Abstract
The acquisition of high-purity impurities is pivotal for structural identification and an origin analysis, thereby laying a critical foundation for subsequent toxicological evaluation, quality standard development, and process optimization. This study investigated the feasibility of using a solvent gradient twin-column recycling chromatography technique for the separation and purification of multiple trace impurities in iohexol. In this approach, a modifier with a weaker elution strength than the mobile phase is introduced between two chromatographic columns to form a step gradient solvent system. This gradient slows down the leading edge of the elution band relative to the rear edge, inducing a band compression effect that counteracts band broadening and enhances the chromatographic resolution. By optimizing parameters such as the mobile phase composition, elution mode, and modifier flow rate, three trace impurities were successfully separated and purified from iohexol. Their respective purities were improved from initial concentrations of 0.36%, 0.35%, and 0.15% to 97.82%, 91.56%, and 81.56%, respectively. Leveraging the band compression effect on the target components, the impurities were simultaneously purified and concentrated. These results demonstrate that the proposed method is highly effective for the rapid isolation and preparation of trace pharmaceutical impurities.
1. Introduction
Pharmaceutical impurities are substances present in drug products that lack therapeutic effects and can compromise drug stability and efficacy or even pose risks to human health [1,2,3,4]. Therefore, the identification and control of impurities are crucial during pharmaceutical development [5,6,7]. While the targeted synthesis of impurities is possible by elucidating their formation pathways during drug processing, the complexity and diversity of impurity profiles, coupled with the incomplete understanding of reaction mechanisms, often hinder the determination of specific impurity generation routes, thereby constraining the feasibility of targeted synthesis strategies [8]. Consequently, the direct isolation and purification of individual impurities from the active pharmaceutical ingredient (API) generally represents a more practical and effective standard approach [9].
Recycling chromatography [10,11,12] is a powerful separation technique characterized by its high resolving power. By switching valves, the analyte is recirculated through a system comprising one or more chromatographic columns (including single-column, twin-column [13,14,15,16,17,18,19], and multi-column configurations). This effectively extends the column length without increasing the system pressure, making this technique particularly valuable for analyzing and separating difficult-to-resolve mixtures [20]. In recent years, recycling chromatography has been increasingly employed to isolate and purify trace-level substances, such as pharmaceutical impurities [21].
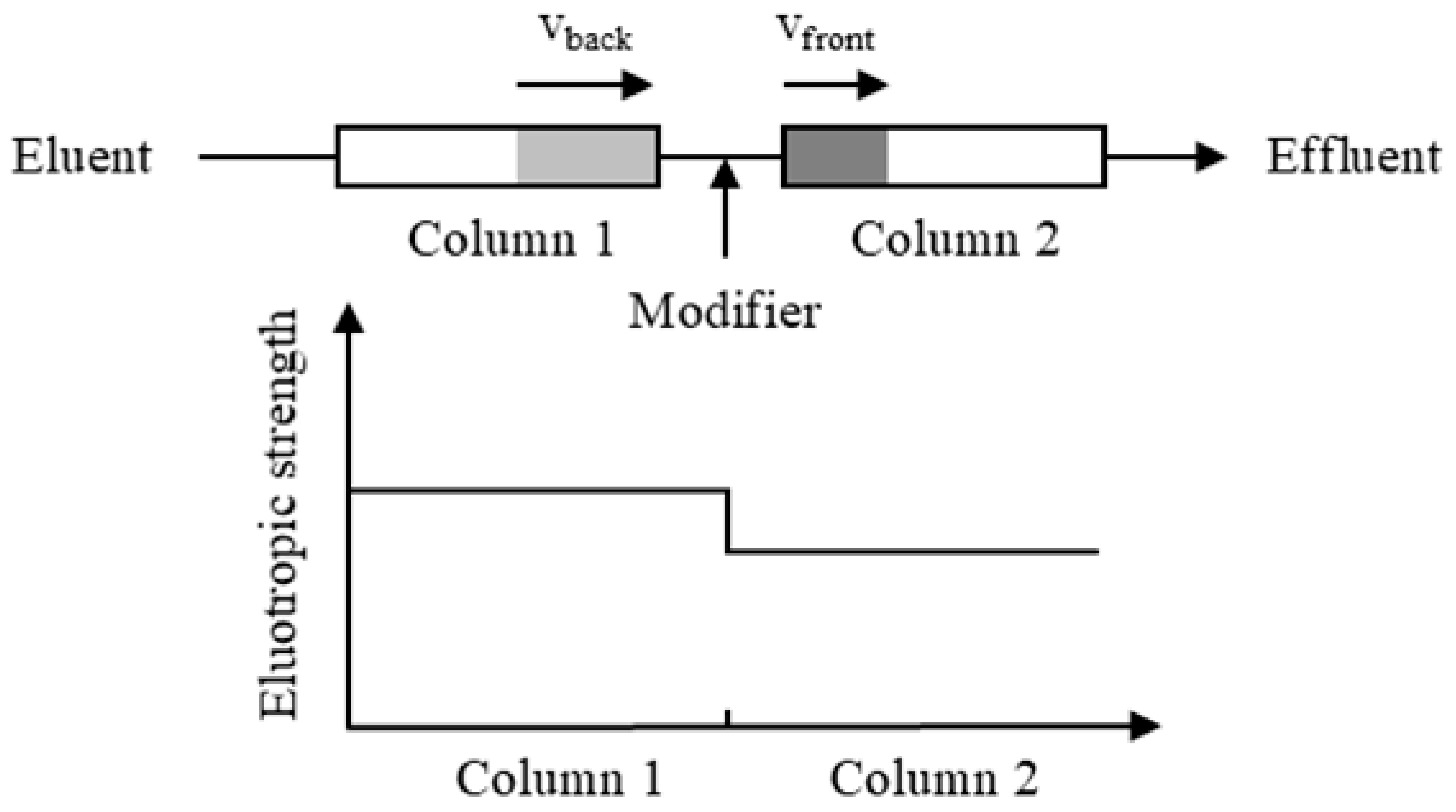
However, in practice, the concentration band of the target component often broadens due to various non-ideal factors, such as the mass transfer resistance [22,23], axial dispersion, and extra-column dead volume [24]. This band broadening not only complicates preparative-scale separations but also dilutes the target component, posing challenges for downstream processing. To address band broadening, our laboratory developed an improved twin-column recycling chromatography (TCRC) system [25,26]. As shown in Figure 1, a modifier with a weaker elution strength than the eluent is continuously introduced at a constant flow rate between two chromatographic columns. This generates a stepwise solvent gradient: the elution strength of the mobile phase is reduced in the downstream column. Consequently, when an impurity band migrates from the upstream to the downstream column, the leading edge slows down so that the trailing edge moves quicker than the leading edge (i.e., vback > vfront), thereby compressing the band [27,28,29]. This band compression counteracts the band broadening, enhances the separation efficiency, and simultaneously concentrates target compounds, making the system particularly advantageous for isolating low-abundance compounds. Using this approach, we successfully isolated individual key impurities from paclitaxel [25] and orlistat [30].

Figure 1.
Schematic diagram of band moving rate changes.
However, APIs and synthetic intermediates often contain multiple trace impurities. Comprehensive pharmaceutical impurity research and rational impurity quality standard establishment typically require the preparation of all individual impurity monomers. The traditional sequential isolation of impurities using TCRC is labor-intensive and time-consuming, requiring substantial amounts of solvents and raw materials. Therefore, the simultaneous isolation of multiple impurities in a single chromatographic run represents a more promising strategy.
Theoretically, after sufficient cycles, multiple impurities can be spatially distributed across the chromatographic system, enabling the sequential collection of the individual impurity at the outlet. However, unlike single-impurity purification, during multiple-impurity separation, the concentration bands of each impurity gradually separate with increasing cycle numbers. This expansion of the spatial footprint occupied by each impurity within the column may exceed the system’s ability to retain all target impurities internally, ultimately leading to the potential loss or leakage of some components. To overcome this limitation, the present study aims to assess the feasibility of the improved TCRC method for the one-step separation of multiple trace impurities in iohexol, focusing on isolating distinct chromatographic peaks (without structural identification) to provide an efficient and low-consumption solution for impurity isolation.
