Abstract
Paracetamol can induce hypothermia in humans and rodents. The study’s aim is to review the mechanisms of paracetamol-induced hypothermia in rodents or the results issued from in vitro studies on the same species’ tissues (in doses that do not produce hepatic impairment) using the latest developments published in scientific journals over the last 15 years. Available human studies are also analysed. An extensive search in PubMed databases exploring the hypothermic response to paracetamol was conducted. 4669 articles about paracetamol’s effects on body temperature in mice or rats were found. After applying additional filters, 20 articles were selected for review, with 9 of them presented in tabular forms. The analysis of these articles found that the hypothermic effect of paracetamol is due to the inhibition of a cyclooxygenase-1 variant, is potentiated by endothelin receptor antagonists, and can be mediated through GABAA receptors and possibly through transient receptor potential cation channel subfamily A member 1 via N-acetyl-p-benzoquinone imine in the central nervous system. Human studies confirm the in vivo and in vitro experiments in rodents regarding the presence of a hypothermic effect after high, non-toxic doses of paracetamol. Further research is required to understand the mechanisms behind paracetamol’s hypothermic effect in humans.
1. Introduction
Paracetamol (acetaminophen) is one of the most commonly prescribed drugs worldwide. Synthetized over 150 years ago, paracetamol is highly efficient as analgesic and antipyretic and is on the list of the World Health Organization’s essential medicines. However, the drug is considered to be devoid of anti-inflammatory properties [1].
High doses of paracetamol pose a serious risk for hepatotoxicity due to the lack of neutralisation of the oxidation metabolite N-acetyl-p-benzoquinone imine (NAPQI), secondary to the depletion of glutathione [2].
Aside from the analgesic and antipyretic effects, paracetamol also presents hypothermic effects. Hypothermia induced by paracetamol was firstly described in the 1960s in rats treated with high doses of the drug in the presence of hepatic failure. A decade later, paracetamol was shown to produce profound hypothermia when administered intracerebroventricularly [3]. In 1982, Massey et al. demonstrated its hypothermic effects in mice treated with non-toxic doses [4].
The serendipitous discovery of the hypothermic effect, together with the clinical implications of the therapeutic induced hypothermia, led to new pharmacodynamic studies to understand the mechanism behind paracetamol’s effect of lowering body temperature. A significant number of studies started their investigations using the methodologies previously employed by researchers to determine the mechanisms of the analgesic effects of paracetamol.
It is thus essential to briefly revisit some of the past scientific perspectives on paracetamol’s analgesic and antipyretic effects for a better understanding of the state of the art on the hypothermic effect of paracetamol.
1.1. Short History of the Research Regarding the Mechanisms of Paracetamol-Induced Analgesic and Antipyretic Effects
For a long time, the analgesic and antipyretic activities of paracetamol were believed to be explained mainly by the inhibition of cyclooxygenase (COX) at the central nervous system (CNS) level [5].
Moreover, an argument for this theory was the fact that paracetamol acts especially in sites with low amounts of peroxides—like those found in the brain tissues—because of the interference of the drug with the peroxidase site of COX (POX-), which leads to paracetamol inactivation [6]. The high amounts of peroxides at the sites of inflammation can also be an explanation of the fact that paracetamol does not present anti-inflammatory properties [7].
More specifically, the analgesic and antipyretic effects of paracetamol were suggested to be produced by inhibition of the COX-1 and COX-2 subtypes [8]. However, data showed that COX-2 inhibition is less involved in the analgesic effects, as Ayoub et al., 2006, demonstrated that paracetamol also has an analgesic effect in COX-2 knockout (K.O.) mice [9].
Chandrasekaran et al., 2002, revealed that the COX-3 subtype (with one intron-retaining gene of COX-1) was the target molecule for paracetamol’s central analgesic mechanism [10], but this mechanism is probably irrelevant in humans and rodents, according to Graham G.G. and Scott K.F., 2005 [11].
In more recent years, the lack of proof that the analgesic effects of paracetamol are dependent only on COX’s central inhibition has made researchers discover and describe other peripheral and non-COX effects [12].
Pini et al., 1996, demonstrated that paracetamol’s analgesic effects also depend on the serotoninergic system, showing that the depletion of serotonin in mice brains (with D,L p-chlorophenylalanine) prevents the antinociceptive effect of paracetamol [13]. Moreover, the opioid and NMDA glutamatergic mechanisms of action were also researched for explaining paracetamol-induced analgesia [14,15,16].
1.2. Recent Developments on Paracetamol’s Analgesic and Antipyretic Effects
In the last 15 years, there have been numerous studies focused on the role of the cannabinoid system in explaining the analgesic effect of paracetamol. Högestätt E.D. et al. (2005) showed that paracetamol is metabolised in two steps: firstly, to p-aminophenol, and secondly, to N-arachidonoyl-phenol amine (AM404), a derivative of the arachidonic acid [17].
AM404 acts as a TRPV1 agonist (transient receptor potential cation channel subfamily V member 1) and an anandamide reuptake inhibitor, which causes an increase in endogenous cannabinoids. Cannabinoids produce anti-nociceptive effects that are primarily mediated by CB1 receptors [17,18,19]. Recent studies have also shown that AM404 may have an additional anti-inflammatory role, inhibiting COX activity and prostaglandin synthesis in the CNS [20].
Another line of research that expanded in recent years is the investigation of serotoninergic involvement in paracetamol’s analgesic effect. Paracetamol increases serotonin levels, especially in the pons and frontal cortex of rats, further involving both 5-HT2 receptors and opioid receptors (µ1 and κ) [21,22]. Moreover, 5-HT2 and 5-HT3 receptors seem to play an important role in the analgesic effect of paracetamol, while the implications of 5-HT1 receptors are still unclear [23,24]. However, it should be noted that there does not seem to be any direct binding of paracetamol with either the 5-HT2 receptors or with the 5-HT1 or 5-HT3 receptors, and the mechanism is likely to be indirect [24,25,26].
