Abstract
Drug hypersensitivity reactions are classified into immediate and delayed types, according to the onset time. In contrast to the immediate type, delayed drug hypersensitivity mainly involves T lymphocyte recognition of the drug antigens and cell activation. The clinical presentations of such hypersensitivity are various and range from mild reactions (e.g., maculopapular exanthema (MPE) and fixed drug eruption (FDE)), to drug-induced liver injury (DILI) and severe cutaneous adverse reactions (SCARs) (e.g., Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP)). The common culprits of delayed drug hypersensitivity include anti-epileptics, antibiotics, anti-gout agents, anti-viral drugs, etc. Delayed drug hypersensitivity is proposed to be initiated by different models of molecular recognition, composed of drug/metabolite antigen and endogenous peptide, HLA presentation, and T cell receptor (TCR) interaction. Increasing the genetic variants of HLA loci and drug metabolic enzymes has been identified to be responsible for delayed drug hypersensitivity. Furthermore, preferential TCR clonotypes, and the activation of cytotoxic proteins/cytokines/chemokines, are also involved in the pathogenesis of delayed drug hypersensitivity. This review provides a summary of the current understanding of the molecular recognition, genetic susceptibility, and immune mediators of delayed drug hypersensitivity.
1. Introduction
Drug hypersensitivity reactions are initiated by exposure to the drug within the therapeutic range, and present in immune-mediated characteristics and symptoms [1,2]. Most of the reactions are unpredictable adverse drug reactions (ADRs) and affect more than 7% of the general population worldwide [3]. Drug hypersensitivity reactions involve specific antibodies or T cell receptors (TCR). According to its definition by the Nomenclature Review Committee of the World Allergy Organization, drug hypersensitivity accounts for 15% of all kinds of drug-related adverse reactions [1,3,4]. The International Consensus on Drug Allergies (ICON) classifies drug hypersensitivity reactions into immediate and non-immediate types, according to the onset time (Table 1). The immediate type usually refers to the symptoms appearing within 1–6 h after exposure to the suspected drugs. The common presentations of immediate-type drug hypersensitivity include angioedema, urticaria and anaphylaxis. The frequent culprits for immediate drug hypersensitivity include non-steroid anti-inflammatory drugs (NSAIDs), neuromuscular blocking agents (NMBA), aspirin, antibiotics, and vaccines (Table 1). By comparison, non-immediate type hypersensitivity frequently presents as a delayed-type drug reaction, in which the onset time is days to weeks after an initial exposure to the culprit drugs [1,5,6,7,8]. Delayed-type drug hypersensitivity frequently involves skin reactions, ranging from mild reactions (e.g., maculopapular exanthema (MPE), and fixed drug eruption (FDE)) to severe cutaneous adverse reactions (SCARs) (e.g., drug reaction with eosinophilia and systemic symptoms (DRESS) (also called DiHS, drug-induced hypersensitivity syndrome), Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and acute generalized exanthematous pustulosis (AGEP)) (Table 1). In addition, there are other delayed drug reactions, such as drug-induced liver injury (DILI), or drug-specific reaction (e.g., abacavir hypersensitivity) [9,10,11]. The mortality rates for SJS and TEN are high, i.e., 5–10% for SJS, 30% for SJS/TEN overlap, and 30–50% for TEN [12,13,14,15]. The mortality rate is approximately 4% for AGEP and 10% for DRESS [10,16]. The common causative drugs for delayed-type drug hypersensitivity are anti-epileptic drugs (AED), antibiotics, allopurinol, anti-viral agents, and NSAIDs (Table 1).

Table 1.
Classification and clinical symptoms of drug hypersensitivity reactions based on the definition by ICON.
2. Classification of Delayed-Type Drug Hypersensitivity
The delayed-type hypersensitivity reaction is also called a type IV reaction, classified by Gell and Coombs, and mainly involves T cell-antigen recognition, accompanied by the activation of other leukocytes [17]. According to the types of mostly involved T cells and their downstream mediators, the type IV reaction is sub-grouped as IVa, IVb, IVc, and IVd [18] (Figure 1). Type IVa, IVb, and IVd are mediated by TH1, TH2, and IL-8-producing TH cells, respectively, and involve other inflammatory cells, such as macrophages, eosinophils, and neutrophils. The type IVa reaction mainly involves TH1 cell activation, which releases chemokines and cytokines, such as IFN-γ and TNF-β, to recruit and activate macrophages to produce inflammatory mediators, such as TNF-α [19]. Type IVa drug hypersensitivity is suggested to be associated with MPE and FDE. The type IVb reaction mainly involves TH2 cell activation, which releases IL4, IL13, IL-5, and eotaxin to activate eosinophils and mast cells. Type IVb drug hypersensitivity is suggested to mediate the pathogenesis of DRESS/DiHS and DILI [20,21,22,23]. The CXCL8/IL-8-producing Th cells mediate the type IVd reaction via producing CXCL8 and GM-CSF to activate neutrophils, which are predominately in drug-related AGEP. By comparison, type IVc drug hypersensitivity mainly involves cytotoxic T cells; here, cytotoxic T lymphocytes (CTL) directly kill target cells by releasing cytotoxic cytokines, including granulysin, granzymes, and perforin, and also by cellular contact through a Fas/FasL pathway [24]. Type IVc drug hypersensitivity is mainly involved in SJS/TEN (Figure 1).
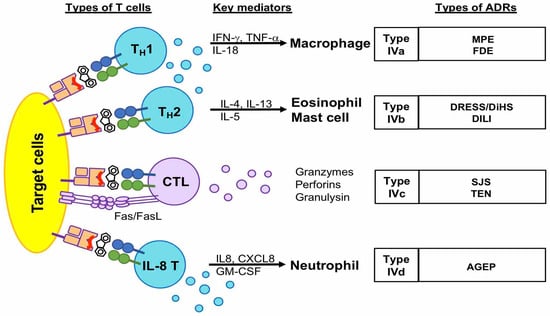
Figure 1.
The characteristics of type IV (delayed-type) drug hypersensitivity. Delayed-type drug hypersensitivity is mostly induced by T cells. They are subdivided into four types (IVa, IVb, IVd, and IVc) according to the types of T cells, such as TH1, TH2, IL8 TH cells, and cytotoxic T cells. The types IVa, IVb, and IVd are mediated by TH cells and the activation of downstream granulocytes, such as macrophages, mast cells, eosinophils, and neutrophils. By comparison, type IVc is mainly mediated by cytotoxic T cells, which induce target cell death by releasing cytokines, such as granulysin, granzyme B, and perforin, or direct interaction of Fas/FasL. Abbreviations: FDE, fixed drug eruption; MPE, maculopapular eruption; AGEP, acute generalized exanthem pustulosis; DRESS, drug reactions with eosinophilia and systemic symptoms; DiHS, drug-induced hypersensitivity syndrome; DILI, drug-induced liver injury; SJS, Stevens–Johnson syndrome; TEN, toxic epidermal necrolysis.
3. Proposed Models of Molecular Recognition in Delayed Drug Hypersensitivity
Delayed drug hypersensitivity reactions are initiated by a T cell receptor (TCR) recognizing the drug/metabolite antigen(s). The drug/metabolite antigen may interact with endogenous peptides covalently or noncovalently. The antigen could be presented with a human leukocyte antigen (HLA) to TCR, in order to induce T cell-mediated hypersensitivity reactions. There are four hypotheses proposed for the molecular recognition of drugs by TCR in delayed-type drug hypersensitivity: (1) the hapten hypothesis, (2) the pharmacological interaction with immune receptors (p-i) concept, (3) the altered peptide repertoire model, and (4) altered TCR repertoire model (Figure 2).
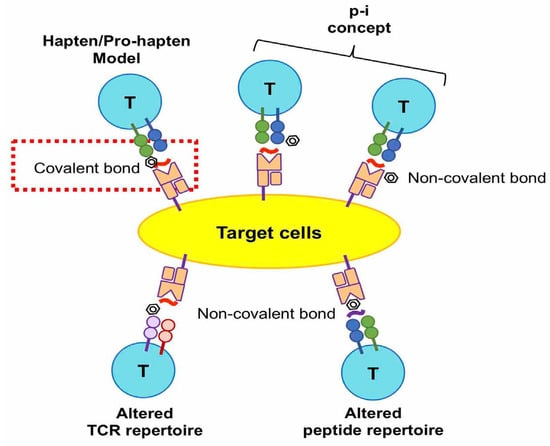
Figure 2.
Four hypotheses proposed for the molecular recognition of drugs by TCR in delayed type drug hypersensitivity. The hapten model hypothesizes that a drug or metabolite covalently binds to an endogenous peptide and leads to an immunogenic response. The other three hypotheses suggest non-covalent interactions between drugs, endogenous peptides, TCR, and HLA. The pharmacological interaction with the immune receptor (p-i) concept hypothesizes that the drug interacts with TCR, HLA, or both non-covalently. Altered TCR repertoire and altered peptide repertoire models propose that the drug non-covalently binds on TCR or HLA in the groove region.
- (1)
- The hapten/pro-hapten hypothesis describes that the causative drugs or the reactive metabolites are too small, with a molecular weight of fewer than 1000 daltons, to be immunogenic and recognized by the immune receptors. The haptens become immunogenic by the covalent binding of drug/metabolite to the endogenous peptides or proteins to form a hapten–carrier complex. The antigenic complex could be recognized by an antibody, or be presented on the HLA molecule and then recognized by TCR, resulting in the induction of drug-specific cellular or humoral immune responses. This hypothesis has been valid in cases of penicillin-induced ADRs [25,26,27]. The major antigenic determinant of penicillin-induced hypersensitivity is penicilloyl polylysine. This structure is formed by the covalent bond of a β-lactam ring to lysine residues in proteins [27,28]. Regarding the delayed drug hypersensitivty, penicilloyl peptides were found to be recognized as T-cell antigenic determinants in the penicillin allergy [25].
- (2)
- The pharmacological interaction with the immune receptor (p-i) concept postulates that drugs may noncovalently interact with the HLA, TCR, or endogenous proteins (or peptides) [29]. Our previous studies showed that carbamazepine (CBZ), one of the aromatic antiepileptic drugs, directly interacts with HLA-B*15:02 protein. This interaction of CBZ presentation on HLA-B*15:02 does not involve intracellular antigen processing or drug metabolism [30]. In addition, we showed another similar example for the interaction between oxypurinol and HLA-B*58:01. Oxypurinol, a reactive metabolite of allopurinol, can directly and immediately activate specific T cells through HLA-B*58:01; this is without intracellular antigen processing [31]. We demonstrated the key residuals of oxypurinol recognition on the HLA-B*58:01 cleft [32].
- (3)
- The altered peptide repertoire model refers to the causative drugs occupying a position in the peptide-binding groove of the HLA protein, the alteration of the properties of the binding cleft, and the peptide specificity of HLA binding. This model has been suggested by studies on abacavir hypersensitivity [33,34]. Abacavir binds to the F-pocket of HLA-B*57:01 and changes the properties of conformation and structure in the antigen-binding cleft. The interaction between the drug and HLA causes the altered peptide repertoire, resulting in TCR recognition, T cell activation, and a drug hypersensitivity reaction. The altered peptide repertoire causes a polyclonal activation of T cells and systemic manifestations resembling an autoimmune response [33,34].
