Molluscicidal Activity of the Crude Extract and Fractions of Myrsine parvifolia
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collect
2.2. Obtention of the Extracts and Fractions for the Bioassay
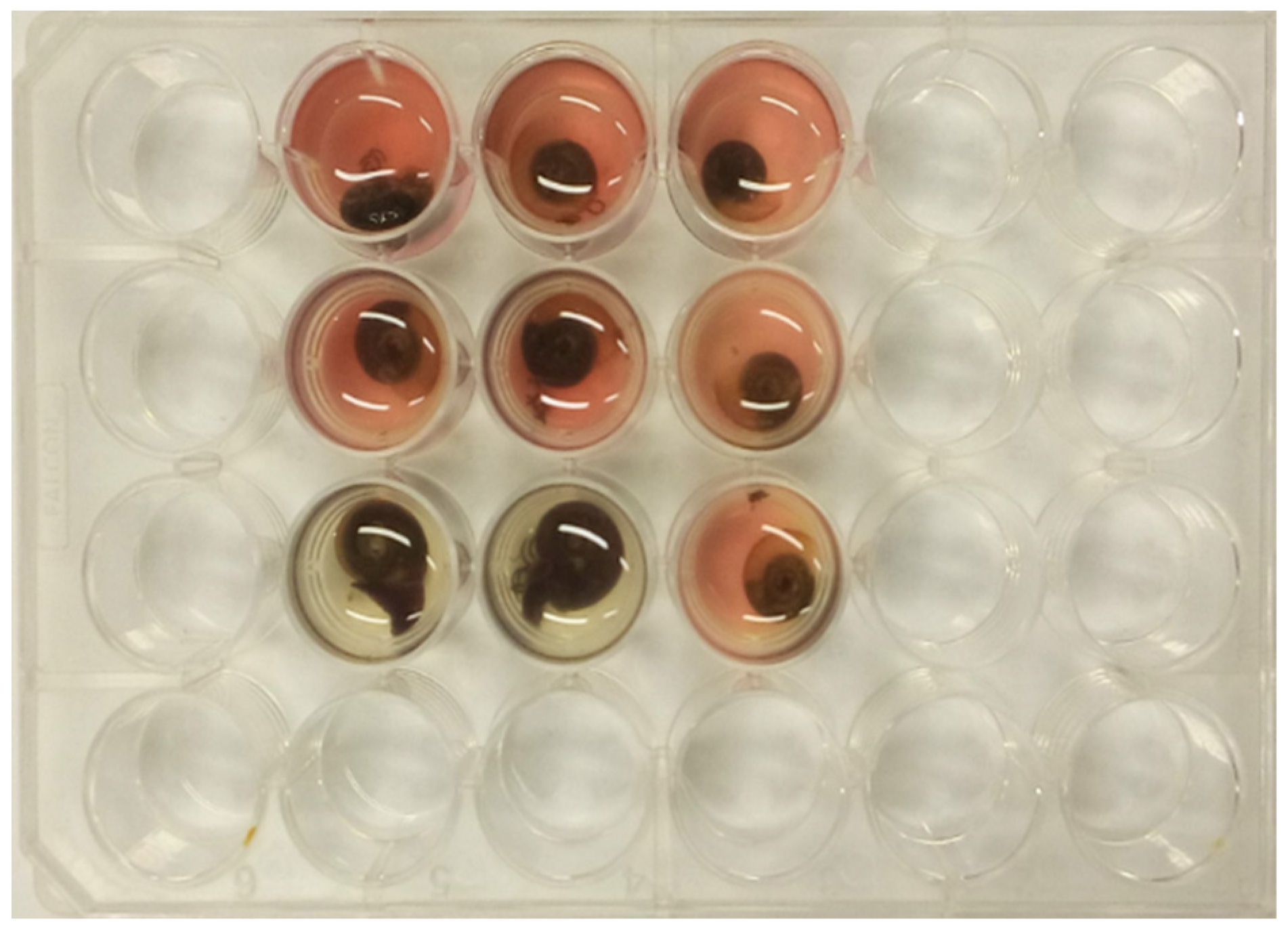
2.3. Molluscicidal Activity Assay with Specie Biomphalaria Glabrata
2.4. Molluscicidal Activity Assay with the Specie Physella sp.
2.5. Statistical Analysis
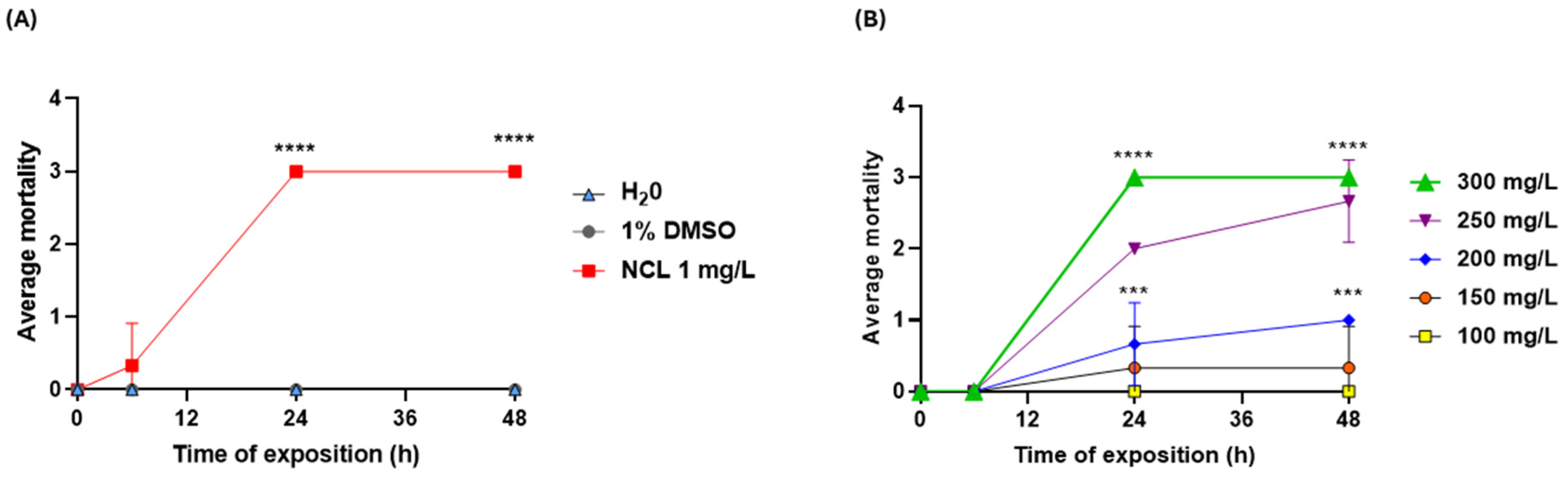
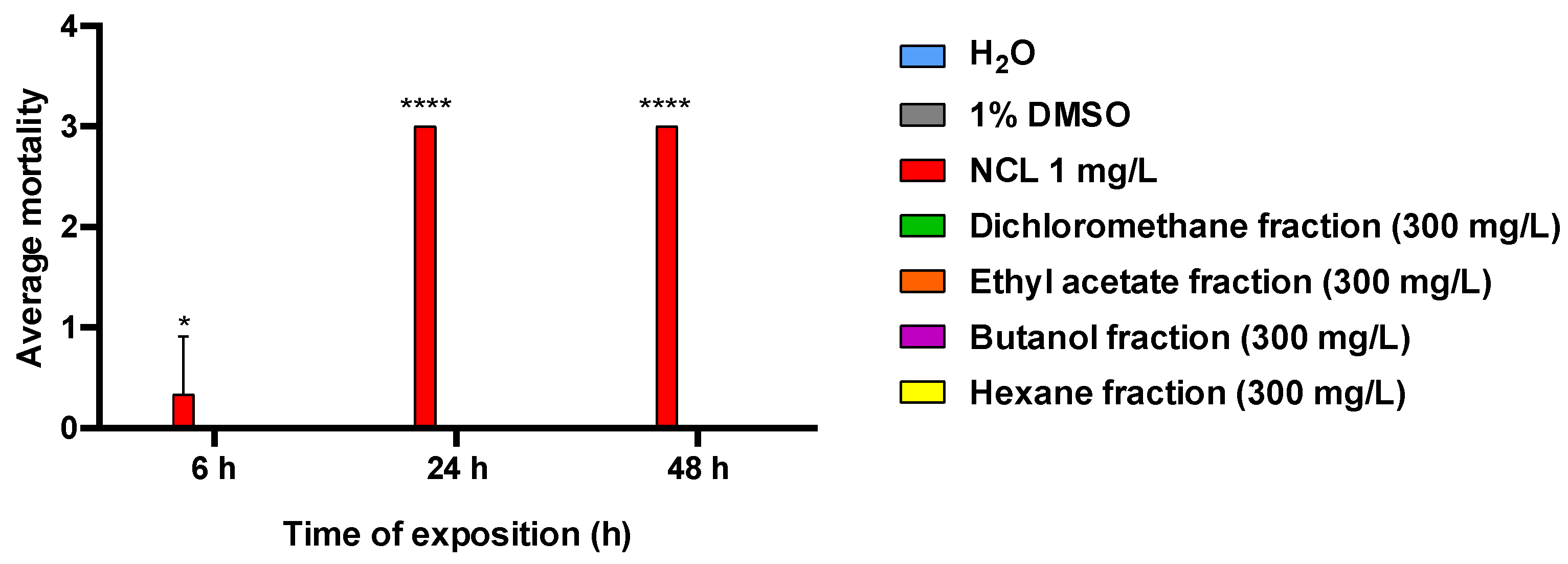
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 10 October 2021).
- Southisavath, P.; Kling, K.; Homsana, A.; Probst-Hensch, N.; Paris, D.H.; Sayasone, S.; Odermatt, P. Elimination of schistosomiasis mekongi in reach for Lao PDR: The last patient with severe disease? Parasitol. Int. 2025, 104, 102976. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde; Secretaria de Vigilância em Saúde; Departamento de Vigilância Epidemiológica. Vigilância da Esquistossomose Mansoni: Diretrizes Técnicas, 4th ed.; Série A. Normas e Manuais Técnicos; Ministério da Saúde: Brasília, Brazil; Secretaria de Vigilância em Saúde: Brasília, Brazil; Departamento de Vigilância das Doenças Transmissíveis: Brasília, Brazil, 2014. [Google Scholar]
- Ministério da Saúde; Secretaria de Vigilância em Saúde e Ambiente; Departamento de Doenças Transmissíveis. Vigilância da Esquistossomose Mansoni: Diretrizes Técnicas [recurso eletrônico]; Ministério da Saúde: Brasília, Brazil; Secretaria de Vigilância em Saúde e Ambiente: Brasília, Brazil; Departamento de Doenças Transmissíveis: Brasília, Brazil, 2024. [Google Scholar]
- Pirzaman, A.T.; Sepidarkish, M.; Alizadeh, F.; Al-Obidy, S.; Ebrahimi, P.; Kianifard, N.; Nooshabadi, M.S.; Tadi, M.J.; Dehkharghani, M.Z.; Mousavi, S.; et al. Prevalence of human Schistosoma mansoni infection in endemic regions (2010–2024): A systematic review and meta-analysis. eClinicalMedicine 2024, 77, 102855. [Google Scholar]
