Elucidating O and Cr Elemental Transfer Behavior in Submerged Arc Welding with Cr2O3-Bearing Fluxes
Abstract
1. Introduction
2. Materials and Methods
2.1. Flux Preparation
2.2. Welding Experiment
2.3. Chemical Composition Analysis
3. Thermodynamic Calculation
3.1. Droplet Zone
- The equilibrium temperature in the submerged arc welding (SAW) process was taken as approximately 2500 °C, corresponding to the typical temperature of the arc plasma.
- The chemical composition of the BM served as the input for the metallic phase.
- An equilibrium simulation was carried out using Fe and O as the representative input elements to estimate the O concentration in the droplet zone. During this calculation, the O partial pressure PO2 (The O2 partial pressure obtained from the previous step) is fixed according to the values listed in Table 4. The resulting O concentrations and simulated PO2 values are also presented in Table 4.
- 4.
- It is well known that metal evaporation tends to occur during the SAW process due to the presence of arc plasma. As noted by Kou [13], thermodynamic analysis alone cannot fully predict such losses. Given that detailed metal evaporation models are still limited in the literature, a recent empirical approach was applied to estimate metal evaporation. As such, the relationships used in Equations (1) and (2) were adopted here to approximate the evaporation behaviors of Mn and Si under high-temperature SAW conditions, which is consistent with previous papers [32,35]. The extent of metal evaporation was evaluated using Equations (1) and (2), as proposed by Zhu et al. [36], where η represents the burn-off ratio of the metal subject to the droplet zone.
- 5.
- Based on the above calculations, the metal input composition for thermodynamic simulation of the weld pool zone is summarized in Table 5.