- (4)
- The altered TCR repertoire model proposes that culprit drugs directly interact with TCR, and not with the peptides nor the HLA molecules. The antigenic molecules bind to specific TCRs and cause conformational change. The antigen-bound TCRs can interact with HLA-endogenous peptide complexes and elicit immune reactions [35]. In this model, the TCR repertoire is altered upon interaction with the drug/metabolite antigen. The drug antigen-bound TCR is essential in this model to induce drug hypersensitivity reactions [35].
In addition to the above four hypotheses, viral infection has been proposed to contribute to HLA/drug/TCR interactions, and viral peptides may be involved in the process of drug presentation and immune recognition, leading to drug hypersensitivity [26].
4. Genetic Susceptibility of Delayed Drug Hypersensitivity
Different approaches have been applied to explore the genetic susceptibility of drug hypersensitivity. The genetic variants involved in controlling (1) the immune response, especially the HLA alleles; (2) the drug metabolism enzymes for drug oxidation, conjugation, hydrolysis, and acetylation; and (3) the drug transporters or receptors, have been proposed to be associated with delayed-type drug hypersensitivity. The genetic susceptibility of delayed drug hypersensitivity showed drug-specific, phenotype-specific, and ethnic variation (Table 2, Table 3, Table 4, Table 5 and Table 6).
4.1. Genetic Susceptibility of Antiepileptics-Induced Hypersensitivity Reactions
We first reported that HLA-B*15:02 is a genetic marker for CBZ-induced SJS/TEN in Han Chinese patient populations in Taiwan in 2004 [36] (Table 2). This association has been further validated in other Asian countries, including Hong Kong, Singapore, Vietnam, Thailand, Malaysia, and India [37,38,39,40,41,42,43] (Table 2). We carried out a prospective study and showed that the genetic screening of HLA-B*15:02 before CBZ administration prevented the occurrence of CBZ-induced SJS/TEN [44]. None of the 4877 recruited patients who received CBZ treatment with preemptive pharmacogenomic testing developed SJS/TEN [44]. Additionally, we found HLA-B*57:01 was associated with CBZ-induced SJS/TEN in Europeans [45] (Table 2). By comparison, HLA-A*31:01 is associated with CBZ-induced MPE and DRESS, which was first reported in Han Chinese in Taiwan in 2006 [46]; this was then validated in different populations, including Europeans and Japanese [45,46,47,48,49,50,51] (Table 2).
Aside from being a risk allele for CBZ-induced SJS/TEN, HLA-B*15:02 has also been associated with SCARs induced by other antiepileptics that have a similar aromatic structure to CBZ, such as oxcarbazepine [52,53], phenytoin [40,53,54,55], and lamotrigine [56] (Table 2). In addition, HLA-A*32:01 was reported to be associated with oxcarbazepine-induced MPE in the Eastern Han Chinese population [57]. HLA-B*13:01 and B*51:01 are suggested to be related to phenytoin-induced SCARs in different studies in Asians, including Han Chinese, Japanese, and Malaysian patient populations [55,58] (Table 2). In addition to HLA alleles, we found that the loss of function in the allele of cytochrome P450 2C9 (CYP2C9), CYP2C9*3, affecting drug metabolism, was responsible for phenytoin-induced SCARs in Taiwan [58]. The genetic association was validated in the patient populations from Thailand and Japan [58,59,60] (Table 2). For lamotrigine-induced SCARs, HLA-A*31:01 and HLA-B*38:01 were reported to be risk alleles in patients of Asian or European descent [49,61] (Table 2).


Table 2.
Genetic variants associated with antiepileptics-induced hypersensitivity reactions.
Table 2.
Genetic variants associated with antiepileptics-induced hypersensitivity reactions.
| Causative Drugs | Reactions | Genetic Factors | Ethnicity | OR (95% CI) | p-Value | Reference |
|---|---|---|---|---|---|---|
| Carbamazepine (CBZ) | SJS/TEN | HLA-B*15:02 | Han Chinese | 2504 (126–49,522) | 3.13 × 10−27 | [36] |
| Thai | 25.5 (2.68–242.61) | 0.0005 | [40] | |||
| 7.27 (2.04–25.97) | 4.46 × 10−13 | [43] | ||||
| Indian | 71.40 (3.0–1698) | 0.0014 | [42] | |||
| Malaysian | 16.15 (4.57–62.4) | 7.87 × 10−6 | [41] | |||
| Vietnamese | 33.78 (7.55–151.03) | <0.0001 | [39] | |||
| Singaporean | 27.20 (2.67–∞) | 0.004 | [38] | |||
| HLA-B*57:01 | European | 9.0 (4.2–19.4) | 9.62 × 10−7 | [45] | ||
| DRESS | HLA-A*31:01 | Han Chinese | 23.0 (4.2–125) | <0.001 | [47] | |
| 6.86 (2.4–19.9) | 2.7 × 10−3 | [50] | ||||
| European | 49.9 (12.9–193.6) | 4.0 × 10−8 | [45] | |||
| 13.2 (8.4–20.8) | <0.001 | [47] | ||||
| 12.41 (1.27–121.03) | 3.5 × 10−8 | [48] | ||||
| 22.00 (1.03–1190.36) | 0.047 | [49] | ||||
| Japanese | 10.8 (5.9–19.6) | 3.64 × 10−15 | [51] | |||
| MPE/DRESS | HLA-A*31:01 | Han Chinese | 17.5 (4.6–66.5) | 0.0022 | [46] | |
| MPE | HLA-B*15:02 | Thai | 7.27 (2.04–25.97) | 0.0022 | [43] | |
| Oxcarbazepine (OXC) | SJS/TEN | HLA-B*15:02 | Han Chinese | 27.90 (7.84–99.23) | 1.12 × 10−9 | [52] |
| 80.7 (3.8–1714.4) | 8.4 × 10−4 | [53] | ||||
| MPE | HLA-A*32:01 | Han Chinese | 15.877 (1.817–138.720) | 0.004 | [57] | |
| Phenytoin (PHT) | SCARs | CYP2C9*3 | Taiwanese | 14.00 (6.75–29.02) | 0.00001 | [58] |
| Japanese | 8.88 (2.20–35.83) | |||||
| Malaysian | 5.60 (0.56–56.20) | |||||
| Thai | 4.30 (1.41–13.09) | <0.05 | [59] | |||
| Taiwanese, Japanese, Thai | 20.86 (9.03–48.20) | 1.22 × 10−13 | [60] | |||
| HLA-B*15:02 | Asian (Han Chinese, Japanese, Malaysian) | 5.0 (2.0–13) | 0.025 | [58] | ||
| SJS/TEN | HLA-B*15:02 | Han Chinese | 5.1 (1.8–15.1) | 0.0041 | [53] | |
| 3.50 (1.10–11.18) | 0.045 | [55] | ||||
| Thai | 18.5 (1.82–188.40) | 0.005 | [40] | |||
| Malaysian | 5.71 (1.41–23.10) | 0.016 | [54] | |||
| Lamotrigine (LTG) | SCARs | HLA-A*31:01 | Korean | 11.43 (1.95–59.77) | 0.0037 | [61] |
| HLA-B*38:01 | European | 147.00 (1.88–483) | 0.001 | [49] | ||
| SJS/TEN | HLA-B*15:02 | Han Chinese | 4.98 (1.43–17.28) | 0.01 | [56] | |
| AEDs (CBZ, LTG, PHT, etc.) | SCARs | HLA-B*15:02 | Han Chinese | 17.6 (2.9–105.2) | 0.001 | [37] |
Abbreviations: SJS/TEN, Stevens–Johnson syndrome/toxic epidermal necrolysis; DRESS, drug reaction with eosinophilia and systemic symptoms; MPE, maculopapular exanthema; OR, odds ratio; SCARs, severe cutaneous adverse reactions.
4.2. Genetic Susceptibility of Allopurinol Hypersensitivity
Allopurinol, a xanthine oxidase inhibitor, is the first-line drug to treat hyperuricemia and gout. Allopurinol is also one of the common culprit drugs to induce drug hypersensitivity. We first found HLA-B*58:01 to be associated with allopurinol-induced SCARs in Han Chinese people in Taiwan in 2005 [62] (Table 3). This association was further replicated and validated among various ethnicities, including European [63,64], Thai [65,66], Japanese [67], Korean [68], and African American [69] (Table 3). HLA-B*58:01 genetic screening has been shown to be a promising strategy for preventing allopurinol SCARs [70]. We further found that renal dysfunction, and increased plasma levels of the metabolite of allopurinol, i.e., oxypurinol, deteriorate the severity of allopurinol hypersensitivity [71]. This might explain the higher mortality rate of allopurinol-induced SCARs in patients with chronic kidney disease, because of the delayed clearance of oxypurinol. Furthermore, allopurinol-induced liver injury (DILI) was found to be associated with HLA-A*34:02, HLA-B*53:01, and HLA-B*58:01 [66,69] (Table 3). Some genomics studies validated the attribution of HLA-B*58:01 and proposed that other genetic variants, out of the HLA region, might also contribute to the development of allopurinol hypersensitivity [67] (Table 3).


Table 3.
Genetic variants associated with allopurinol-induced hypersensitivity reactions.
Table 3.
Genetic variants associated with allopurinol-induced hypersensitivity reactions.
| Reactions | Genetic Factors | Ethnicity | OR (95% CI) | p-Value | Reference |
|---|---|---|---|---|---|
| SJS/TEN | HLA-B*58:01 | Han Chinese | 580.3 (34.4–9780.9) | 4.7 × 10−24 | [62] |
| European | 80 (34–187) | <10−6 | [63] | ||
| Japanese | 62.8 (21.2–185.8) | 5.388 × 10−12 | [67] | ||
| Thai | 348.3 (19.2–6336.9) | 1.6 × 10−13 | [65] | ||
| 579.0 (29.5–11,362.7) | <0.001 | [66] | |||
| DRESS | HLA-B*58:01 | Han Chinese | 47.7 (18.2–125.4) | 1.0 × 10−26 | [71] |
| Thai | 430.3 (22.6–8958.9) | <0.001 | [66] | ||
| SCARs | HLA-B*58:01 | Korean | 97.8 (18.3–521.5) | 2.45 × 10−11 | [68] |
| HLA-B*58:01 | Han Chinese | 44.0 (21.5–90.3) | 2.6 × 10−41 | [71] | |
| European | 39.11 (4.49–340.51) | 5.9 × 10−4 | [64] | ||
| DILI | HLA-B*58:01, HLA-B*53:01 cluster | African-American, Caucasian, Hispanic | NA | 0.0007 | [69] |
| MPE | HLA-B*58:01 | Thai | 144.0 (13.9–1497.0) | <0.001 | [66] |
| Han Chinese | 8.5 (4.2–17.5) | 2.3 × 10−9 | [71] |
Abbreviations: SJS/TEN, Stevens–Johnson syndrome/toxic epidermal necrolysis; DRESS, drug reaction with eosinophilia and systemic symptoms; DILI, drug-induced liver injury; SCARs, severe cutaneous adverse reactions; MPE, maculopapular exanthema; NA, not available; OR, odds ratio.