- Alehegne, K.D.; Mitiku, B.A. Schistosoma mansoni Epidemiology Among Snails, Rodents and Children: A One Health Approach. Infect. Drug Resist. 2022, 15, 5629–5643. [Google Scholar] [CrossRef] [PubMed]
- Rangel, L.S.; Gomes, K.N.F.G.; Santos, J.A.A.; Faria, R.X. Bioactivity of substances isolated from natural products on mollusks Biomphalaria glabrata (SAY, 1818) (Planorbidae): A review. Braz. J. Biol. 2023, 83, e266526. [Google Scholar] [CrossRef] [PubMed]
- Sarquis, O.; Pieri, O.S.; Santos, J.A.A. Effects of bayluscide wp70R on the survival and water-eaving behavior of Biomphalaria straminea, snail hosts of schistosomiasis in northeast Brazil. Memórias Inst. Oswaldo Cruz 1997, 92, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.C.V.A.; da Silva Rangel, L.; Gomes, K.N.F.; de Albuquerque, R.D.D.G.; dos Santos, J.A.A.; Faria, R.X. Effects of Abelmoschus esculentus Extracts and Fractions on Embryos and Adult Individuals of Biomphalaria glabrata (Say, 1818) and on Schistosoma mansoni Cercariae. Separations 2024, 11, 99. [Google Scholar] [CrossRef]
- Cantanhede, S.P.D.; Marques, A.M.; Silva-Souza, N.; Valverde, A.L. Atividade moluscicida de plantas: Uma alternativa profilática. Rev. Bras. Farm. 2010, 20, 282–288. [Google Scholar] [CrossRef]
- Ardpairin, J.; Subkrasae, C.; Dumidae, A.; Pansri, S.; Homkaew, C.; Meesil, W.; Kumchantuek, T.; Phoungpetchara, I.; Dillman, A.R.; Pavesi, C.; et al. Symbiotic bacteria associated with entomopathogenic nematodes showed molluskicidal activity against Biomphalaria glabrata, an intermediate host of Schistosoma mansoni. Parasites Vectors 2024, 17, 529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Araújo, P.S.; Caixeta, M.B.; Nunes, E.D.S.; Gonçalves, B.B.; Rocha, T.L. Green synthesis of silver nanoparticles using Croton urucurana and their toxicity in freshwater snail species Biomphalaria glabrata. Acta Trop. 2024, 255, 107224. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, A.L.; França, H.S.; Tietbohl, L.A.C.; Luna, B.N.; Santos, M.G.; Freitas, M.F.; Oliveira, A.P.; Rocha, L. Volatile constituents of three Myrsine L. Species from Brazil. Rec. Nat. Prod. 2017, 11, 82–87. [Google Scholar]
