Abstract
Background: Antithrombotic therapy plays an important role in acute coronary syndrome (ACS). The combination of anticoagulant and antiplatelet therapy resulted in fewer complications and stronger potency compared to traditional monotherapy. Our net meta-analysis aimed to compare and rank the safety of different treatments used in patients with ACS. Method: We conducted a search for trials in three prominent databases. The main objective of our investigation was to assess hemorrhage. Additional outcomes included mortality, myocardial infarction, stroke, and embolism. We used a frequentist network meta-analysis with a random-effects model to, directly and indirectly, compare safety across different antithrombotic strategies. Result: A total of 30 randomized clinical trials were included in this net meta-analysis with 135,471 ACS patients. In these eight different antithrombotic therapies, SAPT (single-agent platelet inhibitor therapy) showed the lowest risk of bleeding (SUCRA = 0.5%). The highest risk of bleeding was observed in VKA (vitamin K antagonists) + DAPT (dual antiplatelet therapy) (SUCRA = 99.8%). Bleeding among NOAC (non-vitamin K antagonist oral anticoagulants) + DAPT was found to be higher than DAPT (OR = 1.94, 95% CI = 1.42–2.65). NOAC + SAPT significantly reduced the embolism (OR = 1.50, 95% CI = 1.16–1.94) and myocardial infarction (OR = 1.22, 95% CI = 1.08–1.37) events compared with SAPT. In addition, VKA significantly reduced the rate of stroke compared with SAPT (OR = 3.45, 95% CI = 1.17–10.18). However, no significant difference was observed in death events among these eight antithrombotic therapies. Conclusions: We advise against the use of SAPT in ACS due to its elevated risk of embolism, myocardial infarction, and stroke. It is important to mention that the combination of NOAC and SAPT has a lower incidence of myocardial infarction, bleeding and embolism problems. Therefore, the combination of NOAC and SAPT may be the optimal approach to achieve a balance between the risks of bleeding and embolism. This meta-analysis was registered in PROSPERO with the registration number CRD42024542826.
1. Introduction
Acute coronary syndrome arises from the rupture or erosion of unstable atherosclerotic plaques within the coronary arteries, leading to thrombus formation and subsequently causing an acute myocardial ischemic syndrome. Of course, approximately 14% of overall patients with ACS failed to survive [1]. It is notable that the elderly have the highest incidence of cardiovascular disease and frequently present with ACS. However, recent studies showed that increasing incidence and presence were observed in younger individuals with ACS. This emerging phenomenon significantly contributes to the substantial economic burden that ACS imposes on society [2]. Long-term antiplatelet and anticoagulant therapies play a key role in preventing complications associated with ACS. Oral antiplatelet therapy (aspirin or P2Y12 inhibitors) and anticoagulants (unfractionated heparin, low-molecular-weight heparins, direct thrombin inhibitors, or Xa factor inhibitors) are recommended therapeutic approaches in the initial management of ACS, regardless of whether the treatment is invasive or non-invasive [3].
When ACS patients are exposed to antithrombotic therapy, bleeding is a known complication of any oral anticoagulant and antiplatelet medications [4]. Thrombotic risk is higher than bleeding risk in the early phase within the initial 30 days following ACS events. Next, risk factors for bleeding will transcend thrombus formation in the later stage with the implementation of intensified antithrombotic therapies, which results in attenuation of the benefits of anticoagulation. Hence, the chronic management of ACS requires a personalized approach to antithrombotic therapy following the acute phase to balance the risk of bleeding and embolism [5]. Although various studies revealed that novel oral anticoagulants overcome the drawbacks associated with traditional anticoagulants, including unfractionated and low-molecular-weight heparins, and vitamin K antagonists [6], anticoagulant or antithrombotic monotherapy cannot significantly benefit ACS patients compared to their combination [7]. Thus, increasingly, research has explored the safety and efficacy of novel oral anticoagulants in combination with standard antiplatelet therapy for ACS. These studies suggest that the combination of novel oral anticoagulants with antiplatelet therapy significantly reduces the occurrence of fatal bleeding and indicates the importance of incorporating low-dose novel oral anticoagulants in antiplatelet regimens [8]. This network meta-analysis systematically evaluated the safety of various combinations of novel or traditional anticoagulants with dual or single antiplatelet agents in ACS. The findings present novel insights into the treatment of ACS, offering new references for clinical practice.
2. Material and Method
This study conducted a systematic review and network meta-analysis in accordance with the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extended statement for Network Meta-analyses (PRISMA-NMA) [9].
2.1. Search Strategy
This literature research was conducted for clinical trials from inception to 4 April 2025. Our research took place in three major databases, including PubMed/MEDLINE, Cochrane/CENTRAL, and Scopus, and was limited to papers published in the English language. The search strategy was as follows: (apixaban OR edoxaban OR darexaban OR rivaroxaban OR otamixaban OR direct oral anticoagulants) AND (myocardial ischemia OR acute coronary syndrome OR PCI OR coronary disease OR MI) AND (aspirin OR P2Y12 receptor antagonists OR antiplatelet).
2.2. Inclusion and Exclusion Criteria
Studies were included for the following reasons: (1) randomized controlled trials compared anticoagulant, antiplatelet or combination therapy, (intervention group or control group) in patients with coronary heart disease; (2) patients were administered VKA, NOAC, aspirin or P2Y12 receptor antagonists after coronary heart disease; (3) efficacy and safety endpoints.
Studies were excluded for the following reasons: (1) letters to the editor, reviews, and animal studies; (2) there was a combination of heparin or other non-antithrombotic interventions; (3) the studies were duplicates; (4) not RCT studies.
2.3. Outcome Assessment
Bleeding, death, myocardial infarction, stroke, and embolism were the main outcomes in this net meta-analysis. The safety of antithrombotic drugs was assessed by the rate of bleeding induced by antithrombotic drugs including major bleeding and clinically relevant non-major bleeding. Other outcomes (death, myocardial infarction, stroke, and embolism) represented the side effects of the drug.
2.4. Data Extraction and Assessment of Quality
The identified studies were imported into Endnote X9. Initially, we eliminated redundant studies. Two autonomous researchers (Qingsheng Niu and Ziyi Zhu) conducted data extraction and assessed the quality of references, followed by a cross-validation process. A third researcher (Fulin Wang) was included to conduct the discussion and reach a consensus if there was any disagreement. Basic information, inclusion and exclusion criteria, type of research, sample size, type and dosage of intervention treatment, control group, follow-up and outcome indicators were recorded. A method recommended in the Cochrane Handbook 5.1.0 was used to evaluate the quality [10]. The following aspects were examined: (i) allocation concealment, (ii) sequence generation, (iii) blinding of outcome assessment, (iv) blinding of participants and personnel, (v) selective reporting, (vi) incomplete outcome data, and (vii) other bias.