4.3. Genetic Susceptibility of Antibiotics-Induced Hypersensitivity Reactions
Antibiotics can cause either immediate-type or delayed-type drug hypersensitivity. Antibiotic hypersensitivity has shown an immune-related genetic predisposition. The HLA-DRB3*02:02 allele, absent in Europeans, accounts for 83% of amoxicillin-induced MPE cases in Italy [72] (Table 4). A high level of HLA-DRB1*15:01 was observed in Europeans with amoxicillin-clavulanate-induced liver injury [73], and a high level of HLA-B*57:01 was observed in flucloxacillin-induced liver injury [74].
Co-trimoxazole, a combination of sulfamethoxazole (SMX) and trimethoprim (TMP), is associated with delayed drug hypersensitivity. HLA-B*38 was reported to be related to sulfamethoxazole-induced SJS/TEN in Europeans [63]. Kongpan T. et al. reported that carriers with HLA-B*15:02, HLA-C*06:02, or HLA-C*08:01 had an increased risk of co-trimoxazole-induced SJS/TEN (odds ratio: 11) [75]. By whole genome sequencing (WGS), our recent multi-country case-control study showed that HLA-B*13:01 was strongly associated with co-trimoxazole-induced SCARs in patients from Taiwan, Thailand, and Malaysia [76]. Notably, HLA-B*13:01 contributed to 85.4% of patients with co-trimoxazole-induced DRESS [76]. A multicentric study of the Thai population showed that HLA-B*15:02 and HLA-C*08:01 are associated with cotrimoxazole-SJS/TEN and HLA-B*13:01 in DRESS. Additionally, the haplotypes of HLA-A*11:01-B*15:02 and HLA-B*13:01-C*03:04 are associated with co-trimoxazole-induced SJS/TEN and DRESS, respectively [77]. Earlier studies indicate that gene variants involved in drug metabolisms, such as NAT2 [78,79,80,81,82] and GSTM1 [83] null genotypes, were associated with sulfonamide-induced hypersensitivity reactions. However, the associations were weak and lacked validation.
Other significant discoveries, regarding pharmacogenomic associations with antibiotics-induced SCARs, include HLA-A*32:01, is associated with vancomycin-induced DRESS in Caucasians [84], and HLA-B*13:01, associated with dapsone-induced DRESS in north-eastern and south-eastern Asians [85,86,87,88,89,90] (Table 4).


Table 4.
Genetic variants associated with antibiotics-induced hypersensitivity reactions.
Table 4.
Genetic variants associated with antibiotics-induced hypersensitivity reactions.
| Causative Drugs | Reactions | Genetic Factors | Ethnicity | OR (95% CI) | p-Value | Reference |
|---|---|---|---|---|---|---|
| Amoxicillin | MPE | HLA-DRB3*02:02 | European | 8.88 (3.37–23.32) 1 | <0.0001 | [72] |
| Co-amoxiclav | DILI | HLA-DRB1*15:01 | European | 2.59 (1.44–4.68) | 0.002 | [73] |
| Flucloxacillin | DILI | HLA-B*57:01 | European | 80.6 (22.8–284.9) | 8.97 × 10−19 | [74] |
| Sulfamethoxazole (SMX) | SJS/TEN | HLA-B*38 | European | 8.6 (3.5–21) | <0.003 | [63] |
| Co-trimoxazole (SMX/TMP) | SJS/TEN | HLA-B*15:02 | Thai | 3.91 (1.42–10.92) | 0.0037 | [75] |
| HLA-C*06:02 | 11.84 (1.24–566.04) | 0.0131 | ||||
| HLA-C*08:01 | 3.53 (1.21–10.40) | 0.0108 | ||||
| SCARs | HLA-A*11:01-B*15:02 haplotype, | Thai (HIV-infected patients only) | 4.36 (1.43–13.34) | 0.0108 | [77] | |
| HLA-B*13:01-C*03:04 haplotype | 3.77 (1.27–11.19) | 0.0251 | ||||
| HLA-B*13:01 | Han Chinese, Thai, Malaysia | 11.7 (5.7–24) | 1.3 × 10−13 | [76] | ||
| Vancomycin | DRESS | HLA-A*32:01 | European | NA | 1 × 10−8 | [84] |
| Dapsone | SCARs | HLA-B*13:01 | Thai | 39.00 (7.67–198.21) | 5.34 × 10−7 | [85] |
| 54.00 (7.96–366.16) | 0.0001 | [88] | ||||
| DRESS | HLA-B*13:01 | Han Chinese | 20.53 (11.55–36.48) | 6.84 × 10−25 | [86] | |
| Taiwanese, Malaysian | 49.64 (5.89–418.13) | 2.92 × 10−4 | [87] | |||
| HLA-B*13:01 | Korean | 73.67 (2.56–2119.93) | 0.012 | [89] | ||
| HLA-B*13:01 | Papua | 233.46 (1.7–67.7) | 7.11 × 10−9 | [90] |
Abbreviations: SJS/TEN, Stevens–Johnson syndrome/toxic epidermal necrolysis; DRESS, drug reaction with eosinophilia and systemic symptoms; SCARs, severe cutaneous adverse reactions; NA, not available; OR, odds ratio. 1 The odds ratio represents the increased risk of delayed type compared to immediate type reaction.
4.4. Genetic Susceptibility of Antiviral Agents-Induced Hypersensiticity
Abacavir is a nucleoside reverse transcriptase inhibitor, usually used in combined therapy for treating patients with HIV infection. Approximately 5–8% of European patients treated with abacavir developed immune-related ADR during the first six weeks of treatment [91]. HLA-B*57:01 was identified as a genetic predisposition for abacavir-related hypersensitivity in Caucasians in 2002 [92,93] (Table 5). The subsequent randomized clinical trials, recruiting 1956 patients from 19 countries, demonstrated that the carriage of HLA-B*57:01 could be a genetic predictor, in order to prevent abacavir hypersensitivity [94].
Nevirapine, a non-nucleoside reverse transcriptase inhibitor to treat HIV infection, has been reported to be associated with a hypersensitivity reaction; it has the clinical presentations of fever, a skin rash, or hepatitis. HLA-B*35:05 and HLA-Cw*04:01 were found to be related to a nevirapine-induced skin rash in Thailand [95,96] and nevirapine-induced SJS/TEN among Africans, respectively [97] (Table 5). Of note, HLA-Cw*04 was also found to be associated with cutaneous adverse reactions in multiple ethnicities [96] (Table 5). HLA-DRB1*01:01 was proposed to contribute to nevirapine-induced DRESS in patients in West Australia [98] (Table 5). Studies of multiple ethnicities show that the HLA-DRB1*01:01 allele is associated with DILI in white people [96] (Table 5). The other studies showed HLA-Cw*08 was associated with Nevirapine-induced hepatitis in Sardinian and Japanese populations [99,100]. The association between nevirapine-induced hepatotoxicity and HLA-Cw*04 was reported in Han Chinese people; this needs further validation [101]. In addition, an SNP (rs3099844) of the HCP gene was proposed to be associated with nevirapine-SJS/TEN in Africans [102] (Table 5).
Raltegravir, an HIV integrase inhibitor introduced in 2007, was associated with DRESS in Africans. HLA-B*53:01 was implicated as a risk allele of raltegravir-DRESS in the African population [103] (Table 5).


Table 5.
Genetic variants associated with antiviral agents-induced hypersensitivity reactions.
Table 5.
Genetic variants associated with antiviral agents-induced hypersensitivity reactions.
| Causative Drugs | Reactions | Genetic Factors | Ethnicity | OR (95% CI) | p-Value | Reference |
|---|---|---|---|---|---|---|
| Abacavir | DiHS | HLA-B*57:01 | Caucasians | 23.6 (8.0–70.0) | <0.0001 | [92] |
| 117 (29–481) | <0.0001 | [93] | ||||
| NA | NA | [94] | ||||
| Nevirapine | SJS/TEN | rs3099844 (HCP5) | Mozambique | 2.03 (na) | 0.039 | [102] |
| HLA-C*04:01 | African | 4.84 (2.71–8.61) | 8.47 × 10−8 | [97] | ||
| DiHS | HLA-Cw*04 | Thai | 2.43(1.22–4.84) | 0.17 | [96] | |
| NA | 0.0088 | [104] | ||||
| Asians, Blacks, Whites | 2.51 (1.73–3.62) | 8.7 × 10−6 | [96] | |||
| Han Chinese | 3.611 (1.135–11.489) | 0.030 | [101] | |||
| HLA-B*35:05 | Thai | 18.96 (4.87–73.44) | 4.6 × 10−6 | [95] | ||
| HLA-B*35 | Asians | 3.47 (1.58–7.61) | 0.053 | [96] | ||
| HLA-DRB1*01 | Whites | 3.02 (1.66–5.49) | 0.0074 | [96] | ||
| 4.8 (na) | 0.14 | [98] | ||||
| HLA-Cw*08 | Sardinian | NA | 0.05 | [99] | ||
| Japanese | NA | 0.03 | [100] | |||
| Raltegravir | DRESS | HLA-B*53:01 | African | NA | NA | [103] |
Abbreviations: SJS/TEN, Stevens–Johnson syndrome/toxic epidermal necrolysis; DiHS, drug-induced hypersensitivity syndrome; DRESS, drug reaction with eosinophilia and systemic symptoms; NA, not available; OR, odds ratio.
4.5. Genetic Susceptibility of Hypersensitivity Reactions to Anti-Thyroid Drugs and Methazolamide
Anti-thyroid drugs (ATD), including carbimazole and methimazole, have been reported to induce agranulocytosis, and their association with HLA genotypes has been found in different ethnicities. Table 6 lists the genetic variants associated with drug-induced agranulocytosis (DIA). Methimazole-induced agranulocytosis was associated with HLA-DRB1*08:03:02 in Japanese people [105] (Table 6). HLA-B*27:05, HLA-B*38:02, and HLA-DRB1*08:03 alleles were found to be related to ATD-induced agranulocytosis in Taiwan [106] (Table 6). HLA-B*38:02 and HLA-DRB1*08:03 alleles were reported to be associated with ATD-induced agranulocytosis in Han Chinese people [107,108] (Table 6). HLA-B*27:05 was reported in a European population [109], as well as in Han Chinese people from northern China [110] (Table 6).
Methazolamide, an intraocular pressure-lowering drug, may cause SJS/TEN in Asians. HLA-B*59:01 has been proposed to be associated with methazolamide-induced SJS/TEN in Korean, Japanese, and Han Chinese patients [111,112,113,114] (Table 6).


Table 6.
Genetic variants associated with hypersensitivity reactions to anti-thyroid drugs and methazolamide.
Table 6.
