Abstract
Background: Blood lactate is a potentially useful biomarker to predict the mortality and severity of sepsis. The purpose of this study is to systematically review the ability of lactate to predict hierarchical sepsis clinical outcomes and distinguish sepsis, severe sepsis and septic shock. Methods: We conducted an exhaustive search of the PubMed, Embase and Cochrane Library databases for studies published before 1 October 2022. Inclusion criteria mandated the presence of case–control, cohort studies and randomized controlled trials that established the association between before-treatment blood lactate levels and the mortality of individuals with sepsis, severe sepsis or septic shock. Data was analyzed using STATA Version 16.0. Results: A total of 127 studies, encompassing 107,445 patients, were ultimately incorporated into our analysis. Meta-analysis of blood lactate levels at varying thresholds revealed a statistically significant elevation in blood lactate levels predicting mortality (OR = 1.57, 95% CI 1.48–1.65, I2 = 92.8%, p < 0.00001). Blood lactate levels were significantly higher in non-survivors compared to survivors in sepsis patients (SMD = 0.77, 95% CI 0.74–0.79, I2 = 83.7%, p = 0.000). The prognostic utility of blood lactate in sepsis mortality was validated through hierarchical summary receiver operating characteristic curve (HSROC) analysis, yielding an area under the curve (AUC) of 0.72 (95% CI 0.68–0.76), accompanied by a summary sensitivity of 0.65 (95% CI 0.59–0.7) and a summary specificity of 0.7 (95% CI 0.64–0.75). Unfortunately, the network meta-analysis could not identify any significant differences in average blood lactate values’ assessments among sepsis, severe sepsis and septic shock patients. Conclusions: This meta-analysis demonstrated that high-level blood lactate was associated with a higher risk of sepsis mortality. Lactate has a relatively accurate predictive ability for the mortality risk of sepsis. However, the network analysis found that the levels of blood lactate were not effective in distinguishing between patients with sepsis, severe sepsis and septic shock.
1. Introduction
Sepsis, characterized by life-threatening organ dysfunction resulting from a dysregulated host response to infection [1], represents a significant global health concern, impacting millions of individuals worldwide. Several significant infectious conditions and inappropriate treatment may culminate in the progression to severe sepsis or septic shock [2]. To enhance clinical outcomes in these patients, early identification of individuals at risk of mortality and timely optimization of clinical decision-making are of paramount importance [3].
Clinical scoring systems, such as Sequential Organ Failure Assessment (SOFA) (Supplementary Table S1) and systemic inflammatory response syndrome (SIRS) (Supplementary Table S2), have been proposed for predicting sepsis-related outcomes, including mortality [4]. Nevertheless, SOFA entails a time-consuming process that necessitates multiple laboratory and clinical data inputs, while SIRS exhibits limited sensitivity in predicting mortality [5]. In contrast, blood lactate levels offer a rapid and easily obtainable measurement, serving as a surrogate for tissue hypoperfusion in critically ill patients [6]. Combining point-of-care lactate assessment with the qSOFA (Supplementary Table S3) score in rapid bedside assessments more accurately identifies the risk of sepsis-related mortality compared to using the qSOFA score alone [7].
Elevated blood lactate levels may result from tissue hypoxia and anaerobic metabolism; they can also develop in other ways (Figure 1) [8]. Studies consistently demonstrate that the duration and severity of hyperlactatemia are directly correlated with mortality in septic shock patients. Elevated blood lactate levels serve as a valuable marker for assessing the severity of sepsis and predicting patient outcomes [9,10,11]. This is attributed to their ability to accurately and promptly reflect the perfusion status of peripheral tissues in the body and their sensitivity in indicating the presence of cellular hypoxia or tissue hypoperfusion [12,13,14]. However, there exists inconsistency in the reference criteria for predicting the prognosis of sepsis in cases of elevated lactate levels. While certain studies emphasized an initial lactate level greater than 2.0 mmol/L as indicative of elevated lactate levels [15,16], others suggested that elevated lactate levels are defined by an initial lactate level exceeding 4.0 mmol/L [17,18,19]. Furthermore, there is no relevant quantitative meta-analysis focusing on different blood lactate level to predict different outcomes in sepsis, such as severe sepsis and septic shock.
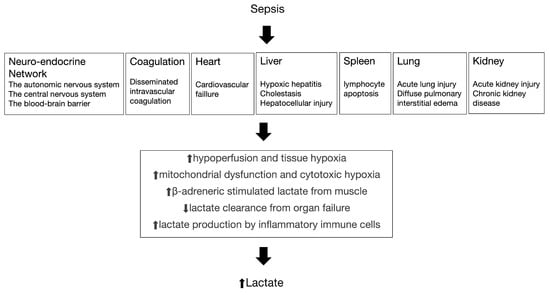
Figure 1.
The pathogenesis of sepsis and the relationship with increased lactate.
For this purpose, we conducted a systematic review and diagnostic systematic review to rigorously and quantitatively assess the accuracy of blood lactate levels in predicting sepsis mortality. Additionally, we performed a network meta-analysis to evaluate whether blood lactate can differentiate between sepsis, severe sepsis and septic shock.
2. Methods
2.1. Study Protocol
This analysis was conducted in accordance with a predetermined protocol following the recommendations of a guideline for systematic reviews of prognostic factor studies. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions was followed for data abstractions. Our study protocol was registered on PROSPERO (CRD449572).
2.2. Search Strategy
We comprehensively searched the PubMed, Cochrane and Embase databases for English-language studies published before 1 October 2022. The strategy was “(“sepsis” [MeSH Terms] OR “sepsis” [All Fields] OR “septic shock” [MeSH Terms] OR “septic shock” [All Fields]) AND (“lactate” [MeSH Terms] OR “lactate” [All Fields] OR “hyperlactatemia” [All Fields])”.
2.3. Study Selection
Titles and abstracts of search results were screened independently (YL). The full texts of the remaining results were assessed independently by another two of us (BZ, RZ) for inclusion based on predetermined criteria. Any discrepancies were resolved through discussion, potentially with a third reviewer. We manually searched the reference lists of included studies and existing systematic reviews as well as all articles citing the included studies on Google Scholar.
In accordance with the objectives of our meta-analysis, we developed a ‘Population, Index prognostic factor, Comparator prognostic factor, Outcome, Timing, Settings’ (PICOTS) framework adapted from the guideline proposed by Riley et al. [20]. Our study inclusion criteria were as follows according to PICOTS framework: (1) population: sepsis patients with a well-defined diagnostic reference standard for sepsis; (2) index prognostic factor: before-treatment blood lactate levels measured; (3) outcome: nonsepsis, sepsis, severe sepsis, sepsis shock and death; (4) if studies were based on overlapping patients, the most completed one was chosen; and (5) studies were restricted to English publications. We used the following criteria for study exclusion: (1) studies lacking relevant outcomes or lactate levels; and (2) conference abstracts, reviews, case reports, and experiment studies.
2.4. Data Collection and Assessment of Study Quality
The relevant articles and eligible data were assessed and extracted by two authors (BZ, RZ), respectively. If a disagreement occurred, it was discussed and the consensus with a third author was reached. The quality of evidence was assessed by the modified Grading of Recommendations Assessment, Development, and Evaluation system (GRADE) by consensus among the authors [21,22].
The following data were collected from each study: first author name, area, publication date, the type of studied design, number of patients, timing of lactate measurements and primary outcome (nonsepsis, sepsis, severe sepsis, sepsis shock and death). When an included study reported different cut-off values, we chose one which made both sensitivity and specificity more than 50% as possible. When an included study reported the same outcome at different follow-up timepoint (e.g., 7-day mortality and 30-day mortality), we chose the earliest one. If the included studies did not report the mean and standard deviation, estimates for these parameters were derived from the sample size, median and quartiles [23,24]. Table 1 presents the baseline characteristics of included studies.

Table 1.
Basic characteristics of included studies.
Two independent reviewers (BZ, RZ) performed quality assessments of selected studies using the QUADAS-2 criteria [25]. This checklist consists of four key domains: patient selection, index test, reference standard, and flow and timing. Within each study, the domains are assessed in terms of risk of bias and the first three of these domains are assessed in terms of concerns about applicability. Signaling questions as specified in the QUADAS-2 tool enable the reviewer to give each domain a rating of high, low or unclear. If the answers to all signaling questions for a domain are “yes”, then risk of bias can be judged low. If any signaling question is answered “no”, potential for bias exists. The “unclear” category should be used only when insufficient data are reported to permit a judgment. When there was a disagreement, the third author made the final decision based on the criteria. Details of the QUADAS-2 criteria are elaborated on Table 2.

Table 2.
Risk of bias using the QUADAS-2.
Using the Newcastle–Ottawa Scale (NOS) [26] for cohort studies, the risk of bias was assessed for each outcome in all included studies. According to the selection of cohort (up to four points), the comparability of cohort design and analysis (up to two points) and the adequacy of result measurement (up to three points), a maximum of nine points will be obtained. Seven to nine points are considered high quality (low risk of bias) (Table 3).

Table 3.
Quality assessment of included studies by Newcastle–Ottawa Scale (NOS).
2.5. Statistical Analysis
The meta-analysis used the combined effects of each result. We calculated odds ratios (ORs) and 95% confidence intervals (CIs) for each result using a random effects model, the between-study heterogeneity was evaluated by the χ2-based Q statistics and I2 test, and a significant heterogeneity was as p (value of Q test) < 0.1 or I2 > 50%. When significant heterogeneity was observed, we would apply the random effects models for analysis. Otherwise, we would apply the fixed effects models. We applied funnel plots as well as Egger’s test [27] to assess publication bias. A two-sided p value of 0.05 was deemed as statistical significance. Based on different classifications of blood lactate levels and the categorization of children and adults, we conducted subgroup analyses.
