Abstract
Adropin—a multifunctional peptide with tissue-protective capacity that regulates energy homeostasis, sensitivity to insulin and inflammatory response—seems to show an inverse association with the presence of cardiovascular and renal diseases, obesity and diabetes mellitus in the general population. The purpose of the study is to elucidate whether adropin may be a plausible predictive biomarker for clinical outcomes in post-ST elevation of myocardial infarction (STEMI) patients with newly diagnosed prediabetes according to the American Diabetes Association criteria. A total of 1214 post-STEMI patients who received percutaneous coronary intervention were identified in a local database of the private hospital “Vita Center” (Zaporozhye, Ukraine). Between November 2020 and June 2024, we prospectively enrolled 498 patients with prediabetes in this open prospective cohort study and followed them for 3 years. The combined clinical endpoint at follow-up was defined as cardiovascular death due to acute myocardial infarction, heart failure, sudden death due to arrhythmia or cardiac surgery, and/or all-cause death. We identified 126 clinical events and found that serum levels of adropin < 2.15 ng/mL (area under the curve = 0.836; 95% confidence interval = 0.745–0.928; sensitivity = 84.9%; specificity = 72.7%; likelihood ratio = 3.11; p = 0.0001) predicted clinical outcomes. Multivariate logistic regression showed that a Gensini score ≥ 32 (Odds ratio [OR] = 1.07; p = 0.001), adropin ≤ 2.15 ng/mL (OR = 1.18; p = 0.001), use of SGLT2i (OR = 0.94; p = 0.010) and GLP-1 receptor agonist (OR = 0.95; p = 0.040) were independent predictors of clinical outcome. Kaplan–Meier plots showed that patients with lower adropin levels (≤2.15 ng/mL) had worse clinical outcomes compared to patients with higher adropin levels (>2.15 ng/mL). In conclusion, low levels of adropin (≤2.15 ng/mL) independently predicted clinical outcomes in post-STEMI patients with newly detected prediabetes and improved the discriminative ability of the Gensini score for 3-year follow-up events. Future clinical studies are needed to clarify whether adropin is a promising molecule to be incorporated into conventional risk scores for the prediction of MACCEs after STEMI.
1. Introduction
Prediabetes, as an intermediate stage between normal glucose homeostasis and established diabetes mellitus (DM), is a common non-cardiovascular outcome after ST elevation of myocardial infarction (STEMI) [1]. Although the global prevalence of prediabetes is approximately 22% (ranging from 9.6% to 37.2%), between 35% and 68% of STEMI patients with no prior diagnosis of DM at discharge from the hospital demonstrate newly detected fasting glucose and/or abnormal glucose tolerance [2,3,4].
Generally, prediabetes is defined as a metabolic condition that is associated with hyperglycemia in the range of 5.6–6.9 mmol/L or glycated hemoglobin (HbA1c) in the range of 5.7–6.4%, which are typically higher than normal ranges but lower than the conventional thresholds for diabetes mellitus [2]. Although the diagnostic criteria for the condition are variable and differ between some clinical guidelines, early diagnosis and thorough screening of prediabetes are considered essential steps not only to prevent diabetes mellitus but also to predict cardiovascular complications [5,6]. Indeed, the concept of “prediabetes” is based on a predictive model that utilized the high composite risk of developing diabetes mellitus and CVD, including acute myocardial infarction, heart failure and asymptomatic atherosclerosis, by potentiating the effects of metabolic abnormalities, such as hyperglycemia, lipid toxicity, insulin resistance and concomitant metabolic condition, including abdominal obesity [2].
Although prediabetes was not found to be a strong predictor of 30-day outcome in STEMI patients without elevated glycosylated hemoglobin (HbA1c) levels on admission (5.7–6.4%), diagnosed prediabetes or DM was independently associated with long-term all-cause and cardiovascular (CV) mortality and hospital readmission [7,8,9,10]. In addition, prediabetes sufficiently increases the risk of complications after STEMI and percutaneous coronary intervention (PCI), including heart failure (HF) and sudden death [11,12,13,14].
The main underlying pathogenic mechanisms by which newly diagnosed prediabetes among non-DM individuals after STEMI intervenes in CV outcomes may include (a) support of angiopathy through low-grade inflammation, oxidative stress, endothelial dysfunction, (b) adverse cardiac remodeling through accumulation of extracellular matrix, acceleration of coronary atherosclerosis, myocardial ischemia/necrosis, neurohumoral activation, (c) attenuation of endogenous reparative potency via genetic/epigenetic regulation of metabolic memory, insulin resistance, lipid toxicity, alteration of mitochondrial structure and function, impairment of cellular energy homeostasis, (d) worsening auto-paracrine cardiac function regulation, (e) potentiating the impact of conventional CV and non-CV risk factors, such as abdominal obesity, hypertension, dyslipidemia, chronic kidney disease, on target organs, PCI efficacy and long-term survival, etc. [15,16,17,18,19,20,21]. Overall, prediabetes rather reflects the continuum of the risk of microvascular and macrovascular outcomes than the stable abnormality of glucose metabolism. Indeed, individuals with known prediabetes had a higher prevalence of adnominal obesity and dyslipidemia with a more atherogenic lipid profile and an elevated risk of atherosclerotic cardiovascular disease. In this context, it should be noted that the multivariate impact of prediabetes on the clinical outcomes of patients after STEMI requires a rational approach to risk stratification.
Previous clinical studies have shown that the conventional scores, clinical signs/symptoms, echocardiographic features (left ventricular ejection fraction and global longitudinal strain) and biomarker models, including natriuretic peptides, cardiac troponins, HbA1c and C-reactive protein, had limited discriminative potency for long-term outcomes in stable prediabetes patients after STEMI effectively treated with PCI [22]. Indeed, only high concentrations of N-terminal brain natriuretic pro-peptide (NT-proBNP) and high-sensitivity troponin T (hs-TnT) predicted incident CV events in patients with prediabetes [23]. Of note, at least 50% of HF individuals, regardless of other CV risk factors, including prediabetes, diabetes, obesity and metabolic syndrome, exhibited near normal concentrations of NT-proBNP [24,25]. On the other hand, it has been established that the combination of echocardiographic parameters and circulating biomarkers along with traditional CV risk factors in individuals who are normoglycemic or with prediabetes may improve the risk prediction for both incident HF and total CV events in asymptomatic/stable CV disease [26,27]. It remains unclear what biomarker models are optimal for patients after STEMI with newly diagnosed prediabetes to be stratified at risk of further outcomes.
