Abstract
Mitochondria serve as an essential component in the maintenance of cardiac function, and targeting them may represent a promising approach to handling heart failure (HF). HF in this review refers to various etiologies, including ischemic cardiomyopathy, dilated cardiomyopathy, and hypertrophic cardiomyopathy, unless otherwise specified. Mitochondrial dysfunction, a distinctive feature of HF, leads to a progressive decrease in bioenergetic reserves due to switching of energy production from oxidation of fatty acids in mitochondria to glycolytic pathways. The main problem in developing methods to improve mitochondrial function lies in the fact that protein preparations injected through the bloodstream cannot enter cells through the plasma membrane. Modern gene therapy involving the delivery of missing genes to cells using adeno-associated virus (AAV) vectors has the potential to improve the function of cardiomyocytes (CMCs). This type of therapy aims to target proteins that have been lost, damaged, or altered due to pathological conditions in the myocardium. This review summarizes pathophysiological mechanisms associated with mitochondrial dysfunction, which is mainly caused by increased oxidative stress and impaired mitochondrial biodynamics under HF progression. It also addresses possible ways to modulate these processes using gene therapy. Special attention is paid to modern characteristics of AAVs that can be used as vectors for the efficient delivery of desired genes to CMCs.
1. Introduction
Targeting mitochondrial biodynamics is a promising approach for preventing and correcting heart failure (HF) progression. Currently, most medications focus on treating the symptoms of this pathological condition rather than the underlying cause of heart remodeling. Mitochondria play a crucial role in maintaining cardiac function, and their targeted intervention could be a more effective strategy for treating HF [1]. Most of the ATP used by the heart (approximately 95%) is produced through oxidative metabolism in mitochondria, utilizing free fatty acids as the primary substrate for oxidation [2]. Mitochondrial function is maintained by several closely coordinated mechanisms, which are collectively referred to as the mitochondrial quality control system. This system includes the following processes: fusion and fission, degradation, and biogenesis [3]. Mitochondrial dysfunction, a hallmark of HF, results in a progressive reduction in bioenergetic reserves due to the shift in energy production from mitochondrial fatty acid oxidation to glycolytic pathways. This process leads to a decrease in fatty acid oxidation [4]. Enhanced glucose uptake and metabolism also serve as growth signals that contribute to pathological remodeling of the heart [5]. In the later stages of HF, ketone bodies can also serve as substrates for mitochondrial oxidation [6]. As a result of changes in the oxidative substrate, oxidative stress increases, inducing cardiomyocyte (CMC) apoptosis. This, in turn, causes dysregulation of calcium homeostasis and damage to proteins and lipids. Moreover, there is a leakage of mitochondrial DNA and inflammation, which ultimately stimulates various signaling pathways leading to cardiac remodeling and HF [6]. In addition, the neurohumoral dysregulation caused by angiotensin II, endothelin-1, and sympatho-adrenergic hyperactivation that occurs in HF stimulates the hypertrophy of ventricular CMCs and exacerbates cellular damage [7].
Mitochondrial DNA (mtDNA) is a key factor in the development of HF. In a study of the left ventricle in patients with end-stage HF, it was found that the mtDNA levels decrease by more than 40%. This reduction in mtDNA leads to a significant decrease in mitochondrial replication, which in turn worsens mitochondrial biogenesis [8]. Furthermore, the depletion of mtDNA is an early indicator of left ventricular hypertrophy during the progression from hypertrophy to insufficiency in individuals with HF [9]. This loss of mtDNA is preceded by oxidative stress, which causes a decrease in the expression of genes encoded by mtDNA and complex respiratory chain enzymes [10]. It is noteworthy that this oxidative stress only affects mtDNA, but not the nuclear genome [10], which potentially arises from the lack of protective histones in mtDNA, which, as a rule, has a less pronounced epigenetic modification [11].
HF is commonly understood as a complex clinical syndrome resulting from structural or functional impairment of ventricular filling or ejection of blood [12]. The term “HF” in this review covers multiple etiologies, including ischemic cardiomyopathy, dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM), and restrictive cardiomyopathy (RCM). The scope of this article primarily encompasses chronic HF with reduced ejection fraction (HFrEF)—a clinically defined HF phenotype characterized by reduced left ventricular systolic function [13,14], in which mitochondrial dysfunction is a well-established and central pathophysiological mechanism [15]. Where relevant, specific conditions are indicated in the text.
The challenge in improving the function of mitochondria within the heart lies in the fact that, when administered systemically via the bloodstream, drugs may be unable to penetrate through the plasma membrane of the cell. At the same time, intracellular protein deficiency, such as heat shock proteins, proteins and enzymes that mediate oxidative phosphorylation processes, triggers changes in CMC metabolism and the formation of “vicious pathological circles”. Modern gene therapy, based on the delivery of genes for missing molecules intracellularly through the adenovirus pathway (AAV-delivery therapy), could improve the functional state of CMCs by targeting proteins that are lost, destroyed, or change their function in response to myocardial alterations [16,17]. Repairing damaged mitochondria and removing dysfunctional ones are essential for maintaining homeostasis and preventing CMC death. Genetic forms of cardiomyopathy, such as mutations in DES, LMNA, or mitochondrial genes, can lead to mitochondrial dysfunction. These cases illustrate that mitochondrial defects are not only secondary to HF but may also result directly from pathogenic gene variants.
In this review, we will discuss the pathophysiological mechanisms associated with mitochondrial dysfunction, which mainly depend on increased oxidative stress and impaired mitochondrial biodynamics related to the progression of HF, and explore ways to modulate these processes using gene therapy.
2. Mitochondrial Biogenesis in HF
In HF, mitochondrial dysfunction contributes not only to energy depletion but also to disruption of ion homeostasis and an increase in ROS production. These inadequate responses exacerbate CMC stress, promote apoptosis, and accelerate pathological remodeling [4]. Oxidative stress is linked to morphological and functional abnormalities, which contribute to the development of cardiac hypertrophy and the progression of HF [18]. It should be noted that these mechanisms are involved in the pathogenesis of HF of different etiologies, including ischemic, hypertensive, and genetic cardiomyopathies and others. The general pattern of mitochondrial dysfunction appears to be consistent across all these conditions.
This decline in bioenergetic reserves occurs regardless of HF etiology, emphasizing that mitochondrial dysfunction is a convergent pathophysiological mechanism in various forms of the disease [2]. Neurohormonal abnormalities, systemic inflammation, and cellular stress contribute to mitochondrial dysfunction in HF, which is followed by an increase in the production of ROS and the release of nitric oxide synthase (NOS). Increased ROS also inhibits mitochondrial oxidative phosphorylation and can trigger mPTP opening, further reducing ATP production. These molecular processes also lead to changes in the cellular regulation of calcium exchange, contributing to cellular apoptosis, fibrosis, and hypertrophy of CMCs. These events ultimately lead to cardiac remodeling and the progression of HF [19,20].
This pathophysiological basis is shared across acquired and genetic forms of HF. In ischemic or hypertensive cardiomyopathy, neurohormonal activation and systemic inflammation suppress mitochondrial oxidative capacity. In inherited disorders, such as desminopathy (caused by DES mutations, OMIM #125660), Barth syndrome (BTHS, OMIM #302060) associated with a mutation in the tafazzin gene (TAZ, OMIM #300394), or LMNA-related cardiomyopathy (LMNA, OMIM #150330), structural or metabolic gene defects directly impair mitochondrial integrity, leading to mislocalization, respiratory chain deficiency, and oxidative stress [2]. It was also found that some pathogenic variants in the DES gene, encoding the structural intermediate filament protein desmin, were associated with mitochondrial defects in individuals suffering from familial left ventricular non-compaction cardiomyopathy (LVNC) [21]. Thus, regardless of origin, HF is mediated by mitochondrial failure as one of the common pathogenetic mechanisms.
HF is characterized by a shift in energy production from the oxidation of fatty acids within mitochondria and towards glycolytic pathways, in order to maintain adequate levels of ATP [22,23]. This metabolic shift reflects a decline in mitochondrial oxidative capacity rather than an efficient substrate adaptation. Importantly, increased reliance on glycolysis does not compensate for impaired fatty acid oxidation, as pyruvate is often inefficiently oxidized due to inhibition of pyruvate dehydrogenase and reduces Krebs cycle activity in failing CMCs. Although glycolysis produces pyruvate, which can be oxidized in mitochondria, the ATP output from glycolysis is insufficient to meet the high-energy demands of CMCs, accounting for less than 5% of total ATP, which explains why this metabolic switch does not prevent energy depletion [24]. In contrast, ketone bodies can be efficiently oxidized by mitochondria in HF, as their utilization bypasses pyruvate dehydrogenase and provides a more oxygen-efficient source of acetyl-CoA, thereby partially preserving mitochondrial ATP production under metabolic stress. Therefore, the sanogenetic mechanisms employed to restore energy balance contribute to the development of pathological pathways resulting in cardiac remodeling [25]. Increased glucose metabolism also suppresses catabolism of branched-chain amino acids (BCAAs), activating mTOR signaling and promoting hypertrophy [26]. Moreover, the inability to oxidize fatty acids can lead to the accumulation of lipotoxic metabolites [27]. In this context, various studies have demonstrated dysregulation of several molecular pathways involved in fatty acid metabolism. Data obtained from both animal and human studies suggest that the levels of peroxisome proliferator-activated receptor α (PPARα), a transcription factor that regulates the transport of fatty acids into mitochondria and peroxisomes, and the coactivator peroxisome-proliferator γ coactivator 1 α (PGC-1α), decrease with HF [28]. Additionally, elevated malonyl-CoA inhibits carnitine palmitoyltransferase 1 (CPT1), limiting fatty acid entry into mitochondria and contributing to energy deficiency [29]. These observations are consistent with findings from both animal models and human tissue studies, emphasizing the translational relevance of PPARα and PGC-1α as potential therapeutic targets. Preclinical studies demonstrate that adeno-associated virus (AAV)-mediated overexpression of CPT1B (OMIM, #601987) in CMCs restores the activity of fatty acid oxidation (FAO), attenuates hypertrophy, and reduces mitochondrial ROS [29,30]. Similarly, strategies to enhance sirtuin activity—either via direct AAV delivery or indirect NAD+ augmentation (e.g., via NMNAT or NAMPT)—could reactivate PGC-1α and improve mitochondrial quality control, though this approach remains underinvestigated in cardiac models. Notably, AAV-driven CPT1B expression bypasses malonyl-CoA inhibition, offering a gene-centric solution to lipotoxicity [29].