We performed meta-analysis by using the hierarchical summary receiver operating characteristic (HSROC) model to estimate and compare SROC curves [28]. Sensitivity and specificity were calculated by true positives, false positives, true negatives, and false negatives. In order to further quantify the lactate level of various outcome, we calculated the frequentist analogue of the surface under the cumulative ranking curve (SUCRA) for each outcome [29].
Data was analyzed using STATA Version 16.0 [30]. The network was evaluated using frequentist multivariate meta-analysis (commands network meta and mvmeta) in Stata 16.0. Additionally, publication bias and sensitivity analysis were also conducted by STATA version 16.0.
3. Results
A total of 6105 articles were initially retrieved from the databases. However, after a rigorous selection process, 127 studies involving a cumulative cohort of 107,445 patients were ultimately incorporated into our analysis (Figure 2). No additional pertinent articles were found in the reference lists of the original publications. Detailed characteristics of the included studies can be found in Table 1. Among the included studies, before-treatment blood lactate measured values had been documented in 78 studies [6,9,10,11,12,13,15,16,17,18,19,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97]. Also, most of them provided data on the association between nonsepsis, sepsis, severe sepsis and septic shock and different blood lactate values. Meanwhile, there were 82 studies that demonstrated the relationship between blood lactate and sepsis induced mortality [9,11,15,19,31,34,36,38,39,40,42,43,44,45,46,47,48,49,50,51,53,55,58,59,62,63,65,67,68,69,70,71,72,74,75,76,78,81,83,85,87,88,89,91,93,95,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132]. Additionally, there were 46 articles reported the diagnostic accuracy of blood lactate levels in determining sepsis and associated clinical prognosis [11,15,31,33,34,38,43,44,45,50,53,56,59,64,65,66,67,69,70,71,72,77,78,85,89,100,102,103,106,107,108,110,111,114,117,121,122,124,133,134,135,136,137,138,139,140]. All included studies were observational studies.
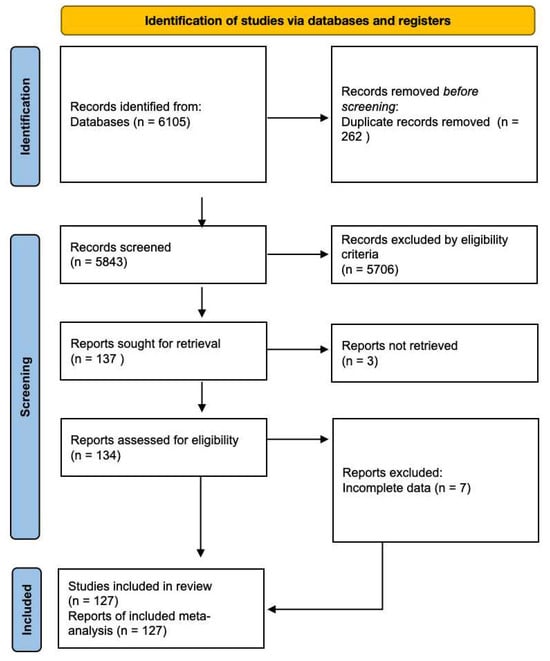
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for study identification and selection.
In the pooled analysis, the recommended threshold for identifying the high level of blood lactate varied across the included studies. Nine studies set a cut-off value for high level blood lactate below 2 mmol/L [33,38,41,85,103,108,124,135,140], while 36 studies recognized the cut-off values for high level blood lactate between 2 and 4 mmol/L [6,11,15,16,34,35,39,43,50,52,53,64,65,66,67,69,70,71,72,76,77,89,94,100,102,106,107,110,111,114,117,121,133,134,138,139]. However, there were 19 studies that used a significant elevated cut-off values in demonstrating high blood lactate levels above 4 mmol/L [6,13,17,18,19,42,43,56,57,63,65,69,78,80,93,94,95,96,97].
3.1. Assessment of Methodological Quality
The QUADAS-2 tool was employed to evaluate the quality of the included studies. The majority of these studies met a significant portion of the criteria outlined in the QUADAS list. Detailed results of the QUADAS assessments are provided in Table 2. Among the enrolled studies, a total of 124 studies were included, with 110 of them achieving a NOS score equal to or greater than seven points, indicating their classification as high-quality studies. Please refer to Table 3 for a comprehensive overview of these high-quality studies.
3.2. Higher Blood Lactate Value Was Associated with Mortality of Sepsis
A total of 78 articles were included in the comparative analysis, assessing the impact of various blood lactate levels on sepsis prognosis. Heterogeneity testing revealed significant heterogeneity across the research findings (I2 = 92.8%, p = 0.000). To account for this heterogeneity, a random effects model was applied for the meta-analysis, confirming the prognostic significance of elevated blood lactate levels in sepsis mortality [OR = 1.57, 95% CI 1.48–1.65] (Figure 3). This finding indicates that higher blood lactate levels are associated with sepsis mortality. Given the substantial heterogeneity, we conducted a subgroup analysis based on different blood lactate cutoff values. Specifically, 13 studies utilized a blood lactate cutoff of ≥2 mmol/L (OR = 2.49, 95% CI 2.00–3.10, I2 = 67.4%, p = 0.000), while 17 studies employed a cutoff of ≥4 mmol/L (OR = 3.48, 95% CI 2.79–4.34, I2 = 55.5%, p = 0.003) (Figure 3). Despite the presence of considerable heterogeneity, the pooled effect sizes remained robust, as confirmed by sensitivity analysis (Supplementary Figure S1). Such data demonstrated the blood lactate value was positive associated with sepsis mortality.
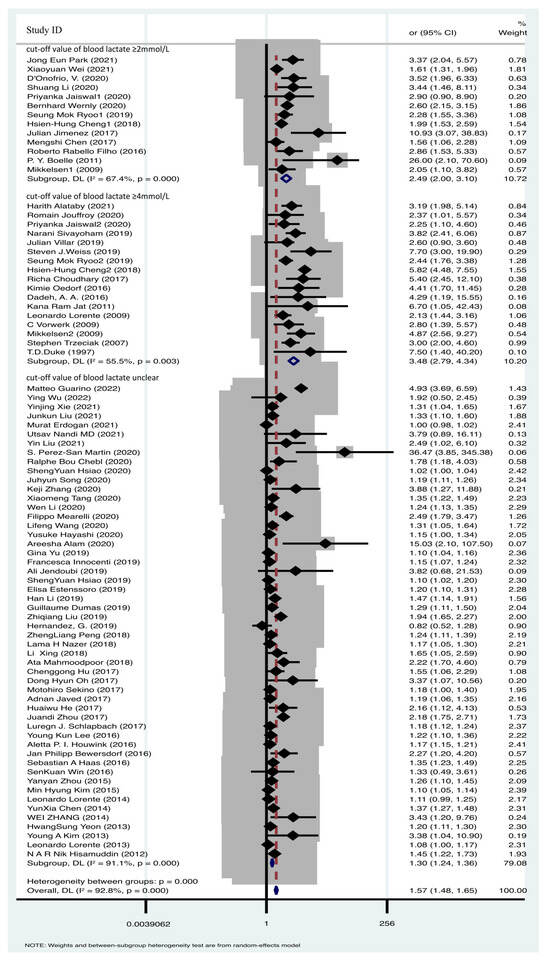
Figure 3.
Forest plot of lactate and sepsis mortality [6,9,10,11,12,13,15,16,17,18,19,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97].
3.3. Blood Lactate Significantly Elevated in Non-Survivors of Sepsis Events
A total of 82 articles, comprising 46,956 participants, provided data on blood lactate levels among both survivors and non-survivors with sepsis. These data unequivocally demonstrated a significant elevation in blood lactate levels among non-survivors (SMD = 0.77, 95% CI 0.74–0.79, I2 = 83.7%, p = 0.000) (Figure 4). To gain further insights, we conducted subgroup analyses, with 11 studies focusing on pediatric patients (SMD = 0.93, 95% CI 0.82–1.03, I2 = 77.3%, p = 0.000) and 71 studies on adult patients (SMD = 0.76, 95% CI 0.73–0.78, I2 = 84.2%, p = 0.000) (Figure 4). Despite substantial heterogeneity, the pooled effect sizes remained robust, as confirmed by sensitivity analysis (Supplementary Figure S2). Examination of funnel plots indicated no evidence of publication bias, as they exhibited a symmetrical distribution (Supplementary Figure S3).
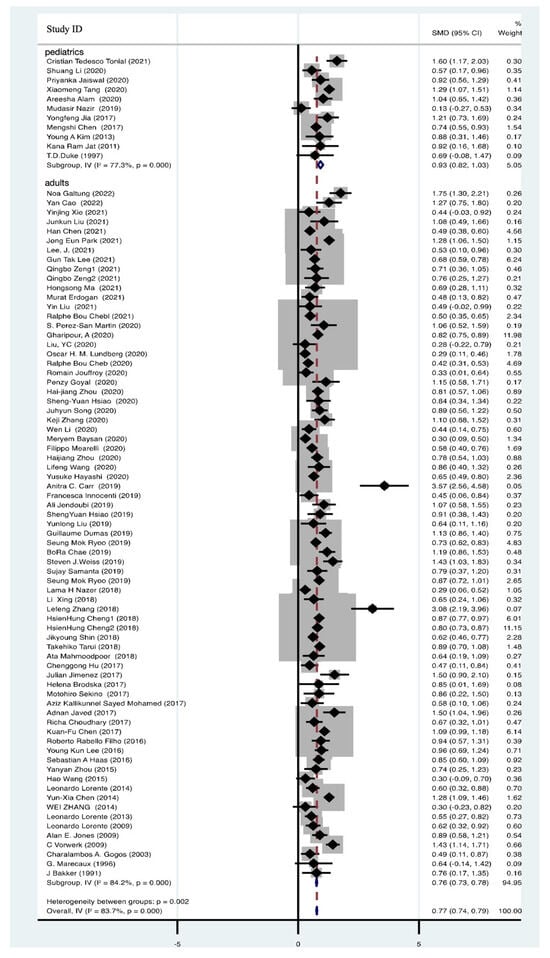
Figure 4.