Genetic variants associated with hypersensitivity reactions to anti-thyroid drugs and methazolamide.
| Causative Drugs | Reactions | Genetic Factors | Ethnicity | OR//break//(95% CI) | p-Value | Reference |
|---|---|---|---|---|---|---|
| Methimazole | DIA | HLA-DRB1*08:03:02 | Japanese | 5.42 (na) | 0.002 | [105] |
| HLA-B*38:02, DRB1*08:03 haplotype | Han Chinese | 48.41 (21.66–108.22) | 3.32 × 10−21 | [107] | ||
| HLA-B*38:02 | 21.48 (11.13–41.48) | 6.75 × 10−32 | ||||
| HLA-DRB1*08:03 | 6.13 (3.28–11.46) | 1.83 × 10−9 | ||||
| Carbimazole/Methimazole | DIA | HLA-B*38:02:01 | Han Chinese | 265.5 (27.87–2528.0) | 2.5 × 10−14 | [108] |
| Carbimazole, Methimazole, Propylthiouracil | DIA | HLA-B*27:05 | Caucasian | 7.30 (3.81–13.96) | 1.91 × 10−9 | [109] |
| Methimazole, Propylthiouracil | DIA | HLA-B*27:05 | Han Chinese | 60.11 (3.27–1104.4) | 1.1 × 10−4 | [110] |
| HLA-B*38:02 | 6.55 (2.11–20.36) | 2.41 × 10−4 | ||||
| HLA-DRB1*08:03 | 3.95 (1.60–9.79) | 1.57 × 10−3 | ||||
| Methazolamide | SJS/TEN | HLA-B*59:01 | Han Chinese | 305.0 (11.3–8259.9) | 6.3 × 10−7 | [111] |
| Korean | 249.8 (13.4–4813.5) | <0.001 | [112] | |||
| Japanese | NA | NA | [114] | |||
| HLA-B*59:01 | Han Chinese | 146.00 (16.12–1321.98) | 6.19 × 10−10 | [113] | ||
| HLA-B*55:02 | 71.00 (7.84–643.10) | 1.43 × 10−4 |
Abbreviations: DIA, drug-induced agranulocytosis; SJS/TEN, Stevens–Johnson syndrome/toxic epidermal necrolysis; NA, not available; OR, odds ratio.
5. T Cell Receptor (TCR) Usage in Delayed Drug Hypersensitivity
In addition to HLA alleles, TCRs also play a crucial role in the pathogenesis of drug hypersensitivity. The preferential usage of TRBV genes and clonally-expanding CDR3 was observed in blister cells from skin lesions, and oxypurinol-cultured peripheral blood mononuclear cells of allopurinol-SCAR patients [115]. Recently, we identified a public αβ T cell receptor (TCR) from the skin blister cells of CBZ-SJS/TEN Asian and European patients [116]. The public TCR was composed of VFDNTDKLI and ASSLAGELF of CDR3 in TRA and TRB chains, respectively [116] (Table 7). This clonotype of TCR showed drug- and phenotype-specificity in an HLA-B*15:02-favored manner. Introducing T cells, with this TCR clonotype, to HLA-B*15:02 transgenic mice via the oral administration of CBZ, resulted in the development of SCARs symptoms. By comparison, HLA-B*15:02 transgenic mice received CBZ, but no adoptive T cell transfer showed SCAR symptoms. The data suggests that specific TCR recognizes the drug antigen and participates in SCAR. In addition, the results also support that HLA is insufficient to induce SCAR, and without the presence of drug-specific TCR, HLA-B*15:02 carriers are tolerant to CBZ [116]. Furthermore, a specific αβTCR pair was observed in HLA-B*13:01-restricted dapsone-induced drug-induced hypersensitivity syndrome (DiHS) [117]. Dapsone interacts with both HLA-B*13:01 and a specific TCR clonotype, the pair of TRAV12-3 and TRBV28 [117] (Table 7). The mode of interaction between dapsone, HLA, and TCR is different from that of abacavir and HLA-B*57:01, but similar to the interaction between oxypurinol and HLA-B*58:01.

Table 7.
TCR clonotype usage in SCARs.
6. Key Immune Mediators Involved in Delayed Drug Hypersensitivity
T lymphocytes-mediated delayed-type drug hypersensitivity reactions trigger the activation and production of many cytokines and chemokines, such as TNFs, IFNs, GM-CSF, TARC/CCL17, IL-6, IL-8/CXCL8, IL-4, IL-5, IL-8, IL-15, IL-36, RANTES, and CXCL8, etc. These cytokines/chemokines could enhance more cytotoxic cells, including macrophages, eosinophils, neutrophils, and mast cells, gathering and functioning in the inflammatory site and leading to tissue damage. These cytokines and chemokines are responsible for the trafficking, proliferation, regulation, or activation of T lymphocytes and other leukocytes. For example, IL4 and IL-5 play the main role in type IVb reactions by regulating the proliferation, migration, and activation of eosinophils [118]. Neutrophils are the main mediator cells in the type IVd reaction and could be activated and recruited by IL-8, CXCL8, GM-CSF, RANTES, MIP-2, and TNF-α [119,120,121,122,123].
For AGEP, the accumulation of neutrophils is the main characteristic, and CXCL8/IL-8 plays an essential role in the neutrophil-forming pustules [122]. IL-7, IL-22, and GM-CSF might synergistically enhance CXCL8/IL-8 production, and prevent neutrophil apoptosis [124]. Neutrophils, macrophages and mast cells could be identified from the skin lesions of patients with AGEP, implicating the involvement of different innate immune cells downstream of delayed drug hypersensitivity [124]. DILI was hypothesized to begin with damage-associated molecular pattern molecules (DAMPs), such as HMGB1 and ATP, and the activation of T cells [125]. DAMPs, activated by innate immune responses, can cause the activation of cytotoxic cells; this releases TNFs, IL-1b, IL-8, IL-6, and CXCL10, thus recruiting more leukocytes [126]. The histology of DILI suggests that the cellular immune response mainly involves TH1 and CD8+ T lymphocytes [127]. In addition, DILI frequently shows eosinophilia, suggesting the involvement of TH2 and IL-5 signaling [128]. These studies suggest that the complex interaction and cross-talk of innate and adaptive immunity are involved in the clinical presentation of delayed drug hypersensitivity [122,126].
Cytotoxic T lymphocytes (CTL) play a major role in type IVc hypersensitivity, and preferential TCR may recognize specific antigens represented by HLA molecules, leading to the direct killing of antigen-presenting cells. The cellular contact of target cells and effector cells (CTL) could induce the death of antigen-presenting cells through two proposed mechanisms, i.e., the delivery of cytotoxic proteins (e.g., granulysin, perforin, and granzyme B) and Fas-FasL signaling [129]. Perforins and granzymes are the major types of cytotoxic proteins released by CTL. The polymer of perforin forms pores in the cell surface of the target cell in the presence of Ca2+, and causes cell lysis and the cell membrane to become permeable for the entry of granzymes [130]. We previously identified a cytotoxic protein, granulysin, as a key mediator for keratinocyte death in SJS/TEN [131]. In addition, the interaction of Fas on the target cell membrane with the Fas ligand, expressed on the CTL cell surface, has been reported to induce caspase-dependent target cell apoptosis in TEN [5,132,133]. The key mediators of type IVc drug hypersensitivity are summarized below.
- (1)
- Granulysin
Granulysin (GNLY), originally known as an anti-microbial peptide, is a member of the saposin-like protein (SAPLIP) family. It is also a component of lytic granules in CTL and nature killer (NK) cells. GNLY was demonstrated to be potently adept in lysing bacteria extracellularly, and to be more efficient with additional perforin and granzyme B in eliminating intracellular bacteria [134]. Opposite to granzyme B, which induces apoptosis via caspase-3 and -9, granulysin causes endoplasmic reticulum stress and activates caspase-7 [135]. We first reported that 15 kDa secretory granulysin serves as a key mediator for the disseminated keratinocyte apoptosis in patients with SJS/TEN [131]. Compared to the perforin, granzyme B, and FasL, the levels of granulysin were significantly increased in SJS/TEN blister fluids. The overexpression or depletion of granulysin was correlated to the cell cytotoxicity in the models of SJS/TEN [131]. Many studies support that granulysin is aggressively enhanced in drug-induced SJS/TEN, FDE, and DRESS/DiHS, but not MPE [136,137,138].
- (2)
- Perforin and granzyme B
The activated drug-specific CTL and NK cells could release perforins to punch pores on the membrane of target cells, which promotes the entry of granzymes to activate the caspase cascade and induce apoptosis [139]. Granzymes are serine proteases with five types (A, B, H, K, and M) in humans, and they could induce cell death in different pathways. Granzyme A and granzyme B are abundantly expressed in CTL and NK cells. They penetrate into target cells through perforin pores and cause cell death in the classical caspase apoptotic pathway by granzyme B or in a caspase-independent pathway by granzyme A [140]. Granzyme B is the greatest pro-apoptotic member in the granzyme family and mainly contributes to DNA fragmentation in the susceptible cell. Granzyme B mediates caspase-dependent cell death by directly activating pro-caspases and cleaving downstream caspase substrates, such as an inhibitor of caspase-activated DNase (ICAD). Despite direct activation of procaspase-3, granzyme B specifically and rapidly cleaves Bid into a truncated form and induces the release of cytochrome c and Smac/Diablo to activate the caspase-3 pathway [141]. The other caspase-independent cell death mechanism implies that granzyme B causes cell death with an abolished caspase activity [142].
- (3)
- Fas/FasL signaling pathway
Fas ligand (FasL), a member of the TNF family, induces apoptosis in susceptible cells in response to the cross-linking of the receptor, Fas. Fas/FasL-induced apoptosis plays an essential role in immune homeostasis and is involved in cytotoxicity in epidermal cells in drug hypersensitivity. The Fas-associated death domain protein (FADD) is recruited to Fas upon the interaction of Fas and FasL. The binding of procaspase-8 to FADD results in the formation of the death-inducing signaling complex, finally leading to the activation of effector caspase-3 through activated caspase-8 [143]. Viard et al. proposed that a suicidal interaction between Fas and FasL, expressed in keratinocytes, resulted in the extensive necrosis of epidermal cells in patients with SJS/TEN [144].
- (4)
- Thymus and activation-regulated chemokine (TARC) and type 2 helper T cells (TH2)
TARC is expressed by monocyte-derived dendritic cells [145] and epithelial cells [146]. It can regulate the migration and activation of the type 2 T helper (TH2) via CCR4 [147]. The serum level of TARC was found to be significantly associated with blood eosinophil counts and the severity of DiHS/DRESS [148,149]. TARC has been suggested to be a prognostic marker for early DiHS/DRESS [150]. Additionally, the finding of eosinophilia, the increase in TH-2-associated cytokines and chemokines (e.g., TARC and macrophage-derived chemokine (MDC)), and the high proportion of IL-4 and IL-13-producing CD4+ T cells in DiHs/DRESS, suggest that TH2 cells play an essential role in the pathogenesis of DiHS/DRESS [148,151,152,153,154].”