2.5. Statistical Analysis
Stata software version 17 (StataCorp LLC, College Station, TX, USA) was used for the data net meta-analysis (NMA). STATA packages used include mvmeta, network, st0411, and sencode package. Assessment of heterogeneity was done using RevMan 5.3. The rates of events with each antiplatelet, anticoagulant or combination treatment were entered as an individual study arm, and data were pooled in a multiple treatment NMA that allows integration of direct and indirect comparisons. Heterogeneity was also quantified using chi-square tests and the inconsistency statistic (I2). Heterogeneity was considered significant for values of p < 0.1 and I2 > 50%. When a moderate or high heterogeneity (I2 > 50% and p-value < 0.1) was observed, a random-effect model was employed; otherwise, a fixed-effect model was applied [11]. Odds ratio (OR) with confidence interval (CI) of 95% was adopted as a representative measure of dichotomous outcomes. The level of statistical significance was set as p < 0.05. The area under the cumulative ranking (SUCRA) showed the possibility of each intervention being the best.
3. Results
3.1. Search Results and Characteristics of Included Studies
A total of 2914 articles were retrieved from Medline, Embase, and Cochrane databases. A number of 909 duplicate articles were eliminated using the EndNote and were excluded. On careful assessment of titles and abstracts, 1975 did not qualify according to the inclusion criteria and were excluded. Eventually, 30 RCTs studies were included in this net meta-analysis. Literature retrieval and screening processes were carried out in accordance with the PRISMA guidelines, the special process screening is shown in Figure 1, eight types of interventions were included: VKA, NOAC, DAPT, SAPT, VKA + DAPT, VKA + SAPT, NOAC + DAPT, NOAC + SAPT.
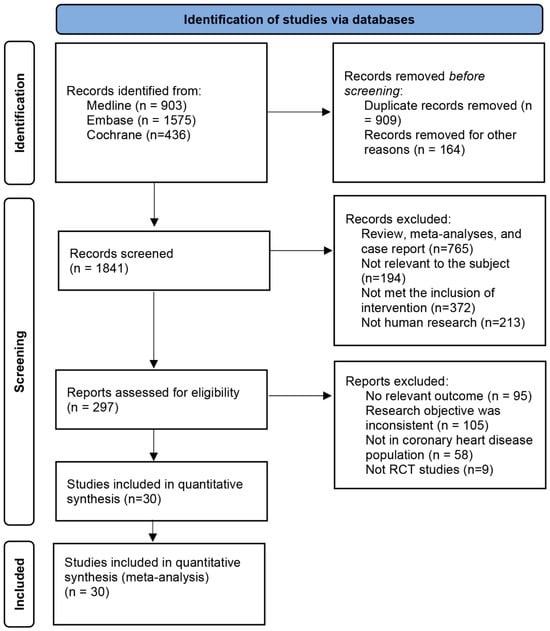
Figure 1.
Process for identifying studies eligible for the network meta-analysis.
Thirty studies with 135,471 patients with atrial fibrillation, ACS, or stable cardiovascular disease were included. The typical characteristics of the studies included are presented in Table 1. The methodological qualities of the included studies are presented in Figure 2. Table 2 illustrates the absolute event rates across various clinical outcomes, highlighting the differences between intervention and control groups.

Table 1.
Basic information of the induced studies.
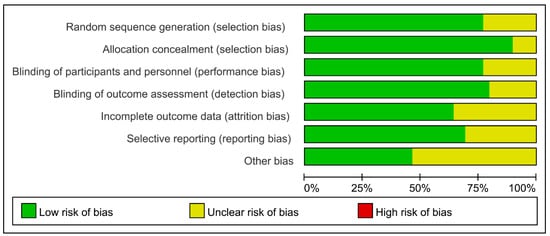
Figure 2.
Methodological quality evaluation of the included studies.

Table 2.
Absolute event rates of different outcomes.
3.2. Effect on Bleeding Event of Different Treatments
Data on the impact of combining anticoagulant medications with antiplatelet therapy on bleeding events were obtained from 29 trials [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] including a total of 97,478 participants. Figure 3 demonstrated a significantly elevated risk of bleeding in VKA + DAPT across all eight treatments, with a SUCRA value of 99.8%. Nevertheless, Figure 4a did not show any notable disparity between SAPT and VKA, with an odds ratio (OR) of 2.12 and a 95% confidence interval (CI) ranging from 0.96 to 4.69. The results indicated that the combination of standard anticoagulants with antiplatelet medications significantly increased the occurrence of bleeding complications. Furthermore, the incidence of bleeding in patients receiving NOAC + DAPT was observed to be greater compared to those receiving NOAC + SAPT, with an odds ratio of 1.53 and a 95% confidence interval ranging from 1.02 to 2.30. The lowest risk of bleeding was observed in SAPT (SUCRA = 0.5%).
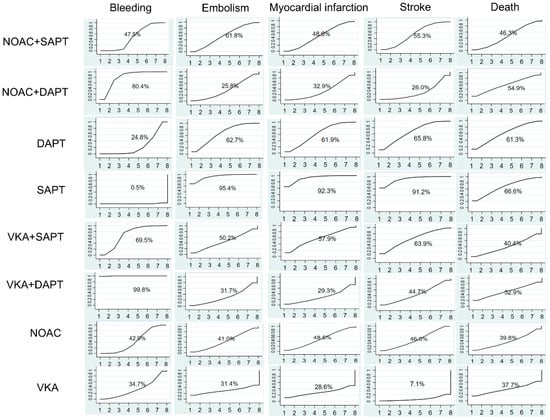
Figure 3.
Ranking of treatment strategies based on probability of their protective effects on outcomes of bleeding, embolism, myocardial infarction, stroke, and death according to the cumulative ranking area (SUCRA).
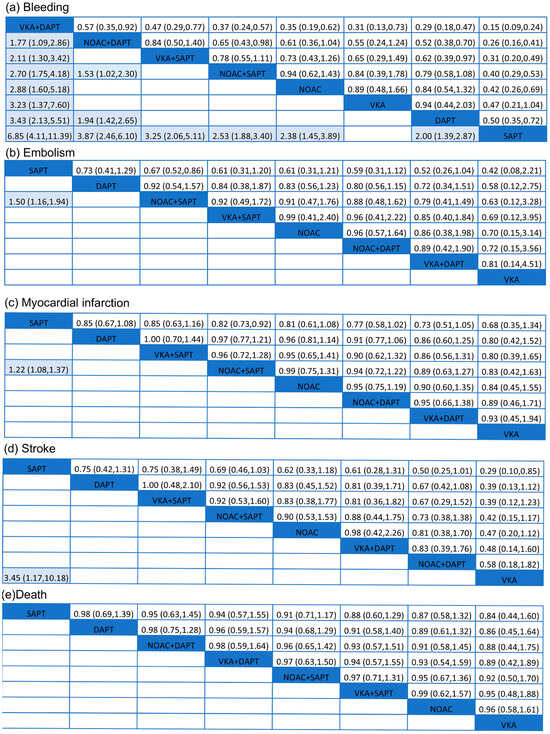
Figure 4.