3.2. Weld Pool Zone
- The thermodynamic databases FToxid, Fstel, and FactPS were applied in this work.
- The molten slag and steel systems were described through the solution models ASlag-liq (all oxides), S (FToxid-SLAGA), and LIQUID (Fstel-Liqu).
- The equilibrium temperature in SAW of 2000 °C was set. All other settings were kept consistent with our previous work.
4. Results and Discussion
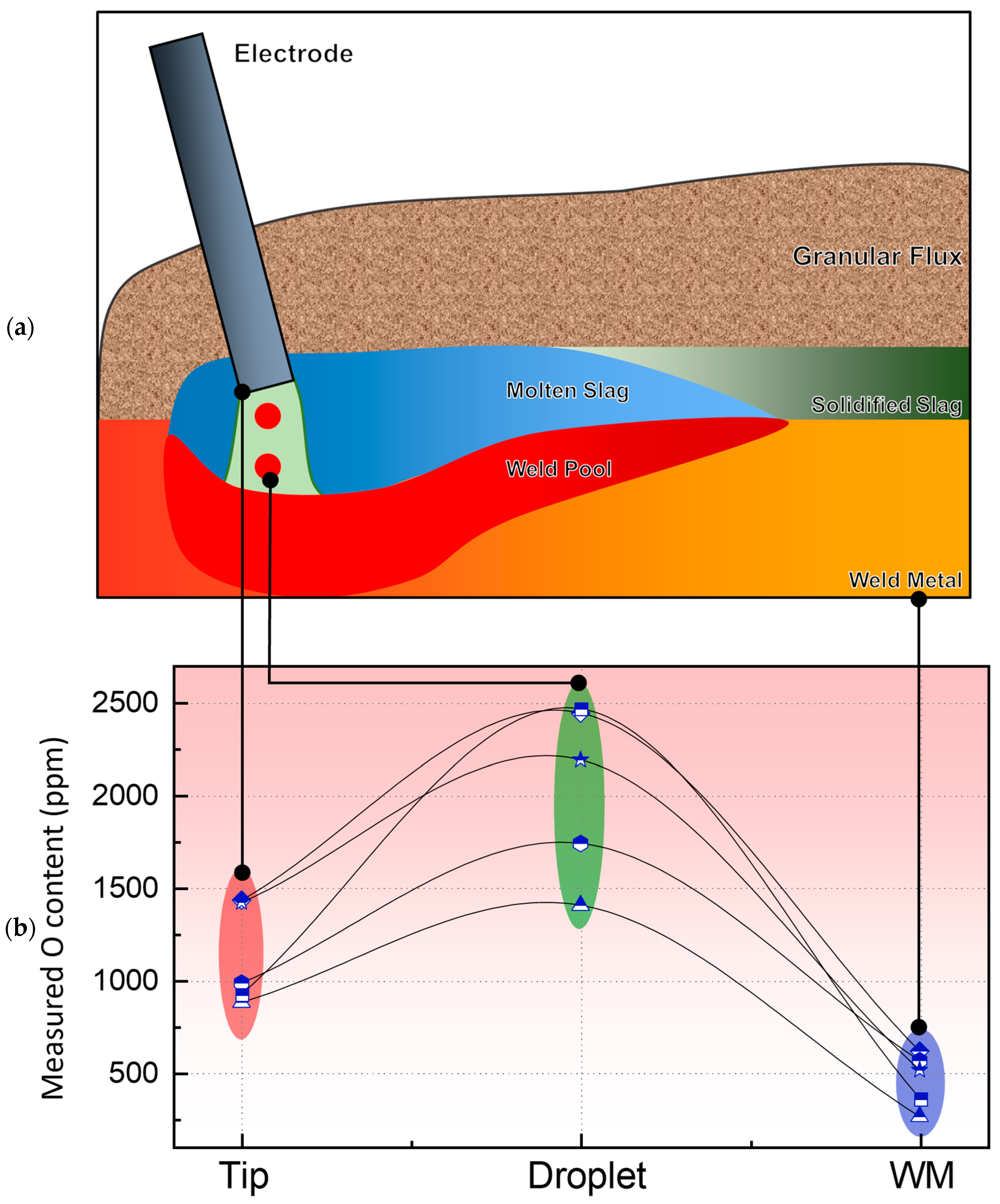
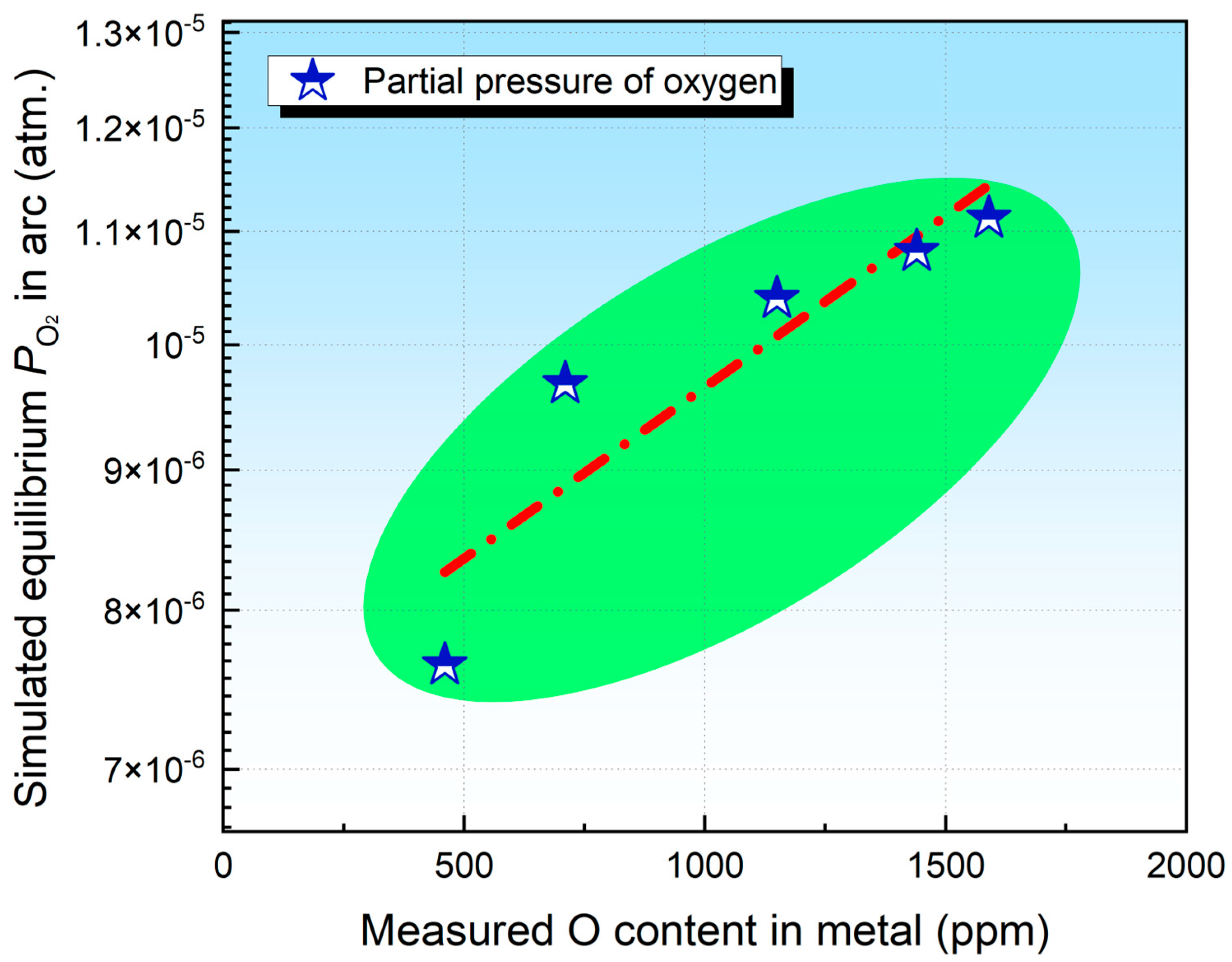
4.1. Transfer of O
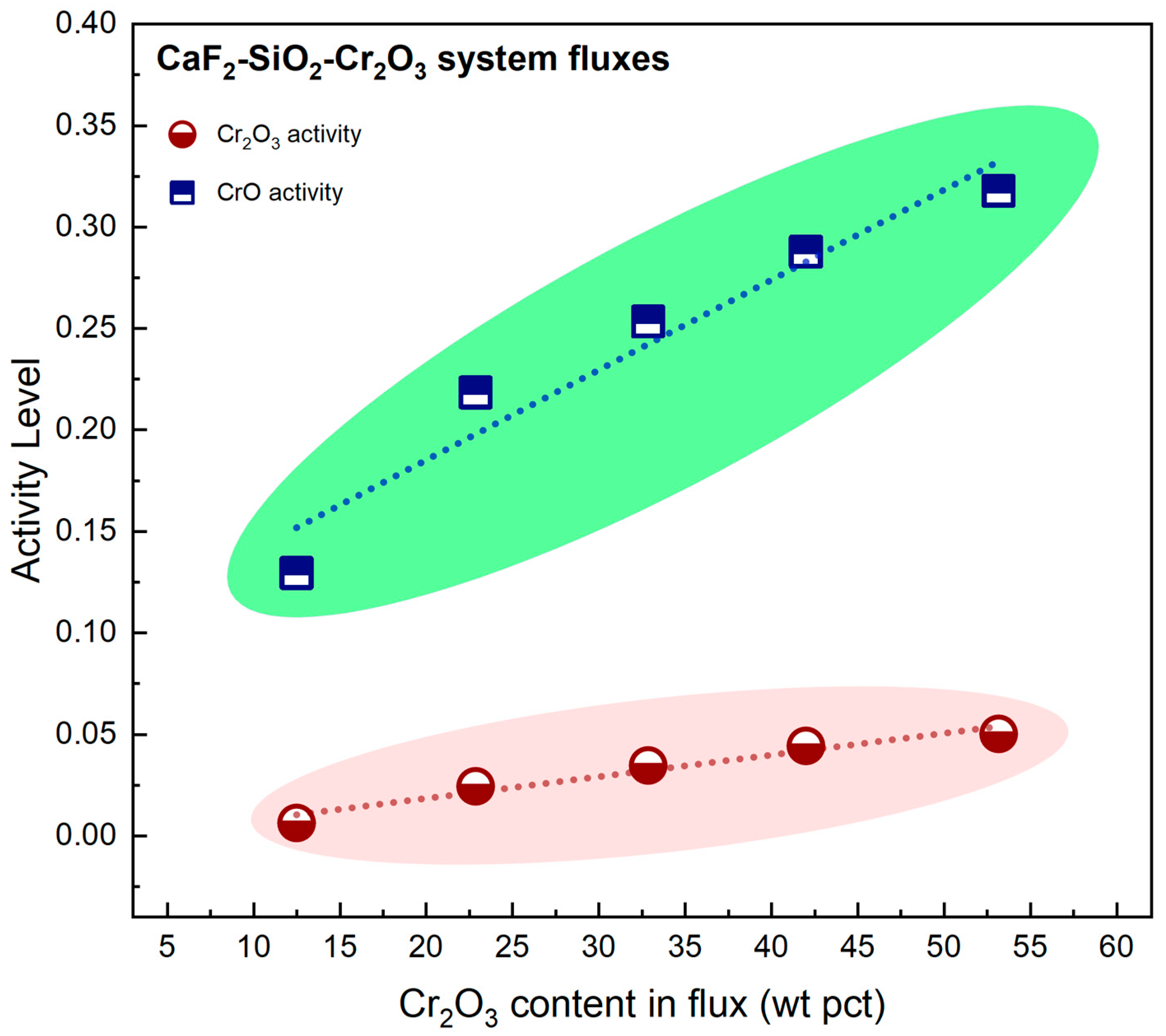
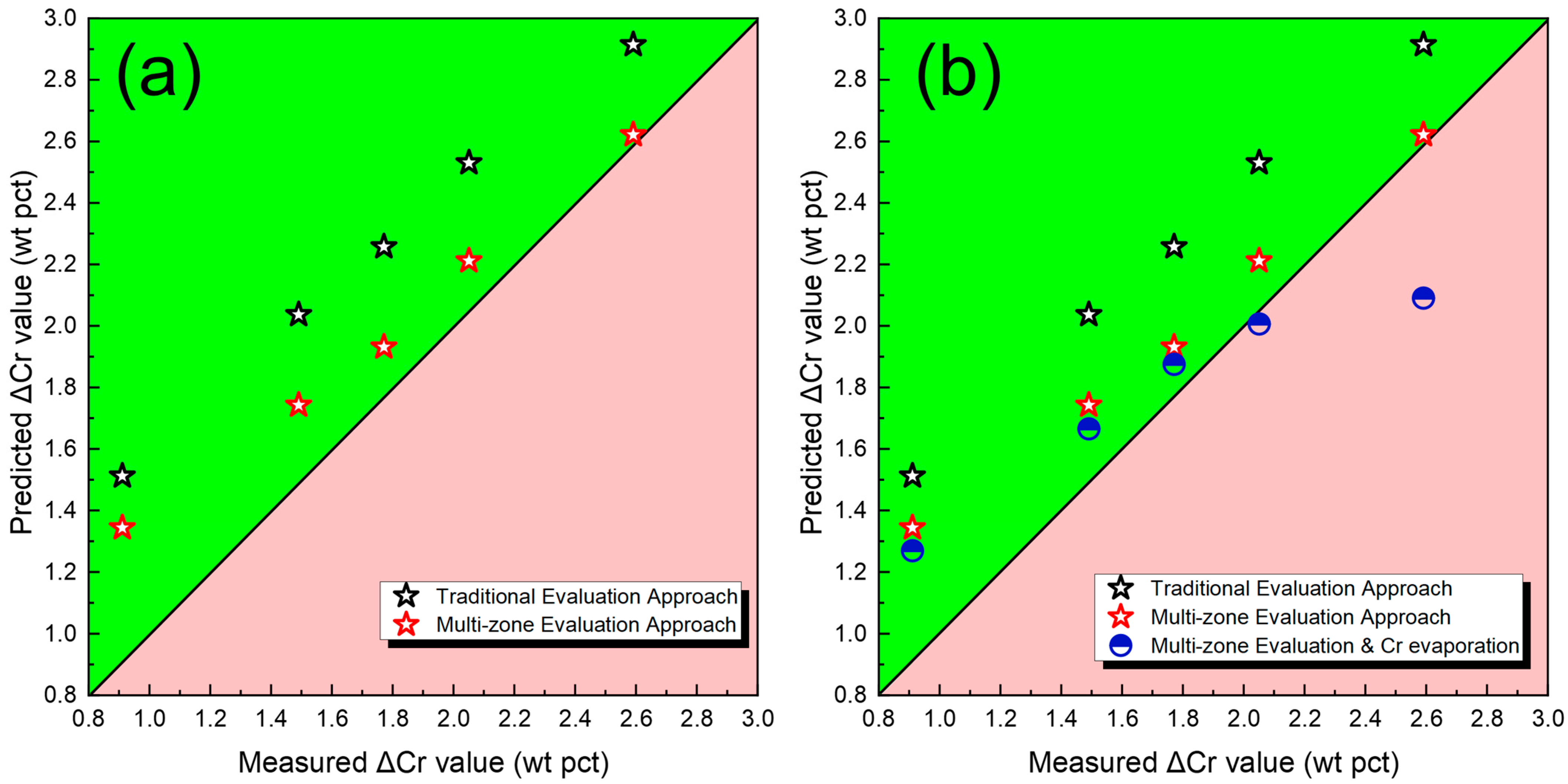
4.2. Transfer of Cr
5. Conclusions
- A multi-zone thermodynamic model is established to simulate element transfer in the droplet and weld pool zones. Compared to the conventional gas–slag–metal equilibrium model, this approach more accurately reflected the actual distribution and evolution of O and Cr during welding.
- The results revealed that O is significantly enriched in the molten droplet zone due to the decomposition of Cr2O3 under high-temperature arc conditions. This O enrichment leads to subsequent deoxidation reactions in the weld pool, which were well captured by the proposed cross-zone model.
- The transfer behavior of Cr was found to be strongly influenced by both thermodynamic factors and evaporation Cr loss. While the activities of Cr2O3 and CrO increase with rising Cr2O3 content in the flux, the accompanying increase in O potential within the droplet zone tends to inhibit Cr transfer into the weld metal. Incorporating evaporation loss into the cross-zone model further improved the accuracy of ΔCr prediction.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sengupta, V.; Havrylov, D.; Mendez, P. Physical Phenomena in the Weld Zone of Submerged Arc Welding—A Review. Weld. J. 2019, 98, 283–313. [Google Scholar]