The latest developments on paracetamol’s analgesic effect are those that involve blocking the CNS T-type Cav3.2 calcium channels and stimulating the Kv7 potassium channels found in the dorsal root ganglion and spinal dorsal horn neurons. These discoveries may represent new targets in analgesia. The latest data is best summarised in a recent review of Przybyła G.W. et al., 2020 [27].
1.3. The Role of Paracetamol in Inducing Hypothermia as a Therapeutic Option
The hypothermic effect of different substances, including paracetamol, became of clinical interest in the early 2000s when two prospective randomized trials indicated that inducing hypothermia may be beneficial for some cardiac arrest patients [28].
Nowadays, inducing a state of mild hypothermia (34–35 °C) named “targeted temperature management” represents an efficacious therapeutic option for neuroprotection in different neurological injuries secondary to ischemic stroke, post-cardiac arrest, post-traumatic brain injury with high intracranial pressure, or to perinatal asphyxia-related cardiac arrest in newborns [29,30,31,32,33].
However, paracetamol alone did not show any significant benefits when investigated in two trials that studied targeted temperature management. High doses of paracetamol induced small decreases in core body temperature (CBT) in normothermic or subfebrile patients with ischaemic acute stroke. Studies that investigate paracetamol in addition to other methods that induce hypothermia are scarce [34,35].
It is thus certain that paracetamol is not efficacious in producing or maintaining the targeted temperature in therapeutic hypothermia, but its hypothermic mechanism of action could be of high interest in developing new pharmacological tools for lowering the body temperature.
A comprehensive review on the role of prostaglandins and nonsteroidal anti-inflammatory drugs in the hypothermic response in animals was published by Aronoff D.M. and Romanovsky A.A. in a volume of Sharma H., 2007 [36].
Since then, not many reviews regarding the hypothermic effect of acetaminophen have been performed, leaving much of the new data in the field unsystematised.
The aim of the study is to review the mechanisms of paracetamol-induced hypothermia in rodents or the results issued from in vitro studies using the same species’ tissues (in doses that do not produce hepatic impairment) using the latest developments published in scientific journals over the last 15 years (1 January 2007–31 December 2021). The available human studies on this subject will also be analysed.
2. Materials and Methods
A literature review of paracetamol’s pharmacological influence on body temperature in rodents (mice and rats) was performed using the “tags” shown below to search the PubMed database.
The tag used initially was “(paracetamol OR acetaminophen) AND (hypothermia OR hypothermic OR antipyretic OR pyrexia OR “body temperature”)”, and it resulted in 20,688 articles (see Figure 1). When using the filter “mice OR rats”, the search returned 4669 results.
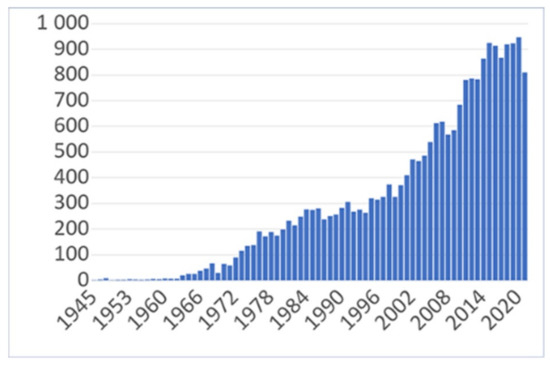
Figure 1.
The evolution of interest regarding paracetamol’s hypothermic and antipyretic effects as reflected in the number of articles published and indexed in PubMed between 1945 and 2021.
The final, slightly more restrictive tag used was as follows:
“(paracetamol OR acetaminophen) AND (hypothermia OR hypothermic OR “body temperature”) AND (mice OR rats)”, limits 1 January 2007–31 December 2021.
The search returned 44 results.
Our purpose was to evaluate in rodents the possible hypothermic mechanism of non-toxic doses of paracetamol (excluding paracetamol-induced hepatic toxicity).
After reading the full text, we excluded 24 articles that did not present the mechanism of action for the hypothermic or antipyretic effects of paracetamol, as well as those that dealt with these mechanisms in hepatic insufficiency settings.
Nine articles dealt with the in vivo mechanism of paracetamol hypothermia and five articles were chosen since they presented in vitro experiments using paracetamol (all five articles were already included in the first nine). The results are presented in Table 1 and Table 2 and in the Discussion section. An additional 11 articles were also screened and included as remarks in the Discussion. Searching inside the references of articles, we identified two studies in humans regarding paracetamol’s hypothermic effect.

Table 1.
Synthesis of the findings of the literature that presents paracetamol’s hypothermic effects in vivo.

Table 2.
Synthesis of the findings of the literature that present paracetamol’s hypothermic effects in vitro/ex vivo.
3. Results
The interest regarding paracetamol’s hypothermic and antipyretic effects is in a positive trend reflected in the number of articles on this subject published and indexed in PubMed between 1945 and 2021 (See Figure 1).
4. Discussion
4.1. Considerations Regarding the Methods Used in the Studies Analysed
The mechanism of hypothermia observed after paracetamol’s hepatic impairment was not analysed because hypothermia appearing in this condition could be induced by hepatic pathology, not by a direct action of paracetamol, and it could induce some biases in the research. From another point of view, we aimed to find potential therapeutic strategies in which inducing hypothermia is necessary in different conditions, such as CNS severe hypoxia. In some cases, hypothermia associated with hepatic impairment is a pathology by itself and cannot be used as a therapeutic method.
Mice and rats have different body temperature regulations and different dose thresholds for paracetamol toxicity. Rats are more resistant to paracetamol-induced liver injury, as the LD50 (lethal dose) for rats is 2404 mg/kg body weight (bw), whereas for mice it is 338 mg/kg bw for the oral route of administration [46].
The temperature measurement methods were different across studies. Some used CBT measurements (intrarectally or by subcutaneous implants, although the last is not a proper CBT measurement), and others used peripherally measured temperature under the hind limb. Moreover, the ambient temperature was also different across studies, and sometimes the temperature was used in disregard of the purpose of the study. For example, some authors used the ambient temperature of 23 °C instead of 30 °C, as in the study in which lipopolysaccharides (LPS) were used to induce pyrexia, although an ambient temperature of 30 °C represents the working standard [37].
