Valorization of the Bioactive Potential of Juniperus communis L. Berry Extracts Using a Box–Behnken Design and Characterization of Kernel Oil Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Collection and Handling
2.2. Experimental Design
2.2.1. Berry Oil Extraction
2.2.2. Berry Polyphenols Extraction
2.3. Reagents and Solvents
2.4. Instrumentation and Software
2.5. Analyses of J. communis Fruit Extracts
2.5.1. Phytochemical Compounds’ Determination
2.5.2. In Vitro Antioxidant Capacity
2.6. Evaluation of J. communis Berry Oil
2.6.1. Peroxide Value (PV) Assay
2.6.2. DPPH•-Scavenging Capacity
2.6.3. Fatty Acid Quantification
2.6.4. Volatile Compound Identification
2.7. Statistics
3. Results and Discussion
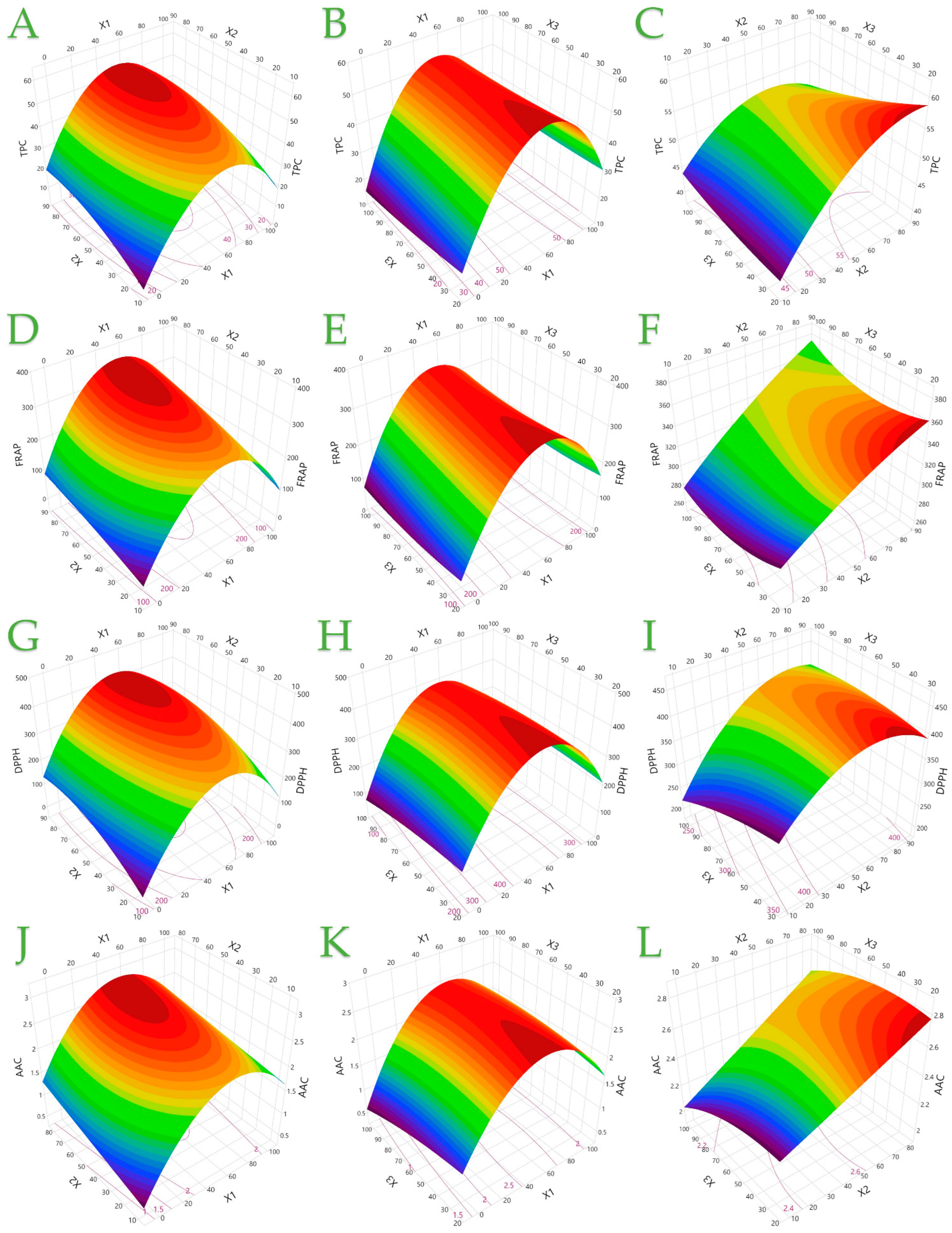
3.1. Extraction Parameter Evaluation
3.2. Model Analysis
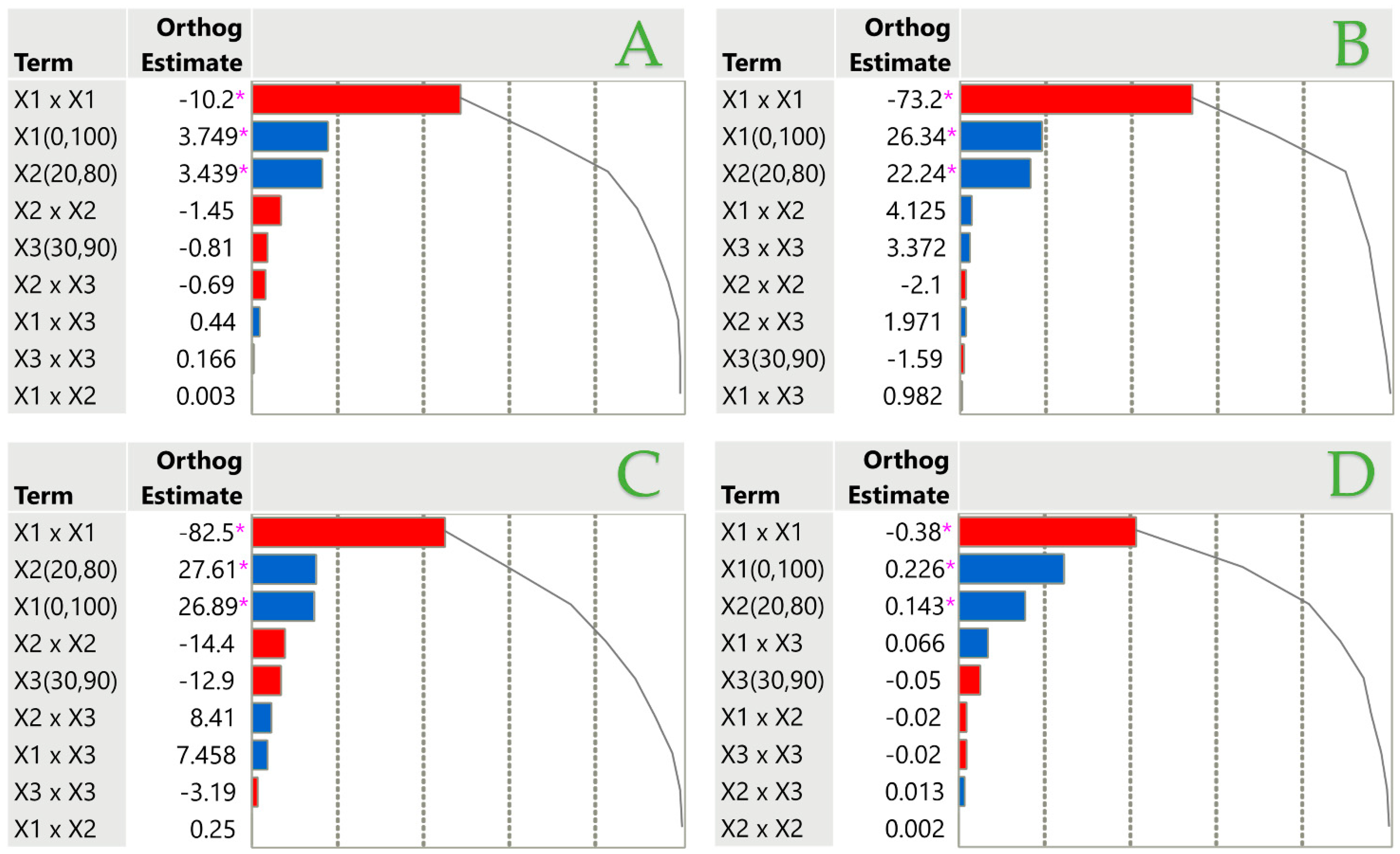
3.3. Effect of Individual Extraction Parameters on Each Assay Through Pareto Plot Analysis
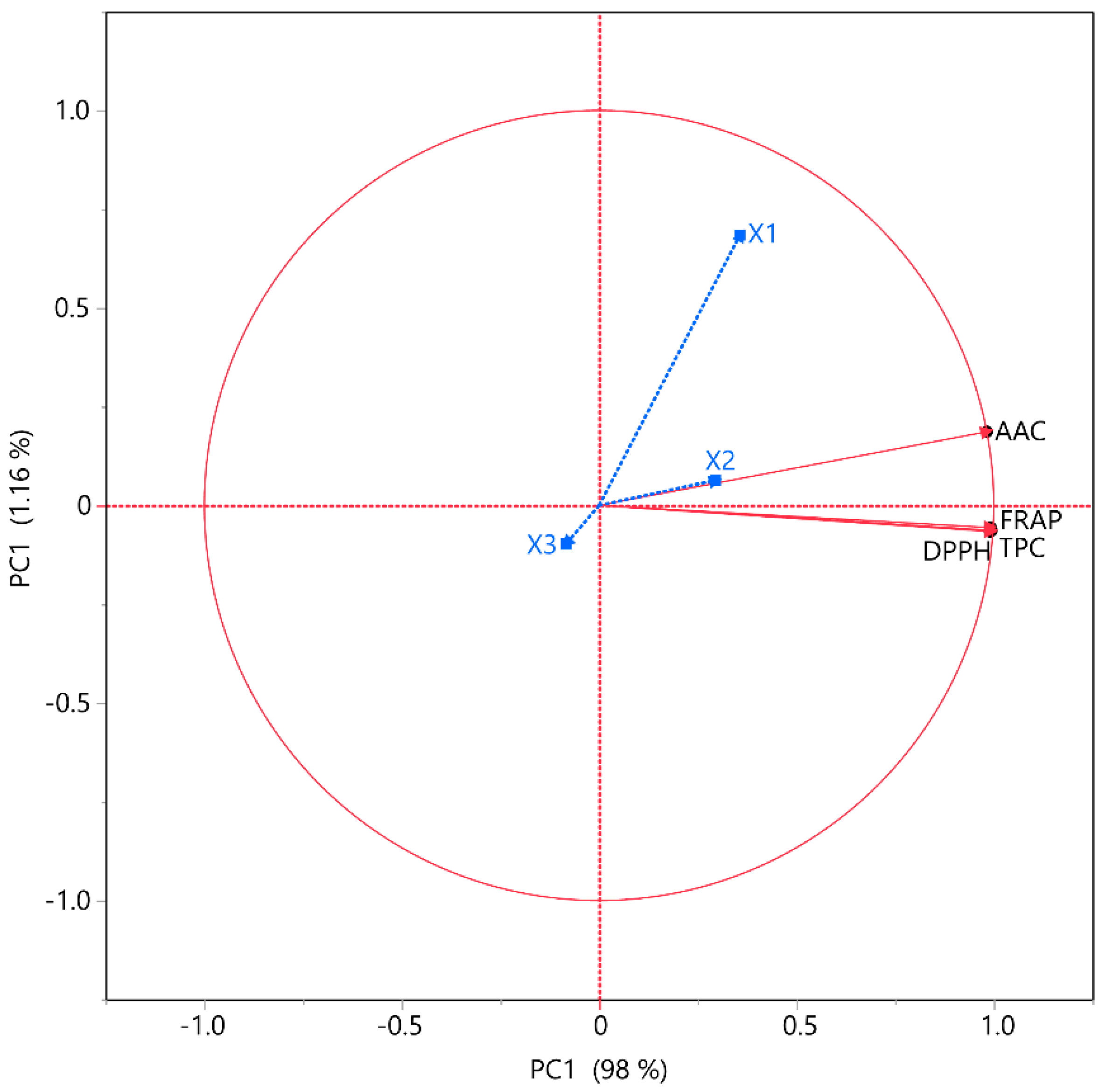
3.4. Correlation Analyses
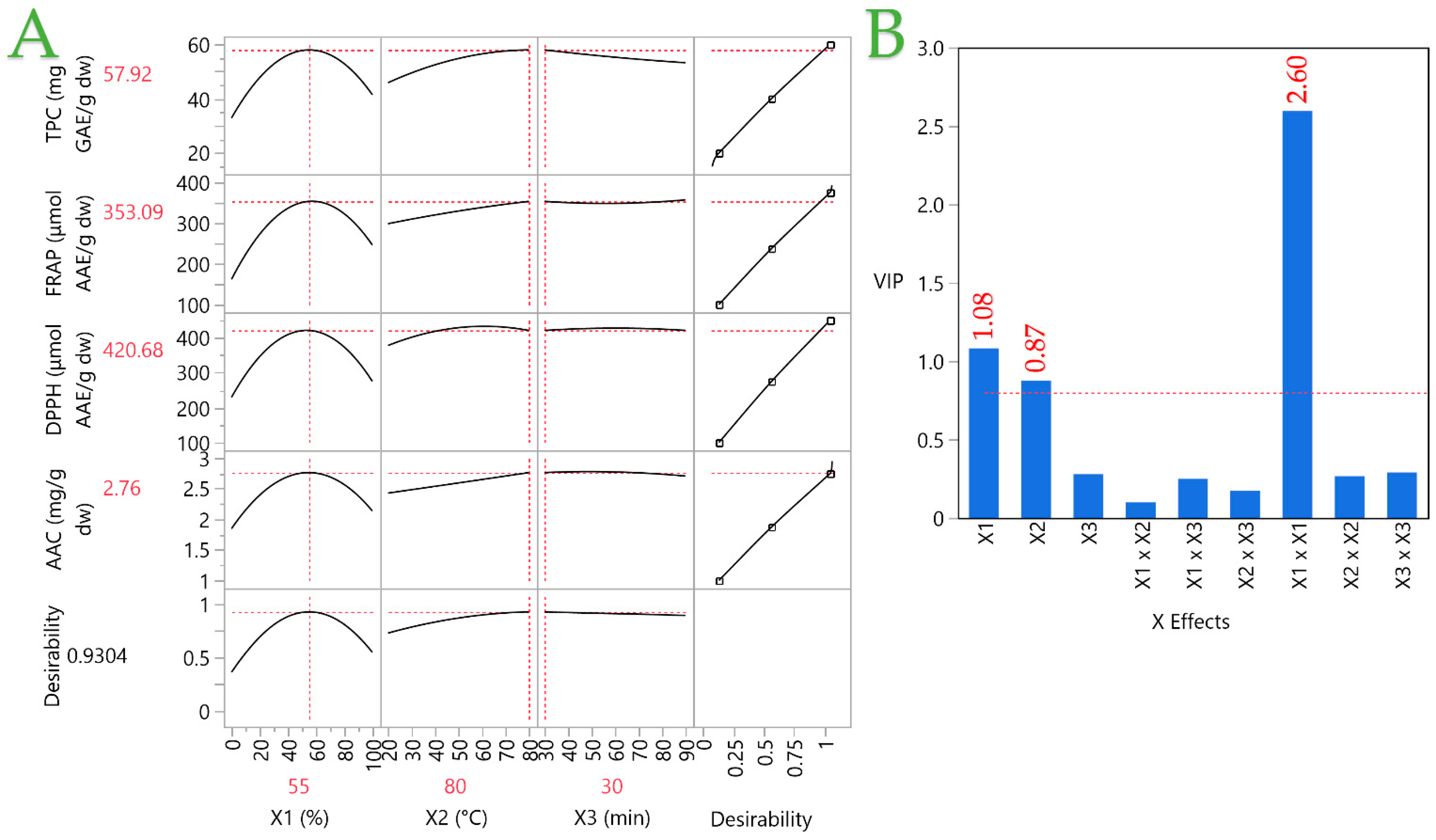
3.5. Partial Least Squares (PLS) Analysis
| Parameters | Partial Least Squares (PLS) Regression | STE Experimental Values |
|---|---|---|
| TPC (mg GAE/g dw) | 57.92 | 55.11 ± 1.54 |
| FRAP (μmol AAE/g dw) | 353.09 | 351.98 ± 20.41 |
| DPPH (μmol AAE/g dw) | 420.68 | 421.37 ± 31.6 |
| AAC (mg AA/g dw) | 2.76 | 2.57 ± 0.07 |
3.6. Berry Oil Analyses
3.6.1. Volatile Compounds
3.6.2. Fatty Acids’ Determination
3.6.3. Oxidative Stability and Antioxidant Capacity of Berries’ Kernel Oil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Polyphenolic Compounds (Standards) | Equation (Linear) | R2 | Retention Time (min) | λmax (nm) | LOD (mg/L) | LOQ (mg/L) |
|---|---|---|---|---|---|---|
| 3-Hydroxytyrosol | y = 183,448.37x − 251,422.04 | 0.997 | 14.552 | 278 | 2.87 | 8.69 |
| Pelargonin chloride | y = 1610.01x − 2626.92 | 0.997 | 18.900 | 275 | 2.84 | 8.61 |
| Catechin | y = 11,920.79x − 128.19 | 0.997 | 20.933 | 278 | 2.54 | 7.71 |
| Homovanillic acid | y = 18,843.08x + 6856.98 | 0.999 | 24.458 | 279 | 1.18 | 3.59 |
| Epicatechin | y = 142,099.00x + 4705.94 | 0.999 | 25.821 | 278 | 0.19 | 0.58 |
| Syringic acid | y = 24,093.04x + 6513.28 | 0.999 | 25.900 | 360 | 3.17 | 9.59 |
| Rutin | y = 46,365.62x − 31,562.74 | 0.997 | 33.777 | 254 | 2.65 | 8.03 |
| Kaempferol-3-glucoside | y = 50,916.85x − 423,988 | 0.996 | 38.724 | 265 | 3.00 | 9.08 |
| Apigenin-7-O-glucoside | y = 64,742.65x + 15,897.94 | 0.998 | 39.854 | 336 | 2.22 | 6.72 |
| Apigenin | y = 95,483.53x − 5214.26 | 0.998 | 55.860 | 227 | 1.03 | 3.13 |
| Kaempferol | y = 93,385.02x − 18,613.03 | 0.999 | 56.883 | 265 | 1.34 | 4.05 |
References
