Evaluation of the Effects of Tetrahydrocannabinol (THC) and Cannabidiol (CBD) on Gingival and Skin Keratinocyte Growth, Migration, Metabolic Activity, and Pro-Inflammatory Cytokine Secretion
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cells and Cell Culture Steps
2.3. Cannabis Derivatives
2.4. Effects of CBD and THC on Keratinocyte Morphology
2.5. Effects of CBD and THC on Keratinocyte Cell Viability and Growth
2.6. Effects of CBD and THC on Cell Migration
2.7. Effect of CBD and THC on Keratinocyte Lactate Dehydrogenase Activity
2.8. Effect of CBD and THC on Mitochondrial Oxygen Consumption and the Rate of Extracellular Acidification
2.8.1. The Mito Stress Assay to Evaluate OCR
2.8.2. The Glyco Stress Assay to Evaluate ECAR
2.9. Effects of CBD and THC on IL-6 and IL-8 Secretion by Keratinocytes Following LPS Stimulation
2.10. Statistical Analyses
3. Results
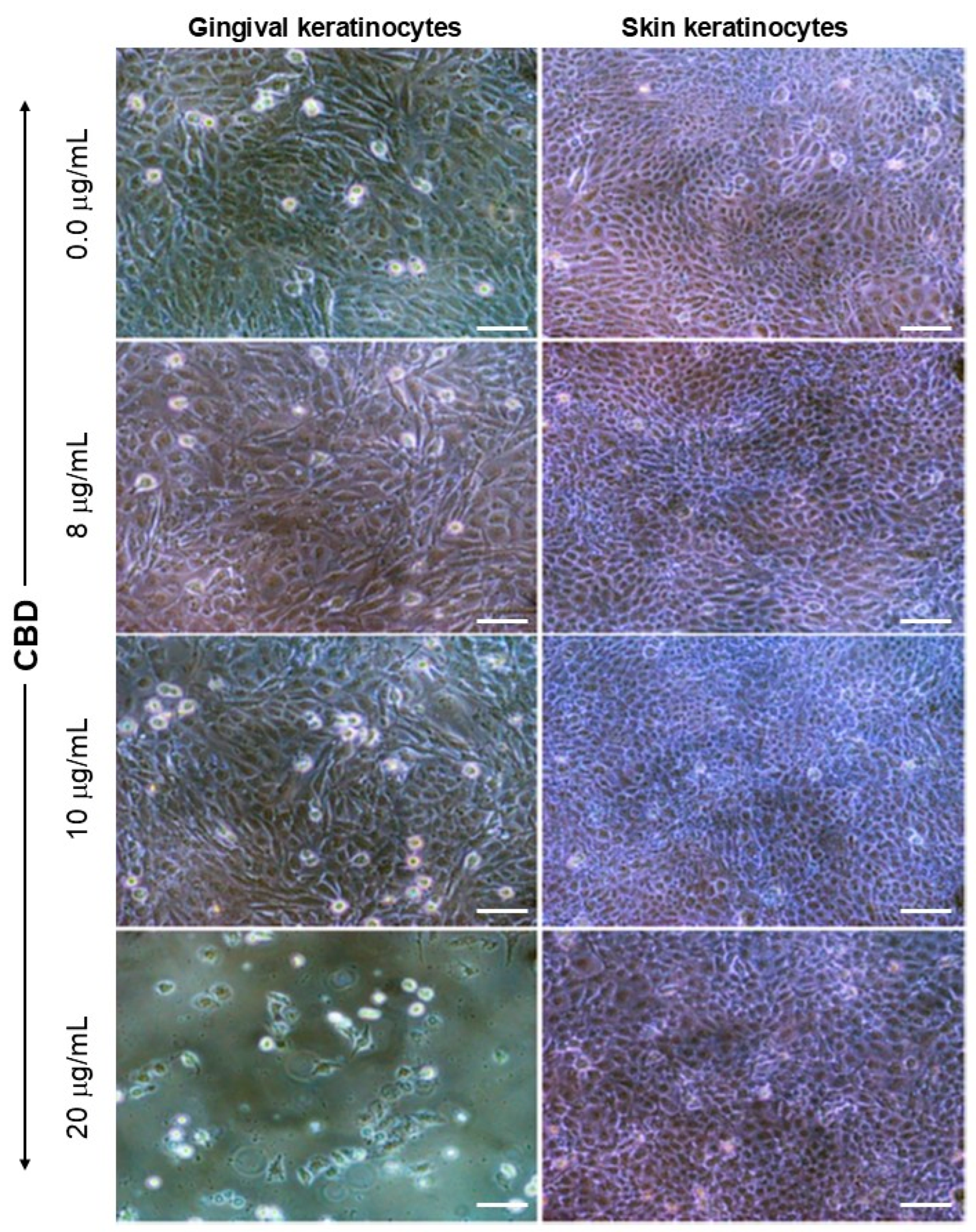
3.1. Exposure to CBD or THC Did Not Affect Keratinocyte Morphology
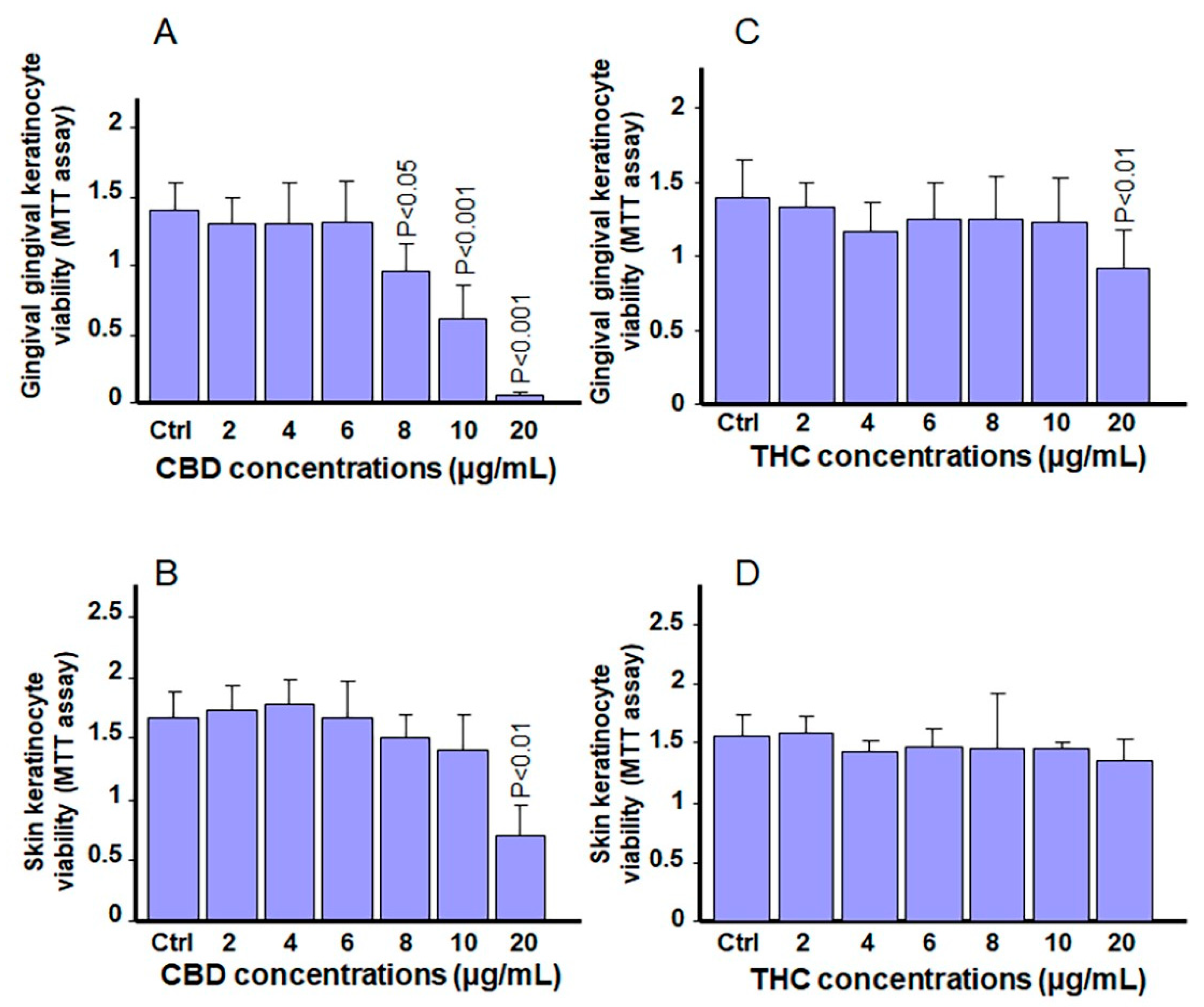
3.2. Exposure to CBD or THC Slightly Affects Cell Viability in Keratinocytes
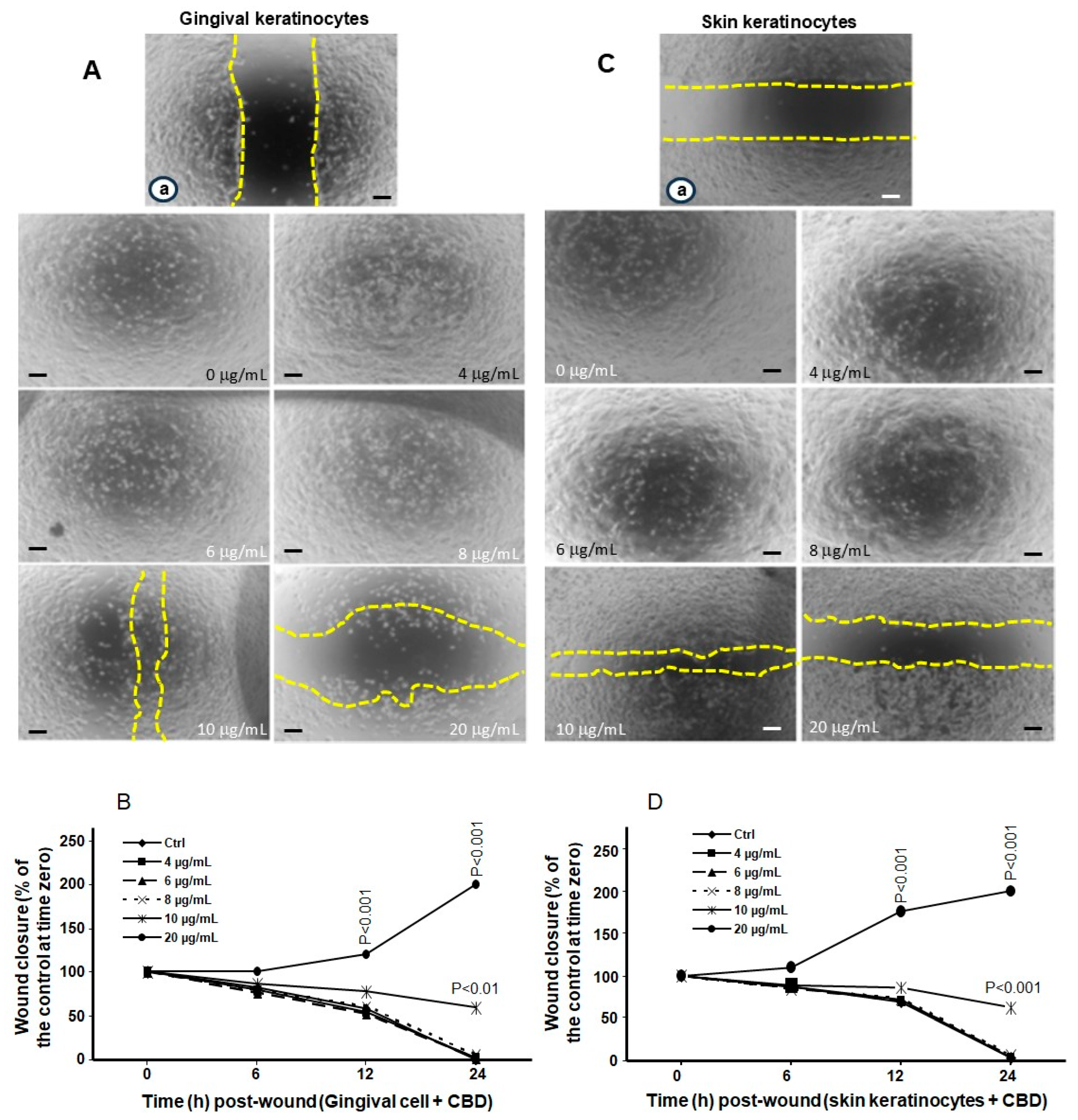
3.3. Low and Mid Concentrations of CBD and THC Have No Adverse Effects on Cell Migration
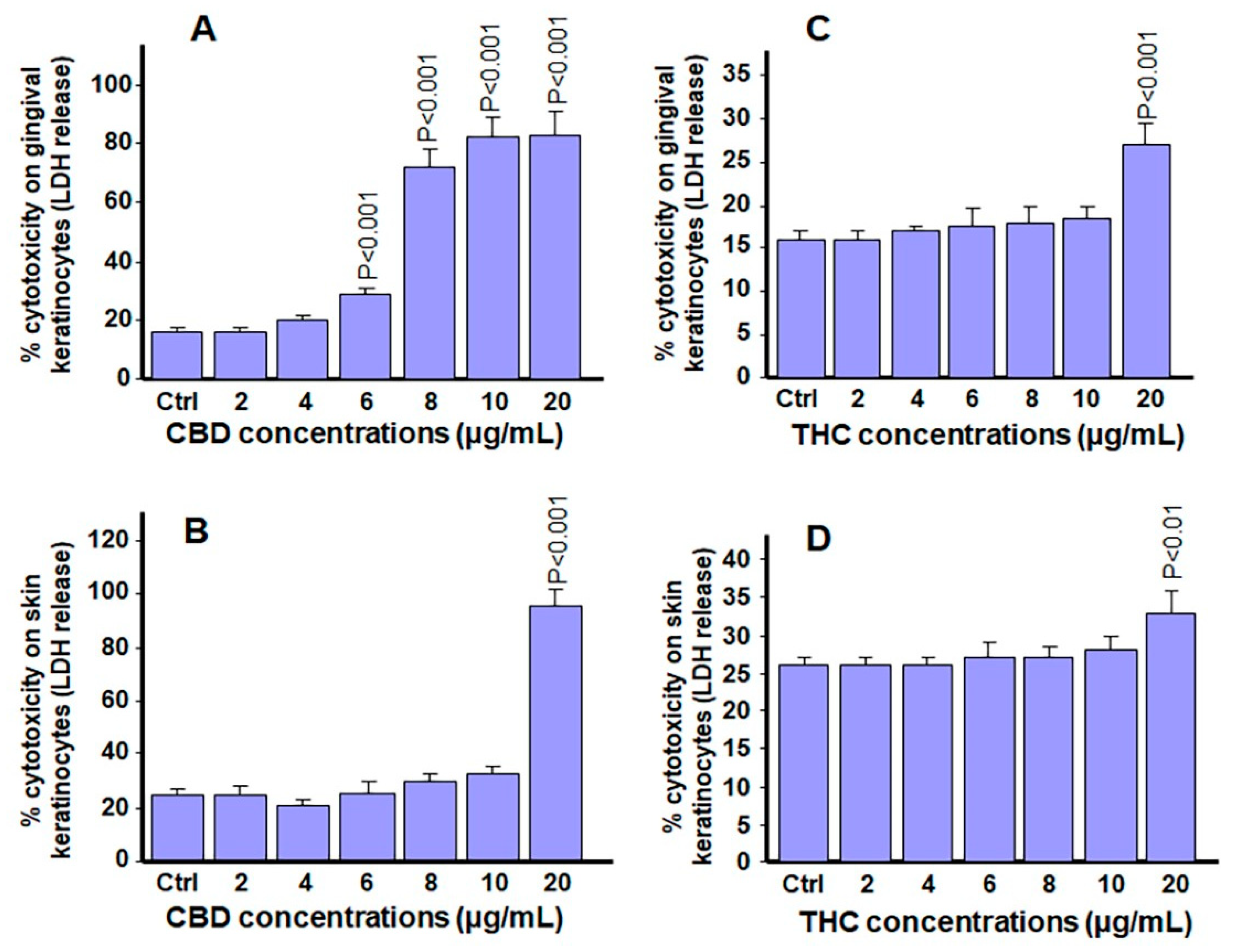
3.4. Low and Mid-Range Concentrations of Cannabinoids Were Not Cytotoxic to Gingival and Skin Keratinocytes
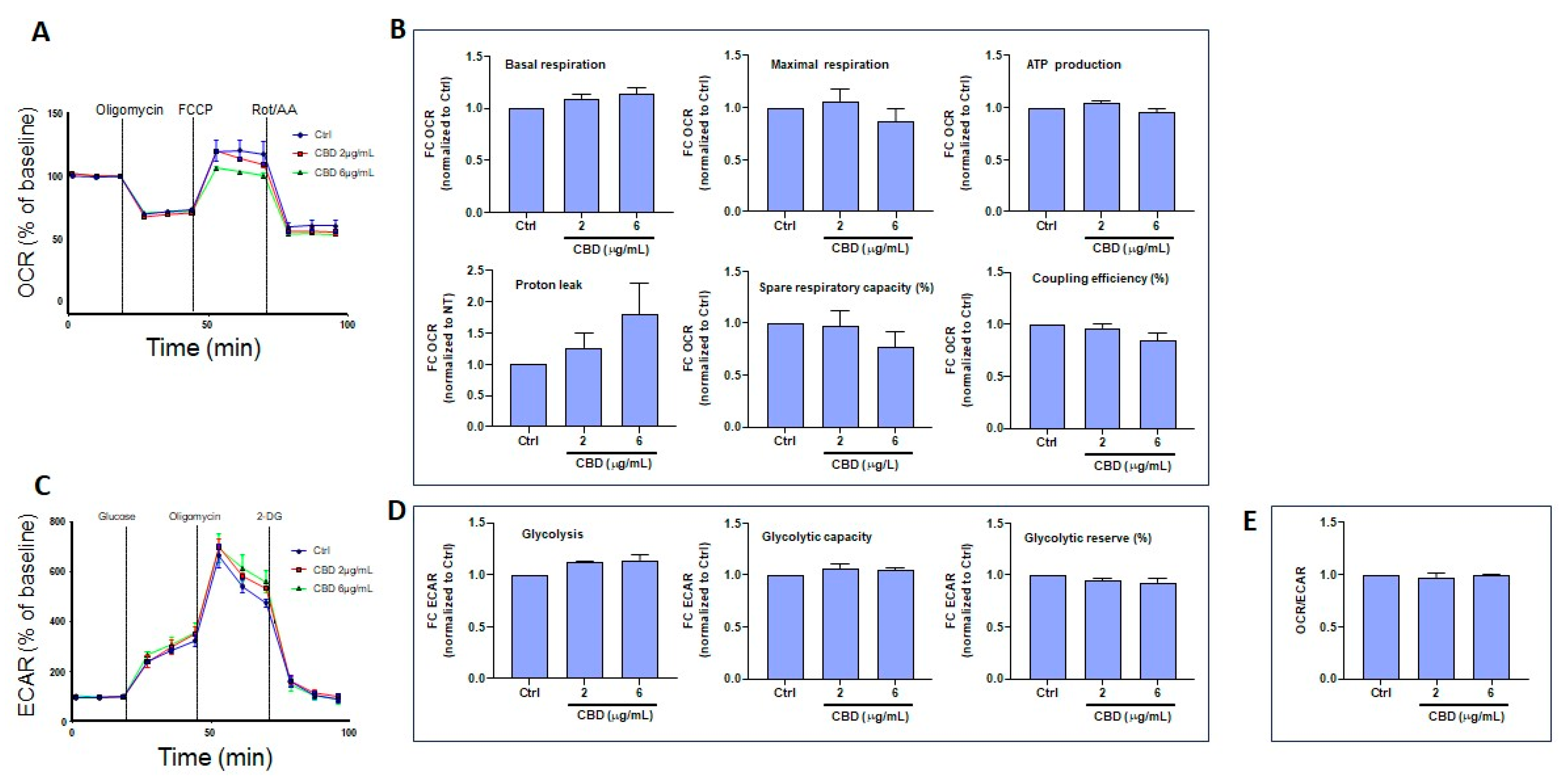
3.5. CBD and THC Exposure Does Not Induce Adverse Effects on Oxygen Consumption or Extracellular Acidification Rates in Gingival Keratinocytes
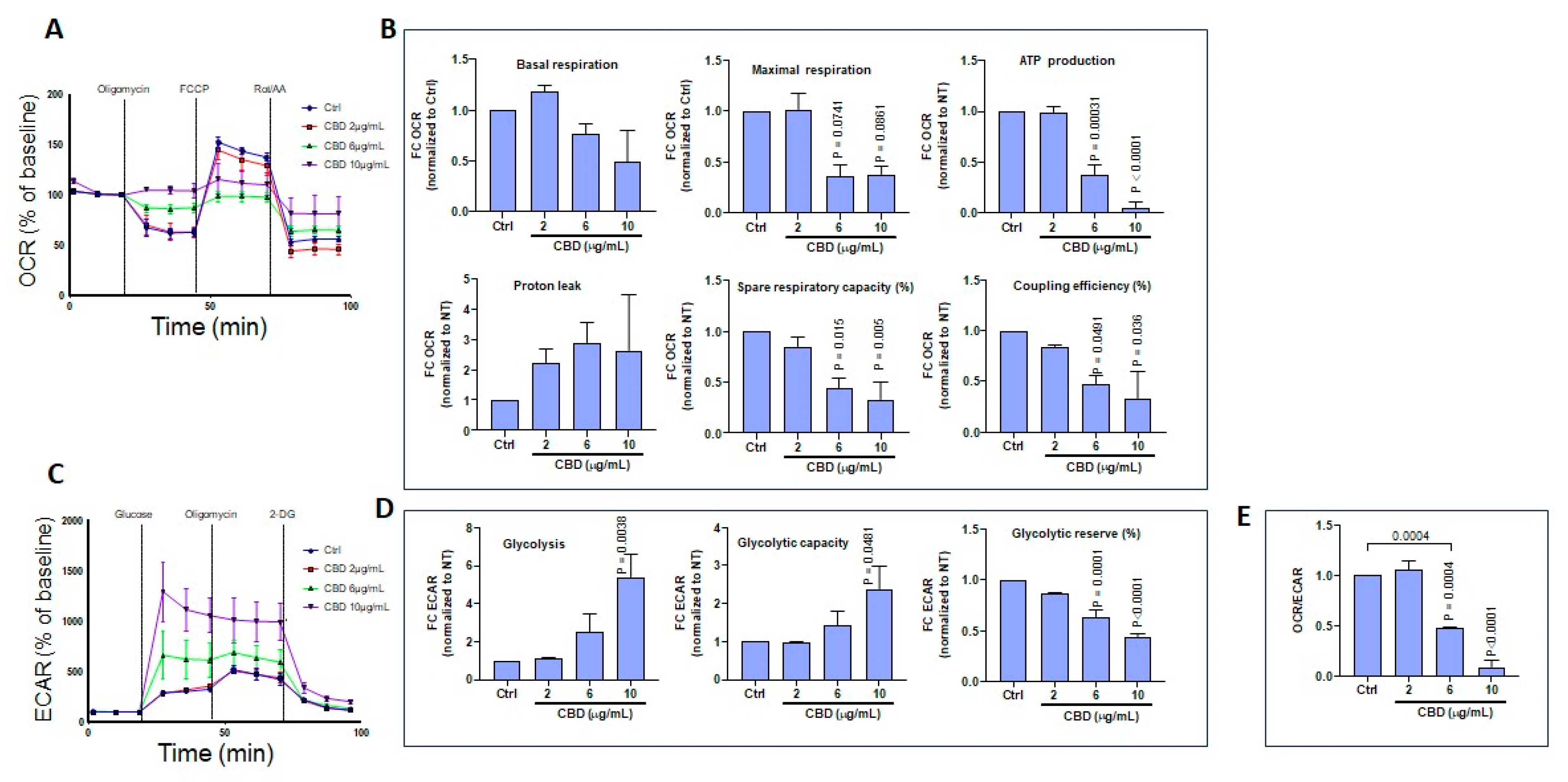
3.6. CBD and THC Treatment Modulates Oxygen Consumption and Extracellular Acidification Rates in Skin Keratinocytes
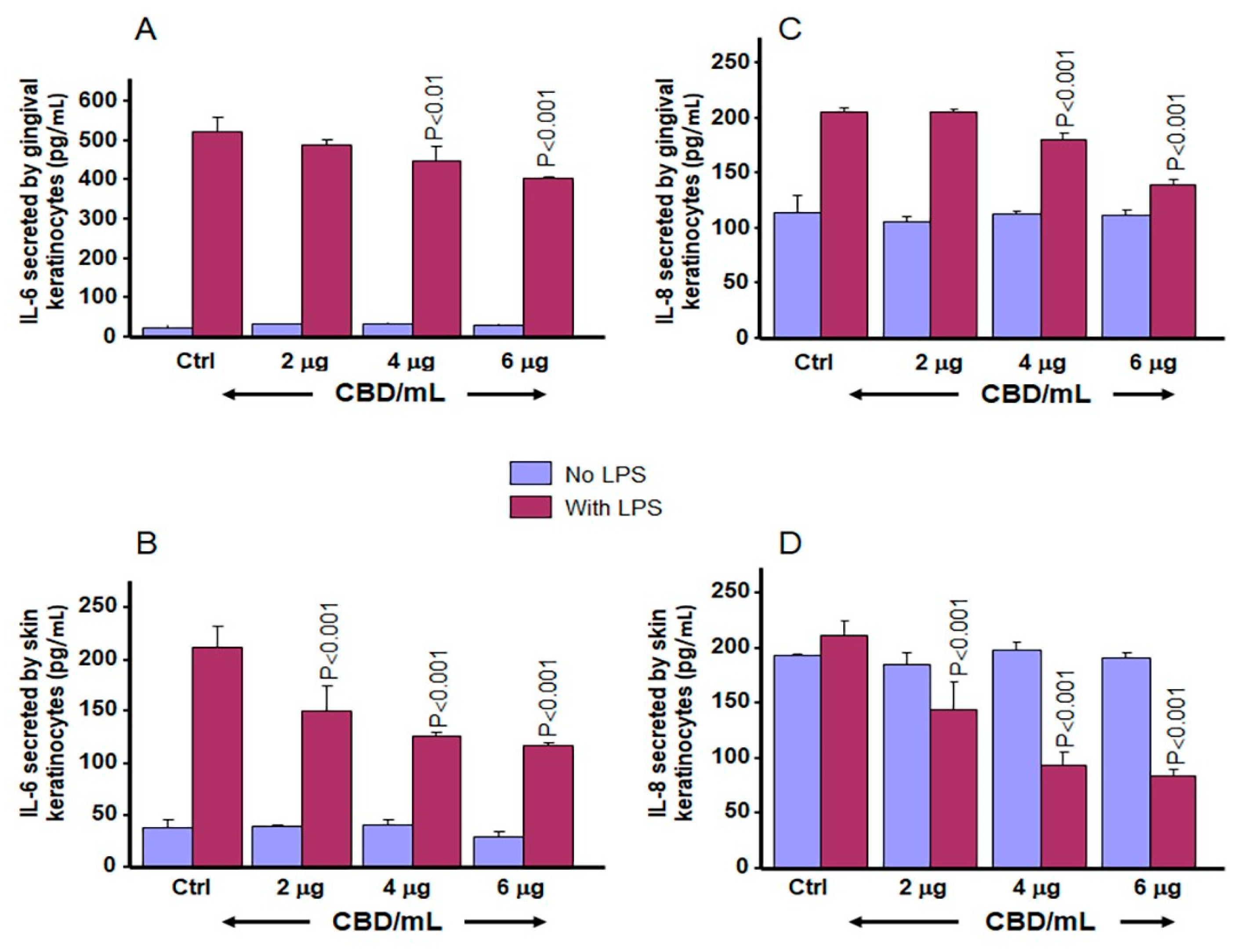
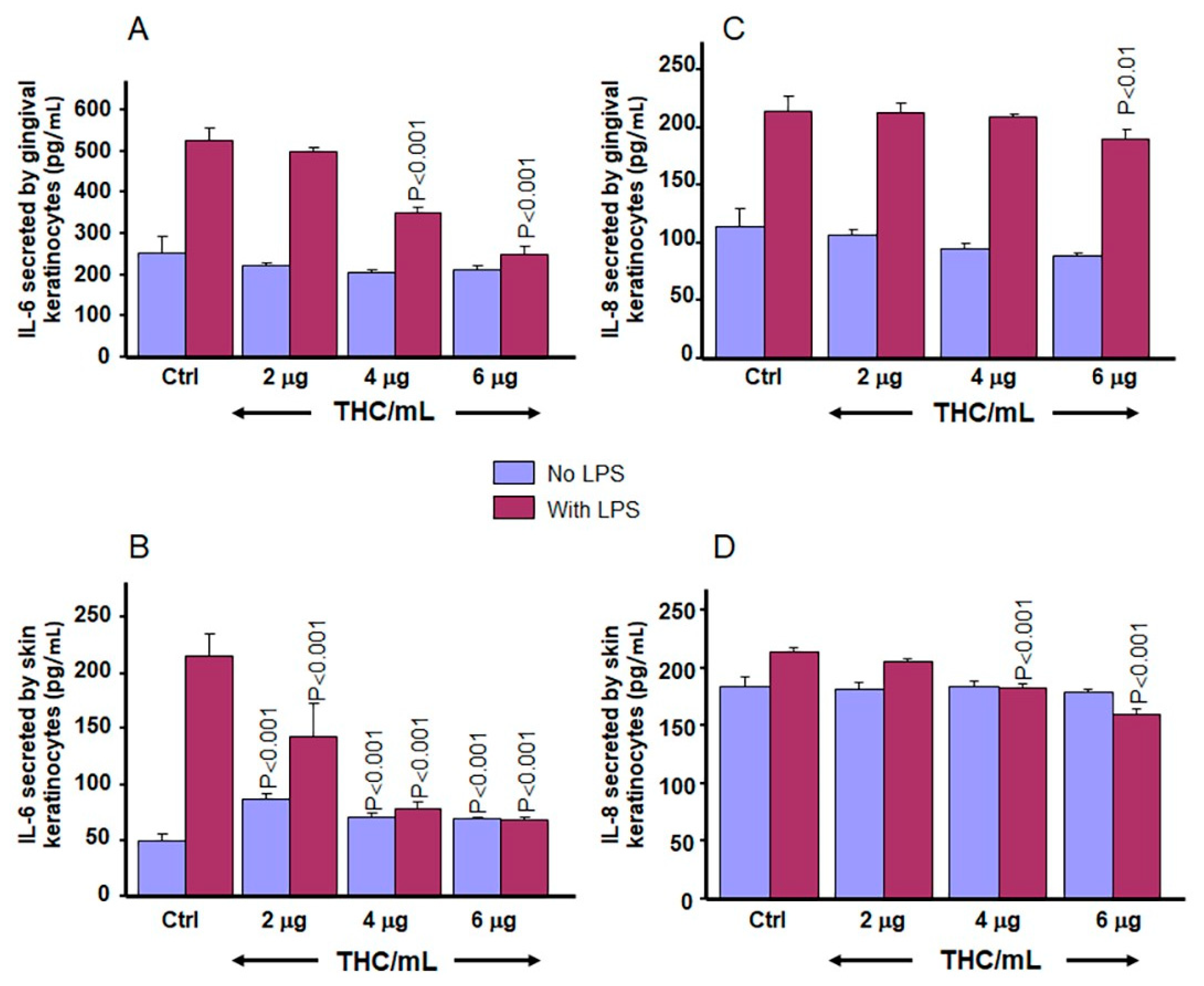
3.7. CBD and THC Decrease LPS-Induced Secretion of IL-6 and IL-8 by Gingival and Skin Keratinocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ElSohly, M.A.; Majumdar, C.G.; Chandra, S.; Radwan, M.M. A 10-year trend in cannabis potency (2013–2022) in different geographical regions of the United States of America. Front. Public Health 2024, 12, 1442522. [Google Scholar] [CrossRef]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, phenolics, terpenes and alkaloids of cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef]
