A Randomised Pilot Trial to Demonstrate the Feasibility of a Prototype Electronic Heating Device in Patients with Meibomian Gland Dysfunction
Abstract
1. Introduction
2. Materials and Methods
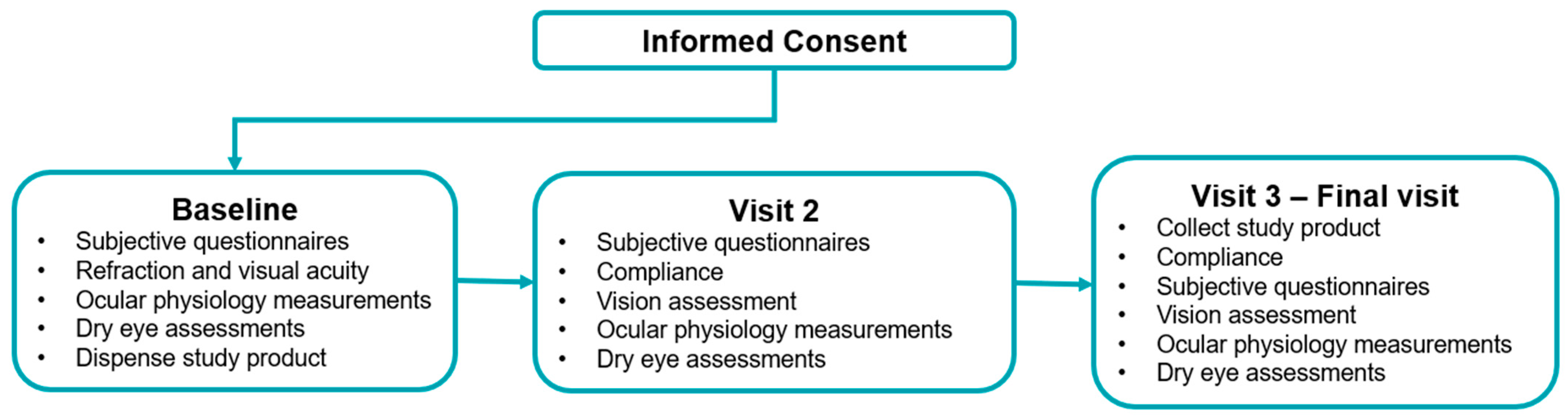
2.1. Study Design
2.2. Study Participants
2.3. Study Treatments
2.4. Study Parameters
2.4.1. Questionnaires
2.4.2. Tear Film Characteristics
2.4.3. Meibomian Gland Function
2.4.4. Safety Measures
2.5. Statistical Analysis
3. Results
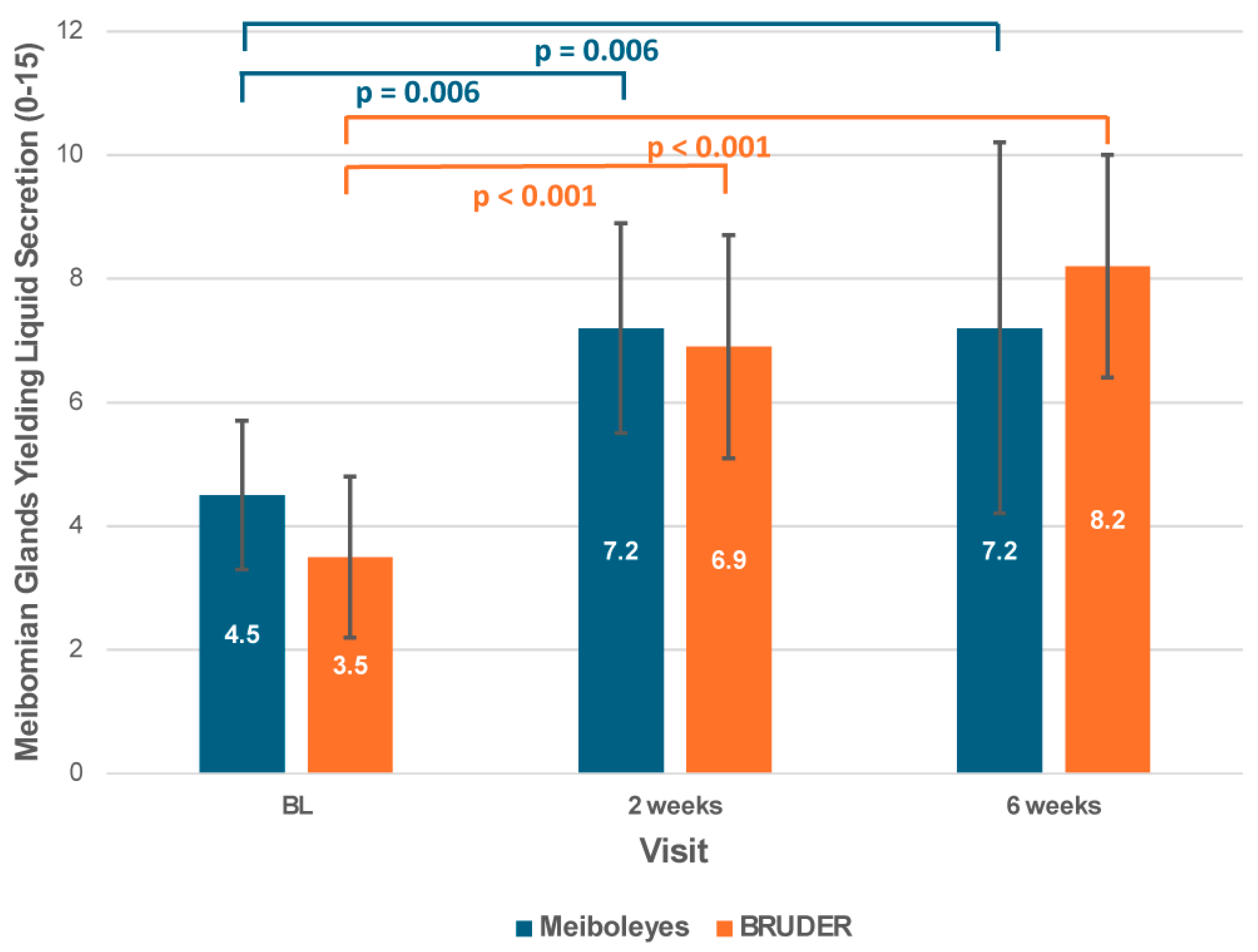
3.1. Meibomian Gland Function
3.2. Tear Break-Up Time
3.3. Ocular Physiology
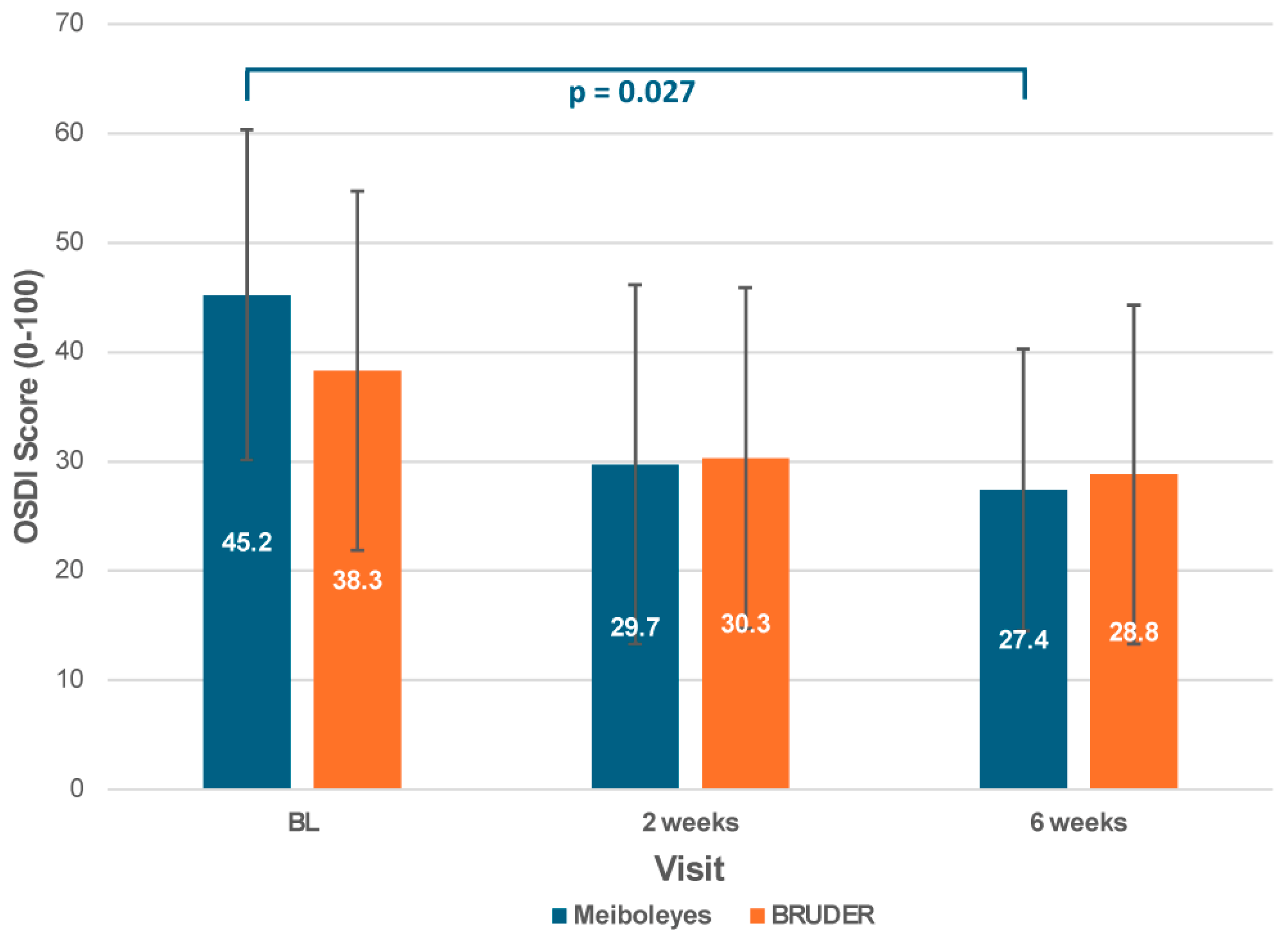
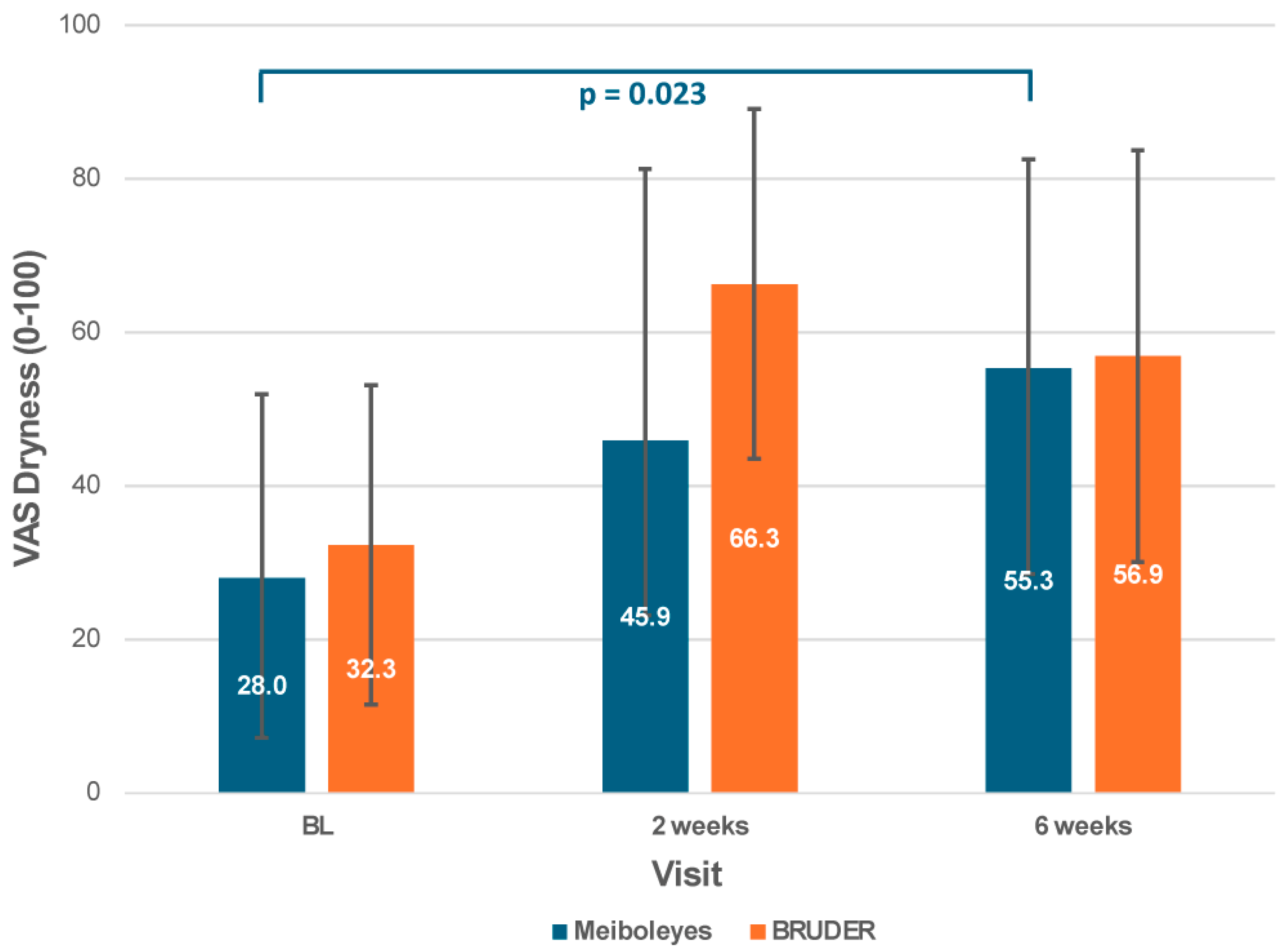
3.4. Subjective Symptoms
3.5. Lipid Layer Thickness
3.6. Adverse Events
3.7. Compliance to Treatment
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MGD | Meibomian Gland Dysfunction |
| OSDI | Ocular Surface Disease Index |
| TBUT | Tear Break-up Time |
| VAS | Visual Analogue Scale |
| MGS | Meibomian Gland Secretion |
| MGYLS | Meibomian Glands Yielding Liquid Secretion |
| IPL | Intense Pulsed Light |
| MGX | Meibomian Gland Expression |
| NIKBUT | Non-Invasive Keratograph Break-Up Time |
References
- Stapleton, F.; Argueso, P.; Asbell, P.; Azar, D.; Bosworth, C.; Chen, W.; Ciolino, J.; Craig, J.P.; Gallar, J.; Galor, A.; et al. TFOS DEWS III Digest Report. Am. J. Ophthalmol. 2025, 279, 451–553. [Google Scholar] [CrossRef]
- Nelson, J.D.; Shimazaki, J.; Benitez-del-Castillo, J.M.; Craig, J.P.; McCulley, J.P.; Den, S.; Foulks, G.N. The international workshop on meibomian gland dysfunction: Report of the definition and classification subcommittee. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1930–1937. [Google Scholar] [CrossRef]
- Schaumberg, D.A.; Nichols, J.J.; Papas, E.B.; Tong, L.; Uchino, M.; Nichols, K.K. The international workshop on meibomian gland dysfunction: Report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1994–2005. [Google Scholar] [CrossRef]
