Abstract
Long-COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome are disabling diseases characterised by ongoing fatigue, post-exertional malaise, cognitive impairment, and autonomic dysfunction. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome typically follows viral infections, whereas Long-COVID exclusively follows SARS-CoV-2 infection, with overlapping but distinct features. This review uses comprehensive searches of online databases to compare their clinical presentations, pathophysiologies, and treatments. Both Long-COVID and ME/CFS appear to involve multifactorial mechanisms, including viral persistence, immune dysregulation, endothelial dysfunction, and autoimmunity, though their relative contributions remain uncertain. Symptom management strategies are consistent, however. Cognitive behaviour therapy has been successful, and there are minimal drug treatments. Graded exercise therapy occupies a contested place, recommending individualised pacing and multidisciplinary rehabilitation. Common and exclusive mechanisms must be identified to formulate valuable therapies. A more significant body of research focusing on immune dysfunction as a pathogenic mechanism for advancing the disease and enabling more effective therapies and diagnostics is needed.
1. Introduction
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) has been a subject of scientific interest for a very long time, as it is a complex illness. Despite the persistent presence of ME/CFS, the literature has consistently presented it as a disorder that remains shrouded in ambiguity. Numerous studies and scientific articles have explored this illness, often from specialised perspectives, indicating a scarcity of unified information. This suggests that a comprehensive understanding of ME/CFS has yet to be achieved.
Some patients who have gone through the acute phase of infection with COVID-19 have not fully recovered. They develop an array of symptoms, which are collectively described as “Long-/Post-COVID syndrome”. It is defined by the World Health Organisation (WHO) as “the continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least 2 months with no other explanation” [1]. There is a resemblance between these symptoms and those typical of the constellation of ME/CFS symptoms.
In the aftermath of the COVID-19 pandemic, the landscape has shifted, offering more specific insights and directing attention towards new avenues of investigation into immunological pathogenesis. Long-COVID has generated renewed interest and attention towards this illness due to the observed parallels in symptoms. This overlap has prompted experts to explore ME/CFS more deeply. ME/CFS has gained prominence because the similarity of its symptoms with those of Long-COVID suggests a new category of disease: infection-triggered chronic illness, described in the literature of PAPIS, PAIS, IACC, and IACI.
Not all patients with Long-COVID exhibit symptoms linked to ME/CFS abnormalities or neurological involvement. This distinction helps in understanding the heterogeneity of Long-COVID, which encompasses a broad range of symptoms. However, many manifestations do not directly involve the central nervous system (CNS), and only a subset of patients exhibit neurological symptoms.
At the same time, post-exertional malaise (PEM) and orthostatic intolerance are the hallmarks of ME/CFS, representing a distinct subset of symptoms. To address their unique symptomatology, patients with Long-COVID who do not fit the ME/CFS criteria might require alternative diagnostic approaches.
We conducted comprehensive research to compare the two conditions—ME/CFS and Long-COVID, to highlight their similar pathophysiology and to propose a research strategy that considers Long-COVID’s viral pathogenesis as a possible etiological mechanism underlying ME/CFS and its associated neuroimmunological features. We searched multiple online databases, including Scopus, Google Scholar, and PubMed/MEDLINE. The key search phrases used were combinations of terms such as “Myalgic Encephalomyelitis” (ME). “Systemic Exertion Intolerance Disease” (SEID), “Chronic Fatigue Syndrome” (CFS), “Chronic Fatigue Immune Dysfunction Syndrome” (CFIDS), and “Post-Viral Fatigue Syndrome” (PVFS). Filters were applied to limit results to studies published between 2018 and 2025 and written in English.
3. Treatment and Management Approaches
More studies are needed to address the marked heterogeneity among patients with ME/CFS and Long-COVID, as well as intragroup variability, despite their seemingly similar pathophysiology. Patients within these groups often differ in presentation and treatment response, suggesting that the understanding of their pathogenesis remains incomplete and poorly unified. Alternatively, these pathological states may arise from diffuse imbalances across multiple systems, with variations in the pathways of dysfunction that ultimately converge into similar symptom profiles. Because of the difficulty in pinpointing a specific mechanism to target as a primary treatment, no conclusive data currently support a standardised therapeutic regimen. In light of these challenges, the following study [75], which evaluates the effectiveness of several treatment approaches, provides valuable insights and general guidance for selecting management strategies for these complex conditions.
Disease severity appears to be the most influential factor affecting treatment effectiveness. However, variables such as sex, age, disease duration, and diagnostic status also seem to significantly influence treatment outcomes, sometimes even more so than the diagnosis of ME/CFS or Long-COVID itself. This observation suggests that individual variability plays a major role in the presumed convergent pathogenesis. In general, Long-COVID patients may be more responsive to the proposed therapeutic interventions.
To better address the diverse therapeutic needs of patients with either condition, it may be useful to classify them according to their predominant symptoms. The study found that the most consistently beneficial interventions across all symptom clusters were activity pacing and fluid/electrolyte management. Other therapies, recommended according to specific symptom clusters, are summarised as follows:
- Cluster 1—Multisystemic Symptomatology: Treatment approaches include manual lymphatic drainage and intravenous or subcutaneous immunoglobulin (IgG) therapy to address immune dysfunction.