Summary of results from network meta-analysis (on the lower triangle) and traditional pairwise meta-analysis (on the upper triangle) on primary outcomes: (a) bleeding, (b) embolism, (c) myocardial infarction, (d) stroke, (e) death. On the lower triangle and upper triangle, the row-defining treatment is compared with the column-defining treatment, and ORs > 1 favor the row-defining treatment. Remarkable results are shown in shadow.
3.3. Effect on Embolism Event of Different Treatments
Embolism events were reported from a total of 16 studies [17,18,19,21,22,23,25,26,27,29,30,31,33,36,37,41] of 75,261 patients. As shown in Figure 3, two effective treatments for reducing embolism were VKA (SUCRA 31.4%) and NOAC + DAPT (SUCRA 25.8%). SAPT (SUCRA 95.4%) increased embolism in patients with ACS. NOAC + SAPT significantly reduced embolism events compared with SAPT (OR = 1.50, 95% CI = 1.16–1.94), and other treatments were not superior to SAPT.
3.4. Effect on Myocardial Infarction Event of Different Treatments
A total of 24 studies [12,13,15,16,17,18,19,21,22,23,24,25,26,27,28,29,30,31,33,35,36,38,40,41] of 93,708 patients showed that SAPT experienced a high rate of myocardial infarction (SUCRA 95.6%). Figure 4c showed that, compared with SAPT, NOAC + SAPT (OR= 1.22, 95% CI = 1.08–1.37) decreased myocardial infarction in patients with ACS. For reduction on myocardial infarction, VKA (SUCRA 28.6%) and VKA + DAPT (SUCRA 29.3%) showed the highest safety ranking. On the contrary, SAPT (SUCRA 92.3%), DAPT (SUCRA 61.9%) showed the lowest safety rankings, because they had the highest possibility of myocardial infarction.
3.5. Effect on Stroke Event of Different Treatments
The results concluding 21 trials [12,13,15,16,17,19,21,22,23,24,25,26,27,28,29,30,31,33,35,36,41] of 87,715 patients were demonstrated. In Figure 4d, VKA significantly reduced the rate of stroke compared with SAPT (OR = 3.45, 95% CI = 1.17–10.18). The results indicated that the SUCRA curve (91.2%) of SAPT was highest in those eight different treatments on ACS. VKA group showed the lowest SUCRA curve (7.1%), which was much better than other treatments.
3.6. Effect on Death of Different Treatments
A total of 26 trials, involving 95,493 people, were conducted to assess the impact of anticoagulant therapy and antiplatelet therapy on mortality. The studies referenced are numbered [12,13,15,16,17,18,19,21,23,24,25,26,27,28,29,30,31,32,33,35,36,37,38,40,41]. According to Figure 4e, the eight treatments did not exhibit any significant differences. However, SAPT (SUCRA 66.6%) was found to be the least effective in preventing death. The VKA treatment, with a SUCRA value of 37.7%, demonstrated the highest level of effectiveness.
3.7. Risks of Bias
The results of publication bias in studies contributing to primary outcomes were displayed in Figure 5, and risks of bias in bleeding, myocardial infarction, embolism and death were low. The scatter plot distribution is relatively concentrated. Risk of bias in stroke was high because there were various studies with small sample sizes included. We performed a meta-regression analysis to assess the association between follow-up duration and stroke risk, and the results demonstrated that follow-up time was not a significant source of heterogeneity for stroke outcomes.
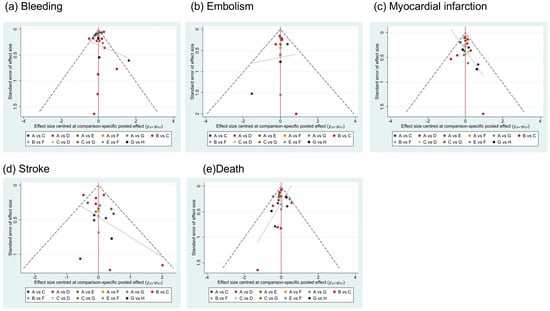
Figure 5.
Publication bias. A = NOAC + SAPT (non-vitamin K antagonist oral anticoagulants plus single-agent platelet inhibitor therapy), B = NOAC + DAPT (non-vitamin K antagonist oral anticoagulants plus dual antiplatelet therapy), C = DAPT (dual antiplatelet therapy), D = SAPT (single-agent platelet inhibitor therapy), E = VKA + SAPT (vitamin K antagonists plus single-agent platelet inhibitor therapy), F = VKA + DAPT (vitamin K antagonists plus dual antiplatelet therapy), G = NOAC (non-vitamin K antagonist oral anticoagulants), H = VKA (vitamin K antagonists).
4. Discussion
This network meta-analysis compared different anticoagulants, antiplatelets, and combinations of both therapies in patients with ACS. The five complications we analyzed in this meta-analysis revealed that NOAC + SAPT could significantly reduce complications and play an important role in treatment effect. However, SAPT showed the lowest effectiveness or safety among these therapies. Although VKA showed the lowest rate of myocardial infarction, stroke, and death, it could not show safety on bleeding. Other treatments showed no significant difference. Overall, our results concluded that NOAC + SAPT shows more safety and effectiveness in the treatment of ACS, which provides new evidence for this field.
Antiplatelet and anticoagulant drugs are central to ACS and post-PCI treatment but increase bleeding risk [42,43]. Balancing bleeding and embolism risks remains a key challenge [44]. Our meta-analysis showed that single antiplatelet therapy (aspirin or P2Y12 inhibitors) in ACS has the highest embolism risk and poorest efficacy, while NOAC monotherapy carries a notable myocardial infarction risk. Although NOACs are generally safer and easier to use than VKAs [45,46], we found no clear benefit over VKAs in ACS, aligning with previous studies [47]. Combination therapies are more effective when monotherapy is insufficient [48]. DAPT lowers bleeding risk but shows considerable embolism and stroke risk, limiting its benefit in ACS. In contrast, VKA or NOAC combined with single antiplatelet therapy offers better thrombosis prevention with higher bleeding risk, but no difference was found between these two combinations. Overall, double antithrombotic therapy is more effective and safer than monotherapy in ACS.
Despite the elevated bleeding risk associated with triple therapy in post-PCI atrial fibrillation patients, European guidelines continue to endorse this approach when the net clinical benefit is favorable [49]. Research by Davide’s team revealed that NOAC plus P2Y12 inhibitor dual therapy had a more favorable safety profile than triple therapy approaches [50]. Toshiki’s meta-analysis showed the risk of stent thrombosis was not significantly different in double antithrombotic therapy vs. triple antithrombotic therapy [51]. Our net meta-analysis analyzed and compared VKA + DAPT and NOAC + DAPT on safety in ACS. NOAC + DAPT trended toward lower rates of stroke compared with VKA + DAPT but did not reach a significantly statistical difference. NOAC + DAPT significantly reduced rate of bleeding compared with VKA + DAPT. Our net meta-analysis indicated that NOAC + DAPT may be a better choice in triple antithrombotic therapy [52,53,54]. Nevertheless, further research is required to determine the effectiveness and safety of VKA + DAPT and NOAC + DAPT. In our analysis, bleeding outcomes were extracted based on the definitions used in the original trials (e.g., TIMI, BARC, GUSTO). Most of the included studies clearly distinguished between major and minor bleeding. However, due to variability in definitions and incomplete reporting across studies, a consistent grading system could not be uniformly applied.