Adropin, a recently identified multifunctional pro-angiogenic neuroendocrine protein that belongs to a group of hepatokines, plays a crucial role in maintaining glucose and lipid homeostasis, regulation of insulin sensitivity and body weight, recruitment of endothelial progenitor cells, immune response, organ protection and attenuation of inflammation [28]. It is mainly produced by hepatocytes and has also been found in the brain, gastrointestinal tract and myocardium [28]. Adropin acts through direct interaction with the G-protein-coupled receptor and activates the NB-3/Notch and vascular endothelial growth factor (VEGF) receptor (VEGFR2)-PI3K-Akt and VEGFR2-Erk1/2 signaling pathways [29]. In STEMI, adropin was able to promote myocardial ischemia, prevent myocardial necrosis, neutrophil/macrophage infiltration of the necrotic area, modulate endothelial function and exert cardioprotective effects [30,31,32].
Adropin was found to be inversely associated with insulin resistance and body mass index [33]. On the other hand, adropin levels were often elevated in chronic HF patients, whereas low adropin levels were found in acute HF due to STEMI [34,35]. However, adropin levels in HF patients appear to be closely related to metabolic comorbidities and chronic kidney disease [36]. Overall, low levels of adropin have been associated with CV complications. In addition, low levels of adropin have been proposed as a novel biomarker for HF, renal outcomes, type 2 DM-induced adverse cardiac remodeling, atherosclerosis and cardiac cachexia [37,38,39]. Therefore, the measurement of adropin is promising in the context of investigating the impact of prediabetes on clinical outcomes after STEMI. The aim of this study is to determine whether adropin is a plausible predictive biomarker for clinical outcomes in post-STEMI patients with newly diagnosed prediabetes.
2. Materials and Methods
2.1. Patient Population and Study Design
A total of 1214 post-STEMI patients who received PCI were identified in a local database of the private hospital “Vita Center” (Zaporozhye, Ukraine). Using the inclusion criteria (male and female aged ≥18 years, newly diagnosed prediabetes within 3–6 months after STEMI treated with successful PCI (TIMI score = 2–3), informed consent to participate in the study), we prospectively enrolled 498 patients with prediabetes in this open prospective cohort study and followed them for 3 years. The inclusion and exclusion criteria, as well as study procedures and determination of clinical outcomes, are outlined in Figure 1. The study was conducted between November 2020 and June 2024.
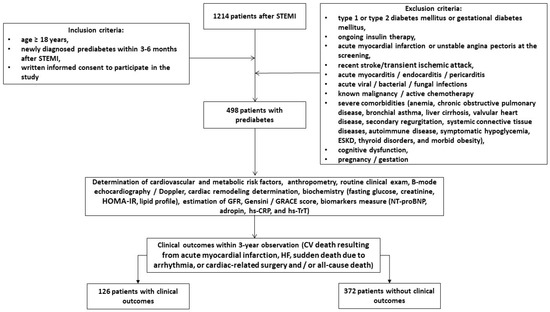
Figure 1.
Flow chart of study design. Abbreviations: CV, cardiovascular; GRACE, Global Registry of Acute Coronary Events; HOMA-IR, homeostatic assessment model of insulin resistance; HF, heart failure; NT-proBNP, N-terminal brain natriuretic pro-peptide; hs-CRP, high-sensitivity C-reactive peptide; GFR, glomerular filtration rate; hs-TrT, high-sensitivity troponin T; STEMI, ST elevation of myocardial infarction; ESKD, end-stage kidney disease.
2.2. Demographics and Anthropomorphic Data Collection
Demographics and anthropomorphic data, basic clinical characteristics and comorbidities were collected at baseline and at the end of the study.
2.3. Clinical Outcomes Determination
The combined clinical endpoint of follow-up was defined as CV death resulting from acute myocardial infarction, HF, sudden death due to arrhythmia, or cardiac-related surgery and/or all-cause death.
2.4. Relevant Medical Data Collection
Over the three-year study period, we collected data on patients from a variety of sources, including medical records, databases, discharge summaries, autopsy reports and direct calls to patients, their relatives and/or their doctors.
2.5. Determination of Prediabetes/Diabetes and Other Comorbidities
The identification of individuals at risk of prediabetes and type 2 diabetes was performed using the American Diabetes Association (ADA) Diabetes Risk Test [40]. We used the ADA criteria for prediabetes: glycosylated hemoglobin (HbA1c) = 5.7–6.4%, fasting plasma glucose = 5.6–6.9 mmol/L, 2-h plasma glucose during a 75 g oral glucose tolerance test (OGTT) = 7.8–11.0 mmol/L.
Chronic kidney disease (CKD) [41], heart failure [42], hypertension [43] and dyslipidemia [44] were assessed according to current clinical guidelines.
2.6. Echocardiography Examination
All patients underwent echocardiographic and Doppler examinations performed by two blinded, highly experienced echocardiographers according to the guidelines of the American Society of Echocardiography [45]. The standard apical 2- and 4-chamber views were acquired at baseline and at the end of the study using a GE Healthcare Vivid E95 scanner (General Electric Company, Horton, Norway). The conventional hemodynamic parameters included left ventricular ejection fraction (LVEF) using Simpson’s method, left ventricular end-diastolic (LVEDV) and end-systolic (LVESV) volumes, left atrial volume index (LAVI), early diastolic blood filling (E) and mean longitudinal strain ratio (e’). The estimated E/e’ ratio was expressed as the ratio of the E wave velocity to the averaged medial and lateral e’ velocities. Left ventricular hypertrophy was defined as a left ventricular mass index (LVMI) ≥ 95 g/m2 in women or ≥115 g/m2 in men. Left ventricular global longitudinal strain (GLS) was obtained via 2D speckle tracking imaging after obtaining high-quality echocardiographic recordings during at least three cardiac cycles. Data were stored in DICOM format for further analysis.
2.7. Glomerular Filtration Rate and Insulin Resistance Determination
The conventional CKD-EPI formula was used to estimate the glomerular filtration rate (eGFR) [46]. The homeostatic assessment model of insulin resistance (HOMA-IR) was used to assess insulin resistance [47].
2.8. Post-STEMI Risk Determination and Assessment of Atherosclerosis Severity
All enrolled patients underwent coronary angiography, and the results were assessed by at least two interventional physicians. The severity of atherosclerosis was assessed using the Gensini score system [48]. The post-discharge Global Registry of Acute Coronary Events (GRACE) score was calculated for all patients with a discriminative survival of 3 years [49].
2.9. Blood Sampling
Venous blood samples (3–5 mL) were collected from fasting patients in Vacutainer tubes at three time points: baseline and end of study. Pooled samples were centrifuged (3000 r/min, 30 min). Sera were collected and immediately frozen and stored at −70 °C until analysis before utilization. All routine biochemical tests were performed using standard biochemical techniques on a Roche P800 analyzer (Basel, Switzerland).
2.10. Biomarker Analysis
NT-proBNP, adropin, hs-TrT and hs-CRP were quantified in the serum using commercial enzyme-linked immunosorbent assay (ELISA) kits manufactured by Elabscience (Houston, TX, USA). The intra- and inter-assay coefficients of variations for all kits were <10%.