- Cluster 2—POTS-Dominant Presentation: Activity pacing and the use of compression stockings are recommended. The use of compression stockings may help manage orthostatic intolerance.
- Cluster 3—Cognitive and Sleep Dysfunction with Increased Pain: Activity pacing and ADHD-type medications may be beneficial for the management of brain fog and neuropsychiatric symptoms.
- Cluster 4—Milder Symptomatology: Activity pacing is recommended to manage PEM and stabilise energy levels.
The study [75] has its limitations; however, it may serve as a valuable foundation for designing future research on ME/CFS and Long-COVID, particularly studies focusing on distinct patient subgroups within each condition.
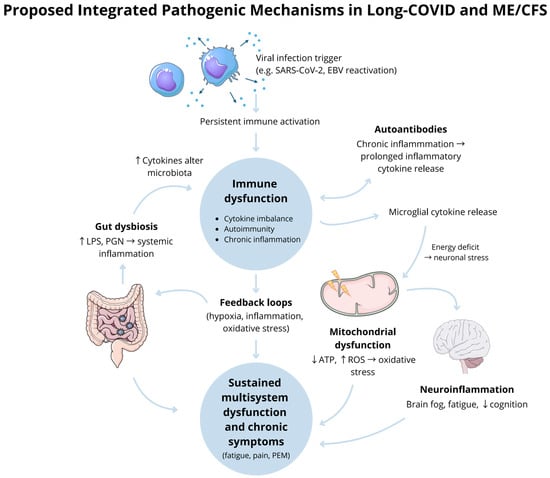
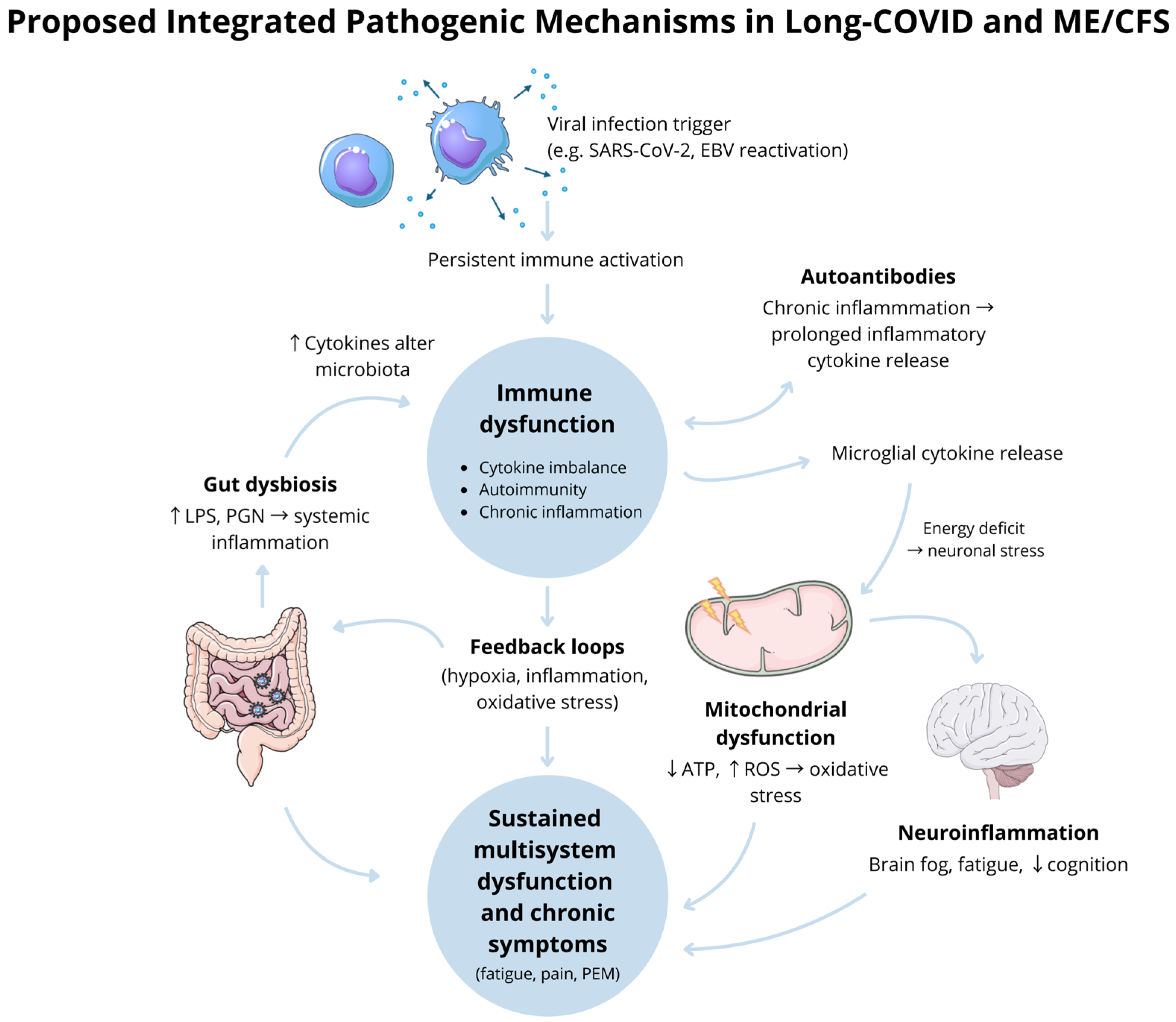
Moreover, the neuroinflammation, gut dysbiosis, and associated neuropsychiatric symptoms observed in both conditions may be mitigated through probiotic therapy. Restoring gut microbial balance, improving barrier function, and reducing intestinal permeability could represent important steps toward managing these chronic, long-lasting conditions [76]. Nevertheless, further studies are required to establish definitive efficacy, and an individualised treatment approach remains advised.
