Abstract
Background: Dotinurad (DOT) has demonstrated beneficial metabolic effects in preclinical models as a selective uric acid reabsorption inhibitor. However, its clinical impact on steatotic liver disease (SLD) with hyperuricemia (HU-SLD) remains unclear. Methods: This observational pilot study evaluated 33 patients with HU-SLD (Metabolic dysfunction-associated steatotic liver disease: n = 20; Metabolic dysfunction-associated alcohol-related liver disease: n = 1; Alcohol-related liver disease: n = 12) treated with DOT for at least 6 months. Laboratory parameters were assessed at baseline and at 6 months. The primary outcomes were changes in serum uric acid levels, hepatobiliary function markers, and renal function markers. Results: DOT significantly reduced serum uric acid levels from 8.4 (7.7–9.0) to 6.0 (5.9–6.8) mg/dL at 6 months (p < 0.001). Regarding hepatobiliary markers, gamma-glutamyl transferase decreased from 47 (30–78) to 43 (27–54) U/L (p = 0.042) and total bilirubin decreased from 0.6 (0.5–1.0) to 0.6 (0.4–0.7) mg/dL (p = 0.023). Significant but modest improvements in renal function were also observed, with serum creatinine decreasing from 1.1 (0.9–1.3) to 1.0 (0.9–1.1) mg/dL (p = 0.010) and estimated glomerular filtration rate increasing from 55.6 (44–67.3) to 56.6 (48.8–71.5) mL/min/1.73 m2 (p = 0.007). No significant changes were observed for aspartate aminotransferase, alanine aminotransferase, fibrosis-related markers, lipid profiles, or glycemic markers. Moreover, no treatment discontinuations or adverse events were recorded during the study period. Conclusions: DOT effectively reduced serum uric acid and modestly improved renal and hepatobiliary parameters in HU-SLD without any patient-reported complications. These real-world findings support the potential of DOT as a well-tolerated therapeutic option beyond urate lowering and warrant further investigation in larger, controlled studies.
1. Introduction
Steatotic liver disease (SLD) encompasses a spectrum of liver disorders characterized by hepatic fat accumulation, including the conditions traditionally classified as non-alcoholic fatty liver disease (NAFLD) and alcohol-related liver disease (ALD). Recent updates in disease nomenclature have led to the reclassification of NAFLD as metabolic dysfunction-associated steatotic liver disease (MASLD), which requires the presence of at least one cardiometabolic risk factor. As a new category, metabolic dysfunction-associated alcohol-related liver disease (MetALD) has also been proposed to designate patients with both metabolic dysfunction and moderate alcohol consumption [1]. Meanwhile, ALD, which is primarily caused by excessive alcohol consumption, continues to be recognized within the broader category of SLD by reflecting the overlapping pathophysiological mechanisms of metabolic dysfunction, oxidative stress, and inflammatory pathways that contribute to liver injury. Beyond liver-specific outcomes, SLD is strongly associated with an increased risk of extrahepatic complications, including cardiovascular disease, chronic kidney disease, and certain malignancies [2,3,4]. The coexistence of hepatic and renal impairment in SLD has garnered increasing attention due to its clinical implications and potential for targeted intervention [5,6].
Serum uric acid (UA) levels are closely associated with obesity, metabolic syndrome, insulin resistance, and the presence of hepatic steatosis [7,8]. Notably, the prevalence of hyperuricemia (HU) differs across SLD subtypes, being approximately 33–35% in MASLD but only 12–13% in ALD, with MetALD showing intermediate rates [8,9,10]. Approved in Japan in January 2020, dotinurad (DOT) is a novel selective UA reabsorption inhibitor (SURI) with high specificity for urate transporter 1 (URAT1). In preclinical mouse models, DOT has demonstrated beneficial effects on obesity, insulin resistance, and diet-induced hepatic steatosis, as well as potential renoprotective actions [11].
This pilot study evaluated the clinical effects of DOT in patients with SLD accompanied by HU (HU-SLD), including MASLD, MetALD, and ALD forms of the disease.
2. Materials and Methods
2.1. Patients and Clinical Examinations
The present retrospective analysis was based on prospectively collected data. This study was approved by Nagano Municipal Hospital (ID number: 0026) and Shinshu University School of Medicine (ID number: 4285), and was performed in adherence to the Helsinki declaration of 1975 (1983 revision). Due to the retrospective study design, the requirement for written informed consent was waived by the respective ethics committees; instead, an opt-out procedure was adopted by disclosing study information on the institutional website to allow patients the opportunity to decline participation. We carefully reviewed the medical records of 33 Japanese SLD patients who had been treated with DOT at Nagano Municipal Hospital (Nagano, Japan) or Shinshu University Hospital (Matsumoto, Japan) between May 2020 and August 2025. The therapeutic effects of DOT administration in HU-SLD patients were investigated using blood test data obtained prior to DOT initiation (baseline) and at 3, 6, and 12 months thereafter. DOT treatment was initiated at 0.5 mg once daily. The dose was increased stepwise from 0.5 mg to 1 mg, and then to 2 mg within 6 months if serum UA remained > 7.0 mg/dL in men or > 6.0 mg/dL in women (Figure 1).