- Sharma, L.; Chhibber, R.; Kumar, V.; Khan, W.N. Element Transfer Investigations on Silica-Based Submerged Arc Welding Fluxes. Silicon 2023, 15, 305–319. [Google Scholar] [CrossRef]
- Bui, H.V.; Trinh, N.Q.; Tashiro, S.; Suga, T.; Kakizaki, T.; Yamazaki, K.; Lersvanichkool, A.; Murphy, A.B.; Tanaka, M. Individual Effects of Alkali Element and Wire Structure on Metal Transfer Process in Argon Metal-Cored Arc Welding. Materials 2023, 16, 3053. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.; Eagar, T. Prediction of Weld-metal Composition during Flux-shielded Welding. J. Mater. Energy Syst. 1983, 5, 160–164. [Google Scholar] [CrossRef]
- Chai, C.; Eagar, T. Slag-metal Equilibrium during Submerged Arc Welding. Metall. Trans. B 1981, 12, 539–547. [Google Scholar] [CrossRef]
- Olson, D.; Liu, S.; Frost, R.; Edwards, G.; Fleming, D. Nature and Behavior of Fluxes Used for Welding. ASM Int. ASM Handb. 1993, 6, 55–63. [Google Scholar]
- Paniagua-Mercado, A.M.; López-Hirata, V.M.; Muñoz, M.L.S. Influence of the Chemical Composition of Flux on the Microstructure and Tensile Properties of Submerged-Arc Welds. J. Mater. Process. Technol. 2005, 169, 346–351. [Google Scholar] [CrossRef]
- Xu, K.; Fang, T.; Zhao, L.; Cui, H.; Lu, F. Effect of Trace Element on Microstructure and Fracture Toughness of Weld Metal. Acta Metall. Sin. (Engl. Lett.) 2020, 33, 425–436. [Google Scholar] [CrossRef]
- Meng, X.; Artinov, A.; Bachmann, M.; Rethmeier, M. Numerical and Experimental Investigation of Thermo-Fluid Flow and Element Transport in Electromagnetic Stirring Enhanced Wire Feed Laser Beam Welding. Int. J. Heat Mass Transf. 2019, 144, 118663. [Google Scholar] [CrossRef]
- Choudhary, S.; Shandley, R.; Kumar, A. Optimization of Agglomerated Fluxes in Submerged Arc Welding. Mater. Today Proc. 2018, 5, 5049–5057. [Google Scholar] [CrossRef]
