Synthesis, Structural Characterization, and In Silico Antiviral Prediction of Novel DyIII-, YIII-, and EuIII-Pyridoxal Helicates
Abstract
1. Introduction
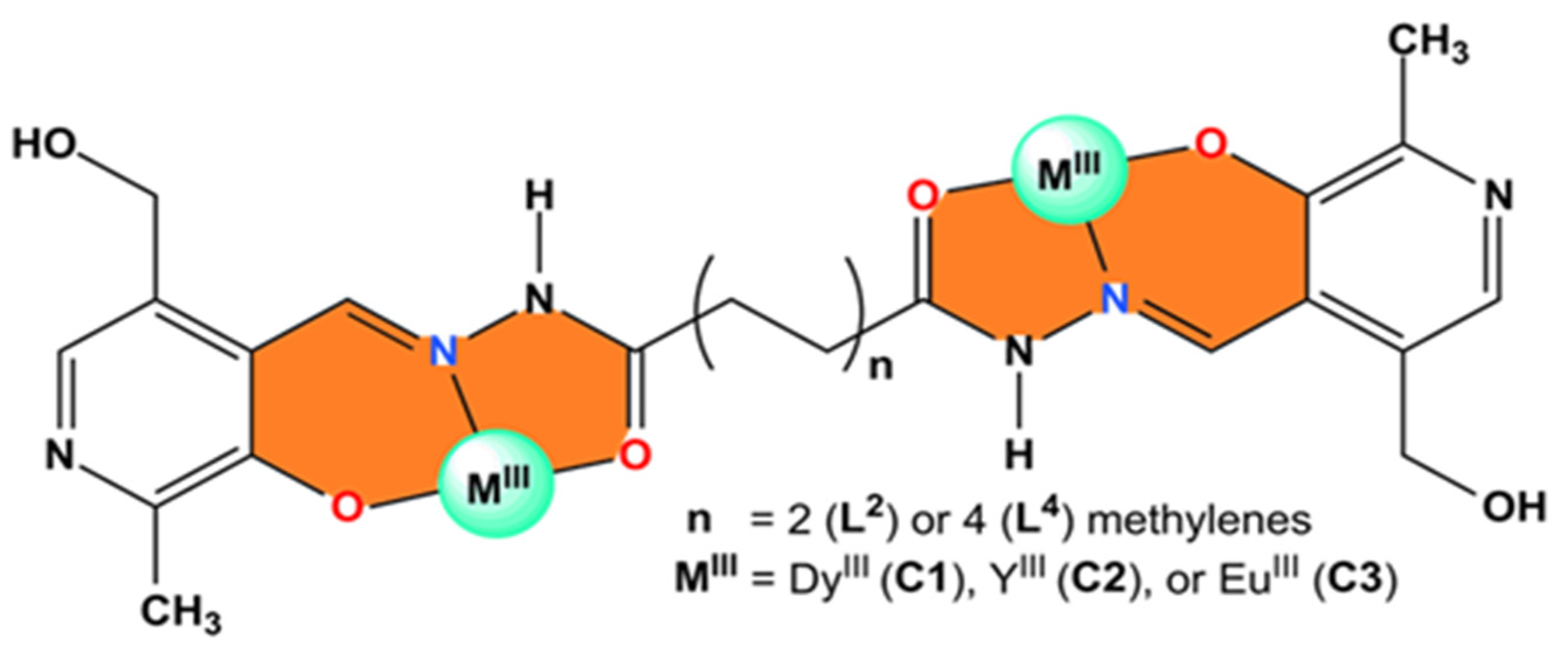
2. Results and Discussion
2.1. X-Ray Crystallography and Solid-State Structures
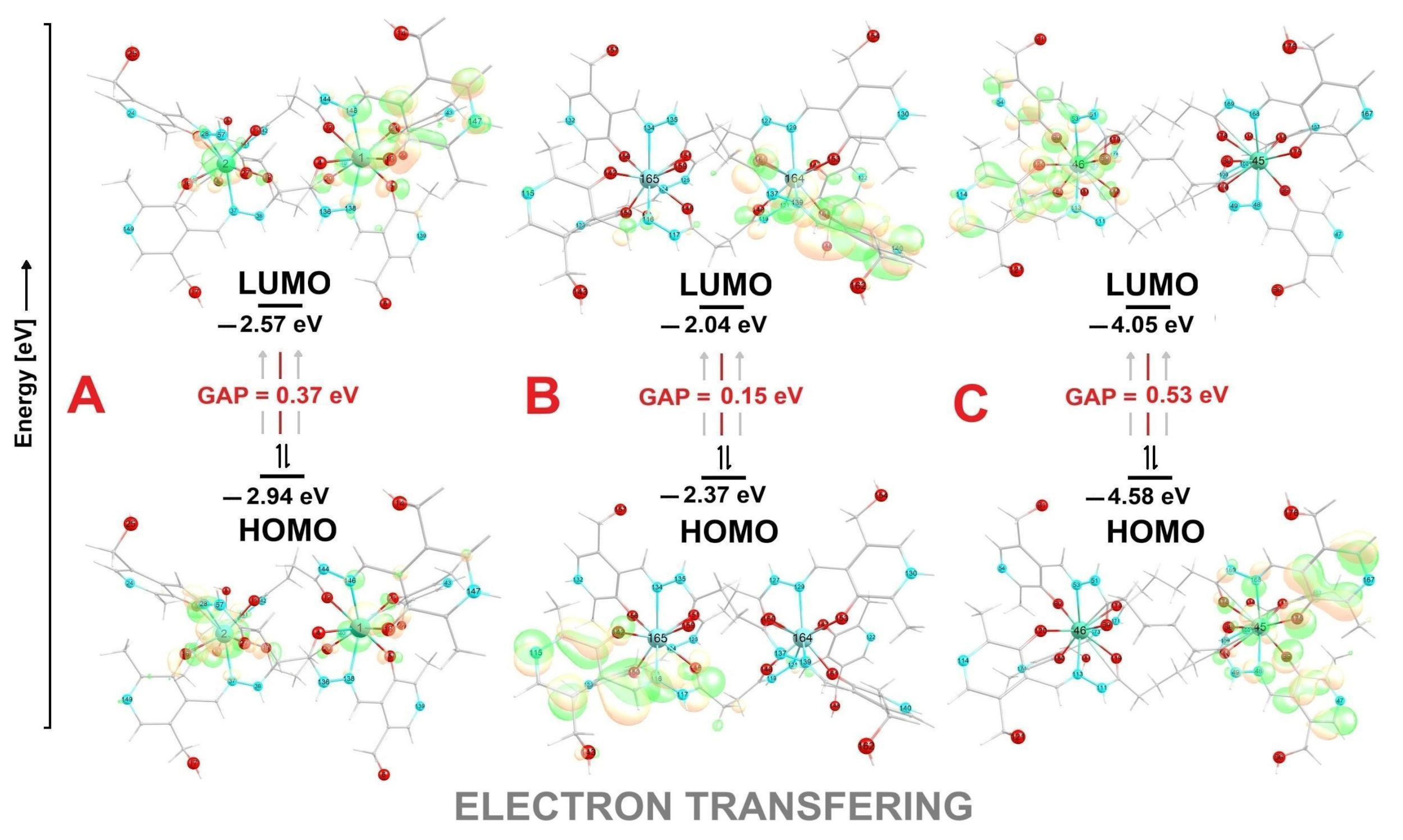
2.2. DFT Analysis
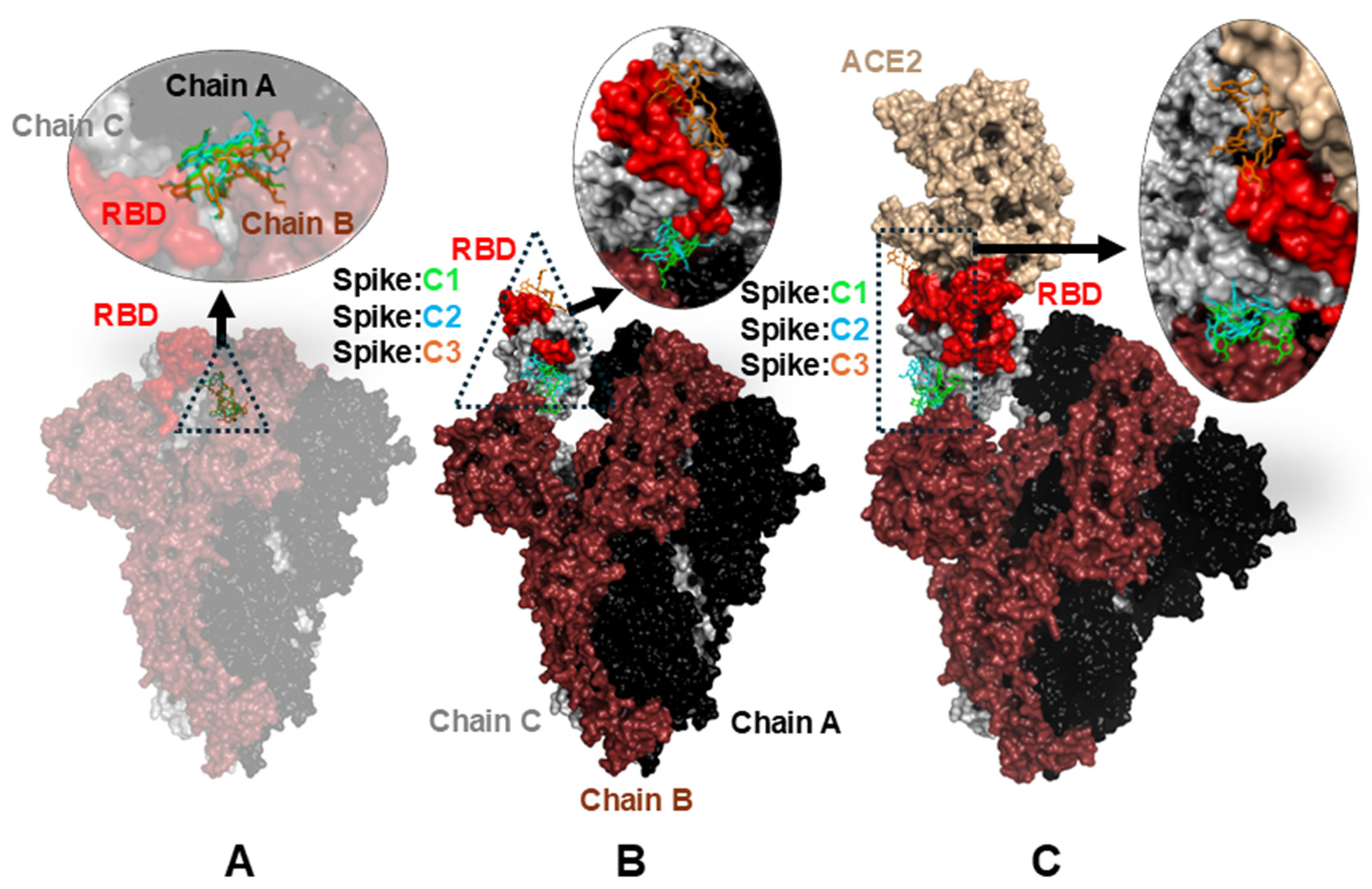
2.3. Molecular Docking Calculations
3. Materials and Methods
3.1. General Characterization and Instrumentation
3.2. X-Ray Crystallography
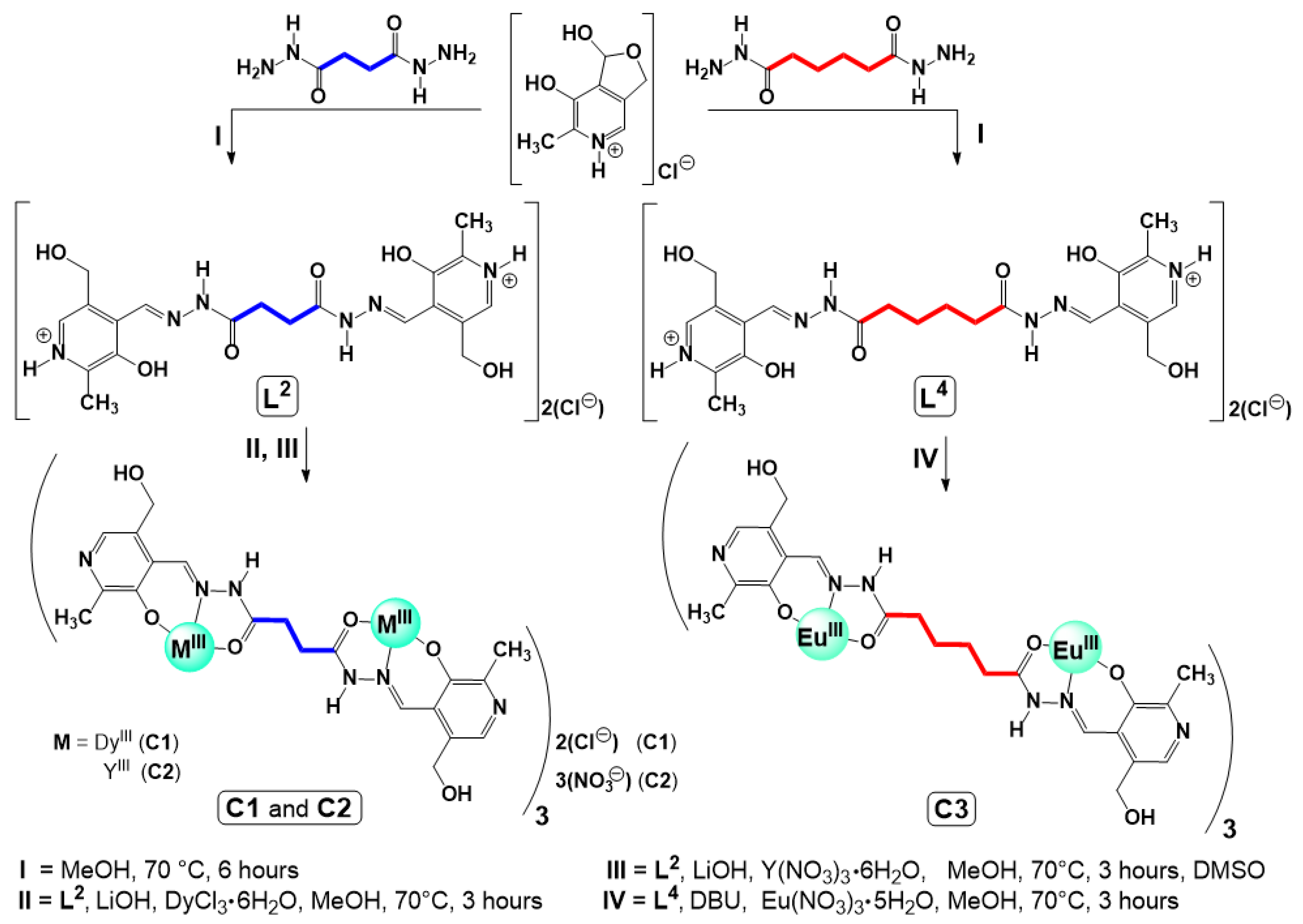
3.3. Synthetic Procedures
3.3.1. Synthesis of Ligands L2 and L4
- •
- Ligand L2 (chloride salt): Yield: 0.466 g, 90%. M.P.: 220 °C (decomposition). Elem. Anal. for C20H26N6O6Cl2 (M.W. = 517.36 g mol−1), Calc. (%): C, 46.43; H, 5.07; N, 16.24. Found (%): C, 46.45; H, 5.09; N, 16.21. FT-IR (ATR, cm−1): 3048 [w, ν(C–H)aromatic], 2962 [w, ν(C–H)aliphatic], 1698 [m, ν(C=O)], and 1658 [w, ν(C=N)]. 1H NMR (D2O, 400 MHz): 3.27 (s, 6H, CH3), 3.53 (s, 4H, CH2), 5.49 (s, 4H, CH2), 8.75 (s, 2H, ArH), 9.21 (s, 2H, CHimine). 13C{1H} NMR (D2O, 100 MHz): 19.52, 36.06, 61.77, 127.70, 131.93, 132.00, 132.53, 148.69, 152.83, 163.13, 177.67.