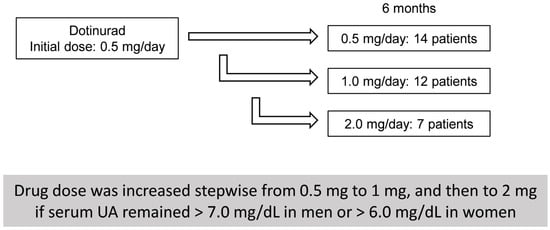
Figure 1.
Dotinurad dose escalation and patient distribution at 6 months.
Patients were included if they met the following criteria: (1) hepatic steatosis on abdominal ultrasonography defined as increased hepatic echogenicity relative to the right renal cortex (i.e., hepatorenal contrast) [12]; (2) HU defined as serum UA > 7.0 mg/dL in men and > 6.0 mg/dL in women [13]; and (3) the availability of follow-up data for at least 6 months after the initiation of DOT treatment. The exclusion criteria were alternative causes of liver dysfunction, including viral hepatitis, drug-induced liver injury, autoimmune liver disease, Wilson’s disease, hereditary hemochromatosis, and citrin deficiency [14].
MASLD was defined according to current consensus guidelines as hepatic steatosis in the presence of at least one cardiometabolic risk factor without excessive alcohol consumption [15]. ALD was judged as hepatic steatosis with a history of sustained alcohol consumption ≥ 60 g/day for men and ≥ 50 g/day for women. MetALD was defined as hepatic steatosis in individuals with at least one cardiometabolic risk factor in addition to alcohol consumption above low-risk thresholds (30–60 g/day for men and 20–50 g/day for women) but below the gender-specific amounts considered diagnostic for MASLD [15]. Patients were defined as having hypertension (HT) if their systolic/diastolic pressure was > 140/90 mmHg or if they were taking anti-hypertensive drugs [16]. Type 2 diabetes mellitus (DM) was diagnosed by a fasting plasma glucose level ≥ 126 mg/dL on two occasions, hemoglobin A1c (HbA1c) ≥ 6.5%, plasma glucose ≥ 200 mg/dL at 2 h during an oral glucose tolerance test, or random plasma glucose ≥ 200 mg/dL with classic symptoms of hyperglycemia, or if the patient was taking insulin or oral hypoglycemic agents [17]. Patients were judged as having dyslipidemia (DL) if their fasting serum levels of total cholesterol, low-density lipoprotein cholesterol, or triglyceride (TG) were ≥ 220 mg/dL, ≥ 140 mg/dL, or ≥ 150 mg/dL, respectively, or if they were taking lipid-lowering drugs [18].
All laboratory data were obtained in a fasting state. Albumin-bilirubin (ALBI) score and fibrosis-4 index (FIB-4) were calculated using the following formulae: ALBI score = (log10 total bilirubin [T-Bil] [mg/dL] × 0.66) + (albumin [g/dL] × −0.085 × 10), and FIB-4 = (age [years] × aspartate aminotransferase [AST] [U/L])/(platelet count [PLT] [×104/μL] × 10 × alanine aminotransferase [ALT] [U/L]1/2). Blood samples were obtained at baseline as well as at 3, 6, and 12 months of treatment. Regarding adverse events, patients were interviewed at 1–3-month intervals, with relevant information also collected through retrospective chart review.
2.2. Statistical Analysis
Clinical data are expressed as the number (percentage) or median (interquartile range [IQR]). Statistical analyses were performed using R software ver. 4.3.0. The Wilcoxon signed-rank test was employed to compare paired continuous variables before and after DOT treatment. Patients were defined as responders if their serum gamma-glutamyl transferase (GGT) levels were decreased ≥30% at 6 months of DOT therapy. The Mann–Whitney U test and Chi-square test were used to compare baseline characteristics between responders and non-responders. To account for multiple comparisons, p-values were adjusted using the Bonferroni method. Univariate and multivariate linear regression analyses were performed to identify factors associated with changes in GGT and estimated glomerular filtration rate (eGFR). Candidate variables were first examined individually in univariate analyses, with relevant variables subsequently employed in multivariate models. Stepwise selection based on the Akaike information criterion was applied to determine the final models. Given its clinical relevance, UA was forcibly included as an explanatory variable in multivariate testing. All statistical tests were evaluated at the 0.05 level of significance. Patients with missing laboratory data at key time points were excluded from the corresponding analyses, i.e., no imputation for missing values was performed.