These variabilities made it impossible for us to superpose the results of different labs and researchers.
4.2. Lack of Hypothermic Effect after COX-1 or/and COX-2 Inhibition, but Possibly after Inhibition of (Some) Cyclooxygenase-1 Variant Enzymes
A number of studies showed that paracetamol-induced hypothermia is correlated with reduced brain PGE2 synthesis [9]. The hypothermic effect in mice is established after 30 min at therapeutic doses of paracetamol (160 mg/kg bw) [37].
The hypothermic action of paracetamol appeared to be linked to the inhibition of a COX-1 variant (probably constitutive), whereas the antipyretic action may be induced by the COX-2 inhibition [47]. Li S. et al., 2008, reported the antipyretic action of paracetamol one hour after LPS administration in COX-1 knockout mice (although, theoretically, it lasts at least 2 h until COX-2 induction by LPS occurs through processes involving the nucleus of cells, COX-2 being an inducible enzyme) [37].
Paracetamol did not change the PGE2 levels in plasma or in the brain of the non-febrile mice, leading to the conclusion that its hypothermic effect is probably not due to its COX-2-inhibiting activity. This effect might be due to the anti-glutamate or antioxidant activities of paracetamol [37]. These results conflict with the hypothesis of Ayoub S.S. et al., 2006, that mention an involvement of COX-1, COX-2 and a hypothetic COX-3 isoenzymes [9].
The hypothermic effect of paracetamol is due to the inhibition of (some) cyclooxygenase-1 variant enzymes, after Ayoub S.S. and Flower R.J., 2019 [45]. Hypothermia induced by paracetamol depends indeed on a COX gene in mice, but its translation product might be different from COX-1 and COX-2 [45]. These results are based on the lack of a hypothermic effect of a COX-1 inhibitor (SC-560) or a COX-2 inhibitor (celecoxib), respectively. The non-selective COX-1 and COX-2 inhibitor indomethacin produced no hypothermia, only a robust antipyretic effect in mice when LPS was administered [45].
The reduction of paracetamol-induced hypothermia in COX-1 K.O. mice was accompanied by a reduction in the paracetamol-induced inhibition of brain PGE2 synthesis [48,49].
LPS treatment induced PGE2 in the brain, and this induction was completely suppressed by paracetamol. Paracetamol also reduced the levels of several other prostanoids in the brain, as well as in the blood. This fact signifies that paracetamol not only blocked induced PGE2, implying an effect on COX-2, but also reduced the levels of other prostaglandins and TXB2. This fact indicates (after Mirrasekhian E. et al., 2018) that paracetamol inhibits both COX-1 and COX-2 and may reveal that paracetamol interacts with the peroxidase site of the cyclooxygenase enzymes, present in both isoforms. This interaction is preferentially seen in tissues with low peroxide concentration, such as the brain [44].
4.3. Trials in Humans Concerning Hypothermia after Paracetamol
Acute paracetamol ingestion (at a dose of 20 mg/kg lean body mass) reduces normal, non-febrile CBT during sub-neutral conditions for thermoregulation, e.g., 120 min passive exposure to 20 °C, 40% relative humidity [50]. The authors conclude that this reduction of CBT induced by paracetamol is due to a decrease of autonomic shivering responses, probably mediated by COX-2 inhibition. In 2008, Hinz et al. found that a standard dose of paracetamol caused an almost complete inhibition of COX-2 in humans, whereas a moderate inhibition of COX-1 was noticed [51]. In accordance with the former data, the same research group [52] found that the same doses of paracetamol reduced CBT (0.16–0.57 °C) after 120 min exposure during acute cold stress (10 °C) but had no effect on CBT at a neutral ambient temperature (25 °C).
Taking into account these results in humans, we can explain the hypothermic effect of paracetamol in mice as being due to the ambient temperature under 30 °C used in the experiments. A temperature of 30 °C is the lowest level that does not produce thermogenesis in mice [53].
4.4. The Serotoninergic, Opioid, Nociceptin, Cannabinoid, Endothelin, GABA-ergic Systems, and Mitochondrial-Related Functions Involvment in Paracetamol’s Hypothermic Effect
Fukushima A. et al., 2017, showed no effect on influencing the hypothermic effect of paracetamol when pre-treating mice with the serotonin synthesis inhibitor DL-p-chlorophenylalanine (PCPA), thus producing a decrease of serotonin in the brain by 70% and significantly inhibiting the analgesic action of paracetamol. They concluded that the hypothermic effect of paracetamol is not mediated by the serotonin system, in contrast with its analgesic effect [43].
The 5-HT7 receptor is a potential defense mechanism in stopping fever, but the antipyretic property of paracetamol is not due to its action on 5-HT7 receptors [54].
The opioid system seems not to be involved either, as Corley G. et al., 2009, showed in rats the lack of effects of opioid receptor antagonists (µ, ĸ, or δ) on paracetamol-induced hypothermia. The authors also demonstrated no effect of cannabinoid CB1 or nociceptin (NOP) receptor antagonists [38].
There is, however, a potentiation of paracetamol’s hypothermic effect when it is co-administered with an endothelin receptor antagonist (the substance codenamed BQ123), as demonstrated by Briyal S. et al., 2010 [39]. This effect might reduce the infarction volume in rats following cerebral ischemia.
The levels of malonyldialdehyde (MDA) increased and glutathione (GSH) decreased in the brains of rats following ischemia. Paracetamol alone did not influence these enzymes. BQ123 alone and in combination with paracetamol decreased MDA and increased GSH levels in the brains of ischaemic rats. MDA levels significantly improved following treatment with both BQ123 and paracetamol compared to BQ123 alone. A decreased level of MDA in the brain of ischemic rats indicates a decrease in the levels of lipid peroxidation. An increase in GSH levels (signifying neuroprotection) in the brain of ischaemic rats treated with BQ123 and paracetamol occurred. This suggests that hypothermia induced by BQ123 plus paracetamol can be responsible for protecting the brain against the oxidative stress in the middle cerebral artery occlusion model [39].
