Taguchi-Based Optimization of Ultrasound-Assisted Valorization of Coffee Silver Skin for Increasing Phenolic Content: Antioxidant Activity, Physical Properties, and Energy Consumption Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Design and Extraction Protocol
2.3. Determination of Temperature Profile of the Extraction Process
2.4. Characterization of Coffee Silver Skin Extract
2.4.1. Physical Properties
Measurement of Total Soluble Solids Content (TSS) and Refractive Index
Color
2.4.2. Chemical Properties
Antioxidant Activity Assay
Total Phenolic Content (TPC) Assay
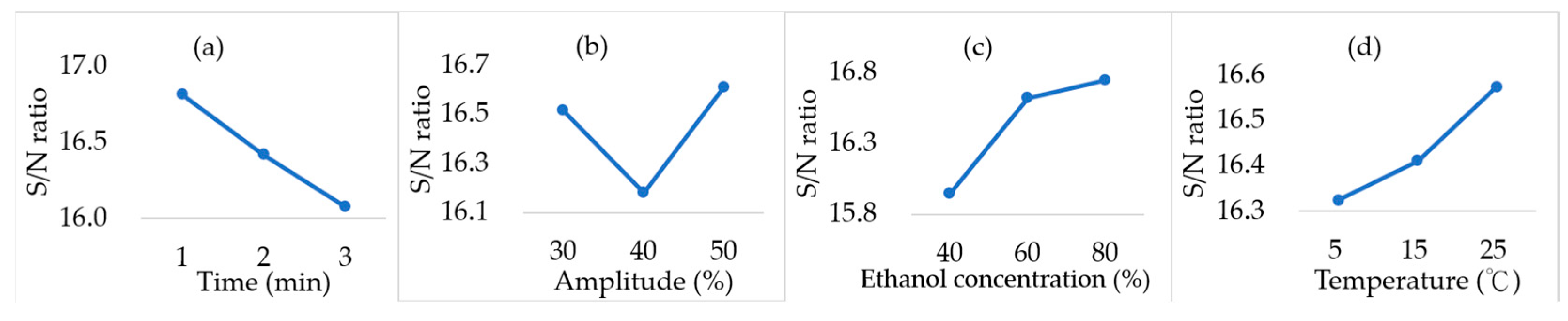
2.5. Process Optimization by the Taguchi Method
2.6. Process Assessment
2.6.1. Energy Requirements for Extraction
Determination of Total Energy Consumption (TEC) and Specific Energy Consumption (SEC)
2.7. Statistical Analysis
3. Results
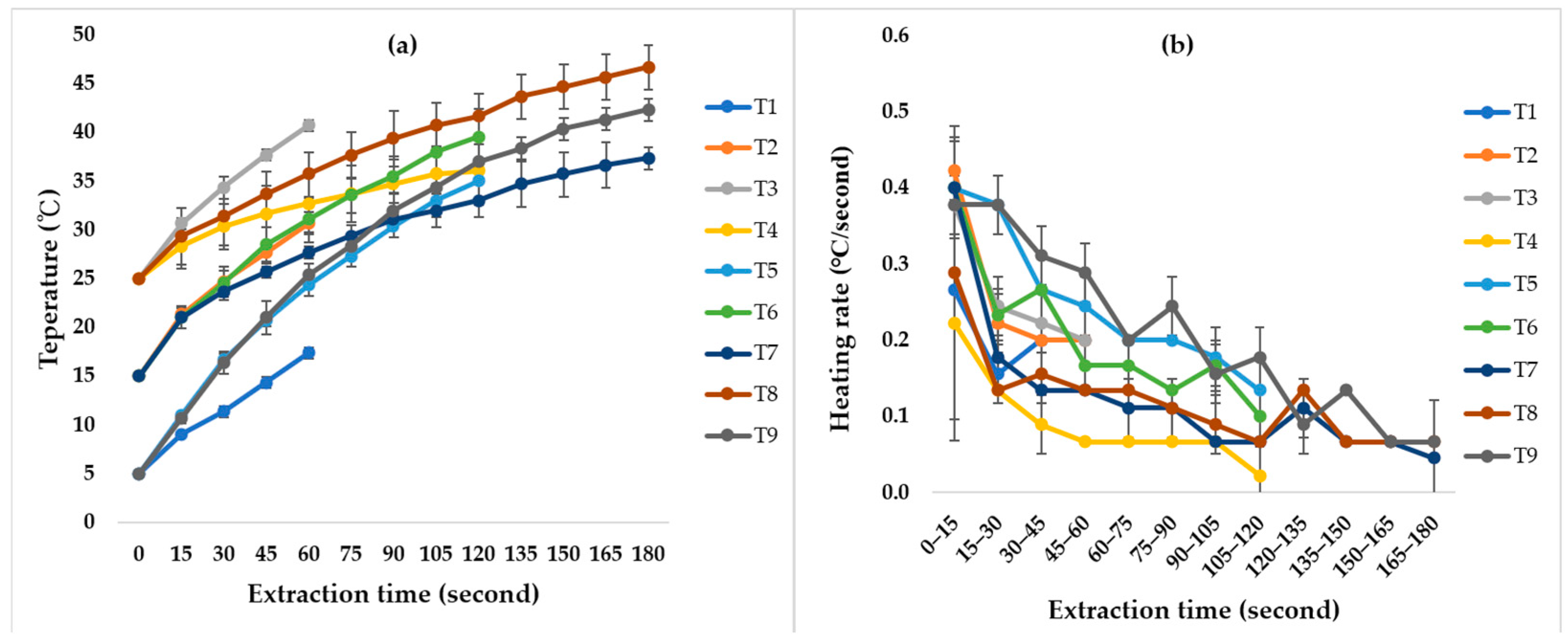