3. Results
3.1. Clinical Characteristics of HU-SLD Patients Treated with DOT
A total of 33 patients diagnosed with HU-SLD were included in this study (Table 1). Patients were classified into the MASLD, MetALD, or ALD groups based on the respective diagnostic criteria. A total of 60.6% of patients (n = 20) were placed into the MASLD group, 3% (n = 1) into the MetALD group, and 36.4% (n = 12) into the ALD group. Median age was 59 years (IQR: 53–71), and 87.9% of patients were male. During the observation period, no hepatoprotective or nephroprotective agents, such as ursodeoxycholic acid, sodium-glucose cotransporter-2 inhibitors, glucagon-like peptide-1 receptor antagonist, spironolactone, tolvaptan, angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors, or pemafibrate, were co-administered. The prevalence of HT, DM, and DL was 54.5%, 36.4%, and 60.6%, respectively. Among the 33 patients with available anthropometric data, median body weight was 74.3 (65.2–76.6) kg, and median body mass index (BMI) was 24.3 (23.8–29.6) kg/m2. In comparisons of baseline characteristics between the ALD and MASLD groups, serum UA levels were significantly higher in the ALD group (9.2 [8.4–9.9] vs. 8.2 [7.4–8.5] mg/dL, p = 0.039) (Supplementary Table S1).

Table 1.
Comparison of Clinical and Laboratory Data Before and at 6 Months of Dotinurad Therapy (n = 33).
3.2. Six-Month Outcomes of DOT Treatment in HU-SLD
At 6 months of DOT therapy, a significant reduction in UA levels was observed versus baseline (8.4 [7.7–9.0] to 6.0 [5.9–6.8] mg/dL, p < 0.001) (Table 1 and Figure 2). No treatment discontinuations due to adverse events were recorded during DOT therapy. In addition to its urate-lowering effect, DOT was associated with an improvement in renal function; serum creatinine levels were significantly decreased (1.1 [0.9–1.3] to 1.0 [0.9–1.1] mg/dL, p = 0.010), and eGFR improved from 55.6 (44–67.3) to 56.6 (48.8–71.5) mL/min/1.73 m2 (p = 0.007). Regarding liver function parameters, DOT treatment led to significant reductions in GGT (47 (30–78) to 43 (27–54) U/L, p = 0.042) and T-Bil (0.6 (0.5–1.0) to 0.6 (0.4–0.7) mg/dL, p = 0.023) levels. However, no significant changes were noted for liver transaminases (AST or ALT), alkaline phosphatase (ALP), PLT, blood urea nitrogen, lipid parameters, fasting glucose, HbA1c, or fibrosis-related markers including FIB-4, hyaluronic acid, type IV collagen, and autotaxin. No adverse events related to DOT treatment, such as gastrointestinal disorders, arthralgia, or dermatitis, were observed.
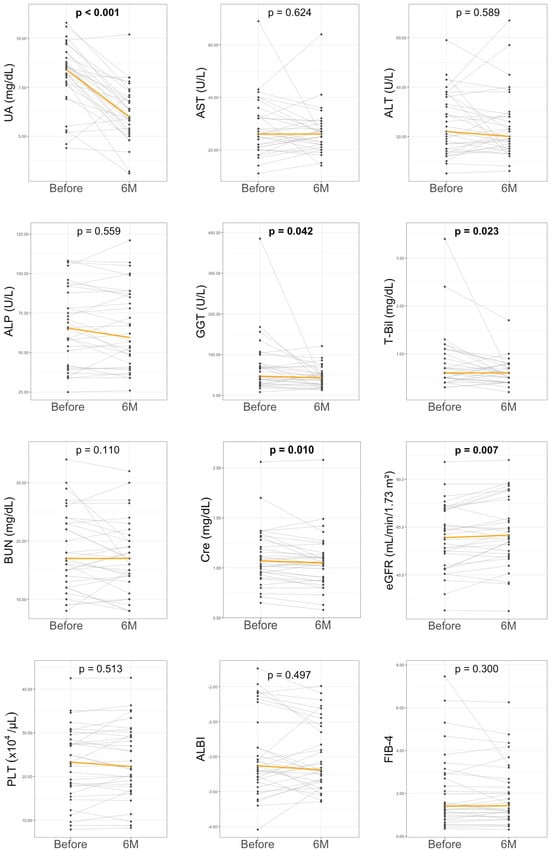
Figure 2.
Changes in laboratory parameters before and at 6 months of dotinurad treatment. ALBI, albumin-bilirubin score; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; Cre, creatinine; eGFR, estimated glomerular filtration rate; FIB-4, fibrosis-4 index; GGT, gamma-glutamyltransferase; PLT, platelet count; T-Bil, total bilirubin; UA, uric acid. The orange line represents the median value. Sample size was n = 33.