When comparing double antithrombotic therapy strategies to triple antithrombotic therapy, it was shown that the risk of bleeding increased with triple antithrombotic therapy, particularly in the combination of VKA and DAPT. In trials that reported an elevated bleeding risk associated with the combination of VKA and DAPT, the bleeding events were predominantly major, as defined by BARC ≥ 3, TIMI major bleeding, or bleeding requiring transfusion or hospitalization. The observed increase in clinically significant major bleeding with this regimen suggests that its use should generally be avoided. Thus, alternative regimens with lower bleeding risk, such as NOAC-based strategies, are often preferred in contemporary practice to optimize safety without compromising efficacy. Significantly, in this comprehensive meta-analysis, there was minimal or nonexistent variation in the majority of outcomes, with the exception of bleeding. In addition, few research studies compared the safety of triple antithrombotic therapy with anticoagulant or antithrombotic monotherapy. In our net meta-analysis, comparing SAPT, NOAC + DAPT, VKA + DAPT, and NOAC + SAPT resulted in a significantly considerable risk of bleeding. The combination of anticoagulant and antiplatelet therapy was more effective than antiplatelet or anticoagulant therapy alone [7]. Moreover, NOAC + SAPT showed a statistically significant low risk of embolism and myocardial infarction. Of course, the above data demonstrated that combined antithrombotic therapy has certain advantages in patients who need long-term antithrombotic therapy [55]. Triple antithrombotic therapy, which combines anticoagulants with dual antiplatelet therapy, can significantly enhance antithrombotic efficacy but is also associated with a substantially increased risk of bleeding. In patients with ACS, the use of triple therapy should be strictly limited to high-risk individuals with a clear indication for anticoagulation, particularly those with atrial fibrillation, mechanical heart valves, or a history of thromboembolic events. Current mainstream strategies recommend using triple therapy only for a short duration (1 week to 1 month after PCI), followed by de-escalation to dual therapy consisting of an oral anticoagulant and a single P2Y12 inhibitor, aiming to balance thrombosis prevention with bleeding risk [49]. Treatment decisions should be individualized, taking into account the patient’s bleeding risk, thrombotic risk, and renal function. Overall, triple therapy should be used cautiously and reserved for selected patients with a clearly demonstrated benefit, with ongoing clinical monitoring and dynamic adjustments to the regimen.
In our network meta-analysis, heterogeneity in stroke outcomes was notable. We conducted a meta-regression to examine the effect of follow-up duration on stroke outcomes. The results showed no significant association, indicating that follow-up time did not contribute to heterogeneity in stroke risk. Therefore, we speculate that this could be partially attributed to the inclusion of several small-sized trials, increasing random variability and potential bias. Moreover, inconsistencies in stroke definitions and adjudication across studies may have contributed to clinical heterogeneity. Differences in patient baseline characteristics, such as atrial fibrillation prevalence, age, and comorbidities, further complicated the comparability of stroke risk. Additionally, different antithrombotic regimens (e.g., NOACs, VKAs, antiplatelets, or combinations) have varied mechanisms of action and safety profiles. Dabigatran, for instance, is known to increase gastrointestinal bleeding (GIB) risk [56], which may indirectly affect stroke outcomes through altered medication adherence or discontinuation. Taken together, these factors highlight the importance of cautious interpretation of stroke outcomes within indirect comparisons, particularly when data quality or event frequency is limited.
There is a wide variety of antiplatelet agents available. According to the latest guidelines, dual antiplatelet therapy consisting of aspirin combined with a P2Y12 inhibitor is recommended. Ticagrelor or prasugrel is the preferred first-line P2Y12 inhibitor. If these agents are contraindicated, not tolerated, unaffordable, or unavailable, clopidogrel may be used as an alternative option [57]. For patients at high risk of bleeding, current guidelines recommend several strategies. In those with a risk of gastrointestinal bleeding, the use of proton pump inhibitors (PPIs) is advised. Among patients receiving DAPT with ticagrelor, transitioning to ticagrelor monotherapy after at least 1 month post-PCI is suggested. Additionally, in patients requiring long-term oral anticoagulation, it is recommended to discontinue aspirin 1 to 4 weeks after PCI and continue with a P2Y12 inhibitor—preferably clopidogrel [58]. When antiplatelet and/or anticoagulant therapy has to be discontinued due to serious bleeding, clinicians are often compelled to resume anticoagulation therapy after a temporary interruption due to GIB. A meta-analysis suggests that the optimal timing for restarting NOACs is between 15 and 30 days after the GIB event [59]. For patients with a history of intracranial hemorrhage, the resumption of antithrombotic therapy may need to be delayed for 4 to 6 weeks or longer. In individuals previously treated with VKAs or NOACs should be preferred over VKAs upon reinitiation unless contraindicated. Among NOACs, apixaban or edoxaban may be especially suitable for elderly patients or those at high risk of gastrointestinal bleeding [60]. It is important to note that before resuming anticoagulation, patients should be receiving appropriate supportive treatments such as proton pump inhibitors, blood pressure control, and avoidance of NSAIDs. In addition, the risk of recurrent bleeding should be closely monitored [61].
In the context of ACS, transition strategies for anticoagulation require careful planning. When switching from parenteral anticoagulants, such as unfractionated heparin or bivalirudin to oral agents, the approach depends on the specific type of oral anticoagulant being initiated [62]. VKAs typically require an overlap period with parenteral anticoagulants until the target INR is achieved. Once adequate anticoagulation is established, NOACs can usually be started 12 to 24 h after discontinuation of the parenteral agents [63]. Conversely, in situations requiring reversal of oral anticoagulants, such as major bleeding or urgent surgery, parenteral anticoagulants can be reintroduced once bleeding is controlled and the clinical condition is stable. The timing and dosage should be individualized based on bleeding risk, thrombotic risk, and renal function [64]. A multidisciplinary approach involving cardiology, hematology, and critical care is often essential to optimize these transitions. For long-term anticoagulation management in patients with coronary artery disease who may require recurrent hospitalizations, interventions, or surgical revascularization, general ACS guidelines recommend aspirin plus a P2Y12 receptor inhibitor for 12 months, followed by aspirin monotherapy. For patients at high bleeding risk, DAPT can be shortened to 3 to 6 months, followed by single antiplatelet therapy. For those with high ischemic risk but low bleeding risk, extended DAPT or low-dose rivaroxaban combined with aspirin may be considered [65].