- Elkins, A.C.; Deseo, M.A.; Rochfort, S.; Ezernieks, V.; Spangenberg, G. Development of a validated method for the qualitative and quantitative analysis of cannabinoids in plant biomass and medicinal cannabis resin extracts obtained by super-critical fluid extraction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1109, 76–83. [Google Scholar] [CrossRef]
- Shehata, I.; Hashim, A.; Elsaeidy, A.; Nair, A.; Urits, I.; Viswanath, O.; Kaye, A.D.; Habib, M. Cannabinoids and Their Role in Chronic Pain Treatment: Current Concepts and a Comprehensive Review. Health Psychol. Res. 2022, 10, 35848. [Google Scholar] [CrossRef]
- Thapa, D.; Patil, M.; Warne, L.N.; Carlessi, R.; Falasca, M. Enhancing Tetrahydrocannabinol’s Therapeutic Efficacy in Inflammatory Bowel Disease: The Roles of Cannabidiol and the Cannabinoid 1 Receptor Allosteric Modulator ZCZ011. Pharmaceuticals 2025, 18, 148. [Google Scholar] [CrossRef]
- Chester, L.A.; Englund, A.; Chesney, E.; Oliver, D.; Wilson, J.; Sovi, S.; Dickens, A.M.; Oresic, M.; Linderman, T.; Hodsoll, J.; et al. Effects of Cannabidiol and Delta-9-Tetrahydrocannabinol on Plasma Endocannabinoid Levels in Healthy Volunteers: A Randomized Double-Blind Four-Arm Crossover Study. Cannabis Cannabinoid Res. 2024, 9, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, D.G. A Personal Retrospective: Elevating Anandamide (AEA) by Targeting Fatty Acid Amide Hydrolase (FAAH) and the Fatty Acid Binding Proteins (FABPs). Front. Pharmacol. 2016, 7, 370. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- ALSalamat, H.A.; Abuarab, S.F.; Salamah, H.M.; Ishqair, A.H.; Dwikat, M.F.; Nourelden, A.Z.; Qandil, A.N.; Barakat, Y.; Barakat, M. Cannabis and cancer: Unveiling the potential of a green ally in breast, colorectal, and prostate cancer. J. Cannabis Res. 2024, 6, 24. [Google Scholar] [CrossRef]
- Motwani, M.P.; Bennett, F.; Norris, P.C.; Maini, A.A.; George, M.J.; Newson, J.; Henderson, A.; Hobbs, A.J.; Tepper, M.; White, B.; et al. Potent Anti-Inflammatory and Pro-Resolving Effects of Anabasum in a Human Model of Self-Resolving Acute Inflammation. Clin. Pharmacol. Ther. 2018, 104, 675–686. [Google Scholar] [CrossRef]