3.1. Extraction Temperature Profile
3.2. Characterization of Coffee Silver Skin Extract
3.2.1. Physical Properties
TSS and Refractive Index
Color
3.2.2. Chemical Properties
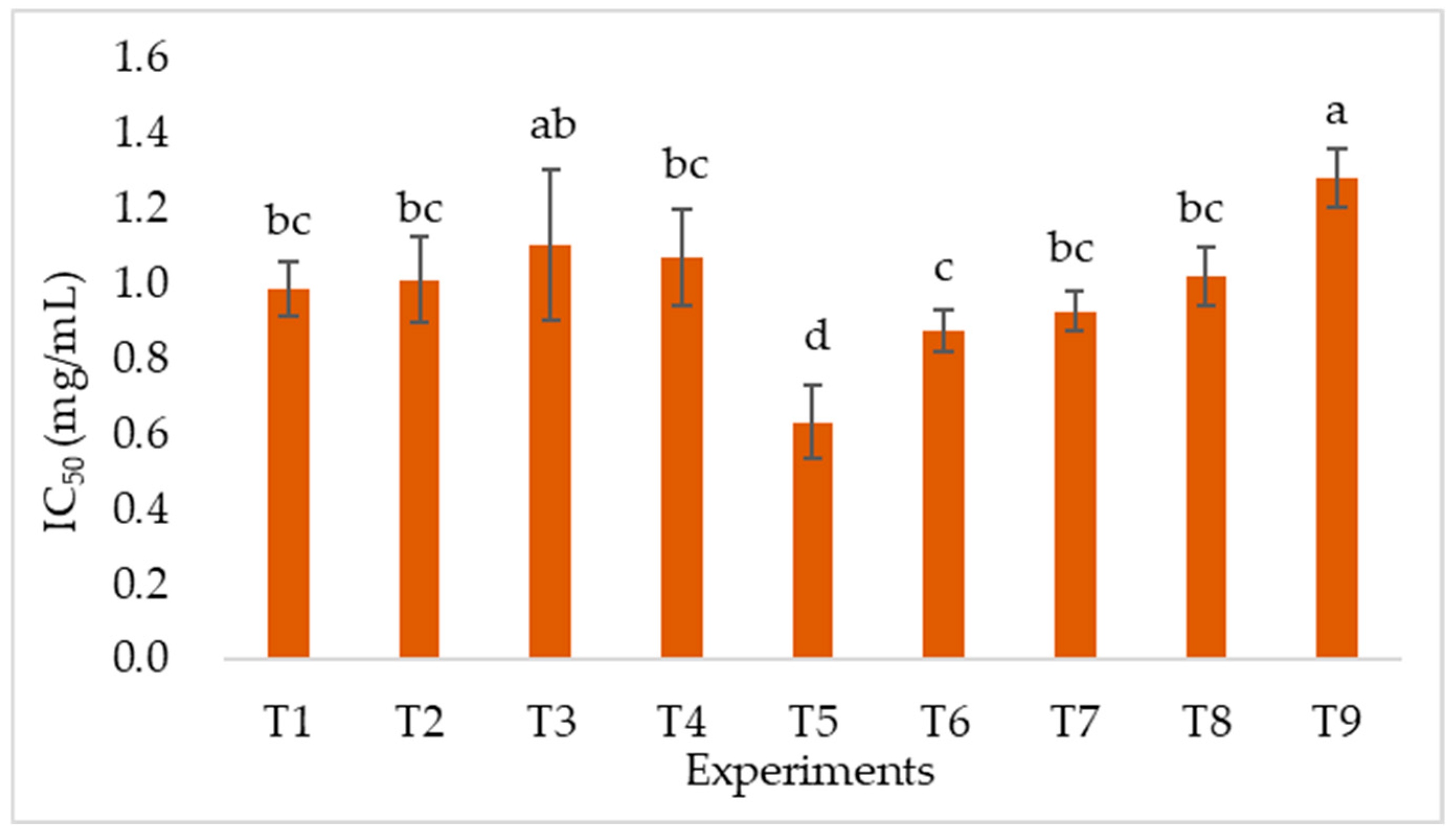
Antioxidant Activity
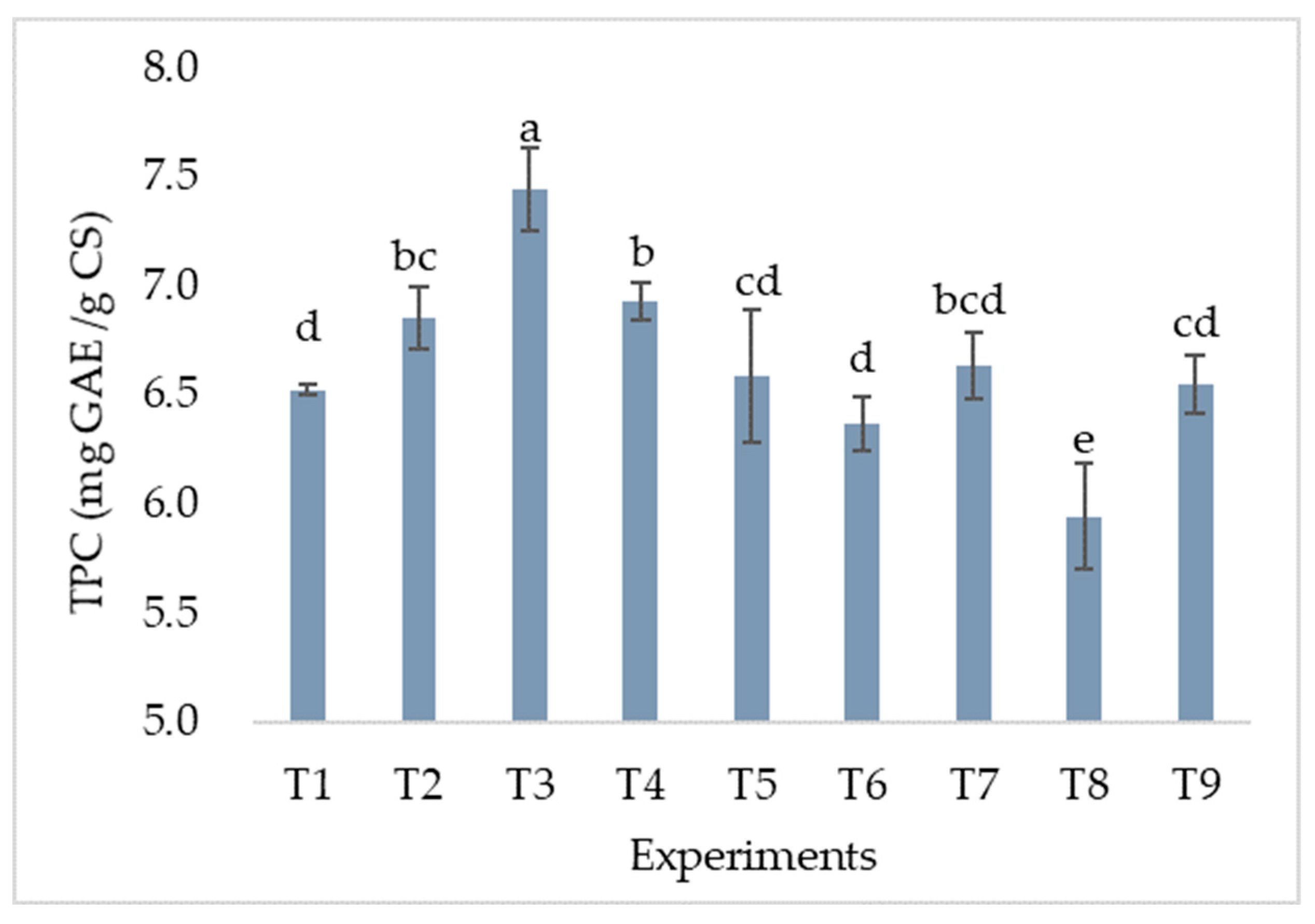
TPC
3.3. Process Assessment
Energy Requirements for Coffee Silver Skin Extraction (TEC and SEC)
4. Discussion
4.1. Temperature Profile of the Extraction Process
4.2. Characterization of Coffee Silver Skin Extract
4.2.1. Physical Properties
Effects of Extraction Parameters on TSS and Refractive Index
Color
4.2.2. Chemical Properties
Effects of Extraction Parameters on Antioxidant Activity
Effects of Extraction Parameters on TPC
4.3. Process Assessment
4.3.1. Energy Requirements for Coffee Silver Skin Extraction
TEC and SEC
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CS | Coffee silver skin |
| TPC | Total phenolic content |
| DPPH | 2,2-Diphenyl-1-picrylhydrazy |
| TEC | Total energy consumption |
| SEC | Specific energy consumption |
| TSS | Total soluble solids |
| R value | Range value |
| S/N | Signal-to-noise |
| IC50 | Half maximal inhibitory concentration |
| LTB | The larger-the-best |
| STB | The smaller-the-best |
| T1–T9 | Treatments 1 to 9 |
| GAE | Gallic acid equivalent |
References
- Özhamamcı, İ.; Sayın, B. Circular Utilization of Coffee Silverskin in Beef Patties: Effects on Technological, Sensory, and Safety Attributes. Food Biosci. 2025, 72, 107436. [Google Scholar] [CrossRef]
- United States Department of Agriculture Foreign Agricultural Service. Coffee: World Markets and Trade; Global Market Analysis. 2025. Available online: https://apps.fas.usda.gov/psdonline/circulars/coffee.pdf (accessed on 13 October 2025).