Aydin M. et al., 2011, describes in immature rats that paracetamol decreased some markers of cellular injuries (apoptosis, heat shock protein 70 – HSP70) and increased healthy cell counts after hyperthermia induced by LPS in the brain [55].
A novel explanation of paracetamol-induced hypothermia in rodents is provided by Bashir S. et al., 2020. Paracetamol significantly attenuated mitochondrial function by up to 30% for complex I and 40% for complex II, in vitro. These data suggest that both the antipyretic and hypothermic effects induced by paracetamol could be attributed to the direct inhibition of lipolysis and mitochondrial function [56].
The cannabinoid hypothesis of the hypothermic effect of paracetamol (via the metabolite AM404 synthesised under the influence of fatty acid amide hydrolase, FAAH) is unsupported by at least four findings [40]:
- −
- The pharmacological inhibition of FAAH did not reduce the hypothermic effect of paracetamol;
- −
- FAAH K.O. mice that received paracetamol presented a similar hypothermic effect to that recorded in wild type (WT) mice;
- −
- The CB1 and TRPV1 antagonists did not reduce the hypothermic effect of paracetamol; and
- −
- When using K.O. mice, CB1R K.O., or TRPV1 K.O., the hypothermic effect was similar to that recorded in WT mice.
However, Ayoub S.S. et al., 2011, made an interesting finding regarding the cannabinoid system and the hypothermic effect of paracetamol: when used in combination with WIN55,212-2—an agonist of CB1 and CB2 receptors—paracetamol demonstrated a supra-additive hypothermic effect [40].
The hypothermic effect of paracetamol was antagonized by picrotoxin (a GABAA receptor antagonist) and flumazenil (a benzodiazepine receptor antagonist). Paracetamol’s hypothermic effect might be mediated somehow through GABAA receptors [42].
Paracetamol reduced brain PGE2 levels in CB1 receptor K.O. mice in a way comparable to non-treated mice of the same strain. This fact is further proof of the lack of CB1 receptors involvement in the induction of hypothermia by paracetamol [40].
4.5. Anti-Hyperthermic or Antipyretic Effects of Paracetamol
Paracetamol, in addition to its demonstrated hypothermic effect at therapeutic (160–200 mg/kg bw) or slightly higher doses (300 mg/kg bw) has been used in vivo at doses of 300 mg/kg bw to interfere with TRPV1 antagonists (the substance codenamed AMG8163, which causes hyperthermia in mice). Paracetamol 300 mg/kg bw decreased body temperature by 1.5 °C, completely reversing hyperthermia. An interesting fact is that low doses of paracetamol (100 or 150 mg/kg) were not effective in reversing AMG8163-induced hyperthermia [57]. It is possible that the same mechanism of action that produces hypothermia can be involved in diminishing hyperthermia induced by TRPV1 antagonists.
In acute stages of fractures in Wistar rats, the administration of paracetamol did not control hyperthermia [58].
Paracetamol partially prevents the febrile response of the body induced by endogenous pyrogens, such as IL1β, including the administration of these pyrogens in brain tissues. Csetényi B. et al., 2017, proposed that prostaglandin-mediated mechanisms have an important role in the mechanism of action of IL1β in the cingulate cortex since paracetamol pre-treatment partially prevented an increase in the body temperature of Wistar rats [59].
On the contrary, paracetamol did not influence the pyrogenic effect of IL1β injection in the nucleus accumbens of Wistar rats, and it seemed that this nucleus is not involved in paracetamol’s antipyretic effects [60].
The ex vivo studies of the research group of Ayoub S.S., 2019, confirmed that LPS-induced fever is PGE2-mediated and COX-2-dependent. At a dose of 15 mg/kg, SC560 (a COX-1 blocker without hypothermic action) reduced brain PGE2 synthesis by 76%. COX-1 inhibition by SC560 did not produce hypothermia by decreasing PGE2 synthesis. The authors also showed that COX-2 inhibition with celecoxib did not produce hypothermia. Since COX-2 K.O. mice fail to develop a fever in response to LPS, COX-2 seemed to be the antipyretic target for paracetamol [45].
The effect of other pyrogens, apart from LPS, could be reversed by paracetamol. Paracetamol (150 mg/kg) reduced the fever associated with zymosan induced experimental arthritis in rats and decreased the concentration of PGE2 in the cerebrospinal fluid, suggesting the involvement of PGE2 in this response [61].
Paracetamol does not interfere with reactive oxygen species (ROS) production, as observed using electron paramagnetic resonance (EPR) studies, although there is an increased formation of ROS in different tissues during LPS-induced fever [62].
4.6. TRPA1′s Role in Paracetamol-Induced Hypothermia
As shown above by Ayoub S.S., 2011, TRPV1 K.O. mice have a similar paracetamol-induced hypothermic response as the WT, suggesting that TRPV1 is not involved in the mechanism of hypothermia in paracetamol [40].
These results are also confirmed by Gentry C. et al., 2015, who used TRPV1 K.O., TRPA1 (transient receptor potential cation channel subfamily A member 1) K.O., and WT mice in their studies. Gentry et al. demonstrated that TRPV1 K.O. mice manifested the same hypothermic effect as their WT littermates. Moreover, resiniferatoxin, an agonist of TRPV1 receptors, had no effect on paracetamol’s hypothermic effect, thus emphasising the lack of TRPV1 involvement in paracetamol-induced hypothermia [41].
In contrast, in TRPA1 K.O. mice, the administration of paracetamol was without an effect on body temperature. In addition, a TRPA1 antagonist inhibited hypothermia, strongly suggesting that TRPA1 mediates paracetamol-evoked hypothermia.