- Monteiro Viana, J.C.; da Silva Gomes, G.E.; Duarte Oliveira, F.J.; Marques de Araújo, L.N.; Teles, G.; Mourão, C.F.; de Vasconcelos Gurgel, B.C. The Role of Different Types of Cannabinoids in Periodontal Disease: An Integrative Review. Pharmaceutics 2024, 16, 893. [Google Scholar] [CrossRef]
- Chaoul, N.; Palazzo, S.; Cinquantasei, A.; Aresta, V.; De Chirico, C.; Albanesi, M. Cannabidiol modulation of immune cell function: In vitro insights and therapeutic implications for atopic dermatitis. Postepy Dermatol. Alergol. 2024, 41, 408–414. [Google Scholar] [CrossRef]
- Paudel, K.S.; Hammell, D.C.; Agu, R.U.; Valiveti, S.; Stinchcomb, A.L. Cannabidiol bioavailability after nasal and transdermal application: Effect of permeation enhancers. Drug Dev. Ind. Pharm. 2010, 36, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Scheau, C.; Badarau, I.A.; Mihai, L.G.; Scheau, A.E.; Costache, D.O.; Constantin, C.; Calina, D.; Caruntu, C.; Costache, R.S.; Caruntu, A. Cannabinoids in the Pathophysiology of Skin Inflammation. Molecules 2020, 25, 652. [Google Scholar] [CrossRef]
- Sunda, F.; Arowolo, A. A molecular basis for the anti-inflammatory and anti-fibrosis properties of cannabidiol. FASEB J. 2020, 34, 14083–14092. [Google Scholar] [CrossRef]
- Henshaw, F.R.; Dewsbury, L.S.; Lim, C.K.; Steiner, G.Z. The Effects of Cannabinoids on Pro- and Anti-Inflammatory Cytokines: A Systematic Review of In Vivo Studies. Cannabis Cannabinoid Res. 2021, 6, 177–195. [Google Scholar] [CrossRef]
- Knapp, A.A.; Lee, D.C.; Borodovsky, J.T.; Auty, S.G.; Gabrielli, J.; Budney, A.J. Emerging Trends in Cannabis Administration Among Adolescent Cannabis Users. J. Adolesc. Health 2019, 64, 487–493. [Google Scholar] [CrossRef]
- Rouabhia, M.; Piché, M.; Hazzi, C.; Corriveau, M.N.; Chakir, J. Effect of cannabis smoke condensate on human nasal epithelial cell adhesion, growth, and migration. Am. J. Otolaryngol. 2023, 44, 103890. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef]
- Gilchrist, E.P.; Moyer, M.P.; Shillitoe, E.J.; Clare, N.; Murrah, V.A. Establishment of a human polyclonal oral epithelial cell line. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000, 90, 340–347. [Google Scholar] [CrossRef]