Increased acetylation of mitochondrial proteins involved in BCAA oxidation, pyruvate and succinate dehydrogenase activity, the malate–aspartate shuttle, the Krebs cycle, and fatty acid oxidation has been observed in HF models. This post-translational modification functionally impairs multiple oxidative pathways, thereby limiting the ability of mitochondria to oxidize both glucose- and lipid-derived substrates. This also contributes to increased susceptibility to the opening of the mitochondrial permeability transition pore (mPTP) [31,32]. Protein acetylation may be the result of an excessive concentration of short-chain acyl-CoA, which can be caused by a decrease in fatty acid oxidation or a decrease in the activity of sirtuin-dependent deacetylation of proteins [33]. Figure 1 schematically summarizes the interplay between metabolic remodeling, mitochondrial protein acetylation, redox imbalance, and mitochondrial dynamics in HF. Indeed, sirtuin activity is dependent on the level of NAD+, which is reduced in the presence of mitochondrial dysfunction and CMC hypertrophy. Reduced NAD+ availability not only diminishes sirtuin-dependent deacetylation but also disrupts mitochondrial redox balance, promoting oxidative stress. Under these conditions, hyperacetylation of mitochondrial proteins and increased ROS production can sensitize mitochondria to mPTP opening, despite the inhibitory effects of matrix NADH under physiological conditions [34]. Various studies have consistently demonstrated the protective effects of sirtuins on various biological processes. Specifically, sirtuins are known to activate the pAMPKa signaling pathway, increase levels of Bcl-2 protein, inhibit nuclear factor κB (NF-κB), reduce levels of phosphoprotein B kinase, regulate energy metabolism via acetylation, suppress expression of Wnt3a, and decrease levels of caspase-3 mRNA and poly(ADP-ribose) polymerase 1 (PARP-1) protein. Sirtuins protect mitochondria by deacetylating key enzymes, enhancing energy metabolism, stabilizing mPTP, and reducing oxidative stress, thus mitigating CMC dysfunction and HF progression [35].
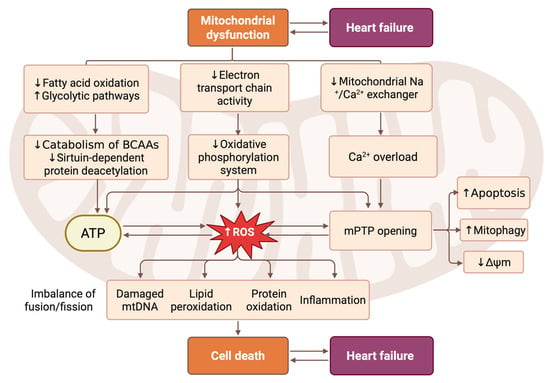
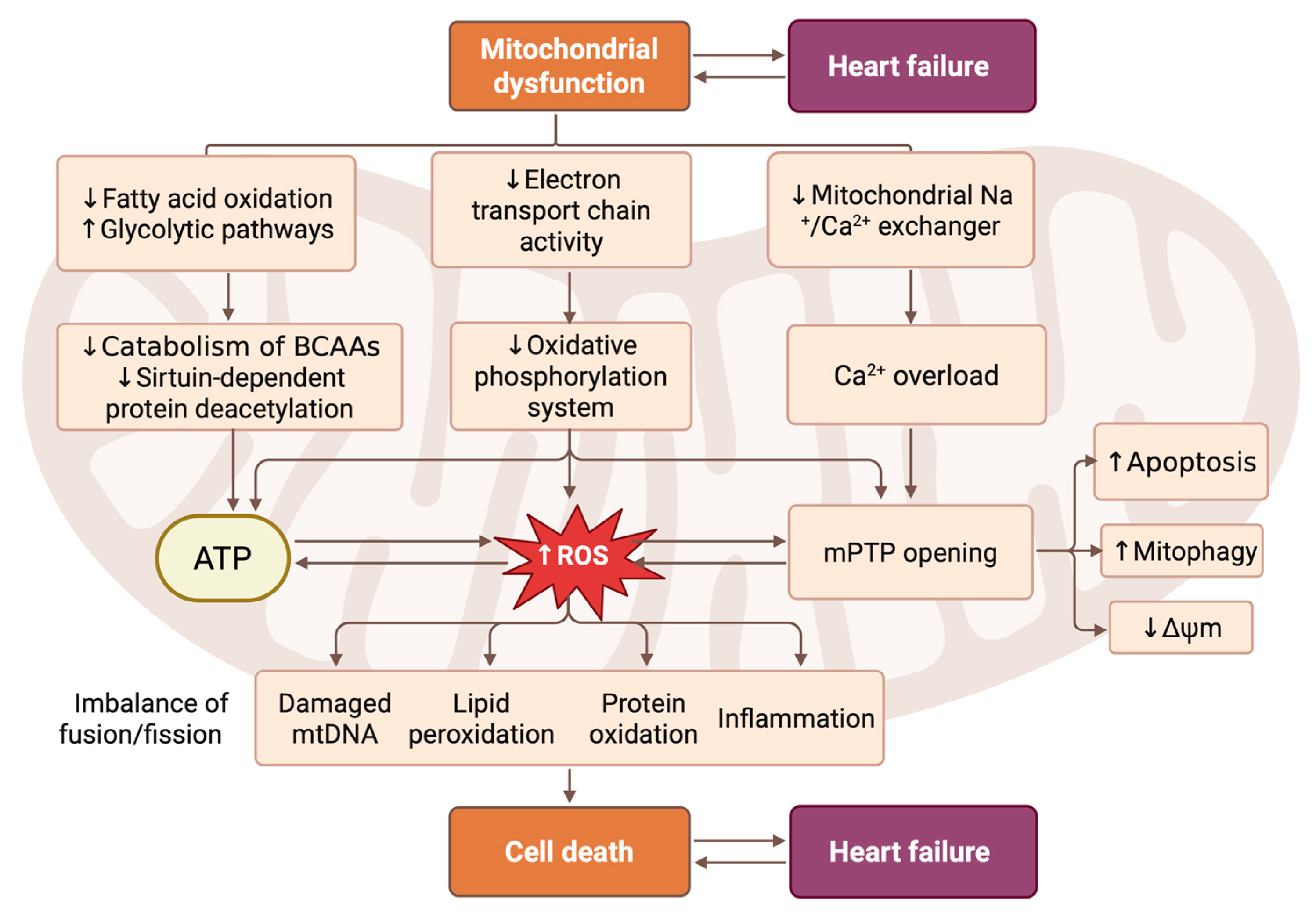
Figure 1.
Pathophysiological cascade linking mitochondrial dysfunction to CMC death and HF progression. This scheme illustrates the central role of mitochondrial dysfunction in driving the pathogenesis of HF through interconnected metabolic, bioenergetic, and signaling pathways. Mitochondrial impairment is initiated with reduced fatty acid oxidation and increased dependence on glycolytic metabolism, accompanied by diminished electron transport chain activity and oxidative phosphorylation. This leads to decreased ATP production, elevation of reactive oxygen species (ROS) levels, and calcium overload due to impaired mitochondrial Na+/Ca2+ exchange. The resulting oxidative stress triggers lipid peroxidation, protein oxidation, mtDNA damage, and inflammation, while also promoting mitochondrial permeability transition pore (mPTP) opening. These events activate subsequent processes, including apoptosis, mitophagy, and loss of mitochondrial membrane potential (Δψm). Concurrently, imbalanced mitochondrial fusion/fission dynamics further exacerbate their dysfunction. Together, these mechanisms converge on CMC death, which directly contributes to cardiac remodeling and the clinical manifestation of HF. Created in BioRender. Abakumova, T. (2026) https://BioRender.com/l7th57b.
Mitochondrial dynamics refers to the continuous process of mitochondrial fission and fusion. This process plays a crucial role in regulating mitochondrial metabolism and maintaining the intracellular environment [36,37]. Mitochondrial function is regulated by two distinct mechanisms: division and fusion. Division, facilitated by proteins such as dynamin-related protein 1 (DRP1), fission 1 (FIS1), mitochondrial fission factor (MFF), and mitochondrial division proteins 49 and 51 (MiD49/51), leads to mitochondrial fragmentation, which is essential for cell division and the removal of damaged mitochondria. Fusion, organized by mitofusins 1 and 2 (MFN1/2) and optic atrophy protein 1 (OPA1), results in the formation of interconnected networks that facilitate the maintenance of mitochondrial DNA integrity through complementation and selective removal of damaged mitochondrial DNA and energy distribution. While cleavage proteins primarily contribute to morphological changes, fusion proteins play various roles in mitophagy, apoptosis, and energy metabolism [38] (Figure 1).
Recent studies suggest that modulating MFN2/OPA1 balance could restore mitochondrial connectivity and improve ATP supply in HF. This provides a basis for gene therapy interventions targeting these fusion/fission proteins. In particular, these pathological processes disrupt intracellular energy metabolism through changes in substrate utilization, leading to substrate hypoxia and further compromising mitochondrial function. This hypoxia is exacerbated by the accumulation of ROS, which primarily arises as a consequence of impaired electron transport and inefficient oxidative phosphorylation, further leading to energy depletion and inhibition of mitochondrial ATP synthesis. The accumulation of ROS also triggers processes of mitophagy, which can lead to an imbalance in mitochondrial fusion and fission. This imbalance can activate programmed cell death processes and further exacerbate the severity of HF.
Remarkably, AAV-based interventions can reconfigure this system. In a murine aortic stenosis model, AAV9-mediated overexpression of ADRB3 (OMIM #109691) restored fission/fusion equilibrium, reduced oxidative stress, and halted HF progression—without directly targeting mitochondrial machinery, but via upstream signaling modulation [39].
Altered substrate utilization in HF leads to substrate hypoxia, further impairing mitochondrial function. Accumulated ROS exacerbates energy depletion, triggers mitophagy, and may disrupt the balance between fission and fusion, promoting cell death and aggravating HF.
Mitochondria in adult CMCs can be classified into three main categories based on their location and function. The majority of mitochondria, known as interfibrillar mitochondria (IFM), are situated adjacent to myofibrils and play a crucial role in calcium signaling and providing energy for contraction. A smaller subset, sarcoplasmic submembrane mitochondria (SSM), is positioned near the membrane of subcoronary arteries and serves to supply energy for ion channel activity and signal transmission within cells. Finally, perinuclear mitochondria (PNM) are located close to the nucleus and contribute to energy requirements for transcriptional processes [40,41,42]. IFM supplies energy for contraction and calcium signaling, SSM supports ion channels and signal transduction, and PNM supports transcriptional processes. Recent research has demonstrated that, despite their distinctive structure, mitochondria are able to alter their shape in adult CMCs [43,44,45,46]. Although various observation techniques allow us to determine the dynamics of mitochondria within CMCs, the findings obtained differ from the fundamental dynamics of these organelles derived from cultured cells.
Additional research on heart tissue is therefore required in order to investigate the dynamics of mitochondrial activity in cardiac and mature CMCs. This will involve assessing the morphological condition of the myocardium, considering the characteristics of the tissue rather than individual CMCs.
Mitochondrial health in cardiac muscle cells is maintained not only through mitochondrial dynamics, but also through mitophagy (the selective isolation of damaged mitochondria through autophagy). Drp1 (dynamin-related protein 1) plays a crucial role in inducing mitophagy. Mitochondrial division mediated by Drp1 is essential for the initiation of mitophagy within the heart [47,48]. The Drp1 C452F mutation in mice results in increased activity of the Drp1 GTPase, as well as resistance to oligomer degradation. This, in turn, leads to impaired mitophagy, mitochondrial depolarization, abnormal calcium handling, reduced ATP production, and activation of a sterile myocardial inflammatory response [49]. In mice with HF caused by transverse aortic constriction (TAC), mitophagy associated with Drp1 is initially activated and then suppressed in the heart in response to pressure overload [50]. In a rat model of HF, CMCs demonstrate increased production of ROS from the mitochondria. It is worth noting that the levels of expression of Mfn2 and Drp1 have been reduced by approximately 50%. This reduction in regulation leads to an accumulation of Parkin in the mitochondria and the subsequent induction of mitophagy. The observed regulation of mitochondrial and mitophagy dynamics mediated by Mfn2 and Drp1 appears to play a crucial role in cardiac protection, possibly through the modulation of ketone body metabolism [51]. A patient with HF with reduced ejection fraction (HFrEF) has elevated levels of Drp1. In terms of clinical relevance, Mfn2 levels in samples from patients with HFrEF remain stable [52]. Therefore, therapeutic strategies aimed at restoring mitochondrial metabolic flexibility, redox balance, and quality control may represent a rational approach to improving cardiac energetics and attenuating the progression of HF [53].