- Belov, T.; Terenzhev, D.; Bushmeleva, K.N.; Davydova, L.; Burkin, K.; Fitsev, I.; Gatiyatullina, A.; Egorova, A.; Nikitin, E. Comparative Analysis of Chemical Profile and Biological Activity of Juniperus communis L. Berry Extracts. Plants 2023, 12, 3401. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Flores-Félix, J.D.; Coutinho, P.; Alves, G.; Silva, L.R. Zimbro (Juniperus communis L.) as a Promising Source of Bioactive Compounds and Biomedical Activities: A Review on Recent Trends. Int. J. Mol. Sci. 2022, 23, 3197. [Google Scholar] [CrossRef]
- Raina, R.; Verma, P.K.; Peshin, R.; Kour, H. Potential of Juniperus Communis L. as a Nutraceutical in Human and Veterinary Medicine. Heliyon 2019, 5, e02376. [Google Scholar] [CrossRef]
- Bais, S.; Gill, N.S.; Kumar, N. Neuroprotective Effect of Juniperus Communis on Chlorpromazine Induced Parkinson Disease in Animal Model. Chin. J. Biol. 2015, 2015, 542542. [Google Scholar] [CrossRef]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef]
- Dursun Capar, T.; Dedebas, T.; Yalcin, H.; Ekici, L. Extraction Method Affects Seed Oil Yield, Composition, and Antioxidant Properties of European Cranberrybush (Viburnum opulus). Ind. Crops Prod. 2021, 168, 113632. [Google Scholar] [CrossRef]
- Tura, M.; Ansorena, D.; Astiasarán, I.; Mandrioli, M.; Toschi, T.G. Evaluation of Hemp Seed Oils Stability under Accelerated Storage Test. Antioxidants 2022, 11, 490. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Cantrell, C.L.; Semerdjieva, I.; Radoukova, T.; Stoyanova, A.; Maneva, V.; Kačániová, M.; Astatkie, T.; Borisova, D.; Dincheva, I.; et al. Essential Oil Composition and Bioactivity of Two Juniper Species from Bulgaria and Slovakia. Molecules 2021, 26, 3659. [Google Scholar] [CrossRef]
- Jeliazkov (Zheljazkov), V.; Kacaniova, M.; Dincheva, I.; Radoukova, T.; Semerdjieva, I.; Astatkie, T.; Schlegel, V. Essential Oil Composition, Antioxidant and Antimicrobial Activity of the Galbuli of Six Juniper Species. Ind. Crops Prod. 2018, 124, 449–458. [Google Scholar] [CrossRef]
- Rawat, P.; Dasila, K.; Singh, M.; Kuniyal, J.C. Influence of Environmental Factors on Phytochemical Compositions and Antioxidant Activity of Juniperus communis L. Discov. Environ. 2025, 3, 11. [Google Scholar] [CrossRef]
- Meringolo, L.; Bonesi, M.; Sicari, V.; Rovito, S.; Passalacqua, N.G.; Loizzo, M.R.; Tundis, R. Essential Oils and Extracts of Juniperus Macrocarpa Sm. and Juniperus oxycedrus L.: Comparative Phytochemical Composition and Anti-Proliferative and Antioxidant Activities. Plants 2022, 11, 1025. [Google Scholar] [CrossRef] [PubMed]
- Tomović, V.; Šojić, B.; Savanović, J.; Kocić-Tanackov, S.; Pavlić, B.; Jokanović, M.; Đorđević, V.; Parunović, N.; Martinović, A.; Vujadinović, D. New Formulation towards Healthier Meat Products: Juniperus communis L. Essential Oil as Alternative for Sodium Nitrite in Dry Fermented Sausages. Foods 2020, 9, 1066. [Google Scholar] [CrossRef]