Although our meta-analysis provides comparative estimates of efficacy and safety, its applicability to high-risk subpopulations, such as the elderly, patients with chronic kidney disease, or those with a prior bleeding history, requires caution. The SWEDEHEART trial evaluated the efficacy and bleeding risk of ticagrelor versus clopidogrel in elderly patients aged ≥ 75 years. The results indicated that ticagrelor was associated with a significantly increased risk of bleeding in this population, while the reduction in ischemic events was limited [66]. The POPular AGE trial showed that clopidogrel significantly reduced bleeding events without a notable difference in ischemic protection. These findings suggest that elderly patients may benefit more from a conservative antiplatelet strategy, and potent antiplatelet agents should be used with caution in this population [67]. The CREST Study indicated that ACS patients with an eGFR < 60 mL/min face elevated risks of both bleeding and major adverse cardiovascular events (MACE) during DAPT, highlighting the need for individualized risk assessment in this population [68]. These groups are frequently encountered in clinical settings but are often underrepresented in randomized controlled trials. Given their increased susceptibility to both ischemic and hemorrhagic complications, individualized antithrombotic strategies may be warranted. Further research leveraging real-world data or patient-level meta-analyses is needed to optimize care in these vulnerable populations.
This meta-analysis has several important limitations that should be acknowledged. First, by focusing on eight specific therapies and excluding heparin or placebo-controlled trials, the strength of our network analysis conclusions may be limited. Second, insufficient primary data prevented subgroup analyses by treatment stages, leaving uncertainty about therapy effects on complications and mortality in ACS patients. Third, many treatment combinations lacked direct comparisons, requiring reliance on indirect effect estimates. Additionally, variability in outcome definitions, follow-up durations, and adjudication criteria across studies may have influenced results. Our classification of drugs by mechanism rather than individual agents may obscure important safety profile differences. A notable limitation is the uncertain duration of triple therapy (NOAC + DAPT), as most protocols transition to dual therapy within 1–2 weeks, yet precise timing data were unavailable. Fourth, our analysis does not actually differentiate between bare-metal stents and drug-eluting stents, despite their known impact on antithrombotic therapy selection and duration in ACS and PCI patients. Therefore, further prospective studies are warranted to evaluate the safety and efficacy of various antithrombotic strategies across different stent types. Finally, while VKA and NOAC showed numerically lower risks of embolism, death, and MI compared to SAPT, these differences were not statistically significant. These limitations underscore the need for standardized outcome reporting, direct comparative studies, and larger-scale investigations to better characterize the efficacy and safety of antithrombotic therapies in ACS patients.
5. Conclusions
This network meta-analysis compared the safety of eight different antithrombotic therapies in patients with ACS. A strategy of dual therapy, especially with NOAC + SAPT, should be a reasonable alternative to triple therapy or monotherapy in patients with ACS indication for chronic anticoagulation. However, future studies are needed to judge the safety and effect of different antithrombotic therapies, due to their drawbacks on other complications.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13082027/s1. References [3,8,48,69,70,71,72] are cited in the supplementary materials.
Author Contributions
Q.N. conducted data acquisition, performed statistical analyses, and contributed to results interpretation. He participated in literature searches, drafted the initial manuscript, and assisted in revising the content for accuracy and clarity. Y.J. played a pivotal role in securing funding and contributed to the study design, particularly in data analysis strategies. He supervised data collection, resolved discrepancies in data interpretation, and validated the analytical results. Y.J. also led the literature synthesis, drafted key manuscript sections, and provided critical revisions to enhance clinical and methodological relevance. Z.Z. was involved in systematic data collection, processing, and preliminary analysis. He contributed to the literature review and supported the integration of findings into the manuscript. F.W. assisted in data curation, quality control of extracted datasets, and preliminary data visualization. He also participated in reviewing relevant literature and verifying result consistency. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Supported by Science and Technology Projects of Xizang Autonomous Region, China (Grant No. XZ202501ZY0120).
Institutional Review Board Statement
None is sought or required as this is a secondary synthesis of already available data.
Informed Consent Statement
Consent does not apply to this review.
Data Availability Statement
The following information was supplied regarding data availability: This is a systematic review/meta-analysis. All data generated or analyzed during this study are included in Supplementary Information Files.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, E93–E621. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Lopes, R.D.; Harrington, R.A. Diagnosis and Treatment of Acute Coronary Syndromes A Review. JAMA J. Am. Med. Assoc. 2022, 327, 662–675. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Hulot, J.S.; Moliterno, D.J.; Harrington, R.A. Antiplatelet and Anticoagulation Therapy for Acute Coronary Syndromes. Circ. Res. 2014, 114, 1929–1943. [Google Scholar] [CrossRef]
- Kamran, H.; Jneid, H.; Kayani, W.T.; Virani, S.S.; Levine, G.N.; Nambi, V.; Khalid, U. Oral Antiplatelet Therapy After Acute Coronary Syndrome: A Review. JAMA J. Am. Med. Assoc. 2021, 325, 1545–1555. [Google Scholar] [CrossRef]
- Rodriguez, F.; Harrington, R.A. Management of Antithrombotic Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2021, 384, 452–460. [Google Scholar] [CrossRef]