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef]
- Jeyalatha, M.V.; Qu, Y.; Liu, Z.; Ou, S.; He, X.; Bu, J.; Li, S.; Reinach, P.S.; Liu, Z.; Li, W. Function of meibomian gland: Contribution of proteins. Exp. Eye Res. 2017, 163, 29–36. [Google Scholar] [CrossRef]
- Jones, L.; Craig, J.P.; Markoulli, M.; Karpecki, P.; Akpek, E.K.; Basu, S.; Bitton, E.; Chen, W.; Dhaliwal, D.K.; Dogru, M.; et al. TFOS DEWS III: Management and Therapy. Am. J. Ophthalmol. 2025, 279, 289–386. [Google Scholar] [CrossRef]
- Geerling, G.; Tauber, J.; Baudouin, C.; Goto, E.; Matsumoto, Y.; O’Brien, T.; Rolando, M.; Tsubota, K.; Nichols, K.K. The international workshop on meibomian gland dysfunction: Report of the subcommittee on management and treatment of meibomian gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2050–2064. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, A.S.; Korb, D.R. Meibomian glands and contact lens wear. Br. J. Ophthalmol. 1981, 65, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D. The optimum temperature for the heat therapy for meibomian gland dysfunction. Ocul. Surf. 2019, 17, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Blackie, C.A.; Solomon, J.D.; Greiner, J.V.; Holmes, M.; Korb, D.R. Inner eyelid surface temperature as a function of warm compress methodology. Optom. Vis. Sci. 2008, 85, 675–683. [Google Scholar] [CrossRef]
- Bilkhu, P.S.; Naroo, S.A.; Wolffsohn, J.S. Effect of a commercially available warm compress on eyelid temperature and tear film in healthy eyes. Optom. Vis. Sci. 2014, 91, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Murakami, D.K.; Blackie, C.A.; Korb, D.R. All Warm Compresses Are Not Equally Efficacious. Optom. Vis. Sci. 2015, 92, e327–e333. [Google Scholar] [CrossRef]
- Bitton, E.; Lacroix, Z.; Leger, S. In-vivo heat retention comparison of eyelid warming masks. Contact Lens Anterior Eye 2016, 39, 311–315. [Google Scholar] [CrossRef]