- Ligasová, A.; Vydržalová, M.; Buriánová, R.; Brůčková, L.; Večeřová, R.; Janošťáková, A.; Koberna, K. A New Sensitive Method for the Detection of Mycoplasmas Using Fluorescence Microscopy. Cells 2019, 8, 1510. [Google Scholar] [CrossRef]
- Bahraminia, M.; Cui, S.; Zhang, Z.; Semlali, A.; Le Roux, É.; Giroux, K.A.; Lajoie, C.; Béland, F.; Rouabhia, M. Effect of cannabidiol (CBD), a cannabis plant derivative, against Candida albicans growth and biofilm formation. Can. J. Microbiol. 2025, 71, 1–13. [Google Scholar] [CrossRef]
- Gaffal, E.; Cron, M.; Glodde, N.; Tüting, T. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy 2013, 68, 994–1000. [Google Scholar] [CrossRef]
- Kozela, E.; Juknat, A.; Kaushansky, N.; Rimmerman, N.; Ben-Nun, A.; Vogel, Z. Cannabinoids decrease the th17 inflammatory autoimmune phenotype. J. Neuroimmune Pharmacol. 2013, 8, 1265–1276. [Google Scholar] [CrossRef]
- Sermet, S.; Finn, B.M.; Crawford, R.B.; Kaminski, N.E. Δ9-Tetrahydrocannabinol and cannabidiol selectively suppress toll-like receptor (TLR) 7- and TLR8-mediated interleukin-1β production by human CD16+ monocytes by inhibiting its post-translational maturation. J. Pharmacol. Exp. Ther. 2025, 392, 103615. [Google Scholar] [CrossRef]
- Viereckl, M.J.; Krutsinger, K.; Apawu, A.; Gu, J.; Cardona, B.; Barratt, D.; Han, Y. Cannabidiol and Cannabigerol Inhibit Cholangiocarcinoma Growth In Vitro via Divergent Cell Death Pathways. Biomolecules 2022, 12, 854. [Google Scholar] [CrossRef]
- Abedin-Do, A.; Zhang, Z.; Douville, Y.; Méthot, M.; Rouabhia, M. Effect of Electrical Stimulation on Diabetic Human Skin Fibroblast Growth and the Secretion of Cytokines and Growth Factors Involved in Wound Healing. Biology 2021, 10, 641. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Rouabhia, M.; Rouabhia, D.; Park, H.J.; Giasson, L.; Zhang, Z. Effect of soft foods on primary human gingival epithelial cell growth and the wound healing process. Food Res. Int. 2017, 100 Pt 1, 433–441. [Google Scholar] [CrossRef]
- Alanazi, H.; Rouabhia, M. Effect of e-cigarette aerosol on gingival mucosa structure and proinflammatory cytokine response. Toxicol. Rep. 2022, 9, 1624–1631. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 465–468. [Google Scholar] [CrossRef]