- Mërtiri, I.; Păcularu-Burada, B.; Stănciuc, N. Phytochemical Characterization and Antibacterial Activity of Albanian Juniperus Communis and Juniperus oxycedrus Berries and Needle Leaves Extracts. Antioxidants 2024, 13, 345. [Google Scholar] [CrossRef]
- Salamon, I.; Otepka, P.; Kryvtsova, M.; Kolesnyk, O.; Hrytsyna, M. Selected Biotopes of Juniperus Communis L. in Slovakia and Their Chemotype Determination. Horticulturae 2023, 9, 686. [Google Scholar] [CrossRef]
- Fejér, J.; Gruľová, D.; Eliašová, A.; Kron, I.; De Feo, V. Influence of Environmental Factors on Content and Composition of Essential Oil from Common Juniper Ripe Berry Cones (Juniperus communis L.). Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2018, 152, 1227–1235. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jayakody, J.T.M.; Kim, J.-I.; Jeong, J.-W.; Choi, K.-M.; Kim, T.-S.; Seo, C.; Azimi, I.; Hyun, J.; Ryu, B. The Influence of Solvent Choice on the Extraction of Bioactive Compounds from Asteraceae: A Comparative Review. Foods 2024, 13, 3151. [Google Scholar] [CrossRef] [PubMed]
- Lapornik, B.; Prošek, M.; Golc Wondra, A. Comparison of Extracts Prepared from Plant By-Products Using Different Solvents and Extraction Time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Kalompatsios, D.; Athanasiadis, V.; Mantiniotou, M.; Lalas, S.I. Optimization of Ultrasonication Probe-Assisted Extraction Parameters for Bioactive Compounds from Opuntia macrorhiza Using Taguchi Design and Assessment of Antioxidant Properties. Appl. Sci. 2024, 14, 10460. [Google Scholar] [CrossRef]
- International Dairy Federation. International IDF Standards 74A:1991: Anhydrous Milkfat—Determination of Peroxide Value; IDF: Brussels, Belgium, 1991. [Google Scholar]
- Kalompatsios, D.; Athanasiadis, V.; Chatzimitakos, T.; Palaiogiannis, D.; Lalas, S.I.; Makris, D.P. Sustainable Exploitation of Waste Orange Peels: Enrichment of Commercial Seed Oils and the Effect on Their Oxidative Stability. Waste 2023, 1, 761–774. [Google Scholar] [CrossRef]
- Kalantzakis, G.; Blekas, G.; Pegklidou, K.; Boskou, D. Stability and Radical-scavenging Activity of Heated Olive Oil and Other Vegetable Oils. Eur. J. Lipid Sci. Technol. 2006, 108, 329–335. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 796/2002 of 6 May 2002 Amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Pomace Oil and on the Relevant Methods of Analysis and the Additional Notes in the Annex to Council Regulation (EEC) No 2658/87 on the Tariff and Statistical Nomenclature and on the Common Customs Tariff. Off. J. Eur. Communities 2002, L128, 14–18. [Google Scholar]