- (5)
- Regulatory T cell (Treg)
The regulatory T cell (Treg) has been suggested to be involved in the pathogenesis of delayed drug hypersensitivity. Takahashi et al. found that the frequency of Tregs in skin lesions was not changed, but the function was impaired in toxic epidermal necrolysis (TEN) patients. Opposite to the observation of TEN, functional Treg dramatically expanded and was abundantly located in skin lesions of DiHS/DRESS patients. The number of Tregs decreases, and Tregs becomes functionally impaired upon the resolution of DiHS/DRESS [155]. Different cytokine expressions in the microenvironment may cause the contraction of Tregs. For example, IL-6, released from CD16+ monocytes, can turn Tregs into TH17 [156]. Such a shift may explain the development of an autoimmune response in a prolonged period of DiHS/DRESS, after clinical resolution [156].
7. Conclusions
Delayed-type hypersensitivity reactions are mainly mediated by the T cell recognition of drug antigens and are accompanied by the activation of downstream leukocytes and immune mediators. These immune responses lead to the diverse clinical presentations of delayed drug hypersensitivity reactions, which range from mild skin reactions (e.g., MPE and FDE) to life-threatening ADRs (e.g., SJS, TEN, DRESS, DiHS, DILI, and AGEP). Several medicines, including anti-epileptics, antibiotics, anti-gout, and anti-viral agents, are associated with delayed drug hypersensitivity reactions. The formation of an immune synapse, composed of TCR, drug/metabolite/peptide, and HLA, may trigger the molecular recognition of delayed drug hypersensitivity. There are four proposed models for the molecular recognition of delayed drug hypersensitivity: the hapten/pro-hapten hypothesis, the pharmacological interaction with immune receptor (p-i) concept, the altered peptide repertoire model, and the altered TCR repertoire model. In these models, the causative drug/metabolite may interact covalently or noncovalently with peptides/protein, and bind to HLA and/or TCR to elicit drug hypersensitivity reactions. Increasing pharmacogenomic studies reveal that genetic variants of HLA loci and drug metabolic enzymes are associated with delayed drug hypersensitivity. Furthermore, preferential TCR clonotypes, and cytotoxic proteins/cytokines/chemokines’ activation, have been reported to contribute to the pathogenesis of delayed drug hypersensitivity. The recent findings, regarding specific T cell receptors in allopurinol-, carbamazepine-, and dapsone-induced SCARs, support the attribution of drug-specific T cells in delayed drug hypersensitivity. Further studies on molecular recognition, genetic susceptibility, and immune mediators provide an important knowledge basis for preventing, diagnosing, and the clinical managing of delayed-type drug hypersensitivity.
Author Contributions
Writing—original draft preparation, M.-T.C. and S.-C.P.; writing—review and editing, S.-I.H. and W.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Ministry of Science and Technology, Taiwan (MOST 108-2320-B-182A-023-MY3, MOST 109-2320-B-182A-008-MY3), and Chang Gung Memorial Hospital (CIRPG3I0041~43, CIRPG3I0021~23, CIRPG3I0031~33, CIRPG2I0011~13).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the support of the members of the Research Center of Drug Hypersensitivity and the Cancer Vaccine and Immune Cell Therapy Core Lab of Chang Gung Memorial Hospital, Taiwan.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AED, antiepileptic drugs; AGEP, acute generalized exanthematous pustulosis; CBZ: carbamazepine; CTL: cytotoxic T lymphocytes; DIA: drug-induced agranulocytosis; DILI, drug-induced liver injury; DiHS, drug-induced hypersensitivity syndrome; DRESS, drug reaction with eosinophilia and systemic symptoms; FDE, fixed drug eruption; HLA: human leukocyte antigen; MPE, maculopapular exanthema; NK: nature killer; NMBAs, neuromuscular blocking agents; NSAIDs, nonsteroid anti-inflammation drugs; SCARs: sever cutaneous adverse drug reactions; SJS, Stevens–Johnson syndrome; TCR: T cell receptor; TEN: toxic epidermal necrolysis.
References
- Demoly, P.; Adkinson, N.F.; Brockow, K.; Castells, M.; Chiriac, A.M.; Greenberger, P.A.; Khan, D.A.; Lang, D.M.; Park, H.S.; Pichler, W.; et al. International Consensus on drug allergy. Allergy 2014, 69, 420–437. [Google Scholar] [CrossRef]
- World Health Organization. International drug monitoring: The role of national centres. Report of a WHO meeting. World Health Organ Tech. Rep. Ser. 1972, 498, 1–25. [Google Scholar]
- Gomes, E.R.; Demoly, P. Epidemiology of hypersensitivity drug reactions. Curr. Opin. Allergy Clin. Immunol. 2005, 5, 309–316. [Google Scholar] [CrossRef]
- Johansson, S.G.; Bieber, T.; Dahl, R.; Friedmann, P.S.; Lanier, B.Q.; Lockey, R.F.; Motala, C.; Ortega Martell, J.A.; Platts-Mills, T.A.; Ring, J.; et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 2004, 113, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.B.; Abe, R.; Pan, R.Y.; Wang, C.W.; Hung, S.I.; Tsai, Y.G.; Chung, W.H. An Updated Review of the Molecular Mechanisms in Drug Hypersensitivity. J. Immunol. Res. 2018, 2018, 6431694. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.F.; Phillips, E.J.; Wiese, M.D.; Heddle, R.J.; Brown, S.G. Immediate-type hypersensitivity drug reactions. Br. J. Clin. Pharmacol. 2014, 78, 1–13. [Google Scholar] [CrossRef]
- Brockow, K.; Ardern-Jones, M.R.; Mockenhaupt, M.; Aberer, W.; Barbaud, A.; Caubet, J.C.; Spiewak, R.; Torres, M.J.; Mortz, C.G. EAACI position paper on how to classify cutaneous manifestations of drug hypersensitivity. Allergy 2019, 74, 14–27. [Google Scholar] [CrossRef]
- Mayorga, C.; Fernandez, T.D.; Montanez, M.I.; Moreno, E.; Torres, M.J. Recent developments and highlights in drug hypersensitivity. Allergy 2019, 74, 2368–2381. [Google Scholar] [CrossRef] [PubMed]
- Lehloenya, R.J.; Peter, J.G.; Copascu, A.; Trubiano, J.A.; Phillips, E.J. Delabeling Delayed Drug Hypersensitivity: How Far Can You Safely Go? J. Allergy Clin. Immunol. Pract. 2020, 8, 2878–2895.E6. [Google Scholar] [CrossRef]
- Mockenhaupt, M. Epidemiology of cutaneous adverse drug reactions. Allergol. Sel. 2017, 1, 96–108. [Google Scholar] [CrossRef]
- Rattay, B.; Benndorf, R.A. Drug-Induced Idiosyncratic Agranulocytosis—Infrequent but Dangerous. Front. Pharmacol. 2021, 12, 727717. [Google Scholar] [CrossRef]
- Andres, E.; Maloisel, F. Idiosyncratic drug-induced agranulocytosis or acute neutropenia. Curr. Opin. Hematol. 2008, 15, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kardaun, S.H.; Sekula, P.; Valeyrie-Allanore, L.; Liss, Y.; Chu, C.Y.; Creamer, D.; Sidoroff, A.; Naldi, L.; Mockenhaupt, M.; Roujeau, J.C.; et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): An original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br. J. Dermatol. 2013, 169, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Sekula, P.; Dunant, A.; Mockenhaupt, M.; Naldi, L.; Bouwes Bavinck, J.N.; Halevy, S.; Kardaun, S.; Sidoroff, A.; Liss, Y.; Schumacher, M.; et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J. Investig. Dermatol. 2013, 133, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Fontana, R.J.; Bonkovsky, H.L.; Watkins, P.B.; Davern, T.; Serrano, J.; Yang, H.; Rochon, J. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008, 135, 1924–1934. [Google Scholar] [CrossRef]
- Chung, W.H.; Wang, C.W.; Dao, R.L. Severe cutaneous adverse drug reactions. J. Dermatol. 2016, 43, 758–766. [Google Scholar] [CrossRef]
- Posadas, S.J.; Pichler, W.J. Delayed drug hypersensitivity reactions—New concepts. Clin. Exp. Allergy 2007, 37, 989–999. [Google Scholar] [CrossRef]
- Pichler, W.J. Delayed drug hypersensitivity reactions. Ann. Intern. Med. 2003, 139, 683–693. [Google Scholar] [CrossRef]
- De Groot, A. Allergic Contact Dermatitis From Topical Drugs: An Overview. Dermatitis 2021, 32, 197–213. [Google Scholar] [CrossRef]
- Kuruvilla, M.; Khan, D.A. Eosinophilic Drug Allergy. Clin. Rev. Allergy Immunol. 2016, 50, 228–239. [Google Scholar] [CrossRef]
- Zirwas, M.J. Contact Dermatitis to Cosmetics. Clin. Rev. Allergy Immunol. 2019, 56, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.A.; Yacoub, M.R.; Ripa, M.; Mannina, D.; Cariddi, A.; Saporiti, N.; Ciceri, F.; Castagna, A.; Colombo, G.; Dagna, L. Eosinophils from Physiology to Disease: A Comprehensive Review. Biomed. Res. Int. 2018, 2018, 9095275. [Google Scholar] [CrossRef] [PubMed]
- Hoofnagle, J.H.; Bjornsson, E.S. Drug-Induced Liver Injury—Types and Phenotypes. N. Engl. J. Med. 2019, 381, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Warrington, R.; Silviu-Dan, F.; Wong, T. Drug allergy. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. 2), 60. [Google Scholar] [CrossRef] [PubMed]
- Padovan, E.; Bauer, T.; Tongio, M.M.; Kalbacher, H.; Weltzien, H.U. Penicilloyl peptides are recognized as T cell antigenic determinants in penicillin allergy. Eur. J. Immunol. 1997, 27, 1303–1307. [Google Scholar] [CrossRef]