4. Modern Characteristics of AAV Vectors for Cardiomyocytes
Adeno-associated viruses (AAVs) represent an effective delivery tool in clinical gene therapy. AAV is a non-enveloped, single-stranded DNA virus belonging to the Dependovirus genus of the Parvoviridae family [79]. The wild-type AAV serotype genome was first successfully cloned in the 1980s, establishing the foundational template for subsequent recombinant AAV (rAAV) vectors [80,81]. The widespread use of rAAVs is attributed to a number of factors. Firstly, the ability of AAVs to infect both dividing and non-dividing cells makes them particularly suitable for targeting post-mitotic cells such as neurons and CMCs [79]. Secondly, AAVs exhibit favorable safety characteristics, including non-pathogenicity, a low frequency of host genome integration, and the capacity for long-term episomal transgene persistence in non-dividing cells. These characteristics are coupled with the inherently high transduction efficiency of certain serotypes [82]. Furthermore, different AAV serotypes exhibit varied cellular and tissue tropism, a trait that is predominantly determined by genomic variations located within the variable regions of the viral capsid, particularly the VP3 domain [83]. For cardiac gene transfer, AAV1, AAV6, and AAV9 have emerged as the most efficacious serotypes, demonstrating successful therapeutic gene delivery to the myocardium in both rodent and large animal models.
It is important to note that while experimental studies on animal models demonstrate efficient myocardial transduction, the results can still vary depending on the species, age, and stage of HF. Careful consideration of these factors is necessary when translating these findings to clinical settings.
AAV1 is one of the most studied and the first viral vector that was approved for the use in gene therapy [84]. This serotype demonstrates one of the broadest tropism profiles among all AAV serotypes, which has led to its widespread use in preclinical and clinical research. AAV1 has been identified as the most efficient serotype for gene delivery to skeletal muscle cells in various animal models [85,86,87], yet it has also demonstrated efficient cardiac transduction and outperforms AAV2 in large-animal myocardium, particularly in case of intramyocardial delivery [88], endothelial and vascular smooth muscle cells [89], and neurons [90].
Another popular serotype for gene delivery is AAV6. This serotype is a natural hybrid resulting from recombination between AAV1 and AAV2 [91], which possesses broadened tropism and enhanced efficiency compared to its parent strains due to its hybrid receptor profile [92]. Owing to the characteristics, AAV6 demonstrates exceptional efficacy in transducing a wide range of tissues including CMCs in various animal models—from mice to sheep [93,94,95]. Moreover, in mice AAV6 demonstrated significant heart expression reaching the peak level with luciferase enzyme activity (7.9 × 105 ± 3.5 × 105 TF (total flux (photons/sec/cm2/sr)) on day 21 after indirect intracoronary injection which was the highest value among AAV1-9 serotypes [93]. Using immunohistochemical assay, it was also shown that up to 100% of CMCs in the LV anterior wall were transduced after recirculating delivery of scAAV6 [95].
Discovered in 2004, the AAV9 serotype has emerged as a leading vector for systemic gene delivery, distinguished primarily by its exceptional tissue tropism [91,96,97]. It demonstrates superior transduction efficiency in critical tissues such as cardiac and skeletal muscle, liver, and pancreas [87]. In mice, systemic delivery of rAAV9 achieves transduction of over 80% of CMCs at a dose of 1.0 × 1011 vg/mouse [98]. Broad targeting capability of this serotype is mediated by its unique receptor profile: it utilizes terminal N-linked galactose as a primary receptor and is hypothesized to interact with a putative integrin, employing the laminin receptor (LR) as a coreceptor. A groundbreaking characteristic of AAV9 is its ability to cross the blood–brain barrier, enabling efficient gene delivery to central nervous system cells, including neurons and astrocytes [99,100]. This capability has established AAV9 as a prime candidate for treating neurodegenerative disorders including spinal muscular atrophy and Parkinson’s disease. Another capacity of AAV9 to cross the vascular endothelium is a key factor in its enhanced cardiac targeting [101]. An additional significant advantage of AAV9 is its low immunogenicity. The prevalence of pre-existing antibodies against AAV9 in the human population is considerably lower than for other serotypes, thereby expanding the potential patient population eligible for gene therapy [102].
While the low immunogenicity of AAV9 is an advantage of this serotype, pre-existing neutralizing antibodies (nAbs) can still limit efficacy. The presentation of AAV antigens by antigen-presenting cells (APCs), primarily dendritic cells, to CD4+ T-helper cells serves as a key signal for initiating the B-cell response, which includes proliferation, affinity maturation, isotype switching, and ultimately, the production of antibodies against the viral capsid [103]. The prevalence of pre-existing anti-AAV nAbs in the human population varies from 2% to 60% depending on the serotype, with antibodies against AAV1 and AAV2 being the most common [104]. The recognition of the AAV capsid or the introduced transgene by the innate immune system activates signaling pathways, such as NF-ĸB, leading to the upregulation of major histocompatibility complex (MHC) genes and the secretion of pro-inflammatory cytokines or type I and III interferons [105]. The released mediators consequently activate genes that suppress viral replication and stimulate the development of the adaptive immune response. Another crucial mechanism is TLR-mediated signaling, primarily via TLR9, which contributes to immune responses against both the transgene and the capsid [106]. TLR9 signaling in APCs leads to antigen presentation on MHC class I and the activation of an AAV-specific cytotoxic CD8+ T-cell response [107,108]. Effector capsid-specific CD8+ T cells migrate to target tissues, where they can induce cell death through the secretion of perforin and granzymes, leading to the loss of the therapeutic transgene and inflammatory tissue damage [108,109]. However, a significant cytotoxic CD8+ T-cell response to the AAV capsid is predominantly observed in the liver [110], in contrast, muscle tissue demonstrates a notable resistance to the elimination of transgene-expressing cells [98,111]. Furthermore, AAV capsids can directly activate the alternative complement pathway, leading to the generation of anaphylatoxins C3a and C5a, opsonization of particles by C3b, and potentially, the formation of the membrane attack complex [112]. Several strategies to mitigate immune responses to AAV vectors, including methods to induce immune tolerance, are currently under investigation. One approach involves transient immunosuppression, which has been explored in multiple studies [113,114]. Another strategy focuses on the use of engineered capsids or capsids with low seroprevalence, aiming to reduce pre-existing immunity [115,116]. Additionally, optimizing the vector dose has been proposed as a way to minimize immune activation while maintaining therapeutic efficacy [117]. Finally, several approaches aim to induce immune tolerance to either the transgene or the capsid itself. They include mechanisms mediated by regulatory T cells, which have shown promising results in a number of studies [118,119].
To improve the efficiency of CMC transduction and advance the clinical application of viral therapies, ongoing research is focused on identifying and developing novel serotypes. These strategies involve identifying new vectors or altering the capsid proteins of existing AAVs to achieve a combination of cardiospecificity and cardiotropism. An example of viral engineering is the development of the chimeric vector AAV2i8, achieved by substituting a hexapeptide in the receptor domain of AAV2 with a sequence derived from AAV8 [120]. The resulting chimeric vector inherited ability of AAV8 for efficient transduction of muscle tissue, including cardiac tissue, while demonstrating significantly reduced accumulation in the liver after systemic administration. Libraries screening of capsids and subsequent directed evolution also is frequently employed to select variants that transduce CMCs most effectively. For example, MyoAAV 1A/2A has been identified as demonstrating record-breaking efficiency in delivering genes to muscles [121]. Furthermore, the process of random mutagenesis of surface-exposed regions of the AAV9 capsid protein not only enabled the creation of new variants of AAV9.45 and AAV9.61 with selectively reduced tropism for the liver, but also identified key sites responsible for liver tropism [122] (Figure 2).
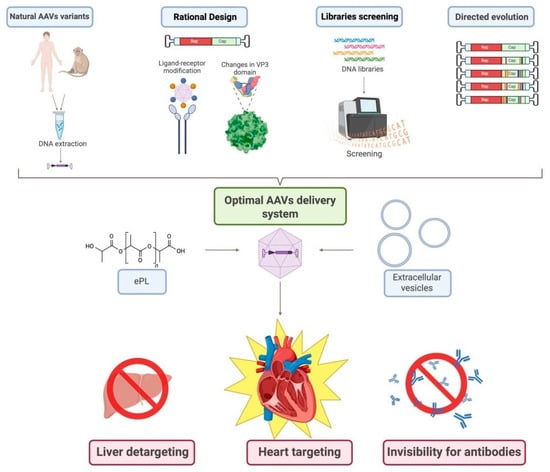
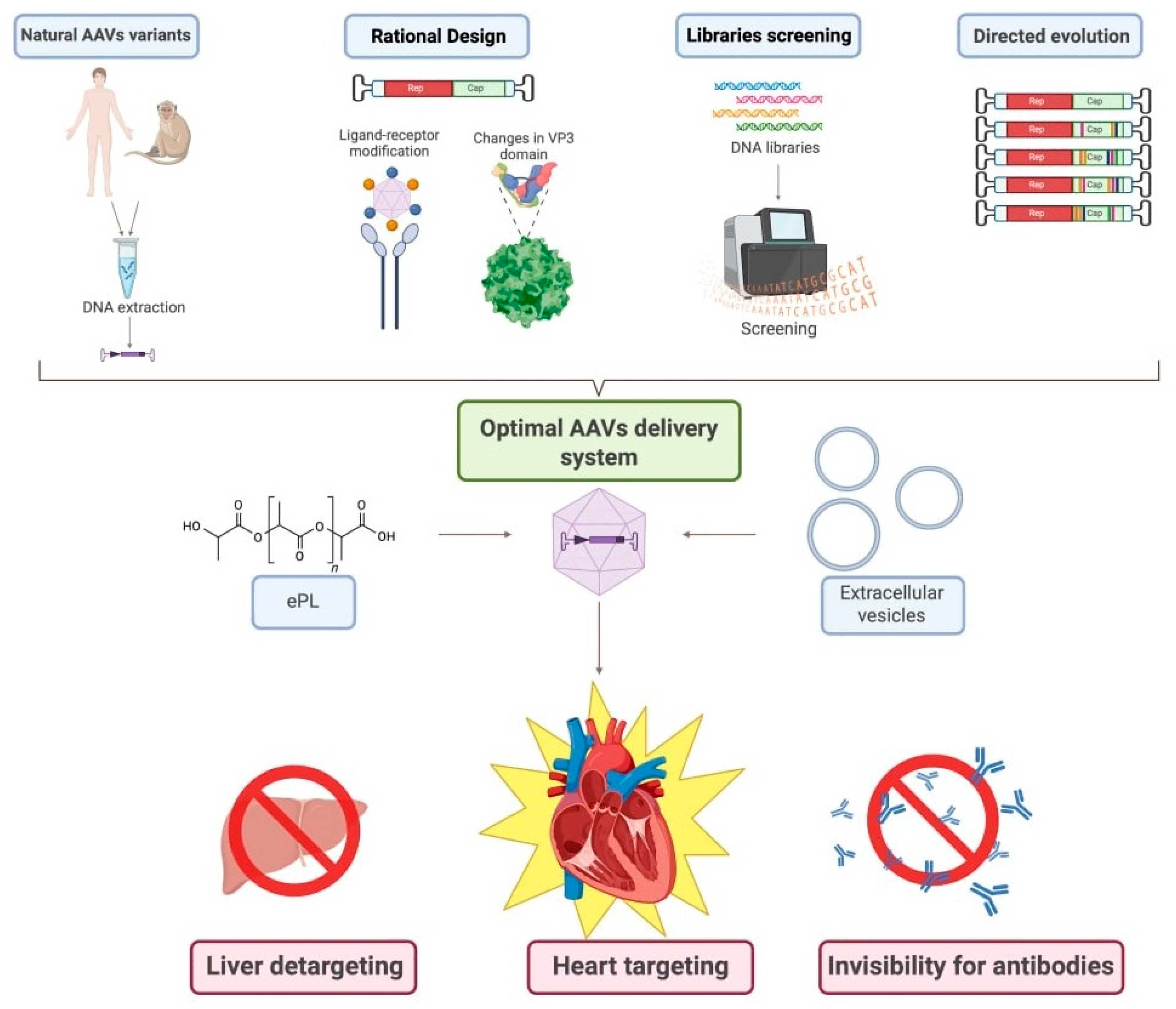
Figure 2.
