Preventive Effect of Peptidoglycan Extracted from Lactobacillus casei ATCC 393 on Dextran Sulfate Sodium-Induced Inflammation in Mice Through Gut Microbiota Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
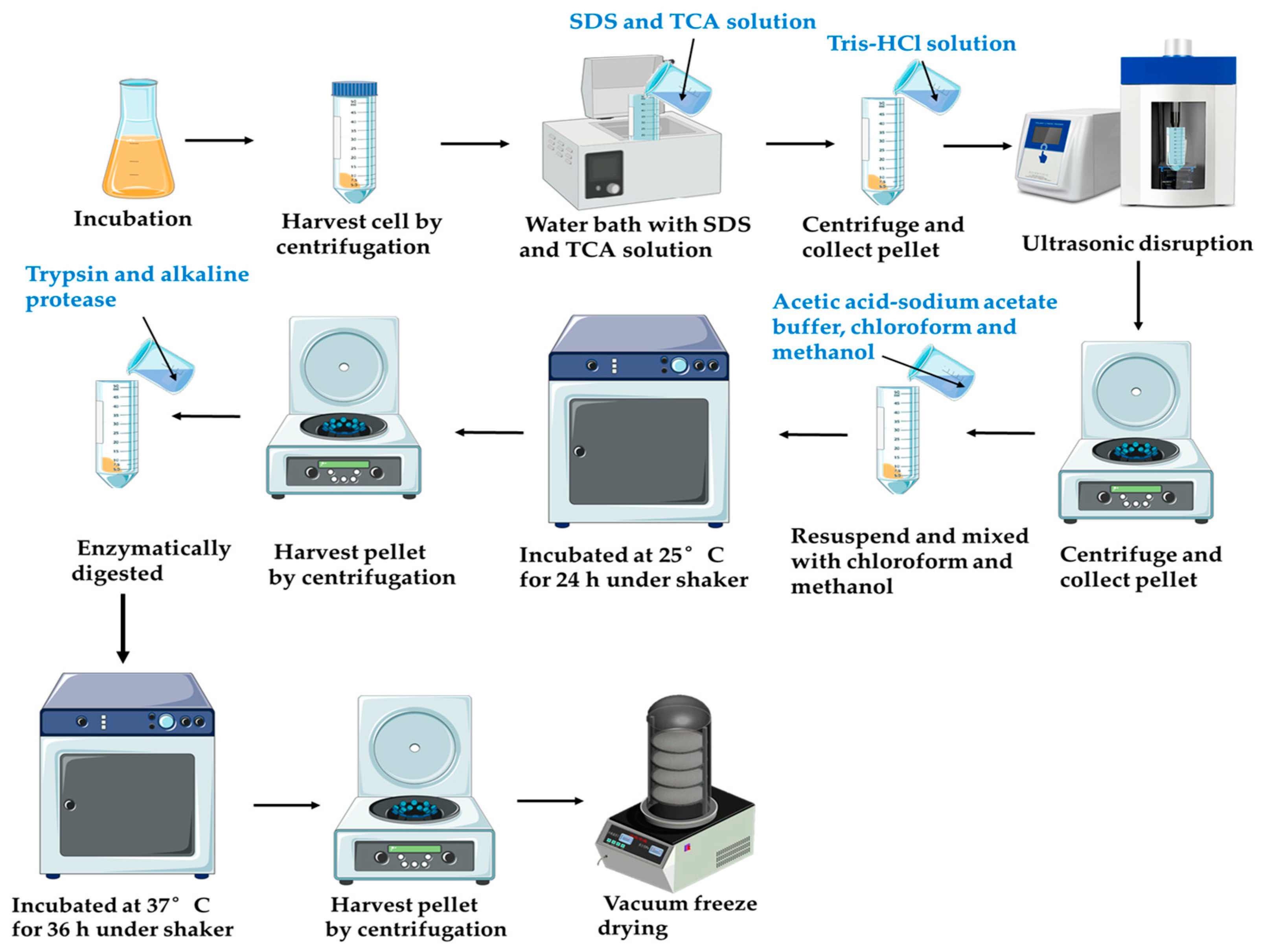
2.2. Peptidoglycan Extraction and Purification
2.3. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
2.4. Method for Detecting Peptidoglycan pH Changes During Enzymatic Hydrolysis
2.5. Helical Conformation Analysis of Peptidoglycan
2.6. Method for Testing Antioxidant Activity
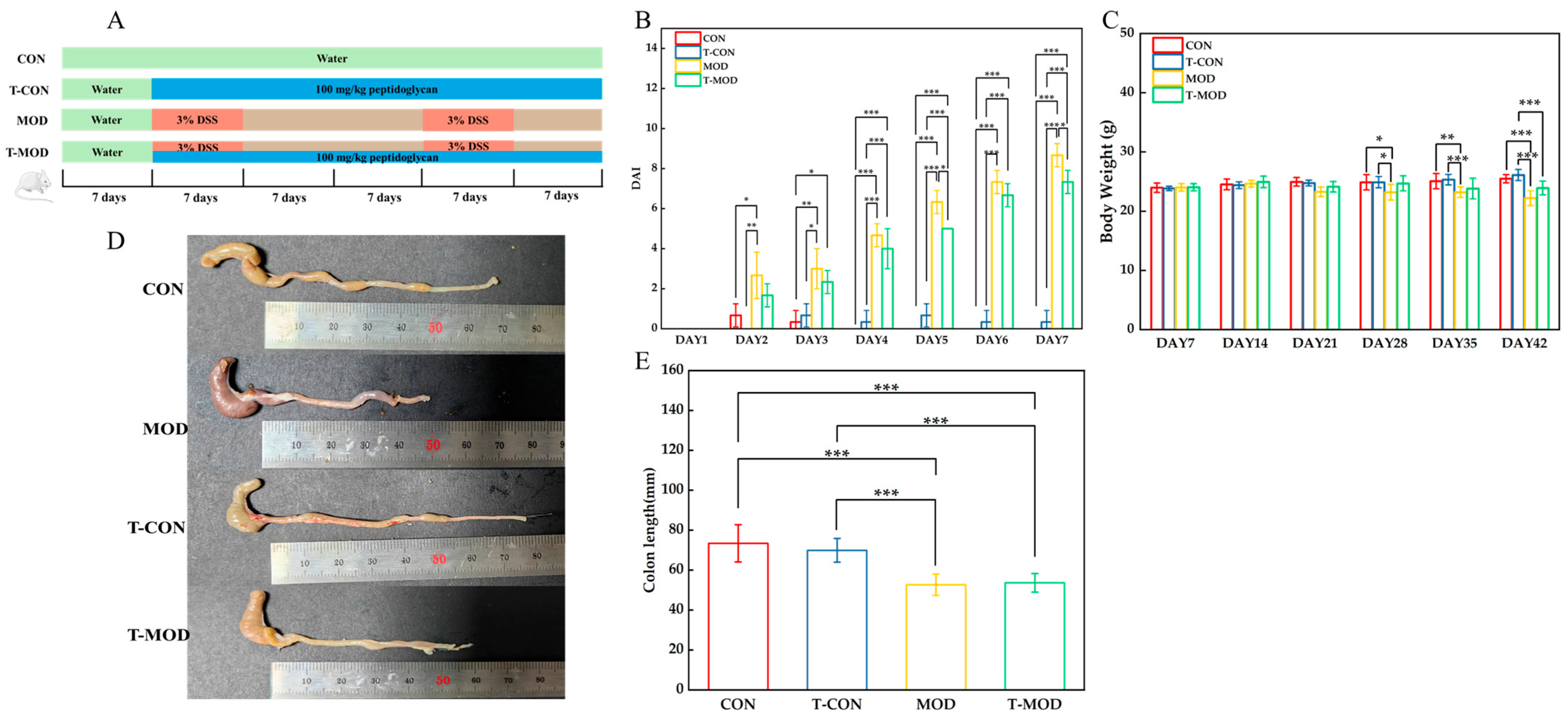
2.7. Animal Experiments
2.8. Assessment of Colitis Symptoms
2.9. Hematoxylin and Eosin (H&E) Staining
2.10. Biochemical Assays
2.11. 16S rRNA Gene and Bioinformatics Analysis
2.12. Short-Chain Fatty Acid Determination
2.13. Statistical Analysis
3. Results and Discussions
3.1. Purification and Characterization of Peptidoglycan
3.2. pH Change of Peptidoglycan During Enzymatic Hydrolysis
3.3. Structure Analysis
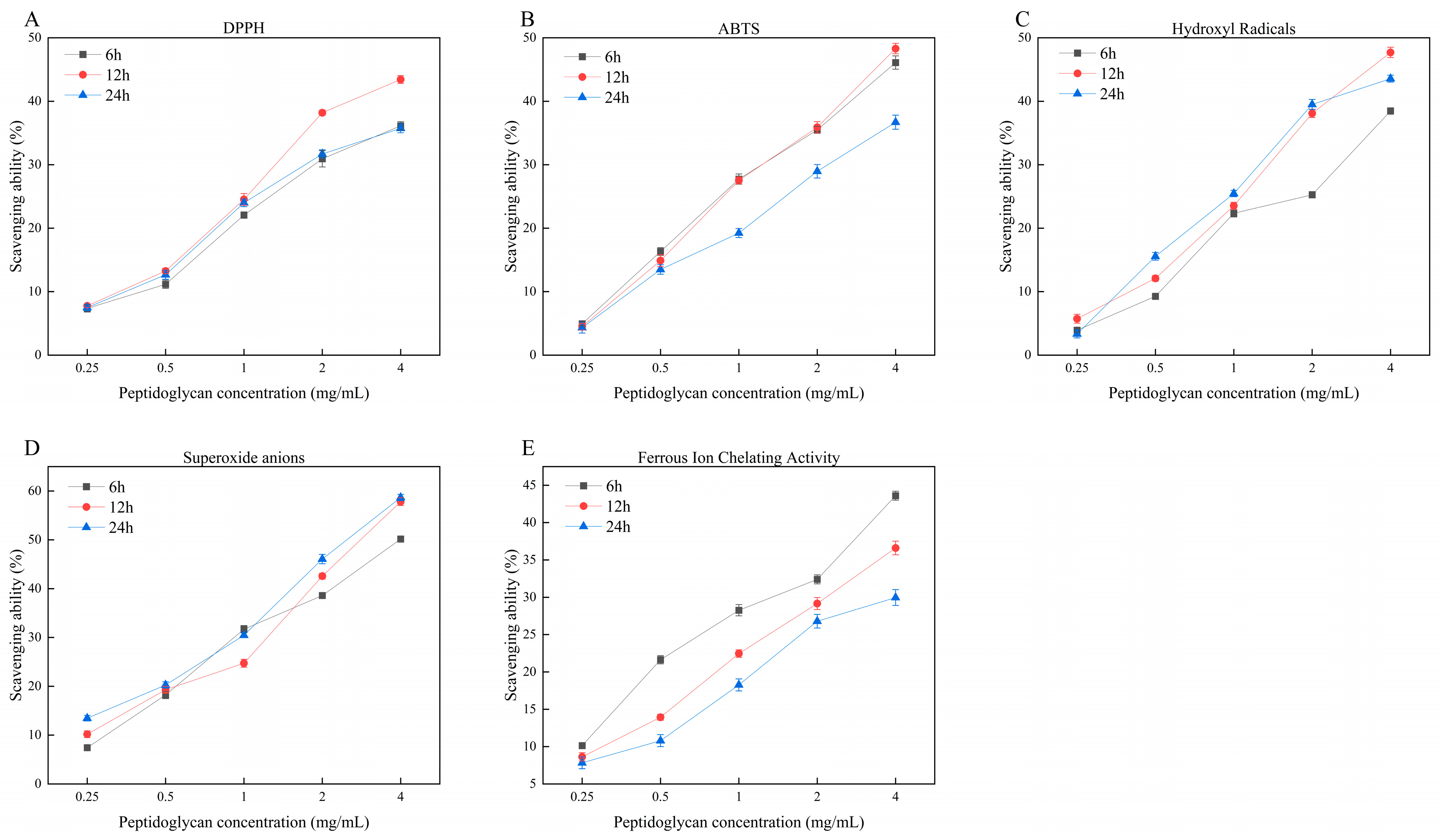
3.4. Antioxidant Activity
3.5. Evaluation of Inflammatory Indicators
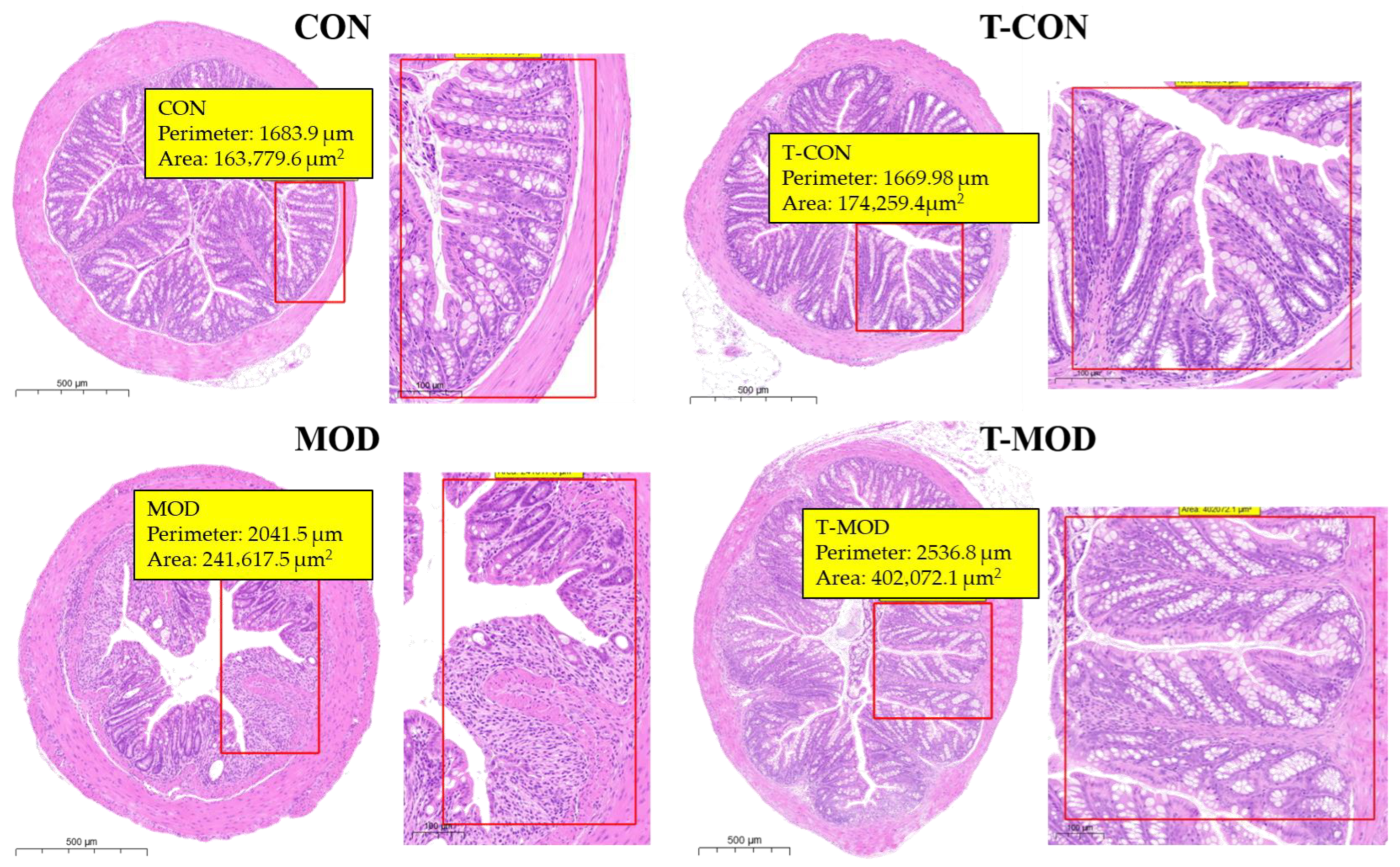
3.5.1. H&E Staining
3.5.2. Changes in Inflammatory Cytokines
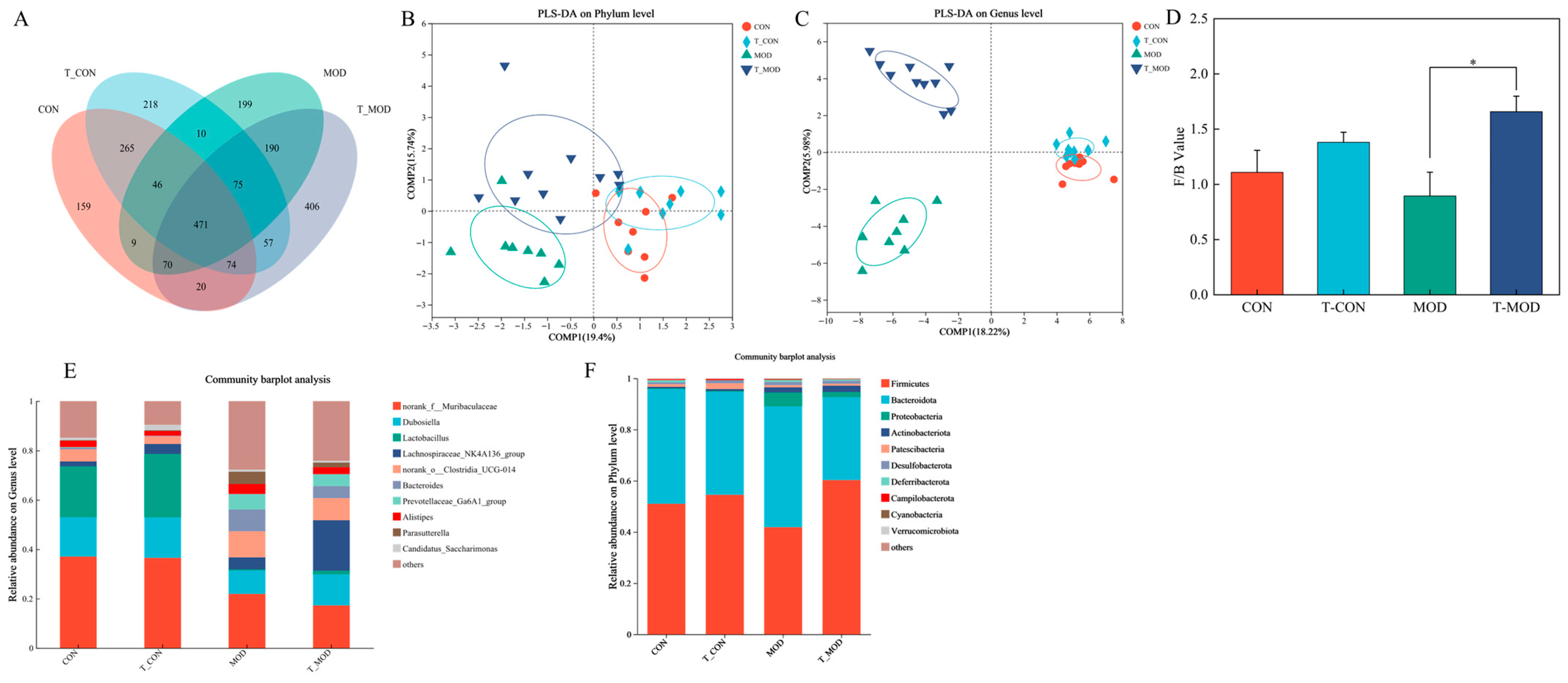
3.5.3. Effect of Peptidoglycan on Mice Gut Microbiota
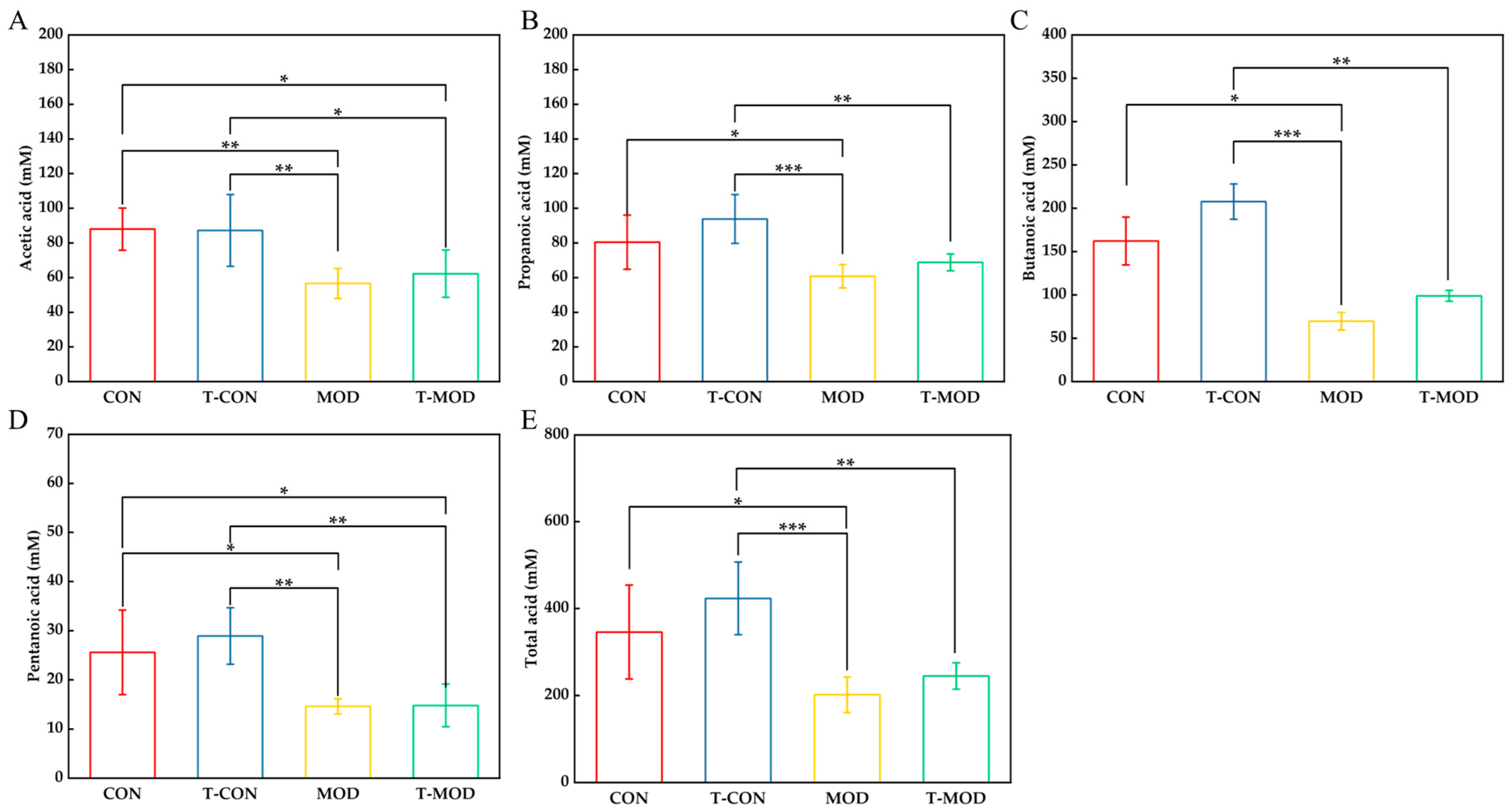
3.5.4. Effect of Peptidoglycan on SCFAs (Short-Chain Fatty Acids)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PG | Peptidoglycan |
References
- Zulfiqar, F.; Hozo, I.; Rangarajan, S.; Mariuzza, R.A.; Dziarski, R.; Gupta, D. Genetic Association of Peptidoglycan Recognition Protein Variants with Inflammatory Bowel Disease. PLoS ONE 2013, 8, e67393. [Google Scholar] [CrossRef] [PubMed]
- Dziarski, R.; Park, S.Y.; Kashyap, D.R.; Dowd, S.E.; Gupta, D. Pglyrp-Regulated Gut Microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii Enhance and Alistipes finegoldii Attenuates Colitis in Mice. PLoS ONE 2016, 11, e0146162. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.D.; Binion, D.G. Silent Inflammatory Bowel Disease. Crohns Colitis 360 2021, 3, otab059. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Lo, B.C.; Núñez, G. Host–microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 2020, 20, 411–426. [Google Scholar] [CrossRef]
- Fernandez, E.M.; Valenti, V.; Rockel, C.; Hermann, C.; Pot, B.; Boneca, I.G.; Grangette, C. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut 2011, 60, 1050–1059. [Google Scholar] [CrossRef]
- El-Araby, A.M.; Fisher, J.F.; Mobashery, S. Bacterial peptidoglycan as a living polymer. Curr. Opin. Chem. Biol. 2025, 84, 102562. [Google Scholar] [CrossRef]