Forest plot of lactate in non-survivors of sepsis [9,11,15,19,31,34,36,38,39,40,42,43,44,45,46,47,48,49,50,51,53,55,58,59,62,63,65,67,68,69,70,71,72,74,75,76,78,81,83,85,87,88,89,91,93,95,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132].
3.4. High Level Blood Lactate Demonstrated a Sufficient Prognostic Value in Determining the Sepsis Mortality
We included 46 articles that focused on prognostic analysis, enabling the conversion of data from these studies into a fourfold table of diagnostic tests to assess the prognostic value of blood lactate in high-risk sepsis populations. A diagnostic meta-analysis was conducted to further investigate the prognostic role of blood lactate. The summary sensitivity was calculated at 0.65 (95% CI 0.59–0.70), with substantial heterogeneity observed (p = 0.00, Q = 2093.9, I2 = 97.85%). Similarly, the summary specificity was 0.7 (95% CI 0.64–0.75), and the pooled estimation indicated significant heterogeneity (p = 0.00, Q = 2584.3, I2 = 98.26%). The Hierarchical Summary Receiver Operating Characteristic (HSROC) curve demonstrated the potential prognostic value of blood lactate levels for high-risk sepsis patients, with an AUC of 0.72 (95% CI 0.68–0.76). Furthermore, the presence of asymmetric distribution in funnel plots, as indicated by Deek’s test (p = 0.00), raised the possibility of publication bias within the studies (Figure 5).
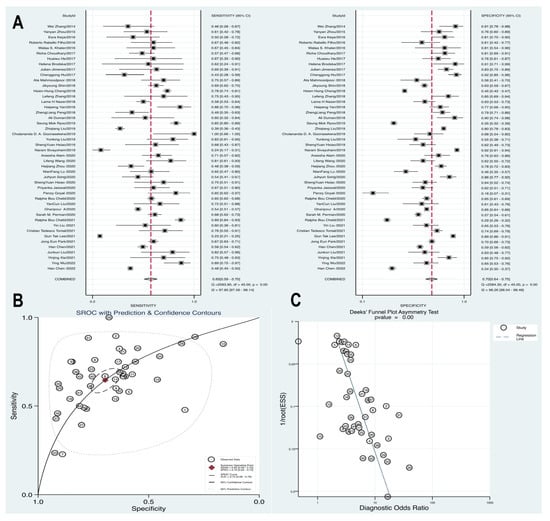
Figure 5.
Forest plot of pooled sensitivity, specificity (A) and HSROC (B) for blood lactate levels predicting mortality in patients with sepsis. (C) Funnel plot with Deek’s test for diagnostic analysis between blood lactate levels and mortality [11,15,31,33,34,38,43,44,45,50,53,56,59,64,65,66,67,69,70,71,72,77,78,85,89,100,102,103,106,107,108,110,111,114,117,121,122,124,133,134,135,136,137,138,139,140].
3.5. Blood Lactate Levels Failed to Distinguish Sepsis, Severe Sepsis and Septic Shock Based on Network Meta-Analysis
As the blood lactate level could indicate the prognosis of sepsis, we attempt to underline whether the value of blood lactate was correlated different outcomes of sepsis (sepsis, severe sepsis and septic shock). For that, network meta-analysis was used to compare the average blood lactate values among non-septic patients, septic patients, severe septic patients and septic shock patients with frequentist statistics (Supplementary Figure S4). In the network meta-analysis, there were significant differences between nonsepsis and sepsis, severe sepsis, septic shock (sepsis vs. nonsepsis, mean 2.03, 95% CI 0.76, 3.30, p < 0.005; severe sepsis vs. nonsepsis, mean 3.03, 95% CI 1.34, 4.72, p < 0.001; septic shock vs. nonsepsis, mean 3.07, 95% CI 1.70, 4.44, p < 0.001), but there were no significant differences among sepsis, severe sepsis and septic shock (severe sepsis vs. sepsis, mean 1.00, 95% CI −0.51, 2.51; septic shock vs. sepsis, mean 1.04, 95% CI −0.05, 2.13; septic shock vs. severe sepsis, mean 0.04, 95% CI −1.58, 1.67) (Figure 6). However, in SUCRA statistical analysis, it still showed the ranked blood lactate levels among patients with sepsis, severe sepsis and septic shock, with blood lactate levels in septic shock patients ranked first, followed by severe sepsis patients and sepsis patients (Figure 7). Funnel plots suggested no publication bias based on its symmetry (Supplementary Figure S4).
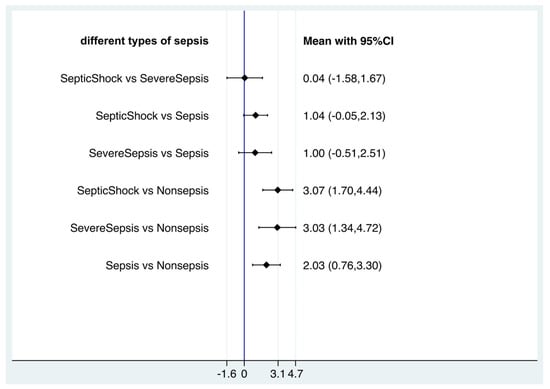
Figure 6.
Forest plot of network meta-analysis of lactate and sepsis, severe sepsis and septic shock.
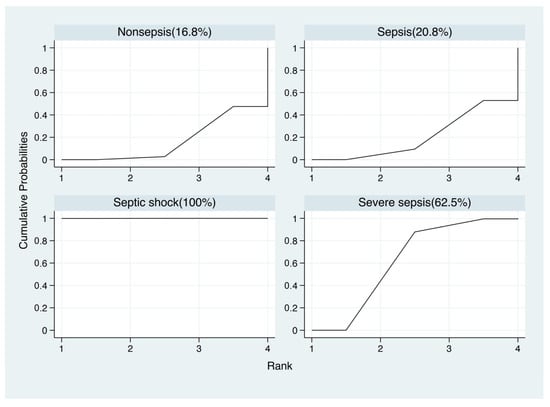
Figure 7.
Results of network rank test and the surface under the cumulative ranking curve (SUCRA).
4. Discussion
Our study conclusively establishes an association between elevated blood lactate levels and increased mortality, providing updated statistical conclusions and risk values. Importantly, this association holds true across diverse demographic factors such as age, gender, race, geographic region, and the specific assay method employed to measure blood lactate. Furthermore, diagnostic systematic review results demonstrate the significant predictive ability of lactate in sepsis mortality. Higher cutoff values for lactate are associated with increased mortality risk. However, this network analysis did not underline any significant differences in average blood lactate levels among individuals with sepsis, severe sepsis and septic shock. In clinical practice, we cannot overly rely on lactate to determine the severity of sepsis. Mildly elevated lactate levels may also progress to severe sepsis or septic shock, and excessively high lactate levels may indicate non-septic infections. These complex clinical scenarios require a more flexible and nuanced approach to assessment.
Several recent studies have highlighted the potential prognostic markers of outcome in severe sepsis, including the central venous minus arterial carbon dioxide pressure to arterial minus central venous oxygen content ratio (Pcv-aCO2/Ca-cvO2 [141,142], the CRP/albumin ratio [86,143], and lactate clearance [65,113,116,144]. The diagnostic performance of the Pcv-aCO2/Ca-cvO2 ratio > 1.696 at 24 h was analyzed using the ROC curve, and it was found to have an AUC of 0.82 (95% CI, 0.661–0.979). The lactate > 1.6 mmol/L had an AUC of 0.853 (95% CI 0.712–0.915) for predicting 28-day mortality [141]. The CRP/albumin ratio had an AUC of 0.621 for predicting mortality [86]. Other biomarkers, such as first urine liver-type fatty acid binding protein (L-FABP), plasma mtDNA also had a predictive value for sepsis mortality [138,145]. The AUC of urine L-FABP and plasma mtDNA for mortality were 0.647 and 0.726, respectively. The AUC was 0.864 for lactate [138]. Lactate had the greatest association with mortality. However, these markers necessitate multiple laboratory measurements, which can be unfavorable for early and accurate assessment. Blood lactate levels are considered sensitive markers for sepsis and septic shock, reflecting cellular metabolism [146]. In our analysis, we included a larger number of studies, enhancing the comprehensiveness and reliability of our results.
In our analysis, we observed heterogeneity among the included studies. The levels of blood lactate varied significantly across different studies, resulting in substantial unmanageable heterogeneity in the pooled effects. This heterogeneity can be attributed, in part, to the diverse sources of arterial and venous blood lactate, variations in measurement equipment and assays, as well as differences in methodologies for lactate measurements and the use of various lactate cut-off values. Moreover, even after conducting subgroup analysis, high heterogeneity persisted due to significant disparities in diagnostic criteria, primary diseases, disease severity, and treatment status, necessitating further validation and analysis of clinical trial results. It is important to note that blood lactate levels in the body are influenced by multiple processes, including lactate generation, transformation, clearance, and recycling [8]. The dynamics of blood lactate levels can provide valuable insights into identifying individuals at high risk for poor clinical outcomes. However, our study has several limitations. It underscores that a single measurement of blood lactate levels in clinical practice may not fully capture the dynamic state of the body. Therefore, dynamic monitoring of blood lactate levels is recommended for more effectively guiding sepsis treatment and assessing prognosis. Additionally, a model incorporating more variables may offer improved predictive capability compared to a single lactate variable. Recently, a study suggested that compared to lactate and albumin alone, the predictor value of the lactate and albumin ratio was outstanding in predicting death and hospital stay (discharge) among sepsis participants, with a sensitivity of 100% and a specificity of 88% [147]. As most of the included studies are observational, they cannot infer causation. Further research, ideally through controlled trials or experimental studies, is needed to establish causal relationships between blood lactate levels and sepsis outcomes. While lactate plays a significant role in predicting the risk of sepsis mortality, in clinical practice, it is essential to consider other clinical indicators and the overall condition of the patient. This comprehensive approach allows for a more thorough evaluation of the patient’s condition and the development of appropriate treatment plans.