2. Materials and Methods
2.1. Materials
Methanol (analytical grade, >99.5%) and acetonitrile (analytical grade, >99.5%) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Chromatographic-grade acetonitrile (>99.9%) was obtained from Macklin Biochemical Co., Ltd. (Shanghai, China). A stock solution of crude iohexol (200 mg·mL−1) was prepared by dissolution in purified water supplied by Wahaha Group Co., Ltd. (Hangzhou, China).
2.2. Analytical Methods
Sample analysis was performed on an EasyChrom high-performance liquid chromatography (HPLC) system (Auno Science and Technology Co., Ltd., Beijing, China) using a MicroPulite XP tC18 column (4.6 mm × 250 mm, 5 μm; WePure Biotech Co., Ltd., Guangzhou, China). The mobile phase comprised acetonitrile and water, and a linear gradient elution was applied to increase the acetonitrile content from 1% to 13% over a 60 min period. Detection was carried out at a wavelength of 254 nm with a flow rate of 0.8 mL·min−1. The column temperature was maintained at 40 °C, and the injection volume was 1 μL.
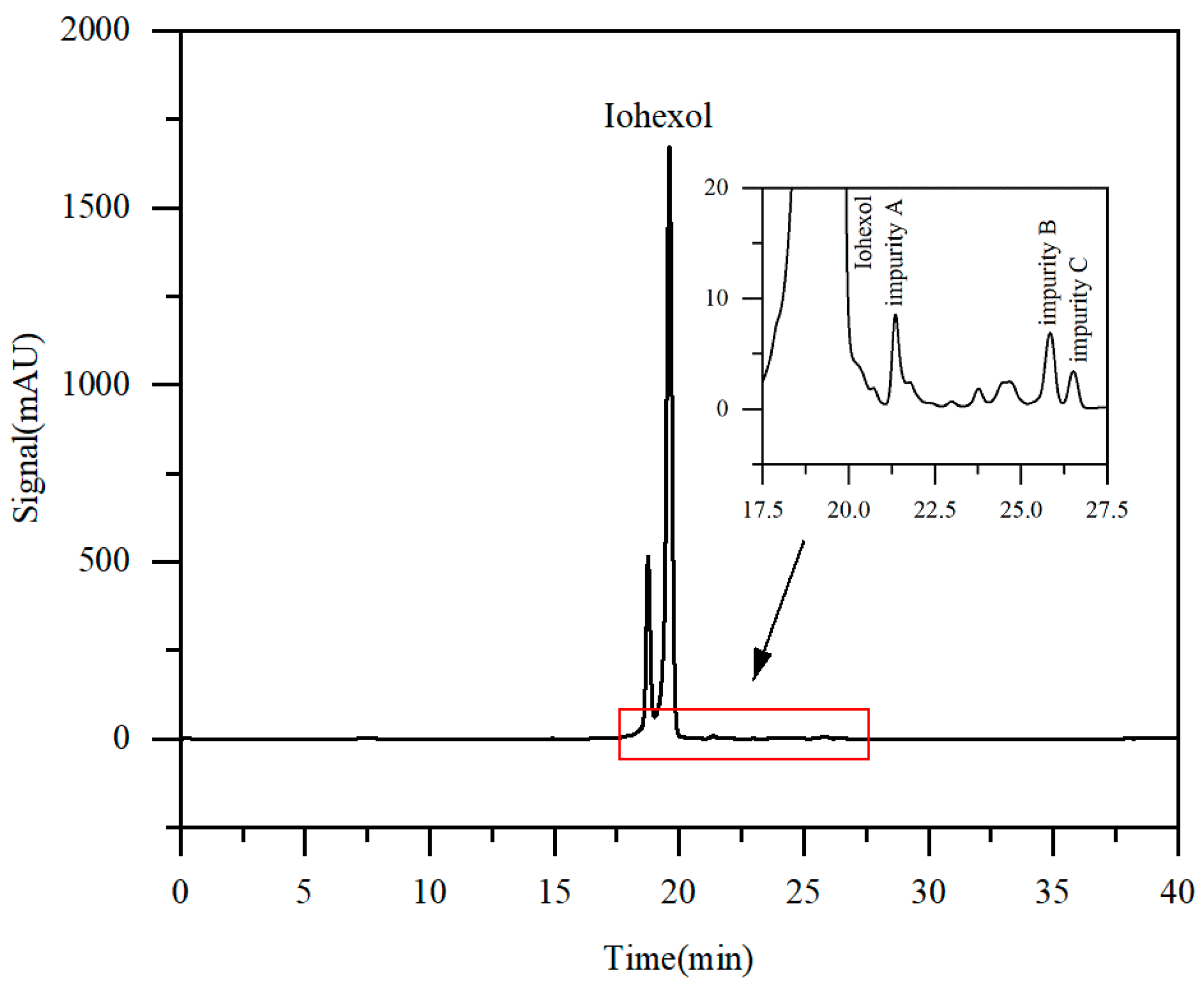
Figure 2 displays the HPLC chromatogram of iohexol crude solution. The primary component, iohexol, constituted 95.86% of the total peak area. Target impurity A was eluted at 21.4 min with a content of 0.36%, while target impurities B and C were eluted at 25.8 min and 26.5 min, with contents of 0.35% and 0.15%, respectively.
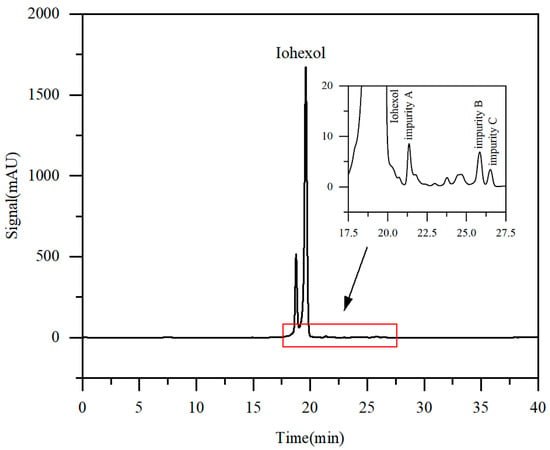
Figure 2.
Chromatogram of iohexol raw material solution (local enlargement of 17.5–27.5 min in inset).