- Grobs, Y.; Romanet, C.; Lemay, S.E.; Bourgeois, A.; Voisine, P.; Theberge, C.; Sauvaget, M.; Breuils-Bonnet, S.; Martineau, S.; El Kabbout, R.; et al. ATP citrate lyase drives vascular remodeling in systemic and pulmonary vascular diseases through metabolic and epigenetic changes. Sci. Transl. Med. 2024, 16, eado7824. [Google Scholar] [CrossRef]
- Jaber, S.M.; Yadava, N.; Polster, B.M. Mapping mitochondrial respiratory chain deficiencies by respirometry: Beyond the Mito Stress Test. Exp. Neurol. 2020, 328, 113282. [Google Scholar] [CrossRef]
- Mazzantini, C.; El Bourji, Z.; Parisio, C.; Davolio, P.L.; Cocchi, A.; Pellegrini-Giampietro, D.E.; Landucci, E. Anti-Inflammatory Properties of Cannabidiol and Beta-Caryophyllene Alone or Combined in an In Vitro Inflammation Model. Pharmaceuticals 2024, 17, 467. [Google Scholar] [CrossRef]
- Derradjia, A.; Alanazi, H.; Park, H.J.; Djeribi, R.; Semlali, A.; Rouabhia, M. α-tocopherol decreases interleukin-1β and -6 and increases human β-defensin-1 and -2 secretion in human gingival fibroblasts stimulated with Porphyromonas gingivalis lipopolysaccharide. J. Periodontal Res. 2016, 51, 295–303. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Yu, S.; Li, C.; Gao, B.; Zhou, X. Cannabidiol attenuates periodontal inflammation through inhibiting TLR4/NF-κB pathway. J. Periodontal Res. 2023, 58, 697–707. [Google Scholar] [CrossRef]
- Christy, S.; Carlsson, A.H.; Larson, D.; Davenport, G.J.; Glenn, J.F.; Brumfield Avina, G., Jr.; Jockheck-Clark, A.; Christy, R.J.; Nuutila, K. Topical Noneuphoric Phytocannabinoid Elixir 14 Reduces Inflammation and Mitigates Burn Progression. J. Surg. Res. 2024, 296, 447–455. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, B.; Park, B.M. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int. J. Dermatol. 2015, 54, e401ee408. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Fumagalli, M.; Pacchetti, B.; Piazza, S.; Magnavacca, A.; Khalilpour, S.; Melzi, G.; Martinelli, G.; Dell’Agli, M. Cannabis sativa L. extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phytother. Res. 2019, 33, 2083–2093. [Google Scholar] [CrossRef]
