Revolutionizing Allogeneic Graft Tolerance Through Chimeric Antigen Receptor-T Regulatory Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Conventional Tregs (Tconv) Have Limited Suppression Capacity
3.2. HLA-A2 as the Basis of Immune Tolerance
3.3. Hypo-Responsive and Metabolic Exhaustion in Tregs
3.4. FOXP3+: A Major Consensus of Treg Function
3.5. Strength and Compatibility of Co-Stimulatory Domain
3.6. Biodistribution of CAR Tregs Key to Early Immune Desensitization
3.7. Complementary Action with Concomitant Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABMR | antibody-mediated rejection |
| ABO | blood group antigen system |
| APC | antigen-presenting cell |
| APRIL | a proliferation-inducing ligand |
| BCMA | B cell maturation antigen |
| CAR | chimeric antigen receptor |
| CTLA | cytotoxic T-lymphocyte-associated protein |
| DC | dendritic cell |
| DTH | delayed-type hypersensitivity |
| EBV | Epstein–Barr virus |
| FKBP | FK506-binding protein |
| GITR | glucocorticoid-induced TNFR-related protein |
| GMP | good manufacturing practice |
| GVHD | graft-versus-host disease |
| HLA | human leukocyte antigen |
| IFN | interferon |
| IL | interleukin |
| IT | intratumoral |
| IV | intravenous |
| LLPC | long-lived plasma cell |
| MHC | major histocompatibility complex |
| mTOR | mammalian target of rapamycin |
| NK | natural killer |
| NSG | NOD scid gamma (mouse strain) |
| NT | no treatment |
| PD-1 | programmed death 1 |
| PTLD | post-transplant lymphoproliferative disorder |
| TCR | T cell receptor |
| TGF-β | transforming growth factor |
| TNF-α | tumor necrosis factor alpha |
| UT | untransduced |
| VCAM | vascular cell adhesion molecule 1 |
References
- Shafiekhani, M.; Shahabinezhad, F.; Tavakoli, Z.; Tarakmeh, T.; Haem, E.; Sari, N.; Nasirabadi, S.; Dehghani, M. Quality of life Associated with Immunosuppressant Treatment Adherence in Liver Transplant Recipients: A Cross-Sectional Study. Front. Pharmacol. 2023, 14, 1051350. [Google Scholar] [CrossRef] [PubMed]
- Allen, U.D.; Preiksaitis, J.K.; AST Infectious Diseases Community of Practice. Post-Transplant Lymphoproliferative Disorders, Epstein-Barr Virus Infection, and Disease in Solid Organ Transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13652. [Google Scholar] [CrossRef] [PubMed]
- Rudensky, A.Y. Regulatory T Cells and Foxp3. Immunol. Rev. 2011, 241, 260. [Google Scholar] [CrossRef] [PubMed]
- Rueda, C.M.; Jackson, C.M.; Chougnet, C.A. Regulatory T-Cell-Mediated Suppression of Conventional T-Cells and Dendritic Cells by Different CAMP Intracellular Pathways. Front. Immunol. 2016, 7, 195682. [Google Scholar] [CrossRef] [PubMed]
- Kaech, S.M.; Wherry, E.J.; Ahmed, R. Effector and Memory T-Cell Differentiation: Implications for Vaccine Development. Nat. Rev. Immunol. 2002, 2, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, E.R.; Lorenz, U.M. Breaking Free of Control: How Conventional T Cells Overcome Regulatory T Cell Suppression. Front. Immunol. 2016, 7, 196858. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhuri, R.; Eil, R.L.; Restifo, N.P. The Interplay of Effector and Regulatory T Cells in Cancer. Curr. Opin. Immunol. 2015, 33, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Lysandrou, M.; Kefala, D.; Vinnakota, J.M.; Savvopoulos, N.; Zeiser, R.; Spyridonidis, A. Regulatory T Cell Therapy for Graft-versus-Host Disease. Bone Marrow Transplant. 2025, 60, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Im, K.I.; Song, Y.; Kim, N.; Nam, Y.S.; Jeon, Y.W.; Cho, S.G. Third-Party Regulatory T Cells Prevent Murine Acute Graft-versus-Host Disease. Korean J. Intern. Med. 2017, 33, 980. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Arany, Z.; Baur, J.A.; Epstein, J.A.; June, C.H. CAR T Therapy beyond Cancer: The Evolution of a Living Drug. Nature 2023, 619, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine Release Syndrome and Associated Neurotoxicity in Cancer Immunotherapy. Nat. Rev. Immunol. 2021, 22, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.; Hennessy, C.; Hester, J.; Issa, F. Chimeric Antigen Receptors and Regulatory T Cells: The Potential for HLA-Specific Immunosuppression in Transplantation. Engineering 2022, 10, 30–43. [Google Scholar] [CrossRef]
- Cassano, A.; Chong, A.S.; Alegre, M.-L. Tregs in Transplantation Tolerance: Role and Therapeutic Potential. Front. Transplant. 2023, 2, 1217065. [Google Scholar] [CrossRef] [PubMed]