2.3. Instrumentation
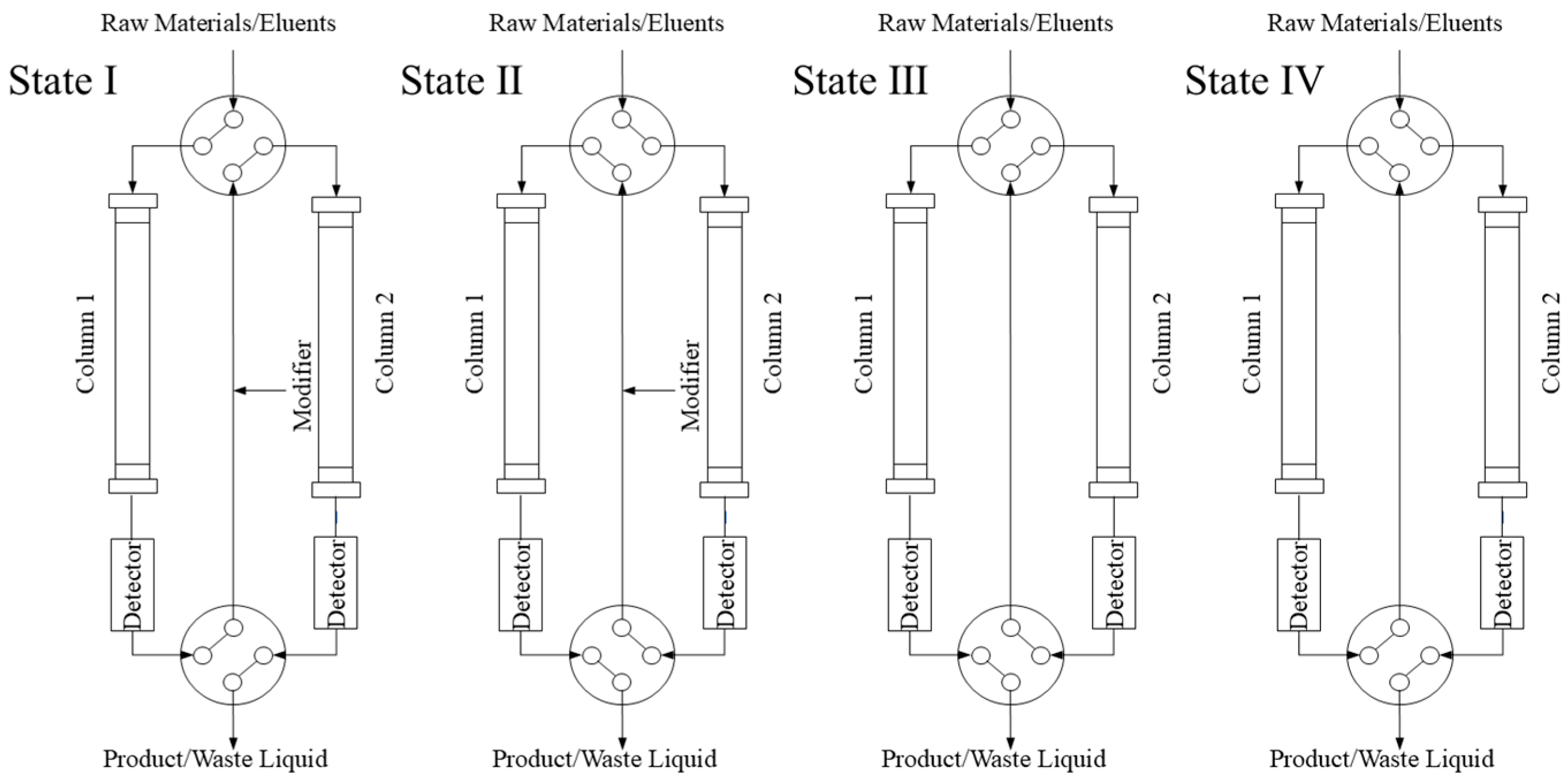
The TCRC system (Auno Science and Technology Co., Ltd., Beijing, China) featured four high-pressure constant-flow pumps: two for binary mobile phase delivery, one for modifier introduction, and one for crude sample solution loading. Two UV detectors (Detector 1 and Detector 2) monitored separation in real-time at Column 1 and Column 2 outlets, respectively. Two four-way/two-position valves were adopted to switch two reversed-phase chromatography columns (10 mm × 250 mm, 30 μm; Auno Technology Co., Ltd., Beijing, China).
As depicted in Figure 3, the system operates through four distinct valve configurations (States I–IV). In State I, the sample flows from Column 1 to Column 2, whereas in State II, the flow direction is reversed, proceeding from Column 2 to Column 1. By alternating between the two states, the components can be continuously circulated in the two columns, thereby effectively increasing the equivalent column length and improving resolution.
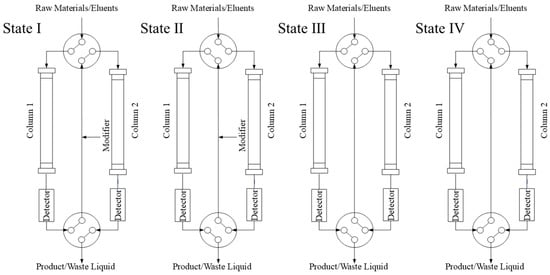
Figure 3.
Schematic diagram of improved twin-column recycling chromatography system.
A modifier with lower elution strength than the mobile phase is continuously introduced between the two columns to induce band compression, counteracting band broadening. If the raw materials contain strongly retained components, they can be eluted separately out of the upstream Column 1 or 2 using a strong eluent under State III or IV, respectively, depending on whether the target compounds are eluted from Column 1 to 2 or from Column 2 to 1.
2.4. Separation Process Design
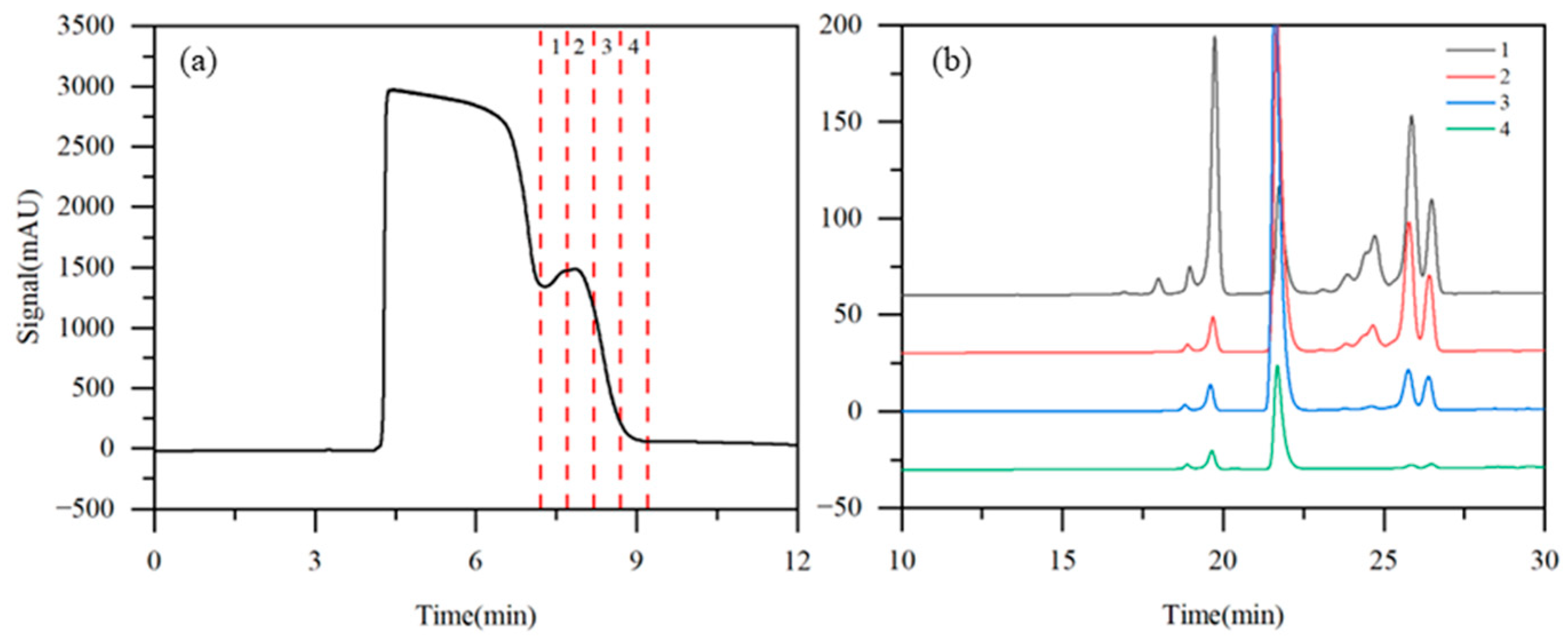
Taking into account both the solubility of the raw material and the chromatographic retention characteristics of the analytes, acetonitrile/water (8:92, v/v) was preliminarily selected as the eluent, with purified water serving as the modifier. To determine the elution order and retention behavior of the components on the semi-preparative column, a single-column operation was first conducted under State III. A 1 mL aliquot of the iohexol crude solution was injected and subsequently eluted with acetonitrile/water (8:92, v/v) at a flow rate of 5 mL·min−1. Four fractions (labeled 1–4) were collected at the column outlet based on the UV detector response (Figure 4a). Further HPLC analysis of these four fractions revealed that iohexol was effectively separated from the later-eluting impurities (Figure 4b). The main component, iohexol, was predominantly eluted before 7.2 min, while the target impurities were detected between 7.2 and 9.2 min. Based on these results, the TCRC operating conditions were preliminarily set as follows: an eluent flow rate of 5 mL·min−1 and a valve switching interval of 10 min (>9.2 min), ensuring complete transfer of all target impurities from the upstream column to the downstream column during each switching cycle.
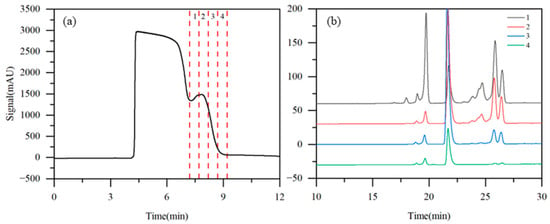
Figure 4.