- McCartney, D.; Kevin, R.C.; Suraev, A.S.; Sahinovic, A.; Doohan, P.T.; Bedoya-Pérez, M.A.; Grunstein, R.R.; Hoyos, C.M.; McGregor, I.S. How long does a single oral dose of cannabidiol persist in plasma? Findings from three clinical trials. Drug Test. Anal. 2023, 15, 334–344. [Google Scholar] [CrossRef]
- Spiera, R.; Hummers, L.; Chung, L.; Frech, T.M.; Domsic, R.; Hsu, V.; Furst, D.E.; Gordon, J.; Mayes, M.; Simms, R.; et al. Safety and Efficacy of Lenabasum in a Phase II, Randomized, Placebo-Controlled Trial in Adults with Systemic Sclerosis. Arthritis Rheumatol. 2020, 72, 1350–1360. [Google Scholar] [CrossRef]
- Billi, M.; Pagano, S.; Pancrazi, G.L.; Valenti, C.; Bruscoli, S.; Di Michele, A.; Febo, M.; Grignani, F.; Marinucci, L. DNA damage and cell death in human oral squamous cell carcinoma cells: The potential biological effects of cannabidiol. Arch. Oral Biol. 2025, 169, 106110. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Gangwar, M.; Mondal, S.C.; Jana, S. Protective effects of tetrahydrocurcumin (THC) on fibroblast and melanoma cell lines in vitro: It’s implication for wound healing. J. Food Sci. Technol. 2017, 54, 1137–1145. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, J.Y.; Kim, D.H.; Kwon, Y.G.; Kim, G.H.; Park, B.J.; Suh, D.H. Potential of cannabidiol as acne and acne scar treatment: Novel insights into molecular pathways of pathophysiological factors. Arch. Dermatol. Res. 2024, 316, 428. [Google Scholar] [CrossRef]
- Tassaneesuwan, N.; Khongkow, M.; Jansrinual, S.; Khongkow, P. Discovering the Potential of Cannabidiol for Cosmeceutical Development at the Cellular Level. Pharmaceuticals 2025, 18, 202. [Google Scholar] [CrossRef]
- Diaz, P.; Katz, T.; Langleben, A.; Rabinovitch, B.; Lewis, E. Healing of a chronic pressure injury in a patient treated with medical cannabis for pain and sleep improvement: A case report. Wound Manag. Prev. 2021, 67, 42–47. [Google Scholar] [CrossRef]
- Almada, M.; Alves, P.; Fonseca, B.M.; Carvalho, F.; Queirós, C.R.; Gaspar, H.; Amaral, C.; Teixeira, N.A.; Correia-da-Silva, G. Synthetic cannabinoids JWH-018, JWH-122, UR-144 and the phytocannabinoid THC activate apoptosis in placental cells. Toxicol. Lett. 2020, 319, 129–137. [Google Scholar] [CrossRef]