- Sicard, A.; Lamarche, C.; Speck, M.; Wong, M.; Rosado-Sánchez, I.; Blois, M.; Glaichenhaus, N.; Mojibian, M.; Levings, M.K. Donor-Specific Chimeric Antigen Receptor Tregs Limit Rejection in Naive but Not Sensitized Allograft Recipients. Am. J. Transplant. 2020, 20, 1562–1573. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh Kozani, P.; Safarzadeh Kozani, P.; Rahbarizadeh, F.; Khoshtinat Nikkhoi, S. Strategies for Dodging the Obstacles in CAR T Cell Therapy. Front. Oncol. 2021, 11, 627549. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Haining, W.N.; Held, W.; Hogan, P.G.; Kallies, A.; Lugli, E.; Lynn, R.C.; Philip, M.; Rao, A.; Restifo, N.P.; et al. Defining ‘T Cell Exhaustion’. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Noyan, F.; Zimmermann, K.; Hardtke-Wolenski, M.; Knoefel, A.; Schulde, E.; Geffers, R.; Hust, M.; Huehn, J.; Galla, M.; Morgan, M.; et al. Prevention of Allograft Rejection by Use of Regulatory T Cells With an MHC-Specific Chimeric Antigen Receptor. Am. J. Transplant. 2017, 17, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Boardman, D.A.; Philippeos, C.; Fruhwirth, G.O.; Ibrahim, M.A.A.; Hannen, R.F.; Cooper, D.; Marelli-Berg, F.M.; Watt, F.M.; Lechler, R.I.; Maher, J.; et al. Expression of a Chimeric Antigen Receptor Specific for Donor HLA Class I Enhances the Potency of Human Regulatory T Cells in Preventing Human Skin Transplant Rejection. Am. J. Transplant. 2017, 17, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Imura, Y.; Ando, M.; Kondo, T.; Ito, M.; Yoshimura, A. CD19-Targeted CAR Regulatory T Cells Suppress B Cell Pathology without GvHD. JCI Insight 2020, 5, e136185. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.M.E.; Migneco, G.; Scott, G.B.; Down, J.; King, S.; Askar, B.; Jennings, V.; Oyajobi, B.; Scott, K.; West, E.; et al. Reovirus-Induced Cell-Mediated Immunity for the Treatment of Multiple Myeloma within the Resistant Bone Marrow Niche. J. Immunother. Cancer 2021, 9, e001803. [Google Scholar] [CrossRef] [PubMed]
- Lamarche, C.; Ward-Hartstonge, K.; Mi, T.; Lin, D.T.S.; Huang, Q.; Brown, A.; Edwards, K.; Novakovsky, G.E.; Qi, C.N.; Kobor, M.S.; et al. Tonic-Signaling Chimeric Antigen Receptors Drive Human Regulatory T Cell Exhaustion. Proc. Natl. Acad. Sci. USA 2023, 120, e2219086120. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.G.; Hoeppli, R.E.; Huang, Q.; Gillies, J.; Luciani, D.S.; Orban, P.C.; Broady, R.; Levings, M.K. Alloantigen-Specific Regulatory T Cells Generated with a Chimeric Antigen Receptor. J. Clin. Investig. 2016, 126, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Pierini, A.; Iliopoulou, B.P.; Peiris, H.; Pérez-Cruz, M.; Baker, J.; Hsu, K.; Gu, X.; Zheng, P.-P.; Erkers, T.; Tang, S.-W.; et al. T Cells Expressing Chimeric Antigen Receptor Promote Immune Tolerance. JCI Insight 2017, 2, e92865. [Google Scholar] [CrossRef] [PubMed]
- Boroughs, A.C.; Larson, R.C.; Choi, B.D.; Bouffard, A.A.; Riley, L.S.; Schiferle, E.; Kulkarni, A.S.; Cetrulo, C.L.; Ting, D.; Blazar, B.R.; et al. Chimeric Antigen Receptor Costimulation Domains Modulate Human Regulatory T Cell Function. JCI Insight 2019, 4, e126194. [Google Scholar] [CrossRef] [PubMed]
- Dawson, N.A.J.; Lamarche, C.; Hoeppli, R.E.; Bergqvist, P.; Fung, V.C.W.; McIver, E.; Huang, Q.; Gillies, J.; Speck, M.; Orban, P.C.; et al. Systematic Testing and Specificity Mapping of Alloantigen-Specific Chimeric Antigen Receptors in Regulatory T Cells. JCI Insight 2019, 4, e123672. [Google Scholar] [CrossRef] [PubMed]
- Bézie, S.; Charreau, B.; Vimond, N.; Lasselin, J.; Gérard, N.; Nerrière-Daguin, V.; Bellier-Waast, F.; Duteille, F.; Anegon, I.; Guillonneau, C. Human CD81 Tregs Expressing a MHC-Specific CAR Display Enhanced Suppression of Human Skin Rejection and GVHD in NSG Mice. Blood Adv. 2019, 3, 3522–3538. [Google Scholar] [CrossRef] [PubMed]
- Dawson, N.A.J.; Rosado-Sánchez, I.; Novakovsky, G.E.; Fung, V.C.W.; Huang, Q.; McIver, E.; Sun, G.; Gillies, J.; Speck, M.; Orban, P.C.; et al. Functional Effects of Chimeric Antigen Receptor Co-Receptor Signaling Domains in Human Regulatory T Cells. Sci. Transl. Med. 2020, 12, eaaz3866. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-K.; Han, J.; Piao, H.; Shin, N.; Jang, J.Y.; Yan, J.-J.; Kim, H.; Chung, J.; Yang, J. Anti-C4d Chimeric Antigen Receptor Regulatory T Cells Suppressed Allograft Rejection in ABO-Incompatible Heart Transplantation. Genes Dis. 2022, 9, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Muller, Y.D.; Ferreira, L.M.R.; Ronin, E.; Ho, P.; Nguyen, V.; Faleo, G.; Zhou, Y.; Lee, K.; Leung, K.L.; Skartsis, N.; et al. Precision Engineering of an Anti-HLA-A2 Chimeric Antigen Receptor in Regulatory T Cells for Transplant Immune Tolerance. Front. Immunol. 2021, 12, 686439. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.C.; Ronin, E.; Ho, P.; Peng, Y.; Tang, Q. Anti-HLA-A2-CAR Tregs Prolong Vascularized Mouse Heterotopic Heart Allograft Survival. Am. J. Transplant. 2022, 22, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Sánchez, I.; Haque, M.; Salim, K.; Speck, M.; Fung, V.C.W.; Boardman, D.A.; Mojibian, M.; Raimondi, G.; Levings, M.K. Tregs Integrate Native and CAR-Mediated Costimulatory Signals for Control of Allograft Rejection. JCI Insight 2023, 8, e167215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Markmann, C.; Yu, M.; Agarwal, D.; Rostami, S.; Wang, W.; Liu, C.; Zhao, H.; Ochoa, T.; Parvathaneni, K.; et al. Immunotherapy Targeting B Cells and Long-Lived Plasma Cells Effectively Eliminates Pre-Existing Donor-Specific Allo-Antibodies. Cell Reports Med. 2023, 4, 101336. [Google Scholar] [CrossRef] [PubMed]
