Abstract
Background: Patients with hormone receptor-positive (HR+)/HER2- metastatic breast cancer (mBC) generally receive hormonal therapy (HT) combined with CDK4/6 inhibitors (CDK4/6i). Despite this treatment, resistance mechanisms to CDK4/6i emerge and the majority of these patients experience disease progression (PD). This highlight the necessity to uncover the resistance mechanism to CDK4/6i through the identification of specific biomarkers. The primary objective is to assess the accuracy and feasibility of a novel multi-gene target panel NGS assay on circulating tumor DNA (ctDNA) to detect molecular alterations of AKT1, ERBB2, ESR1, KRAS, PIK3CA, and TP53 genes in women with BC undergoing HT plus CDK4/6i treatment. Secondarily, the study aims to explore the relationship between genomic profiling and clinical outcomes. Materials and Methods: Plasma samples were collected from 16 patients diagnosed with advanced/locally advanced HR+/HER2- BC at 2 time points: T0 (baseline) and at T1 (3 months after CDK4/6i treatment). Starting from 2 mL of plasma, ctDNA was isolated and libraries were set up using the Plasma-SeqSensei (PQS)® Breast Cancer IVD Kit, sequenced on Nextseq 550 and analyzed using the Plasma-SeqSensei™ IVD Software®. Results: Among the five patients who presented PD, three had PIK3CA mutations and, of these, two showed a higher mutant allele frequency (MAF) at T1. In three patients with stable disease and in eight patients with partial response, the MAF of the detected alterations decreased dramatically or disappeared during CDK4/6i treatment. Conclusions: Based on our findings, the liquid biopsy analysis using the PQS panel seems to be both feasible and accurate, demonstrating a strong sensitivity in detecting mutations. This exploratory analysis of the clinical outcome associated to the mutational status of patients highlights the potential of molecular analysis on liquid biopsy for disease monitoring, although further validation with a larger patient cohort is necessary to confirm these preliminary observations.
1. Introduction
Breast cancer (BC) is the most commonly diagnosed cancer and is a heterogeneous disease, classified into many subgroups according to the presence of hormone receptors, Ki-67, and HER2 expression [1]. About 70% of BCs are estrogen receptor (ER)- and progesterone receptor (PgR)-positive. Endocrine therapy is the gold standard of treatment; however, around 30–50% of patients with BC will relapse [2]. Around 15–20% of ER+ BC patients are intrinsically resistant to treatment, and an additional 30–40% become resistant during treatment. Patients exhibiting resistance show elevated mutation rates and the presence of resistant subclones, suggesting an increased risk of mortality or disease recurrence [3,4,5,6]. Recently, the introduction of CDK4/6 inhibitors (CDK4/6i) has significantly improved the outcome for patients with hormone receptor-positive (HR+) BC in advanced stages, by targeting the cell cycle machinery [4,5]. Approved drugs such as palbociclib, ribociclib, and abemaciclib are now used together with aromatase inhibitors or fulvestrant for the treatment of HR+ metastatic BC patients. Despite these advancements, a substantial number of patients develop disease progression (PD), highlighting the urgent need to explore the underlying mechanisms of resistance to CDK4/6i. The primary objective of this study is to assess the feasibility and accuracy of a new multi-gene next-generation sequencing (NGS) panel designed to identify genomic alterations in AKT1, ERBB2, ESR1, KRAS, PIK3CA, and TP53 genes in circulating tumor DNA (ctDNA) extracted from the plasma of women receiving hormonal therapy (HT) and CDK4/6i. Furthermore, we aim to explore the relationship between genomic profiling and patient clinical outcomes.