- Singh, B.; Khan, Z.A.; Siddiquee, A.N.; Maheshwari, S. Effect of CaF2, FeMn and NiO Additions on Impact Strength and Hardness in Submerged Arc Welding Using Developed Agglomerated Fluxes. J. Alloys Compd. 2016, 667, 158–169. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, G.; Fan, J.; Wang, L.; Zhang, D. A Review on Parallel Development of Flux Design and Thermodynamics Subject to Submerged Arc Welding. Processes 2022, 10, 2305. [Google Scholar] [CrossRef]
- Kou, S. Welding Metallurgy, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 22–122. [Google Scholar]
- Lau, T.; Weatherly, G.; McLean, A. Gas/metal/slag Reactions in Submerged Arc Welding Using CaO-Al2O3 Based Fluxes. Weld. J. 1986, 65, 31–38. [Google Scholar]
- Lau, T.; Weatherly, G.; McLean, A. The Sources of Oxygen and Nitrogen Contamination in Submerged Arc Welding using CaO-Al2O3 Based Fluxes. Weld. J. 1985, 64, 343–347. [Google Scholar]
- Kanjilal, P.; Pal, T.; Majumdar, S. Combined Effect of Flux and Welding Parameters on Chemical Composition and Mechanical Properties of Submerged Arc Weld Metal. J. Mater. Process. Technol 2006, 171, 223–231. [Google Scholar] [CrossRef]
- Jindal, S.; Chhibber, R.; Mehta, N. Effect of Flux Constituents and Basicity Index on Mechanical Properties and Microstructural Evolution of Submerged Arc Welded High Strength Low Alloy Steel. Mater. Sci. Forum 2013, 738–739, 242–246. [Google Scholar] [CrossRef]
- Viano, D.; Ahmed, N.; Schumann, G. Influence of Heat Input and Travel Speed on Microstructure and Mechanical Properties of Double Tandem Submerged Arc High Strength Low Alloy Steel Weldments. Sci. Technol. Weld. Join. 2000, 5, 26–34. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag Metal Reactions during Submerged Arc Welding of Alloy Steels. Metall. Trans. A 1984, 15, 217–227. [Google Scholar] [CrossRef]