- Proics, E.; David, M.; Mojibian, M.; Speck, M.; Lounnas-Mourey, N.; Govehovitch, A.; Baghdadi, W.; Desnouveaux, J.; Bastian, H.; Freschi, L.; et al. Preclinical Assessment of Antigen-Specific Chimeric Antigen Receptor Regulatory T Cells for Use in Solid Organ Transplantation. Gene Ther. 2023, 30, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Henschel, P.; Landwehr-Kenzel, S.; Engels, N.; Schienke, A.; Kremer, J.; Riet, T.; Redel, N.; Iordanidis, K.; Saetzler, V.; John, K.; et al. Supraphysiological FOXP3 Expression in Human CAR-Tregs Results in Improved Stability, Efficacy, and Safety of CAR-Treg Products for Clinical Application. J. Autoimmun. 2023, 138, 103057. [Google Scholar] [CrossRef] [PubMed]
- Arrieta-Bolaños, E.; Hernández-Zaragoza, D.I.; Barquera, R. An HLA Map of the World: A Comparison of HLA Frequencies in 200 Worldwide Populations Reveals Diverse Patterns for Class I and Class II. Front. Genet. 2023, 14, 866407. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Liu, J.; Ren, E.C. Structural and Functional Distinctiveness of HLA-A2 Allelic Variants. Immunol. Res. 2012, 53, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Durgam, S.S.; Rosado-Sánchez, I.; Yin, D.; Speck, M.; Mojibian, M.; Sayin, I.; Hynes, G.E.; Alegre, M.L.; Levings, M.K.; Chong, A.S. CAR Treg Synergy with Anti-CD154 Promotes Infectious Tolerance and Dictates Allogeneic Heart Transplant Acceptance. JCI Insight 2025, 10, e188624. [Google Scholar] [CrossRef] [PubMed]
- Degagné, É.; Donohoue, P.D.; Roy, S.; Scherer, J.; Fowler, T.W.; Davis, R.T.; Reyes, G.A.; Kwong, G.; Stanaway, M.; Vicena, V.L.; et al. High-Specificity CRISPR-Mediated Genome Engineering in Anti-BCMA Allogeneic CAR T Cells Suppresses Allograft Rejection in Preclinical Models. Cancer Immunol. Res. 2024, 12, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.; Berdugo, Y.A.; Tree, T. IL-2-Based Approaches to Treg Enhancement. Clin. Exp. Immunol. 2023, 211, 149. [Google Scholar] [CrossRef] [PubMed]
- Myers, D.R.; Zikherman, J.; Roose, J.P. Tonic Signals: Why Do Lymphocytes Bother? Trends Immunol. 2017, 38, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Mo, F.; McKenna, M.K. Impact of Manufacturing Procedures on CAR T Cell Functionality. Front. Immunol. 2022, 13, 876339. [Google Scholar] [CrossRef] [PubMed]
- Rothe, M.; Suerth, J.; Zychlinski, D.; Meyer, J.; Brendel, C.; Grez, M.; Cavazzana-Calvo, M.; Schambach, A.; Baum, C.; Modlich, U. 487. Large-Scale Expansion of Functional Regulatory T Cells Using a Gas-Permeable Rapid Expansion Cultureware (G-Rex). Mol. Ther. 2012, 20, S189. [Google Scholar] [CrossRef]
- Chakraborty, R.; Mahendravada, A.; Perna, S.K.; Rooney, C.M.; Heslop, H.E.; Vera, J.F.; Savoldo, B.; Dotti, G. Robust and Cost Effective Expansion of Human Regulatory T Cells Highly Functional in a Xenograft Model of Graft-versus-Host Disease. Haematologica 2013, 98, 533. [Google Scholar] [CrossRef] [PubMed]
- Gotti, E.; Tettamanti, S.; Zaninelli, S.; Cuofano, C.; Cattaneo, I.; Rotiroti, M.C.; Cribioli, S.; Alzani, R.; Rambaldi, A.; Introna, M.; et al. Optimization of Therapeutic T Cell Expansion in G-Rex Device and Applicability to Large-Scale Production for Clinical Use. Cytotherapy 2022, 24, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Marín Morales, J.M.; Münch, N.; Peter, K.; Freund, D.; Oelschlägel, U.; Hölig, K.; Böhm, T.; Flach, A.C.; Keßler, J.; Bonifacio, E.; et al. Automated Clinical Grade Expansion of Regulatory T Cells in a Fully Closed System. Front. Immunol. 2019, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Long, A.H.; Haso, W.M.; Shern, J.F.; Wanhainen, K.M.; Murgai, M.; Ingaramo, M.; Smith, J.P.; Walker, A.J.; Kohler, M.E.; Venkateshwara, V.R.; et al. 4-1BB Costimulation Ameliorates T Cell Exhaustion Induced by Tonic Signaling of Chimeric Antigen Receptors. Nat. Med. 2015, 21, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Salter, A.I.; Ivey, R.G.; Kennedy, J.J.; Voillet, V.; Rajan, A.; Alderman, E.J.; Voytovich, U.J.; Lin, C.; Sommermeyer, D.; Liu, L.; et al. Phosphoproteomic Analysis of Chimeric Antigen Receptor Signaling Reveals Kinetic and Quantitative Differences that Affect Cell Function. Sci. Signal. 2018, 11, eaat6753. [Google Scholar] [CrossRef] [PubMed]