Moreover, the latest findings indicate that the hypothermic effect might be due to the production of small quantities of NAPQI in the CNS that could stimulate TRPA1 receptors. Nevertheless, it should be noted that NAPQI is synthesised in the presence of an oxidative pathway that could be different in mice, rats, and humans. The large variations in the oxidative enzymes across species should therefore be accounted for when trying to extrapolate the results to humans.
It is thus certain that TRPA1 receptors are involved in the hypothermic effect of paracetamol, but data suggest that they are not responsible for its antipyretic effect. Gentry C. et al., 2015, demonstrated that TRPA1 K.O. mice respond to antipyretic doses of paracetamol [41]. Mirrasekhian E. et al., 2018, also observed that the antipyretic effects obtained at lower doses of paracetamol, insufficient to induce hypothermia, are not dependent on the TRPA1 receptors’ stimulation [44]. As noted above, for mice, the hypothermic effect is induced in doses of 160 mg/kg bw, while less than 150 mg/kg bw is required for inducing an analgesic effect [44,46]. These findings indicate that the hypothermic and antipyretic activities of paracetamol are influenced by TRPA1 and TRPV1 receptors through different mechanisms.
Other drugs from the NSAIDs group were found to induce a hypothermic effect in rodents, e.g., metamizole [63,64].
5. Conclusions
We present below the conclusions drawn from the articles analysed in this review. Paracetamol’s mechanism of lowering the normal central body temperature is still a subject of debate for researchers. We mention that many of these data are disparate, and some are not confirmed in the further (from a chronological point of view) articles.
- −
- Paracetamol’s hypothermic action is due to the inhibition of a COX-1 variant (probably constitutive), and its antipyretic action is due to the inhibition of COX-2;
- −
- Mitochondrial-related functions are involved in paracetamol’s hypothermic effect;
- −
- Endothelin receptor antagonists potentiate the hypothermic effect of paracetamol;
- −
- Opioid receptor (µ, ĸ, or δ) antagonists or nociceptin (NOP) receptor antagonists have no effect on paracetamol-induced hypothermia;
- −
- Cannabinoid CB1 receptor antagonists do not influence paracetamol-induced hypothermia;
- −
- Paracetamol has no involvement on the serotoninergic system concerning hypothermia (as opposed to its analgesic effect);
- −
- Paracetamol’s hypothermic effect is mediated somehow through GABAA receptors;
- −
- TRPV1 has no effect on paracetamol-induced hypothermia; and
- −
- TRPA1 is involved in the hypothermic response to paracetamol, possibly via NAPQI, a paracetamol metabolite produced in CNS.
The hypothermic mechanism of paracetamol is different from its antipyretic mechanism. More data is needed, but TRPA1 agonists have the potential to be used in clinical practice to induce hypothermia (for targeted temperature management).
Human studies confirm the in vivo and in vitro experiments in rodents regarding the presence of a hypothermic mechanism after high, non-toxic doses of paracetamol (in sub-neutral ambient temperature and humidity conditions).
Taking into account all these statements, we can observe that paracetamol’s hypothermic effect can be regarded in a dual perspective:
- −
- A favorable one, regarding its protective cellular action against brain ischemia; and
- −
- An unfavorable one, regarding its toxicity on mitochondrial function and the inhibition of lipolysis.
Consequently, further research is required to thoroughly understand the mechanisms of action behind paracetamol’s hypothermic effect in humans.
Author Contributions
Conceptualization, L.C. and H.P.; methodology, R.C.Ț. and C.I.V.G.; validation, D.C.; visualization, S.V. and H.P.; supervision, O.A.C.; writing—original draft, L.C. and R.C.Ț.; writing—review and editing, O.A.C. and I.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Botting, R. Paracetamol-Inhibitable COX-2. J. Physiol. Pharmacol. 2000, 51, 609–618. [Google Scholar] [PubMed]
- Hodgman, M.J.; Garrard, A.R. A Review of Acetaminophen Poisoning. Crit. Care Clin. 2012, 28, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.G.; Alderdice, M.T. Inhibition of Leukocytic Pyrogen-Induced Fever by Intracerebroventricular Administration of Salicylate and Acetaminophen in the Cat. Exp. Biol. Med. 1972, 140, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Massey, T.E.; Walker, R.M.; McElligott, T.F.; Racz, W.J. Acetaminophen-Induced Hypothermia in Mice: Evidence for a Central Action of the Parent Compound. Toxicology 1982, 25, 187–200. [Google Scholar] [CrossRef]