4. Conclusions
The onset of ME/CFS after a prior infection and its possible evolution after a SARS-CoV-2 infection suggest a possible shared pathogenesis with Long-COVID, especially considering the similarities in the clinical symptoms. Therefore, a distinction should be made in those cases based on the patient’s history and possible immunological markers.
Long-COVID syndrome and ME/CFS are conditions with overlapping symptoms and potentially converging pathophysiological mechanisms. Both conditions manifest as complex, multisystem illnesses, often with fatigue, post-exertional malaise, and cognitive dysfunction as hallmark features. While ME/CFS has long been poorly defined, the emergence of Long-COVID syndrome offers an opportunity to study chronic illnesses with a defined initiating event, in this case, SARS-CoV-2 infection.
Key parallels, such as immune dysregulation, neuroinflammation, and metabolic disturbances, provide a framework for shared investigation. However, the distinctiveness of each condition must not be overlooked, mainly as only a subset of Long-COVID patients fulfil diagnostic criteria for ME/CFS. Understanding these overlaps and differences will be instrumental in refining the diagnostic tools and treatment approaches.
Further research is needed to elucidate the shared and divergent pathways, focusing on immune markers, mitochondrial dysfunction, and the gut–brain axis. These insights could pave the way for targeted therapies and advancing care for ME/CFS patients and patients with long-term sequelae of Long-COVID.
Author Contributions
M.I., M.M. and H.T. designed the model and framework of presented data. G.A., M.S.H. and M.I. wrote the manuscript with input from all authors. All authors participated in contributing to text and the content of the manuscript, including revisions and edits. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ANS | Autonomic nervous system |
| ATP | Adenosine triphosphate |
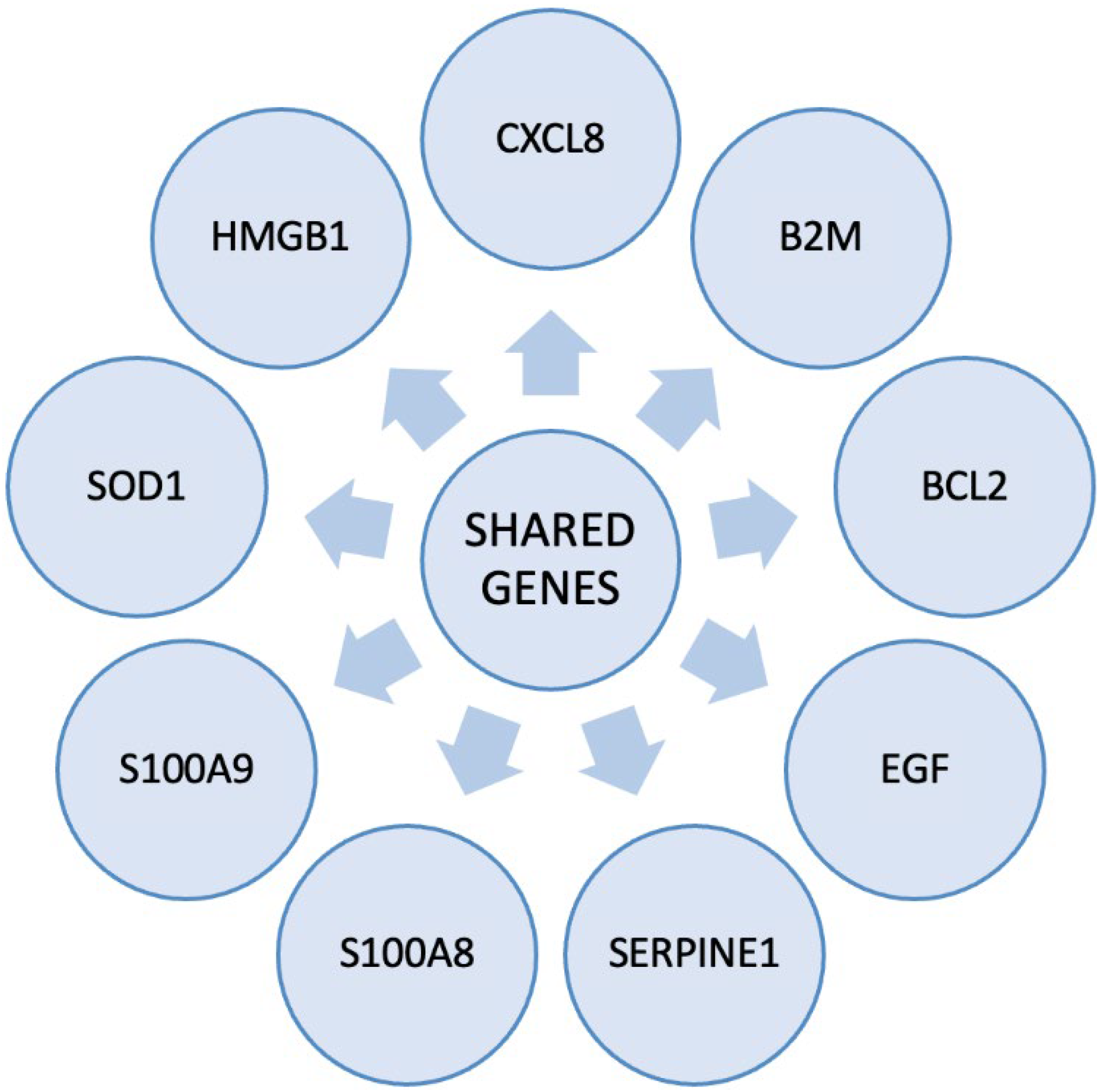
| BCL2 | B-cell lymphoma 2 |
| B2M | Beta-2-microglobulin |
| CD8+ | Cluster of differentiation 8 positive |
| COVID-19 | Coronavirus disease-19 |
| CMV | Cytomegalovirus |
| CNS | Central nervous system |
| CXCL8 | C-X-C Motif Chemokine Ligand 8 |
| EBV | Epstein–Barr Virus |
| EGF | Epidermal Growth Factor |
| ENOS | Endothelial nitric oxide synthase |
| G-CSF | Granulocyte colony-stimulating factor |
| HHV-6 | Human herpesvirus 6 |
| HHV-7 | Human herpesvirus 7 |
| HMGB1 | High mobility group box 1 protein |
| hsCPR | High sensitivity C-reactive protein |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| IFN-γ | Interferon-γ |
| IL-10 | Interleukin 10 |
| LTA | Lymphotoxin- α |
| LPS | Lipopolysaccharides |
| ME/CFS | Myalgic Encephalomyelitis/Chronic Fatigue Syndrome |
| NF-κB | Nuclear factor kappa B |
| NO | Nitric oxide |
| NK cells | Natural killer cells |
| OXPHOS | oxidative phosphorylation |
| PGN | Peptidoglycans |
| PEM | Post-exertional malaise |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus-2 |
| SERPINE1 | Serpin Family E Member 1 |
| SOD1 | Superoxide dismutase type 1 |
| S100A8 | S100 calcium-binding protein A8 |
| S100A9 | S100 calcium-binding protein A9 |
| TCA cycle | Tricarboxylic acid cycle |
| TGFB | Transforming growth factor beta |
| Th cells | T-helper cells |
| TNF-α | Tumor necrosis factor-α |
| TRAIL | Tumor necrosis factor (TNF)-related apoptosis-inducing ligand |
| Tregs | T regulatory cells |
| WHO | World Health Organization |
References
- World Health Organization. Post COVID-19 Condition. Available online: https://www.who.int/teams/health-care-readiness/post-covid-19-condition (accessed on 7 March 2025).