- Corrêa, A.L.; Oliveira, A.P.; Ruppelt, B.M.; de Araújo, E.R.A.; Santos, M.G.; Caldas, G.R.; Muylaert, F.F.; Amendoeira, F.C.; Ferraris, F.K.; de Souza, C.M.V.; et al. Protective effect of Myrsine parvifolia plant extract against the inflammatory process induced by Bothrops jararaca snake venom. Toxicon 2019, 157, 66–76. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Molluscicide Screening and Evolution. Bull. World Health Org. 1965, 33, 567–581. [Google Scholar]
- Santos, J.A.A.; Cavalcante, V.P.; Rangel, L.D.S.; Leite, J.C.V.A.; Faria, R.X. A New Technique Using Low Volumes: A New Technique to Assess the Molluscicidal Activity Using Low Volumes. Evi. Based Complement. Altern. Med. 2017, 2017, 3673197. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.R.R.; Silva, L.D.; Rocha, T.L.; Santos, D.B.; Bezerra, J.C.B.; Machado, K.B.; Paula, J.A.M.; Amaral, V.C.S. Molluscicidal activity of Persea americana Mill. (Lauraceae) stem bark ethanolic extract against the snail Biomphalaria glabrata (Say, 1818): A novel plant-derived molluscicide? An. Braz. Acad. Sci. 2020, 92, e20200715. [Google Scholar] [CrossRef] [PubMed]
- Giovanelli, A.; Silva, C.L.; Medeiros, L.; Vasconcellos, M.C. The molluscicidal activity of niclosamide (Bayluscide WP70(R)) on Melanoides tuberculata (Thiaridae), a snail associated with habitats of Biomphalaria glabrata (Planorbidae). Mem. Inst. Oswaldo Cruz 2002, 97, 743–745. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mott, K. Plant molusccides. In UNDP/World Bank/WHO/TDR; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 1987; Volume 326. [Google Scholar]