- Marzęda, P.; Wróblewska-Łuczka, P.; Drozd, M.; Florek-Łuszczki, M.; Załuska-Ogryzek, K.; Łuszczki, J.J. Cannabidiol Interacts Antagonistically with Cisplatin and Additively with Mitoxantrone in Various Melanoma Cell Lines—An Isobolographic Analysis. Int. J. Mol. Sci. 2022, 23, 6752. [Google Scholar] [CrossRef]
- Walker, O.S.; Gurm, H.; Sharma, R.; Verma, N.; May, L.L.; Raha, S. Delta-9-tetrahydrocannabinol inhibits invasion of HTR8/SVneo human extravillous trophoblast cells and negatively impacts mitochondrial function. Sci. Rep. 2021, 11, 4029. [Google Scholar] [CrossRef]
- Raouf, N.; Darwish, Z.E.; Ramadan, O.; Barakat, H.S.; Elbanna, S.A.; Essawy, M.M. The anticancer potential of tetrahydrocurcumin-phytosomes against oral carcinoma progression. BMC Oral Health 2024, 24, 1126. [Google Scholar] [CrossRef]
- Chan, J.Z.; Duncan, R.E. Regulatory Effects of Cannabidiol on Mitochondrial Functions: A Review. Cells 2021, 10, 1251. [Google Scholar] [CrossRef]
- Jiang, Z.; Jin, S.; Fan, X.; Cao, K.; Liu, Y.; Wang, X.; Ma, Y.; Xiang, L. Cannabidiol Inhibits Inflammation Induced by Cutibacterium acnes-Derived Extracellular Vesicles via Activation of CB2 Receptor in Keratinocytes. J. Inflamm. Res. 2022, 15, 4573–4583. [Google Scholar] [CrossRef]
- Zaiachuk, M.; Suryavanshi, S.V.; Pryimak, N.; Kovalchuk, I.; Kovalchuk, O. The Anti-Inflammatory Effects of Cannabis sativa Extracts on LPS-Induced Cytokines Release in Human Macrophages. Molecules 2023, 28, 4991. [Google Scholar] [CrossRef]
- Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 2018, 23, 2478. [Google Scholar] [CrossRef]
- Ohlsson, A.; Lindgren, J.E.; Andersson, S.; Agurell, S.; Gillespie, H.; Hollister, L.E. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed. Environ. Mass. Spectrom. 1986, 13, 77–83. [Google Scholar] [CrossRef]
- Valiveti, S.; Hammell, D.C.; Earles, D.C.; Stinchcomb, A.L. Transdermal delivery of the synthetic cannabinoid WIN 55,212-2: In vitro/in vivo correlation. Pharm. Res. 2004, 21, 1137–1145. [Google Scholar] [CrossRef]