2.11. Statistics
The Kolmogorov–Smirnov test was used to assess normality and the Levene test to assess homogeneity. Continuous variables were presented as mean (M) and standard deviation (SD) or median (Me) and 25–75% interquartile range (IQR), depending on their distribution. Categorical variables were presented as proportions and percentages of the total. Chi-square, Mann–Whitney U and Kruskal–Wallis tests were used to compare variance according to distribution.
To calculate sample size, we used Equation (1):
Z1-α/2 is 1.96; δ represents allowable error 0.05; p represents sensitivity, which was determined as 0.83 in a previous study of 3-year mortality rate in post-STEMI prediabetic patients treated with PCI, in which clinical outcomes, including death, occurred in 11.2% of patients with prediabetes. Thus, we found that a minimum of >400 patients were needed to obtain concise results in this study. Taking into consideration a possible 20% drop-out rate due to several reasons, we decided to include 480 more patients.
Spearman’s correlation coefficient was calculated to determine the correlation between variables. Plausible predictors of the combined clinical outcome were identified using univariate logistic regression and backward stepwise multivariate logistic regression. An odds ratio (OR) and 95% confidence interval (CI) were calculated for each predictor. The reliability of the predictive models was determined by receiver operating curve (ROC) analysis, with further calculation of area under the curve (AUC), its CI, sensitivity (Se), specificity (Sp) and likelihood ratio (LR) for each predictor. The Youden test was used to estimate the cut-off points for irisin and its trajectory. We compared the incremental prognostic capacity of models using a binary prediction methodology based on the estimation of integrated discrimination indices (IDI) and net reclassification improvement (NRI). The Kaplan–Meyer curve analysis was performed to elucidate a plausible benefit for clinical outcome as a function of adropin levels (≥2.15 ng/mL vs. <2.15 ng/mL). A 2-sided p < 0.05 was considered significant. Variables were tested using SPSS v. 23 (IBM, Armonk, New York, NY, USA) and GraphPad Prism v. 9 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. General Characteristics of the Patients
We identified 126 combined clinical endpoints, which were associated with CV death due to recurrent myocardial infarction (n = 62, 49.2%), acute or acutely decompensated HF (n = 29, 23.0%), sudden death (n = 21, 16.7%) and stroke (n = 14, 11.1%). Accordingly, we divided the entire group into two groups: with (n = 126) and without (n = 372) clinical outcomes.
The study participants had a mean age of 63 years, and 52.8% were male (Table 1). They had a mean body mass index of 26.8 ± 3.76 kg/m2, a mean waist circumference of 99.1 ± 5.22 cm and a mean waist-to-hip ratio of 0.92 ± 0.11 units. The comorbidity profile included dyslipidemia (85.3%), hypertension (70.8%), chronic heart failure (43.4%), atrial fibrillation (18.1%), smoking (42.4%), abdominal obesity (39.0%), left ventricular hypertrophy (93.8%) and chronic kidney disease grades 1–3 (26.3%). Among the enrolled patients, 43.7% had culprit lesion in left artery descending artery; 38.2% had culprit damage in right coronary artery; 10.8% and 7.3% exerted culprit lesion in circumflex coronary artery and left main coronary artery, respectively. Along with this, 60.4% had anterior localization of myocardial infarction. The mean values of GRACE and Gensini scores were 144 ± 37 and 32 (16–45), respectively.

Table 1.
Baseline characteristics of eligible post-STEMI patients.
All patients had stable hemodynamics at baseline, with mean LVEF of 48 (42–53), moderate enlargement of left ventricle, mean LV GLS of −14.9 (−12.1; −16.7), E/e’ of 17 ± 7 units and mean LAVI of 44 (35–55) mL/m2. The mean eGFR was 73 ± 15 mL/min/1.73 m2; the mean HOMA-IR was 7.34 ± 2.9 units; and fasting glucose was 6.10 ± 0.6 mmol/L. The mean level of NT-proBNP was 623 (172–1160) pmol/mL; the mean adropin was 2.96 (1.92–4.30) pg/mL; the mean levels of hs-TnT and hs-CRP were 0.06 (0.02–0.10) ng/mL and 4.32 (2.15–6.70) mg/L, respectively.
All individuals received optimal therapy depending on their clinical state, fasting glucose, lipid profile and comorbidities, which included antihypertensive agents (angiotensin-converting enzyme inhibitors, angiotensin-II receptor antagonists, calcium channel blockers and thiazide-like/loop diuretics), beta-blockers and ivabradine, mineralocorticoid receptor antagonists when needed, antiplatelet agents and statins. Patients with atrial fibrillation were treated with anticoagulants. We did not find significant differences between subgroups in terms of age, gender, anthropometric parameters (BMI, waist circumference, waist-to-hip ratio), the presence of CV risk factors and comorbidities, such as dyslipidemia, hypertension, chronic heart failure (including HFpEF/HFmrEF), atrial fibrillation, smoking, abdominal obesity, left ventricular hypertrophy, chronic kidney disease, GRACE score, Gensini score, systolic and diastolic blood pressure, left ventricular dimensions and ejection fraction, GLS, eGFR, HOMA-IR, fasting glucose, HbA1c, creatinine, serum uric acid, lipids, hs-CRP, NT-proBNP and hs-TnT, as well as concomitant medications apart from mineralocorticoid receptor antagonists, GLP-1 receptor agonist and SGLT2 inhibitors, which were administered frequently in free-event groups. Therefore, the patients from the event group more often had HF with mildly reduced ejection fraction and less frequently had HF with preserved ejection fraction compared with the free-event group. Yet, culprit lesions in left main and right coronary arteries were more frequently detected in the event group than in the free-event group. Of note, the mean levels of adropin were significantly higher in the free-event group compared with the event group.
3.2. Spearman’s Correlation between the Levels of Biomarkers at Baseline and Other Parameters
The NT-proBNP levels were positively associated with E/e’ (r = 0.32, p = 0.001), LAVI (r = 0.32, p = 0.001), LV hypertrophy (r = 0.28, p = 0.001) and inversely with GLS (r = −0.37, p = 0.001), left ventricular ejection fraction (r = −0.36, p = 0.001) and eGFR (r = −0.34, p = 0.001) (Table 2).

Table 2.
Spearman’s correlation coefficients between the levels of biomarkers and other parameters.
Adropin levels correlated positively with left ventricular ejection fraction (r = 0.34, p = 0.001), GLS (r = 0.32, p = 0.001) and negatively with the Gensini score (r = −0.34, p = 0.001), LAVI (r = −0.32, p = 0.001), fasting plasma glucose (r = −0.32, p = 0.001), HOMA-IR (r = −0.29, p = 0.001) and HbA1c (r = −0.26, p = 0.001). The concentrations of hs-CRP were significantly associated with the Gensini score (r = −0.28, p = 0.001) and LAVI (r = −0.30, p = 0.001). The levels of adropin and hs-CRP demonstrated mildly positive associations with GRACE (r = −0.26, p = 0.001 and r = 0.28, p = 0.001, respectively).