- •
- Ligand L4 (chloride salt): Yield: 0.485 g, 89%. M.P.: 180 °C (decomposition). Elem. Anal. for C22H30N6O6Cl2 (M.W. = 545.42 g mol−1), Calc. (%): C, 48.45; H, 5.54; N, 15.41. Found (%): C, 48.44; H, 5.59; N, 15.40. FT-IR (ATR, cm−1): 3119 [w, ν(C–H)aromatic], 2940 [w, ν(C–H)aliphatic], 1686 [s, ν(C=O)], and 1624 [w, ν(C=N)]. 1H NMR (D2O, 400 MHz): 2.13 (s, 4H, CH2), 2.85 (m, 4H, CH2), 2.98 (s, 6H, CH3), 5.20 (s, 4H, CH2), 8.46 (s, 2H, ArH), 8.93 (s, 2H, CHimine). 13C{1H} NMR (D2O, 100 MHz): 19.54, 26.52, 36.09, 61.72, 127.70, 131.93, 132.00, 132.53, 148.69, 152.83, 163.16, 177.67.
3.3.2. Synthesis of Complexes C1–C3
- •
- Complex [Dy2(L2)3]2Cl∙15H2O (C1): Yield: 0.072 g, 54.1%. M.P.: 350 °C (decomposition). FT-IR (ATR, cm−1): 2974 [w, ν(C–H)aliphatic], 1629 [m, ν(C=O)], and 1610 [m, ν(C=N)]. UV-Vis λmax: 303 nm; ε: 40,135 M−1 cm−1 in DMF(5%)/Tris-HCl pH 7.4 buffer mixture. λmax: 290 nm; ε: 14,336 M−1 cm−1 in DMSO solution.