- Mitra, U. Kinetics of Slag Metal Reactions During Submerged Arc Welding of Steel. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1984. [Google Scholar]
- Mitra, U.; Eagar, T. Slag-Metal Reactions During Welding: Part II. Theory. Metall. Trans. B 1991, 22, 73–81. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-Metal Reactions During Welding: Part I. Evaluation and Reassessment of Existing Theories. Metall. Trans. B 1991, 22, 65–71. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-Metal Reactions During Welding: Part III. Verification of the Theory. Metall. Trans. B 1991, 22, 83–100. [Google Scholar] [CrossRef]
- Mitra, U.; Sutton, R.; Eagar, T. Comparison of Theoretically Predicted and Experimentally Determined Submerged Arc Weld Deposit Compositions. Metall. Trans. B 1983, 14, 510–513. [Google Scholar] [CrossRef]
- Quintana, R.; Cruz, A.; Perdomo, L.; Castellanos, G.; García, L.L.; Formoso, A.; Cores, A. Study of the Transfer Efficiency of Alloyed Elements in Fluxes during the Submerged Arc Welding Process. Weld. Int. 2003, 17, 958–965. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q. Probing Element Transfer Behavior During the Submerged Arc Welding Process for CaF2–SiO2–Na2O–Cr2O3 Agglomerated Fluxes: A Thermodynamic Approach. Processes 2022, 10, 1900. [Google Scholar] [CrossRef]
- Block-Bolten, A.; Eagar, T.W. Metal Vaporization from Weld Pools. Metall. Trans. B 1984, 15, 461–469. [Google Scholar] [CrossRef]
- Chai, C.; Eagar, T. Slag-Metal Reactions in Binary CaF2–Metal Oxide Welding Fluxes. Weld. J. 1982, 61, 229–232. [Google Scholar]
- Indacochea, J.E.; Blander, M.; Christensen, N.; Olson, D.L. Chemical Reactions during Submerged Arc Welding with FeO-MnO-SiO2 Fluxes. Metall. Trans. B 1985, 16, 237–245. [Google Scholar] [CrossRef]