Algorithm for developing the most effective pathway for CMC transduction with AAVs. This scheme illustrates the multi-faceted approach to engineering next-generation AAV vectors with enhanced CMC specificity and reduced off-target effects, outlining four primary strategies: (1) isolation and use of natural AAV variants from human or non-human primate tissues followed by DNA extraction and cloning; (2) rational design involving structure-guided capsid modification, including ligand-receptor engineering and targeted mutagenesis of the VP3 domain; (3) high-throughput screening of diverse capsid DNA libraries using platforms such as flow cytometry or next-generation sequencing to identify clones with desired tropism and transduction profiles; and (4) directed evolution through iterative rounds of mutagenesis and selection to generate novel capsids with improved characteristics. The resulting optimized AAVs can be further refined into an advanced delivery system via encapsulation in extracellular vesicles (EVs) or complexation with polymers like ethylenediamine-poly-L-lysine (ePL) to enhance stability, cellular uptake, and immune evasion. The ultimate goal of this integrated pipeline is to achieve simultaneous liver detargeting (minimizing hepatic transduction and associated toxicity), heart targeting (maximizing transduction efficiency in CMCs), and invisibility to pre-existing neutralizing antibodies—thereby improving safety, efficacy, and clinical translatability of mitochondrial-targeted gene therapy for HF. Created in BioRender. Abakumova, T. (2026) https://BioRender.com/yogxpfu.
These engineering approaches directly address concerns regarding off-target transduction and vector loss in peripheral tissues. Optimizing capsid design is critical for maximizing myocardial specificity while minimizing systemic exposure.
Efficacy of myocardial transduction by various AAV serotypes demonstrates significant dependence on delivery methods and dosage. In general, AAVs delivery during the gene therapy can be performed via two ways: surgical and percutaneous techniques [82]. The main surgical technique involves the injection of medication into the heart muscle using thin needles [123] achieving local transduction rates up to 60% of CMCs in dog models after scAAV6 percutaneous transendocardial delivery [124]. This technique offers distinct advantages, including high local vector concentration, circumvention of the endothelial barrier, and minimized off-target organ exposure. However, its applicability is limited by the restricted diffusion radius from the injection site, making it unsuitable for treating diffuse cardiac pathologies, for example, HF [124].
To increase efficiency of transduction, a “gene painting” technique was developed. The method employs direct epicardial application of AAV vectors combined with poloxamer gel to prolong viral contact time and mild trypsinization to enhance viral penetration [125]. This methodology enables targeted gene delivery to atrial tissue with high efficiency [126]. However, both this approach and intramyocardial injection constitute invasive procedures that may precipitate various associated adverse effects, including inflammatory responses and procedural complications.
In addition, researchers are using molecular cardiac surgery with recirculating delivery (MCARD) [127]. This approach is an invasive but extremely effective way of delivering genetic constructs, where the animal is connected to an artificial circulatory system [128]. It enables the cardiac circulation to be completely isolated from the systemic circulation, ensuring that blood containing the vector recirculates exclusively through the coronary system. The primary benefits of MCARD include enhanced myocardial contact time through prolonged intracoronary vector circulation, protection from pre-existing antibody neutralization via the closed-circuit system, and minimized risk of off-target transduction. These findings support the concept that delivery method critically influences therapeutic outcomes. Less invasive but equally efficient delivery techniques remain a major area of active research.
An alternative strategy is transvascular delivery, which involves vector administration into the vascular compartment, and subsequent migration to CMCs. Systemic intravenous administration is the simplest method but the least effective, as it is characterized by massive vector loss in peripheral tissues [129]. Rodents can be effectively infected with AAVs through intravenous administration, but this is much more difficult to achieve in larger animals, and in humans [130].
Antegrade intracoronary injection is the least invasive method of delivering genetic constructs to the myocardium and is widely used in preclinical and clinical studies of HF [131,132,133]. This strategy facilitates the homogeneous distribution of the virus throughout the myocardial tissue; nevertheless, its CMC transduction efficiency remains significantly restricted [130,134]. An alternative strategy to enhance transduction efficiency is retrograde infusion, which involves injection of the viral vector directly into the coronary vein [135]. The contact of the vector with the coronary endothelium is prolonged, which enhances its passage into the tissue, and the increased intravascular pressure increases the proportion of transduced CMCs [136].
For comparison, in rodent models, AAV1 and AAV6 demonstrate optimal efficiency with intramyocardial or intracoronary injection, whereas AAV9 is most suitable for transvascular delivery. The proportion of transduced CMCs is also dose-dependent. At high viral loads, all cardiotropic serotypes exhibit comparable transduction efficacy; however, at medium doses, only AAV9 maintains high myocardial transduction activity [137]. A different trend can be seen in large animal models. Intracoronary administration demonstrates superior efficacy for AAV6 [138]. Systemic intravenous delivery is more complex: while AAV9 achieves substantial skeletal muscle transduction but limited myocardial expression, AAV8 shows robust cardiac transduction [139]. Notably, AAV9 demonstrates superior delivery efficiency in sheep models using MCARD [140]. Therefore, optimal serotype choice in large animals is context-dependent, dictated by the delivery method, dosage, and the specific animal model. To summarize available data, Table 2 provides an overview of cardiac transduction efficiency of AAV serotypes depending on the animal model, administration method, and vector dose.

Table 2.
Comparative cardiac transduction efficiency of various AAV serotypes across animal models, delivery methods and dosing regimens.
Overall, these data emphasize that both vector selection and delivery route are critical determinants of therapeutic success. Interspecific variability highlights the need for thorough preclinical testing before switching to HF therapy in humans.
5. Conclusions and Perspectives
Despite significant progress in the development of gene therapy approaches for the treatment of HF, which are aimed at correcting mitochondrial dysfunction, there are still a number of limitations and challenges that require attention in future research.
Firstly, there are some limitations on the tropism and efficiency of delivery of AAV particles to target cells and tissues in the body. For example, serotypes AAV6 and AAV9 with a high degree of tropism for CMCs have varying degrees of transduction efficiency depending on the method of delivery, the stage of HF development, concomitant pathologies, the type of experimental animal, or individual characteristics of the organism. From a clinical point of view, intravenous administration of the vector is preferable due to the reduced risk of adverse effects, but there is a significant loss of the vector in peripheral tissues, such as the liver, which leads to a decrease in AAV delivery to the myocardium. At the same time, more effective invasive methods of delivering viral vectors to the myocardium, such as MCARD, are not suitable for widespread use in clinical practice. Secondly, even when using AAV9 with low immunogenicity, the injected viral particles may be neutralized by circulating pre-existing antibodies to AAV capsids in the majority of the human population. In addition, successful transduction of CMCs may lead to the development of cellular immunity directed against transgenic CMCs. Thirdly, the effectiveness of transduction may decrease depending on the severity of HF in patients. For example, in conditions of pre-existing extensive cardiosclerosis, severe ischemic cardiomyopathy, hypertrophy or myocardial remodeling, which alter coronary blood flow and tissue structure, contributing to a reduction in successfully transduced functional CMCs. As demonstrated in the treatment of Ndufs6 deficiency, therapeutic intervention was effective only in neonatal HF but had no effect on HF in adult subjects.
Despite the convincing effectiveness of preclinical models, the clinical translation of cardiac gene therapy has faced significant difficulties, especially in the CUPID 2 trial of AAV1-mediated ATP2A2 (SERCA2a, OMIM #108740) delivery for advanced HF. While the earlier CUPID 1 study suggested potential benefits with respect to symptoms and cardiac function, the randomized, double-blind, placebo-controlled CUPID 2 trial did not demonstrate any improvement in primary or secondary endpoints [73]. Subsequent studies have shown that this incongruity between the results obtained on animal models and in humans is due to a variety of interrelated factors, including low efficiency of myocardial transduction resulting from poor vector penetration through the coronary endothelium and limited diffusion into fibrous tissue; the high prevalence of pre-existing neutralizing antibodies against AAV1 that could block cell uptake [141]; intervention at a late stage of the disease, when extensive myocardial remodeling and cardiomyocyte loss left insufficient viable tissues for functional recovery [142]. These findings suggest the following provisional conclusion: even a biologically validated target (e.g., SERCA2a) cannot succeed without optimized vector design, precise delivery, appropriate timing, and rigorous patient selection.
This translation gap also explains why other promising mitochondrial targets, such as CPT1B and CAV3, remain the subject of preclinical studies, despite reliable cardioprotection in rodent and large animal models [74,78]. Firstly, neither CPT1B (OMIM #601987) nor CAV3 (OMIM #601253) are established monogenic causes of common HF, as they lack human genetic verification [142]. Secondly, their mechanisms include metabolic restructuring, which carries risks depending on the context: for example, fatty acid oxidation caused by CPT1B can improve energy supply in pressure-overload HF, but at the same time may increase oxygen demand and ROS production during ischemia [143]. Thirdly, achieving therapeutic effects probably requires a high level of homogeneous expression in the myocardium, which requires higher doses of the vector than ion-controlling proteins such as SERCA2a, increasing the risk of hepatotoxicity and immune activation. Finally, after the CUPID 2 setback, this field has strategically shifted towards next-generation AAV capsids (e.g., MyoAAV), immune modulation protocols and targets with human genetics (e.g., S100A1) [144], which has temporarily redirected translational efforts away from complex metabolic interventions. Nevertheless, with advances in precise phenotyping, cardiotropic vectors, and early intervention strategies, these mitochondrial pathways may still find their translation into clinical practice for carefully defined endotypes of HF.
The complexity of HF treatment can also be explained by the peculiarities of mitochondrial functioning—mitochondrial biodynamics, their division and fusion, and mitophagy are complex molecular processes. Therefore, gene therapy targeting one of these components (e.g., overexpression of Drp1 or Mfn) may only exacerbate mitochondrial dysfunction in the context of chronic heart disease.