- •
- Complex [Y2(L2)3]3(NO3)Cl∙14H2O (without crystallization from DMSO) (C2): Yield: 0.033 g, 02.7%. M.P.: 338 °C (decomposition). FT-IR (ATR, cm−1): 3045 [w, ν(C–H)aromatic], 1646 [s, ν(C=O)], and 1609 [m, ν(C=N)]. UV-Vis λmax: 291 nm; ε: 33,549 M−1 cm−1 in DMF(5%)/Tris-HCl pH 7.4 buffer mixture. λmax: 292 nm; ε: 44,618 M−1 cm−1 in DMSO solution.
- •
- Complex [Eu2(L4)3]∙12H2O (C3): Yield: 0.032 g, 24.9%. M.P.: 350 °C (decomposition). FT-IR (ATR, cm−1): 3047 [w, ν(C–H)aromatic], 1641 [s, ν(C=O)], and 1603 [m, ν(C=N)]. UV-Vis λmax: 300 nm; ε: 106,879 M−1 cm−1 in DMF (5%)/Tris-HCl pH 7.4 buffer mixture. λmax: 291 nm; ε: 55,496 M−1 cm−1 in DMSO solution.
3.4. DFT Calculations
3.5. In Silico Evaluations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ascenzi, P.; Bettinelli, M.; Boffi, A.; Botta, M.; De Simone, G.; Luchinat, C.; Marengo, E.; Mei, H.; Aime, S. Rare Earth Elements (REE) in Biology and Medicine. Rend. Fis. Acc. Lincei 2020, 31, 821–833. [Google Scholar] [CrossRef]
- Zheng, B.; Fan, J.; Chen, B.; Qin, X.; Wang, J.; Wang, F.; Deng, R.; Liu, X. Rare-Earth Doping in Nanostructured Inorganic Materials. Chem. Rev. 2022, 122, 5519–5603. [Google Scholar] [CrossRef] [PubMed]
- Brayshaw, L.L.; Smith, R.C.G.; Badaoui, M.; Irving, J.A.; Price, S.R. Lanthanides Compete with Calcium for Binding to Cadherins and Inhibit Cadherin-Mediated Cell Adhesion. Metallomics 2019, 11, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Pol, A.; Barends, T.R.M.; Dietl, A.; Khadem, A.F.; Eygensteyn, J.; Jetten, M.S.M.; Op Den Camp, H.J.M. Rare Earth Metals Are Essential for Methanotrophic Life in Volcanic Mudpots. Environ. Microbiol. 2014, 16, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Cotton, S.A.; Raithby, P.R. Systematics and Surprises in Lanthanide Coordination Chemistry. Coord. Chem. Rev. 2017, 340, 220–231. [Google Scholar] [CrossRef]
- Patyal, M.; Kaur, K.; Bala, N.; Gupta, N.; Malik, A.K. Innovative Lanthanide Complexes: Shaping the Future of Cancer/Tumor Chemotherapy. J. Trace Elem. Med. Biol. 2023, 80, 127277. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Sun, Y.; Ren, J.; Qu, X. Recent Progress in Lanthanide Complexes for DNA Sensing and Targeting Specific DNA Structures. Inorg. Chim. Acta 2016, 452, 50–61. [Google Scholar] [CrossRef]
- Jastrząb, R.; Nowak, M.; Skrobańska, M.; Tolińska, A.; Zabiszak, M.; Gabryel, M.; Marciniak, Ł.; Kaczmarek, M.T. DNA as a Target for Lanthanide(III) Complexes Influence. Coord. Chem. Rev. 2019, 382, 145–159. [Google Scholar] [CrossRef]
- Franklin, S.J. Lanthanide-Mediated DNA Hydrolysis. Curr. Opin. Chem. Biol. 2001, 5, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, S.; Rocha, S.; Sousa, N.R.; Catarino, C.; Belo, L.; Bronze-da-Rocha, E.; Valente, M.J.; Santos-Silva, A. Toxicity Mechanisms of Gadolinium and Gadolinium-Based Contrast Agents—A Review. Int. J. Mol. Sci. 2024, 25, 4071. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, L.; Nordberg, G.F.; Nurchi, V.M.; Aaseth, J.O. Gadolinium in Medical Imaging—Usefulness, Toxic Reactions and Possible Countermeasures—A Review. Biomolecules 2022, 12, 742. [Google Scholar] [CrossRef] [PubMed]
- Kostelnik, T.I.; Orvig, C. Radioactive Main Group and Rare Earth Metals for Imaging and Therapy. Chem. Rev. 2019, 119, 902–956. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Eder, M. [177Lu]Lu-PSMA-617 (PluvictoTM): The First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer. Pharmaceuticals 2022, 15, 1292. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.-X.; Yang, H.-B. Supramolecular Transformations within Discrete Coordination-Driven Supramolecular Architectures. Chem. Soc. Rev. 2016, 45, 2656–2693. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C.; Housecroft, C.E. Coordination Chemistry: The Scientific Legacy of Alfred Werner. Chem. Soc. Rev. 2013, 42, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Kruger, P.E.; Martin, N.; Nieuwenhuyzen, M. Dinuclear Double Helicates with a Twist: Synthesis, Structure and Supramolecular Entanglement in [M2L2] Metallo-Helices {M = Co(II), Cu(II), H2L = Bis(N-Salicylidene-4,4′-Diaminodiphenyl)Methane}. J. Chem. Soc. Dalton Trans. 2001, 1966–1970. [Google Scholar] [CrossRef]
- Piguet, C.; Bernardinelli, G.; Hopfgartner, G. Helicates as Versatile Supramolecular Complexes. Chem. Rev. 1997, 97, 2005–2062. [Google Scholar] [CrossRef] [PubMed]
- Fazio, E.; Haynes, C.J.E.; De La Torre, G.; Nitschke, J.R.; Torres, T. A Giant M2L3 Metallo-Organic Helicate Based on Phthalocyanines as a Host for Electroactive Molecules. Chem. Commun. 2018, 54, 2651–2654. [Google Scholar] [CrossRef] [PubMed]
- Golla, U.; Adhikary, A.; Mondal, A.K.; Tomar, R.S.; Konar, S. Synthesis, Structure, Magnetic and Biological Activity Studies of Bis-Hydrazone Derived Cu(ii) and Co(ii) Coordination Compounds. Dalton Trans. 2016, 45, 11849–11863. [Google Scholar] [CrossRef] [PubMed]
- Peberdy, J.C.; Malina, J.; Khalid, S.; Hannon, M.J.; Rodger, A. Influence of Surface Shape on DNA Binding of Bimetallo Helicates. J. Inorg. Biochem. 2007, 101, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Glasson, C.R.K.; Meehan, G.V.; Clegg, J.K.; Lindoy, L.F.; Smith, J.A.; Keene, F.R.; Motti, C. Microwave Synthesis of a Rare [Ru2L3]4+ Triple Helicate and Its Interaction with DNA. Chem.-Eur. J. 2008, 14, 10535–10538. [Google Scholar] [CrossRef] [PubMed]
- Mainardi Martins, F.; Chaves, O.A.; Acunha, T.V.; Roman, D.; Iglesias, B.A.; Back, D.F. Helical Water-Soluble NiII Complexes with Pyridoxal Ligand Derivatives: Structural Evaluation and Interaction with Biomacromolecules. J. Inorg. Biochem. 2021, 215, 111307. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.M.; Siqueira, J.D.; Iglesias, B.A.; Chaves, O.A.; Back, D.F. Pyridoxal Water-Soluble Cobalt(II) Helicates: Synthesis, Structural Analysis, and Interactions with Biomacromolecules. J. Inorg. Biochem. 2022, 233, 111854. [Google Scholar] [CrossRef] [PubMed]
- Phongtongpasuk, S.; Paulus, S.; Schnabl, J.; Sigel, R.K.O.; Spingler, B.; Hannon, M.J.; Freisinger, E. Binding of a Designed Anti-Cancer Drug to the Central Cavity of an RNA Three-Way Junction. Angew. Chem. Int. Ed. 2013, 52, 11513–11516. [Google Scholar] [CrossRef] [PubMed]
- Oleksi, A.; Blanco, A.G.; Boer, R.; Usón, I.; Aymamí, J.; Rodger, A.; Hannon, M.J.; Coll, M. Molecular Recognition of a Three-Way DNA Junction by a Metallosupramolecular Helicate. Angew. Chem. Int. Ed. 2006, 45, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Malina, J.; Hannon, M.J.; Brabec, V. Iron(II) Supramolecular Helicates Interfere with the HIV-1 Tat–TAR RNA Interaction Critical for Viral Replication. Sci. Rep. 2016, 6, 29674. [Google Scholar] [CrossRef] [PubMed]