- Chapot-Chartier, M.P.; Kulakauskas, S. Cell wall structure and function in lactic acid bacteria. Microb. Cell Fact. 2014, 13, S9. [Google Scholar] [CrossRef]
- Willmann, R.; Lajunen, H.M.; Erbs, G.; Newman, M.A.; Kolb, D.; Tsuda, K.; Katagiri, F.; Fliegmann, J.; Bono, J.J.; Cullimore, J.V.; et al. Arabidopsis lysin-motif proteins LYM1 LYM3 CERK1 mediate bacterial peptidoglycan sensing and immunity to bacterial infection. Proc. Natl. Acad. Sci. USA 2011, 108, 19824–19829. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Wu, L.L.; Liu, J.W.; Chen, K.X.; Li, Y.M. Iridoids and derivatives from Catalpa ovata with their antioxidant activities. Fitoterapia 2023, 169, 105599. [Google Scholar] [CrossRef]
- Yao, X.; Yi, Z.K.; Xu, M.; Han, Y. A Review on the Extraction, Structural Characterization, Function, and Applications of Peptidoglycan. Macromol. Rapid Commun. 2025, 46, e2400654. [Google Scholar] [CrossRef]
- Wells, J.M. Immunomodulatory mechanisms of lactobacilli. Microb. Cell Fact. 2011, 10, S17. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, F.; Zhang, M.; Xia, Y.; Ai, L.; Wang, G. Effect of D-Ala-Ended Peptidoglycan Precursors on the Immune Regulation of Lactobacillus plantarum Strains. Front. Immunol. 2022, 12, 825825. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, S.H.; Kim, M.G.; Lee, H.J.; Kim, G.B. Lactobacillus plantarum CAU1055 ameliorates inflammation in lipopolysaccharide-induced RAW264.7 cells and a dextran sulfate sodium-induced colitis animal model. J. Dairy Sci. 2019, 102, 6718–6725. [Google Scholar] [CrossRef]
- Arenas, T.; Osorio, A.; David Ginez, L.; Camarena, L.; Poggio, S. Bacterial cell wall quantification by a modified low-volume Nelson-Somogyi method and its use with different sugars. Can. J. Microbiol. 2022, 68, 295–302. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Philpott, D.J. Peptidoglycan: A critical activator of the mammalian immune system during infection and homeostasis. Immunol. Rev. 2011, 243, 40–60. [Google Scholar] [CrossRef]
- Jarchum, I.; Pamer, E.G. Regulation of innate and adaptive immunity by the commensal microbiota. Curr. Opin. Immunol. 2011, 23, 353–360. [Google Scholar] [CrossRef]
- Wolf, A.J. Peptidoglycan-induced modulation of metabolic and inflammatory responses. Immunometabolism 2023, 5, e00024. [Google Scholar] [CrossRef]
- Fuochi, V.; Spampinato, M.; Distefano, A.; Palmigiano, A.; Garozzo, D.; Zagni, C.; Rescifina, A.; Volti, G.L.; Furneri, P.M. Soluble peptidoglycan fragments produced by Limosilactobacillus fermentum with antiproliferative activity are suitable for potential therapeutic development: A preliminary report. Front. Mol. Biosci. 2023, 10, 1082526. [Google Scholar] [CrossRef]
- Garde, S.; Chodisetti, P.K.; Reddy, M. Peptidoglycan: Structure, Synthesis, and Regulation. EcoSal Plus 2021, 9, eESP-0010-2020. [Google Scholar] [CrossRef]