5. Conclusions
Based on the meta-analysis, blood lactate revealed the capability to predict the multiple sepsis mortality. This study demonstrated the high-level blood lactate was associated with an elevated risk of death and predicted higher risk of mortality. However, the levels of blood lactate failed to distinguish sepsis, severe sepsis and septic shock. Large-scale multicenter randomized clinical trials are needed to provide high-level evidence and confirm the optimal cut-off for prognosis of sepsis. In clinical practice, we cannot overly rely on lactate to determine the severity of sepsis; the dynamic monitoring of blood lactate levels is recommended for more effectively guiding sepsis treatment and assessing prognosis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines12020447/s1, Figure S1. Sensitivity analysis of the individual trials on the results for blood lactate level associated with sepsis mortality; Figure S2. Sensitivity analysis of the individual trials on the results for blood lactate level associated survivors and non-survivors of sepsis; Figure S3. Funnel plot with Egger’s test for association between blood lactate levels and mortality; Figure S4. The network meta-analysis of available comparisons of blood lactate levels of patients with various outcomes; Figure S5. Comparison-adjusted funnel plot for blood lactate levels of patients with various clinical outcomes. A: nonsepsis; B: sepsis; C: severe sepsis; D: septic shock; Table S1. Original Sequential Organ Failure Assessment (SOFA) score; Table S2. Systemic Inflammatory Response Syndrome (SIRS) Criteria; Table S3. qSOFA (Quick SOFA) Criteria.
Author Contributions
All the authors contributed equally to the work presented in this article. B.Z. and Y.L. conceived the idea of this study. R.Z. contributed to the data extraction. B.Z., R.Z. and J.Q. computed and evaluated the pooled outcomes. B.Z. contributed to the study protocol and wrote the article. Y.L. revised the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Information Files. The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AUC | Areas under the curve |
| CI | Confidence interval |
| HSROC | Summary receiver operating characteristic curve |
| NOS | Newcastle–Ottawa Scale |
| OR | Odds Ratio |
| QUADAS | Quality assessment of diagnostic accuracy studies |
| SE | Standard error |
| SIRS | Systemic inflammatory response syndrome |
| SMD | Standard mean difference |
| SOFA | Sequential Organ Failure Assessment |
| SUCRA | Surface under the cumulative ranking |
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K.; International Forum of Acute Care, T. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef]
- Font, M.D.; Thyagarajan, B.; Khanna, A.K. Sepsis and Septic Shock—Basics of diagnosis, pathophysiology and clinical decision making. Med. Clin. N. Am. 2020, 104, 573–585. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. Am. Coll. Chest Physicians/Soc. Crit. Care Med. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Mikkelsen, M.E.; Miltiades, A.N.; Gaieski, D.F.; Goyal, M.; Fuchs, B.D.; Shah, C.V.; Bellamy, S.L.; Christie, J.D. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit. Care Med. 2009, 37, 1670–1677. (In English) [Google Scholar] [CrossRef]
- Wright, S.W.; Hantrakun, V.; Rudd, K.E.; Lau, C.Y.; Lie, K.C.; Chau, N.V.V.; Teprarrukkul, P.; West, T.E.; Limmathurotsakul, D. Enhanced bedside mortality prediction combining point-of-care lactate and the quick Sequential Organ Failure Assessment (qSOFA) score in patients hospitalised with suspected infection in southeast Asia: A cohort study. Lancet Glob. Health 2022, 10, e1281–e1288. (In English) [Google Scholar] [CrossRef]
- Garcia-Alvarez, M.; Marik, P.; Bellomo, R. Sepsis-associated hyperlactatemia. Crit. Care 2014, 18, 503. [Google Scholar] [CrossRef]
- Kim, Y.A.; Ha, E.J.; Jhang, W.K.; Park, S.J. Early blood lactate area as a prognostic marker in pediatric septic shock. Intensive Care Med. 2013, 39, 1818–1823. (In English) [Google Scholar] [CrossRef]
- Houwink, A.P.I.; Rijkenberg, S.; Bosman, R.J.; van der Voort, P.H.J. The association between lactate, mean arterial pressure, central venous oxygen saturation and peripheral temperature and mortality in severe sepsis: A retrospective cohort analysis. Crit. Care 2016, 20, 56. (In English) [Google Scholar] [CrossRef]
- Filho, R.R.; Rocha, L.L.; Correa, T.D.; Pessoa, C.M.; Colombo, G.; Assuncao, M.S. Blood Lactate Levels Cutoff and Mortality Prediction in Sepsis-Time for a Reappraisal? a Retrospective Cohort Study. Shock 2016, 46, 480–485. [Google Scholar] [CrossRef]
- Schlapbach, L.J.; MacLaren, G.; Festa, M.; Alexander, J.; Erickson, S.; Beca, J.; Slater, A.; Schibler, A.; Pilcher, D.; Millar, J.; et al. Prediction of pediatric sepsis mortality within 1 h of intensive care admission. Intensive Care Med. 2017, 43, 1085–1096. (In English) [Google Scholar] [CrossRef]
- Oedorf, K.; Day, D.E.; Lior, Y.; Novack, V.; Sanchez, L.D.; Wolfe, R.E.; Kirkegaard, H.; Shapiro, N.I.; Henning, D.J. Serum Lactate Predicts Adverse Outcomes in Emergency Department Patients With and Without Infection. West. J. Emerg. Med. 2017, 18, 258–266. (In English) [Google Scholar] [CrossRef]
- Chen, H.; Zhao, C.Y.; Wei, Y.; Jin, J. Early lactate measurement is associated with better outcomes in septic patients with an elevated serum lactate level. Crit. Care 2019, 23, 351. (In English) [Google Scholar] [CrossRef]
- Park, J.E.; Lee, B.; Yoon, S.J.; Park, C.M.; Jung, C.W.; Ahn, M.J.; Park, H.D.; Hwang, S.Y.; Shin, T.G.; Kang, E.S. Complementary Use of Presepsin with the Sepsis-3 Criteria Improved Identification of High-Risk Patients with Suspected Sepsis. Biomedicines 2021, 9, 1076. [Google Scholar] [CrossRef]
- D’Onofrio, V.; Meersman, A.; Vijgen, S.; Cartuyvels, R.; Messiaen, P.; Gyssens, I.C. Risk Factors for Mortality, Intensive Care Unit Admission, and Bacteremia in Patients Suspected of Sepsis at the Emergency Department: A Prospective Cohort Study. Open Forum Infect. Dis. 2021, 8, ofaa594. (In English) [Google Scholar] [CrossRef]
- Alataby, H.; Nfonoyim, J.; Diaz, K.; Al-Tkrit, A.; Akhter, S.; David, S.; Leelaruban, V.; Gay-Simon, K.S.; Maharaj, V.; Colet, B.; et al. The Levels of Lactate, Troponin, and N-Terminal Pro-B-Type Natriuretic Peptide Are Predictors of Mortality in Patients with Sepsis and Septic Shock: A Retrospective Cohort Study. Med. Sci. Monit. Basic. Res. 2021, 27, e927834. [Google Scholar] [CrossRef]
- Hernandez, G.; Ospina-Tascon, G.A.; Damiani, L.P.; Estenssoro, E.; Dubin, A.; Hurtado, J.; Friedman, G.; Castro, R.; Alegria, L.; Teboul, J.L.; et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA 2019, 321, 654–664. [Google Scholar] [CrossRef]
- Vorwerk, C.; Loryman, B.; Coats, T.J.; Stephenson, J.A.; Gray, L.D.; Reddy, G.; Florence, L.; Butler, N. Prediction of mortality in adult emergency department patients with sepsis. Emerg. Med. J. 2009, 26, 254–258. [Google Scholar] [CrossRef]
- Riley, R.D.; Moons, K.G.M.; Snell, K.I.E.; Ensor, J.; Hooft, L.; Altman, D.G.; Hayden, J.; Collins, G.S.; Debray, T.P.A. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ 2019, 364, k4597. [Google Scholar] [CrossRef]
- Foroutan, F.; Guyatt, G.; Zuk, V.; Vandvik, P.O.; Alba, A.C.; Mustafa, R.; Vernooij, R.; Arevalo-Rodriguez, I.; Munn, Z.; Roshanov, P.; et al. GRADE Guidelines 28: Use of GRADE for the assessment of evidence about prognostic factors: Rating certainty in identification of groups of patients with different absolute risks. J. Clin. Epidemiol. 2020, 121, 62–70. (In English) [Google Scholar] [CrossRef]
- Iorio, A.; Spencer, F.A.; Falavigna, M.; Alba, C.; Lang, E.; Burnand, B.; McGinn, T.; Hayden, J.; Williams, K.; Shea, B.; et al. Use of GRADE for assessment of evidence about prognosis: Rating confidence in estimates of event rates in broad categories of patients. BMJ Brit Med. J. 2015, 350, h870. (In English) [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studie. Ann. Intern. Med. 2011, 155, 529–536. (In English) [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. (In English) [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Rutter, C.M.; Gatsonis, C.A. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evalua-tions. Stat. Med. 2001, 20, 2865–2884. (In English) [Google Scholar] [CrossRef] [PubMed]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zamora, J.; Abraira, V.; Muriel, A.; Khan, K.; Coomarasamy, A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med. Res. Methodol. 2006, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.J.; Li, B.B.; Lin, Y.; Shi, F.; Chen, W.B.; Wu, W.Y.; Zhang, W.J.; Fei, Y.; Zou, S.Q.; Yao, C. Combining Blood-Based Biomarkers to Predict Mortality of Sepsis at Arrival at the Emergency Department. Med. Sci. Monit. 2021, 27, e929527. (In English) [Google Scholar] [CrossRef]