- Natalie, C.A.; Olson, D.L.; Blander, M. Physical and Chemical Behavior of Welding Fluxes. Annu. Rev. Mater. Sci. 1986, 16, 389–413. [Google Scholar] [CrossRef]
- Sharma, L.; Chhibber, R. Design of TiO2–SiO2–MgO and SiO2–MgO–Al2O3-Based Submerged Arc Fluxes for Multipass Bead on Plate Pipeline Steel Welds. J. Press. Vessel Technol. 2019, 141, 041402. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, P.; Zhang, D. Advancing Manganese Content Prediction in Submerged Arc Welded Metal: Development of a Multi-Zone Model via the Calphad Technique. Processes 2023, 11, 1265. [Google Scholar] [CrossRef]
- Bale, C.W.; Chartrand, P.; Degterov, S.; Eriksson, G.; Hack, K.; Mahfoud, R.B.; Melançon, J.; Pelton, A.; Petersen, S. FactSage Thermochemical Software and Databases. Calphad 2002, 26, 189–228. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J. Reprint of: FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, J.; Zhang, D. Advancing Methodologies for Elemental Transfer Quantification in The Submerged Arc Welding Process: A Case Study of CaO-SiO2-MnO Flux. Processes 2024, 12, 137. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Shi, H.; Huang, L.; Mao, Z. Element Loss Behavior and Compensation in Additive Manufacturing of Memory Alloys. Trans. China Weld. Inst. 2022, 43, 50–55. [Google Scholar]
- Mills, A.; Thewlis, G.; Whiteman, J. Nature of Inclusions in Steel Weld Metals and Their Influence on Formation of Acicular Ferrite. Mater. Sci. Technol. 1987, 3, 1051–1061. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D.; Liu, P. Thermodynamic Nature of SiO2 and FeO in Flux O Potential Control Subject to Submerged Arc Welding Process. Processes 2023, 11, 400. [Google Scholar] [CrossRef]
- Chai, C.-S. Slag-Metal Reactions During Flux-Shielded Arc Welding. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1980. [Google Scholar]