- Cardo, L.; Nawroth, I.; Cail, P.J.; McKeating, J.A.; Hannon, M.J. Metallo Supramolecular Cylinders Inhibit HIV-1 TAR-TAT Complex Formation and Viral Replication in Cellulo. Sci. Rep. 2018, 8, 13342. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Fu, K. Latest Development of Approved COVID-19 Drugs and COVID-19 Drugs Undergoing Late Stage Clinical Trials. Front. Drug Discov. 2023, 3, 1304129. [Google Scholar] [CrossRef]
- Yu, W.; Krishnan, M.K.; Weekly, M.; Shanker, R.M.; Doshi, P.; Ragan, J.A.; Greene, R.A.; Gampper, B.; Caron, S.; McKillop, A.; et al. The Unprecedented Paxlovid Journey from Milligrams to Millions of Patient Doses during the COVID-19 Pandemic. Commun. Med. 2025, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, R.E.; Neto, A.M.; Santos, I.A.; Jardim, A.C.; Corbi, P.P.; Bergamini, F.R. What Is Holding Back the Development of Antiviral Metallodrugs? A Literature Overview and Implications for SARS-CoV-2 Therapeutics and Future Viral Outbreaks. Dalton Trans. 2020, 49, 16004–16033. [Google Scholar] [CrossRef] [PubMed]
- Labach, D.S.; Kohio, H.P.; Tse, E.A.; Paparisto, E.; Friesen, N.J.; Pankovich, J.; Bazett, M.; Barr, S.D. The Metallodrug BOLD-100 Is a Potent Inhibitor of SARS-CoV-2 Replication and Has Broad-Acting Antiviral Activity. Biomolecules 2023, 13, 1095. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Shukla, S.N.; Gaur, P. Metallo-Antiviral Aspirants: Answer to the Upcoming Virus Outbreak. Eur. J. Med. Chem. Rep. 2023, 8, 100104. [Google Scholar] [CrossRef] [PubMed]
- Tucci, A.R.; da Rosa, R.M.; Rosa, A.S.; Augusto Chaves, O.; Ferreira, V.N.S.; Oliveira, T.K.F.; Coutinho Souza, D.D.; Borba, N.R.R.; Dornelles, L.; Rocha, N.S.; et al. Antiviral Effect of 5′-Arylchalcogeno-3-Aminothymidine Derivatives in SARS-CoV-2 Infection. Molecules 2023, 28, 6696. [Google Scholar] [CrossRef] [PubMed]
- Gil-Moles, M.; Basu, U.; Büssing, R.; Hoffmeister, H.; Türck, S.; Varchmin, A.; Ott, I. Gold Metallodrugs to Target Coronavirus Proteins: Inhibitory Effects on the Spike–ACE2 Interaction and on PLpro Protease Activity by Auranofin and Gold Organometallics. Chem. Eur. J. 2020, 26, 15140–15144. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wang, R.; Chan, J.F.W.; Zhang, A.J.; Cheng, T.; Chik, K.K.H.; Ye, Z.W.; Wang, S.; Lee, A.C.Y.; Jin, L.; et al. Metallodrug Ranitidine Bismuth Citrate Suppresses SARS-CoV-2 Replication and Relieves Virus-Associated Pneumonia in Syrian Hamsters. Nat. Microbiol. 2020, 5, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Gil-Moles, M.; Türck, S.; Basu, U.; Pettenuzzo, A.; Bhattacharya, S.; Rajan, A.; Ma, X.; Büssing, R.; Wölker, J.; Burmeister, H.; et al. Metallodrug Profiling against SARS-CoV-2 Target Proteins Identifies Highly Potent Inhibitors of the S/ACE2 Interaction and the Papain-like Protease PLpro. Chem. Eur. J. 2021, 27, 17928–17940. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M. “Let’s Twist Again”Double-Stranded, Triple-Stranded, and Circular Helicates. Chem. Rev. 2001, 101, 3457–3498. [Google Scholar] [CrossRef] [PubMed]
- Seitz, M.; Oliver, A.G.; Raymond, K.N. The Lanthanide Contraction Revisited. J. Am. Chem. Soc. 2007, 129, 11153–11160. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.-L.; Xue, S.-F.; Tao, Z.; Zhu, Q.-J.; Lindoy, L.F.; Wei, G. Advances in the Lanthanide Metallosupramolecular Chemistry of the Cucurbit[n]Urils. Coord. Chem. Rev. 2015, 287, 89–113. [Google Scholar] [CrossRef]
- Kurbah, S.D.; Kumar, A.; Syiemlieh, I.; Lal, R.A. Crystal Structure and Biomimetic Activity of Homobinuclear Dioxidovanadium(V) Complexes Containing Succinoyldihydrazones Ligands. Polyhedron 2018, 139, 80–88. [Google Scholar] [CrossRef]
- Noei-Hootkani, H.; Karrari, S.; Hosseini-Monfared, H.; Mayer, P.; Notash, B. Synthesis, Structural Characterization, DFT Studies and Catalytic Properties of Dinuclear Oxidovanadium(V) Complexes Derived from Adipohydrazone Ligands. J. Mol. Struct. 2017, 1143, 452–461. [Google Scholar] [CrossRef]
- Kurbah, S.D.; Kumar, A.; Shangpung, S.; Syiemlieh, I.; Khongjoh, I.; Lal, R.A. Synthesis, Characterization, and Fluorescence Chemosensor Properties of a Cis -Dioxomolybdenum(VI) Complex Containing Multidentate Hydrazone Ligands. Z. Anorg. Allg. Chem. 2017, 643, 794–801. [Google Scholar] [CrossRef]
- Asha, T.M.; Kurup, M.R.P. Synthesis and Characterization of Homobimetallic Molybdenum(VI) Complexes of a Dihydrazone as Efficient Catalysts for the Synthesis of Hexahydroxyquinolines via Multicomponent Hantzsch Reaction. J. Mol. Struct. 2020, 1204, 127553. [Google Scholar] [CrossRef]
- Henderson, W.; Koh, L.L.; Ranford, J.D.; Robinson, W.T.; Svensson, J.O.; Vittal, J.J.; Wang, Y.M.; Xu, Y. Clusters in Bis-Tridentate Tethered Domains of an Iron Chelating Drug. J. Chem. Soc. Dalton Trans. 1999, 19, 3341–3343. [Google Scholar] [CrossRef]
- Adhikary, A.; Jena, H.S.; Konar, S. A Family of Fe3+ Based Double-Stranded Helicates Showing a Magnetocaloric Effect, and Rhodamine B Dye and DNA Binding Activities. Dalton Trans. 2015, 44, 15531–15543. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Su, H.; Xu, G.; Khan, M.A.; Li, H. Synthesis, Crystal Structures, and Luminescent Properties of Zn(ii), Cd(ii), Eu(iii) Complexes and Detection of Fe(iii) Ions Based on a Diacylhydrazone Schiff Base. RSC Adv. 2020, 10, 23372–23378. [Google Scholar] [CrossRef] [PubMed]
- Malviya, A.; Jena, H.S.; Mondal, A.K.; Konar, S. Europium-Based Dinuclear Triple-Stranded Helicate vs. Tetranuclear Quadruple-Stranded Helicate: Effect of Stoichiometric Ratio on the Supramolecular Self-Assembly. Eur. J. Inorg. Chem. 2015, 2015, 2901–2907. [Google Scholar] [CrossRef]
- Wang, B.; Ma, B.; Wei, Z.; Yang, H.; Wang, M.; Yin, W.; Gao, H.; Liu, W. Lanthanide Supermolecular Transformers Induced by K+ and CO2. Inorg. Chem. 2021, 60, 2764–2770. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, J.; Arunachalam, R.; Subramanian, P.S.; Suresh, E.; Valkonen, A.; Rissanen, K.; Albrecht, M. Coordinatively Unsaturated Lanthanide(III) Helicates: Luminescence Sensors for Adenosine Monophosphate in Aqueous Media. Angew. Chem. Int. Ed. 2016, 55, 9625–9629. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.K.; Jena, H.S.; Malviya, A.; Konar, S. Lanthanide-Directed Fabrication of Four Tetranuclear Quadruple Stranded Helicates Showing Magnetic Refrigeration and Slow Magnetic Relaxation. Inorg. Chem. 2016, 55, 5237–5244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ali, B.; Wu, J.; Guo, M.; Yu, Y.; Liu, Z.; Tang, J. Construction of Metallosupramolecular Coordination Complexes: From Lanthanide Helicates to Octahedral Cages Showing Single-Molecule Magnet Behavior. Inorg. Chem. 2019, 58, 3167–3174. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.S.; Couce, M.D.; Sordo, J. Coordination Chemistry of Vitamin B6 and Derivatives: A Structural Overview. Coord. Chem. Rev. 2012, 256, 3036–3062. [Google Scholar] [CrossRef]