Based on the identified problems of therapy for HF associated with mitochondrial dysfunction, several future directions can be proposed to address these gaps and limitations. A promising direction is the engineering of AAV capsids to achieve maximum tropism for human CMCs and minimal tropism for the liver; the use of AAV encapsulation in extracellular vesicles may contribute to a reduction in the development of an immune response and an increase in cell specificity during transduction.
Another promising area is the search for and validation of new genes (in addition to the known SERCA2a, S100A1, ADRB3, CPT1B) that have defects resulting in mitochondrial dysfunction: oxidative stress, biogenesis, ketone body metabolism, and calcium hemostasis. Genes that promote the transition of CMC metabolism from glycolysis to fatty acid oxidation are of particular interest in this context.
From the point of view of practical medicine, the most important strategy is to determine the stage of HF development at which gene therapy will be most effective: future research should focus on studying the use of gene therapy drugs at different stages of HF, from the initial stages of congestion in the small and large circulatory systems to the final dystrophic phase. Different cardiac diseases leading to HF, such as ischemic, diabetic and hypertensive cardiomyopathies, should also be taken into account.
In the future, gene therapy aimed at correcting mitochondrial dysfunction should be tested and refined in combination with pharmacological drugs already in clinical use (beta-blockers, angiotensin-converting enzyme II inhibitors, diuretics, cardiac glycosides) or in combination with cell therapy. The study of the synergistic effects of all these groups of drugs and approaches is one of the most promising areas of future research for HF therapy.
Although gene therapy for mitochondrial dysfunction in HF opens up new prospects, its path to clinical application requires overcoming significant technical and biological limitations. Strategic research aimed at optimizing delivery, overcoming the immune response, searching for new targets, and conducting thorough preclinical studies on relevant models are important aspects of successfully realizing this potential.
Author Contributions
Conceptualization, writing—original draft preparation, M.B. and A.S.; writing—review and editing, R.K., V.O., S.A. and A.M.; editing, S.S. and V.B.; visualization, A.S., V.O. and S.A.; project administration, M.B.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation (RSF), grant number 23-75-10111.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AAV | adeno-associated virus |
| APCs | antigen-presenting cells |
| BCAAs | branched-chain amino acids |
| CAV3 | caveolin 3 |
| CMC | cardiomyocyte |
| CPT1 | carnitine palmitoyltransferase 1 |
| CUPID 1 | Calcium Up-Regulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (Phase 1/2) |
| CUPID 2 | Calcium Up-Regulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (Phase 2b) |
| FAO | fatty acid oxidation |
| HF | heart failure |
| HFpEF | heart failure with preserved ejection fraction |
| HFrEF | heart failure with reduced ejection fraction |
| IFM | interfibrillar mitochondria |
| LR | laminin receptor |
| LVNC | left ventricular non-compaction cardiomyopathy |
| MCARD | molecular cardiac surgery with recirculating delivery |
| MHC | major histocompatibility complex |
| mPTP | mitochondrial permeability transition pore |
| nAbs | neutralizing antibodies |
| NOS | nitric oxide synthase |
| PGC-1α | coactivator peroxisome-proliferator γ coactivator 1 α |
| PNM | perinuclear mitochondria |
| PPARα | peroxisome proliferator-activated receptor α (PPARα) |
| rAAV | recombinant AAV adeno-associated virus |
| SSM | sarcoplasmic submembrane mitochondria |
References
- Raimundo, N. Mitochondrial pathology: Stress signals from the energy factory. Trends Mol. Med. 2014, 20, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef]
- Titus, A.S.; Sung, E.A.; Zablocki, D.; Sadoshima, J. Mitophagy for cardioprotection. Basic Res. Cardiol. 2023, 118, 42. [Google Scholar] [CrossRef]
- Gallo, G.; Rubattu, S.; Volpe, M. Mitochondrial dysfunction in heart failure: From pathophysiological mechanisms to therapeutic opportunities. Int. J. Mol. Sci. 2024, 25, 2667. [Google Scholar] [CrossRef]
- Harris, M.P.; Zhang, Q.J.; Cochran, C.T.; Ponce, J.; Alexander, S.; Kronemberger, A.; Fuqua, J.D.; Zhang, Y.; Fattal, R.; Harper, T.; et al. Perinatal versus adult loss of ULK1 and ULK2 distinctly influences cardiac autophagy and function. Autophagy 2022, 18, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Ritterhoff, J.; Tian, R. Metabolism in cardiomyopathy: Every substrate matters. Cardiovasc. Res. 2017, 113, 411–421. [Google Scholar] [CrossRef]
- Hinton, A., Jr.; Claypool, S.M.; Neikirk, K.; Senoo, N.; Wanjalla, C.N.; Kirabo, A.; Williams, C.R. Mitochondrial structure and function in human heart failure. Circ. Res. 2024, 135, 372–396. [Google Scholar] [CrossRef]
- Liu, M.; Lv, J.; Pan, Z.; Wang, D.; Zhao, L.; Guo, X. Mitochondrial dysfunction in heart failure and its therapeutic implications. Front. Cardiovasc. Med. 2022, 9, 945142. [Google Scholar] [CrossRef]
- Elorza, A.A.; Soffia, J.P. mtDNA Heteroplasmy at the Core of Aging-Associated Heart Failure. An integrative view of OXPHOS and mitochondrial life cycle in cardiac mitochondrial physiology. Front. Cell Dev. Biol. 2021, 9, 625020. [Google Scholar] [CrossRef]
- Hong, Y.S.; Longchamps, R.J.; Zhao, D.; Castellani, C.A.; Loehr, L.R.; Chang, P.P.; Matsushita, K.; Grove, M.L.; Boerwinkle, E.; Arking, D.E.; et al. Mitochondrial DNA copy number and incident heart failure: The atherosclerosis risk in communities (ARIC) study. Circulation 2020, 141, 1823–1825. [Google Scholar] [CrossRef] [PubMed]
- Mongelli, A.; Mengozzi, A.; Geiger, M.; Gorica, E.; Mohammed, S.A.; Paneni, F.; Ruschitzka, F.; Costantino, S. Mitochondrial epigenetics in aging and cardiovascular diseases. Front. Cardiovasc. Med. 2023, 10, 1204483. [Google Scholar] [CrossRef]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032, Erratum in Circulation 2023, 147, e674. https://doi.org/10.1161/CIR.0000000000001142.. [Google Scholar] [CrossRef] [PubMed]
- Rosca, M.G.; Hoppel, C.L. Mitochondrial dysfunction in heart failure. Heart Fail. Rev. 2013, 18, 607–622. [Google Scholar] [CrossRef]
- Soh, J.E.C.; Shimizu, A.; Molla, M.R.; Zankov, D.P.; Nguyen, L.K.C.; Khan, M.R.; Tesega, W.W.; Chen, S.; Tojo, M.; Ito, Y.; et al. RhoA rescues cardiac senescence by regulating Parkin-mediated mitophagy. J. Biol. Chem. 2023, 299, 102993. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yu, D.; Li, J.; Si, L.; Zhu, D.; Li, B.; Gao, Y.; Sun, L.; Wang, X.; Wang, X. Asiatic acid protects against pressure overload-induced heart failure in mice by inhibiting mitochondria-dependent apoptosis. Free Radic. Biol. Med. 2023, 208, 545–554. [Google Scholar] [CrossRef]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur. J. Prev. Cardiol. 2020, 27, 494–510. [Google Scholar] [CrossRef]
- Kumar, A.A.; Kelly, D.P.; Chirinos, J.A. Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circulation 2019, 139, 1435–1450. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Z.; Zhang, W.; Liu, X. Mitochondrial dysfunction and mitochondrial therapies in heart failure. Pharmacol. Res. 2022, 175, 106038. [Google Scholar] [CrossRef]
- Kubánek, M.; Schimerová, T.; Piherová, L.; Brodehl, A.; Krebsová, A.; Ratnavadivel, S.; Stanasiuk, C.; Hansíková, H.; Zeman, J.; Paleček, T.; et al. Desminopathy: Novel desmin variants, a new cardiac phenotype, and further evidence for secondary mitochondrial dysfunction. J. Clin. Med. 2020, 9, 937. [Google Scholar] [CrossRef]
- Cannon, M.V.; Silljé, H.H.; Sijbesma, J.W.; Vreeswijk-Baudoin, I.; Ciapaite, J.; van der Sluis, B.; van Deursen, J.; Silva, G.J.; de Windt, L.J.; Gustafsson, J.Å.; et al. Cardiac LXRα protects against pathological cardiac hypertrophy and dysfunction by enhancing glucose uptake and utilization. EMBO Mol. Med. 2015, 7, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Hou, L. Branched chain amino acids metabolism in heart failure. Front. Nutr. 2023, 10, 1279066. [Google Scholar] [CrossRef]
- Meeran, M.F.N.; Azimullah, S.; Al Ahbabi, M.M.; Jha, N.K.; Lakshmanan, V.K.; Goyal, S.N.; Ojha, S. Nootkatone, a dietary fragrant bioactive compound, attenuates dyslipidemia and intramyocardial lipid accumulation and favorably alters lipid metabolism in a rat model of myocardial injury: An in vivo and in vitro study. Molecules 2020, 25, 5656. [Google Scholar] [CrossRef]
- Zhu, X.X.; Wang, X.; Jiao, S.Y.; Liu, Y.; Shi, L.; Xu, Q.; Wang, J.J.; Chen, Y.E.; Zhang, Q.; Song, Y.T.; et al. Cardiomyocyte peroxisome proliferator-activated receptor α prevents septic cardiomyopathy via improving mitochondrial function. Acta Pharmacol. Sin. 2023, 44, 2184–2200. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Battiprolu, P.K.; Fukushima, A.; Nguyen, K.; Milner, K.; Gupta, A.; Altamimi, T.; Byrne, N.; Mori, J.; et al. Malonyl CoA decarboxylase inhibition improves cardiac function post-myocardial infarction. JACC Basic Transl. Sci. 2019, 4, 385–400. [Google Scholar] [CrossRef]
- Barcena de Arellano, M.L.; Pozdniakova, S.; Kühl, A.A.; Baczko, I.; Ladilov, Y.; Regitz-Zagrosek, V. Sex differences in the aging human heart: Decreased sirtuins, pro-inflammatory shift and reduced anti-oxidative defense. Aging 2019, 11, 1918–1933. [Google Scholar] [CrossRef]
- Fukushima, A.; Milner, K.; Gupta, A.; Lopaschuk, G.D. Myocardial energy substrate metabolism in heart failure: From pathways to therapeutic targets. Curr. Pharm. Des. 2015, 21, 3654–3664. [Google Scholar] [CrossRef]
