteria. Meandros Med. Dent. J. 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Krunić, J.; Stojanović, N.; Đukić, L.; Roganović, J.; Popović, B.; Simić, I.; Stojić, D. Clinical antibacterial effectiveness and biocompatibility of gaseous ozone after incomplete caries removal. Clin. Oral Investig. 2019, 23, 785–792. [Google Scholar] [CrossRef]
- Santos, G.M.; Pacheco, R.L.; Bussadori, S.K.; Santos, E.M.; Riera, R.; de Oliveira Cruz Latorraca, C.; Mota, P.; Benavent Caldas Bellotto, E.F.; Martimbianco, A.L.C. Effectiveness and Safety of Ozone Therapy in Dental Caries Treatment: Systematic Review and Meta-analysis. J. Evid. Based Dent. Pract. 2020, 20, 101472. [Google Scholar] [CrossRef]
- Huth, K.C.; Quirling, M.; Lenzke, S.; Paschos, E.; Kamereck, K.; Brand, K.; Hickel, R.; Ilie, N. Effectiveness of ozone against periodontal pathogenic microorganisms. Eur. J. Oral Sci. 2011, 119, 204–210. [Google Scholar] [CrossRef]
- Garlapati, K. Ozone Therapy-A Revolutionary Noninvasive Therapy in Dentistry. Dentistry 2012, 1, 473. [Google Scholar] [CrossRef]
- Yılmaz, S.; Algan, S.; Gursoy, H.; Noyan, U.; Kuru, B.E.; Kadir, T. Evaluation of the clinical and antimicrobial effects of the Er:YAG laser or topical gaseous ozone as adjuncts to initial periodontal therapy. Photomed. Laser Surg. 2013, 31, 293–298. [Google Scholar] [CrossRef]
- Nicolini, A.C.; Rotta, I.D.S.; Langa, G.P.J.; Friedrich, S.A.; Arroyo-Bonilla, D.A.; Wagner, M.C.; Weidlich, P.; Rösing, C.K.; Cavagni, J. Efficacy of ozonated water mouthwash on early plaque formation and gingival inflammation: A randomized controlled crossover clinical trial. Clin. Oral Investig. 2021, 25, 1337–1344. [Google Scholar] [CrossRef]
- Shichiri-Negoro, Y.; Tsutsumi-Arai, C.; Arai, Y.; Satomura, K.; Arakawa, S.; Wakabayashi, N. Ozone ultrafine bubble water inhibits the early formation of Candida albicans biofilms. PLoS ONE 2021, 16, e0261180. [Google Scholar] [CrossRef]
- Tetè, G.; D’Amicantonio, T.; Polizzi, E. Efficacy Ozone Therapy in Reducing Periodontal Disease. Materials 2023, 16, 2375. [Google Scholar] [CrossRef]
- Colombo, M.; Gallo, S.; Garofoli, A.; Poggio, C.; Arciola, C.R.; Scribante, A. Ozone Gel in Chronic Periodontal Disease: A Randomized Clinical Trial on the Anti-Inflammatory Effects of Ozone Application. Biology 2021, 10, 625. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Topi, S.; Gnoni, A.; Dipalma, G.; Mancini, A.; Di Domenico, M.; Tartaglia, G.M.; Scarano, A.; et al. The Gaseous Ozone Therapy as a Promising Antiseptic Adjuvant of Periodontal Treatment: A Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 985. [Google Scholar] [CrossRef]
- Serafini, G.; De Biase, A.; Lamazza, L.; Mazzucchi, G.; Lollobrigida, M. Efficacy of Topical Treatments for the Management of Symptomatic Oral Lichen Planus: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 1202. [Google Scholar] [CrossRef]
- Putnins, E.E.; Di Giovanni, D.; Bhullar, A.S. Dental unit waterline contamination and its possible implications during periodontal surgery. J. Periodontol. 2001, 72, 393–400. [Google Scholar] [CrossRef]
- Wirthlin, M.R.; Marshall, G.W., Jr.; Rowland, R.W. Formation and decontamination of biofilms in dental unit waterlines. J. Periodontol. 2003, 74, 1595–1609. [Google Scholar] [CrossRef]
- Szymanska, J. Evaluation of mycological contamination of dental unit waterlines. Ann. Agric. Environ. Med. 2005, 12, 153–155. [Google Scholar]