- Sun, J.; Shi, Y.H.; Le, G.W.; Ma, X.Y. Distinct immune response induced by peptidoglycan derived from Lactobacillus sp. World J. Gastroenterol. 2005, 11, 6330–6337. [Google Scholar] [CrossRef]
- Zhang, Z. Functional Properties of Lactic Acid Bacteria Peptidoglycan andits Effect on the Quality of Fresh-Cut Apples. Master’s Thesis, Shandong Agricultural University, Taian, China, 2020. [Google Scholar]
- Cao, Y.; Gao, J.; Zhang, L.; Qin, N.; Zhu, B.; Xia, X. Jellyfish skin polysaccharides enhance intestinal barrier function and modulate the gut microbiota in mice with DSS-induced colitis. Food Funct. 2021, 12, 10121–10135. [Google Scholar] [CrossRef] [PubMed]
- Hyder, A. PG1yRP3 concerts with PPARγ to attenuate DSS-induced colitis in mice. Int. Immunopharmacol. 2019, 67, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Study About the Preventive and Therapeutic Effects of Fucoidan onDSS-Induced Acute Ulcerative Colitis in C57BL/6 Male Mice. Master’s Thesis, Soochow University, Suzhou, China, 2023. [Google Scholar]
- Huang, Z.; Gong, L.; Jin, Y.; Stanton, C.; Ross, R.P.; Zhao, J.; Yang, B.; Chen, W. Different Effects of Different Lactobacillus acidophilus Strains on DSS-Induced Colitis. Int. J. Mol. Sci. 2022, 23, 14841. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Chen, C.-C.; Lin, Y.-T.; Wu, W.-K.; Chang, L.-C.; Lai, C.-H.; Wu, M.-S.; Kuo, C.-H. Evaluation and Optimization of Sample Handling Methods for Quantification of Short-Chain Fatty Acids in Human Fecal Samples by GC–MS. J. Proteome Res. 2019, 18, 1948–1957. [Google Scholar] [CrossRef]
- Xu, M.; Pan, L.; Wang, B.; Zou, X.; Zhang, A.; Zhou, Z.; Han, Y. Simulated Digestion and Fecal Fermentation Behaviors of Levan and Its Impacts on the Gut Microbiota. J. Agric. Food Chem. 2023, 71, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.; Brown, R.R.; Anderle, S.K.; Chetty, C.; Cromartie, W.J.; Gooder, H.; Schwab, J.H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect. Immun. 1982, 35, 1003–1010. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, D.D.; Guo, Y.X.; Zeng, X.Q. Structure and anti-inflammatory capacity of peptidoglycan from Lactobacillus acidophilus in RAW-264.7 cells. Carbohydr. Polym. 2013, 96, 466–473. [Google Scholar] [CrossRef]