3.3. The Reliability of the Predictive Ability of Adropin: The Results of the ROC Curve Analysis
We found that serum levels of adropin < 2.15 ng/mL (area under curve [AUC] = 0.836; 95% confidence interval [CI] = 0.745–0.928; sensitivity = 84.9%; specificity = 72.7%; likelihood ratio = 3.11; p = 0.0001) predicted clinical outcomes (Figure 2).
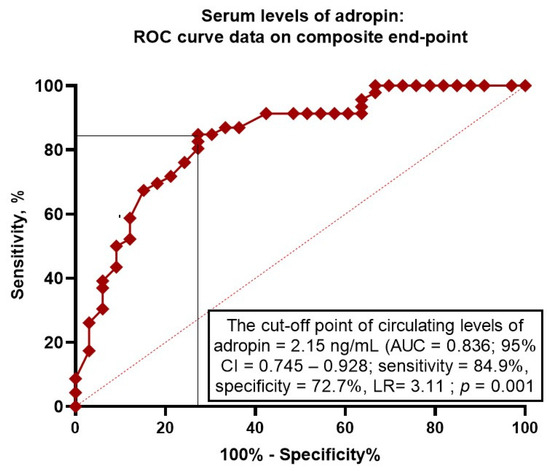
Figure 2.
Receiver operating curve analysis for clinical events: the optimal cut-off points of adropin. Abbreviations: AUC, area under curve; CI, confidence interval; LR, likelihood ratio.
3.4. Predictors for Clinical Events in Post-STEMI Patients with Prediabetes: Univariate and Multivariate Logistic Regression Analysis
For this analysis, we used the median value for the Gensini score, GRACE score, as well as the mean level of NT-proBNP at baseline (Table 3). The univariate logistic regression revealed that clinical events were not predicted by a GRACE score ≥ 144, serum levels of NT-proBNP ≥ 623 pmol/mL and anterior localization of myocardial infarction. On the contrary, a Gensini score ≥ 32 (OR = 1.10; p = 0.001), serum levels of adropin ≤ 2.15 ng/mL (OR = 1.14; p = 0.001), culprit lesion in left main coronary artery (OR = 1.04; p = 0.042), administration of SGLT2i (OR = 0.93; p = 0.016) and GLP-1 receptor agonist (OR = 0.95; p = 0.048) showed the predictive potencies for clinical outcome. The multivariate logistic regression revealed that a Gensini score ≥ 32 (OR = 1.07; p = 0.001), adropin ≤ 2.15 ng/mL (OR = 1.18; p = 0.001), use of SGLT2i (OP = 0.94; p = 0.010) and GLP-1 receptor agonist (OR = 0.95; p = 0.040) were independent predictors for clinical outcome.

Table 3.
Predictors for clinical events: the results of logistic regression.
3.5. Comparison of the Predictive Models
We compared the predictive models for clinical outcome and established that Model 2 (adropin ≤ 2.15 ng/mL) was superior to Model 1 (Gensini score ≥ 32), whereas Model 3 (administration of SGLT2i) and Model 4 (administration of GLP-1 receptor agonist) were not significantly better than the reference value of Model 1 (Table 4). Model 1 + Model 2 demonstrated better discriminative potency in comparison with Model 1.

Table 4.
The comparisons of predictive models for clinical outcomes.
3.6. Survival of the Post-STEMI Patients with Newly Diagnosed Prediabetes Depending on Serum Levels of Adropin
Kaplan–Meier plots showed a significant difference between patient groups with serum levels of adropin lower than 2.15 ng/mL vs. concentrations exceeding 2.15 ng/mL (Figure 3). We found that patients with lower adropin levels had a significant benefit in clinical event occurrence compared with those with higher adropin levels (OR = 2.469; 95% CI = 1.214–5.020; log rank test = 0.0120).
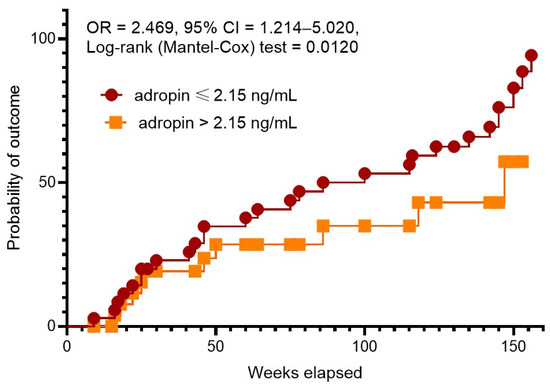
Figure 3.
The Kaplan–Meier analysis of 3-year clinical outcomes in post-STEMI patients with newly diagnosed prediabetes depending on serum levels of adropin. Abbreviations: CI, confidence interval; OR, odds ratio.
4. Discussion
In this study, we found for the first time that among patients at moderate risk of complications after myocardial infarction and newly diagnosed prediabetes, low adropin levels were superior in their prognostic value to the traditional GRACE and Gensini risk scales. Moreover, we found that among these patients, the traditional prognostic markers, such as natriuretic peptides, C-reactive protein and troponins, did not demonstrate their discriminatory potential with respect to the combined end point, which included cardiovascular and total death. Yet, for post-STEMI patients after effective treatment with a modern strategy of complete revascularization with PCI, the risk of prediabetes as a cluster of risk factors worsening prognosis remains, and such patients do not differ from each other in most of the traditional parameters. However, a number of indicators, such as the Gensini score and adropin, retained their predictive value for clinical use. Another feature of our study is that all patients were adequately treated; yet, adropin improved the discriminative potential of Gensini for long-term events.
The prognostic significance of newly diagnosed prediabetes has not been adequately studied in patients after STEMI in contrast to diabetes mellitus, whose role in modulating complications after STEMI/PCI is fairly well established [50,51,52]. Indeed, several multicenter studies, including the post hoc analysis of the BIO-RESORT and BIONYX stent trials, have shown that prediabetes in post-STEMI patients treated with PCI resulted in a significantly higher mortality rate [53,54,55]. However, the study design was not based on newly diagnosed prediabetes after STEMI but on established diabetes before STEMI. Finally, it remains unclear whether the 3-year mortality rate for post-STEMI patients with newly diagnosed prediabetes is the same as for those who had this condition before STEMI. On the other hand, the current strategy of complete revascularization compared with the culprit-lesion-only strategy of revascularization has proven its ability to improve clinical outcomes in patients with and without diabetes mellitus, but there are a lack of data on the contribution of prediabetes to adverse clinical outcomes after complete revascularization [56]. Some clinical studies showed that prediabetes, as determined by the ADA criteria (HbA1c = 5.7%–6.5%), was either not associated with long-term adverse CV outcomes or mildly associated with clinical outcomes in patients with CAD treated with PCI [57,58], whereas ACS patients undergoing PCI, regardless of the type of revascularization (complete or culprit-lesion-only), with prediabetes were correlated with major adverse CV and cerebrovascular events (MACCEs) [59,60]. However, we established that the risk of MACCEs in STEMI patients after PCI may be independently associated with the levels of adropin.