- Dauber, C.; Romero, M.; Chaparro, C.; Ureta, C.; Ferrari, C.; Lans, R.; Frugoni, L.; Echeverry, M.V.; Calvo, B.S.; Trostchansky, A. Cookies enriched with coffee silverskin powder and coffee silverskin ultrasound extract to enhance fiber content and antioxidant properties. Appl. Food Res. 2024, 4, 100373. [Google Scholar] [CrossRef]
- Chemat, A.; Touraud, D.; Müller, R.; Kunz, W.; Fabiano-Tixier, A.-S. Valorization of Coffee Silverskin Using Extraction Cycles and Water as a Solvent: Design of Process. Molecules 2024, 29, 1318. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Peixoto, J.A.; Andrade, N.; Oliveira, M.B.P.; Martel, F.; Alves, R.C. Coffee silverskin: Potential health benefits and current applications. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2025; pp. 829–840. [Google Scholar]
- Inácio, H.P.; Santetti, G.S.; Dacoreggio, M.V.; da Silva Haas, I.C.; Baranzelli, J.; Emanuelli, T.; Hoff, R.B.; Kempka, A.P.; Fritzen Freire, C.B.; de Mello Castanho Amboni, R.D. Effects of different extraction methods on the phenolic profile, antioxidant and antimicrobial activity of the coffee grounds and coffee silverskin (Coffea arabica L.). JSFA Rep. 2023, 3, 354–363. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee silverskin extracts: Quantification of 30 bioactive compounds by a new HPLC-MS/MS method and evaluation of their antioxidant and antibacterial activities. Food Res. Int. 2020, 133, 109128. [Google Scholar] [CrossRef]
- Peixoto, J.A.B.; Andrade, N.; Machado, S.; Costa, A.S.; Puga, H.; Oliveira, M.B.P.; Martel, F.; Alves, R.C. Valorizing coffee silverskin based on its phytochemicals and antidiabetic potential: From lab to a pilot scale. Foods 2022, 11, 1671. [Google Scholar] [CrossRef]
- Kulkarni, R.M.; Mentha, S.S.; R, A.; Mascarenhas, E.W.; Polavarapu, S.; Appaiah, T. Valorization of waste coffee silverskin as a source of antioxidant by extraction with agitation. Environ. Qual. Manag. 2024, 34, e22209. [Google Scholar] [CrossRef]
- Brzezińska, R.; Wirkowska-Wojdyła, M.; Piasecka, I.; Górska, A. Application of response surface methodology to optimize the extraction process of bioactive compounds obtained from coffee silverskin. Appl. Sci. 2023, 13, 5388. [Google Scholar] [CrossRef]
- Vimercati, W.C.; da Silva Araújo, C.; Macedo, L.L.; Pimenta, C.J. Optimal extraction condition for the recovery of bioactive compounds and antioxidants from coffee silverskin. J. Food Process Eng. 2022, 45, e14009. [Google Scholar] [CrossRef]
- Machado, M.; Galrinho, M.F.; Passos, C.P.; Santo, L.E.; Chiș, M.S.; Ranga, F.; Puga, H.; Palmeira, J.; Coimbra, M.A.; Oliveira, M.B.P. Prebiotic potential of a coffee silverskin extract obtained by ultrasound-assisted extraction on Lacticaseibacillus paracasei subsp. paracasei. J. Funct. Foods 2024, 120, 106378. [Google Scholar] [CrossRef]
- Guglielmetti, A.; D’ignoti, V.; Ghirardello, D.; Belviso, S.; Zeppa, G. Optimisation of ultrasound and microwave-assisted extraction of caffeoylquinic acids and caffeine from coffee silverskin using response surface methodology. Ital. J. Food Sci. 2017, 29, 409–423. [Google Scholar] [CrossRef]
- Ginting, A.R.; Kit, T.; Mingvanish, W.; Thanasupsin, S.P. Valorization of coffee silverskin through subcritical water extraction: An optimization based on T-CQA using response surface methodology. Sustainability 2022, 14, 8435. [Google Scholar] [CrossRef]
- Koskinakis, S.E.; Stergiopoulos, C.; Vasileiou, C.; Krokida, M. Sustainable Valorization of Coffee Silverskin Waste: Pressurized Liquid Extraction of Bioactive Compounds. Foods 2025, 14, 615. [Google Scholar] [CrossRef] [PubMed]
- Boateng, I.D. Mechanisms, capabilities, limitations, and economic stability outlook for extracting phenolics from agro-byproducts using emerging thermal extraction technologies and their combinative effects. Food Bioprocess Technol. 2024, 17, 1109–1140. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef] [PubMed]
- Elmas, E.; Şen, F.B.; Kublay, İ.Z.; Baş, Y.; Tüfekci, F.; Derman, H.; Bekdeşer, B.; Aşçı, Y.S.; Capanoglu, E.; Bener, M. Green Extraction of Antioxidants from Hazelnut By-products Using Microwave-Assisted Extraction, Ultrasound-Assisted Extraction, and Pressurized Liquid Extraction. Food Bioprocess Technol. 2025, 18, 5388–5406. [Google Scholar] [CrossRef]