- Kacuráková, M.; Wilson, R.H. Developments in mid-infrared FT-IR spectroscopy of selected carbohydrates. Carbohydr. Polym. 2001, 44, 291–303. [Google Scholar] [CrossRef]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2017, 52, 456–506. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, D.D.; Zeng, X.Q.; Sun, Y.Y.; Cao, J.X. Phosphorylation of peptidoglycan from Lactobacillus acidophilus and its immunoregulatory function. Int. J. Food Sci. Technol. 2016, 51, 664–671. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, D.D.; Guo, Y.X.; Sun, Y.Y.; Zeng, X.Q. Peptidoglycan diversity and anti-inflammatory capacity in Lactobacillus strains. Carbohydr. Polym. 2015, 128, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.Z. Exatration, Structual Analysis and Bioactivity of Peptidoglycan from L. paracasei subp. Paracaseix 12. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2015. [Google Scholar]
- Zhixiang, Y.; Haicheng, Y.; Jinrong, W. Structural characterization and antioxidant activity in vitro of selenized Bacillus subtilis peptidoglycan. J. Henan Univ. Technol. (Nat. Sci. Ed.) 2023, 44, 84–91. [Google Scholar] [CrossRef]
- Wang, K. Preparation, Structual Characterization and Function of Peptidoglycan from Lactic Acid Bacteria. Master’s thesis, Nanjing Agricultural University, Nanjing, China, 2017. [Google Scholar]
- Al-Azzouny, R.A.; Wang, R.; Yoo, S.H. Purification and Characterization of a 6.5 kDa Antioxidant Peptidoglycan Purified from Silk Worm (Bombyx mori) Pupae Extract. Food Sci. Biotechnol. 2011, 20, 243–249. [Google Scholar] [CrossRef]
- Fan, L.P.; Li, J.W.; Deng, K.Q.; Ai, L.Z. Effects of drying methods on the antioxidant activities of polysaccharides extracted from Ganoderma lucidum. Carbohydr. Polym. 2012, 87, 1849–1854. [Google Scholar] [CrossRef]
- Sun, J.; Chen, H.; Kan, J.; Gou, Y.R.; Liu, J.; Zhang, X.; Wu, X.N.; Tang, S.X.; Sun, R.; Qian, C.L.; et al. Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int. J. Biol. Macromol. 2020, 153, 708–722. [Google Scholar] [CrossRef]
- Chen, X.Y.; Li, X.; Sun-Waterhouse, D.; Zhu, B.Y.; You, L.J.; Hileuskaya, K. Polysaccharides from Sargassum fusiforme after UV/H2O2 degradation effectively ameliorate dextran sulfate sodium-induced colitis. Food Funct. 2021, 12, 11747–11759. [Google Scholar] [CrossRef]
- Jing, X.; Zulfiqar, F.; Park, S.Y.; Núñez, G.; Dziarski, R.; Gupta, D. Peptidoglycan Recognition Protein 3 and Nod2 Synergistically Protect Mice from Dextran Sodium Sulfate-Induced Colitis. J. Immunol. 2014, 193, 3055–3069. [Google Scholar] [CrossRef]
- Arora, A.; Sharma, N.; Kakkar, D. Natural polysaccharides for ulcerative colitis: A general overview. Asian Pac. J. Trop. Biomed. 2023, 13, 185–194. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Z.; Cheng, L.; Kang, J.; Liu, Y.; Zhao, Y.; Xiao, M.; Liu, H.; Zhu, Q.; Guo, Q.; et al. Structural Characterization of Water-Soluble Pectin from the Fruit of Diospyros lotus L. and Its Protective Effects against DSS-Induced Colitis in Mice. J. Agric. Food Chem. 2025, 73, 1630–1641. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Salem, M.B.; Elzallat, M.; Mohammed, D.M.; Hammam, O.A.; Abdel-Wareth, M.T.A.; Hassan, M. Helix pomatia mucin alleviates DSS-induced colitis in mice: Unraveling the cross talk between microbiota and intestinal chemokine. Heliyon 2024, 10, e37362. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Wang, L.J.; Tang, L.; Wang, L.; Cao, S.Y.; Wu, Q.; Zhang, Z.; Li, L. Salvianolic acid B alters the gut microbiota and mitigates colitis severity and associated inflammation. J. Funct. Foods 2018, 46, 312–319. [Google Scholar] [CrossRef]
- Liu, X.M.; Chen, Y.J.; Liu, Y.; Hu, Y.; Wang, K.K.; Huang, L.X.; Ke, X.Q.; Peng, L.; Guo, Z.G. Protective effects and mechanisms of extracts of Gleditsia sinensis Lam. Thorn on DSS-induced colitis in mice. J. Ethnopharmacol. 2025, 340, 119244. [Google Scholar] [CrossRef]
- Rong, Y.M.; Zhu, M.H.; Wang, N.; Zhang, F.Y.; Liu, T.J. Photodynamic therapy with a novel photosensitizer inhibits DSS-induced ulcerative colitis in rats via the NF-κB signaling pathway. Front. Pharmacol. 2025, 15, 1539363. [Google Scholar] [CrossRef]
- Li, X.L.; Sun, Q.; Wang, Y.W.; Han, D.Q.; Fan, J.H.; Zhang, J.L.; Yang, C.H.; Ma, X.X.; Sun, Q.S. The regulatory effects of L-plantarum peptidoglycan microspheres on innate and humoral immunity in mouse. J. Microencapsul. 2017, 34, 635–643. [Google Scholar] [CrossRef]
- Gryaznova, M.; Burakova, I.; Smirnova, Y.; Morozova, P.; Chirkin, E.; Gureev, A.; Mikhaylov, E.; Korneeva, O.; Syromyatnikov, M. Effect of Probiotic Bacteria on the Gut Microbiome of Mice with Lipopolysaccharide-Induced Inflammation. Microorganisms 2024, 12, 1341. [Google Scholar] [CrossRef]
- Li, S.C.; Peng, H.H.; Sun, Y.N.; Yang, J.L.; Wang, J.; Bai, F.Q.; Peng, C.Y.; Fang, S.Z.; Cai, H.M.; Chen, G.J. Yeast β-glucan attenuates dextran sulfate sodium-induced colitis: Involvement of gut microbiota and short-chain fatty acids. Int. J. Biol. Macromol. 2024, 280, 135846. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Chen, C.; Zheng, Y.; Wu, Z.-J.; Zhou, M.-Q.; Liu, X.-Y.; Miyashita, K.; Duan, D.-L.; Du, L. Fucoxanthin Alleviates Dextran Sulfate Sodium-Induced Colitis and Gut Microbiota Dysbiosis in Mice. J. Agric. Food Chem. 2024, 72, 4142–4154. [Google Scholar] [CrossRef]
- Shang, L.; Liu, H.; Yu, H.; Chen, M.; Yang, T.; Zeng, X.; Qiao, S. Core Altered Microorganisms in Colitis Mouse Model: A Comprehensive Time-Point and Fecal Microbiota Transplantation Analysis. Antibiotics 2021, 10, 643. [Google Scholar] [CrossRef]
- Joung, J.Y.; Choi, K.; Lee, J.H.; Oh, N.S. Protective Potential of Limosilactobacillus fermentum Strains and Their Mixture on Inflammatory Bowel Disease via Regulating Gut Microbiota in Mice. J. Microbiol. Biotechnol. 2025, 35, e2410009. [Google Scholar] [CrossRef]