- White, K.D.; Chung, W.H.; Hung, S.I.; Mallal, S.; Phillips, E.J. Evolving models of the immunopathogenesis of T cell-mediated drug allergy: The role of host, pathogens, and drug response. J. Allergy Clin. Immunol. 2015, 136, 219–234; quiz 235. [Google Scholar] [CrossRef] [PubMed]
- Maker, J.H.; Stroup, C.M.; Huang, V.; James, S.F. Antibiotic Hypersensitivity Mechanisms. Pharmacy 2019, 7, 122. [Google Scholar] [CrossRef]
- Castells, M.; Khan, D.A.; Phillips, E.J. Penicillin Allergy. N. Engl. J. Med. 2019, 381, 2338–2351. [Google Scholar] [CrossRef]
- Pichler, W.J. The p-i Concept: Pharmacological Interaction of Drugs With Immune Receptors. World Allergy Organ. J. 2008, 1, 96–102. [Google Scholar] [CrossRef]
- Wei, C.Y.; Chung, W.H.; Huang, H.W.; Chen, Y.T.; Hung, S.I. Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens-Johnson syndrome. J. Allergy Clin. Immunol. 2012, 129, 1562–1569.e5. [Google Scholar] [CrossRef]
- Yun, J.; Marcaida, M.J.; Eriksson, K.K.; Jamin, H.; Fontana, S.; Pichler, W.J.; Yerly, D. Oxypurinol directly and immediately activates the drug-specific T cells via the preferential use of HLA-B*58:01. J. Immunol. 2014, 192, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chen, J.K.; Ko, T.M.; Wei, C.Y.; Wu, J.Y.; Chung, W.H.; Chen, S.Y.; Liao, Y.D.; Hung, S.I.; Chen, Y.T. Immunologic basis for allopurinol-induced severe cutaneous adverse reactions: HLA-B*58:01-restricted activation of drug-specific T cells and molecular interaction. J. Allergy Clin. Immunol. 2015, 135, 1063–1065.e5. [Google Scholar] [CrossRef] [PubMed]
- Illing, P.T.; Vivian, J.P.; Dudek, N.L.; Kostenko, L.; Chen, Z.; Bharadwaj, M.; Miles, J.J.; Kjer-Nielsen, L.; Gras, S.; Williamson, N.A.; et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature 2012, 486, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Ostrov, D.A.; Grant, B.J.; Pompeu, Y.A.; Sidney, J.; Harndahl, M.; Southwood, S.; Oseroff, C.; Lu, S.; Jakoncic, J.; de Oliveira, C.A.; et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc. Natl. Acad. Sci. USA 2012, 109, 9959–9964. [Google Scholar] [CrossRef]
- Watkins, S.; Pichler, W.J. Sulfamethoxazole induces a switch mechanism in T cell receptors containing TCRVbeta20-1, altering pHLA recognition. PLoS ONE 2013, 8, e76211. [Google Scholar] [CrossRef]
- Chung, W.H.; Hung, S.I.; Hong, H.S.; Hsih, M.S.; Yang, L.C.; Ho, H.C.; Wu, J.Y.; Chen, Y.T. Medical genetics: A marker for Stevens-Johnson syndrome. Nature 2004, 428, 486. [Google Scholar] [CrossRef] [PubMed]
- Man, C.B.; Kwan, P.; Baum, L.; Yu, E.; Lau, K.M.; Cheng, A.S.; Ng, M.H. Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia 2007, 48, 1015–1018. [Google Scholar] [CrossRef]
- Chong, K.W.; Chan, D.W.; Cheung, Y.B.; Ching, L.K.; Hie, S.L.; Thomas, T.; Ling, S.; Tan, E.C. Association of carbamazepine-induced severe cutaneous drug reactions and HLA-B*1502 allele status, and dose and treatment duration in paediatric neurology patients in Singapore. Arch. Dis. Child. 2014, 99, 581–584. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Chu, H.C.; Nguyen, D.V.; Phan, M.H.; Craig, T.; Baumgart, K.; van Nunen, S. HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in Vietnamese. Asia Pac. Allergy 2015, 5, 68–77. [Google Scholar] [CrossRef]
- Locharernkul, C.; Loplumlert, J.; Limotai, C.; Korkij, W.; Desudchit, T.; Tongkobpetch, S.; Kangwanshiratada, O.; Hirankarn, N.; Suphapeetiporn, K.; Shotelersuk, V. Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B*1502 allele in Thai population. Epilepsia 2008, 49, 2087–2091. [Google Scholar] [CrossRef]
- Chang, C.C.; Too, C.L.; Murad, S.; Hussein, S.H. Association of HLA-B*1502 allele with carbamazepine-induced toxic epidermal necrolysis and Stevens-Johnson syndrome in the multi-ethnic Malaysian population. Int. J. Dermatol. 2011, 50, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Mehta, T.Y.; Prajapati, L.M.; Mittal, B.; Joshi, C.G.; Sheth, J.J.; Patel, D.B.; Dave, D.M.; Goyal, R.K. Association of HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Sukasem, C.; Chaichan, C.; Nakkrut, T.; Satapornpong, P.; Jaruthamsophon, K.; Jantararoungtong, T.; Koomdee, N.; Sririttha, S.; Medhasi, S.; Oo-Puthinan, S.; et al. Association between HLA-B Alleles and Carbamazepine-Induced Maculopapular Exanthema and Severe Cutaneous Reactions in Thai Patients. J. Immunol. Res. 2018, 2018, 2780272. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Lin, J.J.; Lu, C.S.; Ong, C.T.; Hsieh, P.F.; Yang, C.C.; Tai, C.T.; Wu, S.L.; Lu, C.H.; Hsu, Y.C.; et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N. Engl. J. Med. 2011, 364, 1126–1133. [Google Scholar] [CrossRef]
- Mockenhaupt, M.; Wang, C.W.; Hung, S.I.; Sekula, P.; Schmidt, A.H.; Pan, R.Y.; Chen, C.B.; Dunant, A.; Gouvello, S.L.; Schumacher, M.; et al. HLA-B*57:01 confers genetic susceptibility to carbamazepine-induced SJS/TEN in Europeans. Allergy 2019, 74, 2227–2230. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.I.; Chung, W.H.; Jee, S.H.; Chen, W.C.; Chang, Y.T.; Lee, W.R.; Hu, S.L.; Wu, M.T.; Chen, G.S.; Wong, T.W.; et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharm. Genom. 2006, 16, 297–306. [Google Scholar] [CrossRef]
- Genin, E.; Chen, D.P.; Hung, S.I.; Sekula, P.; Schumacher, M.; Chang, P.Y.; Tsai, S.H.; Wu, T.L.; Bellón, T.; Tamouza, R.; et al. HLA-A*31:01 and different types of carbamazepine-induced severe cutaneous adverse reactions: An international study and meta-analysis. Pharmacogenom. J. 2014, 14, 281–288. [Google Scholar] [CrossRef]
- McCormack, M.; Alfirevic, A.; Bourgeois, S.; Farrell, J.J.; Kasperavičiūtė, D.; Carrington, M.; Sills, G.J.; Marson, T.; Jia, X.; De Bakker, P.I.; et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N. Engl. J. Med. 2011, 364, 1134–1143. [Google Scholar] [CrossRef]
- Ramírez, E.; Bellón, T.; Tong, H.Y.; Borobia, A.M.; De Abajo, F.J.; Lerma, V.; Moreno Hidalgo, M.A.; Castañer, J.L.; Cabañas, R.; Fiandor, A.; et al. Significant HLA class I type associations with aromatic antiepileptic drug (AED)-induced SJS/TEN are different from those found for the same AED-induced DRESS in the Spanish population. Pharmacol. Res. 2017, 115, 168–178. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Hui, R.C.; Wu, T.; Chang, W.C.; Hsih, M.S.; Yang, C.H.; Ho, H.C.; Chang, Y.G.; Chen, M.J.; Lin, J.Y.; et al. Genotype-phenotype association between HLA and carbamazepine-induced hypersensitivity reactions: Strength and clinical correlations. J. Dermatol. Sci. 2014, 73, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, T.; Mushiroda, T.; Yowang, A.; Takahashi, A.; Kubo, M.; Shirakata, Y.; Ikezawa, Z.; Iijima, M.; Shiohara, T.; Hashimoto, K.; et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum. Mol. Genet. 2011, 20, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.B.; Hsiao, Y.H.; Wu, T.; Hsih, M.S.; Tassaneeyakul, W.; Jorns, T.P.; Sukasem, C.; Hsu, C.N.; Su, S.C.; Chang, W.C.; et al. Risk and association of HLA with oxcarbazepine-induced cutaneous adverse reactions in Asians. Neurology 2017, 88, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.I.; Chung, W.H.; Liu, Z.S.; Chen, C.H.; Hsih, M.S.; Hui, R.C.; Chu, C.Y.; Chen, Y.T. Common risk allele in aromatic antiepileptic-drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics 2010, 11, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Ng, C.C.; Too, C.L.; Choon, S.E.; Lee, C.K.; Chung, W.H.; Hussein, S.H.; Lim, K.S.; Murad, S. Association of HLA-B*15:13 and HLA-B*15:02 with phenytoin-induced severe cutaneous adverse reactions in a Malay population. Pharmacogenom. J. 2017, 17, 170–173. [Google Scholar] [CrossRef]
- Cheung, Y.K.; Cheng, S.H.; Chan, E.J.; Lo, S.V.; Ng, M.H.; Kwan, P. HLA-B alleles associated with severe cutaneous reactions to antiepileptic drugs in Han Chinese. Epilepsia 2013, 54, 1307–1314. [Google Scholar] [CrossRef]
- Zeng, T.; Long, Y.S.; Min, F.L.; Liao, W.P.; Shi, Y.W. Association of HLA-B*1502 allele with lamotrigine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese subjects: A meta-analysis. Int. J. Dermatol. 2015, 54, 488–493. [Google Scholar] [CrossRef]
- Xu, J.; Shi, X.; Qiu, Y.; Zhang, Y.; Chen, S.; Shi, Y.; Deng, Y. Association between HLA-A*3201 allele and oxcarbazepine-induced cutaneous adverse reactions in Eastern Han Chinese population. Seizure 2019, 65, 25–30. [Google Scholar] [CrossRef]
- Chung, W.H.; Chang, W.C.; Lee, Y.S.; Wu, Y.Y.; Yang, C.H.; Ho, H.C.; Chen, M.J.; Lin, J.Y.; Hui, R.C.; Ho, J.C. Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA 2014, 312, 525–534. [Google Scholar] [CrossRef]
- Tassaneeyakul, W.; Prabmeechai, N.; Sukasem, C.; Kongpan, T.; Konyoung, P.; Chumworathayi, P.; Tiamkao, S.; Khunarkornsiri, U.; Kulkantrakorn, K.; Saksit, N.; et al. Associations between HLA class I and cytochrome P450 2C9 genetic polymorphisms and phenytoin-related severe cutaneous adverse reactions in a Thai population. Pharm. Genom. 2016, 26, 225–234. [Google Scholar] [CrossRef]
- Su, S.C.; Chen, C.B.; Chang, W.C.; Wang, C.W.; Fan, W.L.; Lu, L.Y.; Nakamura, R.; Saito, Y.; Ueta, M.; Kinoshita, S.; et al. HLA Alleles and CYP2C9*3 as Predictors of Phenytoin Hypersensitivity in East Asians. Clin. Pharmacol. Ther. 2019, 105, 476–485. [Google Scholar] [CrossRef]