- Lalas, S.; Gortzi, O.; Athanasiadis, V.; Dourtoglou, E.; Dourtoglou, V. Full Characterisation of Crambe Abyssinica Hochst. Seed Oil. J. Am. Oil Chem. Soc. 2012, 89, 2253–2258. [Google Scholar] [CrossRef]
- Toulaki, A.K.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Roufas, K.; Mantanis, G.I.; Dourtoglou, V.G.; Lalas, S.I. Investigation of Xinomavro Red Wine Aging with Various Wood Chips Using Pulsed Electric Field. Beverages 2024, 10, 13. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the Extraction Method on the Recovery of Bioactive Phenolic Compounds from Food Industry By-Products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef]
- Lo Scalzo, R. Organic Acids Influence on DPPH Scavenging by Ascorbic Acid. Food Chem. 2008, 107, 40–43. [Google Scholar] [CrossRef]
- More, P.R.; Jambrak, A.R.; Arya, S.S. Green, Environment-Friendly and Sustainable Techniques for Extraction of Food Bioactive Compounds and Waste Valorization. Trends Food Sci. Technol. 2022, 128, 296–315. [Google Scholar] [CrossRef]
- Thoo, Y.; Ng, S.Y.; Khoo, M.; Mustapha, W.; Ho, C. A Binary Solvent Extraction System for Phenolic Antioxidants and Its Application to the Estimation of Antioxidant Capacity in Andrographis paniculata Extracts. Int. Food Res. J. 2013, 20, 1103–1111. [Google Scholar]
- Dziedzinski, M.; Kobus-Cisowska, J.; Szymanowska, D.; Stuper-Szablewska, K.; Baranowska, M. Identification of Polyphenols from Coniferous Shoots as Natural Antioxidants and Antimicrobial Compounds. Molecules 2020, 25, 3527. [Google Scholar] [CrossRef]
- Dimitrov, F.; Panghyová, L.; Vargová, V.; Baxa, S.; Polovka, M.; Kopuncová, M.; Tobolková, B.; Hrouzková, S.; Sádecká, J. Gas-Chromatographic Analyses of Volatile Organic Compounds in Essential Oils Extracted from Slovak Juniper Berries and Needles (Juniperus communis L.). J. Food Compos. Anal. 2024, 133, 106419. [Google Scholar] [CrossRef]
- Orav, A.; Koel, M.; Kailas, T.; Müürisepp, M. Comparative Analysis of the Composition of Essential Oils and Supercritical Carbon Dioxide Extracts from the Berries and Needles of Estonian Juniper (Juniperus communis L.). Procedia Chem. 2010, 2, 161–167. [Google Scholar] [CrossRef]
- Baum, S.J.; Kris-Etherton, P.M.; Willett, W.C.; Lichtenstein, A.H.; Rudel, L.L.; Maki, K.C.; Whelan, J.; Ramsden, C.E.; Block, R.C. Fatty Acids in Cardiovascular Health and Disease: A Comprehensive Update. J. Clin. Lipidol. 2012, 6, 216–234. [Google Scholar] [CrossRef]
- Satija, A.; Hu, F.B. Plant-Based Diets and Cardiovascular Health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef]
- Custers; Emma, E.M.; Kiliaan; Amanda, J. Dietary Lipids from Body to Brain. Prog. Lipid Res. 2022, 85, 101144. [Google Scholar] [CrossRef]