- Lee, C.F.; Chavez, J.D.; Garcia-Menendez, L.; Choi, Y.; Roe, N.D.; Chiao, Y.A.; Edgar, J.S.; Goo, Y.A.; Goodlett, D.R.; Bruce, J.E.; et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation 2016, 134, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Bedi, K.C., Jr.; Snyder, N.W.; Brandimarto, J.; Aziz, M.; Mesaros, C.; Worth, A.J.; Wang, L.L.; Javaheri, A.; Blair, I.A.; Margulies, K.B.; et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 2016, 133, 706–716. [Google Scholar] [CrossRef]
- Carrico, C.; Meyer, J.G.; He, W.; Gibson, B.W.; Verdin, E. The mitochondrial acylome emerges: Proteomics, regulation by sirtuins, and metabolic and disease implications. Cell Metab. 2018, 27, 497–512. [Google Scholar] [CrossRef]
- Liu, Y.P.; Wen, R.; Liu, C.F.; Zhang, T.N.; Yang, N. Cellular and molecular biology of sirtuins in cardiovascular disease. Biomed. Pharmacother. 2023, 164, 114931. [Google Scholar] [CrossRef] [PubMed]
- Kleele, T.; Rey, T.; Winter, J.; Zaganelli, S.; Mahecic, D.; Perreten Lambert, H.; Ruberto, F.P.; Nemir, M.; Wai, T.; Pedrazzini, T.; et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 2021, 593, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Kraus, F.; Roy, K.; Pucadyil, T.J.; Ryan, M.T. Function and regulation of the divisome for mitochondrial fission. Nature 2021, 590, 57–66. [Google Scholar] [CrossRef]
- Tokuyama, T.; Yanagi, S. Role of mitochondrial dynamics in heart diseases. Genes 2023, 14, 1876. [Google Scholar] [CrossRef]
- Pun-García, A.; Clemente-Moragón, A.; Villena-Gutierrez, R.; Gómez, M.; Sanz-Rosa, D.; Díaz-Guerra, A.; Prados, B.; Medina, J.P.; Montó, F.; Ivorra, M.D.; et al. Beta-3 adrenergic receptor overexpression reverses aortic stenosis-induced heart failure and restores balanced mitochondrial dynamics. Basic Res. Cardiol. 2022, 117, 62. [Google Scholar] [CrossRef]
- Hoppel, C.L.; Tandler, B.; Fujioka, H.; Riva, A. Dynamic organization of mitochondria in human heart and in myocardial disease. Int. J. Biochem. Cell Biol. 2009, 41, 1949–1956. [Google Scholar] [CrossRef]
- Eldarov, C.M.; Vangely, I.M.; Vays, V.B.; Sheval, E.V.; Holtze, S.; Hildebrandt, T.B.; Kolosova, N.G.; Popkov, V.A.; Plotnikov, E.Y.; Zorov, D.B.; et al. Mitochondria in the nuclei of rat myocardial cells. Cells 2020, 9, 712. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Javadov, S.; Margreiter, R.; Hagenbuchner, J.; Ausserlechner, M.J. Analysis of mitochondrial function, structure, and intracellular organization in situ in cardiomyocytes and skeletal muscles. Int. J. Mol. Sci. 2022, 23, 2252. [Google Scholar] [CrossRef]
- Li, A.; Gao, M.; Jiang, W.; Qin, Y.; Gong, G. Mitochondrial dynamics in adult cardiomyocytes and heart diseases. Front. Cell Dev. Biol. 2020, 8, 584800. [Google Scholar] [CrossRef]
- Ding, Q.; Qi, Y.; Tsang, S.Y. Mitochondrial biogenesis, mitochondrial dynamics, and mitophagy in the maturation of cardiomyocytes. Cells 2021, 10, 2463. [Google Scholar] [CrossRef]
- Wu, L.; Wang, L.; Du, Y.; Zhang, Y.; Ren, J. Mitochondrial quality control mechanisms as therapeutic targets in doxorubicin-induced cardiotoxicity. Trends Pharmacol. Sci. 2023, 44, 34–49. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Xu, Y.; Xu, C.; Wang, H.; Rui, T. Regulation of mitochondrial dynamics in cardiomyocytes: Implications for cardiac health and disease. Front. Cell Dev. Biol. 2025, 13, 1652683. [Google Scholar] [CrossRef]
- Jin, J.Y.; Wei, X.X.; Zhi, X.L.; Wang, X.H.; Meng, D. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol. Sin. 2021, 42, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Mukai, R.; Mareedu, S.; Zhai, P.; Oka, S.I.; Huang, C.Y.; Hsu, C.P.; Yousufzai, F.A.K.; Fritzky, L.; Mizushima, W.; et al. Distinct roles of DRP1 in conventional and alternative mitophagy in obesity cardiomyopathy. Circ. Res. 2023, 133, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Leo, V.; Kelly, M.; Stockenhuber, A.; Kennedy, N.W.; Bao, L.; Cereghetti, G.M.; Harper, A.R.; Czibik, G.; Liao, C.; et al. Resistance of dynamin-related protein 1 oligomers to disassembly impairs mitophagy, resulting in myocardial inflammation and heart failure. J. Biol. Chem. 2015, 290, 25907–25919, Erratum in J. Biol. Chem. 2016, 291, 25762. https://doi.org/10.1074/jbc.A115.665695.. [Google Scholar] [CrossRef]
- Shirakabe, A.; Zhai, P.; Ikeda, Y.; Saito, T.; Maejima, Y.; Hsu, C.P.; Nomura, M.; Egashira, K.; Levine, B.; Sadoshima, J. Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure. Circulation 2016, 133, 1249–1263. [Google Scholar] [CrossRef]
- Thai, P.N.; Seidlmayer, L.K.; Miller, C.; Ferrero, M.; Dorn, G.W., II; Schaefer, S.; Bers, D.M.; Dedkova, E.N. Mitochondrial quality control in aging and heart failure: Influence of ketone bodies and mitofusin-stabilizing peptides. Front. Physiol. 2019, 10, 382. [Google Scholar] [CrossRef]
- Chaanine, A.H.; Joyce, L.D.; Stulak, J.M.; Maltais, S.; Joyce, D.L.; Dearani, J.A.; Klaus, K.; Nair, K.S.; Hajjar, R.J.; Redfield, M.M. Mitochondrial morphology, dynamics, and function in human pressure overload or ischemic heart disease with preserved or reduced ejection fraction. Circ. Heart Fail. 2019, 12, e005131. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.V.; Sandeep, N.; Nair, R.V.; Hu, D.Q.; Zhao, M.; Lan, I.S.; Fajardo, G.; Matkovich, S.J.; Bernstein, D.; Reddy, S. Transcriptomic and functional analyses of mitochondrial dysfunction in pressure overload-induced right ventricular failure. J. Am. Heart Assoc. 2021, 10, e017835. [Google Scholar] [CrossRef]
- Kim, Y.; Landstrom, A.P.; Shah, S.H.; Wu, J.C.; Seidman, C.E.; American Heart Association. Gene therapy in cardiovascular disease: Recent advances and future directions in science: A science advisory from the American Heart Association. Circulation 2024, 150, e471–e480. [Google Scholar] [CrossRef] [PubMed]
- Chaanine, A.H.; LeJemtel, T.H.; Delafontaine, P. Mitochondrial pathobiology and metabolic remodeling in progression to overt systolic heart failure. J. Clin. Med. 2020, 9, 3582. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.B.; Kalkhoran, S.B.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Mitochondrial fusion and fission proteins as novel therapeutic targets for treating cardiovascular disease. Eur. J. Pharmacol. 2015, 763, 104–114. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Qin, Y.; Li, A.; Gao, M.; Liu, H.; Gong, G. Mitochondrial fusion protein Mfn2 and its role in heart failure. Front. Mol. Biosci. 2021, 8, 681237. [Google Scholar] [CrossRef]
- Chen, L.; Qin, Y.; Liu, B.; Gao, M.; Li, A.; Li, X.; Gong, G. PGC-1α-mediated mitochondrial quality control: Molecular mechanisms and implications for heart failure. Front. Cell Dev. Biol. 2022, 10, 871357. [Google Scholar] [CrossRef]
- Werbner, B.; Tavakoli-Rouzbehani, O.M.; Fatahian, A.N.; Boudina, S. The dynamic interplay between cardiac mitochondrial health and myocardial structural remodeling in metabolic heart disease, aging, and heart failure. J. Cardiovasc. Aging 2023, 3, 9. [Google Scholar] [CrossRef]
- Fonseka, O.; Gare, S.R.; Chen, X.; Zhang, J.; Alatawi, N.H.; Ross, C.; Liu, W. Molecular mechanisms underlying heart failure and their therapeutic potential. Cells 2025, 14, 324. [Google Scholar] [CrossRef]
- Gao, S.; Li, D.; Wang, B.; Zhang, H.; Chen, L. Two promising approaches in the treatment of myocardial infarction: Stem cells and gene therapy. Front. Cardiovasc. Med. 2025, 12, 1540066. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.M.; Lees, J.G.; Murray, A.J.; Velagic, A.; Lim, S.Y.; De Blasio, M.J.; Ritchie, R.H. Precision medicine: Therapeutically targeting mitochondrial alterations in heart failure. JACC Basic Transl. Sci. 2025, 10, 101345. [Google Scholar] [CrossRef]
- Cao, Y.; Vergnes, L.; Wang, Y.C.; Pan, C.; Chella Krishnan, K.; Moore, T.M.; Rosa-Garrido, M.; Kimball, T.H.; Zhou, Z.; Charugundla, S.; et al. Sex differences in heart mitochondria regulate diastolic dysfunction. Nat. Commun. 2022, 13, 3850. [Google Scholar] [CrossRef]
- Naumenko, N.; Mutikainen, M.; Holappa, L.; Ruas, J.L.; Tuomainen, T.; Tavi, P. PGC-1α deficiency reveals sex-specific links between cardiac energy metabolism and EC-coupling during development of heart failure in mice. Cardiovasc. Res. 2022, 118, 1520–1534. [Google Scholar] [CrossRef]
- Zincarelli, C.; Soltys, S.; Rengo, G.; Rabinowitz, J.E. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008, 16, 1073–1080. [Google Scholar] [CrossRef]
- Du, L.; Kido, M.; Lee, D.V.; Rabinowitz, J.E.; Samulski, R.J.; Jamieson, S.W.; Weitzman, M.D.; Thistlethwaite, P.A. Differential myocardial gene delivery by recombinant serotype-specific adeno-associated viral vectors. Mol. Ther. 2004, 10, 604–608. [Google Scholar] [CrossRef]
- Pacak, C.A.; Mah, C.S.; Thattaliyath, B.D.; Conlon, T.J.; Lewis, M.A.; Cloutier, D.E.; Zolotukhin, I.; Tarantal, A.F.; Byrne, B.J. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ. Res. 2006, 99, e3–e9. [Google Scholar] [CrossRef]
- Prasad, K.M.; Xu, Y.; Yang, Z.; Acton, S.T.; French, B.A. Robust cardiomyocyte-specific gene expression following systemic injection of AAV: In vivo gene delivery follows a Poisson distribution. Gene Ther. 2011, 18, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jonker, T.; Cervera-Barea, A.; Dong, Z.; Visser, R.N.; Birza, E.E.; Boender, A.R.; Klerk, M.; Yang, Y.; Visser, J.; et al. AAV6 vectors provide superior gene transfer compared to AAV9 vectors following intramyocardial administration. Mol. Ther. Methods Clin. Dev. 2025, 33, 101532. [Google Scholar] [CrossRef] [PubMed]