- Minh, P.S.; Nguyen, V.-T.; Nguyen, V.T.; Uyen, T.M.T.; Do, T.T.; Nguyen, V.T.T. Study on the Fatigue Strength of Welding Line in Injection Molding Products under Different Tensile Conditions. Micromachines 2022, 13, 1890. [Google Scholar] [CrossRef]
- Heuschkel, J. Composition Controlled, High-Strength, Ductile, Tough Steel Weld Metals. Weld. J. 1964, 43, 361s–384s. [Google Scholar]
- Lewis, W.; Faulkner, G.; Rieppel, P. F Flux and Filler Wire Developments for Submerged Arc Welding HY80 steel. Weld. J. 1961, 40, 337s–340s. [Google Scholar]
- Dowden, J.; Kapadia, P. Plasma Arc Welding: A Mathematical Model of the Arc. J. Phys. D Appl. Phys. 1994, 27, 902. [Google Scholar] [CrossRef]
- Nart, E.; Celik, Y. A Practical Approach for Simulating Submerged Arc Welding Process Using FE Method. J. Constr. Steel Res. 2013, 84, 62–71. [Google Scholar] [CrossRef]
- Schönmaier, H.; Krein, R.; Schmitz-Niederau, M.; Schnitzer, R. Influence of the Heat Input on the Dendritic Solidification Structure and the Mechanical Properties of 2.25 Cr-1Mo-0.25 V Submerged-Arc Weld Metal. J. Mater. Eng. Perform. 2021, 30, 7138–7151. [Google Scholar] [CrossRef]
- Amanie, J.; Oguocha, I.; Yannacopoulos, S. Effect of Submerged Arc Welding Parameters on Microstructure of SA516 Steel Weld Metall. Can. Metall. Q. 2012, 51, 48–57. [Google Scholar] [CrossRef]


| Fluxes | Cr2O3 | SiO2 | Na2O | CaF2 |
|---|---|---|---|---|
| F-1 | 12.58 | 7.99 | 0.52 | 78.91 |
| F-2 | 22.89 | 7.56 | 0.48 | 69.07 |
| F-3 | 32.88 | 7.63 | 0.55 | 58.94 |
| F-4 | 41.99 | 7.88 | 0.61 | 49.52 |
| F-5 | 53.19 | 7.66 | 0.49 | 38.66 |
| C | Si | Mn | Ti | Cr | O | Fe | |
|---|---|---|---|---|---|---|---|
| BM | 0.112 | 0.142 | 1.54 | 0.015 | 0.018 | 0.003 | Balanced |
| Electrode | 0.127 | 0.049 | 1.65 | 0.015 | 0.015 | 0.003 | Balanced |