3.3. Longitudinal Changes in Liver Enzymes and UA by 12-Month DOT Therapy
To assess the longitudinal effects of DOT, serial measurements of key laboratory parameters were evaluated at baseline and at 3, 6, and 12 months in 24 patients with available blood test data. Multiple comparisons among time points were adjusted using Bonferroni correction. As illustrated in Figure 3, UA levels displayed a significant and sustained decrease beginning at 3 months, with further reductions maintained throughout the 12-month follow-up period (p = 0.041 at 3 months, p = 0.001 at 6 months, and p < 0.001 at 12 months compared with baseline). Liver enzyme levels, including AST, ALT, and ALP, remained relatively unchanged over the treatment course. In contrast, GGT levels progressively decreased, reaching statistical significance at 12 months (p = 0.009 vs. baseline). T-Bil levels also exhibited a significant reduction at 3 months (p = 0.021 vs. baseline). Renal function parameters showed no significant improvement in this cohort.
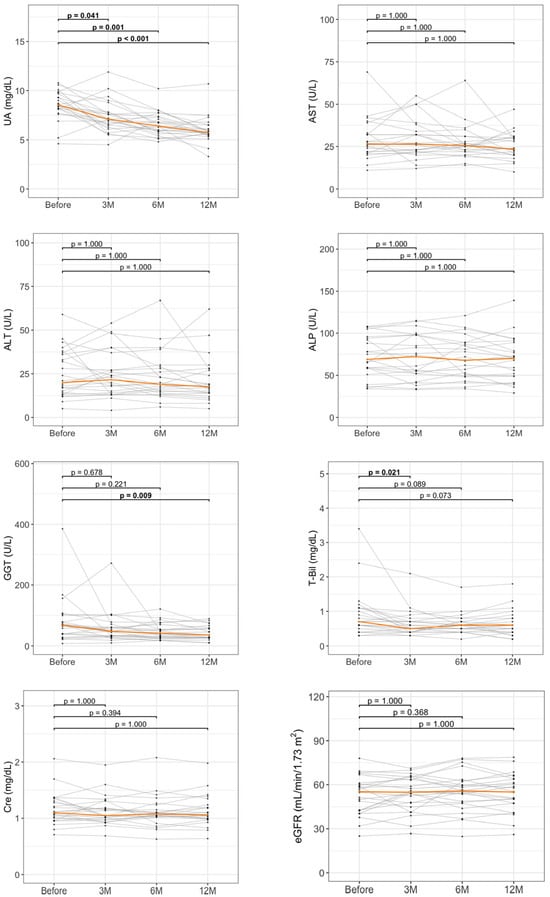
Figure 3.
Longitudinal changes in laboratory parameters during dotinurad treatment. Spaghetti plots show changes in UA and liver-related parameters at baseline, 3 months (3M), 6 months (6M), and 12 months (12M). ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cre, creatinine; eGFR, estimated glomerular filtration rate; GGT, gamma-glutamyltransferase; M, months; T-Bil, total bilirubin; UA, uric acid. The orange line represents the median value. Sample size was n = 24.
3.4. Comparison of Clinical Factors According to GGT ≥ 30% Improvement
To investigate the clinical factors associated with changes in GGT levels under DOT treatment, patients were stratified at 6 months into responders (GGT ≥ 30% improvement) and non-responders. At baseline, responders had a significantly higher prevalence of DM (66.7% vs. 19%, p = 0.010), as well as higher AST (32.5 (27.2–39.2) vs. 25 (20–27) U/L, p = 0.036) and GGT (73.5 (39.8–140.2) vs. 45 (26–67) U/L, p = 0.043) levels. No significant differences were observed for other metabolic parameters, including age and BMI (Table 2). Notably, the proportion of responders did not differ significantly between the MASLD (50% vs. 66.7%, p = 0.465) and ALD (41.7% vs. 33.3%, p = 0.716) groups.

Table 2.
Comparison of Clinical and Laboratory Data Before and at 6 Months of Dotinurad Therapy.
3.5. Correlation Between GGT Improvement and Biochemical Parameters
To further explore potential factors associated with changes in GGT, we analyzed correlations between the 6-month GGT improvement rate (ΔGGT rate) and clinical laboratory parameters (Figure 4). Among liver-related markers, a significant positive correlation was observed between ΔGGT rate and baseline AST levels (r = 0.37, p = 0.034) as well as baseline GGT values (r = 0.529, p = 0.002). In contrast, no significant correlations were observed between ΔGGT rate and ALT, ALP, T-Bil, or UA.