- Harenberg, J.; Marx, S.; Krejczy, M.; Wehling, M. New anticoagulants—Promising and failed developments. Br. J. Pharmacol. 2012, 165, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Hirsh, J. Combined antiplatelet and anticoagulant therapy: Clinical benefits and risks. J. Thromb. Haemost. 2007, 5, 255–263. [Google Scholar] [CrossRef]
- Liu, L.L.; Lei, H.; Hu, J.H.; Tang, Y.; Xu, D.Y. Direct Oral Anticoagulants Combined with Antiplatelet Therapy in the Treatment of Coronary Heart Disease: An Updated Meta-analysis. Drugs 2021, 81, 2003–2016. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M. Cochrane Handbook for Systematic Reviews of Interventions. Am. J. Public Health 2020, 110, 753–754. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Tangelder, M.J.; Frison, L.; Weaver, D.; Wilcox, R.G.; Bylock, A.; Emanuelsson, H.; Held, P.; Oldgren, J. Effect of ximelagatran on ischemic events and death in patients with atrial fibrillation after acute myocardial infarction in the efficacy and safety of the oral direct thrombin inhibitor ximelagatran in patients with recent myocardial damage (ESTEEM) trial. Am. Heart J. 2008, 155, 382–387. [Google Scholar] [CrossRef]
- APPRAISE Steering Committee and Investigators; Alexander, J.H.; Becker, R.C.; Bhatt, D.L.; Cools, F.; Crea, F.; Dellborg, M.; Fox, K.A.; Goodman, S.G.; Harrington, R.A.; et al. Apixaban, an Oral, Direct, Selective Factor Xa Inhibitor, in Combination With Antiplatelet Therapy After Acute Coronary Syndrome Results of the Apixaban for Prevention of Acute Ischemic and Safety Events (APPRAISE) Trial. Circulation 2009, 119, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Mega, J.L.; Braunwald, E.; Mohanavelu, S.; Burton, P.; Poulter, R.; Misselwitz, F.; Hricak, V.; Barnathan, E.S.; Bordes, P.; Witkowski, A.; et al. ATLAS ACS-TIMI 46 study group. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): A randomised, double-blind, phase II trial. Lancet 2009, 374, 29–38. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Antman, E.M.; Widimsky, P.; Ebrahim, I.O.; Kiss, R.G.; Saaiman, A.; Polasek, R.; Contant, C.F.; McCabe, C.H.; Braunwald, E. Otamixaban for the treatment of patients with non-ST-elevation acute coronary syndromes (SEPIA-ACS1 TIMI 42): A randomised, double-blind, active-controlled, phase 2 trial. Lancet 2009, 374, 787–795. [Google Scholar] [CrossRef]
- Oldgren, J.; Budaj, A.; Granger, C.B.; Khder, Y.; Roberts, J.; Siegbahn, A.; Tijssen, J.G.; Van de Werf, F.; Wallentin, L. RE-DEEMInvestigators Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: A randomized double-blind phase II trial. Eur. Heart J. 2011, 32, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.H.; Lopes, R.D.; James, S.; Kilaru, R.; He, Y.; Mohan, P.; Bhatt, D.L.; Goodman, S.; Verheugt, F.W.; Flather, M.; et al. APPRAISE-2 Investigators. Apixaban with Antiplatelet Therapy after Acute Coronary Syndrome. N. Engl. J. Med. 2011, 365, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Steg, P.G.; Mehta, S.R.; Jukema, J.W.; Lip, G.Y.; Gibson, C.M.; Kovar, F.; Kala, P.; Garcia-Hernandez, A.; Renfurm, R.W.; Granger, C.B. RUBY-1 InvestigatorsRUBY-1: A randomized double-blind placebo-controlled trial of the safety tolerability of the novel oral factor Xa inhibitor darexaban (YM150) following acute coronary syndrome. Eur. Heart J. 2011, 32, 2541–2554. [Google Scholar] [CrossRef]
- Mega, J.L.; Braunwald, E.; Wiviott, S.D.; Bassand, J.P.; Bhatt, D.L.; Bode, C.; Burton, P.; Cohen, M.; Cook-Bruns, N.; Fox, K.A.; et al. ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in Patients with a Recent Acute Coronary Syndrome. N. Engl. J. Med. 2012, 366, 9–19. [Google Scholar] [CrossRef]
- Ogawa, H.; Goto, S.; Matsuzaki, M.; Hiro, S.; Shima, D.; Investigators, A.-J. Randomized, Double-Blind Trial to Evaluate the Safety of Apixaban With Antiplatelet Therapy After Acute Coronary Syndrome in Japanese Patients (APPRAISE-J). Circ. J. 2013, 77, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Steg, P.G.; Mehta, S.R.; Pollack, C.V., Jr.; Bode, C.; Cohen, M.; French, W.J.; Hoekstra, J.; Rao, S.V.; Ruzyllo, W.; Ruiz-Nodar, J.M.; et al. TAO Investigators. Anticoagulation With Otamixaban and Ischemic Events in Non-ST-Segment Elevation Acute Coronary Syndromes The TAO Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2013, 310, 1145–1155. [Google Scholar] [CrossRef]
- Dewilde, W.J.; Oirbans, T.; Verheugt, F.W.; Kelder, J.C.; De Smet, B.J.; Herrman, J.P.; Adriaenssens, T.; Vrolix, M.; Heestermans, A.A.; Vis, M.M.; et al. WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013, 381, 1107–1115. [Google Scholar] [CrossRef]
- Gibson, C.M.; Mehran, R.; Bode, C.; Halperin, J.; Verheugt, F.W.; Wildgoose, P.; Birmingham, M.; Ianus, J.; Burton, P.; van Eickels, M.; et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N. Engl. J. Med. 2016, 375, 2423–2434. [Google Scholar] [CrossRef]
- Xu, H.; Ruff, C.T.; Giugliano, R.P.; Murphy, S.A.; Nordio, F.; Patel, I.; Shi, M.; Mercuri, M.; Antman, E.M.; Braunwald, E. Concomitant Use of Single Antiplatelet Therapy With Edoxaban or Warfarin in Patients With Atrial Fibrillation: Analysis From the ENGAGE AF-TIMI48 Trial. J. Am. Heart Assoc. 2016, 5, e002587. [Google Scholar] [CrossRef] [PubMed]
- Ohman, E.M.; Roe, M.T.; Steg, P.G.; James, S.K.; Povsic, T.J.; White, J.; Rockhold, F.; Plotnikov, A.; Mundl, H.; Strony, J.; et al. Clinically significant bleeding with low-dose rivaroxaban versus aspirin, in addition to P2Y12 inhibition, in acute coronary syndromes (GEMINI-ACS-1): A double-blind, multicentre, randomised trial. Lancet 2017, 389, 1799–1808. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. COMPASS Investigators. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef]
- Cannon, C.P.; Bhatt, D.L.; Oldgren, J.; Lip, G.Y.H.; Ellis, S.G.; Kimura, T.; Maeng, M.; Merkely, B.; Zeymer, U.; Gropper, S.; et al. RE-DUAL PCI Steering Committee and Investigators. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N. Engl. J. Med. 2017, 377, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Kopin, D.; Jones, W.S.; Sherwood, M.W.; Wojdyla, D.M.; Wallentin, L.; Lewis, B.S.; Verheugt, F.W.A.; Vinereanu, D.; Bahit, M.C.; Halvorsen, S.; et al. Percutaneous coronary intervention and antiplatelet therapy in patients with atrial fibrillation receiving apixaban or warfarin: Insights from the ARISTOTLE trial. Am. Heart J. 2018, 197, 133–141. [Google Scholar] [CrossRef]