- Flower, R.J.; Vane, J.R. Inhibition of Prostaglandin Synthetase in Brain Explains the Anti-Pyretic Activity of Paracetamol (4-Acetamidophenol). Nature 1972, 240, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Aronoff, D.; Oates, J.; Boutaud, O. New Insights into the Mechanism of Action of Acetaminophen: Its Clinical Pharmacologic Characteristics Reflect Its Inhibition of the Two Prostaglandin H2 Synthases. Clin. Pharmacol. Ther. 2006, 79, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hanel, A.M.; Lands, W.E.M. Modification of Anti-Inflammatory Drug Effectiveness by Ambient Lipid Peroxides. Biochem. Pharmacol. 1982, 31, 3307–3311. [Google Scholar] [CrossRef]
- Schwab, J.M.; Schluesener, H.J.; Laufer, S. COX-3: Just Another COX or the Solitary Elusive Target of Paracetamol? Lancet 2003, 361, 981–982. [Google Scholar] [CrossRef]
- Ayoub, S.S.; Colville-Nash, P.R.; Willoughby, D.A.; Botting, R.M. The Involvement of a Cyclooxygenase 1 Gene-Derived Protein in the Antinociceptive Action of Paracetamol in Mice. Eur. J. Pharmacol. 2006, 538, 57–65. [Google Scholar] [CrossRef]
- Chandrasekharan, N.V.; Dai, H.; Roos, K.L.T.; Evanson, N.K.; Tomsik, J.; Elton, T.S.; Simmons, D.L. COX-3, a Cyclooxygenase-1 Variant Inhibited by Acetaminophen and Other Analgesic/Antipyretic Drugs: Cloning, Structure, and Expression. Proc. Natl. Acad. Sci. USA 2002, 99, 13926–13931. [Google Scholar] [CrossRef]
- Graham, G.G.; Scott, K.F. Mechanism of Action of Paracetamol. Am. J. Ther. 2005, 12, 46–55. [Google Scholar] [CrossRef]
- Bertolini, A.; Ferrari, A.; Ottani, A.; Guerzoni, S.; Tacchi, R.; Leone, S. Paracetamol: New Vistas of an Old Drug. CNS Drug Rev. 2006, 12, 250–275. [Google Scholar] [CrossRef]
- Pini, L.A.; Sandrini, M.; Vitale, G. The Antinociceptive Action of Paracetamol Is Associated with Changes in the Serotonergic System in the Rat Brain. Eur. J. Pharmacol. 1996, 308, 31–40. [Google Scholar] [CrossRef]
- Björkman, R. Central Antinociceptive Effects of Non-Steroidal Anti-Inflammatory Drugs and Paracetamol. Experimental Studies in the Rat. Acta Anaesthesiol. Scand. Suppl. 1995, 103, 1–44. [Google Scholar]
- Cichewicz, D.L. Synergistic Interactions between Cannabinoid and Opioid Analgesics. Life Sci. 2004, 74, 1317–1324. [Google Scholar] [CrossRef]
- Pini, L.A.; Vitale, G.; Ottani, A.; Sandrini, M. Naloxone-Reversible Antinociception by Paracetamol in the Rat. J. Pharmacol. Exp. Ther. 1997, 280, 934–940. [Google Scholar]
- Högestätt, E.D.; Jönsson, B.A.G.; Ermund, A.; Andersson, D.A.; Björk, H.; Alexander, J.P.; Cravatt, B.F.; Basbaum, A.I.; Zygmunt, P.M. Conversion of Acetaminophen to the Bioactive N-Acylphenolamine AM404 via Fatty Acid Amide Hydrolase-Dependent Arachidonic Acid Conjugation in the Nervous System. J. Biol. Chem. 2005, 280, 31405–31412. [Google Scholar] [CrossRef]
- Dani, M.; Guindon, J.; Lambert, C.; Beaulieu, P. The Local Antinociceptive Effects of Paracetamol in Neuropathic Pain Are Mediated by Cannabinoid Receptors. Eur. J. Pharmacol. 2007, 573, 214–215. [Google Scholar] [CrossRef]
- Ohashi, N.; Kohno, T. Analgesic Effect of Acetaminophen: A Review of Known and Novel Mechanisms of Action. Front. Pharmacol. 2020, 11, 1916. [Google Scholar] [CrossRef]
- Saliba, S.W.; Marcotegui, A.R.; Fortwängler, E.; Ditrich, J.; Perazzo, J.C.; Muñoz, E.; de Oliveira, A.C.P.; Fiebich, B.L. AM404, Paracetamol Metabolite, Prevents Prostaglandin Synthesis in Activated Microglia by Inhibiting COX Activity. J. Neuroinflammat. 2017, 14, 246. [Google Scholar] [CrossRef]
- Ruggieri, V.; Vitale, G.; Pini, L.A.; Sandrini, M. Differential Involvement of Opioidergic and Serotonergic Systems in the Antinociceptive Activity of N-Arachidonoyl-Phenolamine (AM404) in the Rat: Comparison with Paracetamol. Naunyn-Schmied Arch. Pharm. 2008, 377, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Vijayakaran, K.; Kesavan, M.; Kannan, K.; Sankar, P.; Tandan, S.K.; Sarkar, S.N. Arsenic Decreases Antinociceptive Activity of Paracetamol: Possible Involvement of Serotonergic and Endocannabinoid Receptors. Environ. Toxicol. Pharmacol. 2014, 38, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, U.A.; Khobragade, R.; Naik, C.; Yegnanarayan, R.; Kale, J. Randomized, Double-Blind, Placebo-Controlled Study to Investigate the Pharmacodynamic Interaction of 5-HT3 Antagonist Ondansetron and Paracetamol in Postoperative Patients Operated in an ENT Department under Local Anesthesia. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Hamurtekin, Y.; Nouilati, A.; Demirbatir, C.; Hamurtekin, E. The Contribution of Serotonergic Receptors and Nitric Oxide Systems in the Analgesic Effect of Acetaminophen: An Overview of the Last Decade. Turk. J. Pharm. Sci. 2020, 17, 119–126. [Google Scholar] [CrossRef]
- Epureanu, F.B.; Păunescu, H.; Ghiță, I.; Costescu, M.; Coman, L.; Fulga, I.; Coman, O.A. New Experimental Data on the Central Effects of an Old Analgesic—Paracetamol. Farmacia 2019, 67, 648–655. [Google Scholar] [CrossRef]