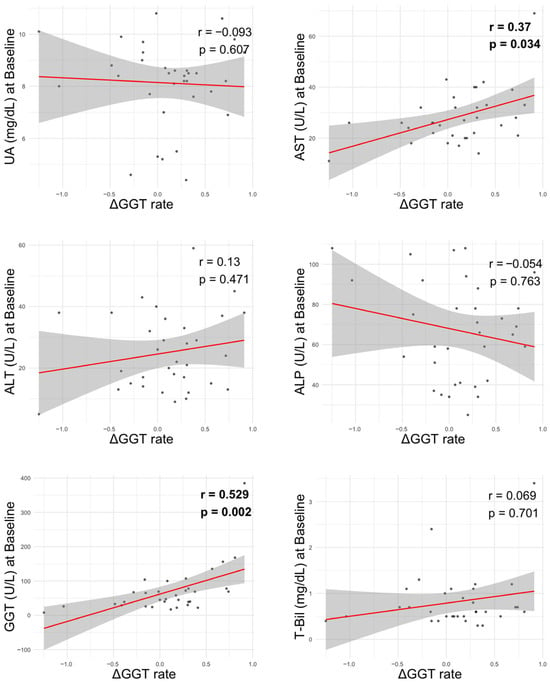
Figure 4.
Correlation between liver-related parameters at baseline and GGT improvement rate. ΔGGT rate: ([baseline GGT-6-month GGT]/baseline GGT) ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase; T-Bil, total bilirubin; UA, uric acid.
3.6. Baseline Predictors Changes in GGT and eGFR During DOT Treatment
In univariate analysis, the 6-month changes in GGT were significantly associated with baseline AST (β 0.020, p = 0.008) and GGT (β 0.004, p < 0.001) levels. In multivariate analysis, ALP (β −0.008, p = 0.007), GGT (β 0.005, p < 0.001), and BUN (β 0.028, p = 0.004) were all independently associated with changes in GGT (Table 3).

Table 3.
Baseline Factors Associated with Changes in GGT: Univariate and Multivariate Analyses.
Regarding the 6-month changes in eGFR, univariate analysis revealed a significant association with baseline albumin (β −0.004, p = 0.003). In multivariate analysis, AST (β −0.011, p = 0.002) and PLT (β −0.006, p = 0.018) were independently associated with changes in eGFR (Table 4).

Table 4.
Baseline Factors Associated with Changes in eGFR: Univariate and Multivariate Analyses.
4. Discussion
4.1. Main Findings
This study evaluated the impact of DOT on liver and kidney function in patients with HU-SLD. DOT treatment led to significant improvements in serum UA and GGT levels. Furthermore, DOT therapy was associated with a modest, but significant, amelioration in renal function as evidenced by changes in creatinine and eGFR levels. These favorable changes in hepatobiliary and renal parameters were observed regardless of alcohol consumption, suggesting a potential therapeutic effect independent of disease etiology. No treatment discontinuations or adverse drug-related events were recorded during the observation period, which demonstrated the safety of DOT.
4.2. Context with Published Literature
In the kidney, DOT acts on URAT1 in the proximal tubules by lowering intracellular UA and promoting urinary UA excretion without significant inhibition of other urate transporters, including glucose transporter 9 (GLUT9), ATP-binding cassette transporter G2 (ABCG2), and organic anion transporter 1/3 (OAT1/3) [19]. By avoiding such inhibition, DOT may minimize off-target metabolic effects and drug–drug interactions. Reduced intracellular UA may limit oxidative stress, endothelial dysfunction, and activation of the NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome [20]. Both in its soluble form and as monosodium urate crystals, UA can act in a damage-associated molecular pattern by triggering NLRP3 activation, IL-1β release, and downstream inflammatory responses, thereby contributing to tissue injury [20]. Recent clinical studies have also demonstrated that DOT significantly reduces serum UA levels and helps preserve renal function across multiple cohorts (Table 5) [21,22,23,24,25]. In line with these reports, our study confirmed a robust reduction in serum UA levels along with modest, but significant, improvements in renal markers during DOT therapy in patients with HU-SLD.

Table 5.
Summary of Recent Clinical Data on Dotinurad.
In the liver and adipose tissue, preclinical studies have shown that URAT1 inhibition ameliorates steatosis, insulin resistance, and mitochondrial dysfunction largely through reductions in oxidative stress, lipogenesis, and pro-inflammatory signaling [11]. UA is also a known activator of the NLRP3 inflammasome, promoting hepatic inflammation and fibrosis via IL-1β and IL-18 release as well as pyroptotic cell death [26]. Additional UA-related mechanisms include endoplasmic reticulum stress-induced activation of sterol regulatory element-binding proteins (SREBP), which leads to lipid accumulation and insulin resistance [27,28]. Our findings of improved GGT without significant improvements in AST or ALT support these experimental observations, suggesting a beneficial contribution to oxidative stress and cholestasis [29]. Biologically, UA lowering may preferentially mitigate oxidative stress and cholestatic pathways rather than hepatocellular injury, which helps explain the greater effect on GGT compared with AST and ALT. Based on the above lines of reasoning (Figure 5), our clinical results demonstrating improvements in both renal and hepatobiliary parameters with DOT lend indirect support to several mechanistic pathways and highlight the potential of urate-lowering therapies in SLD.