- Taylor-Hardy, T.L.; Leonard, R.H., Jr.; Mauriello, S.M.; Swift, E.J., Jr. Effect of dental unit waterline biocides on enamel bond strengths. Gen. Dent. 2001, 49, 421–425. [Google Scholar]
- Kohno, S.; Kawata, T.; Kaku, M.; Fuita, T.; Tsutsui, K.; Ohtani, J.; Tenjo, K.; Motokawa, M.; Tohma, Y.; Shigekawa, M.; et al. Bactericidal effects of acidic electrolyzed water on the dental unit waterline. Jpn. J. Infect. Dis. 2004, 57, 52–54. [Google Scholar]
- Walker, J.T.; Bradshaw, D.J.; Fulford, M.R.; Marsh, P.D. Microbiological evaluation of a range of disinfectant products to control mixed-species biofilm contamination in a laboratory model of a dental unit water system. Appl. Environ. Microbiol. 2003, 69, 3327–3332. [Google Scholar] [CrossRef]
- Isler, S.C.; Uraz, A.; Guler, B.; Ozdemir, Y.; Cula, S.; Cetiner, D. Effects of Laser Photobiomodulation and Ozone Therapy on Palatal Epithelial Wound Healing and Patient Morbidity. Photomed. Laser Surg. 2018, 36, 571–580. [Google Scholar] [CrossRef]
- Al-Omiri, M.K.; Lamfon, H.A.; Al Nazeh, A.A.; Kielbassa, A.M.; Lynch, E. Randomized clinical trial on the comparison of bleaching outcomes using either ozone or hydrogen peroxide. Quintessence Int. 2018, 49, 625–634. [Google Scholar] [CrossRef]
- Durmus, N.; Tok, Y.T.; Kaya, S.; Akcay, M. Effectiveness of the ozone application in two-visit indirect pulp therapy of permanent molars with deep carious lesion: A randomized clinical trial. Clin. Oral Investig. 2019, 23, 3789–3799. [Google Scholar] [CrossRef]
- Uraz, A.; Karaduman, B.; Isler, S.; Gönen, S.; Çetiner, D. Ozone application as adjunctive therapy in chronic periodontitis: Clinical, microbiological and biochemical aspects. J. Dent. Sci. 2019, 14, 27–37. [Google Scholar] [CrossRef]
- Matys, J.; Jaszczak, E.; Flieger, R.; Kostrzewska-Kaminiarz, K.; Grzech-Leśniak, K.; Dominiak, M. Effect of ozone and diode laser (635 nm) in reducing orthodontic pain in the maxillary arch-a randomized clinical controlled trial. Lasers Med. Sci. 2020, 35, 487–496. [Google Scholar] [CrossRef]
- Grocholewicz, K.; Matkowska-Cichocka, G.; Makowiecki, P.; Droździk, A.; Ey-Chmielewska, H.; Dziewulska, A.; Tomasik, M.; Trybek, G.; Janiszewska-Olszowska, J. Effect of nano-hydroxyapatite and ozone on approximal initial caries: A randomized clinical trial. Sci. Rep. 2020, 10, 11192. [Google Scholar] [CrossRef]
- Al-Omiri, M.K.; Alqahtani, N.M.; Alahmari, N.M.; Hassan, R.A.; Al Nazeh, A.A.; Lynch, E. Treatment of symptomatic, deep, almost cariously exposed lesions using ozone. Sci. Rep. 2021, 11, 11166. [Google Scholar] [CrossRef]
- Serag Eldien, A.M.; Fathy Hassabou, N. Clinical and cytological assessment of platelet-rich fibrin versus topical ozonated oil in palatal wound healing after free gingival graft harvesting: Randomized controlled trial. J. Oral Maxillofac. Surg. Med. Pathol. 2022, 34, 343–351. [Google Scholar] [CrossRef]
- Millar, B.J.; Hodson, N. Assessment of the safety of two ozone delivery devices. J. Dent. 2007, 35, 195–200. [Google Scholar] [CrossRef]
- Di Filippo, C.; Cervone, C.; Rossi, C.; di Ronza, C.; Marfella, R.; Capodanno, P.; Luongo, C.; Rossi, F.; D’Amico, M. Antiarrhythmic effect of acute oxygen-ozone administration to rats. Eur. J. Pharmacol. 2010, 629, 89–95. [Google Scholar] [CrossRef]
- Kafoury, R.M.; Huang, M.J. Application of quantitative structure activity relationship (QSAR) models to predict ozone toxicity in the lung. Environ. Toxicol. 2005, 20, 441–448. [Google Scholar] [CrossRef]