- Bateman, L.; Bested, A.C.; Bonilla, H.F.; Chheda, B.V.; Chu, L.; Curtin, J.M.; Dempsey, T.T.; Dimmock, M.E.; Dowell, T.G.; Felsenstein, D.; et al. Myalgic encephalomyelitis/chronic fatigue syndrome: Essentials of diagnosis and management. Mayo Clin. Proc. 2021, 96, 2861–2878. [Google Scholar] [CrossRef]
- Wong, T.L.; Weitzer, D.J. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—A systemic review and comparison of clinical presentation and symptomatology. Medicina 2021, 57, 418. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Rodriguez, L.; Morris, G. Is a diagnostic blood test for chronic fatigue syndrome on the horizon? Expert Rev. Mol. Diagn. 2019, 19, 1049–1051. [Google Scholar] [CrossRef]
- Almulla, A.; Al-Hakeim, H.K.; Maes, M. Chronic fatigue and affective symptoms in acute and long COVID are attributable to immune-inflammatory pathways. Psychiatry Clin. Neurosci. 2023, 77, 125–126. [Google Scholar] [CrossRef]
- Haffke, M.; Freitag, H.; Rudolf, G.; Seifert, M.; Doehner, W.; Scherbakov, N.; Hanitsch, L.G.; Wittke, K.; Bauer, S.; Konietschke, F.; et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2022, 20, 138. [Google Scholar] [CrossRef]
- Komaroff, A.L.; Lipkin, W.I. Insights from Myalgic Encephalomyelitis/Chronic Fatigue Syndrome May Help Unravel the Pathogenesis of Post-Acute COVID-19 Syndrome. Trends Mol. Med. 2021, 27, 895–906. [Google Scholar] [CrossRef]
- Stanculescu, D.; Larsson, L.; Bergquist, J. Hypothesis: Mechanisms That Prevent Recovery in Prolonged ICU Patients Also Underlie Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Front. Med. 2021, 8, 628029. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, T.; Cai, J.; Huang, C.; Zhan, S.; Liu, J. Bioinformatics and systems biology approach to identify the pathogenetic link of long COVID and myalgic encephalomyelitis/chronic fatigue syndrome. Front. Immunol. 2022, 13, 952987. [Google Scholar] [CrossRef]
- Yin, K.; Peluso, M.J.; Luo, X.; Thomas, R.; Shin, M.-G.; Neidleman, J.; Andrew, A.; Young, K.C.; Ma, T.; Hoh, R.; et al. Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nat. Immunol. 2024, 25, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.M.; Kell, D.B.; Pretorius, E. Cardiovascular and haematological pathology in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A role for viruses. Blood Rev. 2023, 60, 101075. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Quincy Brown, J.; Vander Heide, R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef]
- Can, F.K.; Özkurt, F.; Özturk, N.; Sezan, S. Effect of IL-6, IL-8/CXCL8, IP-10/CXCL 10 levels on the severity in COVID 19 infection. Int. J. Clin. Pract. 2021, 75, e14970. [Google Scholar] [CrossRef]
- Tate, W.; Walker, M.; Sweetman, E.; Helliwell, A.; Peppercorn, K.; Edgar, C.; Blair, A.; Chatterjee, A. Molecular mechanisms of neuroinflammation in ME/CFS and long COVID to sustain disease and promote relapses. Front. Neurol. 2022, 13, 877772. [Google Scholar] [CrossRef]
- Urano, T.; Suzuki, Y.; Iwaki, T.; Sano, H.; Honkura, N.; Castellino, F.J. Recognition of plasminogen activator inhibitor type 1 as the primary regulator of fibrinolysis. Curr. Drug Targets 2019, 20, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Fricke-Galindo, I.; Buendia-Roldan, I.; Chavez-Galan, L.; Pérez-Rubio, G.; Hernández-Zenteno, R.d.J.; Ramos-Martinez, E.; Zazueta-Márquez, A.; Reyes-Melendres, F.; Alarcón-Dionet, A.; Guzmán-Vargas, J.; et al. SERPINE1 rs6092 variant is related to plasma coagulation proteins in patients with severe COVID-19 from a tertiary care hospital. Biology 2022, 11, 595. [Google Scholar] [CrossRef]