In a previous meta-analysis, the levels of adropin were found to be decreased in overweight/obese patients vs. normal-weight individuals [61]. Aligned with this, the adropin levels were lower in patients with diabetes mellitus compared with healthy volunteers [62]. Moreover, low levels of adropin were correlated with metabolic syndrome, and perhaps this is the adaptive regulator counteracting the development of metabolic syndrome and prediabetes [63,64]. Indeed, previous studies revealed that adropin is closely related to upregulation of the endothelial nitric oxide synthase expression and peroxisome-proliferator-activated receptor-gamma in vasculature and inhibition of atherosclerosis via modulating the expression of vascular endothelial growth factor receptor 2, vascular cell adhesion molecule 1 and intercellular adhesion molecule 1, as well as a shift of the macrophage phenotype to anti-inflammatory M2 [65,66]. Moreover, regulation of adropin histone deacetylation in vivo and in vitro enhanced atherosclerosis development [67]. Although adropin was not found to trigger low-oxidative lipids’ efflux and had no significant effects on oxidized low-density lipoprotein-induced foam cell formation, it attenuated the development of plaque and its vulnerability through suppression of the inflammatory reaction [67].
In our study, we detected low levels of adropin in the enrolled patients, but we did not find an association between the levels of adropin and inflammatory biomarkers, such as hs-CRP, age and gender, whereas in the study by Butler AA et al. (2012) [64], the levels of adropin were negatively associated with age, and they were lower in females. However, there was an inverse link between the Gensini score and circulating levels of adropin. Taking into consideration these findings, we suggested that adropin seemed to be an adaptive co-regulator of metabolic homeostasis with a vasoactive component, which ensures a tissue-protective effect. In this connection, the decreased levels of adropin in post-STEMI individuals with newly diagnosed prediabetes can be considered a new pathogenetic factor contributing to microvascular inflammation, plaque instability, endothelial dysfunction, acceleration of atherosclerosis and post-conditioning [29,30,32]. Indeed, adropin is involved in downregulation of the mRNA expression of several endothelial cell markers, such as leukocyte differentiation antigen 31 and vascular endothelial cadherin, which play a pivotal role in endothelial dysfunction and plaque instability [68]. Therefore, adropin acting via transforming growth factor-beta suppresses oxidative stress and modulates lipid profiles and fibroblast activation, leading to accumulation of extracellular matrix and fibrosis [69]. Taken together, low levels of adropin, which are a result of adipose tissue inflammation and counter-regulation of inflammation, are associated with acceleration of atherosclerosis, plaque rapture, endothelial dysfunction, cardiac fibrosis and impaired vascular integrity [70]. Thus, a deficiency in adropin is probably the maladaptive mechanism that links local and systemic inflammation induced by impaired glucose homeostasis, lipid toxicity and mitochondrial dysfunction and supports functional and structural changes in coronary artery and myocardium with MACCEs [71].
Finally, in the study, we established that post-STEMI patients treated with PCI may demonstrate a sufficient difference in survival depending on the levels of adropin. Patients with lower levels of adropin benefited in terms of MACCEs compared with those with higher levels. These findings require a clear elucidation in a large clinical trial because the conventional risk scores, such as GRACE and Gensisni, did not reproduce their discriminative potencies for these individuals. Thus, newly diagnosed prediabetes in well-treated patients after STEMI is likely to be a sufficient risk factor for long-term survival. This is the basis for further adaptation of the currently available approach to the prediction of clinical CV outcomes in the patient population. However, clear molecular mechanisms via which adropin may be involved in tissue reparation and protection are not fully understood.
In summary, we believe that the measurement of serum adropin levels is a simple and reasonably reliable tool for predicting the cumulative three-year risk of death. Although this is a rapid and inexpensive method of cardiovascular risk assessment that probably complements clinical assessment well and may be an effective guide for patient stratification, it needs validation in a large clinical trial setting among the full spectrum of patients with acute coronary syndrome and myocardial infarction.
Study Limitations
The study has several limitations. First, during admission to hospital with STEMI, we did not assess the patients’ glycemia status and used findings, which were available through the discharge reports. Second, all individuals were well treated with a combination of diet and contemporary agents to reach optimal glycemia control after the determination of newly diagnosed prediabetes. Third, in the study, we did not investigate the role of SGLT2i and GLP-1 receptor agonists in the dynamics of adropin in connection with MACCE occurrence. Finally, in the study, we included patients who underwent complete and culprit-lesion-only revascularization. Overall, we believe that these limitations will not intervene with the findings’ interpretation.
5. Conclusions
We established that low levels of adropin (≤2.15 ng/mL) independently predicted clinical outcomes in post-STEMI patients treated with PCI with newly detected prediabetes and improved the discriminative ability of the Gensini score for 3-year follow-up events. Future clinical studies are needed to clarify whether adropin is a promising molecule to be incorporated into conventional risk scores for the prediction of MACCEs after STEMI.
Author Contributions
Conceptualization, A.E.B.; methodology, M.L. and A.E.B.; software, A.E.B. and O.O.B.; validation, T.A.B., A.E.B., M.L. and U.C.H.; formal analysis, O.O.B., T.A.B. and A.E.B.; investigation, O.O.B. and T.A.B.; resources, T.A.B. and O.O.B.; data curation, A.E.B. and T.A.B.; writing—original draft preparation, T.A.B., O.O.B., U.C.H., M.L. and A.E.B.; writing—review and editing, T.A.B., O.O.B., U.C.H., M.L. and A.E.B.; visualization, O.O.B. and T.A.B.; supervision, A.E.B.; project administration, T.A.B. and O.O.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of Zaporozhye Medical Academy of Post-graduate Education (protocol number: 8; date of approval: 10 October 2020).
Informed Consent Statement
Informed consent was received from all individuals involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy restrictions.