2. Materials and Methods
Plasma samples were retrospectively collected from 16 patients with advanced/locally advanced HR+/HER2- BC, enrolled at IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori” (Meldola, Italy). The cohort of patients was selected based on CDK4/6i treatment and in relation to response after 3 months of treatment. The samples were collected at two time points: at baseline (T0) and after three months of CDK4/6i treatment (T1). CtDNA was isolated from 2 mL of plasma using QIAamp Circulating Nucleic Acid Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Plasma-Seqsensei (PQS)® Breast Cancer IVD Kit (Sysmex Inostics, GmbH, Hamburg, Germany) was used for library preparation, which covers 3623 different COSMIC mutations within selected hotspots (3070 in TP53), and sequenced using Illumina NEXTSEQ550 platform (Illumina, San Diego, CA, USA). The NGS data were analyzed using Plasma-SeqSensei™ IVD Software® (v1.2.2) (Supplementary Table S1). Sequences were aligned to the human reference genome hg19 (GRCh37), and an average read of 21765306 assigned to UID families with sufficient size was reached. The software classified the alterations according to somatic or germline origin. Mutations were listed by the software as potential germline mutations when they were present at an MAF >40% to ≤60% (heterozygous) or ≥90% (homozygous). Box plots and KM plots were generated using SRplot [7].
3. Results
Starting from a very low input of DNA (average 15.7 ng), 96.9% of the sequenced samples passed the four essential parameters (positive and negative controls, sequencing depth, and DNA quantification) quality control (QC) of the Plasma-SeqSensei™ IVD Software®. The test achieved a sensitivity of 95.2% and an optimal percentage in terms of specificity (Supplementary Table S2 and Figure S1). A robust quantification of tumor-specific sequences over a broad dynamic range was achieved using the PQS technology with an internal quantifier, which allowed a detection limit of up to six mutant molecules (MM). Of 16 patients, 5 presented PD, 3 presented stable disease (SD), and 8 presented partial response (PR) after 3 months of CDK4/6i, as shown in Table 1.

Table 1.
Patient and tumor characteristics.
Interestingly, of the five patients with PD, three had mutations in PIK3CA and one in ESR1 genes in the T0 sample, as described in Figure 1A. Of these three PIK3CA-mutated patients with PD, two exhibited an increased mutant allele frequency (MAF) or MM in their T1 samples. One of these patients showed a similar trend in terms of MAF improvement for both PIK3CA and ERBB2 mutations, while the other patient developed 2 novel TP53 mutations during treatment (Figure 1A,B and Supplementary Figure S2).
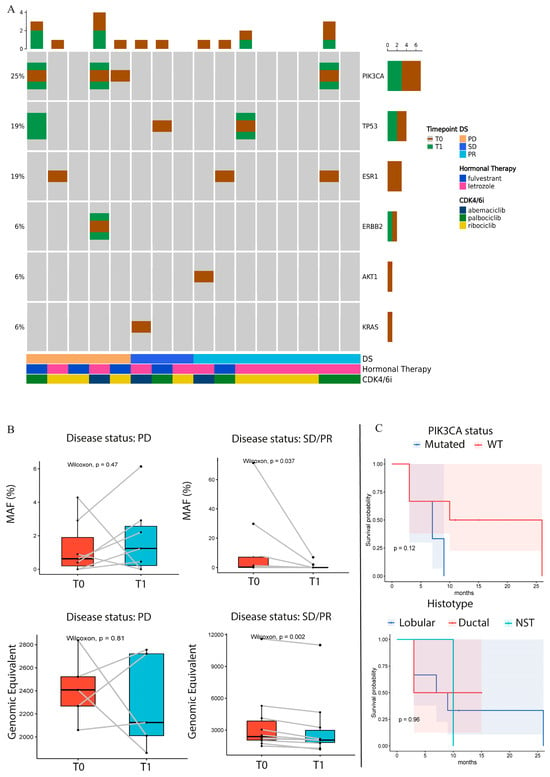
Figure 1.