- Fukao, Y.; Nagasawa, H.; Nihei, Y.; Hiki, M.; Naito, T.; Kihara, M.; Gohda, T.; Ueda, S.; Suzuki, Y. COVID-19-induced acute renal tubular injury associated with elevation of serum inflammatory cytokine. Clin. Exp. Nephrol. 2021, 25, 1240–1246. [Google Scholar] [CrossRef]
- Žarković, N.; Jastrząb, A.; Jarocka-Karpowicz, I.; Orehovec, B.; Baršić, B.; Tarle, M.; Kmet, M.; Lukšić, I.Ł.W.; Skrzydlewska, E. The impact of severe COVID-19 on plasma antioxidants. Molecules 2022, 27, 5323. [Google Scholar] [CrossRef]
- André, S.; Picard, M.; Cezar, R.; Roux-Dalvai, F.; Alleaume-Butaux, A.; Soundaramourty, C.; Cruz, A.S.; Mendes-Frias, A.; Gotti, C.; Leclercq, M.; et al. T cell apoptosis characterizes severe COVID-19 disease. Cell Death Differ. 2022, 29, 1486–1499. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Jayakumar, M.N.; Saleh, M.; Kannan, M.; Halwani, R.; Qaisar, R.; Ahmad, F. SARS-CoV-2 infection- induced growth factors play differential roles in COVID-19 pathogenesis. Life Sci. 2022, 304, 120703. [Google Scholar] [CrossRef] [PubMed]
- Monserrat, J.; Gómez-Lahoz, A.M.; Ortega, M.A.; Sanz, J.; Munoz, B.I.; Arévalo-Serrano, J.; Rodríguez, M.; Gasalla, J.M.; Gasulla, Ó.; Arranz, A.; et al. Role of innate and adaptive cytokines in the survival of COVID-19 patients. Int. J. Mol. Sci. 2022, 23, 10344. [Google Scholar] [CrossRef]
- Mellett, L.; Khader, S.A. S100A8/A9 in COVID-19 pathogenesis: Impact on clinical outcomes. Cytokine Growth Factor Rev. 2021, 63, 90–97. [Google Scholar] [CrossRef]
- Al-kuraishy, H.M.; Al-Gareeb, A.I.; Alkazmi, L.; Habotta, O.A.; Batiha, G.E.S. High-mobility group box 1 (HMGB1) in COVID-19: Extrapolation of dangerous liaisons. Inflammopharmacology 2022, 30, 811–820. [Google Scholar] [CrossRef]
- Sukocheva, O.A.; Maksoud, R.; Beeraka, N.M.; Madhunapantula, S.V.; Sinelnikov, M.; Nikolenko, V.N.; Neganova, M.E.; Klochkov, S.G.; Amjad Kamal, M.; Staines, D.R.; et al. Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome. J. Adv. Res. 2022, 40, 179–196. [Google Scholar] [CrossRef]
- Sepúlveda, N.; Carneiro, J.; Lacerda, E.M.; Nacul, L. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome as a Hyper-Regulated Immune System Driven by an Interplay Between Regulatory T Cells and Chronic Human Herpesvirus Infections. Front. Immunol. 2019, 10, 2684. [Google Scholar] [CrossRef]
- Rivas, J.L.; Palencia, T.; Fernández, G.; García, M. Association of T and NK Cell Phenotype with the Diagnosis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Front. Immunol. 2018, 9, 1028. [Google Scholar] [CrossRef]
- Dhawan, M.; Rabaan, A.A.; Alwarthan, S.; Alhajri, M.; Halwani, M.A.; Alshengeti, A.; Najim, M.A.; Alwashmi, A.S.S.; Alshehri, A.A.; Alshamrani, S.A.; et al. Regulatory T Cells (Tregs) and COVID-19: Unveiling the Mechanisms, and Therapeutic Potentialities with a Special Focus on Long COVID. Vaccines 2023, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- König, R.S.; Albrich, W.C.; Kahlert, C.R.; Bahr, L.S.; Löber, U.; Vernazza, P.; Scheibenbogen, C.; Forslund, S.K. The gut microbiome in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS). Front. Immunol. 2022, 12, 628741. [Google Scholar] [CrossRef]
- Low, R.; Akrami, A. A review of cytokine-based pathophysiology of Long COVID symptoms. Front. Med. 2023, 10, 1011936. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rojas, M.; Rodríguez, Y.; Zapata, E.; Ramírez-Santana, C.; Anaya, J.M. Persistent autoimmune activation and proinflammatory state in post-coronavirus disease 2019 syndrome. J. Infect. Dis. 2022, 225, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Chang XHan, Z. The effect of LTA gene polymorphisms on cancer risk: An updated systematic review and meta- analysis. Biosci. Rep. 2020, 40, BSR20192320. [Google Scholar] [CrossRef]