- Djurovic, S.; Iversen, N.; Jeansson, S.; Hoover, F.; Christensen, G. Comparison of nonviral transfection and adeno-associated viral transduction on cardiomyocytes. Mol. Biotechnol. 2004, 28, 21–32. [Google Scholar] [CrossRef]
- Zhang, H.; Zhan, Q.; Huang, B.; Wang, Y.; Wang, X. AAV-mediated gene therapy: Advancing cardiovascular disease treatment. Front. Cardiovasc. Med. 2022, 9, 952755. [Google Scholar] [CrossRef]
- Zsebo, K.; Yaroshinsky, A.; Rudy, J.J.; Wagner, K.; Greenberg, B.; Jessup, M.; Hajjar, R.J. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: Analysis of recurrent cardiovascular events and mortality. Circ. Res. 2014, 114, 101–108. [Google Scholar] [CrossRef]
- Greenberg, B.; Butler, J.; Felker, G.M.; Ponikowski, P.; Voors, A.A.; Desai, A.S.; Barnard, D.; Bouchard, A.; Jaski, B.; Lyon, A.R.; et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): A randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 2016, 387, 1178–1186. [Google Scholar] [CrossRef]
- Kliesow Remes, A.; Ruf, T.; Zurashvili, T.; Ding, L.; Meyer-Jens, M.; Schwab, D.M.; Hille, S.; Matzen, A.; Michalewski, S.; Kilian, L.; et al. AAV-mediated overexpression of CPT1B protects from cardiac hypertrophy and heart failure in a murine pressure overload model. Basic Res. Cardiol. 2025, 120, 975–989. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, L.; Li, C.; Yang, J.; Duan, F.; Su, Q.; Zhang, Y.; Kou, M.; Zhou, X.; Guo, L.; et al. Gene therapy prevents onset of mitochondrial cardiomyopathy in neonatal mice with Ndufs6 deficiency. Cell Death Discov. 2025, 11, 249. [Google Scholar] [CrossRef]
- Pleger, S.T.; Shan, C.; Ksienzyk, J.; Bekeredjian, R.; Boekstegers, P.; Hinkel, R.; Schinkel, S.; Leuchs, B.; Ludwig, J.; Qiu, G.; et al. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci. Transl. Med. 2011, 3, 92ra64. [Google Scholar] [CrossRef] [PubMed]
- Brinks, H.; Rohde, D.; Voelkers, M.; Qiu, G.; Pleger, S.T.; Herzog, N.; Rabinowitz, J.; Ruhparwar, A.; Silvestry, S.; Lerchenmüller, C.; et al. S100A1 genetically targeted therapy reverses dysfunction of human failing cardiomyocytes. J. Am. Coll. Cardiol. 2011, 58, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Hu, S.; Liu, X.; He, M.; Li, J.; Ma, T.; Huang, M.; Fang, Q.; Wang, Y. CAV3 alleviates diabetic cardiomyopathy via inhibiting NDUFA10-mediated mitochondrial dysfunction. J. Transl. Med. 2024, 22, 390. [Google Scholar] [CrossRef] [PubMed]
- Samulski, R.J.; Muzyczka, N. AAV-mediated gene therapy for research and therapeutic purposes. Annu. Rev. Virol. 2014, 1, 427–451. [Google Scholar] [CrossRef]
- Samulski, R.J.; Berns, K.I.; Tan, M.; Muzyczka, N. Cloning of adeno-associated virus into pBR322: Rescue of intact virus from the recombinant plasmid in human cells. Proc. Natl. Acad. Sci. USA 1982, 79, 2077–2081. [Google Scholar] [CrossRef]
- Laughlin, C.A.; Tratschin, J.D.; Coon, H.; Carter, B.J. Cloning of infectious adeno-associated virus genomes in bacterial plasmids. Gene 1983, 23, 65–73. [Google Scholar] [CrossRef]
- Ishikawa, K.; Weber, T.; Hajjar, R.J. Human cardiac gene therapy. Circ. Res. 2018, 123, 601–613. [Google Scholar] [CrossRef]
- Wang, J.H.; Gessler, D.J.; Zhan, W.; Gallagher, T.L.; Gao, G. Adeno-associated virus as a delivery vector for gene therapy of human diseases. Signal Transduct. Target Ther. 2024, 9, 78. [Google Scholar] [CrossRef]
- Huang, L.Y.; Patel, A.; Ng, R.; Miller, E.B.; Halder, S.; McKenna, R.; Asokan, A.; Agbandje-McKenna, M. Characterization of the adeno-associated virus 1 and 6 sialic acid binding site. J. Virol. 2016, 90, 5219–5230. [Google Scholar] [CrossRef]
- Hauck, B.; Xiao, W. Characterization of tissue tropism determinants of adeno-associated virus type 1. J. Virol. 2003, 77, 2768–2774. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Choi, Y.K.; Campbell-Thompson, M.; Li, C.; Tang, Q.; Crawford, J.M.; Flotte, T.R.; Song, S. Therapeutic level of functional human alpha 1 antitrypsin (hAAT) secreted from murine muscle transduced by adeno-associated virus (rAAV1) vector. J. Gene Med. 2006, 8, 730–735. [Google Scholar] [CrossRef]
- Issa, S.S.; Shaimardanova, A.A.; Solovyeva, V.V.; Rizvanov, A.A. Various AAV serotypes and their applications in gene therapy: An overview. Cells 2023, 12, 785. [Google Scholar] [CrossRef]
- Su, H.; Yeghiazarians, Y.; Lee, A.; Huang, Y.; Arakawa-Hoyt, J.; Ye, J.; Orcino, G.; Grossman, W.; Kan, Y.W. AAV serotype 1 mediates more efficient gene transfer to pig myocardium than AAV serotype 2 and plasmid. J. Gene Med. 2008, 10, 33–41. [Google Scholar] [CrossRef]
- Chen, S.; Kapturczak, M.; Loiler, S.A.; Zolotukhin, S.; Glushakova, O.Y.; Madsen, K.M.; Samulski, R.J.; Hauswirth, W.W.; Campbell-Thompson, M.; Berns, K.I.; et al. Efficient transduction of vascular endothelial cells with recombinant adeno-associated virus serotype 1 and 5 vectors. Hum. Gene Ther. 2005, 16, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, C.M.; Clark, K.R.; Sferra, T.J. Recombinant AAV serotype 1 transduction efficiency and tropism in the murine brain. Gene Ther. 2003, 10, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Asokan, A.; Samulski, R.J. Adeno-associated virus serotypes: Vector toolkit for human gene therapy. Mol. Ther. 2006, 14, 316–327. [Google Scholar] [CrossRef]
- Wu, Z.; Asokan, A.; Grieger, J.C.; Govindasamy, L.; Agbandje-McKenna, M.; Samulski, R.J. Single amino acid changes can influence titer, heparin binding, and tissue tropism in different adeno-associated virus serotypes. J. Virol. 2006, 80, 11393–11397. [Google Scholar] [CrossRef]
- Zincarelli, C.; Soltys, S.; Rengo, G.; Koch, W.J.; Rabinowitz, J.E. Comparative cardiac gene delivery of adeno-associated virus serotypes 1-9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin. Transl. Sci. 2010, 3, 81–89. [Google Scholar] [CrossRef]
- Bish, L.T.; Sleeper, M.M.; Reynolds, C.; Gazzara, J.; Withnall, E.; Singletary, G.E.; Buchlis, G.; Hui, D.; High, K.A.; Gao, G.; et al. Cardiac gene transfer of short hairpin RNA directed against phospholamban effectively knocks down gene expression but causes cellular toxicity in canines. Hum. Gene Ther. 2011, 22, 969–977. [Google Scholar] [CrossRef]
- White, J.D.; Thesier, D.M.; Swain, J.B.; Katz, M.G.; Tomasulo, C.; Henderson, A.; Wang, L.; Yarnall, C.; Fargnoli, A.; Sumaroka, M.; et al. Myocardial gene delivery using molecular cardiac surgery with recombinant adeno-associated virus vectors in vivo. Gene Ther. 2011, 18, 546–552. [Google Scholar] [CrossRef]
- DiMattia, M.A.; Nam, H.J.; Van Vliet, K.; Mitchell, M.; Bennett, A.; Gurda, B.L.; McKenna, R.; Olson, N.H.; Sinkovits, R.S.; Potter, M.; et al. Structural insight into the unique properties of adeno-associated virus serotype 9. J. Virol. 2012, 86, 6947–6958. [Google Scholar] [CrossRef]
- Gao, G.; Vandenberghe, L.H.; Alvira, M.R.; Lu, Y.; Calcedo, R.; Zhou, X.; Wilson, J.M. Clades of Adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004, 78, 6381–6388. [Google Scholar] [CrossRef]
- Flotte, T.R.; Trapnell, B.C.; Humphries, M.; Carey, B.; Calcedo, R.; Rouhani, F.; Campbell-Thompson, M.; Yachnis, A.T.; Sandhaus, R.A.; McElvaney, N.G.; et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: Interim results. Hum. Gene Ther. 2011, 22, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Foust, K.D.; Nurre, E.; Montgomery, C.L.; Hernandez, A.; Chan, C.M.; Kaspar, B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009, 27, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bevan, A.K.; Duque, S.; Foust, K.D.; Morales, P.R.; Braun, L.; Schmelzer, L.; Chan, C.M.; McCrate, M.; Chicoine, L.G.; Coley, B.D.; et al. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol. Ther. 2011, 19, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Vandendriessche, T.; Thorrez, L.; Acosta-Sanchez, A.; Petrus, I.; Wang, L.; Ma, L.; DE Waele, L.; Iwasaki, Y.; Gillijns, V.; Wilson, J.M.; et al. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J. Thromb. Haemost. 2007, 5, 16–24. [Google Scholar] [CrossRef]
- Boutin, S.; Monteilhet, V.; Veron, P.; Leborgne, C.; Benveniste, O.; Montus, M.F.; Masurier, C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010, 21, 704–712. [Google Scholar] [CrossRef]
- Chirmule, N.; Xiao, W.; Truneh, A.; Schnell, M.A.; Hughes, J.V.; Zoltick, P.; Wilson, J.M. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J. Virol. 2000, 74, 2420–2425. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, M.; Osborne, A.; Yu-Wai-Man, P.; Martin, K. Humoral immune responses to AAV gene therapy in the ocular compartment. Biol. Rev. Camb. Philos. Soc. 2021, 96, 1616–1644. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.L.; Suzuki, M.; Zolotukhin, I.; Markusic, D.M.; Morel, L.M.; Lee, B.; Ertl, H.C.; Herzog, R.W. Unique roles of TLR9- and MyD88-dependent and -independent pathways in adaptive immune responses to AAV-mediated gene transfer. J. Innate Immun. 2015, 7, 302–314. [Google Scholar] [CrossRef]
- Huang, X.; Yang, Y. Targeting the TLR9-MyD88 pathway in the regulation of adaptive immune responses. Expert. Opin. Ther. Targets 2010, 14, 787–796. [Google Scholar] [CrossRef]
- Rogers, G.L.; Shirley, J.L.; Zolotukhin, I.; Kumar, S.R.P.; Sherman, A.; Perrin, G.Q.; Hoffman, B.E.; Srivastava, A.; Basner-Tschakarjan, E.; Wallet, M.A.; et al. Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8+ T cells. Blood 2017, 129, 3184–3195. [Google Scholar] [CrossRef] [PubMed]
- Shirley, J.L.; Keeler, G.D.; Sherman, A.; Zolotukhin, I.; Markusic, D.M.; Hoffman, B.E.; Morel, L.M.; Wallet, M.A.; Terhorst, C.; Herzog, R.W. Type I IFN sensing by cDCs and CD4+ T cell help are both requisite for cross-priming of AAV capsid-specific CD8+ T cells. Mol. Ther. 2020, 28, 758–770. [Google Scholar] [CrossRef]