- Huang, B.; Wang, L.; Liu, M.; Wu, X.; Lu, Q.; Liu, R. The underlying mechanism of A-type procyanidins from peanut skin on DSS-induced ulcerative colitis mice by regulating gut microbiota and metabolism. J. Food Biochem. 2022, 46, e14103. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-W.; Xie, Y.; Huang, Z.-C.; Yang, K.; Wang, Z.-G.; Hu, H.-L. Study of the therapeutic effect of raw and processed Vladimiriae Radix on ulcerative colitis based on intestinal flora, metabolomics and tissue distribution analysis. Phytomedicine 2021, 85, 153538. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hu, G.Q.; Fu, S.P.; Qin, D.; Song, Z.Y. Phillyrin ameliorates DSS-induced colitis in mice via modulating the gut microbiota and inhibiting the NF-κB/MLCK pathway. Microbiol. Spectr. 2025, 13, e0200624. [Google Scholar] [CrossRef]
- Li, Q.; Wu, Z.; Sun, R.; Liu, X.; Luo, J.; Chen, X.; Zheng, J.; Liu, R. Improvement in DSS induced acute enteritis in mice with supplementation of bifidobacteria. Acta Aliment. 2025, 54, 84–95. [Google Scholar] [CrossRef]
- Dai, W.; Lv, Y.J.; Quan, M.; Ma, M.F.; Shang, Q.S.; Yu, G.L. Bacteroides salyersiae Is a Candidate Probiotic Species with Potential Anti-Colitis Properties in the Human Colon: First Evidence from an In Vivo Mouse Model. Nutrients 2024, 16, 2918. [Google Scholar] [CrossRef]
- Zhang, Y.; Tu, S.; Ji, X.; Wu, J.; Meng, J.; Gao, J.; Shao, X.; Shi, S.; Wang, G.; Qiu, J.; et al. Dubosiella newyorkensis modulates immune tolerance in colitis via the L-lysine-activated AhR-IDO1-Kyn pathway. Nat. Commun. 2024, 15, 1333. [Google Scholar] [CrossRef]
- Sun, Q.Q.; Yu, Z.F.; Luo, L.; Li, S.; Guan, X.; Sun, Z.L. Modulation of Inflammation Levels and the Gut Microbiota in Mice with DSS-Induced Colitis by a Balanced Vegetable Protein Diet. Plant Foods Hum. Nutr. 2025, 80, 19. [Google Scholar] [CrossRef]
- Su, Y.; Cui, Z.Y.; Chen, C.; Yang, X.Y.; Jiang, Y.J.; Zhang, W.; Zhang, Y.; Man, C.X. Lactobacillus paracasei JY062 with its exopolysaccharide ameliorates intestinal inflammation on DSS-induced experimental colitis through TLR4/MyD88/NF-κB signaling pathway. Food Biosci. 2025, 63, 105689. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, J.Z.; Xie, A.W.; Yang, H.Q.; Li, Y.J.; Mei, Y.X.; Li, J.S.; Xiao, L.; Liu, Y.Y.; Liang, Y.X. Enhancing butyrate synthesis and intestinal epithelial energy supply through mixed probiotic intervention in dextran sulfate sodium-induced colitis. Food Biosci. 2025, 63, 105727. [Google Scholar] [CrossRef]
- Lv, L.; Maimaitiming, M.; Yang, J.C.; Xia, S.L.; Li, X.; Wang, P.Y.; Liu, Z.Q.; Wang, C.Y. Quinazolinone Derivative MR2938 Protects DSS-Induced Barrier Dysfunction in Mice Through Regulating Gut Microbiota. Pharmaceuticals 2025, 18, 123. [Google Scholar] [CrossRef]