Acknowledgments
We thank all patients who gave their consent to participate in the study and all administrative staff and doctors at the Private Hospital “Vita-Center” for assistance with the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tian, L.; Zhu, J.; Liu, L.; Liang, Y.; Li, J.; Yang, Y. Prediabetes and Short-Term Outcomes in Nondiabetic Patients after Acute ST-Elevation Myocardial Infarction. Cardiology 2013, 127, 55–61. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Januzzi, J.L.; Bruemmer, D.; Butalia, S.; Green, J.B.; Horton, W.B.; Knight, C.; Levi, M.; Rasouli, N.; Richardson, C.R. Heart Failure: An Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care 2022, 45, 1670–1690. [Google Scholar] [CrossRef]
- Ritsinger, V.; Tanoglidi, E.; Malmberg, K.; Näsman, P.; Rydén, L.; Tenerz, Å.; Norhammar, A. Sustained prognostic implications of newly detected glucose abnormalities in patients with acute myocardial infarction: Long-term follow-up of the Glucose Tolerance in Patients with Acute Myocardial Infarction cohort. Diabetes Vasc. Dis. Res. 2014, 12, 23–32. [Google Scholar] [CrossRef]
- Norhammar, A.; Tenerz, A.; Nilsson, G.; Hamsten, A.; Efendíc, S.; Rydén, L.; Malmberg, K. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: A prospective study. Lancet 2002, 359, 2140–2144. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.L.; Hilliard, M.E.; Isaacs, D.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2022, 46, S19–S40. [Google Scholar] [CrossRef]
- Davidson, M.B. Historical review of the diagnosis of prediabetes/intermediate hyperglycemia: Case for the international criteria. Diabetes Res. Clin. Pract. 2022, 185, 109219. [Google Scholar] [CrossRef]
- Hermanides, R.S.; Kennedy, M.W.; Kedhi, E.; van Dijk, P.R.; Timmer, J.R.; Ottervanger, J.P.; Dambrink, J.-H.; Gosselink, A.M.; Roolvink, V.; Miedema, K.; et al. Impact of elevated HbA1c on long-term mortality in patients presenting with acute myocardial infarction in daily clinical practice: Insights from a ‘real world’ prospective registry of the Zwolle Myocardial Infarction Study Group. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 616–625. [Google Scholar] [CrossRef]
- Karayiannides, S.; Djupsjö, C.; Kuhl, J.; Hofman-Bang, C.; Norhammar, A.; Holzmann, M.J.; Lundman, P. Long-term prognosis in patients with acute myocardial infarction and newly detected glucose abnormalities: Predictive value of oral glucose tolerance test and HbA1c. Cardiovasc. Diabetol. 2021, 20, 122. [Google Scholar] [CrossRef]
- Lenzen, M.; Ryden, L.; Öhrvik, J.; Bartnik, M.; Malmberg, K.; Reimer, W.S.O.; Simoons, M.L.; on behalf of the Euro Heart Survey Investigators. Diabetes known or newly detected, but not impaired glucose regulation, has a negative influence on 1-year outcome in patients with coronary artery disease: A report from the Euro Heart Survey on diabetes and the heart. Eur. Heart J. 2006, 27, 2969–2974. [Google Scholar] [CrossRef]
- Mai, L.; Wen, W.; Qiu, M.; Liu, X.; Sun, L.; Zheng, H.; Cai, X.; Huang, Y. Association between prediabetes and adverse outcomes in heart failure. Diabetes Obes. Metab. 2021, 23, 2476–2483. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef]
- Cai, X.; Liu, X.; Sun, L.; He, Y.; Zheng, S.; Zhang, Y.; Huang, Y. Prediabetes and the risk of heart failure: A meta-analysis. Diabetes Obes. Metab. 2021, 23, 1746–1753. [Google Scholar] [CrossRef]
- Sinha, A.; Ning, H.; Ahmad, F.S.; Bancks, M.P.; Carnethon, M.R.; O’brien, M.J.; Allen, N.B.; Wilkins, J.T.; Lloyd-Jones, D.M.; Khan, S.S. Association of fasting glucose with lifetime risk of incident heart failure: The Lifetime Risk Pooling Project. Cardiovasc. Diabetol. 2021, 20, 66. [Google Scholar] [CrossRef]
- Hsu, J.-C.; Yang, Y.-Y.; Chuang, S.-L.; Lin, L.-Y.; Chen, T.H.-H. Prediabetes as a risk factor for new-onset atrial fibrillation: The propensity-score matching cohort analyzed using the Cox regression model coupled with the random survival forest. Cardiovasc. Diabetol. 2023, 22, 35. [Google Scholar] [CrossRef]
- Ritchie, R.H.; Abel, E.D. Basic Mechanisms of Diabetic Heart Disease. Circ. Res. 2020, 126, 1501–1525. [Google Scholar] [CrossRef]
- Lind, V.; Hammar, N.; Lundman, P.; Friberg, L.; Talbäck, M.; Walldius, G.; Norhammar, A. Impaired fasting glucose: A risk factor for atrial fibrillation and heart failure. Cardiovasc. Diabetol. 2021, 20, 227. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A.; Lichtenauer, M. Emerging Role of Adipocyte Dysfunction in Inducing Heart Failure Among Obese Patients with Prediabetes and Known Diabetes Mellitus. Front. Cardiovasc. Med. 2020, 7, 583175. [Google Scholar] [CrossRef]
- Brannick, B.; Dagogo-Jack, S. Prediabetes and Cardiovascular Disease. Endocrinol. Metab. Clin. N. Am. 2018, 47, 33–50. [Google Scholar] [CrossRef]
- Berezin, A. Metabolic memory phenomenon in diabetes mellitus: Achieving and perspectives. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S176–S183. [Google Scholar] [CrossRef]
- Johansson, J.S.; Boström, K.B.; Hjerpe, P.; Mourtzinis, G.; Kahan, T.; Ljungman, C. Prediabetes and incident heart failure in hypertensive patients: Results from the Swedish Primary Care Cardiovascular Database. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2803–2810. [Google Scholar] [CrossRef]
- Berezin, A.A. Impaired function of fibroblast growth factor 23/Klotho protein axis in prediabetes and diabetes mellitus: Promising predictor of cardiovascular risk. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2549–2556. [Google Scholar] [CrossRef]