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, J.D.; Rudensky, A.Y. An Essential Role for the IL-2 Receptor in Treg Cell Function. Nat. Immunol. 2016, 17, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.; Jeffery, L.E.; Sansom, D.M. Understanding the CD28/CTLA-4 (CD152) Pathway and Its Implications for Costimulatory Blockade. Am. J. Transplant. 2014, 14, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Golovina, T.N.; Mikheeva, T.; Suhoski, M.M.; Aqui, N.A.; Tai, V.C.; Shan, X.; Liu, R.; Balcarcel, R.R.; Fisher, N.; Levine, B.L.; et al. CD28 Costimulation Is Essential for Human T Regulatory Expansion and Function. J. Immunol. 2008, 181, 2855. [Google Scholar] [CrossRef] [PubMed]
- Lamarthée, B.; Marchal, A.; Charbonnier, S.; Blein, T.; Leon, J.; Martin, E.; Rabaux, L.; Vogt, K.; Titeux, M.; Delville, M.; et al. Transient MTOR Inhibition Rescues 4-1BB CAR-Tregs from Tonic Signal-Induced Dysfunction. Nat. Commun. 2021, 12, 6446. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, E.; Omori, H.; Tabata, Y.; Akieda, Y.; Watanabe, S.; Ogawa, S.; Abe, R. CD28 Co-Stimulation Is Dispensable for the Steady State Homeostasis of Intestinal Regulatory T Cells. Int. Immunol. 2018, 30, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Lightman, S.M.; Utley, A.; Lee, K.P. Survival of Long-Lived Plasma Cells (LLPC): Piecing Together the Puzzle. Front. Immunol. 2019, 10, 965. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted Role of MTOR (Mammalian Target of Rapamycin) Signaling Pathway in Human Health and Disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Vafadari, R.; Kraaijeveld, R.; Weimar, W.; Baan, C.C. Tacrolimus Inhibits NF-KB Activation in Peripheral Human T Cells. PLoS ONE 2013, 8, 60784. [Google Scholar] [CrossRef] [PubMed]
- Efe, O.; Gassen, R.B.; Morena, L.; Ganchiku, Y.; Jurdi, A.A.; Lape, I.T.; Ventura-Aguiar, P.; LeGuern, C.; Madsen, J.C.; Shriver, Z.; et al. A Humanized IL-2 Mutein Expands Tregs and Prolongs Transplant Survival in Preclinical Models. J. Clin. Investig. 2024, 134, e173107. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, S.K.; Daccache, A.; Azzi, J.R. Chimeric Antigen Receptor Treg Therapy in Transplantation. Trends Immunol. 2024, 45, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Mansilla-Soto, J.; Eyquem, J.; Haubner, S.; Hamieh, M.; Feucht, J.; Paillon, N.; Zucchetti, A.E.; Li, Z.; Sjöstrand, M.; Lindenbergh, P.L.; et al. HLA-Independent T Cell Receptors for Targeting Tumors with Low Antigen Density. Nat. Med. 2022, 28, 345. [Google Scholar] [CrossRef] [PubMed]
- Gee, A.P. GMP CAR-T Cell Production. Best Pract. Res. Clin. Haematol. 2018, 31, 126–134. [Google Scholar] [CrossRef] [PubMed]

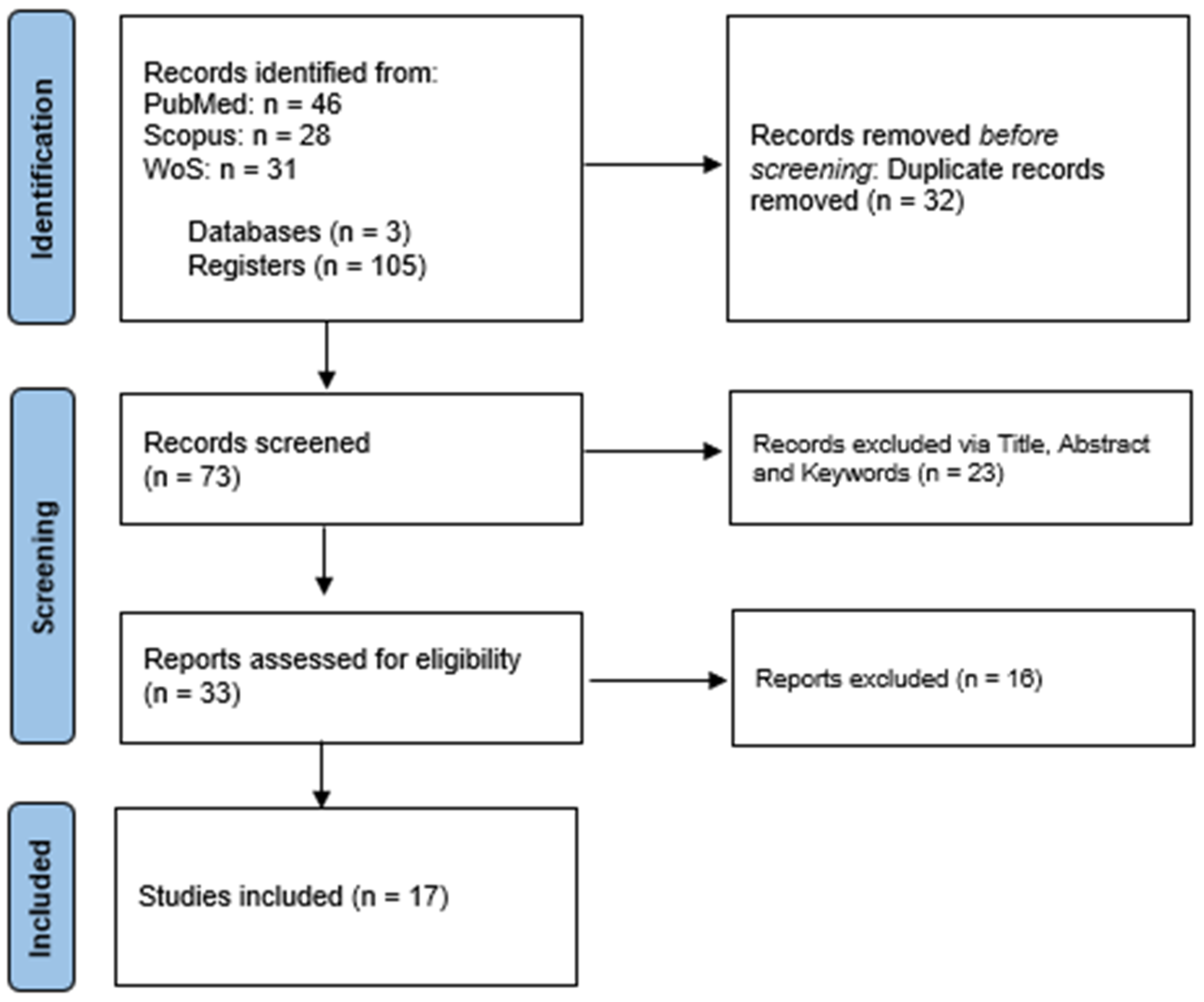
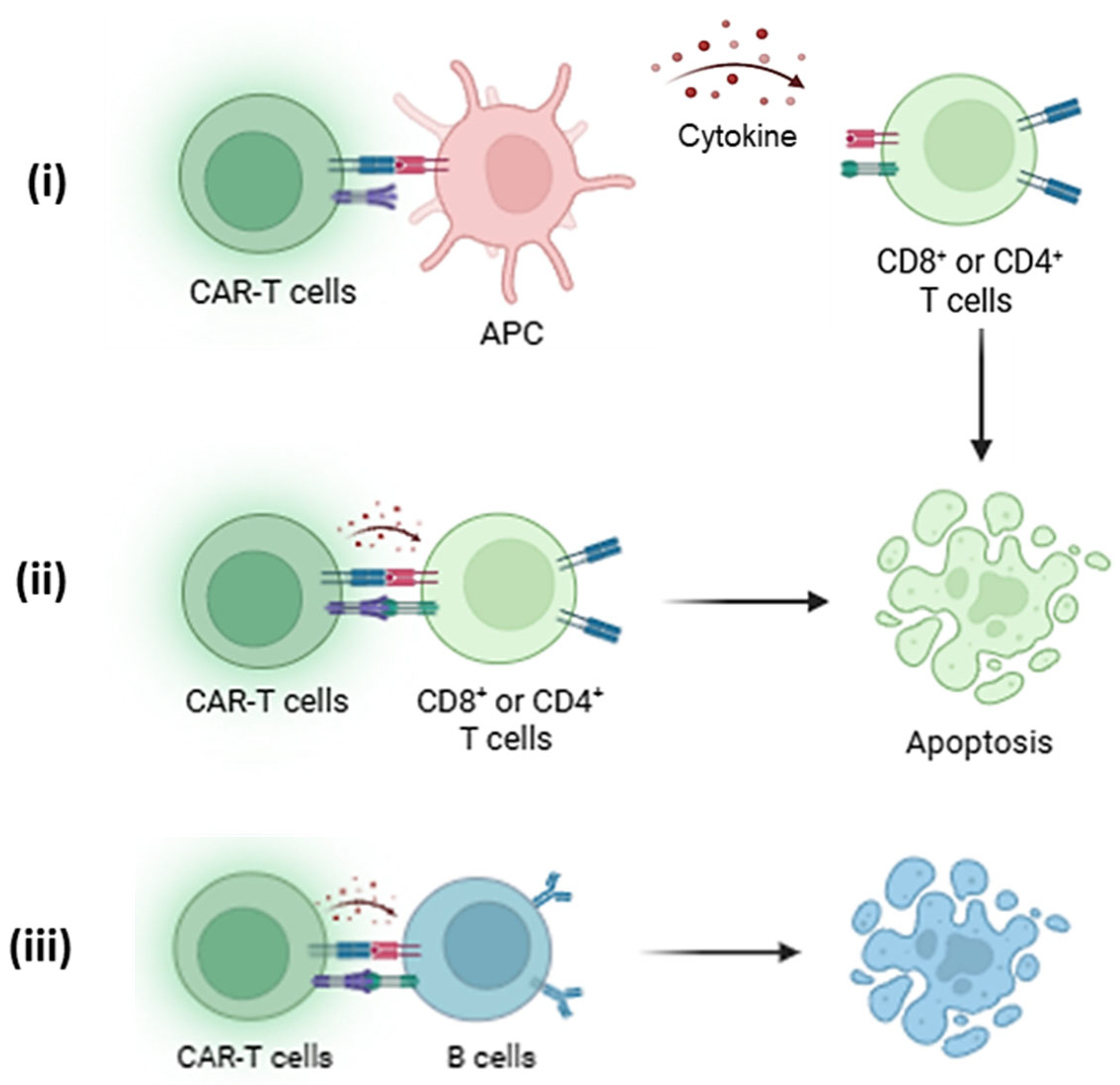
| First Author & Year | Animal Model | Mode of Induced GvHD | CAR T Cell Model and Administered Dose | Results or Outcome |
|---|---|---|---|---|
| Macdonald et al., 2016 [22] | 8 to 12-week-old NSG mice (n = 4/group). | hPBMC (1 × 107 cells/mice) injection from 3 to 4 healthy donors. | HLA-A2-CAR CD4+ Tregs at 0.5 or 1 × 107 cells, once per study. | Survival of mice treated with A2-CAR Tregs was significantly higher compared to HER2-CAR Tregs. Mice treated with A2-CAR Tregs had a delayed onset of GVHD and lower weight loss. In blood samples of treated mice, A2-CAR Tregs showed higher FOXP3 expression and persisted longer (2 weeks) than HER2-CAR Tregs. |
| Noyan et al., 2017 [17] | 8 to 10-week-old NRG mice (n = 3 to 7/group). | Human skin transplant followed by hPBMC (7.5 × 106 cells/mice) injection from healthy donors. | HLA-A2-CAR CD4+ Tregs at 1 × 106 cells, once per study. | A2-CAR Tregs significantly reduced delayed-type hypersensitivity (DTH) response compared to non-transduced Tregs. The ear-swelling response was significantly lower by 70% (p < 0.01) than polyclonal Tregs. In immune-reconstituted humanized NRG mice, A2-CAR Tregs completely prevented the rejection of HLA-A*02-positive target cells, unlike control CAR Tregs or polyclonal Tregs. |
| Boardman et al., 2017 [18] | 10 to 11-week-old BRG mice (n = 2 to 3/group). | Human skin transplant followed by hPBMC (5 × 106 cells/mice) injection from 16 healthy donors. | HLA-A2-CAR CD4+ Tregs with or without CD28-CD3ζ signalling domain at 1 × 106 cells, once per study. | Unlike CAR-modified Teffs, CAR Tregs did not exhibit cytotoxicity against HLA-A2+ cells. They produced low levels of IFN-γ and high levels of IL-10 (p < 0.05), contributing to an immunosuppressive environment. In a human skin xenograft model, CAR Tregs provided superior protection against alloimmune-mediated skin graft rejection compared to polyclonal Tregs. |