- Olafsson, J.; Lai, X.; Landsend, E.C.S.; Olafsson, S.; Parissi, E.; Utheim, O.A.; Raeder, S.; Badian, R.A.; Lagali, N.; Dartt, D.A.; et al. TheraPearl Eye Mask and Blephasteam for the treatment of meibomian gland dysfunction: A randomized, comparative clinical trial. Sci. Rep. 2021, 11, 22386. [Google Scholar] [CrossRef]
- Rossi, C.; Vaccaro, S.; Borselli, M.; Carnovale Scalzo, G.; Toro, M.D.; Scorcia, V.; Giannaccare, G. Noninvasive Ocular Surface Workup in Patients with Meibomian Gland Dysfunction Using Microwave-Heated Eye Bag. Clin. Ophthalmol. 2024, 18, 53–858. [Google Scholar] [CrossRef]
- Sim, H.S.; Petznick, A.; Barbier, S.; Tan, J.H.; Acharya, U.R.; Yeo, S.; Tong, L. Collaborative Research Initiative for Meibomian Gland Dysfunction (CORIM). A Randomized, Controlled Treatment Trial of Eyelid-Warming Therapies in Meibomian Gland Dysfunction. Ophthalmol. Ther. 2014, 3, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.S.; DuBiner, H.B.; Epstein, R.J.; Ernest, P.H.; Greiner, J.V.; Hardten, D.R.; Holland, E.J.; Lemp, M.A.; McDonald, J.E., II; Silbert, D.I.; et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea 2012, 31, 396–404. [Google Scholar] [CrossRef]
- Craig, J.P.; Chen, Y.H.; Turnbull, P.R. Prospective trial of intense pulsed light for the treatment of meibomian gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1965–1970. [Google Scholar] [CrossRef]
- Chelnis, J.; Garcia, C.N.; Hamza, H. Multi-Frequency RF Combined with Intense Pulsed Light Improves Signs and Symptoms of Dry Eye Disease Due to Meibomian Gland Dysfunction. Clin. Ophthalmol. 2023, 17, 3089–3102. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.E.; Lau, B.S.R.; Lim, B.X.H.; Du, R.; Giannaccare, G.; Tong, L.; Stapleton, F.; Lim, C.H.L. Low-level light therapy and intense pulse light therapy in meibomian gland dysfunction. A systematic review and meta-analysis. Contact Lens Anterior Eye 2025, 48, 102344. [Google Scholar] [CrossRef]