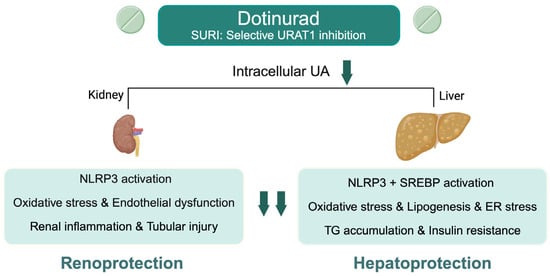
Figure 5.
Proposed molecular mechanisms of renal and hepatic protection by dotinurad through selective URAT1 inhibition in hyperuricemia-associated steatotic liver disease. ER, endoplasmic reticulum; NLRP3, NOD-like receptor family pyrin domain-containing 3; SREBP, sterol regulatory element-binding protein; TG, triglyceride; UA, uric acid; URAT1, urate transporter 1. Created in BioRender. Kimura, T. (2025) https://BioRender.com/gmchkc8.
4.3. Strengths and Limitations
A key strength of this study is the longitudinal assessment of liver- and renal-related biomarkers over a 12-month period, offering insights into treatment dynamics over time. The inclusion of patients with MASLD, MetALD, and ALD enhances the generalizability of the findings as well. To our knowledge, this is among the first clinical reports to suggest potential hepatoprotective effects of DOT in SLD patients, particularly on GGT, alongside its known UA lowering action.
However, several limitations should be noted. This was a retrospective observational study with a small sample size and no control group, which limited causal inferences. Some analyses were based on reduced subsamples, which weakened robustness and generalizability. Nutritional and lifestyle factors, including diet and physical activity, were not systematically assessed [30,31]. Furthermore, attrition bias was possible due to incomplete follow-up and missing data. Finally, no histological or imaging assessments (e.g., liver biopsy, FibroScan, or MRI-PDFF) were available to validate the changes in steatosis or fibrosis.
4.4. Future Implications
The current findings suggest that DOT, beyond its primary indication for HU, may also confer hepatorenal-protective effects in patients with SLD. To validate these results, additional prospective, randomized controlled trials are warranted in other ethnicities. Such studies should evaluate hepatic and metabolic outcomes using endpoints such as MRI-PDFF, transient elastography, and histological assessment. Given its favorable safety profile and potential hepatorenal benefits, DOT may represent a promising treatment option for patients with HU-SLD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13112716/s1, Supplementary Table S1 Clinical and Laboratory Data at Baseline in ALD and MASLD Groups.
Author Contributions
Y.K., T.I., T.K. and H.K. (Hideo Kunimoto) conceptualized and designed the study and drafted the manuscript. K.F., T.O., S.-i.W., H.K. (Hiroyuki Kobayashi) and T.Y. contributed to data collection and analysis. N.T. supervised the research and provided critical revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
T. Kimura is supported by grants from the Japan Agency for Medical Research and Development (AMED) (JP23fk0210125, JP24fk0210125, and 256f0137007j0001) for conducting this research.
Institutional Review Board Statement
This study was reviewed and approved by the Institutional Review Board of Nagano Municipal Hospital (ID number: 0026) and Shinshu University School of Medicine (ID number: 4285), and conducted according to the principles of the Declaration of Helsinki.
Data Availability Statement
The data supporting the findings of this study are not publicly available due to privacy and ethical restrictions but are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank Trevor Ralph for his assistance in English editing.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
ALBI, albumin-bilirubin; ALD, alcohol-related liver disease; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DM, diabetes mellitus; DL, dyslipidemia; DOT, dotinurad; eGFR, estimated glomerular filtration rate; FIB-4, fibrosis-4 index; GGT, gamma-glutamyl transferase; HbA1c, hemoglobin A1c; HT, hypertension; HU, hyperuricemia; HU-SLD, hyperuricemia-associated steatotic liver disease; IQR, interquartile range; MASLD, metabolic dysfunction-associated steatotic liver disease; MetALD, metabolic dysfunction-associated alcohol-related liver disease; MRI-PDFF, magnetic resonance imaging proton density fat fraction; NAFLD, non-alcoholic fatty liver disease; NLRP3, NOD-like receptor family pyrin domain-containing 3; PLT, platelet count; SLD, steatotic liver disease; SURI, selective uric acid reabsorption inhibitor; T-Bil, total bilirubin; TG, triglyceride; UA, uric acid; URAT1, urate transporter 1; ΔGGT rate, GGT improvement rate.