- Guarino, M.; Perna, B.; De Giorgi, A.; Gambuti, E.; Alfano, F.; Catanese, E.M.; Volpato, S.; Strada, A.; Caio, G.; Contini, C.; et al. A 2-year retrospective analysis of the prognostic value of MqSOFA compared to lactate, NEWS and qSOFA in patients with sepsis. Infection 2022, 50, 941–948. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, C.; Zhou, Y.; He, Z.M.; Zhang, W.; Fan, J.; Sun, Y. Risk stratification and prognostic value of serum neutrophil gelatinase-associated lipocalin (sNGAL) in sepsis patients. Acta Biochim. Pol. 2022, 69, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bai, C.; Li, B.; Shan, A.; Shi, F.; Yao, C.; Zhang, Y.; Wang, J.; Chen, W.; Xie, M.; et al. Mortality prediction using a novel combination of biomarkers in the first day of sepsis in intensive care units. Sci. Rep. 2021, 11, 1275. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.Y.; Min, Y.; Yu, J.C.; Wang, Q.L.; Wang, H.; Li, S.; Su, L. Admission Blood Glucose Is Associated With the 30-Days Mortality in Septic Patients: A Retrospective Cohort Study. Front. Med. 2021, 8, 757061. (In English) [Google Scholar] [CrossRef]
- Erdogan, M.; Findikli, H.A. Novel biomarker for predicting sepsis mortality: Vitamin D receptor. J. Int. Med. Res. 2021, 49, 3000605211034733. [Google Scholar] [CrossRef]
- Nandi, U.; Jones, A.E.; Puskarich, M.A. Group IIA secretory phospholipase 2 independently predicts mortality and positive blood culture in emergency department sepsis patients. J. Am. Coll. Emerg. Physicians Open 2021, 2, e12460. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, R.Z.; Cheng, J.; Wu, J.F.; Zhang, S.H. Ratio of serum procalcitonin to monocytic HLA-DR as a reliable parameter in prognosis prediction of sepsis. Clin. Chim. Acta 2021, 519, 94–100. (In English) [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Chen, F.; Cai, K.; Tan, J.; Xie, W.; Qian, R.; Liu, X.; Zhang, W.; Du, H.; et al. A risk score based on pediatric sequential organ failure assessment predicts 90-day mortality in children with Klebsiella pneumoniae bloodstream infection. BMC Infect. Dis. 2020, 20, 916. [Google Scholar] [CrossRef]
- Perez-San Martin, S.; Suberviola, B.; Garcia-Unzueta, M.T.; Lavin, B.A.; Campos, S.; Santibanez, M. Prognostic value of plasma pentraxin 3 levels in patients with septic shock admitted to intensive care. PLoS ONE 2020, 15, e0243849. [Google Scholar] [CrossRef]
- Bou Chebl, R.; Jamali, S.; Mikati, N.; Al Assaad, R.; Abdel Daem, K.; Kattouf, N.; Safa, R.; Makki, M.; Tamim, H.; Abou Dagher, G. Relative Hyperlactatemia in the Emergency Department. Front. Med. 2020, 7, 561. [Google Scholar] [CrossRef]
- Jouffroy, R.; Leguillier, T.; Gilbert, B.; Tourtier, J.P.; Bloch-Laine, E.; Ecollan, P.; Bounes, V.; Boularan, J.; Gueye-Ngalgou, P.; Nivet-Antoine, V.; et al. Pre-Hospital Lactatemia Predicts 30-Day Mortality in Patients with Septic Shock-Preliminary Results from the LAPHSUS Study. J. Clin. Med. 2020, 9, 3290. [Google Scholar] [CrossRef]
- Jaiswal, P.; Dewan, P.; Gomber, S.; Banerjee, B.D.; Kotru, M.; Malhotra, R.K.; Tyagi, V. Early lactate measurements for predicting in-hospital mortality in paediatric sepsis. J. Paediatr. Child. Health 2020, 56, 1570–1576. [Google Scholar] [CrossRef]
- Hsiao, S.Y.; Kung, C.T.; Su, C.M.; Lai, Y.R.; Huang, C.C.; Tsai, N.W.; Wang, H.C.; Cheng, B.C.; Su, Y.J.; Lin, W.C.; et al. Impact of oxidative stress on treatment outcomes in adult patients with sepsis: A prospective study. Medicine 2020, 99, e20872. [Google Scholar] [CrossRef]
- Song, J.; Moon, S.; Park, D.W.; Cho, H.J.; Kim, J.Y.; Park, J.; Cha, J.H. Biomarker combination and SOFA score for the prediction of mortality in sepsis and septic shock: A prospective observational study according to the Sepsis-3 definitions. Medicine 2020, 99, e20495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lv, D.; Deng, Y.; Zhu, C.; Gao, Y.; Huang, Y.; Xu, X. STAPLAg: A convenient early warning score for use in infected patients in the intensive care unit. Medicine 2020, 99, e20274. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.M.; Shao, L.J.; Dou, J.Y.; Zhou, Y.P.; Chen, M.; Cui, Y.; Zhang, Y.C.; Wang, C.X. Fibrinogen as a Prognostic Predictor in Pediatric Patients with Sepsis: A Database Study. Mediat. Inflamm. 2020, 2020, 9153620. (In English) [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, M.; Zhu, B.; Zhu, Y.; Xi, X. Prediction of median survival time in sepsis patients by the SOFA score combined with different predictors. Burn. Trauma. 2020, 8, tkz006. [Google Scholar] [CrossRef] [PubMed]
- Mearelli, F.; Barbati, G.; Casarsa, C.; Giansante, C.; Breglia, A.; Spica, A.; Moras, C.; Olivieri, G.; Occhipinti, A.A.; De Nardo, M.; et al. The Integration of qSOFA with Clinical Variables and Serum Biomarkers Improves the Prognostic Value of qSOFA Alone in Patients with Suspected or Confirmed Sepsis at ED Admission. J. Clin. Med. 2020, 9, 1205. (In English) [Google Scholar] [CrossRef]
- Wang, L.; Zhou, W.; Wang, K.; He, S.; Chen, Y. Predictive value of circulating plasma mitochondrial DNA for Sepsis in the emergency department: Observational study based on the Sepsis-3 definition. BMC Emerg. Med. 2020, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Endoh, H.; Kamimura, N.; Tamakawa, T.; Nitta, M. Lactate indices as predictors of in-hospital mortality or 90-day survival after admission to an intensive care unit in unselected critically ill patients. PLoS ONE 2020, 15, e0229135. (In English) [Google Scholar] [CrossRef] [PubMed]
- Wernly, B.; Heramvand, N.; Masyuk, M.; Rezar, R.; Bruno, R.R.; Kelm, M.; Niederseer, D.; Lichtenauer, M.; Hoppe, U.C.; Bakker, J.; et al. Acidosis predicts mortality independently from hyperlactatemia in patients with sepsis. Eur. J. Intern. Med. 2020, 76, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Gupta, S. Lactate Measurements and Their Association With Mortality in Pediatric Severe Sepsis in India: Evidence That 6-Hour Level Performs Best. J. Intensive Care Med. 2021, 36, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yoo, S.J.; Lee, S.H.; Kim, J.S.; Jung, S.; Kim, Y.J.; Kim, W.Y.; Ryoo, S.M. Utility of the early lactate area score as a prognos-tic marker for septic shock patients in the emergency department. Acute Crit. Care 2019, 34, 126–132. (In English) [Google Scholar] [CrossRef]
- Innocenti, F.; Meo, F.; Giacomelli, I.; Tozzi, C.; Ralli, M.L.; Donnini, C.; Tassinari, I.; Caldi, F.; Zanobetti, M.; Pini, R. Prognostic value of serial lactate levels in septic patients with and without shock. Intern. Emerg. Med. 2019, 14, 1321–1330. (In English) [Google Scholar] [CrossRef]
- Sivayoham, N.; Blake, L.A.; Tharimoopantavida, S.E.; Chughtai, S.; Hussain, A.N.; Cecconi, M.; Rhodes, A. The REDS score: A new scoring system to risk-stratify emergency department suspected sepsis: A derivation and validation study. BMJ Open 2019, 9, e030922. [Google Scholar] [CrossRef]
- Villar, J.; Short, J.H.; Lighthall, G. Lactate Predicts Both Short- and Long-Term Mortality in Patients With and Without Sepsis. Infect. Dis. 2019, 12, 1178633719862776. [Google Scholar] [CrossRef]
- Jendoubi, A.; Jerbi, S.; Maamar, E.; Abbess, A.; Samoud, Z.; Kanzari, L.; Boutiba, I.; Ghedira, S.; Houissa, M. Prognostic Value of High-sensitivity Troponin I in Patients with Septic Shock: A Prospective Observational Study. Indian J. Crit. Care Med. 2019, 23, 320–325. [Google Scholar] [CrossRef]
- Hsiao, S.Y.; Lai, Y.R.; Kung, C.T.; Tsai, N.W.; Su, C.M.; Huang, C.C.; Wang, H.C.; Cheng, B.C.; Su, Y.J.; Lin, W.C.; et al. alpha-1-Acid Glycoprotein Concentration as an Outcome Predictor in Adult Patients with Sepsis. Biomed. Res. Int. 2019, 2019, 3174896. (In English) [Google Scholar] [CrossRef] [PubMed]