- Uthaithammarat, L.; Kasetsuwan, N.; Reinprayoon, U.; Chongpison, Y.; Quanchareonsap, W.; Dissaneevate, P. Randomized, double-masked, sham-controlled trial of efficacy and safety of quantum molecular resonance for treating meibomian gland dysfunction. Eye 2025, 39, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, S.; Liu, X. Efficacy and safety of a vectored thermal pulsation system (Lipiflow®) in the treatment of meibomian gland dysfunction: A systematic review and meta-analysis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 25–39. [Google Scholar] [CrossRef]
- Leng, X.; Shi, M.; Liu, X.; Cui, J.; Sun, H.; Lu, X. Intense pulsed light for meibomian gland dysfunction: A systematic review and meta-analysis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, Z.; Leger, S.; Bitton, E. Ex-vivo heat retention of different eyelid warming masks. Contact Lens Anterior Eye 2015, 38, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.L.; Walt, J.G.; Mink, D.R.; Satram-Hoang, S.; Wilson, S.E.; Perry, H.D.; Asbell, P.A.; Pflugfelder, S.C. Minimal clinically important difference for The Ocular Surface disease index. Arch. Ophthalmol. 2010, 128, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Pult, H.; Riede-Pult, B. Comparison of subjective grading and objective assessment in meibography. Contact Lens Anterior Eye 2013, 36, 22–27. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, C.L.; Tong, L. Intra-observer and inter-observer repeatability of ocular surface interferometer in measuring lipid layer thickness. BMC Ophthalmol. 2015, 15, 53. [Google Scholar] [CrossRef]
- Mooi, J.K.; Wang, M.T.M.; Lim, J.; Muller, A.; Craig, J.P. Minimising instilled volume reduces the impact of fluorescein on clinical measurements of tear film stability. Contact Lens Anterior Eye 2017, 40, 170–174. [Google Scholar] [CrossRef]