- Zardo, D.M.; Alvarez, L.V.H.; Los, F.G.B.; Ito, V.C.; Travalini, A.P.; Cardoso, T.; Wojeicchoski, J.P.; Alberti, A.; Zielinski, A.A.F.; Esmerino, L.; et al. In Vitro Assessment of the Antibacterial and Antioxidant Properties of Essential Oils. Curr. Bioact. Compd. 2019, 15, 592–599. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Kouřimská, L. Assessment of the Nutritional Quality of Plant Lipids Using Atherogenicity and Thrombogenicity Indices. Nutrients 2022, 14, 3795. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Kalompatsios, D.; Mantiniotou, M.; Lalas, S.I. Investigation into the Reduction of Palm Oil in Foods by Blended Vegetable Oils through Response Surface Methodology and Oxidative Stability Tests. Antioxidants 2024, 13, 929. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]

| Independent Variables | Coded Units | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Ethanol concentration (C, % v/v) | X1 | 0 | 50 | 100 |
| Temperature (T, °C) | X2 | 20 | 50 | 80 |
| Extraction time (t, min) | X3 | 30 | 60 | 90 |
| Design Point | Independent Variables | Actual Responses * | |||||
|---|---|---|---|---|---|---|---|
| X1 (C, %) | X2 (T, °C) | X3 (t, min) | TPC (mg GAE/g dw) | FRAP (μmol AAE/g dw) | DPPH (μmol AAE/g dw) | AAC (mg AA/g dw) | |
| 1 | 1 (100) | 0 (50) | 1 (90) | 39.65 | 223.95 | 269.17 | 2.14 |
| 2 | −1 (0) | 1 (80) | 0 (60) | 31.51 | 168.56 | 233.22 | 1.84 |
| 3 | −1 (0) | 0 (50) | −1 (30) | 27.77 | 137.12 | 244.50 | 1.62 |
| 4 | 0 (50) | 1 (80) | 1 (90) | 52.22 | 351.29 | 413.89 | 2.64 |
| 5 | 0 (50) | 0 (50) | 0 (60) | 51.32 | 314.25 | 436.20 | 2.51 |
| 6 | 0 (50) | −1 (20) | −1 (30) | 46.21 | 299.39 | 380.51 | 2.45 |
| 7 | 1 (100) | 1 (80) | 0 (60) | 38.16 | 235.48 | 304.76 | 2.28 |
| 8 | 0 (50) | 0 (50) | 0 (60) | 51.39 | 312.31 | 390.55 | 2.61 |
| 9 | −1 (0) | −1 (20) | 0 (60) | 21.13 | 116.79 | 134.84 | 1.23 |
| 10 | −1 (0) | 0 (50) | 1 (90) | 24.05 | 126.81 | 163.57 | 1.16 |
| 11 | 1 (100) | 0 (50) | −1 (30) | 39.96 | 226.65 | 292.33 | 2.09 |
| 12 | 0 (50) | 1 (80) | −1 (30) | 57.32 | 345.85 | 399.83 | 2.65 |
| 13 | 0 (50) | 0 (50) | 0 (60) | 56.58 | 328.68 | 423.75 | 2.55 |
| 14 | 1 (100) | −1 (20) | 0 (60) | 27.76 | 151.76 | 204.44 | 1.82 |
| 15 | 0 (50) | −1 (20) | 1 (90) | 46.44 | 289.56 | 329.43 | 2.34 |
| Factor | TPC | FRAP | DPPH | AAC |
|---|---|---|---|---|
| Least Squares regression | ||||
| Intercept | 53.1 * | 318.4 * | 416.8 * | 2.557 * |
| X1—solvent concentration | 5.134 * | 36.07 * | 36.82 * | 0.31 * |
| X2—temperature | 4.709 * | 30.46 * | 37.81 * | 0.196 * |
| X3—extraction time | −1.11 | −2.18 | −17.6 | −0.07 |
| X1X2 | 0.005 | 7.988 | 0.485 | −0.04 |
| X1X3 | 0.852 | 1.902 | 14.44 | 0.128 |
| X2X3 | −1.33 | 3.817 | 16.28 | 0.025 |
| X12 | −20.6 * | −147 * | −168 * | −0.77 * |