- Özdemir, M.; Yıldırım, R.; Yurttaş, R.; Başargan, D.; Hakcı, M.B. A Review of ultrasound-assisted extraction of bioactive compounds from coffee waste. Gıda 2025, 50, 56–73. [Google Scholar] [CrossRef]
- Sheu, S.-C.; Su, Y.-Y.; Gavahian, M. Valorization of banana inflorescence with integrated blanching and Taguchi-optimized ultrasound extraction. Qual. Assur. Saf. Crops Foods 2025, 17, 161–172. [Google Scholar] [CrossRef]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green extraction techniques of bioactive compounds: A state-of-the-art review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Wang, H.; Deng, L.; Huang, G. Ultrasound-assisted extraction and value of active substances in Muxu. Ultrason. Sonochem. 2025, 113, 107220. [Google Scholar] [CrossRef]
- Mgoma, S.T.; Basitere, M.; Mshayisa, V.V.; De Jager, D. A systematic review on sustainable extraction, preservation, and enhancement in food processing: The advancement from conventional to green technology through ultrasound. Processes 2025, 13, 965. [Google Scholar] [CrossRef]
- Gavahian, M.; Yang, Y.-H.; Tsai, P.-J. Power ultrasound for valorization of Citrus limon (cv. Eureka) waste: Effects of maturity stage and drying method on bioactive compounds, antioxidant, and anti-diabetic activity. Innov. Food Sci. Emerg. Technol. 2022, 79, 103052. [Google Scholar] [CrossRef]
- de Lima Pena, F.; de Souza, M.C.; Sanches, V.L.; Viganó, J.; Mancini, M.C.S.; Brito-Oliveira, T.C.; dos Santos, B.G.T.; de Queiroz, G.H.A.; Tamborlin, L.; Simabuco, F.M. Phenolic profile, bioactivity and cytotoxicity of plant extracts from thyme, ginger, garlic, ground roasted coffee and coffee silverskin. Int. J. Food Prop. 2025, 28, 2519843. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Rai, D.; Sun, D.W.; Tiwari, B.K. Ultrasound-assisted extraction (UAE) of bioactive compounds from coffee silverskin: Impact on phenolic content, antioxidant activity, and morphological characteristics. J. Food Process Eng. 2019, 42, e13191. [Google Scholar] [CrossRef]
- Taweekayujan, S.; Somngam, S.; Pinnarat, T. Optimization and kinetics modeling of phenolics extraction from coffee silverskin in deep eutectic solvent using ultrasound-assisted extraction. Heliyon 2023, 9, e17942. [Google Scholar] [CrossRef]
- Biondić Fučkar, V.; Nutrizio, M.; Grudenić, A.; Djekić, I.; Režek Jambrak, A. Sustainable ultrasound assisted extractions and valorization of coffee silver skin (CS). Sustainability 2023, 15, 8198. [Google Scholar] [CrossRef]
- Zhang, Z.; Poojary, M.M.; Choudhary, A.; Rai, D.K.; Lund, M.N.; Tiwari, B.K. Ultrasound processing of coffee silver skin, brewer’s spent grain and potato peel wastes for phenolic compounds and amino acids: A comparative study. J. Food Sci. Technol. 2021, 58, 2273–2282. [Google Scholar] [CrossRef]
- Chang, M.-H.; Chen, W.-H.; Wu, D.-R.; Ghorbani, M.; Rajendran, S.; Daud, W.M.A.W. Analysis of vacuum operation on hydrogen separation from H2/H2O mixture via Pd membrane using Taguchi method, response surface methodology, and multivariate adaptive regression splines. Energy Convers. Manag. X 2024, 23, 100645. [Google Scholar] [CrossRef]
- Tzani, A.; Lymperopoulou, T.; Pitterou, I.; Karetta, I.; Belfquih, F.; Detsi, A. Development and optimization of green extraction process of spent coffee grounds using natural deep eutectic solvents. Sustain. Chem. Pharm. 2023, 34, 101144. [Google Scholar] [CrossRef]
- Malkawi, R.; Battah, K.; Alkhreisat, M. Pharmaceutical Insights into Ammi and Parsley: Evaluating Antioxidant Activity, Total Phenolic Content, and Kidney Stone Disintegration Properties. Adv. Pharmacol. Pharm. Sci. 2025, 2025, 5522905. [Google Scholar] [CrossRef]
- Fraley, S.; Zalewski, J.; Oom, M.; Terrien, B. 14.1: Design of experiments via taguchi methods-orthogonal arrays. In Chemical Process Dynamics and Controls; Woolf, P., Ed.; 2022; pp. 14.1.1–14.1.10. Available online: https://eng.libretexts.org/Bookshelves/Industrial_and_Systems_Engineering/Chemical_Process_Dynamics_and_Controls_(Woolf)/14%3A_Design_of_Experiments/14.01%3A_Design_of_Experiments_via_Taguchi_Methods_-_Orthogonal_Arrays (accessed on 13 October 2025).