- Gupta, S. Recent Reports on Pyridoxal Derived Schiff Base Complexes. Rev. Inorg. Chem. 2022, 42, 161–177. [Google Scholar] [CrossRef]
- Sapozhnikov, S.V.; Shtyrlin, N.V.; Kayumov, A.R.; Zamaldinova, A.E.; Iksanova, A.G.; Nikitina, E.V.; Krylova, E.S.; Grishaev, D.Y.; Balakin, K.V.; Shtyrlin, Y.G. New Quaternary Ammonium Pyridoxine Derivatives: Synthesis and Antibacterial Activity. Med. Chem. Res. 2017, 26, 3188–3202. [Google Scholar] [CrossRef]
- Hussain, A.; Mariappan, K.; Cork, D.C.; Lewandowski, L.D.; Shrestha, P.K.; Giri, S.; Wang, X.; Sykes, A.G. A Highly Selective Pyridoxal-Based Chemosensor for the Detection of Zn(ii) and Application in Live-Cell Imaging; X-Ray Crystallography of Pyridoxal-TRIS Schiff-Base Zn(ii) and Cu(ii) Complexes. RSC Adv. 2021, 11, 34181–34192. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-Y.; Cho, J.-H.; Lee, J.S.; Kim, H.J.; Kim, Y.-C. Synthesis and Structure–Activity Relationships of Carboxylic Acid Derivatives of Pyridoxal as P2X Receptor Antagonists. Bioorg. Med. Chem. 2013, 21, 2643–2650. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qiang, X.; Li, Y.; Luo, L.; Xu, R.; Zheng, Y.; Cao, Z.; Tan, Z.; Deng, Y. Pyridoxine-Resveratrol Hybrids Mannich Base Derivatives as Novel Dual Inhibitors of AChE and MAO-B with Antioxidant and Metal-Chelating Properties for the Treatment of Alzheimer’s Disease. Bioorg. Chem. 2017, 71, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Back, D.F.; Bonfada, É.; De Oliveira, G.M.; Lang, E.S. Chelation of ThIV, EuIII and NdIII by Dianionic N,N′-Bis(Pyridoxylideneiminato)Ethylene, (Pyr2en)2−. On the Search of Feasible Modelings for Heavy Metals Damage Inhibition in Living Beings. J. Inorg. Biochem. 2007, 101, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Puntus, L.; Zhuravlev, K.; Lyssenko, K.; Antipin, M.; Pekareva, I. Luminescence and Structural Properties of Lanthanide Complexes of Schiff Bases Derived from Pyridoxal and Amino Acids. Dalton Trans. 2007, 36, 4079. [Google Scholar] [CrossRef] [PubMed]
- Manzoni De Oliveira, G.; Bortolotto, R.; Ribas, D.G.; Faoro, E.; Schulz Lang, E.; Burrow, R.A. Chelation of CeIII and PrIII by N,N′ -Bis(Pyridoxylideneiminato)Ethylene, a Schiff Base Ligand Derived from Vitamin B6: Synthesis, Structural Features and TGA Evaluations of [M(Hpyr2En)(Pyr2En)]· nH2O (M = CeIII, n = 2; PrIII, n = 8). Z. Anorg. Allg. Chem. 2009, 635, 484–487. [Google Scholar] [CrossRef]
- Chakraborty, M.; Mondal, S.; Cardin, C.; Rheingold, A.L.; Das Mukhopadhyay, C.; Kumar Chattopadhyay, S. Yb(III), Sm(III) and La(III) Complexes of a Tetradentate Pyridoxal Schiff Base Ligand: Their DNA-Binding Activity and Bio-Imaging Applications. Polyhedron 2020, 175, 114167. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural Basis for the Recognition of SARS-CoV-2 by Full-Length Human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M. How Do They Know? Influencing the Relative Stereochemistry of the Complex Units of Dinuclear Triple-Stranded Helicate-Type Complexes. Chem. Eur. J. 2000, 6, 3485–3489. [Google Scholar] [CrossRef] [PubMed]
- Carlotto, S.; Armelao, L.; Rancan, M. Helicate versus Mesocate in Quadruple-Stranded Lanthanide Cages: A Computational Insight. Int. J. Mol. Sci. 2022, 23, 10619. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Xu, G.-F.; Zhao, L.; Guo, Y.-N.; Guo, Y.; Tang, J. Observation of Slow Magnetic Relaxation in Triple-Stranded Lanthanide Helicates. Dalton Trans. 2011, 40, 8213. [Google Scholar] [CrossRef] [PubMed]
- Loukopoulos, E.; Griffiths, K.; Akien, G.; Kourkoumelis, N.; Abdul-Sada, A.; Kostakis, G. Dinuclear Lanthanide (III) Coordination Polymers in a Domino Reaction. Inorganics 2015, 3, 448–466. [Google Scholar] [CrossRef]
- Mansell, S.; Liddle, S. Rare Earth and Actinide Complexes. Inorganics 2016, 4, 31. [Google Scholar] [CrossRef]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A Review of the Beneficiation of Rare Earth Element Bearing Minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Arunachalam, R.; Chinnaraja, E.; Valkonen, A.; Rissanen, K.; Sen, S.K.; Natarajan, R.; Subramanian, P.S. Enantiomeric Resolution of Asymmetric-Carbon-Free Binuclear Double-Stranded Cobalt(III) Helicates and Their Application as Catalysts in Asymmetric Reactions. Inorg. Chem. 2018, 57, 11414–11421. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Li, S.; Jia, C.; Mathieson, J.S.; Cronin, L.; Yang, X.-J.; Wu, B. Anion-Dependent Formation of Helicates versus Mesocates of Triple-Stranded M2L3 (M = Fe2+, Cu2+ ) Complexes. Inorg. Chem. 2012, 51, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Huang, P.; Shui, Y.; Wu, G. Coordination Assembly of Helicate and Mesocate with Two-Armed Carbohydrozine Ligand: Synthesis, Structure and Fluorescence. Inorg. Chem. Comunn. 2013, 29, 205–209. [Google Scholar] [CrossRef]
- Albrecht, M.; Kotila, S. Formation of a “ Meso -Helicate” by Self-Assembly of Three Bis(Catecholate) Ligands and Two Titanium(IV) Ions. Angew. Chem. Int. Ed. Engl. 1995, 34, 2134–2137. [Google Scholar] [CrossRef]
- Albrecht, M. Catecholate-Based Helicates. Eur. J. Inorg. Chem. 2020, 2020, 2227–2237. [Google Scholar] [CrossRef]
- Mayans, J.; Font-Bardia, M.; Di Bari, L.; Arrico, L.; Zinna, F.; Pescitelli, G.; Escuer, A. From Mesocates to Helicates: Structural, Magnetic and Chiro-Optical Studies on Nickel(II) Supramolecular Assemblies Derived from Tetradentate Schiff Bases. Chem.–Eur. J. 2018, 24, 7653–7663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dolphin, D. A Triple-Stranded Helicate and Mesocate from the Same Metal and Ligand. Chem. Commun. 2009, 131, 6931. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.G.; Baxter, P.N.W.; Lehn, J.-M. Self-Assembly in Self-Organized Inorganic Systems: A View of Programmed Metallosupramolecular Architectures. J. Braz. Chem. Soc. 2001, 12, 471–483. [Google Scholar] [CrossRef]