| X22 | −2.88 | −3.69 | −29.5 | 0.002 |
| X32 | 0.334 | 6.797 | −6.42 | −0.04 |
| ANOVA | ||||
| F-value (model) | 23.8 | 47.32 | 26.02 | 23.9 |
| F-value (lack of fit) | 1.036 | 4.171 | 1.022 | 9.628 |
| p-Value (model) | 0.0014 * | 0.0003 * | 0.0011 * | 0.0014 * |
| p-Value (lack of fit) | 0.5253 ns | 0.1994 ns | 0.5292 ns | 0.0955 ns |
| R2 | 0.977 | 0.988 | 0.979 | 0.977 |
| Adjusted R2 | 0.936 | 0.968 | 0.941 | 0.936 |
| RMSE | 3.05 | 15.24 | 23.75 | 0.125 |
| MR | 40.76 | 241.9 | 308.1 | 2.129 |
| PRESS | 493.7 | 16,374 | 29,824 | 1.182 |
| CV | 29.26 | 34.74 | 31.54 | 23.03 |
| DF (total) | 14 | 14 | 14 | 14 |
| Parameters | X1 (C, %) | X2 (T, °C) | X3 (t, min) | Desirability | Least Squares Regression |
|---|---|---|---|---|---|
| TPC (mg GAE/g dw) | 55 | 80 | 30 | 0.9316 | 57.92 ± 6.81 |
| FRAP (μmol AAE/g dw) | 57 | 80 | 90 | 0.9191 | 357.19 ± 34.09 |
| DPPH (μmol AAE/g dw) | 54 | 63 | 38 | 0.9349 | 433.03 ± 33.42 |
| AAC (mg AA/g dw) | 58 | 80 | 60 | 0.9903 | 2.78 ± 0.2 |
| Responses | TPC | FRAP | DPPH | AAC |
|---|---|---|---|---|
| TPC | – | 0.9877 | 0.9834 | 0.9650 |
| FRAP | – | 0.9753 | 0.9635 | |
| DPPH | – | 0.9609 | ||
| AAC | – |
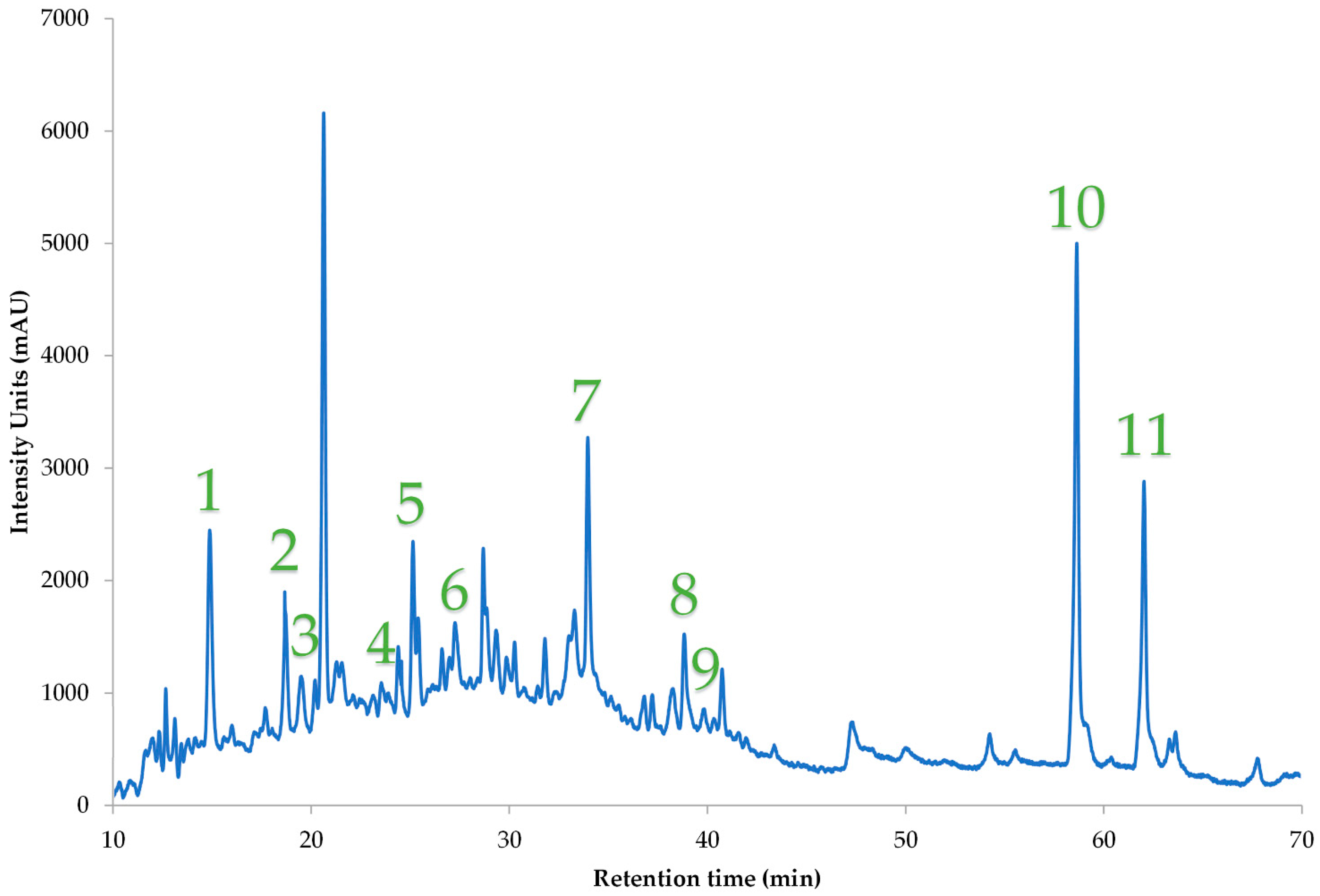
| A/A | Polyphenolic Compounds | Concentration (mg/g dw) |
|---|---|---|
| 1. | 3-Hydroxytyrosol | 0.31 ± 0.01 |
| 2. | Pelargonin chloride | 2.33 ± 0.13 |
| 3. | Catechin | 1.14 ± 0.07 |
| 4. | Homovanillic acid | 0.16 ± 0.01 |
| 5. | Epicatechin | 0.18 ± 0.01 |
| 6. | Syringic acid | 0.27 ± 0.02 |
| 7. | Rutin | 0.32 ± 0.01 |
| 8. | Kaempferol-3-glucoside | 0.21 ± 0.01 |
| 9. | Apigenin-7-O-glucoside | <LOQ |
| 10. | Apigenin | 0.32 ± 0.02 |
| 11. | Kaempferol | 0.16 ± 0 |
| Total identified | 5.41 ± 0.27 |
| A/A | RT (min) | Compound | Chemical Group | CAS Number | Area (%) |
|---|---|---|---|---|---|
| 1. | 9.329 | Tricyclene | Monoterpenes | 508-32-7 | 0.05 ± 0 |
| 2. | 10.249 | (1R)-α-Pinene | Monoterpenes | 7785-70-8 | 9.32 ± 0.34 |
| 3. | 12.228 | (-)-β-Pinene | Monoterpenes | 18172-67-3 | 1.27 ± 0.09 |
| 4. | 14.047 | Myrcene | Monoterpenes | 123-35-3 | 11.91 ± 0.48 |
| 5. | 14.6 | (-)-α-Pinene | Monoterpenes | 7785-26-4 | 0.02 ± 0 |
| 6. | 15.103 | m-Cymene | Alkylbenzenes | 535-77-3 | 0.07 ± 0 |
| 7. | 15.838 | D-Limonene | Monoterpenes | 5989-27-5 | 2.9 ± 0.17 |
| 8. | 16.524 | (E)-β-Ocimene | Monoterpenes | 3779-61-1 | 0.02 ± 0 |
| 9. | 17.201 | α-Ocimene | Monoterpenes | 502-99-8 | 0.01 ± 0 |
| 10. | 17.6 | γ-Terpinene | Monoterpenes | 99-85-4 | 0.03 ± 0 |
| 11. | 17.91 | cis-Sabinene hydrate | Oxygenated Monoterpenoids | 15537-55-0 | 0.12 ± 0.01 |
| 12. | 19.141 | p-Cymenene | Alkylbenzenes | 1195-32-0 | 0.04 ± 0 |
| 13. | 19.547 | Terpinolene | Monoterpenes | 586-62-9 | 0.33 ± 0.02 |