- Gavahian, M.; Chu, R. Design, development, and performance evaluation of an ohmic extractor to valorize fruit by-products based on Taguchi method: Reduced energy consumption and enhanced total phenolics. J. Food Process Eng. 2022, 45, e13825. [Google Scholar] [CrossRef]
- Nutter, J.; Fernandez, M.V.; Jagus, R.J.; Agüero, M.V. Development of an aqueous ultrasound-assisted extraction process of bioactive compounds from beet leaves: A proposal for reducing losses and increasing biomass utilization. J. Sci. Food Agric. 2021, 101, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Chi, M.-H.; Hong, I.-K. Effect of ultrasound irradiation on solvent extraction process. J. Ind. Eng. Chem. 2009, 15, 919–928. [Google Scholar] [CrossRef]
- Ahmed, I.I.; Atta, M.; Mostafa, H. Optimization of natural food preservatives extraction from spices and herbs. Food Technol. Res. J. 2023, 1, 1–8. [Google Scholar] [CrossRef]
- Sikorska-Zimny, K.; Mielicki, W.; Kocik, A.; Kozioł, M.; Ziarkowska, M.; Wojciechowska, M. The Effect of Different Ethanol Concentrations on Functional Properties in Apple Macerates. J. Nutr. Food Secur. 2025, 10, 423–432. [Google Scholar] [CrossRef]
- Özhamamcı, İ. Coffee silverskin as a fat replacer in chicken patty formulation and its effect on physicochemical, textural, and sensory properties. Appl. Sci. 2024, 14, 6442. [Google Scholar] [CrossRef]
- Martuscelli, M.; Esposito, L.; Di Mattia, C.D.; Ricci, A.; Mastrocola, D. Characterization of coffee silver skin as potential food-safe ingredient. Foods 2021, 10, 1367. [Google Scholar] [CrossRef]
- Pourfarzad, A.; Mahdavian-Mehr, H.; Sedaghat, N. Coffee silverskin as a source of dietary fiber in bread-making: Optimization of chemical treatment using response surface methodology. LWT-Food Sci. Technol. 2013, 50, 599–606. [Google Scholar] [CrossRef]
- Buyong, N.L.; Nillian, E. Physiochemical properties of Sarawak’s adapted Liberica coffee silverskin utilizing varying solvents. Food Sci. Nutr. 2023, 11, 6052–6059. [Google Scholar] [CrossRef]
- Expósito-Almellón, X.; Munguía-Ubierna, Á.; Duque-Soto, C.; Borrás-Linares, I.; Quirantes-Piné, R.; Lozano-Sánchez, J. Optimized Ultrasound-Assisted Extraction for Enhanced Recovery of Valuable Phenolic Compounds from Olive By-Products. Antioxidants 2025, 14, 938. [Google Scholar] [CrossRef]
- Garcia-Larez, F.L.; Esquer, J.; Guzmán, H.; Zepeda-Quintana, D.S.; Moreno-Vásquez, M.J.; Rodríguez-Félix, F.; Del-Toro-Sánchez, C.L.; López-Corona, B.E.; Tapia-Hernández, J.A. Effect of Ultrasound-Assisted Extraction (UAE) parameters on the recovery of polyphenols from pecan nutshell waste biomass and its antioxidant activity. Biomass Convers. Biorefinery 2025, 15, 10977–10995. [Google Scholar] [CrossRef]
- Böger, B.R.; Salviato, A.; Valezi, D.F.; Di Mauro, E.; Georgetti, S.R.; Kurozawa, L.E. Optimization of ultrasound-assisted extraction of grape-seed oil to enhance process yield and minimize free radical formation. J. Sci. Food Agric. 2018, 98, 5019–5026. [Google Scholar] [CrossRef] [PubMed]
- Sayem, A.; Ahmed, T.; Mithun, M.U.K.; Rashid, M.; Rana, M.R. Optimising ultrasound-assisted extraction conditions for maximising phenolic, flavonoid content and antioxidant activity in hog plum peel and seed: A response surface methodology approach. J. Agric. Food Res. 2024, 18, 101312. [Google Scholar] [CrossRef]