- Liu, C.-L.; Zhou, L.-P.; Tripathy, D.; Sun, Q.-F. Self-Assembly of Stable Luminescent Lanthanide Supramolecular M4L6 Cages with Sensing Properties toward Nitroaromatics. Chem. Commun. 2017, 53, 2459–2462. [Google Scholar] [CrossRef] [PubMed]
- Mary, Y.S.; Raju, K.; Panicker, C.Y.; Al-Saadi, A.A.; Thiemann, T. Molecular Conformational Analysis, Vibrational Spectra, NBO Analysis and First Hyperpolarizability of (2E)-3-(3-Chlorophenyl)Prop-2-Enoic Anhydride Based on Density Functional Theory Calculations. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2014, 131, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.; Ali, S.; Shahzadi, S.; Sharma, S.K.; Qanungo, K.; Shahid, M. Synthesis and Characterization of Hetero-Bimetallic Complexes with 2-Mercapto-5-Methyl-Benzimidazole: Theoretical Study and Biological Activities. J. Coord. Chem. 2015, 68, 2434–2448. [Google Scholar] [CrossRef]
- Yin, H.-Y.; Tang, J.; Zhang, J.-L. Sulfur Speciation Defined Subcellular Localization of Coumarin Derivatives: Correlation of Structural Relationship to Biological Behaviors. Chin. Chem. Lett. 2017, 29, 267–270. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Peralta-Inga, Z. Molecular Surface Electrostatic Potentials in Relation to Noncovalent Interactions in Biological Systems. Int. J. Quantum Chem. 2001, 85, 676–684. [Google Scholar] [CrossRef]
- Chaves, O.A.; Sueth-Santiago, V.; Pinto, D.C.d.A.; Netto-Ferreira, J.C.; Decote-Ricardo, D.; Lima, M.E.F.d. 2-Chloro-4,6-bis{(E)-3-methoxy-4-[(4-methoxybenzyl)oxy]styryl}pyrimidine: Synthesis, Spectroscopic and Computational Evaluation. Molbank 2021, 2021, M1276. [Google Scholar] [CrossRef] [PubMed]
- Savin, A.; Nesper, R.; Wengert, S.; Fässler, T.F. ELF: The Electron Localization Function. Angew. Chem. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Souza, J., Jr.; Martins, E.; Souza, H.; de Oliveira, R.; Alves, F.; Lima, E.; Cordeiro, L.; Trindade, E.; Lira, B.; Rocha, G.; et al. Synthesis, Spectroscopic Characterization, DFT Calculations and Preliminary Antifungal Activity of New Piperine Derivatives. J. Braz. Chem. Soc. 2021, 32, 490–502. [Google Scholar] [CrossRef]
- Akbari, Z.; Stagno, C.; Iraci, N.; Efferth, T.; Omer, E.A.; Piperno, A.; Montazerozohori, M.; Feizi-Dehnayebi, M.; Micale, N. Biological Evaluation, DFT, MEP, HOMO-LUMO Analysis and Ensemble Docking Studies of Zn(II) Complexes of Bidentate and Tetradentate Schiff Base Ligands as Antileukemia Agents. J. Mol. Struct. 2024, 1301, 137400. [Google Scholar] [CrossRef]
- Milusheva, M.; Todorova, M.; Gledacheva, V.; Stefanova, I.; Feizi-Dehnayebi, M.; Pencheva, M.; Nedialkov, P.; Tumbarski, Y.; Yanakieva, V.; Tsoneva, S.; et al. Novel Anthranilic Acid Hybrids—An Alternative Weapon against Inflammatory Diseases. Pharmaceuticals 2023, 16, 1660. [Google Scholar] [CrossRef] [PubMed]
- Milusheva, M.; Gledacheva, V.; Stefanova, I.; Feizi-Dehnayebi, M.; Mihaylova, R.; Nedialkov, P.; Cherneva, E.; Tumbarski, Y.; Tsoneva, S.; Todorova, M.; et al. Synthesis, Molecular Docking, and Biological Evaluation of Novel Anthranilic Acid Hybrid and Its Diamides as Antispasmodics. Int. J. Mol. Sci. 2023, 24, 13855. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Szentpály, L.v.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Al-Farraj, E.S.; Qasem, H.A.; Aouad, M.R.; Al-Abdulkarim, H.A.; Alsaedi, W.H.; Khushaim, M.S.; Feizi-Dehnayebi, M.; Al-Ghamdi, K.; Abu-Dief, A.M. Synthesis, Structural Determination, DFT Calculation and Biological Evaluation Supported by Molecular Docking Approach of Some New Complexes Incorporating (E)-N’-(3,5-Di-Tert-Butyl-2-Hydroxybenzylidene) Isonicotino Hydrazide Ligand. Appl. Organomet. Chem. 2024, 39, e7768. [Google Scholar] [CrossRef]
- Rossi, A.; Stagno, C.; Piperno, A.; Iraci, N.; Panseri, S.; Montesi, M.; Feizi-Dehnayebi, M.; Bassi, G.; Letizia, M.; Micale, N. Anticancer Activity and Morphological Analysis of Pt (II) Complexes: Their DFT Approach, Docking Simulation, and ADME-Tox Profiling. Appl. Organomet. Chem. 2024, 38, e7403. [Google Scholar] [CrossRef]
- Anwer, K.E.; Hamza, Z.K.; Ramadan, R.M. Synthesis, Spectroscopic, DFT Calculations, Biological Activity, SAR, and Molecular Docking Studies of Novel Bioactive Pyridine Derivatives. Sci. Rep. 2023, 13, 15598. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Iacopetta, D.; Sinicropi, M.S.; Andreu, I.; Mariconda, A.; Saturnino, C.; Giuzio, F.; Longo, P.; Aquaro, S.; Catalano, A. Drugs for COVID-19: An Update. Molecules 2022, 27, 8562. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; Yuan, S.; Chu, H.; Sridhar, S.; Yuen, K.-Y. COVID-19 Drug Discovery and Treatment Options. Nat. Rev. Microbiol. 2024, 22, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Ngoepe, M.P.; Tapala, K.C.; Clayton, H.S. Prophylactic and Therapeutic Potential Zinc Metallodrugs Drug Discovery: Identification of SARS-CoV-2 Replication and Spike/ACE2 Inhibitors. Curr. Comput.-Aided Drug Des. 2022, 18, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Rodriguez, S.; Ramírez-Contreras, D.; Noriega, L.; García-García, A.; Sánchez-Gaytán, B.L.; Melendez, F.J.; Castro, M.E.; de Azevedo, W.F., Jr.; González-Vergara, E. Interaction of Copper Potential Metallodrugs with TMPRSS2: A Comparative Study of Docking Tools and Its Implications on COVID-19. Front. Chem. 2023, 11, 1128859. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.A.; Martins, F.M.; Siqueira, J.D.; Serpa, C.; Chaves, O.A.; Back, D.F. Synthesis of Cobalt(III) Complexes Derived from Pyridoxal: Structural Cleavage Evaluations and in Silico Calculations for Biological Targets. Inorganics 2024, 12, 171. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, S.; Halim, S.A.; Waqas, M.; Ali, B.; Ataya, F.S.; El-Sabbagh, N.M.; Batiha, G.E.S.; Avula, S.K.; Csuk, R.; et al. Identification of New Pharmacophore against SARS-CoV-2 Spike Protein by Multi-Fold Computational and Biochemical Techniques. Sci. Rep. 2024, 14, 3590. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. ORTEP -3 for Windows-a Version of ORTEP -III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Diamond-Crystal and Molecular Structure Visualization; Crystal Impact-Dr. H. Putz & Dr. K. Brandenburg GbR: Bonn, Germany. Available online: https://www.crystalimpact.de/diamond (accessed on 17 July 2025).
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Gaussian 09, Revision A.02, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, and D. J. Fox, Gaussian, Inc., Wallingford CT, 2016. The calculations were performed employing a B3LYP.