| 14. | 20.434 | (±)-Linalool | Terpenoid Alcohols | 78-70-6 | 0.2 ± 0.01 |
| 15. | 21.106 | α-Campholenal | Terpenoid Aldehydes | 4501-58-0 | 0.1 ± 0 |
| 16. | 21.476 | 1-Octen-3-yl acetate | Terpenoid Esters | 2442-10-6 | 0.15 ± 0.01 |
| 17. | 22.253 | Sabinol | Terpenoid Alcohols | 471-16-9 | 0.08 ± 0 |
| 18. | 23.207 | Pinocarvone | Ketones | 30460-92-5 | 0.03 ± 0 |
| 19. | 25.045 | Terpinen-4-ol | Terpenoid Alcohols | 562-74-3 | 0.13 ± 0.01 |
| 20. | 25.288 | (±)-Myrtenal | Terpenoid Aldehydes | 564-94-3 | 0.05 ± 0 |
| 21. | 25.895 | (-)-α-Terpineol | Terpenoid Alcohols | 10482-56-1 | 0.06 ± 0 |
| 22. | 26.27 | (±)-Verbenone | Ketones | 80-57-9 | 0.08 ± 0 |
| 23. | 27.845 | Carveol | Terpenoid Alcohols | 99-48-9 | 0.03 ± 0 |
| 24. | 28.249 | Fenchyl acetate | Terpenoid Esters | 13851-11-1 | 0.05 ± 0 |
| 25. | 28.661 | (-)-Carvone | Monoterpenoid Ketones | 6485-40-1 | 0.03 ± 0 |
| 26. | 29.325 | Citronellol | Terpenoid Alcohols | 106-22-9 | 0.02 ± 0 |
| 27. | 30.553 | Terpinen-4-ol acetate | Terpenoid Esters | 4821-04-9 | 0.05 ± 0 |
| 28. | 31.273 | α-Fenchene | Oxygenated Monoterpenoids | 471-84-1 | 0.03 ± 0 |
| 29. | 32.456 | (±)-Bornyl acetate | Terpenoid Esters | 76-49-3 | 0.67 ± 0.05 |
| 30. | 33.118 | p-Cymene | Alkylbenzenes | 99-87-6 | 0.11 ± 0 |
| 31. | 33.488 | (±)-α-Pinene | Monoterpenes | 80-56-8 | 0.02 ± 0 |
| 32. | 33.752 | 1,2-Diethylbenzene | Alkylbenzenes | 135-01-3 | 0.02 ± 0 |
| 33. | 33.989 | Neodihydrocarveol | Terpenoid Alcohols | 18675-34-8 | 0.01 ± 0 |
| 34. | 34.883 | Myrtenyl acetate | Terpenoid Esters | 1079-01-2 | 0.05 ± 0 |
| 35. | 35.277 | Perillyl acetate | Terpenoid Esters | 15111-96-3 | 0.02 ± 0 |
| 36. | 35.772 | α-Terpinene | Monoterpenes | 99-86-5 | 0.05 ± 0 |
| 37. | 36.048 | (±)-Camphene | Oxygenated Monoterpenoids | 79-92-5 | 0.06 ± 0 |
| 38. | 36.674 | 2-Carene | Oxygenated Monoterpenoids | 554-61-0 | 0.13 ± 0 |
| 39. | 37.331 | α-Cubebene | Sesquiterpenoids | 17699-14-8 | 0.48 ± 0.02 |
| 40. | 38.783 | Copaene | Sesquiterpenes | 3856-25-5 | 0.26 ± 0.02 |
| 41. | 39.268 | Neryl acetate | Terpenoid Esters | 141-12-8 | 0.22 ± 0.01 |
| 42. | 39.836 | γ-Cadinene | Sesquiterpenes | 39029-41-9 | 1.45 ± 0.06 |
| 43. | 42.093 | (-)-Isocaryophyllene | Sesquiterpenes | 118-65-0 | 24.5 ± 1.47 |
| 44. | 42.889 | γ-Muurolene | Sesquiterpenoids | 30021-74-0 | 0.18 ± 0.01 |
| 45. | 43.981 | Humulene | Sesquiterpenes | 6753-98-6 | 15.93 ± 0.46 |
| 46. | 45.73 | Germacrene D | Sesquiterpenes | 23986-74-5 | 17.43 ± 0.94 |
| 47. | 46.095 | α-Longipinene | Sesquiterpenoids | 5989-08-2 | 0.76 ± 0.03 |
| 48. | 46.684 | α-Muurolene | Sesquiterpenoids | 31983-22-9 | 3.16 ± 0.15 |
| 49. | 47.13 | α-Amorphene | Sesquiterpenoids | 23515-88-0 | 1.22 ± 0.09 |
| 50. | 47.841 | δ-Cadinene | Sesquiterpenes | 483-76-1 | 1.46 ± 0.07 |