Detector signal curves (a) and HPLC chromatogram (b) of iohexol in single-column test (1: 7.2–7.7 min; 2: 7.7–8.2 min; 3: 8.2–8.7 min; and 4: 8.7–9.2 min).
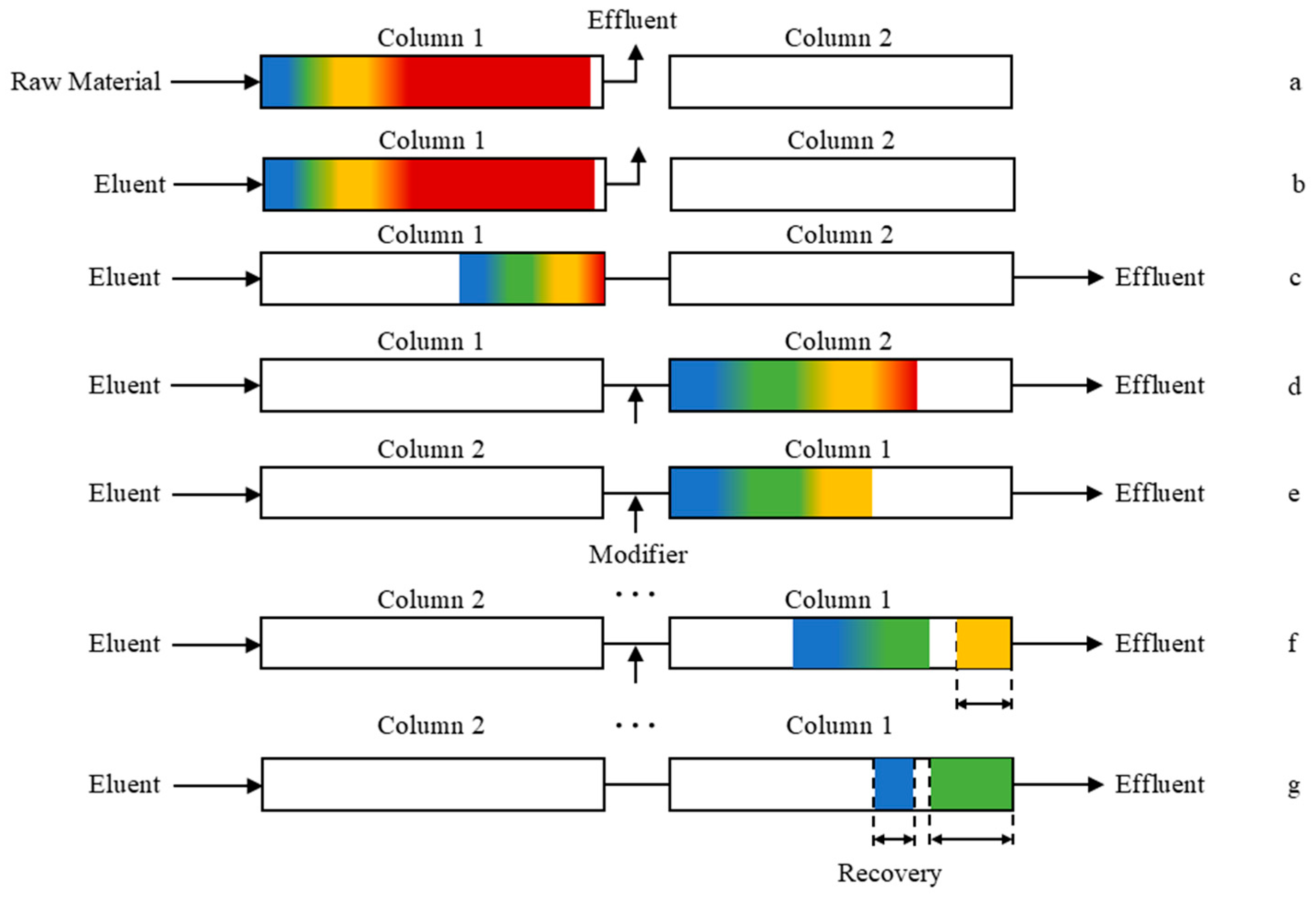
Based on the separation behavior of iohexol crude material on a single chromatographic column, the separation procedure was designed as shown in Figure 5. First, under State III, the crude sample solution was introduced into Column 1 (Figure 5a). After sample loading, the sample pump was stopped, and the elution pump was activated (Figure 5b). Due to their weaker retention, the early-eluting impurities and the main component exited Column 1. When the UV signal at the outlet of Column 1 began to decline, it indicated that the trailing edge of the main component band had mostly eluted, and the target impurities were approaching the column outlet. At this point, the system was swiftly switched to State I, redirecting the flow from Column 1 to Column 2 (Figure 5c,d). Once the target impurities had fully entered Column 2, the system was switched to State II, reversing the flow direction to transfer the target impurities from Column 2 back to Column 1 (Figure 5e). This valve-switching cycle allowed the target impurities to continuously circulate between the two columns, while non-target components were gradually eluted from the system. Impurity A, which is relatively easy to separate, could be collected at the system outlet after a few cycles (Figure 5f). In contrast, impurities B and C required more switching cycles to achieve sufficient resolution; their respective fractions were selectively collected by cutting the eluent stream at appropriate time intervals (Figure 5g).

Figure 5.
The operation process of the twin-column recycling chromatography with a solvent gradient (red represents front impurities and the main component; yellow denotes impurity A; green indicates impurity B; and blue corresponds to impurity C), (a) feeding phase; (b) elution of non-target components; (c,d) transfer of target impurities to column 2; (e) valve switching to elute target impurities from Column 2 to Column 1; (f) recovery of impurity A; (g) recovery of impurities B and C.
However, due to non-ideal factors, such as mass transfer resistance and axial dispersion, the concentration bands of target impurities tend to broaden progressively during the recycling process, leading to dilution and reduced separation efficiency. To address this issue, purified water was continuously introduced as a modifier between the two columns to reduce the elution strength of the mobile phase in the downstream column. As the target concentration band migrated from the upstream to the downstream column, the leading edge advanced more slowly with a longer retention time, while the trailing edge moved faster with a shorter retention time. The target band was compressed to counteract band broadening accordingly [28].
2.5. Evaluation of Separation Performance
To comprehensively evaluate the separation performance, three key parameters were selected: product purity (Pu), solvent consumption (SC), and concentration factor (CRel). Pu was determined by chromatographic analysis of the product fractions, while SC and CRel were calculated using Equations (1) and (2), respectively, as defined below:
where F1 and FM represent the flow rates of the eluent and the modifier, respectively; tRun denotes the total duration of the chromatographic separation process; CF is the concentration of the target component in the crude sample; VF stands for the injection volume of the feed solution; SP and SF are the chromatographic peak areas of the target component in the product fraction and feed solution, respectively; and Vp and Vf are the analytical volumes of the product and feed solutions, respectively.
3. Results
A total of six experimental runs were conducted to investigate the effects of the eluent composition, elution mode, and modifier flow rate on the separation of impurities. The operating conditions and the corresponding separation results are summarized in Table 1.

Table 1.
A summary of the experimental conditions and results of Runs 1–6.