- Watson, S.L.; Jones, L.W.; Stapleton, F.; Hinds, M.; Ng, A.; Tan, J.; Alster, Y.; Bosworth, C.; Rafaeli, O.; DePuy, V.; et al. Efficacy and safety of AZR-MD-001 selenium sulfide ophthalmic ointment in adults with meibomian gland dysfunction: A vehicle-controlled, randomized clinical trial. Ocul. Surf. 2023, 29, 537–546. [Google Scholar] [CrossRef]
- Arita, R. Meibography: A Japanese Perspective. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES48–DES55. [Google Scholar] [CrossRef]
- Terry, R.L.; Schnider, C.M.; Holden, B.A.; Cornish, R.; Grant, T.; Sweeney, D.; La Hood, D.; Back, A. CCLRU standards for success of daily and extended wear contact lenses. Optom. Vis. Sci. 1993, 70, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.D.; Case, C.L.; Greiner, J.V.; Blackie, C.A.; Herman, J.P.; Korb, D.R. Warm compress induced visual degradation and Fischer-Schweitzer polygonal reflex. Optom. Vis. Sci. 2007, 84, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Tichenor, A.A.; Cox, S.M.; Ziemanski, J.F.; Ngo, W.; Karpecki, P.M.; Nichols, K.K.; Nichols, J.J. Effect of the Bruder moist heat eye compress on contact lens discomfort in contact lens wearers: An open-label randomized clinical trial. Contact Lens Anterior Eye 2019, 42, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Ngo, W.; Srinivasan, S.; Jones, L. An Eyelid Warming Device for the Management of Meibomian Gland Dysfunction. J. Optom. 2019, 12, 120–130. [Google Scholar] [CrossRef]
- Rong, B.; Tang, Y.; Tu, P.; Liu, R.; Qiao, J.; Song, W.; Toyos, R.; Yan, X. Intense Pulsed Light Applied Directly on Eyelids Combined with Meibomian Gland Expression to Treat Meibomian Gland Dysfunction. Photomed. Laser Surg. 2018, 36, 326–332. [Google Scholar] [CrossRef]