- Kim, B.K.; Jung, J.W.; Kim, T.B.; Chang, Y.S.; Park, H.S.; Moon, J.; Lee, S.T.; Jung, K.H.; Jung, K.Y.; Chu, K.; et al. HLA-A*31:01 and lamotrigine-induced severe cutaneous adverse drug reactions in a Korean population. Ann. Allergy Asthma Immunol. 2017, 118, 629–630. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.I.; Chung, W.H.; Liou, L.B.; Chu, C.C.; Lin, M.; Huang, H.P.; Lin, Y.L.; Lan, J.L.; Yang, L.C.; Hong, H.S.; et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc. Natl. Acad. Sci. USA 2005, 102, 4134–4139. [Google Scholar] [CrossRef] [PubMed]
- Lonjou, C.; Borot, N.; Sekula, P.; Ledger, N.; Thomas, L.; Halevy, S.; Naldi, L.; Bouwes-Bavinck, J.N.; Sidoroff, A.; de Toma, C.; et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharm. Genom. 2008, 18, 99–107. [Google Scholar] [CrossRef]
- Goncalo, M.; Coutinho, I.; Teixeira, V.; Gameiro, A.R.; Brites, M.M.; Nunes, R.; Martinho, A. HLA-B*58:01 is a risk factor for allopurinol-induced DRESS and Stevens-Johnson syndrome/toxic epidermal necrolysis in a Portuguese population. Br. J. Dermatol. 2013, 169, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Tassaneeyakul, W.; Jantararoungtong, T.; Chen, P.; Lin, P.Y.; Tiamkao, S.; Khunarkornsiri, U.; Chucherd, P.; Konyoung, P.; Vannaprasaht, S.; Choonhakarn, C.; et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharm. Genom. 2009, 19, 704–709. [Google Scholar] [CrossRef]
- Sukasem, C.; Jantararoungtong, T.; Kuntawong, P.; Puangpetch, A.; Koomdee, N.; Satapornpong, P.; Supapsophon, P.; Klaewsongkram, J.; Rerkpattanapipat, T. HLA-B (*) 58:01 for Allopurinol-Induced Cutaneous Adverse Drug Reactions: Implication for Clinical Interpretation in Thailand. Front. Pharmacol. 2016, 7, 186. [Google Scholar] [CrossRef]
- Tohkin, M.; Kaniwa, N.; Saito, Y.; Sugiyama, E.; Kurose, K.; Nishikawa, J.; Hasegawa, R.; Aihara, M.; Matsunaga, K.; Abe, M.; et al. A whole-genome association study of major determinants for allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Pharmacogenom. J. 2013, 13, 60–69. [Google Scholar] [CrossRef]
- Kang, H.R.; Jee, Y.K.; Kim, Y.S.; Lee, C.H.; Jung, J.W.; Kim, S.H.; Park, H.W.; Chang, Y.S.; Jang, I.J.; Cho, S.H.; et al. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharm. Genom. 2011, 21, 303–307. [Google Scholar] [CrossRef]
- Fontana, R.J.; Li, Y.J.; Phillips, E.; Saeed, N.; Barnhart, H.; Kleiner, D. Allopurinol hepatotoxicity is associated with human leukocyte antigen Class I alleles. Liver Int. 2021, 41, 1884–1893. [Google Scholar] [CrossRef]
- Ko, T.M.; Tsai, C.Y.; Chen, S.Y.; Chen, K.S.; Yu, K.H.; Chu, C.S.; Huang, C.M.; Wang, C.R.; Weng, C.T.; Yu, C.L.; et al. Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: National prospective cohort study. BMJ 2015, 351, h4848. [Google Scholar] [CrossRef]
- Ng, C.Y.; Yeh, Y.T.; Wang, C.W.; Hung, S.I.; Yang, C.H.; Chang, Y.C.; Chang, W.C.; Lin, Y.J.; Chang, C.J.; Su, S.C.; et al. Impact of the HLA-B(*)58:01 Allele and Renal Impairment on Allopurinol-Induced Cutaneous Adverse Reactions. J. Investig. Dermatol. 2016, 136, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Oussalah, A.; Chery, C.; Gueant-Rodriguez, R.M.; Gaeta, F.; Cornejo-Garcia, J.A.; Rouyer, P.; Josse, T.; Mayorga, C.; Torres, M.J.; et al. Next-generation sequencing and genotype association studies reveal the association of HLA-DRB3*02:02 with delayed hypersensitivity to penicillins. Allergy 2021, 77, 1827–1834. [Google Scholar] [CrossRef]
- Donaldson, P.T.; Daly, A.K.; Henderson, J.; Graham, J.; Pirmohamed, M.; Bernal, W.; Day, C.P.; Aithal, G.P. Human leucocyte antigen class II genotype in susceptibility and resistance to co-amoxiclav-induced liver injury. J. Hepatol. 2010, 53, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.K.; Donaldson, P.T.; Bhatnagar, P.; Shen, Y.; Pe’er, I.; Floratos, A.; Daly, M.J.; Goldstein, D.B.; John, S.; Nelson, M.R.; et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat. Genet. 2009, 41, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Kongpan, T.; Mahasirimongkol, S.; Konyoung, P.; Kanjanawart, S.; Chumworathayi, P.; Wichukchinda, N.; Kidkeukarun, R.; Preechakul, S.; Khunarkornsiri, U.; Bamrungram, W.; et al. Candidate HLA genes for prediction of co-trimoxazole-induced severe cutaneous reactions. Pharm. Genom. 2015, 25, 402–411. [Google Scholar] [CrossRef]
- Wang, C.W.; Tassaneeyakul, W.; Chen, C.B.; Chen, W.T.; Teng, Y.C.; Huang, C.Y.; Sukasem, C.; Lu, C.W.; Lee, Y.S.; Choon, S.E.; et al. Whole genome sequencing identifies genetic variants associated with co-trimoxazole hypersensitivity in Asians. J. Allergy Clin. Immunol. 2021, 147, 1402–1412. [Google Scholar] [CrossRef]
- Sukasem, C.; Pratoomwun, J.; Satapornpong, P.; Klaewsongkram, J.; Rerkpattanapipat, T.; Rerknimitr, P.; Lertpichitkul, P.; Puangpetch, A.; Nakkam, N.; Konyoung, P.; et al. Genetic Association of Co-Trimoxazole-Induced Severe Cutaneous Adverse Reactions Is Phenotype-Specific: HLA Class I Genotypes and Haplotypes. Clin. Pharmacol. Ther. 2020, 108, 1078–1089. [Google Scholar] [CrossRef]
- Wolkenstein, P.; Carriere, V.; Charue, D.; Bastuji-Garin, S.; Revuz, J.; Roujeau, J.C.; Beaune, P.; Bagot, M. A slow acetylator genotype is a risk factor for sulphonamide-induced toxic epidermal necrolysis and Stevens-Johnson syndrome. Pharmacogenetics 1995, 5, 255–258. [Google Scholar] [CrossRef]
- Pirmohamed, M.; Alfirevic, A.; Vilar, J.; Stalford, A.; Wilkins, E.G.; Sim, E.; Park, B.K. Association analysis of drug metabolizing enzyme gene polymorphisms in HIV-positive patients with co-trimoxazole hypersensitivity. Pharmacogenetics 2000, 10, 705–713. [Google Scholar] [CrossRef]
- Zielinska, E.; Niewiarowski, W.; Bodalski, J.; Rebowski, G.; Skretkowicz, J.; Mianowska, K.; Sekulska, M. Genotyping of the arylamine N-acetyltransferase polymorphism in the prediction of idiosyncratic reactions to trimethoprim-sulfamethoxazole in infants. Pharm. World Sci. 1998, 20, 123–130. [Google Scholar] [CrossRef]
- O’Neil, W.M.; MacArthur, R.D.; Farrough, M.J.; Doll, M.A.; Fretland, A.J.; Hein, D.W.; Crane, L.R.; Svensson, C.K. Acetylator phenotype and genotype in HIV-infected patients with and without sulfonamide hypersensitivity. J. Clin. Pharmacol. 2002, 42, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Alfirevic, A.; Stalford, A.C.; Vilar, F.J.; Wilkins, E.G.; Park, B.K.; Pirmohamed, M. Slow acetylator phenotype and genotype in HIV-positive patients with sulphamethoxazole hypersensitivity. Br. J. Clin. Pharmacol. 2003, 55, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Delomenie, C.; Mathelier-Fusade, P.; Longuemaux, S.; Rozenbaum, W.; Leynadier, F.; Krishnamoorthy, R.; Dupret, J.M. Glutathione S-transferase (GSTM1) null genotype and sulphonamide intolerance in acquired immunodeficiency syndrome. Pharmacogenetics 1997, 7, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Konvinse, K.C.; Trubiano, J.A.; Pavlos, R.; James, I.; Shaffer, C.M.; Bejan, C.A.; Schutte, R.J.; Ostrov, D.A.; Pilkinton, M.A.; Rosenbach, M.; et al. HLA-A*32:01 is strongly associated with vancomycin-induced drug reaction with eosinophilia and systemic symptoms. J. Allergy Clin. Immunol. 2019, 144, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Satapornpong, P.; Pratoomwun, J.; Rerknimitr, P.; Klaewsongkram, J.; Nakkam, N.; Rungrotmongkol, T.; Konyoung, P.; Saksit, N.; Mahakkanukrauh, A.; Amornpinyo, W.; et al. HLA-B*13:01 Is a Predictive Marker of Dapsone-Induced Severe Cutaneous Adverse Reactions in Thai Patients. Front. Immunol. 2021, 12, 661135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.R.; Liu, H.; Irwanto, A.; Fu, X.A.; Li, Y.; Yu, G.Q.; Yu, Y.X.; Chen, M.F.; Low, H.Q.; Li, J.H.; et al. HLA-B*13:01 and the dapsone hypersensitivity syndrome. N. Engl. J. Med. 2013, 369, 1620–1628. [Google Scholar] [CrossRef]
- Chen, W.T.; Wang, C.W.; Lu, C.W.; Chen, C.B.; Lee, H.E.; Hung, S.I.; Choon, S.E.; Yang, C.H.; Liu, M.T.; Chen, T.J.; et al. The Function of HLA-B*13:01 Involved in the Pathomechanism of Dapsone-Induced Severe Cutaneous Adverse Reactions. J. Investig. Dermatol. 2018, 138, 1546–1554. [Google Scholar] [CrossRef]
- Tempark, T.; Satapornpong, P.; Rerknimitr, P.; Nakkam, N.; Saksit, N.; Wattanakrai, P.; Jantararoungtong, T.; Koomdee, N.; Mahakkanukrauh, A.; Tassaneeyakul, W.; et al. Dapsone-induced severe cutaneous adverse drug reactions are strongly linked with HLA-B*13: 01 allele in the Thai population. Pharm. Genom. 2017, 27, 429–437. [Google Scholar] [CrossRef]
- Park, H.J.; Park, J.W. The HLA-B*13:01 and the dapsone hypersensitivity syndrome in Korean and Asian populations: Genotype- and meta-analyses. Expert Opin. Drug Saf. 2020, 19, 1349–1356. [Google Scholar] [CrossRef]
- Krismawati, H.; Irwanto, A.; Pongtiku, A.; Irwan, I.D. Validation study of HLA-B*13:01 as a biomarker of dapsone hypersensitivity syndrome in leprosy patients in Indonesia. PLoS Negl. Trop. Dis. 2020, 14, e0008746. [Google Scholar] [CrossRef]