- Pien, G.C.; Basner-Tschakarjan, E.; Hui, D.J.; Mentlik, A.N.; Finn, J.D.; Hasbrouck, N.C.; Zhou, S.; Murphy, S.L.; Maus, M.V.; Mingozzi, F.; et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J. Clin. Investig. 2009, 119, 1688–1695. [Google Scholar] [CrossRef]
- Brantly, M.L.; Chulay, J.D.; Wang, L.; Mueller, C.; Humphries, M.; Spencer, L.T.; Rouhani, F.; Conlon, T.J.; Calcedo, R.; Betts, M.R.; et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 16363–16368. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, A.K.; Cotter, M.J.; White, L.R.; Clark, S.A.; Wong, N.C.; Holers, V.M.; Bartlett, J.S.; Muruve, D.A. Complement is an essential component of the immune response to adeno-associated virus vectors. J. Virol. 2008, 82, 2727–2740. [Google Scholar] [CrossRef]
- Manning, W.C.; Zhou, S.; Bland, M.P.; Escobedo, J.A.; Dwarki, V. Transient immunosuppression allows transgene expression following readministration of adeno-associated viral vectors. Hum. Gene Ther. 1998, 9, 477–485. [Google Scholar] [CrossRef]
- Vrellaku, B.; Hassan, I.S.; Howitt, R.; Webster, C.P.; Harriss, E.; McBlane, F.; Betts, C.; Schettini, J.; Lion, M.; Mindur, J.E.; et al. A systematic review of immunosuppressive protocols used in AAV gene therapy for monogenic disorders. Mol. Ther. 2024, 32, 3220–3259. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.; Scheideler, O.; Schaffer, D. Engineering the AAV capsid to evade immune responses. Curr. Opin. Biotechnol. 2019, 60, 99–103. [Google Scholar] [CrossRef]
- Ito, M.; Takino, N.; Nomura, T.; Kan, A.; Muramatsu, S.I. Engineered adeno-associated virus 3 vector with reduced reactivity to serum antibodies. Sci. Rep. 2021, 11, 9322. [Google Scholar] [CrossRef]
- Rajwade, A.; Liu, S.; Kumar, M.; Ghobrial, A.; Hahn, S.; Shah, D.K. Investigation of dose-exposure-response relationship for adeno-associated virus-mediated delivery of antibody genes. J. Pharmacol. Exp. Ther. 2025, 392, 103601. [Google Scholar] [CrossRef]
- Arjomandnejad, M.; Sylvia, K.; Blackwood, M.; Nixon, T.; Tang, Q.; Muhuri, M.; Gruntman, A.M.; Gao, G.; Flotte, T.R.; Keeler, A.M. Modulating immune responses to AAV by expanded polyclonal T-regs and capsid specific chimeric antigen receptor T-regulatory cells. Mol. Ther. Methods Clin. Dev. 2021, 23, 490–506. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Melero, M.; Biswas, M. Role of FoxP3+ regulatory T cells in modulating immune responses to adeno-associated virus gene therapy. Hum. Gene Ther. 2024, 35, 439–450. [Google Scholar] [CrossRef]
- Asokan, A.; Conway, J.C.; Phillips, J.L.; Li, C.; Hegge, J.; Sinnott, R.; Yadav, S.; DiPrimio, N.; Nam, H.J.; Agbandje-McKenna, M.; et al. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat. Biotechnol. 2010, 28, 79–82. [Google Scholar] [CrossRef]
- Tabebordbar, M.; Lagerborg, K.A.; Stanton, A.; King, E.M.; Ye, S.; Tellez, L.; Krunnfusz, A.; Tavakoli, S.; Widrick, J.J.; Messemer, K.A.; et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell 2021, 184, 4919–4938.e22. [Google Scholar] [CrossRef]
- Pulicherla, N.; Shen, S.; Yadav, S.; Debbink, K.; Govindasamy, L.; Agbandje-McKenna, M.; Asokan, A. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol. Ther. 2011, 19, 1070–1078. [Google Scholar] [CrossRef]
- Kastrup, J.; Jørgensen, E.; Rück, A.; Tägil, K.; Glogar, D.; Ruzyllo, W.; Bøtker, H.E.; Dudek, D.; Drvota, V.; Hesse, B.; et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris: A randomized double-blind placebo-controlled study: The Euroinject One trial. J. Am. Coll. Cardiol. 2005, 45, 982–988. [Google Scholar] [CrossRef]
- Katz, M.G.; Fargnoli, A.S.; Weber, T.; Hajjar, R.J.; Bridges, C.R. Use of adeno-associated virus vector for cardiac gene delivery in large-animal surgical models of heart failure. Hum. Gene Ther. Clin. Dev. 2017, 28, 157–164. [Google Scholar] [CrossRef]
- Kikuchi, K.; McDonald, A.D.; Sasano, T.; Donahue, J.K. Targeted modification of atrial electrophysiology by homogeneous transmural atrial gene transfer. Circulation 2005, 111, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Scott, L., Jr.; Campbell, H.M.; Pan, X.; Alsina, K.M.; Reynolds, J.; Philippen, L.E.; Hulsurkar, M.; Lagor, W.R.; Li, N.; et al. Atrial-specific gene delivery using an adeno-associated viral vector. Circ. Res. 2019, 124, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.G.; Fargnoli, A.S.; Kendle, A.P.; Bridges, C.R. Molecular cardiac surgery with recirculating delivery (MCARD): Procedure and vector transfer. Methods Mol. Biol. 2017, 1521, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Fargnoli, A.S.; Katz, M.G.; Yarnall, C.; Isidro, A.; Petrov, M.; Steuerwald, N.; Ghosh, S.; Richardville, K.C.; Hillesheim, R.; Williams, R.D.; et al. Cardiac surgical delivery of the sarcoplasmic reticulum calcium ATPase rescues myocytes in ischemic heart failure. Ann. Thorac. Surg. 2013, 96, 586–995. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inagaki, K.; Fuess, S.; Storm, T.A.; Gibson, G.A.; Mctiernan, C.F.; Kay, M.A.; Nakai, H. Robust systemic transduction with AAV9 vectors in mice: Efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 2006, 14, 45–53. [Google Scholar] [CrossRef]
- Chamberlain, K.; Riyad, J.M.; Weber, T. Cardiac gene therapy with adeno-associated virus-based vectors. Curr. Opin. Cardiol. 2017, 32, 275–282. [Google Scholar] [CrossRef]
- Kawase, Y.; Ly, H.Q.; Prunier, F.; Lebeche, D.; Shi, Y.; Jin, H.; Hadri, L.; Yoneyama, R.; Hoshino, K.; Takewa, Y.; et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J. Am. Coll. Cardiol. 2008, 51, 1112–1119. [Google Scholar] [CrossRef]
- Ishikawa, K.; Fish, K.M.; Tilemann, L.; Rapti, K.; Aguero, J.; Santos-Gallego, C.G.; Lee, A.; Karakikes, I.; Xie, C.; Akar, F.G.; et al. Cardiac I-1c overexpression with reengineered AAV improves cardiac function in swine ischemic heart failure. Mol. Ther. 2014, 22, 2038–2045. [Google Scholar] [CrossRef]
- Fish, K.M.; Ladage, D.; Kawase, Y.; Karakikes, I.; Jeong, D.; Ly, H.; Ishikawa, K.; Hadri, L.; Tilemann, L.; Muller-Ehmsen, J.; et al. AAV9.I-1c delivered via direct coronary infusion in a porcine model of heart failure improves contractility and mitigates adverse remodeling. Circ. Heart Fail. 2013, 6, 310–317. [Google Scholar] [CrossRef]
- Jaski, B.E.; Jessup, M.L.; Mancini, D.M.; Cappola, T.P.; Pauly, D.F.; Greenberg, B.; Borow, K.; Dittrich, H.; Zsebo, K.M.; Hajjar, R.J. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J. Card. Fail. 2009, 15, 171–181. [Google Scholar] [CrossRef]
- Weber, C.; Neacsu, I.; Krautz, B.; Schlegel, P.; Sauer, S.; Raake, P.; Ritterhoff, J.; Jungmann, A.; Remppis, A.B.; Stangassinger, M.; et al. Therapeutic safety of high myocardial expression levels of the molecular inotrope S100A1 in a preclinical heart failure model. Gene Ther. 2014, 21, 131–138. [Google Scholar] [CrossRef]
- Raake, P.; von Degenfeld, G.; Hinkel, R.; Vachenauer, R.; Sandner, T.; Beller, S.; Andrees, M.; Kupatt, C.; Schuler, G.; Boekstegers, P. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins: Comparison with surgical and percutaneous intramyocardial gene delivery. J. Am. Coll. Cardiol. 2004, 44, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Bish, L.T.; Morine, K.; Sleeper, M.M.; Sanmiguel, J.; Wu, D.; Gao, G.; Wilson, J.M.; Sweeney, H.L. Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum. Gene Ther. 2008, 19, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Raake, P.W.; Hinkel, R.; Müller, S.; Delker, S.; Kreuzpointner, R.; Kupatt, C.; Katus, H.A.; Kleinschmidt, J.A.; Boekstegers, P.; Müller, O.J. Cardio-specific long-term gene expression in a porcine model after selective pressure-regulated retroinfusion of adeno-associated viral (AAV) vectors. Gene Ther. 2008, 15, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yue, Y.; Zhang, K.; Lostal, W.; Shin, J.H.; Duan, D. Long-term robust myocardial transduction of the dog heart from a peripheral vein by adeno-associated virus serotype-8. Hum. Gene Ther. 2013, 24, 584–594. [Google Scholar] [CrossRef]
- Katz, M.G.; Fargnoli, A.S.; Williams, R.D.; Steuerwald, N.M.; Isidro, A.; Ivanina, A.V.; Sokolova, I.M.; Bridges, C.R. Safety and efficacy of high-dose adeno-associated virus 9 encoding sarcoplasmic reticulum Ca2+ adenosine triphosphatase delivered by molecular cardiac surgery with recirculating delivery in ovine ischemic cardiomyopathy. J. Thorac. Cardiovasc. Surg. 2014, 148, 1065–1073.e2; discussion1072–3. [Google Scholar] [CrossRef]
- Greenberg, B.; Butler, J.; Felker, G.M.; Ponikowski, P.; Voors, A.A.; Pogoda, J.M.; Provost, R.; Guerrero, J.; Hajjar, R.J.; Zsebo, K.M. Prevalence of AAV1 neutralizing antibodies and consequences for a clinical trial of gene transfer for advanced heart failure. Gene Ther. 2016, 23, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Hulot, J.S.; Ishikawa, K.; Hajjar, R.J. Gene therapy for the treatment of heart failure: Promise postponed. Eur. Heart J. 2016, 37, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Ritterhoff, J.; McMillen, T.S.; Villet, O.; Young, S.; Kolwicz, S.C., Jr.; Senn, T.; Caudal, A.; Tian, R. Increasing fatty acid oxidation elicits a sex-dependent response in failing mouse hearts. J. Mol. Cell. Cardiol. 2021, 158, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; La Salvia, S.; Liang, Y.; Adamiak, M.; Kohlbrenner, E.; Jeong, D.; Chepurko, E.; Ceholski, D.; Lopez-Gordo, E.; Yoon, S.; et al. Extracellular vesicle-encapsulated adeno-associated viruses for therapeutic gene delivery to the heart. Circulation 2023, 148, 405–425. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.