- Sivadasan, D.; Madkhali, O.A. The Design Features, Quality by Design Approach, Characterization, Therapeutic Applications, and Clinical Considerations of Transdermal Drug Delivery Systems-A Comprehensive Review. Pharmaceuticals 2024, 17, 1346. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, H.J.; Han, E.T.; Han, J.H.; Park, W.S.; Kwon, Y.S.; Chun, W. 3,4,5-Trihydroxycinnamic acid suppresses phorbol-12-myristate-13-acetate and A23187-induced mast cell activation in RBL-2H3 cells. Exp. Ther. Med. 2023, 25, 227. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Zaiachuk, M.; Pryimak, N.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids Alleviate the LPS-Induced Cytokine Storm via Attenuating NLRP3 Inflammasome Signaling and TYK2-Mediated STAT3 Signaling Pathways In Vitro. Cells 2022, 11, 1391. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahraminia, M.; Laaboudi, F.-Z.; Romanet, C.; Zhang, Z.; Chakir, J.; Béland, F.; Rouabhia, M. Evaluation of the Effects of Tetrahydrocannabinol (THC) and Cannabidiol (CBD) on Gingival and Skin Keratinocyte Growth, Migration, Metabolic Activity, and Pro-Inflammatory Cytokine Secretion. Biomedicines 2025, 13, 2541. https://doi.org/10.3390/biomedicines13102541
Bahraminia M, Laaboudi F-Z, Romanet C, Zhang Z, Chakir J, Béland F, Rouabhia M. Evaluation of the Effects of Tetrahydrocannabinol (THC) and Cannabidiol (CBD) on Gingival and Skin Keratinocyte Growth, Migration, Metabolic Activity, and Pro-Inflammatory Cytokine Secretion. Biomedicines. 2025; 13(10):2541. https://doi.org/10.3390/biomedicines13102541
Chicago/Turabian StyleBahraminia, Maryam, Fatima-Zahrae Laaboudi, Charlotte Romanet, Ze Zhang, Jamila Chakir, François Béland, and Mahmoud Rouabhia. 2025. "Evaluation of the Effects of Tetrahydrocannabinol (THC) and Cannabidiol (CBD) on Gingival and Skin Keratinocyte Growth, Migration, Metabolic Activity, and Pro-Inflammatory Cytokine Secretion" Biomedicines 13, no. 10: 2541. https://doi.org/10.3390/biomedicines13102541
APA StyleBahraminia, M., Laaboudi, F.-Z., Romanet, C., Zhang, Z., Chakir, J., Béland, F., & Rouabhia, M. (2025). Evaluation of the Effects of Tetrahydrocannabinol (THC) and Cannabidiol (CBD) on Gingival and Skin Keratinocyte Growth, Migration, Metabolic Activity, and Pro-Inflammatory Cytokine Secretion. Biomedicines, 13(10), 2541. https://doi.org/10.3390/biomedicines13102541