- Estenssoro, E.; Loudet, C.I.; Edul, V.S.K.; Osatnik, J.; Rios, F.G.; Vasquez, D.N.; Pozo, M.O.; Lattanzio, B.; Palizas, F.; Klein, F.; et al. Health inequi-ties in the diagnosis and outcome of sepsis in Argentina: A prospective cohort study. Crit. Care 2019, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, S.S.; Kang, J.Q.; Yang, L.; Liu, F. Predictive value of C-reactive protein and NT-pro-BNP levels in sepsis pa-tients older than 75 years: A prospective, observational study. Aging Clin. Exp. Res. 2020, 32, 389–397. (In English) [Google Scholar] [CrossRef]
- Dumas, G.; Lavillegrand, J.R.; Joffre, J.; Bige, N.; de-Moura, E.B.; Baudel, J.L.; Chevret, S.; Guidet, B.; Maury, E.; Amorim, F.; et al. Mottling score is a strong predictor of 14-day mortality in septic patients whatever vasopressor doses and other tissue perfusion parameters. Crit. Care 2019, 23, 211. (In English) [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.J.; Guerrero, A.; Root-Bowman, C.; Ernst, A.; Krumperman, K.; Femling, J.; Froman, P. Sepsis alerts in EMS and the results of pre-hospital ETCO2. Am. J. Emerg. Med. 2019, 37, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Meng, Z.B.; Li, Y.F.; Zhao, J.Y.; Wu, S.H.; Gou, S.M.; Wu, H.S. Prognostic accuracy of the serum lactate level, the SOFA score and the qSOFA score for mortality among adults with Sepsis. Scand. J. Trauma. Resus 2019, 27, 51. (In English) [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.M.; Lee, J.; Lee, Y.S.; Lee, J.H.; Lim, K.S.; Huh, J.W.; Hong, S.B.; Lim, C.M.; Koh, Y.; Kim, W.Y. Lactate Level Versus Lactate Clearance for Predicting Mortality in Patients With Septic Shock Defined by Sepsis-3. Crit. Care Med. 2018, 46, e489–e495. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.L.; Huang, L.W.; Yin, J.; Zhang, K.N.; Xiao, K.; Qing, G.Z. Association between early serum cholinesterase activity and 30-day mortality in sepsis-3 patients: A retrospective cohort study. PLoS ONE 2018, 13, e0203128. (In English) [Google Scholar] [CrossRef] [PubMed]
- Nazer, L.H.; Rimawi, D.; Hawari, F.I. Evaluating the Predictive Value of Lactate in Patients With Cancer Having Septic Shock. J. Intensive Care Med. 2020, 35, 789–796. (In English) [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, Z.; Zhou, T.; Cao, X.; Lu, T.; Liang, Y.; He, J.; Liu, C.; Dou, Z.; Shen, B. Early increases in serum FGF21 levels predict mortality of septic patients. Cytokine 2018, 111, 428–433. [Google Scholar] [CrossRef]
- Cheng, H.H.; Chen, F.C.; Change, M.W.; Kung, C.T.; Cheng, C.Y.; Tsai, T.C.; Hsiao, S.Y.; Su, C.M. Difference between elderly and non-elderly patients in using serum lactate level to predict mortality caused by sepsis in the emergency department. Medicine 2018, 97, e0209. (In English) [Google Scholar] [CrossRef]
- Mahmoodpoor, A.; Shadvar, K.; Saghaleini, S.H.; Koleini, E.; Hamishehkar, H.; Ostadi, Z.; Nader, N.D. Which one is a better predictor of ICU mortality in septic patients? Comparison between serial serum lactate concentrations and its re-moval rate. J. Crit. Care 2018, 44, 51–56. (In English) [Google Scholar] [CrossRef]
- Hu, C.G.; Zhou, Y.F.; Liu, C.; Kang, Y. Pentraxin-3, procalcitonin and lactate as prognostic markers in patients with sepsis and septic shock. Oncotarget 2018, 9, 5125–5136. (In English) [Google Scholar] [CrossRef]
- Julian-Jimenez, A.; Yanez, M.C.; Gonzalez-del Castillo, J.; Salido-Mota, M.; Mora-Ordonez, B.; Arranz-Nieto, M.J.; Chano-vas-Borras, M.R.; Llopis-Roca, F.; Modol-Deltell, J.M.; Munoz, G.; et al. Prognostic power of biomarkers for short-term mortality in the elderly patients seen in Emergency Departments due to infections. Enferm. Infecc. Microbiol. Clin. 2019, 37, 11–18. (In Spanish) [Google Scholar] [CrossRef]
- Oh, D.H.; Kim, M.H.; Jeong, W.Y.; Kim, Y.C.; Kim, E.J.; Song, J.E.; Jung, I.Y.; Jeong, S.J.; Ku, N.S.; Choi, J.Y.; et al. Risk factors for mortality in patients with low lactate level and septic shock. J. Microbiol. Immunol. Infect. 2019, 52, 418–425. [Google Scholar] [CrossRef]
- Sekino, M.; Funaoka, H.; Sato, S.; Okada, K.; Inoue, H.; Yano, R.; Matsumoto, S.; Ichinomiya, T.; Higashijima, U.; Matsumoto, S.; et al. Intestinal fatty acid-binding protein level as a predictor of 28-day mortality and bowel ischemia in patients with septic shock: A preliminary study. J. Crit. Care 2017, 42, 92–100. (In English) [Google Scholar] [CrossRef]
- Javed, A.; Guirgis, F.W.; Sterling, S.A.; Puskarich, M.A.; Bowman, J.; Robinson, T.; Jones, A.E. Clinical predictors of early death from sepsis. J. Crit. Care 2017, 42, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lu, X.; Hu, L.; Liu, P.; Zhao, W.; Yan, H.; Tang, L.; Zhu, Y.; Xiao, Z.; Chen, L.; et al. Development and validation of a mortality risk model for pediatric sepsis. Medicine 2017, 96, e6923. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Long, Y.; Liu, D.; Wang, X.; Tang, B. The Prognostic Value of Central Venous-to-Arterial CO2 Differ-ence/Arterial-Central Venous O2 Difference Ratio in Septic Shock Patients with Central Venous O2 Saturation >/=80. Shock 2017, 48, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.; Sitaraman, S.; Choudhary, A. Lactate clearance as the predictor of outcome in pediatric septic shock. J. Emerg. Trauma. Shock 2017, 10, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.D.; Song, J.; Gong, S.J.; Li, L.; Zhang, H.X.; Wang, M.J. Persistent hyperlactatemia-high central venous-arterial carbon dioxide to arterial-venous oxygen content ratio is associated with poor outcomes in early resuscitation of septic shock. Am. J. Emerg. Med. 2017, 35, 1136–1141. (In English) [Google Scholar] [CrossRef] [PubMed]
- Dadeh, A.A.; Wuthisuthimethawee, P. Serum Lactate Levels as a Prognostic Predictor of Septic Shock in Emergency Department Patients with Systemic Inflammatory Responpse Syndrome (SIRS) at Songklanagarind Hospital. J. Med. Assoc. Thai 2016, 99, 913–918. [Google Scholar] [PubMed]
- Lee, Y.K.; Hwang, S.Y.; Shin, T.G.; Jo, I.J.; Suh, G.Y.; Jeon, K. Prognostic Value of Lactate and Central Venous Oxygen Satura-tion after Early Resuscitation in Sepsis Patients. PLoS ONE 2016, 11, e0153305. [Google Scholar] [CrossRef]
- Bewersdorf, J.P.; Hautmann, O.; Kofink, D.; Abdul Khalil, A.; Zainal Abidin, I.; Loch, A. The SPEED (sepsis patient evalua-tion in the emergency department) score: A risk stratification and outcome prediction tool. Eur. J. Emerg. Med. 2017, 24, 170–175. [Google Scholar] [CrossRef]
- Haas, S.A.; Lange, T.; Saugel, B.; Petzoldt, M.; Fuhrmann, V.; Metschke, M.; Kluge, S. Severe hyperlactatemia, lactate clear-ance and mortality in unselected critically ill patients. Intensive Care Med. 2016, 42, 202–210. [Google Scholar] [CrossRef]
- Kuan, W.S.; Ibrahim, I.; Leong, B.S.; Jain, S.; Lu, Q.; Cheung, Y.B.; Mahadevan, M. Emergency Department Management of Sepsis Patients: A Randomized, Goal-Oriented, Noninvasive Sepsis Trial. Ann. Emerg. Med. 2016, 67, 367–378.e3. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, H.; Zhong, Y.; Huang, J.; Lv, J.; Li, J. The Cold-Inducible RNA-Binding Protein (CIRP) Level in Peripheral Blood Predicts Sepsis Outcome. PLoS ONE 2015, 10, e0137721. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Ahn, J.Y.; Song, J.E.; Choi, H.; Ann, H.W.; Kim, J.K.; Kim, J.H.; Jeon, Y.D.; Kim, S.B.; Jeong, S.J.; et al. The C-Reactive Protein/Albumin Ratio as an Independent Predictor of Mortality in Patients with Severe Sepsis or Septic Shock Treated with Early Goal-Directed Therapy. PLoS ONE 2015, 10, e0132109. (In English) [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Martin, M.M.; Gonzalez-Rivero, A.F.; Ferreres, J.; Sole-Violan, J.; Labarta, L.; Diaz, C.; Jimenez, A.; Borreguero-Leon, J.M. Serum levels of caspase-cleaved cytokeratin-18 and mortality are associated in severe septic patients: Pilot study. PLoS ONE 2014, 9, e109618. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Li, C.S. Arterial lactate improves the prognostic performance of severity score systems in septic patients in the ED. Am. J. Emerg. Med. 2014, 32, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, X.W.; Huang, L.; Lu, N.; Zhou, L.; Wu, G.J.; Chen, Y.G. Severe sepsis: Low expression of the ren-in-angiotensin system is associated with poor prognosis. Exp. Ther. Med. 2014, 7, 1342–1348. (In English) [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Shin, T.G.; Jo, I.J.; Jeon, K.; Suh, G.Y.; Lee, T.R.; Cha, W.C.; Sim, M.S.; Song, K.J.; Jeong, Y.K. Association between He-modynamic Presentation and Outcome in Sepsis Patients. Shock 2014, 42, 205–210. (In English) [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Martin, M.M.; Abreu-Gonzalez, P.; Dominguez-Rodriguez, A.; Labarta, L.; Diaz, C.; Sole-Violan, J.; Ferreres, J.; Borreguero-Leon, J.M.; Jimenez, A.; et al. Prognostic value of malondialdehyde serum levels in severe sepsis: A multicenter study. PLoS ONE 2013, 8, e53741. [Google Scholar] [CrossRef] [PubMed]