- Handra, C.; Coman, O.A.; Coman, L.; Enache, T.; Stoleru, S.; Sorescu, A.-M.; Ghita, I.; Fulga, I. The Connection between Different Neurotransmitters Involved in Cognitive Processes. FARMACIA 2019, 67, 193–201. [Google Scholar] [CrossRef]
- Przybyła, G.W.; Szychowski, K.A.; Gmiński, J. Paracetamol—An Old Drug with New Mechanisms of Action. Clin. Exp. Pharm. Physiol. 2021, 48, 3–19. [Google Scholar] [CrossRef]
- Writing Group; Nolan, J.P.; Morley, P.T.; Vanden Hoek, T.L.; Hickey, R.W.; Members of the Advanced Life Support Task Force; Kloeck, W.G.J.; Billi, J.; Böttiger, B.W.; Morley, P.T.; et al. Therapeutic Hypothermia After Cardiac Arrest: An Advisory Statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation. Circulation 2003, 108, 118–121. [Google Scholar] [CrossRef]
- Callaway, C.W.; Donnino, M.W.; Fink, E.L.; Geocadin, R.G.; Golan, E.; Kern, K.B.; Leary, M.; Meurer, W.J.; Peberdy, M.A.; Thompson, T.M.; et al. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015, 132, S465–S482. [Google Scholar] [CrossRef]
- Fink, E.L.; Beers, S.R.; Russell, M.L.; Bell, M.J. Acute Brain Injury and Therapeutic Hypothermia in the PICU: A Rehabilitation Perspective. J. Pediatr. Rehabil. Med. 2009, 2, 309–319. [Google Scholar] [CrossRef]
- Flynn, L.M.C.; Rhodes, J.; Andrews, P.J.D. Therapeutic Hypothermia Reduces Intracranial Pressure and Partial Brain Oxygen Tension in Patients with Severe Traumatic Brain Injury: Preliminary Data from the Eurotherm3235 Trial. Hypothermia Temp. Manag. 2015, 5, 143–151. [Google Scholar] [CrossRef]
- Hong, J.M. Targeted Temperature Management for Ischemic Stroke. J. Neurocrit. Care 2019, 12, 67–73. [Google Scholar] [CrossRef]
- Ma, H.; Sinha, B.; Pandya, R.S.; Lin, N.; Popp, A.J.; Li, J.; Yao, J.; Wang, X. Therapeutic Hypothermia as a Neuroprotective Strategy in Neonatal Hypoxic-Ischemic Brain Injury and Traumatic Brain Injury. Curr. Mol. Med. 2012, 12, 1282–1296. [Google Scholar] [CrossRef]
- Dippel, D.W.J.; van Breda, E.J.; van der Worp, H.B.; van Gemert, H.M.A.; Meijer, R.J.; Kappelle, L.J.; Koudstaal, P.J. PISA-Investigators Effect of Paracetamol (Acetaminophen) and Ibuprofen on Body Temperature in Acute Ischemic Stroke PISA, a Phase II Double-Blind, Randomized, Placebo-Controlled Trial [ISRCTN98608690]. BMC Cardiovasc. Disord. 2003, 3, 2. [Google Scholar] [CrossRef]
- Kasner, S.E.; Wein, T.; Piriyawat, P.; Villar-Cordova, C.E.; Chalela, J.A.; Krieger, D.W.; Morgenstern, L.B.; Kimmel, S.E.; Grotta, J.C. Acetaminophen for Altering Body Temperature in Acute Stroke: A Randomized Clinical Trial. Stroke 2002, 33, 130–134. [Google Scholar] [CrossRef]
- Aronoff, D.M.; Romanovsky, A.A. Eicosanoids in Non-Febrile Thermoregulation. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2007; Volume 162, pp. 15–25. ISBN 978-0-444-51926-9. [Google Scholar] [CrossRef]
- Li, S.; Dou, W.; Tang, Y.; Goorha, S.; Ballou, L.R.; Blatteis, C.M. Acetaminophen: Antipyretic or Hypothermic in Mice? In Either Case, PGHS-1b (COX-3) Is Irrelevant. Prostaglandins Other Lipid Mediat. 2008, 85, 89–99. [Google Scholar] [CrossRef]
- Corley, G.; Rawls, S.M. Opioid, Cannabinoid CB1 and NOP Receptors Do Not Mediate APAP-Induced Hypothermia in Rats. Pharmacol. Biochem. Behav. 2009, 92, 503–507. [Google Scholar] [CrossRef]
- Briyal, S.; Gulati, A. Endothelin-A Receptor Antagonist BQ123 Potentiates Acetaminophen Induced Hypothermia and Reduces Infarction Following Focal Cerebral Ischemia in Rats. Eur. J. Pharmacol. 2010, 644, 73–79. [Google Scholar] [CrossRef]
- Ayoub, S.S.; Pryce, G.; Seed, M.P.; Bolton, C.; Flower, R.J.; Baker, D. Paracetamol-Induced Hypothermia Is Independent of Cannabinoids and Transient Receptor Potential Vanilloid-1 and Is Not Mediated by AM404. Drug Metab. Dispos. 2011, 39, 1689–1695. [Google Scholar] [CrossRef]
- Gentry, C.; Andersson, D.A.; Bevan, S. TRPA1 Mediates the Hypothermic Action of Acetaminophen. Sci. Rep. 2015, 5, 12771. [Google Scholar] [CrossRef]
- Ahangar, N.; Esam, Z.; Bekhradnia, A.; Ebrahimzadeh, M.A. Hypothermic Activity of Acetaminophen; Involvement of GABAA Receptor, Theoretical and Experimental Studies. Iran. J. Basic Med. Sci. 2016, 19, 470–475. [Google Scholar] [PubMed]
- Fukushima, A.; Sekiguchi, W.; Mamada, K.; Tohma, Y.; Ono, H. Serotonergic System Does Not Contribute to the Hypothermic Action of Acetaminophen. Biol. Pharm. Bull. 2017, 40, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Mirrasekhian, E.; Nilsson, J.L.Å.; Shionoya, K.; Blomgren, A.; Zygmunt, P.M.; Engblom, D.; Högestätt, E.D.; Blomqvist, A. The Antipyretic Effect of Paracetamol Occurs Independent of Transient Receptor Potential Ankyrin 1-Mediated Hypothermia and Is Associated with Prostaglandin Inhibition in the Brain. FASEB J. 2018, 32, 5751–5759. [Google Scholar] [CrossRef]
- Ayoub, S.S.; Flower, R.J. Loss of Hypothermic and Anti-Pyretic Action of Paracetamol in Cyclooxygenase-1 Knockout Mice Is Indicative of Inhibition of Cyclooxygenase-1 Variant Enzymes. Eur. J. Pharmacol. 2019, 861, 172609. [Google Scholar] [CrossRef] [PubMed]