| Pierini et al., 2017 [23] | 2 to 4-month-old BALB/c mice (n = 5/group). | GvHD induced by Tcons (1 × 106 cells/mice) injection from allogeneic donor mice. Allogeneic islet transplant from FVB/N mice. | FITC-H-2Dd-mABCAR CD4+ Tregs or T cells at 0.5 or 1 × 106 cells, once per study. | Survival probability and GvHD score of mice treated with mAbCAR Tregs were significantly higher compared to untreated mice (p < 0.001). The mAbCAR Tregs increased islet allograft survival compared to control Tregs, also indicated by the significant reduction in CD8+ T cell infiltration (p < 0.05). |
| Boroughs et al., 2019 [24] | 8 to 10-week-old NSG mice (n = 5/group). | Human skin transplant from 4 healthy donors. | CD19-28ζ or EGFR-28ζ CAR CD4+ Tregs or CD8+ Teff cells or both at 2 × 106 cells each, once per study. | Although both models were superior to Tconv, the CD28 CAR-Tregs expressed significantly less tissue destruction (p < 0.0001) and CD8+ T cell infiltration of graft (p < 0.0001) compared to 4-1BB CAR-Tregs. Thus, it suppressed Teff-mediated tissue damage, leading to prolonged graft survival in a skin xenograft model. |
| Dawson et al., 2019 [25] | 8 to 12-week-old NSG mice (n = 4/group). | hPBMC (8 × 106 cells/mice) injection from 4 healthy donors. Allogeneic skin graft (NSG-A2 mice) and xenogeneic (HLA-A2+) from healthy donors. | HLA-A2 CAR Tregs (4 × 106 cells/mice), once per study. | In the xenogeneic GvHD model, the A2-CAR Tregs improved survival (p < 0.001) and reduced human CD45+ cell engraftment by ~60% (p < 0.01) compared to control groups. Additionally, the bioluminescence imaging revealed lesser formation of keratinocytes and involucrin destruction, prompting better survival of the graft in the A2-CAR Treg-treated mice. |
| Bézie et al., 2019 [26] | 8 to 12-week-old NSG mice (n = 3 to 9/group). | hPBMC (1.5 × 107 cells/mice) injection from 4 healthy donors. Human skin transplant from healthy donors. | HER2- or HLA-A2 CAR CD8+ Tregs (Ratio of 1:1 or 3:1 of PBMC:Tregs), once per study. | The A2-CAR CD8+ Tregs significantly inhibited GvHD compared to HER2 CAR Tregs at 100% and 25% survival rate (p < 0.01), respectively. The A2-CAR CD8+ Tregs prolonged skin graft survival for over 100 days (p < 0.001). The effects of the immunotherapy also behaved in a dose-dependent manner. |
| Dawson et al., 2020 [27] | 8 to 12-week-old NSG mice (n = 3 to 9/group). | HLA-A2+ PBMC (10 × 107 cells/mice) | CD28wt-, CD28mut, ICOS, CTLA-4wt, CTLA-4mut, PD-1, OX40, GITR, 4-1BB, or TNFR2 CAR CD4+ Tregs at 2.5 or 5.0 × 106 Tregs, once per study. | Among the 10 CAR models, exploring different co-stimulatory domains, the CD28wt produced the best survival outcome for the GvHD animal model. Furthermore, the results show significantly higher FoxP3+ and Helios expression of cells in circulation—indicating CAR Treg stability and persistence in vivo. |
| Sicard et al., 2020 [14] | 8 to 16-week-old C57BL/6 mice (n = 5 to 11/group). | Skin grafts from syngeneic and/or HLA-A2+ mice. | HER2- or HLA-A2 CAR CD4+ Tregs at 1 × 106 (equivalent to 30–50 × 106/kg) Tregs, once per study. | The dsCAR-Tregs (14 days) significantly median survival times (p < 0.0001) for the grafts when compared to irrCAR-Tregs (8.5 days) and PBS-treated control (8.0 days). Additionally, dsCAR-Tregs significantly (p < 0.05) reduced the generation of DSAs and the number of DSA-secreting cells in naive recipients, compared to irrCAR-Tregs. |
| Imura et al., 2020 [19] | 8 to 11-week-old NSG mice (n = 4 to 7/group). | hPBMC (5 × 106 cells/mice) injection from 5 healthy donors. | HER2- or CD19- CAR CD4+ Tregs at 2 × 106 cells at 4 to 6 hrs or 7 days after. | In vivo, the CD19-CAR Tregs significantly reduced serum levels of IgG and IgM levels (p < 0.05), suppressed CD20+ B cell expansion, and reduced GvHD scores (p < 0.01) more effectively than polyclonal Tregs. Compared to CAR CD8+ T cells, the CAR Tregs had significantly lower (p < 0.05) IL-6 levels and greater Tregs, preventing further weight loss and exacerbating GvHD. |
| Lee et al., 2022 [28] | Unspecified C57BL/6J mice (n = 3/group). | Heart transplant from healthy BALB/c mice. | C4d-CAR CD4+ Tregs at 1 × 106 cells, once per study. | In a mouse model of ABO-incompatible heart transplantation, recipients treated with anti-C4d CAR Tregs had significantly prolonged (p < 0.05) allograft survival compared to those treated with control CAR Tregs. Histological analysis showed that anti-C4d CAR Tregs reduced inflammation in the heart graft, as evidenced by lower expression of pro-inflammatory cytokines IFN-γ and TNF-α. |
| Muller et al., 2021 [29] | 7 to 8-week-old NSG mice (n = 2 to 6/group). | hPBMC (5 × 106 cells/mice) injection from deceased, de-identified donors. 500 mouse islets or 3000 human islet equivalents (IEQs) transplanted. | HLA-A2-TCRdeficient CD4+ Tconv or Tregs at 2.5 × 106 cells/mice, once per study. | The A2-CAR Tregs demonstrated the antigen specificity of the CAR by significantly improving (p < 0.01) the survival of HLA-A2+ mice compared to control mice, which expired approximately 13 days post-transplant. Also, the A2-CAR Tregs were trafficked specifically to the graft site and prevented the onset of rejection for up to 11 days compared to Tconv after 6 days (p < 0.001). |