- Maes, M.; Almulla, A.; Zhou, B.; Algon, A.; Sodsai, P. In major dysmood disorder, physiosomatic, chronic fatigue and fibromyalgia symptoms are driven by immune activation and increased immune-associated neurotoxicity. Sci. Rep. 2024, 14, 7344. [Google Scholar] [CrossRef]
- Almulla, A.; Jaleel, A.; Algon, A.; Tunvirachaisakul, C.; Hassoun, H.; Al-Hakeim, H.; Maes, M. Mood Symptoms and Chronic Fatigue Syndrome Due to Relapsing-Remitting Multiple Sclerosis Are Associated with Immune Activation and Aberrations in the Erythron. Brain Sci. 2023, 13, 1073. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Gubbi, S.; Koch, C.A. COVID-19 and chronic fatigue syndrome: An endocrine perspective. J. Clin. Transl. Endocrinol. 2021, 27, 100284. [Google Scholar] [CrossRef] [PubMed]
- Shimba, A.; Ejima, A.; Ikuta, K. Pleiotropic effects of glucocorticoids on the immune system in circadian rhythm and stress. Front. Immunol. 2021, 12, 706951. [Google Scholar] [CrossRef]
- Fluge, Ø.; Tronstad, K.J.; Mella, O. Pathomechanisms and possible interventions in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Clin. Investig. 2021, 131, e150377. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, N.; Szklarski, M.; Hartwig, J.; Sotzny, F.; Lorenz, S.; Meyer, A.; Grabowski, P.; Doehner, W.; Scheibenbogen, C. Peripheral endothelial dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome. ESC Heart Fail. 2020, 7, 1064–1071. [Google Scholar] [CrossRef]
- Wirth, K.; Scheibenbogen, C. A unifying hypothesis of the pathophysiology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): Recognitions from the finding of autoantibodies against ß2-adrenergic receptors. Autoimmun. Rev. 2020, 19, 102527. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. The potential role of ischaemia–reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, Long COVID, and ME/CFS: Evidence, mechanisms, and therapeutic implications. Biochem. J. 2022, 479, 1653–1708. [Google Scholar] [CrossRef]
- Bertinat, R.; Villalobos-Labra, R.; Hofmann, L.; Blauensteiner, J.; Sepúlveda, N.; Westermeier, F. Decreased NO production in endothelial cells exposed to plasma from ME/CFS patients. Vasc. Pharmacol. 2022, 143, 106953. [Google Scholar] [CrossRef]
- Massardo, T.; Carlos, J.; Jaimovich, R.; Sáez, C.G.; Risco, L.; Liberman, C.; Verónica Araya, A.; Galleguillos, T.; Castro-Mora, G.; Pereira, J. Regional brain perfusion is associated with endothelial dysfunction markers in major depressive disorder. Neuropsychobiology 2020, 80, 214–224. [Google Scholar] [CrossRef]
- Kell, D.B.; Khan, M.A.; Pretorius, E. Fibrinaloid Microclots in Long COVID: Assessing the Actual Evidence Properly. Res. Pract. Thromb. Haemost. 2024, 8, 102566. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Jubran, A.S.; Almulla, A.F.; Moustafa, S.R.; Maes, M. Increased insulin resistance due to Long COVID is associated with depressive symptoms and partly predicted by the inflammatory response during acute infection. Braz. J. Psychiatry 2023, 45, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Borren, N.Z.; Plichta, D.R.; Joshi, A.D.; Bonilla, G.; Peng, V.; Colizzo, F.; Luther, J.; Khalili, H.; Garber, J.J.; van der Woude, C.J.; et al. Alterations in fecal microbiomes and serum metabolomes of fatigued patients with quiescent inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2021, 19, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Kitami, T.; Fukuda, S.; Kato, T.; Yamaguti, K.; Nakatomi, Y.; Yamano, E.; Kataoka, Y.; Mizuno, K.; Tsuboi, Y.; Kogo, Y.; et al. Deep phenotyping of myalgic encephalomyelitis/chronic fatigue syndrome in Japanese population. Sci. Rep. 2020, 10, 19933. [Google Scholar] [CrossRef]