- Pattanaik, B.; Jetwa, D.; Pattanaik, S.; Manglekar, S.; Naitam, D.; Dani, A. Ozone therapy in dentistry: A literature review. J. Interdiscip. Dent. 2011, 1, 87–92. [Google Scholar] [CrossRef]
- Smith, N.L.; Wilson, A.L.; Gandhi, J.; Vatsia, S.; Khan, S.A. Ozone therapy: An overview of pharmacodynamics, current research, and clinical utility. Med. Gas Res. 2017, 7, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Zambello, A.; Bianchi, M.; Bruno, F. Sicurezza in ozonoterapia. Riv. Ital. Ossigeno-Ozonoterapia 2004, 3, 25–34. [Google Scholar]
- Vipin, T.; Thakkar, H. Ozone (O3): An excellent adjunctive tool in medical and surgical management of patient. Int. J. Res. Med. Sci. 2014, 2, 1257. [Google Scholar] [CrossRef][Green Version]
- Nogales, C.G.; Ferrari, P.H.; Kantorovich, E.O.; Lage-Marques, J.L. Ozone therapy in medicine and dentistry. J. Contemp. Dent. Pract. 2008, 9, 75–84. [Google Scholar] [CrossRef]
- Bocci, V. Oxygen-Ozone Therapy: A Critical Evaluation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Johansson, E.; Andersson-Wenckert, I.; Hagenbjork-Gustafsson, A.; Van Dijken, J.W. Ozone air levels adjacent to a dental ozone gas delivery system. Acta Odontol. Scand. 2007, 65, 324–330. [Google Scholar] [CrossRef]

| Authors and Publication Date | Study Title | Journal | Sample Size | Conclusion | Reference |
|---|---|---|---|---|---|
| Fitzpatrick et al., 2018 | Ozone therapy for the treatment of chronic wounds: a systematic review | Int. Wound. J. | Nine studies (n = 453 patients) | Compared with standard care, ozone therapy as an advanced wound care treatment may improve the proportion of chronic wounds healed in a shorter amount of time, but further research is required | [58] |
| Andrade et al., 2019 | Effectiveness of ozone therapy compared to other therapies for low back pain: a systematic review with meta-analysis of randomized clinical trials | Braz. J. Anesthesiol. | Six clinical trials | Ozone therapy used for six months for lumbar pain relief is more effective than other therapies | [59] |
| Sconza et al., 2020 | Oxygen–ozone therapy for the treatment of knee osteoarthritis: a systematic review of randomized controlled trials | Arthroscopy | 11 studies involving 858 patients in total (629 female and 229 male) were included | On the basis of the data available, oxygen–ozone therapy has, however, proven to be a safe approach with encouraging effects in pain control and functional recovery in the short–middle term | [60] |
| Sconza et al., 2021 | Oxygen–ozone therapy for the treatment of low back pain: a systematic review of randomized controlled trials | Eur. Rev. Med. Pharmacol. Sci. | 15 studies involving 2597 patients in total were included | Oxygen–ozone therapy has proven to be a safe treatment with beneficial effects in pain control and functional recovery at short- to medium-term follow-up | [61] |
| Serra et al., 2023 | The role of ozone treatment as integrative medicine. an evidence and gap map | Front. Public. Health. | 26 systematic reviews were characterized | Ozone treatment contributes to controlling pain, infections, inflammation, and wound healing, as well as increasing the quality of life. No serious adverse effects were related | [62] |