- Zannad, F.; Anker, S.D.; Byra, W.M.; Cleland, J.G.F.; Fu, M.; Gheorghiade, M.; Lam, C.S.P.; Mehra, M.R.; Neaton, J.D.; Nessel, C.C.; et al. COMMANDER HF Investigators. Rivaroxaban in Patients with Heart Failure, Sinus Rhythm, and Coronary Disease. N. Engl. J. Med. 2018, 379, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Hellkamp, A.S.; Becker, R.C.; Berkowitz, S.D.; Breithardt, G.; Fox, K.A.A.; Hacke, W.; Halperin, J.L.; Hankey, G.J.; Mahaffey, K.W.; et al. Impact of polyvascular disease on patients with atrial fibrillation: Insights from ROCKET AF. Am. Heart J. 2018, 200, 102–109. [Google Scholar] [CrossRef]
- Lopes, R.D.; Heizer, G.; Aronson, R.; Vora, A.N.; Massaro, T.; Mehran, R.; Goodman, S.G.; Windecker, S.; Darius, H.; Li, J.; et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N. Engl. J. Med. 2019, 380, 1509–1524. [Google Scholar] [CrossRef]
- Vranckx, P.; Valgimigli, M.; Eckardt, L.; Tijssen, J.; Lewalter, T.; Gargiulo, G.; Batushkin, V.; Campo, G.; Lysak, Z.; Vakaliuk, I.; et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): A randomised, open-label, phase 3b trial. Lancet 2019, 394, 1335–1343. [Google Scholar] [CrossRef]
- Matsumura-Nakano, Y.; Shizuta, S.; Komasa, A.; Morimoto, T.; Masuda, H.; Shiomi, H.; Goto, K.; Nakai, K.; Ogawa, H.; Kobori, A.; et al. OAC-ALONE Study Investigators. Open-Label Randomized Trial Comparing Oral Anticoagulation with and Without Single Antiplatelet Therapy in Patients with Atrial Fibrillation and Stable Coronary Artery Disease Beyond 1 Year After Coronary Stent Implantation: OAC-ALONE Study. Circulation 2019, 139, 604–616. [Google Scholar] [CrossRef]
- Franchi, F.; Rollini, F.; Garcia, E.; Rivas Rios, J.; Rivas, A.; Agarwal, M.; Kureti, M.; Nagaraju, D.; Wali, M.; Briceno, M.; et al. Effects of Edoxaban on the Cellular and Protein Phase of Coagulation in Patients with Coronary Artery Disease on Dual Antiplatelet Therapy with Aspirin and Clopidogrel: Results of the EDOX-APT Study. Thromb. Haemost. 2020, 120, 83–93. [Google Scholar] [CrossRef]
- Akao, M.; Yasuda, S.; Kaikita, K.; Ako, J.; Matoba, T.; Nakamura, M.; Miyauchi, K.; Hagiwara, N.; Kimura, K.; Hirayama, A.; et al. Rivaroxaban monotherapy versus combination therapy according to patient risk of stroke and bleeding in atrial fibrillation and stable coronary disease: AFIRE trial subanalysis. Am. Heart J. 2021, 236, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.B.; Wang, L.L.; Zhou, M.C.; Feng, L.L. Efficacy and safety of rivaroxaban and ticagrelor in elderly patients with atrial fibrillation undergoing percutaneous coronary intervention. Contemp. Clin. Trials 2021, 104, 106365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Si, D.; Zhang, Q.; Jin, L.; Zheng, H.; Qu, M.; Yu, M.; Jiang, Z.; Li, D.; Li, S.; et al. Prophylactic Rivaroxaban Therapy for Left Ventricular Thrombus After Anterior ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Interv. 2022, 15, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Frigoli, E.; Vranckx, P.; Ozaki, Y.; Morice, M.C.; Chevalier, B.; Onuma, Y.; Windecker, S.; Delorme, L.; Kala, P.; et al. Impact of Medication Nonadherence in a Clinical Trial of Dual Antiplatelet Therapy. J. Am. Coll. Cardiol. 2022, 80, 766–778. [Google Scholar] [CrossRef]
- Kanagaratnam, P.; Francis, D.P.; Chamie, D.; Coyle, C.; Marynina, A.; Katritsis, G.; Paiva, P.; Szigeti, M.; Cole, G.; de Andrade Nunes, D.; et al. A randomized controlled trial to investigate the use of acute coronary syndrome therapy in patients hospitalized with COVID-19: The COVID-19 Acute Coronary Syndrome trial. J. Thromb. Haemost. 2023, 21, 2213–2222. [Google Scholar] [CrossRef]
- Gue, Y.X.; Memtsas, V.; Kanji, R.; Wellsted, D.M.; Busby, A.; Smith, M.; Vilar, E.; Ryding, A.; Arachchillage, D.J.; Gorog, D.A. Impact of very low dose rivaroxaban in addition to dual antiplatelet therapy on endogenous fibrinolysis in acute coronary syndrome: The VaLiDate-R study. Thromb. Res. 2024, 236, 144–154. [Google Scholar] [CrossRef]
- Ge, Z.; Kan, J.; Gao, X.; Raza, A.; Zhang, J.J.; Mohydin, B.S.; Gao, F.; Shao, Y.; Wang, Y.; Zeng, H.; et al. Ticagrelor alone versus ticagrelor plus aspirin from month 1 to month 12 after percutaneous coronary intervention in patients with acute coronary syndromes (ULTIMATE-DAPT): A randomised, placebo-controlled, double-blind clinical trial. Lancet 2024, 403, 1866–1878. [Google Scholar] [CrossRef] [PubMed]
- Tersalvi, G.; Biasco, L.; Cioffi, G.M.; Pedrazzini, G. Acute Coronary Syndrome, Antiplatelet Therapy, and Bleeding: A Clinical Perspective. J. Clin. Med. 2020, 9, 2064. [Google Scholar] [CrossRef]
- Albaladejo, P.; Samama, C.M.; Sié, P.; Kauffmann, S.; Mémier, V.; Suchon, P.; Viallon, A.; David, J.S.; Gruel, Y.; Bellamy, L.; et al. Management of Severe Bleeding in Patients Treated with Direct Oral Anticoagulants. Anesthesiology 2017, 127, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Gall, E.; Lafont, A.; Varenne, O.; Dumas, F.; Cariou, A.; Picard, F. Balancing thrombosis and bleeding after out-of-hospital cardiac arrest related to acute coronary syndrome: A literature review. Arch. Cardiovasc. Dis. 2021, 114, 667–679. [Google Scholar] [CrossRef]
- Moon, J.Y.; Nagaraju, D.; Franchi, F.; Rollini, F.; Angiolillo, D.J. The role of oral anticoagulant therapy in patients with acute coronary syndrome. Ther. Adv. Hematol. 2017, 8, 353–366. [Google Scholar] [CrossRef]
- Byrne, R.A.; Colleran, R.; Kastrati, A. Omission of aspirin after ACS or stenting in patients with oral anticoagulation—Why have the goalposts moved? Eurointervention 2019, 14, 1793–1795. [Google Scholar] [CrossRef]
- Abadie, B.Q.; Cannon, C.P.; Cavender, M.A. Novel Oral Anticoagulants Following Percutaneous Coronary Intervention. Circ-Cardiovasc Interv. 2020, 13, e008465. [Google Scholar] [CrossRef]
- Dimitriadis, K.; Soulaidopoulos, S.; Doundoulakis, I.; Iliakis, P.; Tsiachris, D.; Tsioufis, P.; Beneki, E.; Sakalidis, A.; Pagkalidou, E.; Tsiamis, E.; et al. A network meta-analysis of the antithrombotic strategies in patients with atrial fibrillation and percutaneous coronary interventions: Focus on bleeding. Hell. J. Cardiol. 2023, 73, 69–72. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Bhatt, D.L.; Cannon, C.P.; Eikelboom, J.W.; Gibson, C.M.; Goodman, S.G.; Granger, C.B.; Holmes, D.R.; Lopes, R.D.; Mehran, R.; et al. Antithrombotic Therapy in Patients with Atrial Fibrillation Treated with Oral Anticoagulation Undergoing Percutaneous Coronary Intervention a North American Perspective: 2021 Update. Circulation 2021, 143, 583–596. [Google Scholar] [CrossRef]