3.1. Effect of Eluent Composition
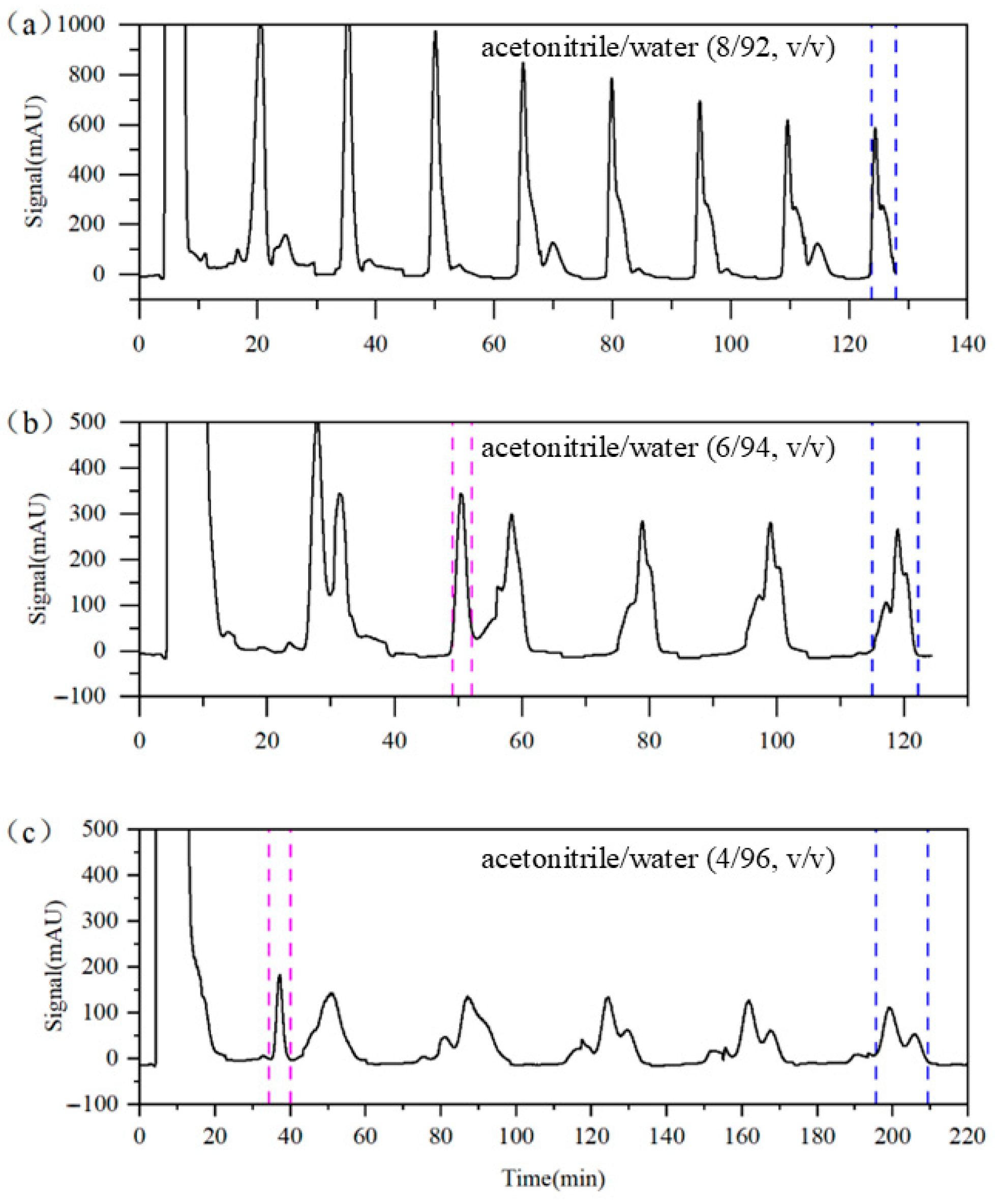
In chromatographic separation systems, the composition of the eluent directly influences the migration rate of concentration bands and is therefore a critical factor affecting the separation performance. Experimental Runs 1 to 3 were designed to examine the impact of eluent compositions on the separation of target impurities. In Run 1, the eluent contained 92% water. As shown in Figure 6a, the separation among components is suboptimal; the purity levels of impurities A, B, and C reach only 90.22%, 69.93%, and 60.87%, respectively, even after 15 switching cycles. When the water content was increased to 94% (Run 2), the separation of impurity A improved significantly. As shown in Figure 6b, impurity A achieved a purity of 96.91% after only two switching cycles; however, impurities B and C remained difficult to resolve, requiring nine switching cycles to achieve purities of only 77.52% and 67.01%, respectively. Further increasing the water content to 96% (Run 3) enabled the isolation of impurity A with a 95.90% purity after only one switching cycle. After nine switching cycles, the purities of impurities B and C increased to 91.57% and 81.63%, respectively. These results demonstrate that increasing the water content in the eluent significantly enhances the separation efficiency of the target impurities. However, the increased water content also prolongs the retention of the target components, leading to longer switching intervals and extended run times, which in turn result in higher solvent consumption. For instance, the solvent consumption in Run 3 was nearly 70% higher than in Run 2. Considering that impurity A is relatively easy to separate, the further optimization of the elution strategy may enable simultaneous improvements in both the product purity and solvent consumption.
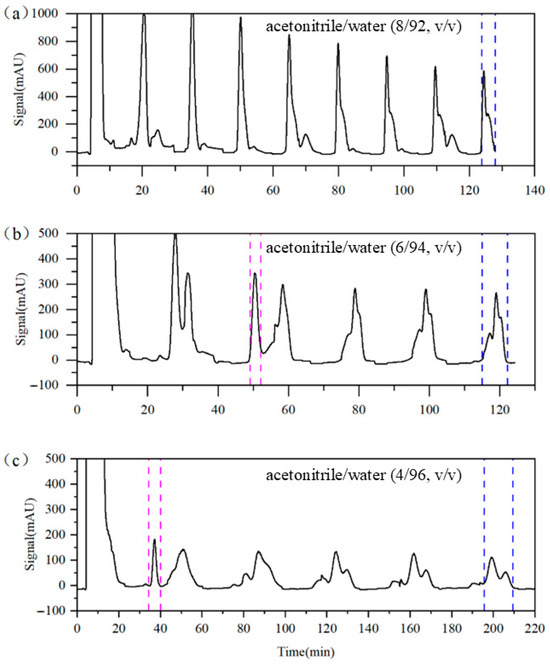
Figure 6.
Detector signal curves of Column 1 in Runs 1–3. (a) Run 1, (b) Run 2, and (c) Run 3. Pink dashed line: recovery of impurity A; blue dashed line: recovery of impurities B and C.
3.2. Effect of Elution Mode
Runs 4 and 5 adopted a stepwise elution strategy: initially using acetonitrile/water (6:94, v/v) as the eluent to purify the readily separable impurity A, followed by switching to a more aqueous eluent to enhance the separation of the more challenging impurities B and C.