- Hisamuddin, N.A.; Azlan, K. The use of laboratory and physiological parameters in predicting mortality in sepsis in-duced hypotension and septic shock patients attending the emergency department. Med. J. Malays. 2012, 67, 259–264. [Google Scholar]
- Jat, K.R.; Jhamb, U.; Gupta, V.K. Serum lactate levels as the predictor of outcome in pediatric septic shock. Indian J. Crit. Care Med. 2011, 15, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Ait-Oufella, H.; Lemoinne, S.; Boelle, P.Y.; Galbois, A.; Baudel, J.L.; Lemant, J.; Joffre, J.; Margetis, D.; Guidet, B.; Maury, E.; et al. Mottling score predicts survival in septic shock. Intensive Care Med. 2011, 37, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Martin, M.M.; Labarta, L.; Diaz, C.; Sole-Violan, J.; Blanquer, J.; Orbe, J.; Rodriguez, J.A.; Jimenez, A.; Borreguero-Leon, J.M.; et al. Matrix metalloproteinase-9,-10, and tissue inhibitor of matrix metalloproteinases-1 blood levels as biomarkers of severity and mortality in sepsis. Crit. Care 2009, 13, R158. (In English) [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, S.; Dellinger, R.P.; Chansky, M.E.; Arnold, R.C.; Schorr, C.; Milcarek, B.; Hollenberg, S.M.; Parrillo, J.E. Serum lactate as a predictor of mortality in patients with infection. Intensive Care Med. 2007, 33, 970–977. (In English) [Google Scholar] [CrossRef]
- Duke, T.D.; Butt, W.; South, M. Predictors of mortality and multiple organ failure in children with sepsis. Intensive Care Med. 1997, 23, 684–692. [Google Scholar] [CrossRef]
- Galtung, N.; Diehl-Wiesenecker, E.; Lehmann, D.; Markmann, N.; Bergstrom, W.H.; Wacker, J.; Liesenfeld, O.; Mayhew, M.; Buturovic, L.; Luethy, R.; et al. Prospective validation of a transcriptomic severity classifier among patients with suspected acute infection and sepsis in the emergency department. Eur. J. Emerg. Med. 2022, 29, 357–365. (In English) [Google Scholar] [CrossRef]
- Cao, Y.; Ma, W.; Liu, Z.; Pei, Y.; Zhu, Y.; Chen, F.; Zou, L.; Jiang, Y.; Liu, X.; Huang, J.; et al. Early predictive value of platelet function for clinical outcome in sepsis. J. Infect. 2022, 84, 628–636. [Google Scholar] [CrossRef]
- Chen, H.; Gong, S.R.; Yu, R.G. Association between normalized lactate load and mortality in patients with septic shock: An analysis of the MIMIC-III database. BMC Anesth. 2021, 21, 16. (In English) [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.; Kim, K.H.; Jeong, N.R.; Kim, S.C.; Oh, E.J. The Association between Dynamic Changes in Serum Presepsin Levels and Mortality in Immunocompromised Patients with Sepsis: A Prospective Cohort Study. Diagnostics 2021, 11, 60. (In English) [Google Scholar] [CrossRef] [PubMed]
- Lee, G.T.; Hwang, S.Y.; Park, J.E.; Jo, I.J.; Kim, W.Y.; Chung, S.P.; Jo, Y.H.; Suh, G.J.; Choi, S.H.; Shin, T.G.; et al. Diagnostic accuracy of lactate levels after initial fluid resuscitation as a predictor for 28 day mortality in septic shock. Am. J. Emerg. Med. 2021, 46, 392–397. (In English) [Google Scholar] [CrossRef]
- Tonial, C.T.; Costa, C.A.D.; Andrades, G.R.H.; Crestani, F.; Bruno, F.; Piva, J.P.; Garcia, P.C.R. Performance of prognostic markers in pediatric sepsis. J. Pediatr. 2021, 97, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; He, L.; Zhang, N.; Lin, Q.; Zhong, L.; Song, J. Prediction of 90-Day Mortality among Sepsis Patients Based on a Nomogram Integrating Diverse Clinical Indices. Biomed. Res. Int. 2021, 2021, 1023513. [Google Scholar] [CrossRef]
- Ma, H.; Liu, H.; Wu, C.; Huang, L. Diagnostic Value of Serum Heparin Binding Protein, Blood Lactic Acid Combined with hs-CRP in Sepsis and Its Relationship with Prognosis. Evid. Based Complement. Alternat Med. 2021, 2021, 5023733. [Google Scholar] [CrossRef]
- Bou Chebl, R.; Geha, M.; Assaf, M.; Kattouf, N.; Haidar, S.; Abdeldaem, K.; Halawi, N.; Khamis, M.; Makki, M.; Tamim, H.; et al. The prognostic value of the lactate/albumin ratio for predicting mortality in septic patients presenting to the emergency department: A prospective study. Ann. Med. 2021, 53, 2268–2277. [Google Scholar] [CrossRef] [PubMed]
- Gharipour, A.; Razavi, R.; Gharipour, M.; Mukasa, D. Lactate/albumin ratio: An early prognostic marker in critically ill patients. Am. J. Emerg. Med. 2020, 38, 2088–2095. [Google Scholar] [CrossRef]
- Liu, Y.C.; Jiang, T.Y.; Chen, Z.S.; Qi, A.L.; Gao, Y.L.; Li, S.X.; Yu, M.M.; Chai, Y.F.; Shou, S.T. Thyroid hormone disorders: A predictor of mortality in patients with septic shock defined by Sepsis-3? Intern. Emerg. Med. 2021, 16, 967–973. [Google Scholar] [CrossRef]
- Lundberg, O.H.M.; Lengquist, M.; Spangfors, M.; Annborn, M.; Bergmann, D.; Schulte, J.; Levin, H.; Melander, O.; Frigyesi, A.; Friberg, H. Circulating bioactive adrenomedullin as a marker of sepsis, septic shock and critical illness. Crit. Care 2020, 24, 636. (In English) [Google Scholar] [CrossRef]
- Bou Chebl, R.; Jamali, S.; Sabra, M.; Safa, R.; Berbari, I.; Shami, A.; Makki, M.; Tamim, H.; Abou Dagher, G. Lactate/Albumin Ratio as a Predictor of In-Hospital Mortality in Septic Patients Presenting to the Emergency Department. Front. Med. 2020, 7, 550182. (In English) [Google Scholar] [CrossRef]
- Goyal, P.; Agarwal, R.; Srivastava, H.; Kar, R.; Sikka, M.; Mohta, M. Serial Serum Lactic Acid in Pregnancy-Associated Sepsis for Maternal Outcome. J. Obstet. Gynaecol. India 2020, 70, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.J.; Lan, T.F.; Guo, S.B. Outcome prediction value of National Early Warning Score in septic patients with community-acquired pneumonia in emergency department: A single-center retrospective cohort study. World J. Emerg. Med. 2020, 11, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Baysan, M.; Baroni, G.D.; van Boekel, A.M.; Steyerberg, E.W.; Arbous, M.S.; van der Bom, J.G. The Added Value of Lactate and Lactate Clearance in Prediction of In-Hospital Mortality in Critically Ill Patients With Sepsis. Crit. Care Explor. 2020, 2, e0087. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lan, T.; Guo, S. Prognostic Prediction Value of qSOFA, SOFA, and Admission Lactate in Septic Patients with Community-Acquired Pneumonia in Emergency Department. Emerg. Med. Int. 2020, 2020, 7979353. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Spencer, E.; Hoskin, T.S.; Rosengrave, P.; Kettle, A.J.; Shaw, G. Circulating myeloperoxidase is elevated in septic shock and is associated with systemic organ failure and mortality in critically ill patients. Free Radic. Biol. Med. 2020, 152, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Wani, W.; Dar, S.A.; Mir, I.H.; Charoo, B.A.; Ahmad, Q.I.; Wajid, S. Lactate clearance prognosticates outcome in pediatric septic shock during first 24 h of intensive care unit admission. J. Intensive Care Soc. 2019, 20, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, J.; Zhang, D.; Jing, L. Neutrophil-lymphocyte ratio and plasma lactate predict 28-day mortality in patients with sepsis. J. Clin. Lab. Anal. 2019, 33, e22942. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.M.; Ahn, R.; Shin, T.G.; Jo, Y.H.; Chung, S.P.; Beom, J.H.; Choi, S.H.; Yoon, Y.H.; Ko, B.S.; Lee, H.J.; et al. Lactate normalization within 6 hours of bundle therapy and 24 hours of delayed achievement were associated with 28-day mortality in septic shock patients. PLoS ONE 2019, 14, e0217857. (In English) [Google Scholar] [CrossRef]