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Fuentealba, P.; Preuss, H.; Stoll, H.; Von Szentpály, L. A Proper Account of Core-Polarization with Pseudopotentials: Single Valence-Electron Alkali Compounds. Chem. Phys. Lett. 1982, 89, 418–422. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]

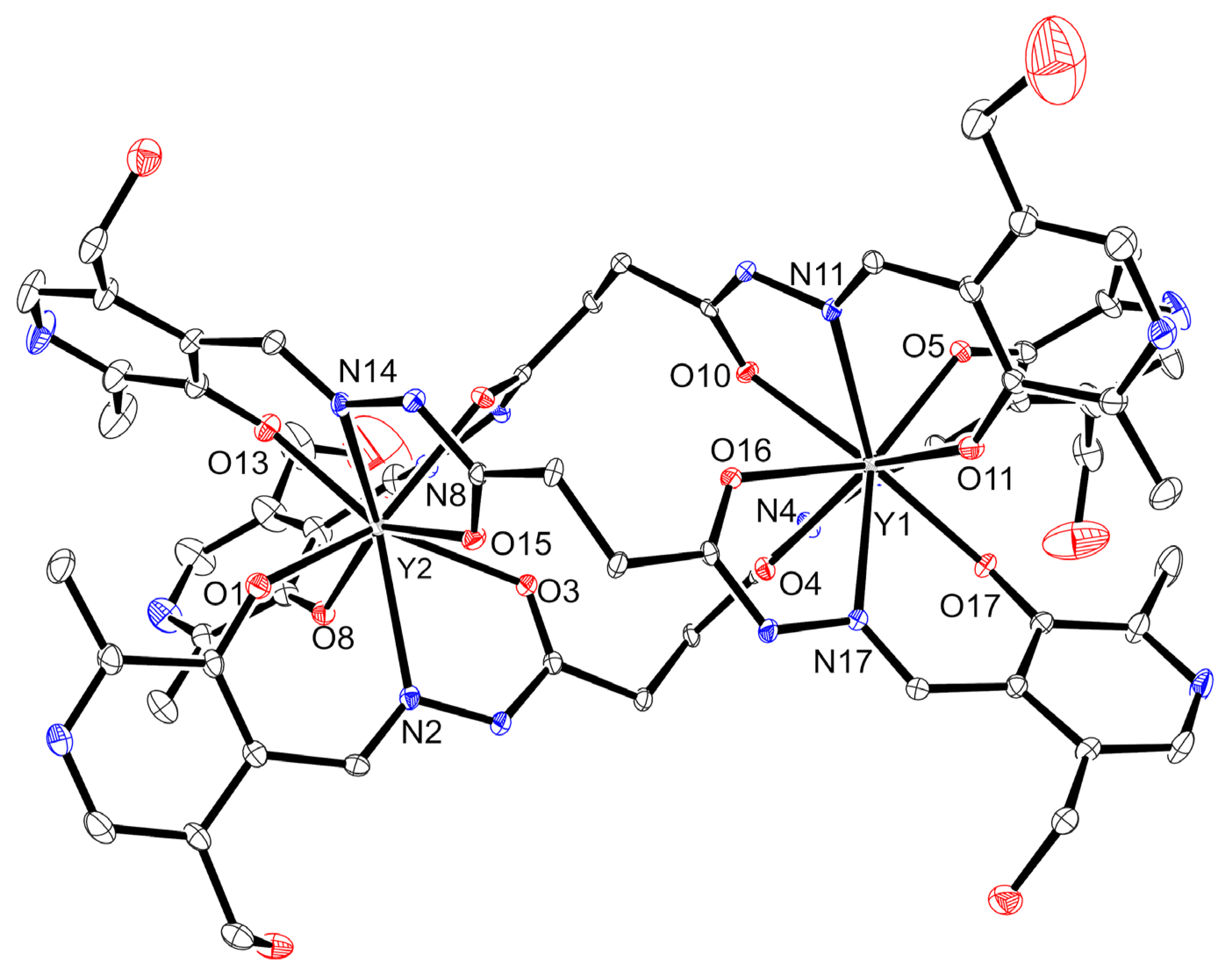

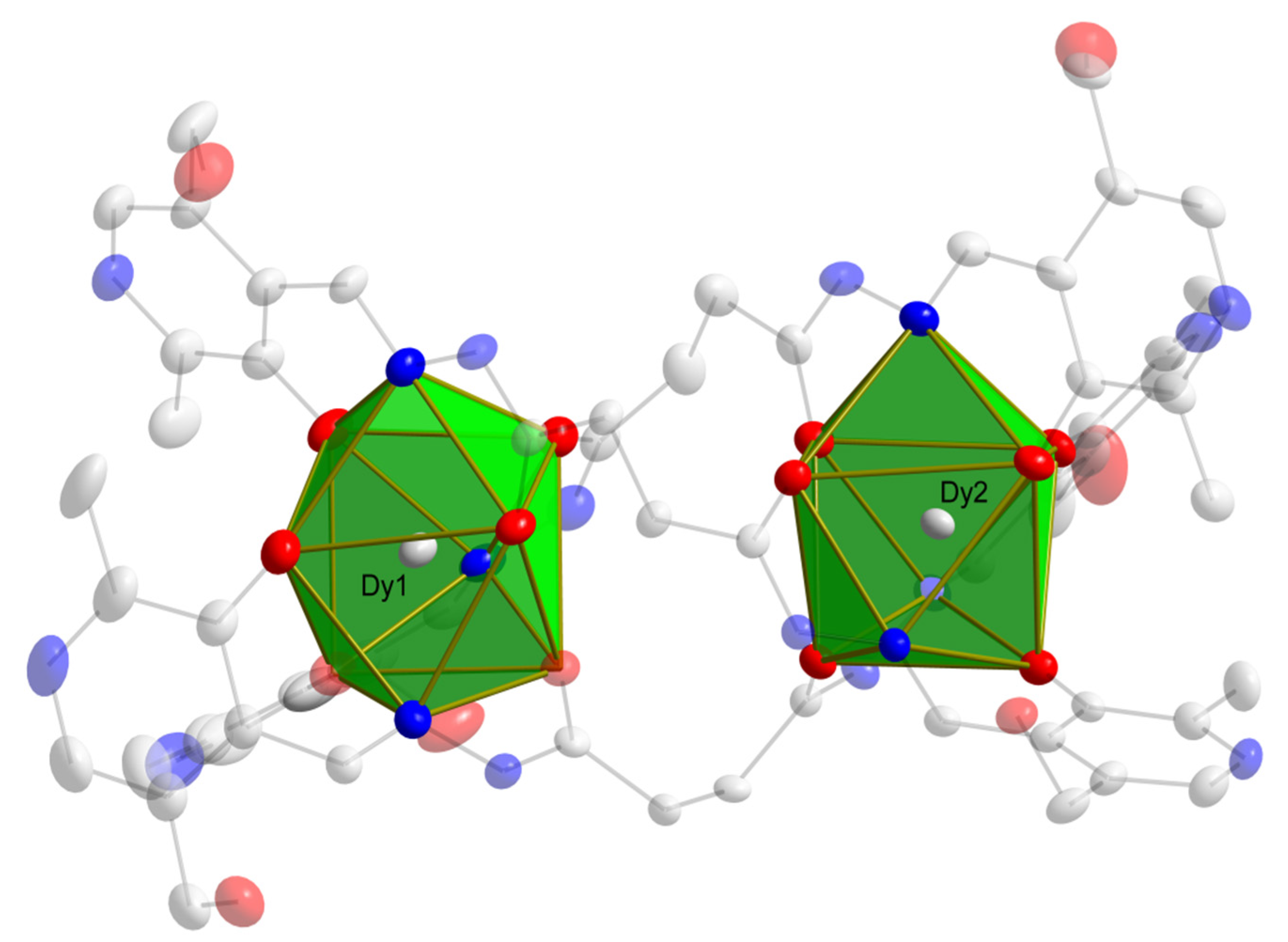
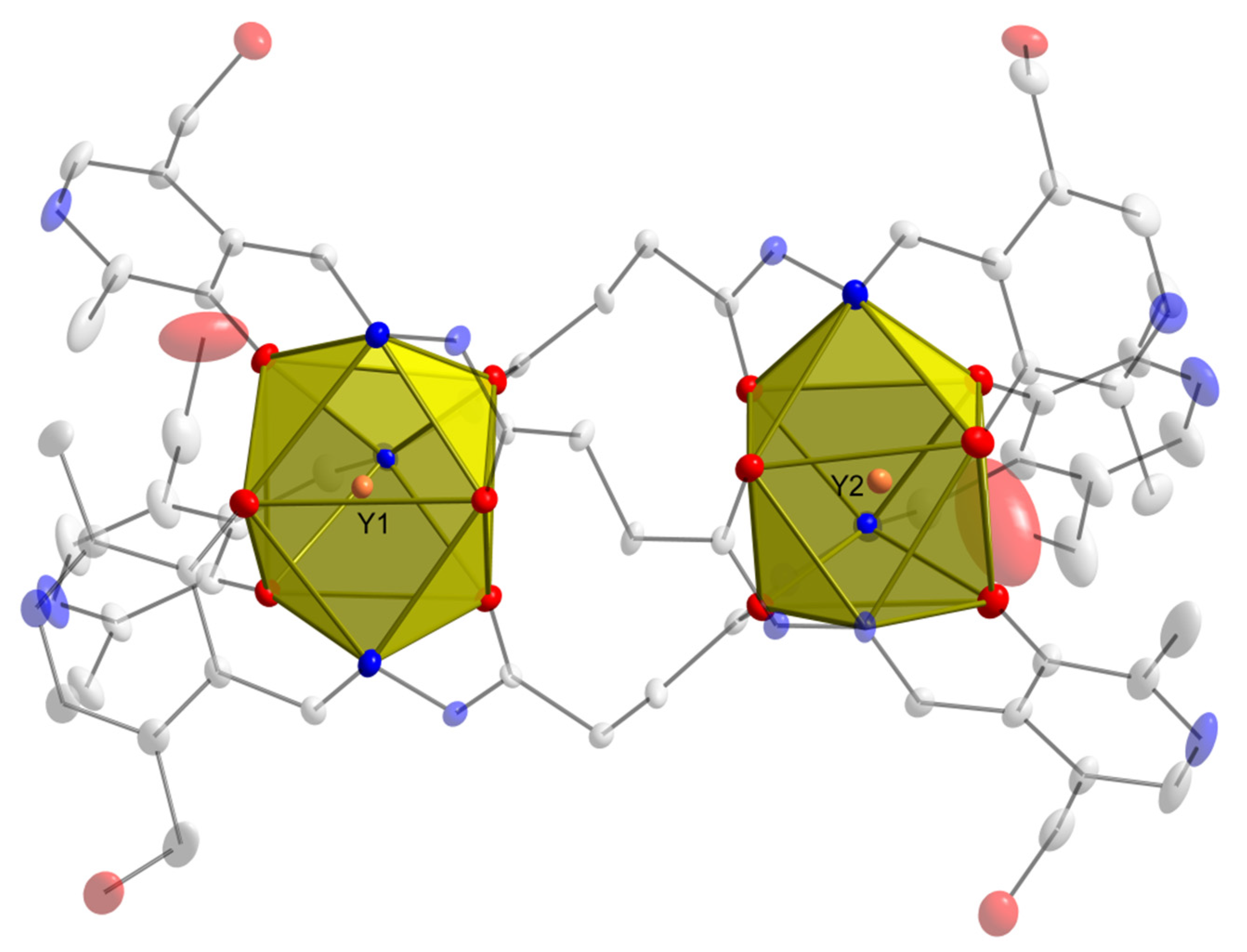
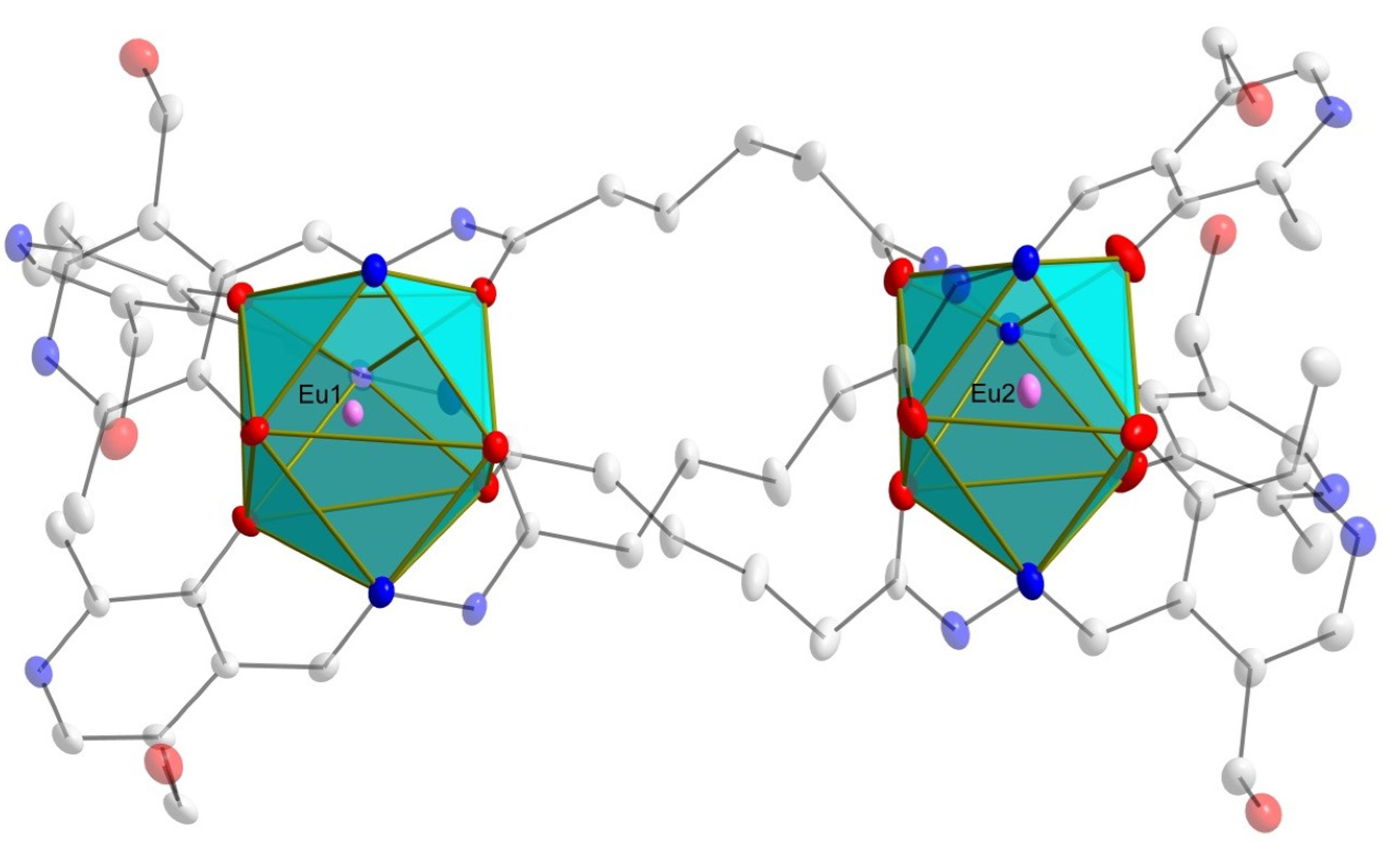


| Bond Length Values (Å) | |||
| Bond | C1 | C2 | C3 * |
| MIII–N(imine) | 2.595(4), 2.604(4), 2.627(4), 2.579(5), 2.580(5), 2.593(5) | 2.575(4), 2.585(4), 2.592(4), 2.593(4), 2.567(4), 2.584(4) | 2.668(4), 2.621(4), 2.679(5), 2.631(7) |
| MIII–O(phenolate) | 2.246(4), 2.259(4), 2.274(4), 2.246(4), 2.260(4), 2.291(4) | 2.266(3), 2.234(3), 2.269(3), 2.239(3), 2.269(3), 2.260(3) | 2.290(4), 2.310(3), 2.279(4), 2.297(5) |
| MIII–O(carbonyl) | 2.410(4), 2.412(4), 2.443(4), 2.424(4), 2.429(4), 2.434(4) | 2.395(3), 2.403(3), 2.416(3), 2.422(3), 2.408(3), 2.390(3) | 2.433(4), 2.483(3), 2.465(4), 2.461(6) |
| N(imine)–N(amide)(H) | 1.380(7), 1.386(6), 1.386(6), 1.384(6), 1.378(6), 1.390(8) | 1.382(5), 1.388(5), 1.377(5), 1.383(5), 1.378(5), 1.391(5) | 1.382(6), 1.390(5), 1.389(6), 1.384(8) |
| C–N(amide) | 1.342(6), 1.343(8), 1.347(6), 1.340(7), 1.344(7), 1.348(8) | 1.349(6), 1.352(6), 1.341(5), 1.341(6), 1.343(6), 1.347(5) | 1.348(7), 1.338(6), 1.321(8), 1.346(14) |
| C=O(carbonyl) | 1.224(7), 1.233(6), 1.234(6), 1.229(6), 1.232(7), 1.238(6) | 1.235(5), 1.230(5), 1.233(5), 1.236(5), 1.236(5), 1.245(5) | 1.230(7), 1.234(6), 1.232(8), 1.237(12) |
| Bonds Angle Values (°) | |||
| Angle | C1 | C2 | C3 * |
| N(imine)–N(amido)(H)–C(O) | 115.9(4), 118.4(4), 118.4(5), 117.1(4), 117.4(4), 117.9(5) | 116.9(4), 117.3(4), 117.8(4), 116.9(4), 117.4(4), 117.7(4) | 117.6(5), 116.8(4), 117.7(6), 117.5(9) |