- Mott, K.E. (Ed.) Plant molluscicides. In Proceedings of the Scientific Working Group on Plant Molluscicides, UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, Geneva, Switzerland, 31 January–2 February 1983. [Google Scholar]
- Ibrahim, A.M.; Ghoname, S.I. Molluscicidal impacts of Anagallis arvensis aqueous extract on biological, hormonal, histological and molecular aspects of Biomphalaria alexandrina snails. Exp. Parasitol. 2018, 192, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Thoa, N.T.; Son, N.T. The genus Myrsine: A review of phytochemistry, pharmacology, and toxicology. Fitoterapia 2024, 177, 106121. [Google Scholar] [CrossRef] [PubMed]
- Abou El-Nour, F. Avaliação das atividades moluscicida, miracicida e cercaricida de extratos aquosos brutos de Origanum majorana, Ziziphus spina-christi e Salvia fruticosa sobre Schistosoma mansoni e Schistosoma hematobium. Rev. Egípcia Biol. Aquática Pesca 2021, 25, 913–933. [Google Scholar] [CrossRef]
- Hostettmann, K.; Kizu, H.; Tomimori, T. Molluscicidal Properties of various saponins. Plant. Med. 1982, 44, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.; Khagta, S.; Guleria, K.; Arya, V. Plantas como moluscicidas: Uma atualização recente. Int. J. Bot. Stud. 2019, 4, 50–56. [Google Scholar]
- Bagalwa, M.K.; Chifundera. Avaliação do impacto ambiental do extrato da casca do caule de Maesa lanceolata usado na República Democrática do Congo. J. Etnofarmacol. 2017, 114, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Winder, O.; Friedrich, C.; Jumba, N.; Griengl, H.; Kartnig, T. Cyclamin, um novo moluscicida dos tubérculos de Cyclamen purpurascens Mill. testado contra o caracol Biomphalaria glabrata (Say). Ann. Limnol. Int. J. Limnol. 1995, 31, 229–232. [Google Scholar] [CrossRef][Green Version]
- Lemma, A. Laboratory and Field Evaluation of the Molluscicidal Properties of Phytolacca dodecandra. Bull. Org. Mond. Sante’ Bull. World Health Org. 1970, 42, 597–612. [Google Scholar][Green Version]