| Wagner et al., 2022 [30] | Unspecified C57BL/6J mice (n = 3 to 7/group). | Heart transplants from single A2-mismatch, haplo-mismatch, and syngeneic donor mice. | HLA-A2 CAR CD4+ Tregs at 1 × 106 cells/mice at 1, 2, or 4 × 106 cells/mice, once per study at Day 1 or 2 post-transplant. | The administration of A2-CAR Tregs increased graft survival (p < 0.05) 99 and 35 days, respectively, compared to without treatment at 23 days. Despite the incremental doses, there were no significant differences reported. With or without rapamycin, the strongly immunogenic, haplo-mismatched heart transplantation model survival was extended from 14 days to 100 days (p < 0.05). |
| Rosado-Sánchez et al., 2023 [31] | 10 to 14-week-old C57BL/6 mice (n = 3 to 13/group). | Skin grafts from syngeneic and/or HLA-A2+ mice. | HLA-A2 with or without CD28 co-stimulatory domain of CAR CD4+ Tregs at 1 × 106 cells/mice, once per study. | CAR Tregs with TNFR family domains (OX40, 4-1BB) did not affect DSA levels, causing failed graft engraftment. Conversely, CD28 and PD-1 CAR Tregs reduced DSA levels, which improved median graft survival (p < 0.01) to 20 days and 19.5 days, respectively, from 14 days untreated. Interestingly, the absence of the CD28 co-stimulatory domain on the A2-CAR Treg still enabled allograft tolerance, compensated by endogenous APCs or dendritic cells. |
| Zhang et al., 2023 [32] | 8 to 12-week-old C57BL/6J mice (n = 3/group). | Skin graft from BALB/cJ mice. Islet transplant from BALB/cJ mice. | CD19 and/or APRIL CAR CD8+ T cells at no specified dose. | The combination of CART-19 and APRIL-CAR (Combo-CART) was highly effective at depleting B cells and PCs in the bone marrow and spleens by approximately 70% (p < 0.05) compared to CART-19 monotherapy. Similarly, the Combo-CART-protected islet allograft up to 30 days (p < 0.01), comparable to the non-sensitized group receiving standard treatment. Interestingly, the eventual depletion of CAR T cells’ activity and resurgence of B cells that returned levels of IgGs to normal but without DSAs had likely displaced endogenous B cells and PCs with graft-tolerant versions. |
| Proics et al., 2023 [33] | 7 to 8-week-old NSG mice (n = 3 to 10/group). | hPBMC (5 × 106 cells/mice) from 2 healthy donors. Human skin transplant from a healthy donor. | HLA-A2 or TX200-TR101 CAR CD4+ Tregs at 1 × 106 (equivalent to 30–50 × 106/kg) Tregs, once per study. | The group treated with TX200-TR101 Tregs exhibited significantly lower (p < 0.05) GvHD scores (0.6) and improved survival (p < 0.05) compared to control groups. TX200-TR101 Tregs localized at human skin grafts and significantly improved delayed rejection (p < 0.001), resulting in a median survival rate of 35 days compared to 16 days in the control group. |
| Lamarche et al., 2023 [21] | 8 to 12-week-old NSG mice (n = 5 to 7/group). | hPBMC (6 × 106 cells/mice) from 3–5 healthy donors. | CD19 or GD2 CAR CD4+ Tregs at 3 × 106 Tregs, once per study. | Despite demonstrating immunosuppressive capacity in vitro, TS-CAR Tregs failed to confer protection against GvHD in vivo. This loss of function was associated with Treg exhaustion, as evidenced by the increased graft immune infiltration and tissue loss. The survival outcomes were comparable to the untreated group and significantly worse than those of animals treated with untransduced Tregs (p < 0.01), indicating that Treg exhaustion critically impairs their regulatory function in the allogeneic setting. |
| Henschel et al., 2023 [34] | Unspecified NRG mice (n = 3 to 6/group). | hPBMC (5 × 105 cells/mice) from 2–4 healthy donors, three times per week. | HLA-A2 or HLA-A2-FOXP3 CAR CD4+ CD45RA+ Tregs at 5 × 105 Tregs, once per study. | The FOXP3-CAR Tregs effectively prevented (p < 0.05) immune-mediated destruction of allogeneic target cells compared to the control group. Even in severely high inflammatory and IL-2-deprived conditions, the FOXP3-CAR Tregs retained ~75% (p < 0.001) of their FOXP3 expression and nearly two-fold more compared to the control group. |
| First Author & Year | CAR Structure | CAR Generation Efficiency or Viability (%) | Implication of CAR Design | ||||
|---|---|---|---|---|---|---|---|
| Binding | Hinge | Transmembrane | Co-Stimulatory | Signal | |||
| Macdonald et al., 2016 [22] | αHLA-A2 or αHER2 | CD8α-CD28 | CD28 | - | CD3ζ | 86–96% | A2-CAR Tregs was successfully generated with a consistent phenotype of high expression of FOXP3, CD25, and CTLA-4 (p < 0.05). Through mixed lymphocyte reactions, A2-CAR Tregs greatly suppressed the proliferation of HLA-A2+ T cells compared to HER2-CAR Tregs (p < 0.001). Other regulatory markers like suppressive markers, CTLA-4 and GARP, were greatly expressed (p < 0.05) in the CAR Tregs than the TCR-stimulated Tregs (p < 0.05). |