- Chae, B.R.; Kim, Y.J.; Lee, Y.S. Prognostic accuracy of the sequential organ failure assessment (SOFA) and quick SOFA for mortality in cancer patients with sepsis defined by systemic inflammatory response syndrome (SIRS). Support. Care Cancer 2020, 28, 653–659. [Google Scholar] [CrossRef]
- Samanta, S.; Singh, R.K.; Baronia, A.K.; Mishra, P.; Poddar, B.; Azim, A.; Gurjar, M. Early pH Change Predicts Intensive Care Unit Mortality. Indian J. Crit. Care Med. 2018, 22, 697–705. [Google Scholar] [CrossRef]
- Zhang, L.F.; Zhang, X.H. Serum sTREM-1, PCT, CRP, Lac as biomarkers for death risk within 28 days in patients with severe sepsis. Open Life Sci. 2018, 13, 42–47. (In English) [Google Scholar] [CrossRef]
- Shin, J.; Hwang, S.Y.; Jo, I.J.; Kim, W.Y.; Ryoo, S.M.; Kang, G.H.; Kim, K.; Jo, Y.H.; Chung, S.P.; Joo, Y.S.; et al. Prognostic Value of The Lac-tate/Albumin Ratio for Predicting 28-Day Mortality in Critically ILL Sepsis Patients. Shock 2018, 50, 545–550. [Google Scholar] [CrossRef]
- Tarui, T.; Yamaguchi, Y.; Suzuki, K.; Tsuruta, R.; Ikeda, H.; Ogura, H.; Kushimoto, S.; Kotani, J.; Shiraishi, S.I.; Suzuki, Y.; et al. Early evaluation of severity in patients with severe sepsis: A comparison with “septic shock”—Subgroup analysis of the Japanese Association for Acute Medicine Sepsis Registry (JAAM-SR). Acute Med. Surg. 2017, 4, 426–431. [Google Scholar] [CrossRef]
- Brodska, H.; Valenta, J.; Pelinkova, K.; Stach, Z.; Sachl, R.; Balik, M.; Zima, T.; Drabek, T. Diagnostic and prognostic value of presepsin vs. established biomarkers in critically ill patients with sepsis or systemic inflammatory response syndrome. Clin. Chem. Lab. Med. 2018, 56, 658–668. [Google Scholar] [CrossRef]
- Jia, Y.F.; Wang, Y.; Yu, X.H. Relationship between blood lactic acid, blood procalcitonin, C-reactive protein and neonatal sepsis and corresponding prognostic significance in sick children. Exp. Ther. Med. 2017, 14, 2189–2193. (In English) [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.K.S.; Mehta, A.A.; James, P. Predictors of mortality of severe sepsis among adult patients in the medical Intensive Care Unit. Lung India 2017, 34, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.F.; Liu, S.H.; Li, C.H.; Wu, C.C.; Chaou, C.H.; Tzeng, I.S.; Hsieh, Y.H.; Blaney, G.N.; Liu, Z.Y.; Han, S.T.; et al. Develop-ment and validation of a parsimonious and pragmatic CHARM score to predict mortality in patients with suspect-ed sepsis. Am. J. Emerg. Med. 2017, 35, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.; Yin, M.; Chen, X.M.; Ding, S.F.; Li, C.; Zhai, Q.; Li, Y.; Liu, H.; Wu, D.W. Combination of Acute Physiology and Chronic Health Evaluation II score, early lactate area, and N-terminal prohormone of brain natriuretic peptide lev-els as a predictor of mortality in geriatric patients with septic shock. J. Crit. Care 2015, 30, 304–309. (In English) [Google Scholar] [CrossRef] [PubMed]
- Jones, A.E.; Trzeciak, S.; Kline, J.A. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit. Care Med. 2009, 37, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Gogos, C.A.; Lekkou, A.; Papageorgiou, O.; Siagris, D.; Skoutelis, A.; Bassaris, H.P. Clinical prognostic markers in patients with severe sepsis: A prospective analysis of 139 consecutive cases. J. Infect. 2003, 47, 300–306. [Google Scholar] [CrossRef]
- Marecaux, G.; Pinsky, M.R.; Dupont, E.; Kahn, R.J.; Vincent, J.L. Blood lactate levels are better prognostic indicators than TNF and IL-6 levels in patients with septic shock. Intensive Care Med. 1996, 22, 404–408. (In English) [Google Scholar] [CrossRef]
- Bakker, J.; Coffernils, M.; Leon, M.; Gris, P.; Vincent, J.L. Blood Lactate Levels Are Superior to Oxygen-Derived Variables in Predicting Outcome in Human Septic Shock. Chest 1991, 99, 956–962. (In English) [Google Scholar] [CrossRef]
- Chen, H.; Gong, S.R.; Yu, R.G. Increased normalized lactate load is associated with higher mortality in both sepsis and non-sepsis patients: An analysis of the MIMIC-IV database. BMC Anesth. 2022, 22, 79. (In English) [Google Scholar] [CrossRef]
- Perman, S.M.; Mikkelsen, M.E.; Goyal, M.; Ginde, A.; Bhardwaj, A.; Drumheller, B.; Sante, S.C.; Agarwal, A.K.; Gaieski, D.F. The sensitivity of qSOFA calculated at triage and during emergency department treatment to rapidly identify sepsis patients. Sci. Rep. 2020, 10, 20395. (In English) [Google Scholar] [CrossRef]
- Lu, N.F.; Jiang, L.; Zhu, B.; Yang, D.G.; Zheng, R.Q.; Shao, J.; Xi, X.M. Elevated plasma histone H4 level predicts increased risk of mortality in patients with sepsis. Ann. Palliat. Med. 2020, 9, 1084–1091. [Google Scholar] [CrossRef]
- Goonasekera, C.D.A.; Carcillo, J.A.; Deep, A. Oxygen Delivery and Oxygen Consumption in Pediatric Fluid Refractory Septic Shock During the First 42 h of Therapy and Their Relationship to 28-Day Outcome. Front. Pediatr. 2018, 6, 314. (In English) [Google Scholar] [CrossRef]
- Duman, A.; Turkdogan, K.A.; Avcil, M.; Yenisey, C.; Ture, M.; Akoz, A.; Dagli, B.; Kapci, M.; Orun, S. The predictive value of the inflammatory markers P-selectin and MCP1 in determining the length of stay and 30-day survival in the differ-entiation of sepsis patients. J. Pak. Med. Assoc. 2018, 68, 1321–1326. [Google Scholar] [PubMed]
- Yan, H.P.; Li, M.; Lu, X.L.; Zhu, Y.M.; Ou-Yang, W.X.; Xiao, Z.H.; Qiu, J.; Li, S.J. Use of plasma mitochondrial DNA levels for determining disease severity and prognosis in pediatric sepsis: A case control study. BMC Pediatr. 2018, 18, 267. [Google Scholar] [CrossRef] [PubMed]
- Khater, W.S.; Salah-Eldeen, N.N.; Khater, M.S.; Saleh, A.N. Role of suPAR and Lactic Acid in Diagnosing Sepsis and Pre-dicting Mortality in Elderly Patients. Eur. J. Microbiol. Immunol. 2016, 6, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Keçe, E.; Yaka, E.; Yılmaz, S.; Doğan, N.; Alyeşil, C.; Pekdemir, M. Comparison of diagnostic and prognostic utility of lactate and procalcitonin for sepsis in adult cancer patients presenting to emergency department with systemic in-flammatory response syndrome. Turk. J. Emerg. Med. 2016, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Madabhushi, S.; Trikha, A.; Anand, R.K.; Ramachandran, R.; Singh, P.M.; Rewari, V. Temporal Evolution of the PcvCO(2)-PaCO(2)/CaO(2)-CcvO(2) Ratio vs Serum Lactate during Resuscitation in Septic Shock. Indian J. Crit. Care Med. 2021, 25, 1370–1376. (In English) [Google Scholar] [CrossRef]
- Dubin, A.; Loudet, C.I.; Hurtado, F.J.; Pozo, M.O.; Comande, D.; Gibbons, L.; Cairoli, F.R.; Bardach, A. Comparison of central venous minus arterial carbon dioxide pressure to arterial minus central venous oxygen content ratio and lactate levels as predictors of mortality in critically ill patients: A systematic review and meta-analysis. Rev. Bras. Ter. Intensiv. 2022, 34, 279–286. [Google Scholar] [CrossRef]
- Basile-Filho, A.; Lago, A.F.; Menegueti, M.G.; Nicolini, E.A.; Rodrigues, L.A.B.; Nunes, R.S.; Auxiliadora-Martins, M.; Ferez, M.A. The use of APACHE II, SOFA, SAPS 3, C-reactive protein/albumin ratio, and lactate to predict mortality of surgical critically ill patients: A retrospective cohort study. Medicine 2019, 98, e16204. (In English) [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.R.; Wernly, B.; Binneboessel, S.; Baldia, P.; Duse, D.A.; Erkens, R.; Kelm, M.; Mamandipoor, B.; Osmani, V.; Jung, C. Failure of Lactate Clearance Predicts the Outcome of Critically Ill Septic Patients. Diagnostics 2020, 10, 1105. (In English) [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, S.; Sugaya, T.; Hossain, M.I.; Islam, M.M.; Chisti, M.J.; Kamoda, T.; Fukushima, T.; Wagatsuma, Y.; Sumazaki, R.; Ahmed, T. Urinary L-FABP as a mortality predictor in < 5-year-old children with sepsis in Bangladesh. Pediatr. Int. 2016, 58, 185–191. (In English) [Google Scholar] [CrossRef] [PubMed]
- Suetrong, B.; Walley, K.R. Lactic Acidosis in Sepsis: It’s Not All Anaerobic: Implications for Diagnosis and Management. Chest 2016, 149, 252–261. (In English) [Google Scholar] [CrossRef]
- Kabra, R.; Acharya, S.; Shukla, S.; Kumar, S.; Wanjari, A.; Mahajan, S.; Gaidhane, S.A.; Bhansali, P.J.; Wasnik, P. Serum Lac-tate-Albumin Ratio: Soothsayer for Outcome in Sepsis. Cureus 2023, 15, e36816. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).