- Craig, J.P.; Muntz, A.; Wang, M.T.M.; Luensmann, D.; Tan, J.; Trave Huarte, S.; Xue, A.L.; Jones, L.; Willcox, M.D.P.; Wolffsohn, J.S. Developing evidence-based guidance for the treatment of dry eye disease with artificial tear supplements: A six-month multicentre, double-masked randomised controlled trial. Ocul. Surf. 2021, 20, 62–69. [Google Scholar] [CrossRef]
- Tan, J.; Ho, L.; Wong, K.; La, A.; Lee, S.; Park, S.; Tran, L.; Stapleton, F. The effects of a hydrating mask compared to traditional warm compresses on tear film properties in meibomian gland dysfunction. Contact Lens Anterior Eye 2018, 41, 83–87. [Google Scholar] [CrossRef]
- Arciniega, J.C.; Uchiyama, E.; Butovich, I.A. Disruption and destabilization of meibomian lipid films caused by increasing amounts of ceramides and cholesterol. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1352–1360. [Google Scholar] [CrossRef]
- Bilkhu, P.S.; Naroo, S.A.; Wolffsohn, J.S. Randomised masked clinical trial of the MGDRx EyeBag for the treatment of meibomian gland dysfunction-related evaporative dry eye. Br. J. Ophthalmol. 2014, 98, 1707–1711. [Google Scholar] [CrossRef]
- Greiner, J.V. Long-term (12-month) improvement in meibomian gland function and reduced dry eye symptoms with a single thermal pulsation treatment. Clin. Exp. Ophthalmol. 2013, 41, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Blackie, C.A.; Coleman, C.A.; Holland, E.J. The sustained effect (12 months) of a single-dose vectored thermal pulsation procedure for meibomian gland dysfunction and evaporative dry eye. Clin. Opthalmol 2016, 10, 1385–1396. [Google Scholar] [CrossRef] [PubMed]


| Study Product | Manufacturer | Composition |
|---|---|---|
| Meiboleyes® | nthalmic Pty Ltd. | Electronic device containing heating eye cups |
| BRUDER Moist Heat Eye Compress | Bruder Healthcare Company LLC | Fabric eye mask containing MediBeads |
| Baseline Characteristics | Meiboleyes® (n = 10) | BRUDER (n = 10) | p-Values |
|---|---|---|---|
| Gender (Male: Female) | 7:3 | 3:7 | − |