(A) Oncoprint of identified variants (T0: baseline, T1: after 3 months, DS: disease status at T1). Columns represent individual plasma samples and are sorted by disease status (DS) and time points. Each gene affected by alterations is reported and sorted by frequency, which indicates the proportion of patients in whom the gene is mutated on the total number of patients (indicated on the left). The different colors represent the different time points (brown for T0, green for T1). The bar plots at the top and right of the Oncoprint indicate the count of events found, respectively, in each sample and in each gene. In the lower part of the Oncoprint, the disease status (DS), hormonal therapy, and CDK4/6i are indicated for each sample. PD: disease progression, SD: stable disease, PR: partial response. (B) The box plot shows the Wilcoxon test applied to the MAF% trend of alterations between T0 and T1 in patients who presented PD and patients with SD or PR. The box plot in the lower part of the figure shows the Wilcoxon test applied to the genomic equivalent of T0 and T1 samples in patients with PR or SD/PR. (C) Patient response in terms of PD or SD/PR. The cohort was divided into PI3KCA-mutated or WT in the upper KM plot and into lobular, ductal, and NST in the lower KM plot. T0: baseline; T1: collection time after 3 months of CDK4/6i treatment; PD: disease progression; SD: stable disease; PR: partial response; DS: disease status at T1; MAF: molecules allele frequency; WT: wild-type; NST: no special type.
On the contrary, patients with SD and PR showed a significant decrease in the MAF of T1; in particular, the AKT1 and the ESR1 mutations disappeared during treatment and the same trend was observed for TP53 and PI3KCA (Figure 1B, Supplementary Table S1 and Figure S2). The genomic equivalent calculated by the software, showed a significant decrease in patients with SD or PR, compared to the patients with PD. Interestingly, one patient with PD was wild-type (WT) for the analyzed genes, but the GE increased from T0 to T1 (Figure 1B). The patient in PR with PI3KCA alteration showed three novel ESR1 mutations in the plasma sample collected during treatment. Finally, patients with alterations in PIK3CA were significantly associated with worse PFS, and the same trend was observed in the OS of the BC population treated with HT in online resources (Supplementary Figure S3). In addition, we performed a PFS analysis of the case series classified into different histotypes; lobular subtypes showed the best outcome (Figure 1C).
4. Discussion and Conclusions
The recent exploratory analyses of the PALOMA-3 trial indicate that OS improvement was maintained (>6 years of follow up) in HR+/HER2- advanced BC patients treated with palbociclib and fulvestrant, supporting this combination as a standard of care for these patients [8]. While the introduction of CDK4/6i (palbociclib, ribociclib, and abemaciclib) has led to improved progression-free survival in HR+/HER2- BC patients, research has shown that the presence of mutations involved in resistance pathways can result in reduced sensitivity to the related drugs [4,5,6]. Then, there is the need for the identification of molecular biomarkers that may be able to select responsive patients. The PADA-1 trial was the first trial that demonstrated the clinical utility of monitoring ESR1 mutations in liquid biopsy, with a doubled PFS in ESR1-mutated patients who switched from AI-palbociclib to fulvestrant–palbociclib [9]. For the first time in Italy, we analyzed the accuracy of this novel NGS target panel assay (AKT1, ERBB2, ESR1, KRAS, PIK3CA, and TP53) on the plasma ctDNA of mBC patients treated with HT plus CDK4/6i. In the present study, we identified four alterations in PIK3CA, making it the most altered gene. Interestingly, as expected, three out of four of the PIK3CA mutations were found in patients with PD. We analyzed the survival trend of our entire cohort, considering alterations in PIK3CA as well as a larger online dataset of patients treated with HT. In both cases, the trend of response in patients with PIK3CA mutations was worse compared to those with WT PIK3CA. Consistent with the literature data, approximately 40% of HR+ BC cases exhibit activating mutations in the PIK3CA subunit of the PI3K gene, such as p.E545K, p.E542K, and p.H1047R [10,11]. Additionally, for the PD subgroup of patients, we observed a trend of improvement in the MAF value from T0 to T1, contrarily to patients with SD and PR. This trend in patients with PD involved both PIK3CA and ERBB2 genes, supporting the association between treatment response and the increase in resistance alterations detected in liquid biopsies during patient monitoring [12,13]. For the patient with double mutations in PIK3CA and ERBB2 genes, the increase in MAF during HT plus CDK4/6i may have significant and immediate clinical implications due to the novel targeted therapies available for HER2+ BC, such as trastuzumab deruxtecan [14,15].