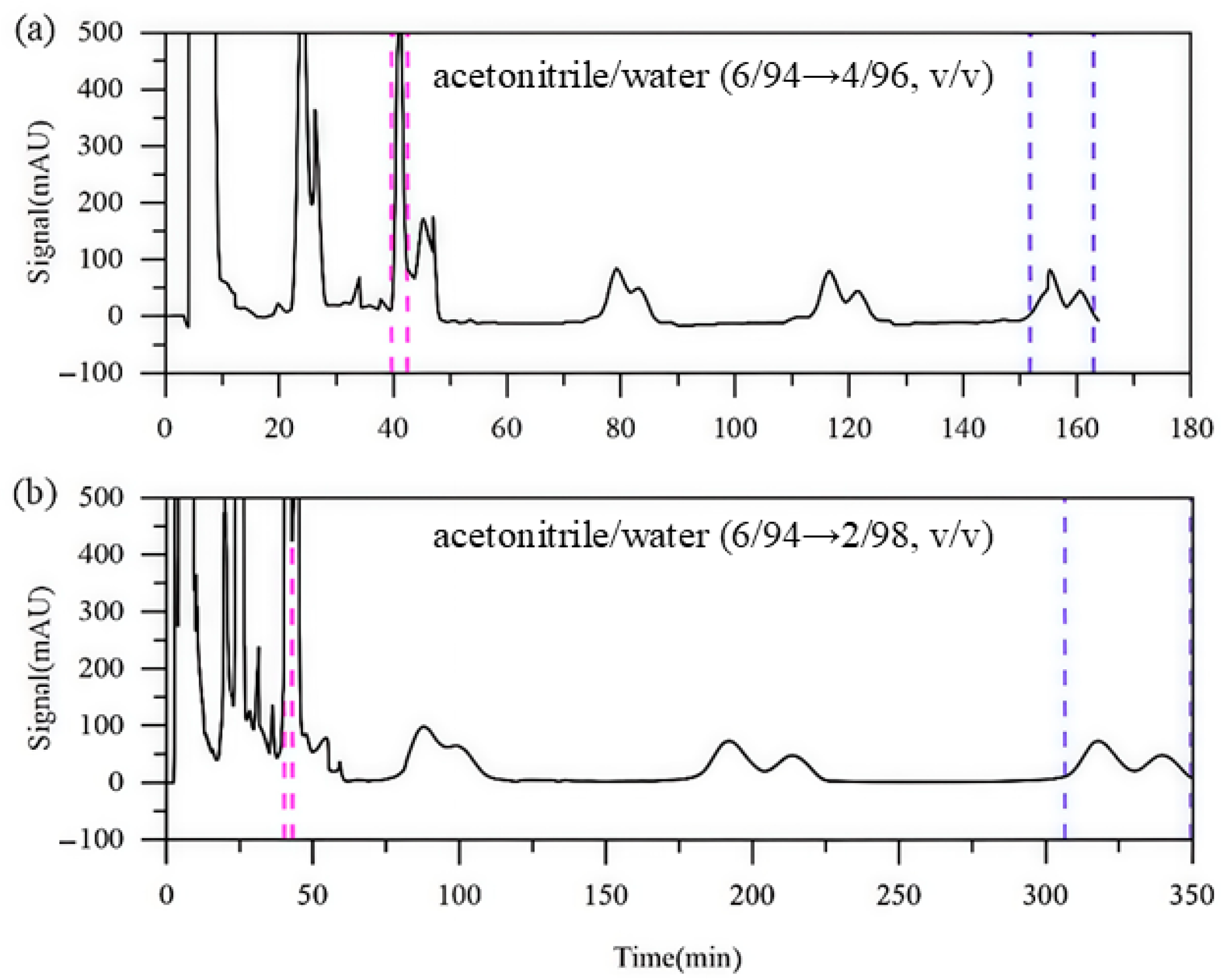
In Run 4, the second-step eluent contained 96% water. As shown in Figure 7a, impurity A was collected during the second switching cycle with a purity of 97.93%, which exceeds that achieved in Run 3 during its first switching cycle. The purities of impurities B and C remained largely unchanged. However, the overall run time was reduced to 162 min, which is significantly shorter than the 210 min required in Run 3. This indicates that the stepwise elution strategy can effectively reduce the solvent consumption without compromising the separation quality. In Run 5, the water content of the second-step eluent was further increased to 98%. As illustrated in Figure 7b, a slight improvement in the separation of all impurities is observed. However, the run time increased substantially, leading to higher solvent consumption. These results suggest that further increasing the water content offers limited improvement in the separation of impurities B and C.
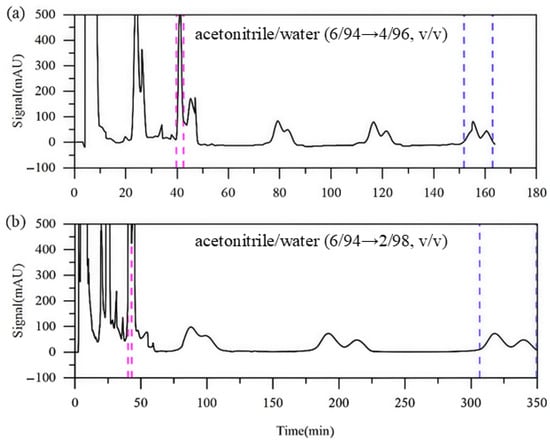
Figure 7.
Detector signal curves of Run 4 and Run 5 ((a): Run 4; (b): Run 5). Pink dashed line: recovery of impurity A; blue dashed line: recovery of impurities B and C.
3.3. Effect of Modifier Flow Rate
In Runs 1 to 5, the CRel values for impurities A, B, and C were all significantly greater than one, indicating that the concentrations of the target impurities in the product fractions were higher than those in the feed solution. This observation confirms that the introduction of a modifier not only facilitates separation but also enables the simultaneous enrichment of target impurities. However, the migration rate of the leading edge is influenced by both the composition and the flow rate of the mobile phase. On one hand, the addition of a modifier increases the water content in the downstream mobile phase, thereby decelerating the migration rate of the leading edge and inducing band compression. On the other hand, the increased flow rate of the downstream mobile phase can accelerate the movement of the leading edge, resulting in band broadening. Thus, the introduction of a modifier does not always lead to a band compression effect and may not necessarily enhance the separation performance.
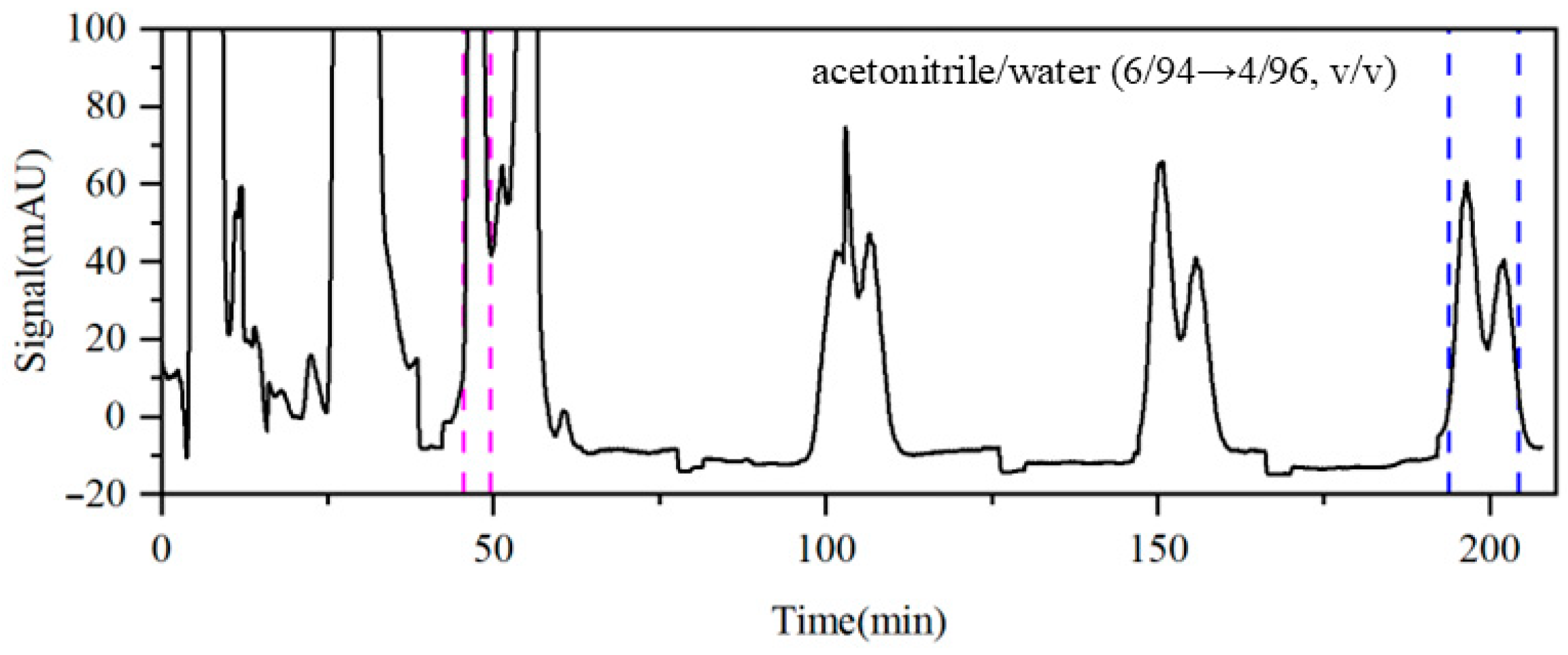
To further explore these two competing effects, Run 6 increased the modifier flow rate from 1.0 mL·min−1 to 1.5 mL·min−1 based on the optimized conditions in Run 4. As shown in Figure 8, the concentration factor of impurity A increases from 5.6 to 6.2, facilitating the identification and collection of the qualified product fraction, with the resulting purity reaching 98.41%. For impurities B and C, the concentration factors increased only slightly from 3.2 to 3.4 and from 5.8 to 5.9, respectively. This limited enhancement may be attributed to their prolonged circulation within the column system. Furthermore, no significant improvements in their purities were observed, suggesting that the acetonitrile/water system has inherent limitations in separating and purifying impurities B and C. Therefore, it may be necessary to modify the composition of the eluent or combine other separation strategies, such as the temperature and pH gradients, to further enhance the separation purity.