| Age (years) | 38.7 ± 14.5 | 38.9 ± 14.8 | 0.853 |
| Age range—min to max (years) | 28−74 | 28−75 | − |
| Ethnicity (Non-Asian: Asian) | 1:9 | 1:9 | − |
| Ocular Surface Disease Index score (mean ± SD) | 45.2 ± 15.1 | 38.3 ± 16.4 | 0.332 * |
| Visual Analogue Scale-Dryness (0–100) | 28.0 ± 23.9 | 32.3 ± 2.08 | 0.529 |
| Average lipid layer thickness (nm) | 56.8 ± 15.0 | 65.4 ± 28.0 | 0.496 |
| Average non-invasive keratography break-up time (s) | 9.3 ± 4.8 | 10.1 ± 5.8 | 1.000 |
| Tear break-up time (s) | 5.5 ± 1.8 | 7.2 ± 1.7 | 0.052 |
| Meibomian gland secretion score (0–45) | 9.5 ± 2.8 | 8.9 ± 2.2 | 0.631 |
| Number of meibomian glands yielding liquid secretion (0–15) | 4.5 ± 1.2 | 3.5 ± 1.3 | 0.105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.; Jia, T.; Qamar, S.; Diec, J.; Stapleton, F. A Randomised Pilot Trial to Demonstrate the Feasibility of a Prototype Electronic Heating Device in Patients with Meibomian Gland Dysfunction. Biomedicines 2025, 13, 2952. https://doi.org/10.3390/biomedicines13122952
Tan J, Jia T, Qamar S, Diec J, Stapleton F. A Randomised Pilot Trial to Demonstrate the Feasibility of a Prototype Electronic Heating Device in Patients with Meibomian Gland Dysfunction. Biomedicines. 2025; 13(12):2952. https://doi.org/10.3390/biomedicines13122952
Chicago/Turabian StyleTan, Jacqueline, Tianni Jia, Sidra Qamar, Jennie Diec, and Fiona Stapleton. 2025. "A Randomised Pilot Trial to Demonstrate the Feasibility of a Prototype Electronic Heating Device in Patients with Meibomian Gland Dysfunction" Biomedicines 13, no. 12: 2952. https://doi.org/10.3390/biomedicines13122952
APA StyleTan, J., Jia, T., Qamar, S., Diec, J., & Stapleton, F. (2025). A Randomised Pilot Trial to Demonstrate the Feasibility of a Prototype Electronic Heating Device in Patients with Meibomian Gland Dysfunction. Biomedicines, 13(12), 2952. https://doi.org/10.3390/biomedicines13122952