| Weld Metals | WM-1 | WM-2 | WM-3 | WM-4 | WM-5 |
|---|---|---|---|---|---|
| Fluxes | F-1 | F-2 | F-3 | F-4 | F-5 |
| ΔO | 460 | 710 | 1150 | 1440 | 1590 |
| ΔCr | 1.034 | 1.603 | 1.783 | 2.063 | 2.604 |
| Flux | PO2 | O Concentration in Droplet |
|---|---|---|
| F-1 | 7.64 × 10−6 | 3642 |
| F-2 | 9.68 × 10−6 | 4100 |
| F-3 | 1.04 × 10−5 | 4250 |
| F-4 | 1.08 × 10−5 | 4331 |
| F-5 | 1.11 × 10−5 | 4391 |
| C | Si | Mn | Ti | Cr | O | Fe | |
|---|---|---|---|---|---|---|---|
| F-1 | 0.12 | 0.06 | 0.82 | 0.015 | 0.017 | 0.184 | Balanced |
| F-2 | 0.12 | 0.06 | 0.82 | 0.015 | 0.017 | 0.207 | Balanced |
| F-3 | 0.12 | 0.06 | 0.82 | 0.015 | 0.017 | 0.214 | Balanced |
| F-4 | 0.12 | 0.06 | 0.82 | 0.015 | 0.017 | 0.218 | Balanced |
| F-5 | 0.12 | 0.06 | 0.82 | 0.015 | 0.017 | 0.221 | Balanced |
| Flux | ΔDO | ΔWO |
|---|---|---|
| F-1 | 3612 | −1732 |
| F-2 | 4070 | −1470 |
| F-3 | 4220 | −1440 |
| F-4 | 4301 | −1461 |
| F-5 | 4361 | −1611 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Fan, J.; Zhang, D. Elucidating O and Cr Elemental Transfer Behavior in Submerged Arc Welding with Cr2O3-Bearing Fluxes. Processes 2025, 13, 4046. https://doi.org/10.3390/pr13124046
Zhang J, Fan J, Zhang D. Elucidating O and Cr Elemental Transfer Behavior in Submerged Arc Welding with Cr2O3-Bearing Fluxes. Processes. 2025; 13(12):4046. https://doi.org/10.3390/pr13124046
Chicago/Turabian StyleZhang, Jin, Jun Fan, and Dan Zhang. 2025. "Elucidating O and Cr Elemental Transfer Behavior in Submerged Arc Welding with Cr2O3-Bearing Fluxes" Processes 13, no. 12: 4046. https://doi.org/10.3390/pr13124046
APA StyleZhang, J., Fan, J., & Zhang, D. (2025). Elucidating O and Cr Elemental Transfer Behavior in Submerged Arc Welding with Cr2O3-Bearing Fluxes. Processes, 13(12), 4046. https://doi.org/10.3390/pr13124046