| Compound | Eg | HOMO | LUMO | ω | η |
|---|---|---|---|---|---|
| C1 | 0.37 | −2.94 | −2.57 | 20.51 | 0.18 |
| C2 | 0.15 | −2.37 | −2.04 | 14.73 | 0.16 |
| C3 | 0.53 | −4.58 | −4.05 | 35.13 | 0.26 |
| Complex | Down Conformation (Kcal/mol) | Up Conformation (Kcal/mol) | Complex ACE2 (Kcal/mol) |
|---|---|---|---|
| C1 | −8.963 | −8.392 | −9.506 |
| C2 | −9.191 | −7.403 | −9.348 |
| C3 | −9.129 | −7.664 | −9.170 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, F.M.; Sokolovicz, Y.C.A.; Oliveira, M.M.; Serpa, C.; Chaves, O.A.; Back, D.F. Synthesis, Structural Characterization, and In Silico Antiviral Prediction of Novel DyIII-, YIII-, and EuIII-Pyridoxal Helicates. Inorganics 2025, 13, 252. https://doi.org/10.3390/inorganics13080252
Martins FM, Sokolovicz YCA, Oliveira MM, Serpa C, Chaves OA, Back DF. Synthesis, Structural Characterization, and In Silico Antiviral Prediction of Novel DyIII-, YIII-, and EuIII-Pyridoxal Helicates. Inorganics. 2025; 13(8):252. https://doi.org/10.3390/inorganics13080252
Chicago/Turabian StyleMartins, Francisco Mainardi, Yuri Clemente Andrade Sokolovicz, Morgana Maciél Oliveira, Carlos Serpa, Otávio Augusto Chaves, and Davi Fernando Back. 2025. "Synthesis, Structural Characterization, and In Silico Antiviral Prediction of Novel DyIII-, YIII-, and EuIII-Pyridoxal Helicates" Inorganics 13, no. 8: 252. https://doi.org/10.3390/inorganics13080252
APA StyleMartins, F. M., Sokolovicz, Y. C. A., Oliveira, M. M., Serpa, C., Chaves, O. A., & Back, D. F. (2025). Synthesis, Structural Characterization, and In Silico Antiviral Prediction of Novel DyIII-, YIII-, and EuIII-Pyridoxal Helicates. Inorganics, 13(8), 252. https://doi.org/10.3390/inorganics13080252