References
- Kanwal, F.; Neuschwander-Tetri, B.A.; Loomba, R.; Rinella, M.E. Metabolic dysfunction–associated steatotic liver disease: Update and impact of new nomenclature on the American Association for the Study of Liver Diseases practice guidance on nonalcoholic fatty liver disease. Hepatology 2024, 79, 1212–1219. [Google Scholar] [CrossRef]
- Tamaki, N.; Kimura, T.; Wakabayashi, S.I.; Umemura, T.; Kurosaki, M.; Loomba, R.; Izumi, N. Long-term clinical outcomes in steatotic liver disease and incidence of liver-related events, cardiovascular events and all-cause mortality. Aliment. Pharmacol. Ther. 2024, 60, 61–69. [Google Scholar] [CrossRef]
- Lai, M.; Lai, J.C.; Allegretti, A.S.; Patidar, K.R.; Cullaro, G. Investigating the Association between Steatotic Liver Disease and CKD in a Nationally Representative Sample. Kidney360 2024, 5, 1844–1852. [Google Scholar] [CrossRef]
- Kimura, T.; Tamaki, N.; Wakabayashi, S.-I.; Tanaka, N.; Umemura, T.; Izumi, N.; Loomba, R.; Kurosaki, M. Colorectal Cancer Incidence in Steatotic Liver Disease (MASLD, MetALD, and ALD). Clin. Gastroenterol. Hepatol. 2025, 23, 2197–2204.e2. [Google Scholar] [CrossRef]
- Bilson, J.; Mantovani, A.; Byrne, C.D.; Targher, G. Steatotic liver disease, MASLD and risk of chronic kidney disease. Diabetes Metab. 2024, 50, 101506. [Google Scholar] [CrossRef]
- Theodorakis, N.; Nikolaou, M. Integrated Management of Cardiovascular-Renal-Hepatic-Metabolic Syndrome: Expanding Roles of SGLT2is, GLP-1RAs, and GIP/GLP-1RAs. Biomedicines 2025, 13, 135. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Yu, C.; Xu, L.; Miao, M. Association of serum uric acid level with non-alcoholic fatty liver disease: A cross-sectional study. J. Hepatol. 2009, 50, 1029–1034. [Google Scholar] [CrossRef]
- Fukuda, T.; Akihisa, T.; Okamoto, T.; Fukaishi, T.; Kawakami, A.; Tanaka, M.; Yamada, T.; Monzen, K. Association of uric acid levels with the development of metabolic dysfunction-associated and metabolic and alcohol-related/associated steatotic liver disease: A study on Japanese participants undergoing health checkups. Endocr. J. 2025, 72, 671–687. [Google Scholar] [CrossRef]
- Hernández-Rubio, A.; Sanvisens, A.; Bolao, F.; Cachón-Suárez, I.; Garcia-Martín, C.; Short, A.; Bataller, R.; Muga, R. Prevalence and associations of metabolic syndrome in patients with alcohol use disorder. Sci. Rep. 2022, 12, 2625. [Google Scholar] [CrossRef]
- Hernández-Rubio, A.; Sanvisens, A.; Bolao, F.; Pérez-Mañá, C.; García-Marchena, N.; Fernández-Prendes, C.; Muñoz, A.; Muga, R. Association of hyperuricemia and gamma glutamyl transferase as a marker of metabolic risk in alcohol use disorder. Sci. Rep. 2020, 10, 20060. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nagoshi, T.; Takahashi, H.; Oi, Y.; Yoshii, A.; Kimura, H.; Ito, K.; Kashiwagi, Y.; Tanaka, T.D.; Yoshimura, M. URAT1-selective inhibition ameliorates insulin resistance by attenuating diet-induced hepatic steatosis and brown adipose tissue whitening in mice. Mol. Metab. 2022, 55, 101411. [Google Scholar] [CrossRef]
- Manley, J.A.; O’Neill, W.C. How echogenic is echogenic? Quantitative acoustics of the renal cortex. Am. J. Kidney Dis. 2001, 37, 706–711. [Google Scholar] [CrossRef]
- Jin, M.; Yang, F.; Yang, I.; Yin, Y.; Luo, J.J.; Wang, H.; Yang, X.F. Uric acid, hyperuricemia and vascular diseases. Front. Biosci. (Landmark Ed.) 2012, 17, 656–669. [Google Scholar] [CrossRef]
- Komatsu, M.; Kimura, T.; Yazaki, M.; Tanaka, N.; Yang, Y.; Nakajima, T.; Horiuchi, A.; Fang, Z.Z.; Joshita, S.; Matsumoto, A.; et al. Steatogenesis in adult-onset type II citrullinemia is associated with down-regulation of PPARalpha. Biochim. Biophys. Acta 2015, 1852, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Umemura, S.; Arima, H.; Arima, S.; Asayama, K.; Dohi, Y.; Hirooka, Y.; Horio, T.; Hoshide, S.; Ikeda, S.; Ishimitsu, T. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens. Res. 2019, 42, 1235–1481. [Google Scholar] [CrossRef]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K. Japanese clinical practice guideline for diabetes 2019. Diabetol. Int. 2020, 11, 165–223. [Google Scholar] [CrossRef]