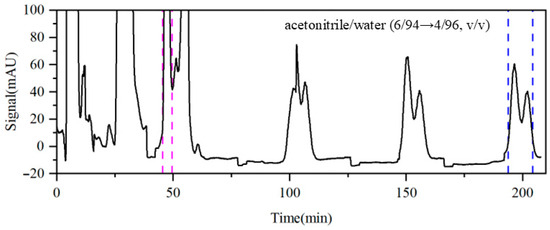
Figure 8.
Detector signal curves of Run 6. Pink dashed line: recovery of impurity A; blue dashed line: recovery of impurities B and C.
3.4. Summary of Optimal Operating Conditions
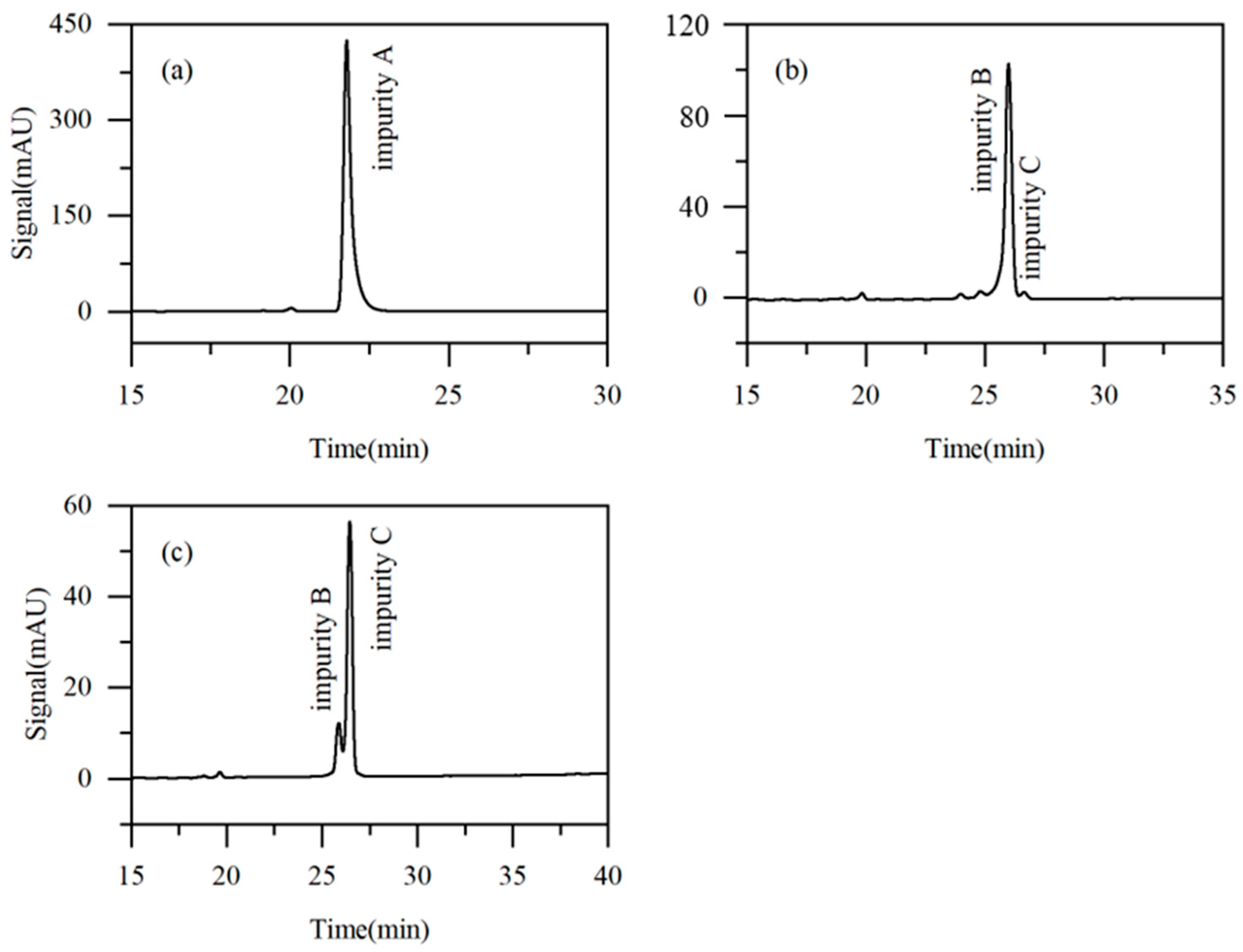
Taking into account the influence of key parameters, including the eluent composition, elution mode, and modifier flow rate on the impurity purification efficiency, solvent consumption, and separation time, Run 4 was identified to offer the optimal separation conditions. Specifically, the initial eluent composition was acetonitrile/water (6:94, v/v), with impurity A (the most readily separable) collected during the second switching cycle. Starting from the third cycle, the eluent was adjusted to acetonitrile/water (4:96, v/v), and the flow rates of the eluent and the modifier (pure water) were set at 5 mL·min−1 and 1 mL·min−1, respectively. The obtained purities of impurities A, B, and C were 97.82%, 91.54%, and 81.56%, respectively. As shown in Figure 9, where the injection volume is 10 μL, the signal peaks for impurities A, B, and C are approximately 423 mAU, 104 mAU, and 53 mAU, respectively. In comparison, the corresponding peaks in Figure 2 (injection volume 1 μL) are all below 10 mAU. This result clearly demonstrates that a significant enrichment of the target impurities was achieved during the recycling process. The final concentrations of impurities A, B, and C in the final collected fractions were increased by 5.6-, 3.2-, and 5.8-fold, respectively, compared to their initial concentrations in the raw material.
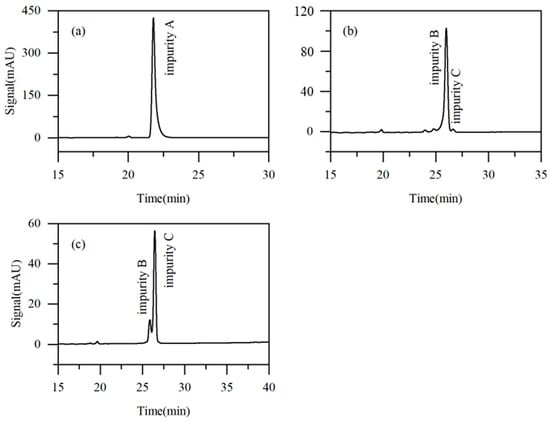
Figure 9.
Chromatograms of Run 5 fractions ((a): fraction of impurity A, (b): fraction of impurity B, and (c): fraction of impurity C).
4. Conclusions
In this study, a solvent gradient twin-column recycling chromatography (TCRC) system was successfully employed for the simultaneous separation and purification of multiple trace impurities (A, B, and C) present in crude iohexol. Crucially, unlike previous solvent gradient TCRC approaches typically limited to single target compounds, this work demonstrates the feasibility and efficiency of isolating multiple distinct impurities in a single chromatographic run. To achieve this multi-component resolution, a novel dynamic gradient switching strategy was developed and implemented, effectively addressing the challenge posed by their differing polarities. By systematically optimizing key operational parameters (i.e., eluent composition, elution strategy, and modifier flow rate), the purity levels of impurities A, B, and C were increased from initial concentrations of 0.36%, 0.35%, and 0.15% to 97.82%, 91.54%, and 81.56%, respectively. Simultaneously, the concentrations of these impurities in the collected fractions were enriched by factors of 5.6, 3.2, and 5.8, respectively, compared to their original levels in the feed solution. These findings demonstrate that the present solvent gradient TCRC method, characterized by its capability for multi-impurity separation and the application of a dynamic gradient control, enables the efficient and rapid separation of multiple trace impurities with a simplified operation and low solvent consumption. This enhanced methodology offers a robust and scalable strategy for the preparative isolation of pharmaceutical impurities, providing strong technical support for subsequent structural characterization, toxicological evaluation, and regulatory-compliant drug registration.