According to MAF value changes, the GE values (3.3 pg of human DNA) also followed the same trend both in the PD and in the SD/PR subgroups of patients, characterized by a consistent increase and decrease in GE values, respectively. Notably, the only patient with PD and an absence of gene alterations was characterized by an increase in GE amount from T0 to T1. The literature data show that ctDNA fluctuations are associated with treatment response [16,17]. Despite not analyzing the ctDNA fraction in relation to patient response, our pilot study suggests that changes in GE values may also indicate treatment response during monitoring. Despite the significant decrease in MAF and GE values for SD/PR subgroups, 67% of patients with SD and 50% of patients with PR showed alterations in the 6 genes analyzed. KRAS and AKT1 mutations were present only in these subgroups, with MAF values of 1.35% and 8.91%, respectively. Both KRAS and AKT1 alterations, as a part of the PI3K/AKT/mTOR signaling transduction pathway, are associated with resistance mechanisms that arise during HT plus CDK4/6i treatments [18,19]. Nevertheless, these alterations disappeared during treatment, supporting the hypothesis of a good response to treatments, as reported in the literature for KRAS alterations [20]. We are aware that our panel permits the evaluation of only six genes; however, they are among the most frequently altered genes in patients who developed resistance mechanisms to HT plus CDK4/6i and for whom therapeutic drugs are available. Larger genomic analyses could help in understanding the interactions among the possible genes involved. Finally, consistent with literature data, our preliminary findings showed a better survival in patients with lobular BC than ductal BC. Despite this result, the assessment of molecular evaluation during treatments is necessary for ductal and lobular BC patients, considering the problem of the late recurrence also for the latter [21,22,23].
Considering the tumor heterogeneity and the small size of our population, testing for mutations in 6 genes may not be sufficient to have a full knowledge of all mechanisms of resistance given that other genes may be involved [24]. Despite these limitations, the liquid biopsy analysis using PQS was feasible and accurate. The assay passed several QC checks and permitted the detection of gene alterations up to 0.06% of the MAF value. Our exploratory secondary aim was to investigate the association between genomic alterations and clinical outcomes. Our findings suggest a trend of association between PD and SD/PR subgroups and the presence or absence of mutations. Consistent with literature data, the presence of PIK3CA alterations was associated with worse treatment response. Decreased and increased MAF values showed a promising role for disease monitoring. Our preliminary findings suggest that molecular analysis in liquid biopsy by using the novel NGS assay was feasible and accurate, and can be useful for a real-time evaluation of the disease. An enlarged case series of patients is needed to validate our preliminary results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines12102183/s1, Figure S1: Quality parameters generated by Plasma-SeqSensei™ IVD Software. Figure S2: Box plot of mutant molecules in T0 and T1 in PD and SD/PR patients. Figure S3: OS of PIK3CA-mutated and WT BC population. Table S1: Patients’ clinical and genomic data. Table S2. Quality parameters of sequencing.
Author Contributions
Conceptualization, S.B., P.U. and U.D.G.; Methodology, A.V.; software, A.V. and D.A.; validation, A.V., D.A. and S.B.; formal analysis, A.V. and S.B.; investigation, A.V., S.B. and R.M.; resources, C.G., R.M., M.P. and F.M.; data curation, A.V. and S.B.; writing—original draft preparation, A.V. and S.B.; writing—review and editing, A.V., C.G., M.P., D.A., F.M., R.M., P.U. and S.B.; visualization, A.V., D.A. and S.B.; supervision, P.U., G.M., U.D.G. and S.B.; project administration, S.B.; funding acquisition, S.B. and U.D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All methods used in our studies involving human participants adhered strictly to the ethical guidelines of our institutional research committee, aligning with the principles outlined in the 1964 Helsinki declaration and its subsequent modifications or equivalent ethical norms. This prospective study, with protocol code IRST B114 (L3P2290), was approved on 10/06/2020 by the Institutional Review Board of IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori”, Meldola, Italy.