| 51. | 48.12 | Cubenene | Sesquiterpenes | 29837-12-5 | 0.17 ± 0.01 |
| 52. | 48.436 | (-)-α-Cadinene | Sesquiterpenes | 24406-05-1 | 0.27 ± 0.01 |
| 53. | 50.597 | Clovene | Sesquiterpenes | 469-92-1 | 2.7 ± 0.14 |
| 54. | 51.267 | (-)-α-Himachalene | Sesquiterpenoids | 3853-83-6 | 0.3 ± 0.02 |
| 55. | 51.918 | (-)-Humulene epoxide II | Oxygenated Sesquiterpenoids | 19888-34-7 | 0.83 ± 0.02 |
| 56. | 55.263 | Alloaromadendrene | Sesquiterpenes | 25246-27-9 | 0.13 ± 0.01 |
| 57. | 57.578 | β-Oplopenone | Oxygenated Sesquiterpenoids | 28305-60-4 | 0.06 ± 0 |
| 58. | 63.651 | Sclarene | Oxygenated Sesquiterpenoids | 511-02-4 | 0.16 ± 0.01 |
| Fatty Acids | Percentage (%) |
|---|---|
| C16:0 (Palmitic acid) | 6.22 ± 0.4 |
| C16:1 (Palmitoleic acid) | 0.56 ± 0.03 |
| C18:0 (Stearic acid) | 2.13 ± 0.08 |
| C18:1 (Oleic acid) | 58.75 ± 2.76 |
| C18:2 (Linoleic acid) | 23.42 ± 1.5 |
| C18:3 (α-Linolenic acid) | 8.92 ± 0.46 |
| ∑ SFA 1 | 8.35 ± 0.48 |
| ∑ MUFA 2 | 59.31 ± 2.79 |
| ∑ PUFA 3 | 32.34 ± 1.96 |
| ∑ ω-3 FA | 8.92 ± 0.46 |
| ∑ ω-6 FA | 23.42 ± 1.5 |
| ∑ ω-9 FA | 58.75 ± 2.76 |
| ∑ UFA 4 | 91.65 ± 4.76 |
| ω-3:ω-6 ratio | 0.38 ± 0 |
| (SFA + MUFA):PUFA ratio | 2.09 ± 0.03 |
| COX 5 | 4.93 ± 0.28 |
| IA 6 | 0.07 ± 0 |
| IT 7 | 0.12 ± 0 |
| HH 8 | 14.65 ± 0.18 |
| HPI 9 | 14.74 ± 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsitsirigka, T.; Kalompatsios, D.; Athanasiadis, V.; Bozinou, E.; Sfougaris, A.I.; Lalas, S.I. Valorization of the Bioactive Potential of Juniperus communis L. Berry Extracts Using a Box–Behnken Design and Characterization of Kernel Oil Compounds. Separations 2025, 12, 209. https://doi.org/10.3390/separations12080209
Tsitsirigka T, Kalompatsios D, Athanasiadis V, Bozinou E, Sfougaris AI, Lalas SI. Valorization of the Bioactive Potential of Juniperus communis L. Berry Extracts Using a Box–Behnken Design and Characterization of Kernel Oil Compounds. Separations. 2025; 12(8):209. https://doi.org/10.3390/separations12080209
Chicago/Turabian StyleTsitsirigka, Theofania, Dimitrios Kalompatsios, Vassilis Athanasiadis, Eleni Bozinou, Athanassios I. Sfougaris, and Stavros I. Lalas. 2025. "Valorization of the Bioactive Potential of Juniperus communis L. Berry Extracts Using a Box–Behnken Design and Characterization of Kernel Oil Compounds" Separations 12, no. 8: 209. https://doi.org/10.3390/separations12080209
APA StyleTsitsirigka, T., Kalompatsios, D., Athanasiadis, V., Bozinou, E., Sfougaris, A. I., & Lalas, S. I. (2025). Valorization of the Bioactive Potential of Juniperus communis L. Berry Extracts Using a Box–Behnken Design and Characterization of Kernel Oil Compounds. Separations, 12(8), 209. https://doi.org/10.3390/separations12080209