| Noyan et al., 2017 [17] | αHLA-A2 | CD8α | CD28 | - | CD3ζ | 55–95% | No significant alterations to the regulatory phenotype of A2-CAR Tregs as FOXP3, CCR7, CD45RO, CD45RA, and CD39 expression remained stable. A2-CAR Tregs produced higher suppression of Teff proliferation compared to control CAR Tregs. Even at a low (1:64) ratio, the CAR Tregs continued to suppress >60% of Teff proliferation (p < 0.01). |
| Boardman et al., 2017 [18] | αHLa-A2 | CD28 | CD28 | - | CD3ζ | 40–80% | After transduction, eGFP, FOXP3, and CTLA-4 remained highly expressed by up to >90% in CAR Tregs. CAR-mediated antigen-specific suppression was confirmed through lesser suppression of effector T cells (p < 0.01) when further cocultured with HLA-A2+ APCs. In spite of that, the CAR Treg did not elicit cytotoxic effects against non-target HLA-A2+ cells. Modified secretion of lower IFN-γ and higher IL-10 was also detected from the CAR Tregs (p < 0.05). |
| Pierini et al., 2017 [23] | αFITC-H-2D | CD28 | CD28 | - | CD3ζ | 30% | A modular CAR system (mAbCAR) that specifically binds FITC and was successfully expressed in both T cells and Tregs. Activation leads to T cell activation (CD69 and CD25 expression) and the generation of an effector memory phenotype (CD44+CD62L-). On the other hand, FITC-stimulated mAbCAR Tregs retained their suppressive function through high FOXP3 expression and increased CD69 and PD-1 expression (p < 0.05), preventing GvHD while maintaining immune tolerance. |
| Boroughs et al., 2019 [24] | αCD19 or αEGFR | CD8 | CD3ζ | CD28 or 4-1BB | CD3ζ; | >50% | Although no significant changes occurred for FOXP3 expression, CD69 expression and IL-10 secretion were significantly higher (p < 0.05 and p < 0.01, respectively) in the CD28 CAR-Tregs vs. 4-1BB CAR-Tregs. The pro-inflammatory cytokines TNF-α, GM-CSF, IL-2, and IFN-γ) were also significantly reduced (p < 0.05) in the CD28 CAR-Tregs groups. As a result, CD28 CAR-Tregs suppressed Teff proliferation by 75%, while 4-1BB CAR-Tregs showed only 20% suppression (p < 0.01). |
| Dawson et al., 2019 [25] | αHLA-A2 or αHER2 | CD8α | CD28 | - | CD3ζ | Not specified | From the twenty variants of hA2-CARs generated, the ten selected had significantly higher (p < 0.01) CD69 and CD71 expression than non-transduced controls. Additionally, only seven hA2-CARs showed robust activation of Tregs upon stimulation with HLA-A2–positive cells. Thus, immunosuppression of effector T cells was 40% more than control Tregs (p < 0.01). Also, the cross-reactive HLA-A alleles were reduced up to 90% (p < 0.05) in hA2-CAR Tregs compared to mA2-CAR, highlighting the potential significance of xenogeneic factors influencing immunity. |
| Bézie et al., 2019 [26] | αHLA-A2 or αHER2 | - | CD28 | - | CD3ζ | 20% | A2-CAR expression was stable after 14 days of culture, retaining its regulatory phenotype after CAR expression. The transduced cells expressed FOXP3, IL-10, and IFN-γ, without gaining cytotoxic markers such as perforin and FASL. Unlike A2-CAR CD8+ Teffs, A2-CAR Tregs did not cause cytotoxicity (p < 0.01) in HLA-A*02+ endothelial cells (ECs). The A2-CAR also suppressed (p < 0.01) effector T cell proliferation by up to 80%, succeeding HER2-CAR Tregs and non-transduced Tregs. |
| Dawson et al., 2020 [27] | αHLA-A2 or αHER2 | - | CD28, ICOS, OX40, GITR, 4-1BB, or TNFR2 | CD28, CD28 (Y173F), ICOS, CTLA-4, CTLA-4 (Y165G), PD-1, OX40, GITR, 4-1BB, or TNFR2. | CD3ζ | >50% | CD28wt and CD28mut stimulated the highest CD69, CD71, LAP, CTLA-4, and GARP expression following antigen-specific stimulation (p < 0.01). CD28wt, 4-1BB, and TNFR2 CARs induced proliferation, but only CD28wt preserved FOXP3 and Helios expression after 12 days of CAR stimulation. Helios loss correlated with instability, confirmed by increased FOXP3 TSDR methylation in TNFR2-CAR Tregs (p < 0.01). |
| Sicard et al., 2020 [14] | αHLA-A2 or αHER2 | CD8a | CD28 | - | CD3ζ | 75% | Transduced donor-specific (ds) Tregs of anti-HLA-A2-specific CAR were successful, maintaining expression of FoxP3, CD4, and regulatory markers. The dsCAR-Tregs suppressed (p < 0.05) the proliferation of HLA-A2+ antigen-presenting cells (APCs) and CD4+ effector T cell proliferation in vitro compared to irrelevant CAR-Tregs (irrCAR-Tregs). |
| Imura et al., 2020 [19] | αCD19 or αHER2 | CD28 | CD28 | - | CD3ζ | 50% | The generated CD19-CAR Tregs maintained proliferation capacity and expression of regulatory markers, including FoxP3, Helios, and CTLA-4, similar to polyclonal Tregs. However, the former showed higher levels (p < 0.01) of anti-inflammatory cytokines like IL-10 and lower levels of IFN-γ and IL-2. The CD19-CAR Tregs also reduced (p < 0.01) B cell proliferation by up to 70% and IgG production (p < 0.05). |