- Ali, T.H. Effects of niclosamide and plant extract molluscicides on the different developmental stages of freshwater snails Physa acuta. Res. Rev. Biosci. Biosci. Regul. Pap. RRBS 2011, 5, 180–185. [Google Scholar][Green Version]


| Substance | Time (h) | LC50 (mg/L) | LC90 (mg/L) |
|---|---|---|---|
| Crude ethanolic extract | 24 | 223.6 | 281.5 |
| 48 | 207.4 | 256.2 | |
| Niclosamide | 48 | 0.06 | 0.2 |
| Substance | Time (h) | LC50 (mg/L) | LC90 (mg/L) |
|---|---|---|---|
| Crude ethanolic extract | 48 | 118 | 136.5 |
| Niclosamide | 24 | 0.106 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, K.N.F.; Rangel, L.d.S.; Leite, J.C.V.Á.; Caldas, G.R.; Corrêa, A.L.; Santos, M.G.; Rocha, L.M.; Santos, J.A.A.d.; Faria, R.X. Molluscicidal Activity of the Crude Extract and Fractions of Myrsine parvifolia. Separations 2025, 12, 208. https://doi.org/10.3390/separations12080208
Gomes KNF, Rangel LdS, Leite JCVÁ, Caldas GR, Corrêa AL, Santos MG, Rocha LM, Santos JAAd, Faria RX. Molluscicidal Activity of the Crude Extract and Fractions of Myrsine parvifolia. Separations. 2025; 12(8):208. https://doi.org/10.3390/separations12080208
Chicago/Turabian StyleGomes, Keyla Nunes Farias, Leonardo da Silva Rangel, João Claudio Vitoria Ático Leite, Gabriel Rocha Caldas, Arthur Luiz Corrêa, Marcelo Guerra Santos, Leandro Machado Rocha, José Augusto Albuquerque dos Santos, and Robson Xavier Faria. 2025. "Molluscicidal Activity of the Crude Extract and Fractions of Myrsine parvifolia" Separations 12, no. 8: 208. https://doi.org/10.3390/separations12080208
APA StyleGomes, K. N. F., Rangel, L. d. S., Leite, J. C. V. Á., Caldas, G. R., Corrêa, A. L., Santos, M. G., Rocha, L. M., Santos, J. A. A. d., & Faria, R. X. (2025). Molluscicidal Activity of the Crude Extract and Fractions of Myrsine parvifolia. Separations, 12(8), 208. https://doi.org/10.3390/separations12080208