- Capodanno, D.; Huber, K.; Mehran, R.; Lip, G.Y.H.; Faxon, D.P.; Granger, C.B.; Vranckx, P.; Lopes, R.D.; Montalescot, G.; Cannon, C.P.; et al. Management of Antithrombotic Therapy in Atrial Fibrillation Patients Undergoing PCI State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Kuno, T.; Ueyama, H.; Takagi, H.; Bangalore, S. The risk of stent thrombosis of dual antithrombotic therapy for patients who require oral anticoagulant undergoing percutaneous coronary intervention: Insights of a meta-analysis of randomized trials. Scand. Cardiovasc. J. 2022, 56, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Shurrab, M.; Danon, A.; Alnasser, S.; Glover, B.; Kaoutskaia, A.; Henderson, M.; Newman, D.; Crystal, E.; Ko, D. Dual-Antithrombotic Therapy with DOACs After Acute Coronary Syndrome or Percutaneous Coronary Intervention in Atrial Fibrillation: A Meta-analysis of Randomized Controlled Trials. Can. J. Cardiol. 2020, 36, 135–142. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, L.; Liu, F.; Wan, R.; Shen, Y.; Lip, G.Y.H.; Hong, K. Efficacy and safety of triple versus dual antithrombotic therapy in atrial fibrillation and ischemic heart disease: A systematic review and meta-analysis. Oncotarget 2017, 8, 81154–81166. [Google Scholar] [CrossRef]
- Shin, D.; Mohanty, B.D.; Lee, E.S. Dual versus triple antithrombotic therapy after percutaneous coronary intervention or acute coronary syndrome in patients with indication for anticoagulation: An updated meta-analysis. Coron. Artery Dis. 2018, 29, 670–680. [Google Scholar] [CrossRef]
- Bor, W.; Gorog, D.A. Antithrombotic Therapy in Patients with Atrial Fibrillation and Acute Coronary Syndrome. J. Clin. Med. 2020, 9, 2020. [Google Scholar] [CrossRef]
- Niu, Q.; Yang, G.; Liu, P.; Jiang, Y. A review on increasing risk for gastrointestinal bleeding associated with dabigatran. Signa Vitae 2024, 20, 13–21. [Google Scholar] [CrossRef]
- Bainey, K.R.; Marquis-Gravel, G.; Belley-Côté, E.; Turgeon, R.D.; Ackman, M.L.; Babadagli, H.E.; Bewick, D.; Boivin-Proulx, L.A.; Cantor, W.J.; Fremes, S.E.; et al. Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology 2023 Focused Update of the Guidelines for the Use of Antiplatelet Therapy. Can. J. Cardiol. 2024, 40, 160–181. [Google Scholar] [CrossRef]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients With Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2025, 151, e771–e862. [Google Scholar] [CrossRef] [PubMed]
- Slouha, E.; Jensen, H.; Fozo, H.; Raj, R.; Thomas, S.; Gorantla, V. Re-starting anticoagulation and antiplatelets after gastrointestinal bleeding: A systematic review. F1000Research 2023, 12, 806. [Google Scholar] [CrossRef] [PubMed]
- Carlin, S.; Eikelboom, J. Restarting Anticoagulation After Major Bleeding in Patients with Atrial Fibrillation. Can. J. Cardiol. 2024, 40, 1291–1293. [Google Scholar] [CrossRef]
- Tomaselli, G.F.; Mahaffey, K.W.; Cuker, A.; Dobesh, P.P.; Doherty, J.U.; Eikelboom, J.W.; Florido, R.; Gluckman, T.J.; Hucker, W.J.; Mehran, R.; et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020, 76, 594–622. [Google Scholar] [CrossRef]
- Smythe, M.A.; Priziola, J.; Dobesh, P.P.; Wirth, D.; Cuker, A.; Wittkowsky, A.K. Guidance for the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 165–186. [Google Scholar] [CrossRef]
- Ballestri, S.; Romagnoli, E.; Arioli, D.; Coluccio, V.; Marrazzo, A.; Athanasiou, A.; Di Girolamo, M.; Cappi, C.; Marietta, M.; Capitelli, M. Risk and Management of Bleeding Complications with Direct Oral Anticoagulants in Patients with Atrial Fibrillation and Venous Thromboembolism: A Narrative Review. Adv. Ther. 2023, 40, 41–66. [Google Scholar] [CrossRef]
- Yang, J.; Jing, J.; Chen, S.; Liu, X.; Wang, J.; Pan, C.; Tang, Z. Reversal and resumption of anticoagulants in patients with anticoagulant-associated intracerebral hemorrhage. Eur. J. Med. Res. 2024, 29, 252. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. ESCScientific Document Group 2020 ESCGuidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367, Erratum in: Eur Heart J. 2021, 42, 1908. [Google Scholar] [CrossRef] [PubMed]
- Szummer, K.; Montez-Rath, M.E.; Alfredsson, J.; Erlinge, D.; Lindahl, B.; Hofmann, R.; Ravn-Fischer, A.; Svensson, P.; Jernberg, T. Comparison Between Ticagrelor and Clopidogrel in Elderly Patients with an Acute Coronary Syndrome: Insights From the SWEDEHEART Registry. Circulation 2020, 142, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Gimbel, M.; Qaderdan, K.; Willemsen, L.; Hermanides, R.; Bergmeijer, T.; de Vrey, E.; Heestermans, T.; Tjon Joe Gin, M.; Waalewijn, R.; Hofma, S.; et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): The randomised, open-label, non-inferiority trial. Lancet 2020, 395, 1374–1381. [Google Scholar] [CrossRef]
- Baber, U.; Chandrasekhar, J.; Sartori, S.; Aquino, M.; Kini, A.S.; Kapadia, S.; Weintraub, W.; Muhlestein, J.B.; Vogel, B.; Faggioni, M.; et al. Associations Between Chronic Kidney Disease and Outcomes With Use of Prasugrel Versus Clopidogrel in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention: A Report From the PROMETHEUS Study. JACC Cardiovasc. Interv. 2017, 10, 2017–2025. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cai, Z.; Dong, S.; Liu, H.; Pang, X.; Chen, Q.; Yuan, J.; Geng, Q. Comparative efficacy and safety of antiplatelet or anticoagulant therapy in patients with chronic coronary syndromes after percutaneous coronary intervention: A network meta-analysis of randomized controlled trials. Front. Pharmacol. 2022, 13, 992376. [Google Scholar] [CrossRef]
- Capodanno, D.; Di Maio, M.; Greco, A.; Bhatt, D.L.; Gibson, C.M.; Goette, A.; Lopes, R.D.; Mehran, R.; Vranckx, P.; Angiolillo, D.J. Safety and Efficacy of Double Antithrombotic Therapy With Non-Vitamin K Antagonist Oral Anticoagulants in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e017212. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A.; Mhanna, M.; Alyosif, M.; Pena, C.; Jabr, A.; Alsughayer, A.; Alfatlawi, H.; Safi, M.; Aldhafeeri, A.; Patel, N.; et al. Safety and Efficacy of Direct Oral Anticoagulant in Addition to Antiplatelet Therapy After Acute Coronary Syndrome: A Systemic Review and Meta-analysis of 53,869 Patients. Clin. Ther. 2024, 46, e1–e6. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).