- Håkansson, Å.; Tormo-Badia, N.; Baridi, A.; Xu, J.; Molin, G.; Hagslätt, M.L.; Karlsson, C.; Jeppsson, B.; Cilio, C.M.; Ahrné, S. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin. Exp. Med. 2015, 15, 107–120. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xiong, H.; Cai, Y.; Chen, W.; Shi, M.; Liu, L.; Wu, K.; Deng, X.; Deng, X.; Chen, T. Clostridium butyricum ameliorates post-gastrectomy insulin resistance by regulating the mTORC1 signaling pathway through the gut-liver axis. Microbiol. Res. 2025, 297, 128154. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.J.; Tchaptchet, S.Y.; Arsene, D.; Mishima, Y.; Liu, B.; Sartor, R.B.; Carroll, I.M.; Miao, E.A.; Fodor, A.A.; Hansen, J.J. Environmental Factors Modify the Severity of Acute DSS Colitis in Caspase-11-Deficient Mice. Inflamm. Bowel. Dis. 2018, 24, 2394–2403. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, M.; Zhou, L.; Ling, S.; Li, Y.; Kong, B.; Huang, P. Dietary fiber intake and risks of proximal and distal colon cancers A meta-analysis. Medicine 2018, 97, e11678. [Google Scholar] [CrossRef]
- Li, C.Y.; Liang, Y.Q.; Qiao, Y. Messengers From the Gut: Gut Microbiota-Derived Metabolites on Host Regulation. Front. Microbiol. 2022, 13, 863407. [Google Scholar] [CrossRef]
- Li, Y.; Faden, H.S.; Zhu, L. The Response of the Gut Microbiota to Dietary Changes in the First Two Years of Life. Front. Pharmacol. 2020, 11, 334. [Google Scholar] [CrossRef]
- Chen, C.; Li, H. The Inhibitory Effect of Gut Microbiota and Its Metabolites on Colorectal Cancer. J. Microbiol. Biotechnol. 2020, 30, 1607–1613. [Google Scholar] [CrossRef]
- Gao, X.X.; Zhao, J.X.; Zhang, H.; Chen, W.; Zhai, Q.X. Modulation of gut health using probiotics: The role of probiotic effector molecules. J. Future Foods 2022, 2, 1–12. [Google Scholar] [CrossRef]
- Tripathy, A.; Dash, J.; Kancharla, S.; Kolli, P.; Mahajan, D.; Senapati, S.; Jena, M.K. Probiotics: A Promising Candidate for Management of Colorectal Cancer. Cancers 2021, 13, 3178. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Zeng, H.; Taussig, D.P.; Cheng, W.-H.; Johnson, L.K.; Hakkak, R. Butyrate Inhibits Cancerous HCT116 Colon Cell Proliferation but to a Lesser Extent in Noncancerous NCM460 Colon Cells. Nutrients 2017, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, M.M.; Xu, H.X.; Wang, W.J.; Yin, Z.P.; Zhang, Q.F. Retrograded starch as colonic delivery carrier of taxifolin for treatment of DSS-induced ulcerative colitis in mice. Int. J. Biol. Macromol. 2025, 288, 138602. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.H.; Lam, W.; Ma, D.L.; Gullen, E.A.; Cheng, Y.C. Butyrate mediates nucleotide-binding and oligomerisation domain (NOD) 2-dependent mucosal immune responses against peptidoglycan. Eur. J. Immunol. 2009, 39, 3529–3537. [Google Scholar] [CrossRef]