- Myhre, P.L.; Lyngbakken, M.N.; Berge, T.; Røysland, R.; Aagaard, E.N.; Pervez, O.; Kvisvik, B.; Brynildsen, J.; Norseth, J.; Tveit, A.; et al. Diagnostic Thresholds for Pre–Diabetes Mellitus and Diabetes Mellitus and Subclinical Cardiac Disease in the General Population: Data from the ACE 1950 Study. J. Am. Heart Assoc. 2021, 10, e020447. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Fan, W.; Bertoni, A.; Budoff, M.J.; Defilippi, C.; Lombardo, D.; Maisel, A.; Szklo, M.; Wong, N.D. N-terminal Pro B-type Natriuretic Peptide and High-sensitivity Cardiac Troponin as Markers for Heart Failure and Cardiovascular Disease Risks According to Glucose Status (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol. 2020, 125, 1194–1201. [Google Scholar] [CrossRef]
- Jia, X.; Al Rifai, M.; Hoogeveen, R.; Echouffo-Tcheugui, J.B.; Shah, A.M.; Ndumele, C.E.; Virani, S.S.; Bozkurt, B.; Selvin, E.; Ballantyne, C.M.; et al. Association of Long-term Change in N-Terminal Pro–B-Type Natriuretic Peptide with Incident Heart Failure and Death. JAMA Cardiol. 2023, 8, 222–230. [Google Scholar] [CrossRef]
- Bachmann, K.N.; Gupta, D.K.; Xu, M.; Brittain, E.; Farber-Eger, E.; Arora, P.; Collins, S.; Wells, Q.S.; Wang, T.J. Unexpectedly Low Natriuretic Peptide Levels in Patients with Heart Failure. JACC Heart Fail. 2021, 9, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Iqbal, N.; Wu, Y.; Hazen, S.L. Usefulness of Cardiac Biomarker Score for Risk Stratification in Stable Patients Undergoing Elective Cardiac Evaluation Across Glycemic Status. Am. J. Cardiol. 2012, 111, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Buchan, T.A.; Ching, C.; Foroutan, F.; Malik, A.; Daza, J.F.; Hing, N.N.F.; Siemieniuk, R.; Evaniew, N.; Orchanian-Cheff, A.; Ross, H.J.; et al. Prognostic value of natriuretic peptides in heart failure: Systematic review and meta-analysis. Heart Fail. Rev. 2021, 27, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Mushala, B.A.S.; Scott, I. Adropin: A hepatokine modulator of vascular function and cardiac fuel metabolism. Am. J. Physiol. Circ. Physiol. 2020, 320, H238–H244. [Google Scholar] [CrossRef] [PubMed]
- Lovren, F.; Pan, Y.; Quan, A.; Singh, K.K.; Shukla, P.C.; Gupta, M.; Al-Omran, M.; Teoh, H.; Verma, S. Adropin Is a Novel Regulator of Endothelial Function. Circulation 2010, 122, S185–S192. [Google Scholar] [CrossRef]
- Wu, L.; Fang, J.; Yuan, X.; Xiong, C.; Chen, L. Adropin reduces hypoxia/reoxygenation-induced myocardial injury via the reperfusion injury salvage kinase pathway. Exp. Ther. Med. 2019, 18, 3307–3314. [Google Scholar] [CrossRef]
- Altamimi, T.R.; Gao, S.; Karwi, Q.G.; Fukushima, A.; Rawat, S.; Wagg, C.S.; Zhang, L.; Lopaschuk, G.D. Adropin regulates cardiac energy metabolism and improves cardiac function and efficiency. Metabolism 2019, 98, 37–48. [Google Scholar] [CrossRef]
- Aydin, S.; Eren, M.; Yilmaz, M.; Kalayci, M.; Yardim, M.; Alatas, O.; Kuloglu, T.; Balaban, H.; Cakmak, T.; Kobalt, M.; et al. Adropin as a potential marker of enzyme-positive acute coronary syndrome. Cardiovasc. J. Afr. 2017, 28, 40–47. [Google Scholar] [CrossRef][Green Version]
- Jasaszwili, M.; Billert, M.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Adropin as A Fat-Burning Hormone with Multiple Functions—Review of a Decade of Research. Molecules 2020, 25, 549. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.; Gu, X.; Qin, Y.; Zheng, X. Elevated Plasma Levels of Adropin in Heart Failure Patients. Intern. Med. 2011, 50, 1523–1527. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Zhao, P.; Wu, M.-C.; Liu, J.; Yin, W. Serum adropin levels are decreased in patients with acute myocardial infarction. Regul. Pept. 2014, 190–191, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-P.; Xu, W.-T.; Wang, L.; You, T.; Chan, S.-P.; Zhao, X.; Yang, X.-J. Serum Adropin Level in Patients with Stable Coronary Artery Disease. Heart Lung Circ. 2015, 24, 975–979. [Google Scholar] [CrossRef]
- Berezina, T.A.; Fushtey, I.M.; Pavlov, S.V.; Berezin, A.E. Predictors of Kidney Function Outcomes and Their Relation to SGLT2 Inhibitor Dapagliflozin in Patients with Type 2 Diabetes Mellitus Who Had Chronic Heart Failure. Adv. Ther. 2023, 41, 292–314. [Google Scholar] [CrossRef]
- Berezin, A.A.; Obradovic, Z.; Novikov, E.V.; Boxhammer, E.; Lichtenauer, M.; Berezin, A.E. Interplay between Myokine Profile and Glycemic Control in Type 2 Diabetes Mellitus Patients with Heart Failure. Diagnostics 2022, 12, 2940. [Google Scholar] [CrossRef]
- Kalkan, A.K.; Cakmak, H.A.; Erturk, M.; Kalkan, K.E.; Uzun, F.; Tasbulak, O.; Diker, V.O.; Aydin, S.; Celik, A. Adropin and Irisin in Patients with Cardiac Cachexia. Arq. Bras. Cardiol. 2018, 111, 39–47. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Care in Diabetes—2023 Abridged for Primary Care Providers. Clin. Diabetes 2023, 41, 4–31. [Google Scholar] [CrossRef]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726, Erratum in Eur. Heart J. 2021, 42, 4901. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2018, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Gensini, G.G. A more meaningful scoring system for determining the severity of coronary heart disease. Am. J. Cardiol. 1983, 51, 606. [Google Scholar] [CrossRef]
- Fox, K.A.A.; Dabbous, O.H.; Goldberg, R.J.; Pieper, K.S.; Eagle, K.A.; Van de Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; Anderson, F.A.; et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (GRACE). BMJ 2006, 333, 1091. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-Y.; Park, J.-S.; Seo, K.-W.; Yang, H.-M.; Lim, H.-S.; Choi, B.-J.; Choi, S.-Y.; Yoon, M.-H.; Hwang, G.-S.; Tahk, S.-J.; et al. Impact of new-onset diabetes on clinical outcomes after ST segment-elevated myocardial infarction. Scand. Cardiovasc. J. 2019, 53, 379–384. [Google Scholar] [CrossRef]