| Lee et al., 2022 [28] | αC4d | CD8 | CD28 | - | CD3ζ | Not specified | There was no significant difference in the expression of regulatory markers (FoxP3, CD25, CTLA-4, and GITR) between anti-C4d CAR Tregs and NT Tregs. However, C4d-CAR Tregs showed enhanced activation by increased CD69 expression and IL-10 secretion compared to both control CAR and NT Tregs (p < 0.01). When cocultured with effector T cells, the anti-C4d CAR Tregs suppressed T cell proliferation significantly more (p < 0.05) than NT Tregs. |
| Muller et al., 2021 [29] | αHLA-A2 | IgG4 | CD28 | - | CD3ζ | 50% | Using CRISPR/Cas9, the endogenous TCR was successfully knocked out, and the A2-CAR construct was integrated into the TRAC locus at 85% and 91% efficiency (p < 0.01), respectively. The TCR-deficient A2-CAR Tregs maintained high levels of regulatory markers such as FoxP3, Helios, and CD25 (p < 0.05). A2-CAR Tregs specifically suppressed the proliferation of HLA-A2+ effector T cells by 80% (p < 0.01) compared to polyclonal Tregs. |
| Wagner et al., 2022 [30] | αHLA-A2 | CD8 | CD28 | - | CD3ζ | 60–80% | The A2-CAR Tregs exhibited enhanced proliferation by 8- to 16-fold higher (p < 0.0001) compared to non-transduced Tregs. Furthermore, their immunosuppressive function in the presence of HLA-A2-expressing cells, marked by CD69 and CD25 activation, was upregulated (p < 0.01). |
| Rosado-Sánchez et al., 2023 [31] | αHLA-A2 | CD28 | CD28 | ICOS; -PD-1; -GITR, and -TNFR family proteins | CD3ζ | 70% | Eight different HLA-A2–specific CAR variants with distinct co-stimulatory domains, Tregs expressing the CD28-based CAR demonstrated the strongest proliferative capacity (p < 0.001) in vitro when cocultured with HLA-A2–expressing cells. As a result, immunoregulatory response, including cytokine secretion like IL-10, was highest in CD28-CAR Tregs (p < 0.01) vs. other variants. |
| Zhang et al., 2023 [32] | αCD19 or αAPRIL | - | CD28 | - | CD3ζ | Not specified | Among the cohort of five multiple myeloma patients with pre-existing DSAs, Combo-CART therapy (CART-BCMA and CART-19) significantly reduced allo-antibodies in three subjects by 47% to 97%. The decline in allo-antibodies was statistically significant (p < 0.05) and was accompanied by total reduction of IgM, IgA, and IgG levels (p < 0.05). |
| Proics et al., 2023 [33] | αHLA-A2 or TX200-TR101 | - | CD28 | - | CD3ζ or without | 90% | TX200-TR101 Tregs retained a high expression (>89%; p < 0.05) of Treg markers, such as FOXP3 and CD25, and were stable after expansion. TX200-TR101 Tregs achieved 52.7% suppression (p < 0.05) compared to the 19.2% suppression of Tconv by control Tregs. |
| Lamarche et al., 2023 [21] | αCD19 or αGD2 | - | CD28 | - | CD3ζ | 75% | Compared to untransduced or non-tonic-signalling (non-TS) groups, the proportion of TS-CAR Tregs expressing inhibitory receptors—the likes of PD-1, TIM-3, LAG-3, GITR, and 4-1BB—was significantly higher (p < 0.05). Higher glycolysis and acidification (p < 0.05) were also observed, relevant to the exhaustive state of TS-CAR Tregs. Despite that, it remained highly suppressive (>70%) in vitro against CD4+ and CD8+ effector T cells (p < 0.01) vs. UT and non-TS-CAR Tregs. |
| Henschel et al., 2023 [34] | αHLA-A2 with or without -FOXP3 | - | CD28 | - | CD3ζ | 75% | CAR modification confirmed a 40% higher level of FOXP3 expression vs. control CAR-Tregs (p < 0.001). Supra-expression of FOXP3 resulted in a low-level cytokine secretion profile: TNF-α, IFN-γ, IL-2, and IL-17 (p < 0.0001); suppression of T-effector cells by 70% (p < 0.01); retention of FOXP3+ subpopulation under IL-2 deprived conditions (p < 0.001). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, A.M.L.; Sakthiswary, R.; Lokanathan, Y. Revolutionizing Allogeneic Graft Tolerance Through Chimeric Antigen Receptor-T Regulatory Cells. Biomedicines 2025, 13, 1757. https://doi.org/10.3390/biomedicines13071757
Chan AML, Sakthiswary R, Lokanathan Y. Revolutionizing Allogeneic Graft Tolerance Through Chimeric Antigen Receptor-T Regulatory Cells. Biomedicines. 2025; 13(7):1757. https://doi.org/10.3390/biomedicines13071757
Chicago/Turabian StyleChan, Alvin Man Lung, Rajalingham Sakthiswary, and Yogeswaran Lokanathan. 2025. "Revolutionizing Allogeneic Graft Tolerance Through Chimeric Antigen Receptor-T Regulatory Cells" Biomedicines 13, no. 7: 1757. https://doi.org/10.3390/biomedicines13071757
APA StyleChan, A. M. L., Sakthiswary, R., & Lokanathan, Y. (2025). Revolutionizing Allogeneic Graft Tolerance Through Chimeric Antigen Receptor-T Regulatory Cells. Biomedicines, 13(7), 1757. https://doi.org/10.3390/biomedicines13071757