- Teramoto, T.; Sasaki, J.; Ishibashi, S.; Birou, S.; Daida, H.; Dohi, S.; Egusa, G.; Hiro, T.; Hirobe, K.; Iida, M. Diagnostic criteria for dyslipidemia executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan—2012 version. J. Atheroscler. Thromb. 2013, 20, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Iida, S.; Katsuyama, H. A Possible Therapeutic Application of the Selective Inhibitor of Urate Transporter 1, Dotinurad, for Metabolic Syndrome, Chronic Kidney Disease, and Cardiovascular Disease. Cells 2024, 13, 450. [Google Scholar] [CrossRef]
- Braga, T.T.; Forni, M.F.; Correa-Costa, M.; Ramos, R.N.; Barbuto, J.A.; Branco, P.; Castoldi, A.; Hiyane, M.I.; Davanso, M.R.; Latz, E.; et al. Soluble Uric Acid Activates the NLRP3 Inflammasome. Sci. Rep. 2017, 7, 39884. [Google Scholar] [CrossRef]
- Tanaka, A.; Taguchi, I.; Hisauchi, I.; Yoshida, H.; Shimabukuro, M.; Hongo, H.; Ishikawa, T.; Kadokami, T.; Yagi, S.; Sata, M.; et al. Clinical effects of a selective urate reabsorption inhibitor dotinurad in patients with hyperuricemia and treated hypertension: A multicenter, prospective, exploratory study (DIANA). Eur. J. Med. Res. 2023, 28, 238. [Google Scholar] [CrossRef]
- Amano, H.; Kobayashi, S.; Terawaki, H. Dotinurad restores exacerbated kidney dysfunction in hyperuricemic patients with chronic kidney disease. BMC Nephrol. 2024, 25, 97. [Google Scholar] [CrossRef]
- Motomura, T.; Higashi, M.; Hattori, A.; Akiyama, R.; Kai, H. Efficacy and Safety of Dotinurad in Patients with Advanced Chronic Kidney Disease. Intern. Med. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Takata, T.; Taniguchi, S.; Mae, Y.; Kageyama, K.; Fujino, Y.; Iyama, T.; Hikita, K.; Sugihara, T.; Isomoto, H. Comparative assessment of the effects of dotinurad and febuxostat on the renal function in chronic kidney disease patients with hyperuricemia. Sci. Rep. 2025, 15, 8990. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. The Long-Term Effects of the Selective Inhibitor of Urate Transporter 1, Dotinurad, on Metabolic Parameters and Renal Function in Japanese Patients With Asymptomatic Hyperuricemia. J. Clin. Med. Res. 2025, 17, 320–333. [Google Scholar] [CrossRef]
- Wan, X.; Xu, C.; Lin, Y.; Lu, C.; Li, D.; Sang, J.; He, H.; Liu, X.; Li, Y.; Yu, C. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J. Hepatol. 2016, 64, 925–932. [Google Scholar] [CrossRef]
- Choi, Y.J.; Shin, H.S.; Choi, H.S.; Park, J.W.; Jo, I.; Oh, E.S.; Lee, K.Y.; Lee, B.H.; Johnson, R.J.; Kang, D.H. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab. Investig. 2014, 94, 1114–1125. [Google Scholar] [CrossRef]
- Nagaya, T.; Tanaka, N.; Suzuki, T.; Sano, K.; Horiuchi, A.; Komatsu, M.; Nakajima, T.; Nishizawa, T.; Joshita, S.; Umemura, T.; et al. Down-regulation of SREBP-1c is associated with the development of burned-out NASH. J. Hepatol. 2010, 53, 724–731. [Google Scholar] [CrossRef]
- Irie, M.; Sohda, T.; Iwata, K.; Kunimoto, H.; Fukunaga, A.; Kuno, S.; Yotsumoto, K.; Sakurai, K.; Iwashita, H.; Hirano, G. Levels of the oxidative stress marker γ-glutamyltranspeptidase at different stages of nonalcoholic fatty liver disease. J. Int. Med. Res. 2012, 40, 924–933. [Google Scholar] [CrossRef]
- De Matteis, C.; Crudele, L.; Di Buduo, E.; Cantatore, S.; Gadaleta, R.M.; Cariello, M.; Suppressa, P.; Antonica, G.; Berardi, E.; Graziano, G.; et al. Hyperhomocysteinemia is linked to MASLD. Eur. J. Intern. Med. 2025, 131, 49–57. [Google Scholar] [CrossRef]
- De Matteis, C.; Novielli, F.; Di Buduo, E.; Arconzo, M.; Gadaleta, R.M.; Cariello, M.; Moschetta, A.; Crudele, L. Atherogenic index of plasma identifies subjects with severe liver steatosis. Sci. Rep. 2025, 15, 9136. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).