- Mehta, S.R.; Wood, D.A.; Storey, R.F.; Mehran, R.; Bainey, K.R.; Nguyen, H.; Meeks, B.; Di Pasquale, G.; López-Sendón, J.; Faxon, D.P.; et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N. Engl. J. Med. 2019, 381, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Bainey, K.R.; Mehta, S.R.; Lai, T.; Welsh, R.C. Complete vs culprit-only revascularization for patients with multivessel disease undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: A systematic review and meta-analysis. Am. Heart J. 2013, 167, 1–14.e2. [Google Scholar] [CrossRef] [PubMed]
- Ploumen, E.H.; Pinxterhuis, T.H.; Zocca, P.; Roguin, A.; Anthonio, R.L.; Schotborgh, C.E.; Benit, E.; Aminian, A.; Danse, P.W.; Doggen, C.J.M.; et al. Impact of prediabetes and diabetes on 3-year outcome of patients treated with new-generation drug-eluting stents in two large-scale randomized clinical trials. Cardiovasc. Diabetol. 2021, 20, 217. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Her, A.-Y.; Jeong, M.H.; Kim, B.-K.; Hong, S.-J.; Kim, S.; Ahn, C.-M.; Kim, J.-S.; Ko, Y.-G.; Choi, D.; et al. Effects of prediabetes on long-term clinical outcomes of patients with acute myocardial infarction who underwent PCI using new-generation drug-eluting stents. Diabetes Res. Clin. Pract. 2019, 160, 107994. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Her, A.-Y.; Jeong, M.H.; Kim, B.-K.; Hong, S.-J.; Kim, S.; Ahn, C.-M.; Kim, J.-S.; Ko, Y.-G.; Choi, D.; et al. Two-Year Clinical Outcomes Between Prediabetic and Diabetic Patients with STEMI and Multivessel Disease Who Underwent Successful PCI Using Drug-Eluting Stents. Angiology 2020, 72, 50–61. [Google Scholar] [CrossRef]
- Oqab, Z.; Kunadian, V.; Wood, D.A.; Storey, R.F.; Rao, S.V.; Mehran, R.; Pinilla-Echeverri, N.; Mani, T.; Boone, R.H.; Kassam, S.; et al. Complete Revascularization Versus Culprit-Lesion-Only PCI in STEMI Patients with Diabetes and Multivessel Coronary Artery Disease: Results from the COMPLETE Trial. Circ. Cardiovasc. Interv. 2023, 16, e012867. [Google Scholar] [CrossRef] [PubMed]
- Cueva-Recalde, J.F.; Ruiz-Arroyo, J.R.; Roncalés García-Blanco, F. Prediabetes and coronary artery disease: Outcome after revascularization procedures. Endocrinol. Nutr. 2016, 63, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.J.; Latif, A.; Ahmad, S.; Willman, C.; Lateef, N.; Shabbir, M.A.; Ahsan, M.Z.; Yousaf, A.; Riasat, M.; Ghali, M.; et al. Outcomes of Prediabetes Compared with Normoglycaemia and Diabetes Mellitus in Patients Undergoing Percutaneous Coronary Intervention: A Systematic Review and Meta-analysis. Heart Int. 2023, 17, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Behnoush, A.H.; Maleki, S.; Arzhangzadeh, A.; Khalaji, A.; Pezeshki, P.S.; Vaziri, Z.; Esmaeili, Z.; Ebrahimi, P.; Ashraf, H.; Masoudkabir, F.; et al. Prediabetes and major adverse cardiac events after acute coronary syndrome: An overestimated concept. Clin. Cardiol. 2024, 47. [Google Scholar] [CrossRef]
- Hosseini, K.; Khalaji, A.; Behnoush, A.H.; Soleimani, H.; Mehrban, S.; Amirsardari, Z.; Najafi, K.; Sabet, M.F.; Mohammadi, N.S.H.; Shojaei, S.; et al. The association between metabolic syndrome and major adverse cardiac and cerebrovascular events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Sci. Rep. 2024, 14, 697. [Google Scholar] [CrossRef]
- Soltani, S.; Kolahdouz-Mohammadi, R.; Aydin, S.; Yosaee, S.; Clark, C.C.T.; Abdollahi, S. Circulating levels of adropin and overweight/obesity: A systematic review and meta-analysis of observational studies. Hormones 2021, 21, 15–22. [Google Scholar] [CrossRef]
- Soltani, S.; Beigrezaei, S.; Malekahmadi, M.; Clark, C.C.T.; Abdollahi, S. Circulating levels of adropin and diabetes: A systematic review and meta-analysis of observational studies. BMC Endocr. Disord. 2023, 23, 73. [Google Scholar] [CrossRef]
- Yosaee, S.; Khodadost, M.; Esteghamati, A.; Speakman, J.R.; Shidfar, F.; Nazari, M.N.; Bitarafan, V.; Djafarian, K. Metabolic Syndrome Patients Have Lower Levels of Adropin When Compared with Healthy Overweight/Obese and Lean Subjects. Am. J. Men’s Health 2016, 11, 426–434. [Google Scholar] [CrossRef]
- Butler, A.A.; Tam, C.S.; Stanhope, K.L.; Wolfe, B.M.; Ali, M.R.; O’Keeffe, M.; St-Onge, M.-P.; Ravussin, E.; Havel, P.J. Low Circulating Adropin Concentrations with Obesity and Aging Correlate with Risk Factors for Metabolic Disease and Increase after Gastric Bypass Surgery in Humans. J. Clin. Endocrinol. Metab. 2012, 97, 3783–3791. [Google Scholar] [CrossRef]
- Niepolski, L.; Grzegorzewska, A.E. Salusins and adropin: New peptides potentially involved in lipid metabolism and atherosclerosis. Adv. Med. Sci. 2016, 61, 282–287. [Google Scholar] [CrossRef]
- Sato, K.; Yamashita, T.; Shirai, R.; Shibata, K.; Okano, T.; Yamaguchi, M.; Mori, Y.; Hirano, T.; Watanabe, T. Adropin Contributes to Anti-Atherosclerosis by Suppressing Monocyte-Endothelial Cell Adhesion and Smooth Muscle Cell Proliferation. Int. J. Mol. Sci. 2018, 19, 1293. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, W. LncRNA HDAC11-AS1 Suppresses Atherosclerosis by Inhibiting HDAC11-Mediated Adropin Histone Deacetylation. J. Cardiovasc. Transl. Res. 2022, 15, 1256–1269. [Google Scholar] [CrossRef]
- Ying, T.; Wu, L.; Lan, T.; Wei, Z.; Hu, D.; Ke, Y.; Jiang, Q.; Fang, J. Adropin inhibits the progression of atherosclerosis in ApoE-/-/Enho-/- mice by regulating endothelial-to-mesenchymal transition. Cell Death Discov. 2023, 9, 402. [Google Scholar] [CrossRef]
- Močnik, M.; Varda, N.M. Current Knowledge of Selected Cardiovascular Biomarkers in Pediatrics: Kidney Injury Molecule-1, Salusin-α and -β, Uromodulin, and Adropin. Children 2022, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Rooban, S.; Senghor, K.A.; Vinodhini, V.; Kumar, J. Adropin: A crucial regulator of cardiovascular health and metabolic balance. Metab. Open 2024, 23, 100299. [Google Scholar] [CrossRef]
- A Berezina, T.; Hoppe, U.C.; Lichtenauer, M.; Berezin, A.A. Methods to predict heart failure in diabetes patients. Expert Rev. Endocrinol. Metab. 2024, 241–256. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).