- Niu, B.; Li, F.; Lv, X.; Xiao, Y.; Zhu, J.; Zhao, J.; Lu, W.; Chen, W. Unveiling the therapeutic potential and mechanism of inulin in DSS-induced colitis mice. Int. J. Biol. Macromol. 2024, 280, 135861. [Google Scholar] [CrossRef]
- Hosono, T.; Saito, K.; Arima, Y.; Ozaki-Masuzawa, Y.; Seki, T. Inulin exacerbates disease severity in a mouse model of ulcerative colitis by causing osmotic diarrhea. Biosci. Biotechnol. Biochem. 2025, 89, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Ariaee, A.; Koentgen, S.; Wardill, H.R.; Hold, G.L.; Prestidge, C.A.; Armstrong, H.K.; Joyce, P. Prebiotic selection influencing inflammatory bowel disease treatment outcomes: A review of the preclinical and clinical evidence. eGastroenterology 2024, 2, e100055. [Google Scholar] [CrossRef]
- Hiengrach, P.; Visitchanakun, P.; Finkelman, M.A.; Chancharoenthana, W.; Leelahavanichkul, A. More Prominent Inflammatory Response to Pachyman than to Whole-Glucan Particle and Oat-β-Glucans in Dextran Sulfate-Induced Mucositis Mice and Mouse Injection through Proinflammatory Macrophages. Int. J. Mol. Sci. 2022, 23, 4026. [Google Scholar] [CrossRef]



| Scores | Weight Loss (%) | Fecal Occult Blood |
|---|---|---|
| 0 | 0 | Normal |
| 1 | 1–3 | Mild blood |
| 2 | 3–6 | Moderate blood |
| 3 | 6–9 | Severe blood |
| 4 | >10 | Only blood |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Sun, J.; Yao, X.; Xu, M.; Xiao, H.; Hong, W.; Han, Y. Preventive Effect of Peptidoglycan Extracted from Lactobacillus casei ATCC 393 on Dextran Sulfate Sodium-Induced Inflammation in Mice Through Gut Microbiota Regulation. Processes 2025, 13, 3978. https://doi.org/10.3390/pr13123978
Li R, Sun J, Yao X, Xu M, Xiao H, Hong W, Han Y. Preventive Effect of Peptidoglycan Extracted from Lactobacillus casei ATCC 393 on Dextran Sulfate Sodium-Induced Inflammation in Mice Through Gut Microbiota Regulation. Processes. 2025; 13(12):3978. https://doi.org/10.3390/pr13123978
Chicago/Turabian StyleLi, Ruiyi, Jing Sun, Xu Yao, Min Xu, Huazhi Xiao, Wanjing Hong, and Ye Han. 2025. "Preventive Effect of Peptidoglycan Extracted from Lactobacillus casei ATCC 393 on Dextran Sulfate Sodium-Induced Inflammation in Mice Through Gut Microbiota Regulation" Processes 13, no. 12: 3978. https://doi.org/10.3390/pr13123978
APA StyleLi, R., Sun, J., Yao, X., Xu, M., Xiao, H., Hong, W., & Han, Y. (2025). Preventive Effect of Peptidoglycan Extracted from Lactobacillus casei ATCC 393 on Dextran Sulfate Sodium-Induced Inflammation in Mice Through Gut Microbiota Regulation. Processes, 13(12), 3978. https://doi.org/10.3390/pr13123978