- Algan-Cavuldak, Ö. Green Extraction of Antioxidant Polyphenols from Strawberry Tree (Arbutus unedo L.) Fruits and Leaves: Application of Deep Eutectic Solvents in the Ultrasound-Assisted Extraction. An. Da Acad. Bras. De Ciências 2025, 97, e20241084. [Google Scholar] [CrossRef]
- Ha, S.Y.; Jung, J.Y.; Kim, H.C.; Yang, J.-K. Optimization of Antioxidant Activity and Phenolic Extraction from Ainsliaea acerifolia Stem Using Ultrasound-Assisted Extraction Technology. BioResources 2024, 19, 6325–6338. [Google Scholar] [CrossRef]
- Frumuzachi, O.; Nicolescu, A.; Babotă, M.; Mocan, A.; Sisea, C.-R.; Hera, O.; Sturzeanu, M.; Rohn, S.; Lucini, L.; Crișan, G. D-Optimal Design-Based Ultrasound-Assisted Extraction Optimization and Extensive Bio-Structural Analysis of Phenolic Compounds from Romanian Cornelian Cherry (Cornus mas L.) Genotypes. Food Bioprocess Technol. 2025, 18, 7915–7932. [Google Scholar] [CrossRef]
- Goh, W.W.; Sultana, S.; Azlan, A. Antioxidant properties of Lemuni leaves (Vitex trifolia var. purpurea) in different concentrations of ethanol-water solvent extraction. Indones. J. Agric. Res. 2024, 7, 106–118. [Google Scholar] [CrossRef]
- Nguyen, N.H.K.; Le Ngoc, T.; PhanThiKieu, L.; Tran Thanh, T.; Mai Huynh, C. Bioactive compounds from red cabbage by microwave-assisted extraction: Anthocyanins, total phenolic compounds and the antioxidant activity. Asian Life Sci 2020, 12, 172–184. [Google Scholar]
- Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Angeloni, S.; Mustafa, A.M.; Vittori, S.; Maggi, F.; Caprioli, G. Chemical composition, antioxidant and enzyme inhibitory properties of different extracts obtained from spent coffee ground and coffee silverskin. Foods 2020, 9, 713. [Google Scholar] [CrossRef]
- Andrade, C.; Perestrelo, R.; Câmara, J.S. Bioactive compounds and antioxidant activity from spent coffee grounds as a powerful approach for its valorization. Molecules 2022, 27, 7504. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, W.; Niu, Y.; Li, W.; Lu, W.; Yu, L. Chemometric Classification and Bioactivity Correlation of Black Instant Coffee and Coffee Bean Extract by Chlorogenic Acid Profiling. Foods 2024, 13, 4016. [Google Scholar] [CrossRef]
- Alnsour, L.; Issa, R.; Awwad, S.; Albals, D.; Al-Momani, I. Quantification of total phenols and antioxidants in coffee samples of different origins and evaluation of the effect of degree of roasting on their levels. Molecules 2022, 27, 1591. [Google Scholar] [CrossRef]
- Silva, M.D.O.; Honfoga, J.N.B.; Medeiros, L.L.D.; Madruga, M.S.; Bezerra, T.K.A. Obtaining bioactive compounds from the coffee husk (Coffea arabica L.) using different extraction methods. Molecules 2020, 26, 46. [Google Scholar] [CrossRef]
- Kobus, Z.; Krzywicka, M.; Pecyna, A.; Buczaj, A. Process efficiency and energy consumption during the ultrasound-assisted extraction of bioactive substances from hawthorn berries. Energies 2021, 14, 7638. [Google Scholar] [CrossRef]

| Independent Variables | Unit | Actual Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Time (X1) | min | 1 | 2 | 3 |
| Amplitude (X2) * | % | 30 | 40 | 50 |
| Ethanol concentration (X3) | % | 40 | 60 | 80 |
| Initial temperature (X4) | °C | 5 | 15 | 25 |