- Walum, E. Acute Oral Toxicity. Environ. Health Perspect 1998, 106, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Engström Ruud, L.; Wilhelms, D.B.; Eskilsson, A.; Vasilache, A.M.; Elander, L.; Engblom, D.; Blomqvist, A. Acetaminophen Reduces Lipopolysaccharide-Induced Fever by Inhibiting Cyclooxygenase-2. Neuropharmacology 2013, 71, 124–129. [Google Scholar] [CrossRef]
- Ayoub, S.S.; Botting, R.M.; Goorha, S.; Colville-Nash, P.R.; Willoughby, D.A.; Ballou, L.R. Acetaminophen-Induced Hypothermia in Mice Is Mediated by a Prostaglandin Endoperoxide Synthase 1 Gene-Derived Protein. Proc. Natl. Acad. Sci. USA 2004, 101, 11165–11169. [Google Scholar] [CrossRef]
- Ayoub, S.S. Paracetamol (Acetaminophen): A Familiar Drug with an Unexplained Mechanism of Action. Temperature 2021, 8, 351–371. [Google Scholar] [CrossRef]
- Foster, J.; Mauger, A.; Thomasson, K.; White, S.; Taylor, L. Effect of Acetaminophen Ingestion on Thermoregulation of Normothermic, Non-Febrile Humans. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef][Green Version]
- Hinz, B.; Cheremina, O.; Brune, K. Acetaminophen (Paracetamol) Is a Selective Cyclooxygenase-2 Inhibitor in Man. FASEB J. 2008, 22, 383–390. [Google Scholar] [CrossRef]
- Foster, J.; Mauger, A.R.; Govus, A.; Hewson, D.; Taylor, L. Acetaminophen (Paracetamol) Induces Hypothermia During Acute Cold Stress. Clin. Drug Investig. 2017, 37, 1055–1065. [Google Scholar] [CrossRef]
- Gordon, C.J.; Aydin, C.; Repasky, E.A.; Kokolus, K.M.; Dheyongera, G.; Johnstone, A.F.M. Behaviorally Mediated, Warm Adaptation: A Physiological Strategy When Mice Behaviorally Thermoregulate. J. Biol. 2014, 44, 41–46. [Google Scholar] [CrossRef]
- Kose, D.; Cadirci, E.; Halici, Z.; Sirin, B.; Dincer, B. The Investigation of Possible Roles of Central 5-HT7 Receptors in Antipyretic Effect Mechanism of Paracetamol in LPS-Induced Hyperthermia Model of Mice. Inflammopharmacology 2019, 27, 1169–1178. [Google Scholar] [CrossRef]
- Aydin, M.; Kislal, F.M.; Ayar, A.; Demirol, M.; Kabakus, N.; Canatan, H.; Bulmus, O.; Ozercan, R.; Yilmaz, B.; Sen, Y.; et al. The Effects of Lipopolysaccharide-Induced Endogenous Hyperthermia and Different Antipyretic Treatment Modalities on Rat Brain. Bratisl. Lek. Listy 2011, 112, 227–234. [Google Scholar]
- Bashir, S.; Elegunde, B.; Morgan, W.A. Inhibition of Lipolysis: A Novel Explanation for the Hypothermic Actions of Acetaminophen in Non-Febrile Rodents. Biochem. Pharmacol. 2020, 172, 113774. [Google Scholar] [CrossRef]
- Gavva, N.R.; Bannon, A.W.; Hovland, D.N.; Lehto, S.G.; Klionsky, L.; Surapaneni, S.; Immke, D.C.; Henley, C.; Arik, L.; Bak, A.; et al. Repeated Administration of Vanilloid Receptor TRPV1 Antagonists Attenuates Hyperthermia Elicited by TRPV1 Blockade. J. Pharm. Exp. 2007, 323, 128–137. [Google Scholar] [CrossRef]
- Yeh, K.-T.; Wu, W.-T.; Subeq, Y.-M.; Niu, C.-C.; Liao, K.-W.; Chen, I.-H.; Wang, J.-H.; Lee, R.-P. Non-Steroid Anti-Inflammatory Drugs Are Better than Acetaminophen on Fever Control at Acute Stage of Fracture. PLoS ONE 2015, 10, e0137225. [Google Scholar] [CrossRef]
- Csetényi, B.; Hormay, E.; Szabó, I.; Takács, G.; Nagy, B.; László, K.; Karádi, Z. Food and Water Intake, Body Temperature and Metabolic Consequences of Interleukin-1β Microinjection into the Cingulate Cortex of the Rat. Behav. Brain Res. 2017, 331, 115–122. [Google Scholar] [CrossRef]
- Takács, G.; Papp, S.; Lukáts, B.; Szalay, C.; Nagy, B.; Fotakos, D.; Karádi, Z. Homeostatic Alterations after IL-1beta Microinjection into the Nucleus Accumbens of the Rat. Appetite 2010, 54, 354–362. [Google Scholar] [CrossRef]
- Kanashiro, A.; Pessini, A.C.; Machado, R.R.; Malvar, D.D.C.; Aguiar, F.A.; Soares, D.M.; do Vale, M.L.; de Souza, G.E.P. Characterization and Pharmacological Evaluation of Febrile Response on Zymosan-Induced Arthritis in Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1631–R1640. [Google Scholar] [CrossRef]
- Gomes, B.R.B.; Firmino, M.; Jorge, J.S.; Ferreira, M.L.O.; Rodovalho, T.M.; Weis, S.N.; Souza, G.E.P.; Morais, P.C.; Sousa, M.V.; Souza, P.E.N.; et al. Increase of Reactive Oxygen Species in Different Tissues during Lipopolysaccharide-Induced Fever and Antipyresis: An Electron Paramagnetic Resonance Study. Free Radic. Res. 2018, 52, 351–361. [Google Scholar] [CrossRef]
- Schlosburg, J.E.; Radanova, L.; Di Marzo, V.; Imming, P.; Lichtman, A.H. Evaluation of the Endogenous Cannabinoid System in Mediating the Behavioral Effects of Dipyrone (Metamizol) in Mice. Behav. Pharm. 2012, 23, 722–726. [Google Scholar] [CrossRef]
- Coman, L.; Păunescu, H.; Coman, O.A.; Chiuțu, L.C. Experimental Research on Possible Interactions of High Doses of Metamizole Sodium with the Endogenous Cannabinoid or Dopaminergic Systems. Farmacia 2014, 62, 79–92. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).