- Lupo, G.F.D.; Rocchetti, G.; Lucini, L.; Lorusso, L.; Manara, E.; Bertelli, M.; Puglisi, E.; Capelli, E. Potential role of microbiome in chronic fatigue syndrome/myalgic encephalomyelits (CFS/ME). Sci. Rep. 2021, 11, 7043. [Google Scholar] [CrossRef]
- Newberry, F.; Hsieh, S.Y.; Wileman, T.; Carding, S.R. Does the microbiome and virome contribute to myalgic encephalomyelitis/chronic fatigue syndrome? Clin. Sci. 2018, 132, 523–542. [Google Scholar] [CrossRef]
- Zhou, B.; Pang, X.; Wu, J.; Liu, T.; Wang, B.; Cao, H. Gut microbiota in COVID-19: New insights from inside. Gut Microbes 2023, 15, 2201157. [Google Scholar] [CrossRef]
- Marasco, G.; Maida, M.; Raffaella, B.M.; Stanghellini, V.; Barbara, G. Meta-analysis: Post-COVID-19 functional dyspepsia and irritable bowel syndrome. Aliment. Pharmacol. Ther. 2023, 58, 6–15. [Google Scholar] [CrossRef]
- Clerbaux, L.; Fillipovska, J.; Muñoz, A.; Petrillo, M.; Coecke, S.; Amorim, M.; Grenga, L. Mechanisms Leading to Gut Dysbiosis in COVID-19: Current Evidence and Uncertainties Based on Adverse Outcome Pathways. J. Clin. Med. 2022, 11, 5400. [Google Scholar] [CrossRef]
- Brown, G. The endotoxin hypothesis of neurodegeneration. J. Neuroinflamm. 2019, 16, 180. [Google Scholar] [CrossRef]
- Plummer, A.; Matos, Y.; Lin, H.; Ryman, S.; Birg, A.; Quinn, D.; Parada, A.; Vakhtin, A. Gut-brain pathogenesis of post-acute COVID-19 neurocognitive symptoms. Front. Neurosci. 2023, 17, 1232480. [Google Scholar] [CrossRef]
- Zuo, W.; He, D.; Liang, C.; Du, S.; Hua, Z.; Nie, Q.; Zhou, X.; Yang, M.; Tan, H.; Xu, J.; et al. The persistence of SARS-CoV-2 in tissues and its association with long COVID symptoms: A cross-sectional cohort study in China. Lancet Infect. Dis. 2024, 24, 845–855. [Google Scholar] [CrossRef]
- Tate, W.; Walker, M.; Peppercorn, K.; Blair, A.; Edgar, C. Towards a Better Understanding of the Complexities of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Long COVID. Int. J. Mol. Sci. 2023, 24, 5124. [Google Scholar] [CrossRef] [PubMed]
- Manosso, L.; Arent, C.; Borba, L.; Ceretta, L.; Quevedo, J.; Réus, G. Microbiota-Gut-Brain Communication in the SARS-CoV-2 Infection. Cells 2021, 10, 1993. [Google Scholar] [CrossRef] [PubMed]
- Tomas, C.; Elson, J.; Strassheim, V.; Newton, J.; Walker, M. The effect of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) severity on cellular bioenergetic function. PLoS ONE 2020, 15, e0231136. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Al-Hadrawi, D.S.; Almulla, A.F.; Maes, M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: A proof of concept and mechanism study. Mol. Psychiatry 2023, 28, 564–578. [Google Scholar] [CrossRef]
- Martini, A.L.; Carli, G.; Kiferle, L.; Piersanti, P.; Palumbo, P.; Morbelli, S.; Calcagni, M.L.; Perani, D.; Sestini, S. Time-dependent recovery of brain hypometabolism in neuro-COVID-19 patients. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 90–102. [Google Scholar] [CrossRef]
- Paul, B.; Lemle, M.; Komaroff, A.; Snyder, S. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2021, 118, e2024358118. [Google Scholar] [CrossRef]
- Faghy, A.; Ashton, R.; McNeils, R.; Arena, R.; Duncan, R. Attenuating post-exertional malaise in Myalgic encephalomyelitis/chronic fatigue syndrome and long-COVID: Is blood lactate monitoring the answer? Curr. Probl. Cardiol. 2024, 49, 102554. [Google Scholar] [CrossRef] [PubMed]
- Cash, A.; Kaufman, D. Oxaloacetate Treatment For Mental And Physical Fatigue In Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Long-COVID fatigue patients: A non-randomized controlled clinical trial. J. Transl. Med. 2022, 20, 295. [Google Scholar] [CrossRef]