| Setyo Budi et al., 2022 | Ozone as an adjuvant therapy for COVID-19: a systematic review and meta-analysis | Int. Immunopharmacol. | 13 studies were included in this review | The beneficial effect of ozone in COVID-19 management is limited to the improvements of laboratory parameters among severe patients. Additionally, no serious adverse event was reported following ozone therapy, suggesting its high safety profile | [63] |
| Authors and Publication Date | Study Title | Journal | Sample Size | Conclusion | Reference |
|---|---|---|---|---|---|
| Isler et al., 2018 | Effects of laser photobiomodulation and ozone therapy on palatal epithelial wound healing and patient morbidity | Photomed. Laser. Surg. | 36 patients | Significantly improves the healing of palatal lesions | [105] |
| Al-Omiri et al., 2018 | Randomized clinical trial on the comparison of bleaching outcomes using either ozone or hydrogen peroxide | Quintessence. Int. | 32 participants | Teeth containing similar bleaching outcomes | [106] |
| Durmus et al., 2019 | Effectiveness of the ozone application in two-visit indirect pulp therapy of permanent molars with deep carious lesion: a randomized clinical trial | Clin. Oral. Investig. | 105 lower first molar teeth | Significant effect in reducing microorganisms | [107] |
| Uraz et al., 2019 | Ozone application as adjunctive therapy in chronic periodontitis: clinical, microbiological and biochemical aspects | J. Dent. Sci. | 18 periodontitis patients | Ozone therapy had no further benefits in terms of clinical, microbiological, or biochemical markers | [108] |
| Matys et al., 2020 | Effect of ozone and diode laser (635 nm) in reducing orthodontic pain in the maxillary arch: a randomized clinical controlled trial | Lasers. Med. Sci. | 76 patients | Diode lasers had a significant pain-relieving effect while ozone did not relieve pain | [109] |
| Grocholewicz et al., 2020 | Effect of nano-hydroxyapatite and ozone on approximal initial caries: a randomized clinical trial | Sci. Rep. | 92 participants | A combination of nano-hydroxyapatite gel and ozone therapy produced the best effect in remineralizing enamel | [110] |
| Al-Omiri et al., 2021 | Treatment of symptomatic, deep, almost cariously exposed lesions using ozone | Sci. Rep. | 84 participants | Ozone applied to partially excised cavities prior to repair alleviates pain | [111] |
| M. Serag Eldien and Fathy Hassabou, 2022 | Clinical and cytological assessment of platelet-rich fibrin versus topical ozonated oil in palatal wound healing after free gingival graft harvesting: randomized controlled trial | J. Oral. Maxillofac. Surg. Med. Patho. | 39 patients | It has a significant improvement in wound healing re-epithelialization | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Meligy, O.A.; Elemam, N.M.; Talaat, I.M. Ozone Therapy in Medicine and Dentistry: A Review of the Literature. Dent. J. 2023, 11, 187. https://doi.org/10.3390/dj11080187
El Meligy OA, Elemam NM, Talaat IM. Ozone Therapy in Medicine and Dentistry: A Review of the Literature. Dentistry Journal. 2023; 11(8):187. https://doi.org/10.3390/dj11080187
Chicago/Turabian StyleEl Meligy, Omar A., Noha M. Elemam, and Iman M. Talaat. 2023. "Ozone Therapy in Medicine and Dentistry: A Review of the Literature" Dentistry Journal 11, no. 8: 187. https://doi.org/10.3390/dj11080187
APA StyleEl Meligy, O. A., Elemam, N. M., & Talaat, I. M. (2023). Ozone Therapy in Medicine and Dentistry: A Review of the Literature. Dentistry Journal, 11(8), 187. https://doi.org/10.3390/dj11080187






_Talaat.jpg)