| Experiment | Variables | |||
| X1 | X2 | X3 | X4 | |
| T1 | 1 | 30 | 40 | 5 |
| T2 | 1 | 40 | 60 | 15 |
| T3 | 1 | 50 | 80 | 25 |
| T4 | 2 | 30 | 60 | 25 |
| T5 | 2 | 40 | 80 | 5 |
| T6 | 2 | 50 | 40 | 15 |
| T7 | 3 | 30 | 80 | 15 |
| T8 | 3 | 40 | 40 | 25 |
| T9 | 3 | 50 | 60 | 5 |
| Experiment | TSS * (°Brix) | Refractive Index |
|---|---|---|
| T1 | 14.2 ± 0.3 f ** | 1.3542 ± 0.0005 e |
| T2 | 18.3 ± 0.2 d | 1.3607 ± 0.0003 cd |
| T3 | 19.7 ± 0.3 c | 1.3631 ± 0.0005 b |
| T4 | 18.3 ± 0.0 d | 1.3608 ± 0.0001 c |
| T5 | 20.1 ± 0.1 ab | 1.3638 ± 0.0002 a |
| T6 | 13.3 ± 0.2 g | 1.3527 ± 0.0004 f |
| T7 | 19.9 ± 0.3 bc | 1.3635 ± 0.0005 ab |
| T8 | 13.3 ± 0.3 g | 1.3526 ± 0.0004 f |
| T9 | 17.9 ± 0.0 e | 1.3601 ± 0.0001 d |
| Experiment | L* | a* | b* |
|---|---|---|---|
| T1 | 23.43 ± 0.05 a * | −3.83 ± 0.31 a | −2.79 ± 0.02 f |
| T2 | 23.10 ± 0.60 a | −3.96 ± 0.97 a | −2.80 ± 0.02 f |
| T3 | 23.08 ± 0.13 a | −7.01 ± 0.19 c | −2.27 ± 0.06 c |
| T4 | 20.77 ± 0.04 c | −10.94 ± 0.09 e | −1.91 ± 0.02 b |
| T5 | 21.47 ± 0.53 b | −8.15 ± 0.89 d | −2.46 ± 0.05 d |
| T6 | 20.69 ± 0.04 c | −11.27 ± 0.07 e | −1.84 ± 0.01 a |
| T7 | 21.91 ± 0.01 b | −7.60 ± 0.04 cd | −2.46 ± 0.01 d |
| T8 | 20.97 ± 0.02 c | −10.78 ± 0.03 e | −1.90 ± 0.01 b |
| T9 | 23.21 ± 0.07 a | −5.92 ± 0.04 b | −2.45 ± 0.03 d |
| Variables | R Value of DPPH | R Value of TPC * |
|---|---|---|
| Time (min) | 2.046 | 0.738 |
| Amplitude (%) | 1.847 | 0.430 |
| Ethanol concentration (%) | 2.174 | 0.795 |
| Initial temperature (°C) | 1.208 | 0.249 |
| Experiment | TEC (kWh) | SEC (kWh/mg GAE/g) |
|---|---|---|
| T1 | 0.0479 ± 0.0005 i * | 0.0073 ± 0.0000 e |
| T2 | 0.0488 ± 0.0005 h | 0.0071 ± 0.0002 e |
| T3 | 0.0500 ± 0.0003 g | 0.0067 ± 0.0002 f |
| T4 | 0.0507 ± 0.0003 f | 0.0073 ± 0.0001 e |
| T5 | 0.0524 ± 0.0003 e | 0.0080 ± 0.0004 d |
| T6 | 0.0542 ± 0.0003 c | 0.0085 ± 0.0002 c |
| T7 | 0.0529 ± 0.0002 d | 0.0080 ± 0.0002 d |
| T8 | 0.0564 ± 0.0003 b | 0.0095 ± 0.0004 a |
| T9 | 0.0593 ± 0.0003 a | 0.0090 ± 0.0002 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-X.; Gavahian, M. Taguchi-Based Optimization of Ultrasound-Assisted Valorization of Coffee Silver Skin for Increasing Phenolic Content: Antioxidant Activity, Physical Properties, and Energy Consumption Assessment. Processes 2025, 13, 3957. https://doi.org/10.3390/pr13123957
Chen Y-X, Gavahian M. Taguchi-Based Optimization of Ultrasound-Assisted Valorization of Coffee Silver Skin for Increasing Phenolic Content: Antioxidant Activity, Physical Properties, and Energy Consumption Assessment. Processes. 2025; 13(12):3957. https://doi.org/10.3390/pr13123957
Chicago/Turabian StyleChen, Yu-Xuan, and Mohsen Gavahian. 2025. "Taguchi-Based Optimization of Ultrasound-Assisted Valorization of Coffee Silver Skin for Increasing Phenolic Content: Antioxidant Activity, Physical Properties, and Energy Consumption Assessment" Processes 13, no. 12: 3957. https://doi.org/10.3390/pr13123957
APA StyleChen, Y.-X., & Gavahian, M. (2025). Taguchi-Based Optimization of Ultrasound-Assisted Valorization of Coffee Silver Skin for Increasing Phenolic Content: Antioxidant Activity, Physical Properties, and Energy Consumption Assessment. Processes, 13(12), 3957. https://doi.org/10.3390/pr13123957