Informed Consent Statement
All patients, with the exception of those who died or were lost to follow-up, gave written informed consent for the use of their medical record data for retrospective research.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Acknowledgments
This research was partially supported by Sysmex in terms of reagents. We give thanks for the contribution of Ricerca Corrente by the Italian Ministry of Health within the research line 3.
Conflicts of Interest
U.D.G.: Consultant: Janssen, Astellas Pharma, Sanofi, Bayer, Pfizer, Bristol-Myers Squibb, Novartis, Ipsen, Merck; Institutional funding: Roche, Sanofi, AstraZeneca. M.P.: reports honoraria for educational events/materials from Novartis, Daiichy-Sankyo, Gilead and travel, accommodations, and/or expenses from grants from Novartis, AstraZeneca, and Lilly.
References
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Szostakowska, M.; Trębińska-Stryjewska, A.; Grzybowska, E.A.; Fabisiewicz, A. Resistance to endocrine therapy in breast cancer: Molecular mechanisms and future goals. Breast Cancer Res. Treat. 2019, 173, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.P.; Chan, T.E.; Lombardo, Y.; Corleone, G.; Rotmensz, N.; Bravaccini, S.; Rocca, A.; Pruneri, G.; McEwen, K.R.; Coombes, R.C.; et al. Single-cell transcriptomics reveals multi-step adaptations to endocrine therapy. Nat. Commun. 2019, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Palleschi, M.; Maltoni, R.; Ravaioli, S.; Vagheggini, A.; Mannozzi, F.; Fanini, F.; Pirini, F.; Tumedei, M.M.; Barzotti, E.; Cecconetto, L.; et al. Ki67 and PR in Patients Treated with CDK4/6 Inhibitors: A Real-World Experience. Diagnostics 2020, 10, 573. [Google Scholar] [CrossRef]
- Maltoni, R.; Roncadori, A.; Balzi, W.; Mazza, M.; Nicolini, F.; Palleschi, M.; Ulivi, P.; Bravaccini, S. An Italian Real-World Study Highlights the Importance of Some Clinicopathological Characteristics Useful in Identifying Metastatic Breast Cancer Patients Resistant to CDK4/6 Inhibitors and Hormone Therapy. Biomedicines 2024, 12, 498. [Google Scholar] [CrossRef]
- Barozzi, I.; Slaven, N.; Canale, E.; Lopes, R.; Barbosa, I.A.M.; Bleu, M.; Ivanoiu, D.; Pacini, C.; Mensa, E.; Chambers, A.; et al. A functional survey of the regulatory landscape of estrogen-receptor-positive breast cancer evolution. Cancer Discov. 2024, 14, 1612–1630. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Rugo, H.S.; Im, S.-A.; Slamon, D.J.; Harbeck, N.; Bondarenko, I.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Women with HR+/HER2− ABC: Updated Exploratory Analyses of PALOMA-3, a Double-blind, Phase III Randomized Study. Clin. Cancer Res. 2022, 28, 3433–3442. [Google Scholar] [CrossRef]
- Bidard, F.C.; Hardy-Bessard, A.-C.; Dalenc, F.; Bachelot, T.; Pierga, J.-Y.; Sabatier, R.; Dubot, C.; Frenel, J.-S.; Ferrero, J.M.; Ladoire, S.; et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022, 23, 1367–1377. [Google Scholar] [CrossRef]
- Shimoi, T.; Hamada, A.; Yamagishi, M.; Hirai, M.; Yoshida, M.; Nishikawa, T.; Sudo, K.; Shimomura, A.; Noguchi, E.; Yunokawa, M.; et al. PIK3CA mutation profiling in patients with breast cancer, using a highly sensitive detection system. Cancer Sci. 2018, 109, 2558–2566. [Google Scholar] [CrossRef]