- Teodoro, T.; Edwards, M.J.; Isaacs, J.D. A unifying theory for cognitive abnormalities in functional neurological disorders, fibromyalgia and chronic fatigue syndrome: Systematic review. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1308–1319. [Google Scholar] [CrossRef]
- van Campen, C.L.M.C.; Rowe, P.C.; Verheugt, F.W.A.; Visser, F.C. Cognitive function declines following orthostatic stress in adults with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Front. Neurosci. 2020, 14, 688. [Google Scholar] [CrossRef] [PubMed]
- Azcue, N.; Gómez-Esteban, J.C.; Acera, M.; Tijero, B.; Fernandez, T.; Ayo-Mentxakatorre, N.; Pérez-Concha, T.; Murueta-Goyena, A.; Lafuente, J.V.; Prada, Á.; et al. Brain fog of post-COVID-19 condition and chronic fatigue syndrome, same medical disorder? J. Transl. Med. 2022, 20, 569. [Google Scholar] [CrossRef]
- Mantovani, E.; Mariotto, S.; Gabbiani, D.; Dorelli, G.; Bozzetti, S.; Federico, A.; Zanzoni, S.; Girelli, D.; Crisafulli, E.; Ferrari, S.; et al. Chronic fatigue syndrome: An emerging sequela in COVID-19 survivors? J. Neurovirol. 2021, 27, 631–637. [Google Scholar] [CrossRef]
- Woo, M.S.; Shafiq, M.; Fitzek, A.; Dottermusch, M.; Altmeppen, H.; Mohammadi, B.; Mayer, C.; Can, B.L.; Raich, L.; Matschke, J.; et al. Vagus nerve inflammation contributes to dysautonomia in COVID-19. Acta Neuropathol. 2023, 146, 387–394. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 21, 408. [Google Scholar] [CrossRef] [PubMed]
- Azcue, N.; Teijeira-Portas, S.; Tijero-Merino, B.; Acera, M.; Fernández-Valle, T.; Ayala, U.; Barrenechea, M.; Murueta-Goyena, A.; Lafuente, J.V.; Lopez, A.; et al. Small Fiber Neuropathy in the post-COVID Condition and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Clinical Significance and Diagnostic Challenges. Eur. J. Neurol. 2025, 32, e70016. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Lin, J.C.; Sheriff, S.; Maudsley, A.A.; Younger, J.W. Evidence of widespread metabolite abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome: Assessment with whole-brain magnetic resonance spectroscopy. Brain Imaging Behav. 2019, 14, 562–572. [Google Scholar] [CrossRef]
- Stein, J.A.; Kaes, M.; Smola, S.; Schulz-Schaeffer, W.J. Neuropathology in COVID-19 Autopsies Is Defined by Microglial Activation and Lesions of the White Matter with Emphasis in Cerebellar and Brain Stem Areas. Front. Neurol. 2023, 14, 1229641. [Google Scholar] [CrossRef]
- Felger, J.C. Imaging the role of inflammation in mood and anxiety-related disorders. Curr. Neuropharmacol. 2018, 16, 533–558. [Google Scholar] [CrossRef]
- Reinhold, D.; Farztdinov, V.; Yan, Y.; Meisel, C.; Sadlowski, H.; Kühn, J.; Perschel, F.H.; Endres, M.; Düzel, E.; Vielhaber, S.; et al. The brain reacting to COVID-19: Analysis of the cerebrospinal fluid proteome, RNA and inflammation. J. Neuroinflamm. 2023, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Abed, A.; Moustafa, S.R.; Almulla, A.; Maes, M.; Kiaei, M.; Kubera, M.; Merino, J.; Pandey, M. Tryptophan catabolites, inflammation, and insulin resistance as determinants of chronic fatigue syndrome and affective symptoms in long COVID. Front. Mol. Neurosci. 2023, 16, 1194769. [Google Scholar] [CrossRef] [PubMed]
- Servier Medical Art. SMART—Servier Medical ART. Available online: https://smart.servier.com (accessed on 7 March 2025).
- Eckey, M.; Li, P.; Morrison, B.; Bergquist, J.; Davis, R.W.; Xiao, W. Patient-reported treatment outcomes in ME/CFS and long COVID. Proc. Natl. Acad. Sci. USA 2025, 122, e2426874122. [Google Scholar] [CrossRef]
- Jurek, J.M.; Castro-Marrero, J. A Narrative Review on Gut Microbiome Disturbances and Microbial Preparations in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Implications for Long COVID. Nutrients 2024, 16, 1545. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).