Author Contributions
Conceptualization, J.F. and F.W.; methodology, F.W.; validation, J.F.; formal analysis, J.F.; investigation, J.F.; resources, F.W.; data curation, J.F.; writing—original draft preparation, J.F.; writing—review and editing, J.F. and F.W.; supervision, F.W.; project administration, F.W.; funding acquisition, F.W. All authors have read and agreed to the published version of the manuscript.
Funding
National Natural Science Foundation of China: no. 22078281.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| TCRC | Twin-column recycling chromatography |
References
- Jacobson-Kram, D.; McGovern, T. Toxicological overview of impurities in pharmaceutical products. Adv. Drug Delivery Rev. 2007, 59, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Q.; Chen, T.K.; Mcguire, M.A.; Kord, A.S. Analytical control of genotoxic impurities in the pazopanib hydrochloride manufacturing process. J. Pharm. Biomed. Anal. 2009, 50, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Manchuri, K.M.; Shaik, M.A.; Gopireddy, V.S.R.; Sultana, N.; Gogineni, S. Analytical methodologies to detect N-Nitrosamine impurities in active pharmaceutical ingredients, drug products and other matrices. Chem. Res. Toxicol. 2024, 37, 1456–1483. [Google Scholar] [CrossRef] [PubMed]
- Khandavilli, U.B.R.; Keshavarz, L.; Elika, S.; René, R.E.S.; Frawley, P.J. Organic salts of pharmaceutical impurity p-Aminophenol. Molecules 2020, 25, 1910. [Google Scholar] [CrossRef]
- Mcgovern, T.; Jacobson-Kram, D. Regulation of genotoxic and carcinogenic impurities in drug substances and products. TrAC 2006, 25, 790–795. [Google Scholar] [CrossRef]
- Roy, J. Pharmaceutical impurities--a mini-review. AAPS PharmSciTech 2002, 3, E6. [Google Scholar] [CrossRef]
- EMA. ICH guideline. In Impurities in New Drug Substances Q3A(R2); EMA: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Lu, H.; Xu, G.; Kou, J.; Wu, S.; Zeng, J.; Liu, Y.; Lin, J.; Xu, Y.; Shang, W.; Li, Y.; et al. Identification, synthesis, characterization, and control strategy establishment for process impurities of baloxavir marboxil. Org. Process Res. Dev. 2024, 28, 1170–1185. [Google Scholar] [CrossRef]
- EMA. ICH guideline. In Impurities in New Drug Substances Q3B(R2); EMA: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Biesenberger, J.A.; Tan, M.; Duvdevan, I. Recycle Gel Permeation Chromatography 2. Analysis of Epoxy Resins. Appl. Polym. Sci. 1971, 15, 1549–1552. [Google Scholar] [CrossRef]
- Biesenberger, J.A.; Tan, M.; Duvdevan, I.; Maurer, T. Recycle Gel Permeation Chromatography 1. Recycle Principle and Design. J. Polym. Sci. Part B 1971, 9, 353–357. [Google Scholar] [CrossRef]
- Duvdevani, I.; Biesenberger, J.A.; Tan, M. Recycle Gel Permeation Chromatography 3. Design Modifications and Some Results with Polycarbonate. J. Polym. Sci. Part B 1971, 9, 429–434. [Google Scholar] [CrossRef]
- Gritti, F. Automated High-Resolution Semi-Preparative Gradient Recycling Liquid Chromatography: Principles, Design, and Applications. LC-GC Eur. 2021, 34, 455–461. [Google Scholar] [CrossRef]
- Shi, C.; Gao, Z.Y.; Zhang, Q.L.; Yao, S.J.; Slater, N.K.H.; Lin, D.Q. Model-based process development of continuous chromatography for antibody capture: A case study with twin-column system. J. Chromatogr. A 2020, 1619, 460936. [Google Scholar] [CrossRef]
- Webber, K.G.I.; Truong, T.; Johnston, S.M.; Zapata, S.E.; Liang, Y.R.; Davis, J.M.; Buttars, A.D.; Smith, F.B.; Jones, H.E.; Mahoney, A.C. Label-Free Profiling of up to 200 Single-Cell Proteomes per Day Using a Dual-Column Nanoflow Liquid Chromatography Platform. Anal. Chem. 2022, 94, 6017–6025. [Google Scholar] [CrossRef] [PubMed]
- Sivo, A.; Kim, T.K.; Ruta, V.; Luisi, R.; Osorio-Tejada, J.; Escriba-Gelonch, M.; Hessel, V.; Sponchioni, M.; Vilé, G. Enhanced flow synthesis of small molecules by in-line integration of sequential catalysis and benchtop twin-column continuous chromatography. React. Chem. Eng. 2022, 7, 2650–2658. [Google Scholar] [CrossRef]
- Rudenko, B.A. Circulation chromatography. J. Anal. Chem. 2009, 64, 547–552. [Google Scholar] [CrossRef]
- Weldon, R.; Lill, J.; Olbrich, M.; Schmidt, P.; Müller-Späth, T. Purification of a GalNAc-cluster-conjugated oligonucleotide by reversed-phase twin-column continuous chromatography. J. Chromatogr. A 2022, 1663, 462734. [Google Scholar] [CrossRef]
- Jing, S.Y.; Shi, C.; Leong, H.Y.; Yuan, J.J.; Gao, D.; Wang, H.B.; Yao, S.J.; Lin, D.Q. A novel twin-column continuous chromatography approach for separation and enrichment of monoclonal antibody charge variants. Eng. Life Sci. 2021, 21, 382–391. [Google Scholar] [CrossRef]
- Zhao, W.Q.; Yang, G.; Zhong, F.Y.; Yang, N.; Zhao, X.; Qi, Y.P.; Fan, G.R. Isolation and purification of diastereoisomeric flavonolignans from silymarin by binary-column recycling preparative high-performance liquid chromatography. J. Sep. Sci. 2014, 37, 2300–2306. [Google Scholar] [CrossRef]
- Lievore, G.; Weldon, R.; Catani, M.; Cavazzini, A.; Müller-Späth, T. Enrichment and recovery of oligonucleotide impurities by N-Rich twin-column continuous chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1209, 123439. [Google Scholar] [CrossRef]
- Horváth, C.; Lin, H.J. Movement and band spreading of unsorbed solutes in liquid chromatography. J. Chromatogr. A 1976, 126, 401–420. [Google Scholar] [CrossRef]
- Cavazzini, A.; Gritti, F.; Kaczmarski, K.; Marchetti, N.; Guiochon, G. Mass-transfer kinetics in a shell packing material for chromatography. Anal. Chem. 2007, 79, 5972–5979. [Google Scholar] [CrossRef]
- Minarik, M.; Franc, M.; Minarik, M. High performance liquid chromatography column efficiency enhancement by zero dead volume recycling and practical approach using park and recycle arrangement. J. Chromatogr. A 2018, 1554, 1–7. [Google Scholar] [CrossRef]
- Xie, W.; Wu, Y.X.; Jin, G.X.; Sang, J.R.; Wei, F. Enrichment and purification of trace substances from yew extracum by twin-column recycling chromatography with a step solvent gradient. J. Chromatogr. B 2025, 1252, 124467. [Google Scholar] [CrossRef]
- Fu, J.; Wu, Y.; Wei, F. Separation and purification of 10-dab III by twin-column recycling chromatography with a step solvent gradient. Ind. Eng. Chem. Res. 2025, 64, 3511–3519. [Google Scholar] [CrossRef]
- Wei, F.; Yang, Z.W.; Zhao, Y.X.; Wang, Q. A twin-column recycling chromatography with solvent gradient for reinforcing the isolation of minor impurities. AIChE J. 2019, 65, 702–711. [Google Scholar] [CrossRef]
- Wei, F.; Sang, J.R.; Zhao, Y.X. Theoretical study of twin-column recycling chromatography with a solvent-gradient for preparative binary separations. J. Chromatogr. A 2021, 1651, 462306. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Hu, Y.; Zhao, Y.; Wu, Y.X.; Jin, G.X. Purification and concentration of minor impurity in the bulk drug by step-gradient twin-column recycling chromatography. Ind. Eng. Chem. Res. 2023, 62, 6241–6250. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, F.; Zhao, Y.X.; Wang, Q. Fine optimization of twin-column recycling chromatography with a solvent gradient for the removal of minor impurities. J. Chromatogr. A 2020, 1609, 460443. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).