- Mosele, F.; Stefanovska, B.; Lusque, A.; Dien, A.T.; Garberis, I.; Droin, N.; Le Tourneau, C.; Sablin, M.-P.; Lacroix, L.; Enrico, D.; et al. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann. Oncol. 2020, 31, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Lastraioli, E.; Bettiol, A.; Iorio, J.; Limatola, E.; Checcacci, D.; Parisi, E.; Bianchi, C.; Arcangeli, A.; Iannopollo, M.; Di Costanzo, F.; et al. Evaluation of RAS Mutational Status in Liquid Biopsy to Monitor Disease Progression in Metastatic Colorectal Cancer Patients. Cells 2023, 12, 1458. [Google Scholar] [CrossRef]
- Elez, E.; Chianese, C.; Sanz-García, E.; Martinelli, E.; Noguerido, A.; Mancuso, F.M.; Caratù, G.; Matito, J.; Grasselli, J.; Cardone, C.; et al. Impact of circulating tumor DNA mutant allele fraction on prognosis in RAS-mutant metastatic colorectal cancer. Mol. Oncol. 2019, 13, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E., Jr.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus Adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Assaf, Z.J.F.; Zou, W.; Fine, A.D.; Socinski, M.A.; Young, A.; Lipson, D.; Freidin, J.F.; Kennedy, M.; Polisecki, E.; Nishio, M.; et al. A longitudinal circulating tumor DNA-based model associated with survival in metastatic non-small-cell lung cancer. Nat. Med. 2023, 29, 859–868. [Google Scholar] [CrossRef]
- Amato, O.; Giannopoulou, N.; Ignatiadis, M. Circulating tumor DNA validity and potential uses in metastatic breast cancer. NPJ Breast Cancer 2024, 10, 21. [Google Scholar] [CrossRef]
- Asghar, U.S.; Kanani, R.; Roylance, R.; Mittnacht, S. Systematic Review of Molecular Biomarkers Predictive of Resistance to CDK4/6 Inhibition in Metastatic Breast Cancer. JCO Precis. Oncol. 2022, 6, e2100002. [Google Scholar] [CrossRef]
- Scheidemann, E.R.; Shajahan-Haq, A.N. Resistance to CDK4/6 Inhibitors in Estrogen Receptor-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 12292. [Google Scholar] [CrossRef]
- Raimondi, L.; Raimondi, F.M.; Pietranera, M.; Di Rocco, A.; Di Benedetto, L.; Miele, E.; Lazzeroni, R.; Cimino, G.; Spinelli, G.P. Assessment of Resistance Mechanisms and Clinical Implications in Patients with KRAS Mutated-Metastatic Breast Cancer and Resistance to CDK4/6 Inhibitors. Cancers 2021, 13, 1928. [Google Scholar] [CrossRef]
- Iorfida, M.; Maiorano, E.; Orvieto, E.; Maisonneuve, P.; Bottiglieri, L.; Rotmensz, N.; Montagna, E.; Dellapasqua, S.; Veronesi, P.; Galimberti, V.; et al. Invasive lobular breast cancer: Subtypes and outcome. Breast Cancer Res. Treat. 2012, 133, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lei, C.; Zhang, Y.; Zhang, J.; Ji, F.; Pan, W.; Zhang, L.; Gao, H.; Yang, M.; Li, J.; et al. Comparison of Overall Survival Between Invasive Lobular Breast Carcinoma and Invasive Ductal Breast Carcinoma: A Propensity Score Matching Study Based on SEER Database. Front. Oncol. 2020, 10, 590643. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Ishiguro, J.; Kotani, H.; Hisada, T.; Ichikawa, M.; Gondo, N.; Yoshimura, A.; Kondo, N.; Hattori, M.; Sawaki, M.; et al. Comparison of clinical outcomes between luminal invasive ductal carcinoma and luminal invasive lobular carcinoma. BMC Cancer 2016, 16, 248. [Google Scholar] [CrossRef] [PubMed]
- Brett, J.O.; Spring, L.M.; Bardia, A.; Wander, S.A. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021, 23, 85. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).