- Hetherington, S.; McGuirk, S.; Powell, G.; Cutrell, A.; Naderer, O.; Spreen, B.; Lafon, S.; Pearce, G.; Steel, H. Hypersensitivity reactions during therapy with the nucleoside reverse transcriptase inhibitor abacavir. Clin. Ther. 2001, 23, 1603–1614. [Google Scholar] [CrossRef]
- Hetherington, S.; Hughes, A.R.; Mosteller, M.; Shortino, D.; Baker, K.L.; Spreen, W.; Lai, E.; Davies, K.; Handley, A.; Dow, D.J.; et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet 2002, 359, 1121–1122. [Google Scholar] [CrossRef] [PubMed]
- Mallal, S.; Nolan, D.; Witt, C.; Masel, G.; Martin, A.M.; Moore, C.; Sayer, D.; Castley, A.; Mamotte, C.; Maxwell, D.; et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet 2002, 359, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Mallal, S.; Phillips, E.; Carosi, G.; Molina, J.M.; Workman, C.; Tomazic, J.; Jägel-Guedes, E.; Rugina, S.; Kozyrev, O.; Cid, J.F.; et al. HLA-B*5701 screening for hypersensitivity to abacavir. N. Engl. J. Med. 2008, 358, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Chantarangsu, S.; Mushiroda, T.; Mahasirimongkol, S.; Kiertiburanakul, S.; Sungkanuparph, S.; Manosuthi, W.; Tantisiriwat, W.; Charoenyingwattana, A.; Sura, T.; Chantratita, W.; et al. HLA-B*3505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai patients. Pharm. Genom. 2009, 19, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Guo, S.; Hall, D.; Cammett, A.M.; Jayadev, S.; Distel, M.; Storfer, S.; Huang, Z.; Mootsikapun, P.; Ruxrungtham, K.; et al. Toxicogenomics of nevirapine-associated cutaneous and hepatic adverse events among populations of African, Asian, and European descent. AIDS 2011, 25, 1271–1280. [Google Scholar] [CrossRef]
- Carr, D.F.; Bourgeois, S.; Chaponda, M.; Takeshita, L.Y.; Morris, A.P.; Castro, E.M.; Alfirevic, A.; Jones, A.R.; Rigden, D.J.; Haldenby, S.; et al. Genome-wide association study of nevirapine hypersensitivity in a sub-Saharan African HIV-infected population. J. Antimicrob. Chemother. 2017, 72, 1152–1162. [Google Scholar] [CrossRef]
- Martin, A.M.; Nolan, D.; James, I.; Cameron, P.; Keller, J.; Moore, C.; Phillips, E.; Christiansen, F.T.; Mallal, S. Predisposition to nevirapine hypersensitivity associated with HLA-DRB1*0101 and abrogated by low CD4 T-cell counts. AIDS 2005, 19, 97–99. [Google Scholar] [CrossRef]
- Littera, R.; Carcassi, C.; Masala, A.; Piano, P.; Serra, P.; Ortu, F.; Corso, N.; Casula, B.; La Nasa, G.; Contu, L.; et al. HLA-dependent hypersensitivity to nevirapine in Sardinian HIV patients. AIDS 2006, 20, 1621–1626. [Google Scholar] [CrossRef]
- Gatanaga, H.; Yazaki, H.; Tanuma, J.; Honda, M.; Genka, I.; Teruya, K.; Tachikawa, N.; Kikuchi, Y.; Oka, S. HLA-Cw8 primarily associated with hypersensitivity to nevirapine. AIDS 2007, 21, 264–265. [Google Scholar] [CrossRef]
- Gao, S.; Gui, X.E.; Liang, K.; Liu, Z.; Hu, J.; Dong, B. HLA-dependent hypersensitivity reaction to nevirapine in Chinese Han HIV-infected patients. AIDS Res. Hum. Retroviruses 2012, 28, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Borgiani, P.; Di Fusco, D.; Erba, F.; Marazzi, M.C.; Mancinelli, S.; Novelli, G.; Palombi, L.; Ciccacci, C. HCP5 genetic variant (RS3099844) contributes to Nevirapine-induced Stevens Johnsons Syndrome/Toxic Epidermal Necrolysis susceptibility in a population from Mozambique. Eur. J. Clin. Pharmacol. 2014, 70, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Hopkins, C.; Duffy, E.; Lee, D.; Loulergue, P.; Ripamonti, D.; Ostrov, D.A.; Phillips, E. Association of the HLA-B*53:01 Allele With Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS) Syndrome During Treatment of HIV Infection With Raltegravir. Clin. Infect. Dis. 2017, 64, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Likanonsakul, S.; Rattanatham, T.; Feangvad, S.; Uttayamakul, S.; Prasithsirikul, W.; Tunthanathip, P.; Nakayama, E.E.; Shioda, T. HLA-Cw*04 allele associated with nevirapine-induced rash in HIV-infected Thai patients. AIDS Res. Ther. 2009, 6, 22. [Google Scholar] [CrossRef]
- Tamai, H.; Sudo, T.; Kimura, A.; Mukuta, T.; Matsubayashi, S.; Kuma, K.; Nagataki, S.; Sasazuki, T. Association between the DRB1*08032 histocompatibility antigen and methimazole-induced agranulocytosis in Japanese patients with Graves disease. Ann. Intern. Med. 1996, 124, 490–494. [Google Scholar] [CrossRef]
- Chen, W.T.; Chi, C.C. Associations of HLA genotypes with antithyroid drug-induced agranulocytosis: A systematic review and meta-analysis of pharmacogenomics studies. Br. J. Clin. Pharmacol. 2019, 85, 1878–1887. [Google Scholar] [CrossRef]
- Chen, P.L.; Shih, S.R.; Wang, P.W.; Lin, Y.C.; Chu, C.C.; Lin, J.H.; Chen, S.C.; Chang, C.C.; Huang, T.S.; Tsai, K.S.; et al. Genetic determinants of antithyroid drug-induced agranulocytosis by human leukocyte antigen genotyping and genome-wide association study. Nat. Commun. 2015, 6, 7633. [Google Scholar] [CrossRef]
- Cheung, C.L.; Sing, C.W.; Tang, C.S.; Cheng, V.K.; Pirmohamed, M.; Choi, C.H.; Hung, C.S.; Lau, E.Y.; Lee, K.F.; Mak, M.W.; et al. HLA-B*38:02:01 predicts carbimazole/methimazole-induced agranulocytosis. Clin. Pharmacol. Ther. 2016, 99, 555–561. [Google Scholar] [CrossRef]
- Hallberg, P.; Eriksson, N.; Ibanez, L.; Bondon-Guitton, E.; Kreutz, R.; Carvajal, A.; Lucena, M.I.; Ponce, E.S.; Molokhia, M.; Martin, J.; et al. Genetic variants associated with antithyroid drug-induced agranulocytosis: A genome-wide association study in a European population. Lancet Diabetes Endocrinol. 2016, 4, 507–516. [Google Scholar] [CrossRef]
- He, Y.; Zheng, J.; Zhang, Q.; Hou, P.; Zhu, F.; Yang, J.; Li, W.; Chen, P.; Liu, S.; Zhang, B.; et al. Association of HLA-B and HLA-DRB1 polymorphisms with antithyroid drug-induced agranulocytosis in a Han population from northern China. Sci. Rep. 2017, 7, 11950. [Google Scholar] [CrossRef]
- Yang, F.; Xuan, J.; Chen, J.; Zhong, H.; Luo, H.; Zhou, P.; Sun, X.; He, L.; Chen, S.; Cao, Z.; et al. HLA-B*59:01: A marker for Stevens-Johnson syndrome/toxic epidermal necrolysis caused by methazolamide in Han Chinese. Pharm. J. 2016, 16, 83–87. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, M.; Lee, K.W.; Kim, S.H.; Kang, H.R.; Park, H.W.; Jee, Y.K. HLA-B*5901 is strongly associated with methazolamide-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. Pharmacogenomics 2010, 11, 879–884. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, F.; Zhang, L.; Xu, D.; Jia, Y.; Cheng, Y.; Han, S.; Wang, T.; Chen, Z.; Su, Y.; et al. Unique motif shared by HLA-B*59:01 and HLA-B*55:02 is associated with methazolamide-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 873–880. [Google Scholar] [CrossRef]
- Shirato, S.; Kagaya, F.; Suzuki, Y.; Joukou, S. Stevens-Johnson syndrome induced by methazolamide treatment. Arch. Ophthalmol. 1997, 115, 550–553. [Google Scholar] [CrossRef]
- Chung, W.H.; Pan, R.Y.; Chu, M.T.; Chin, S.W.; Huang, Y.L.; Wang, W.C.; Chang, J.Y.; Hung, S.I. Oxypurinol-Specific T Cells Possess Preferential TCR Clonotypes and Express Granulysin in Allopurinol-Induced Severe Cutaneous Adverse Reactions. J. Investig. Dermatol. 2015, 135, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.Y.; Chu, M.T.; Wang, C.W.; Lee, Y.S.; Lemonnier, F.; Michels, A.W.; Schutte, R.; Ostrov, D.A.; Chen, C.B.; Phillips, E.J.; et al. Identification of drug-specific public TCR driving severe cutaneous adverse reactions. Nat. Commun. 2019, 10, 3569. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, C.W.; Wang, Z.; Dai, Y.; Zhu, Y.; Lee, Y.S.; Cao, Y.; Chung, W.H.; Ouyang, S.; Wang, H. Functional and structural characteristics of HLA-B*13:01-mediated specific T cells reaction in dapsone-induced drug hypersensitivity. J. Biomed. Sci. 2022, 29, 58. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Paoletti, G.; Puggioni, F.; Racca, F.; Pelaia, G.; Canonica, G.W.; Heffler, E. Interleukin-5 in the Pathophysiology of Severe Asthma. Front. Physiol. 2019, 10, 1514. [Google Scholar] [CrossRef]
- Britschgi, M.; Pichler, W.J. Acute generalized exanthematous pustulosis, a clue to neutrophil-mediated inflammatory processes orchestrated by T cells. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 325–331. [Google Scholar] [CrossRef]
- Biedermann, T.; Kneilling, M.; Mailhammer, R.; Maier, K.; Sander, C.A.; Kollias, G.; Kunkel, S.L.; Hültner, L.; Röcken, M. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J. Exp. Med. 2000, 192, 1441–1452. [Google Scholar] [CrossRef]
- Gleeson, P.; Tanaka, T.I.; Alawi, F.; Alhendi, F.; Fadugba, O. Fixed Drug Eruption of the Tongue Due to Trimethoprim-Sulfamethoxazole. J. Allergy Clin. Immunol. Pract. 2020, 8, 328–329.e1. [Google Scholar] [CrossRef]
- Feldmeyer, L.; Heidemeyer, K.; Yawalkar, N. Acute Generalized Exanthematous Pustulosis: Pathogenesis, Genetic Background, Clinical Variants and Therapy. Int. J. Mol. Sci. 2016, 17, 1214. [Google Scholar] [CrossRef]
- Vallejo-Yague, E.; Martinez-De la Torre, A.; Mohamad, O.S.; Sabu, S.; Burden, A.M. Drug Triggers and Clinic of Acute Generalized Exanthematous Pustulosis (AGEP): A Literature Case Series of 297 Patients. J. Clin. Med. 2022, 11, 397. [Google Scholar] [CrossRef]
- Kabashima, R.; Sugita, K.; Sawada, Y.; Hino, R.; Nakamura, M.; Tokura, Y. Increased circulating Th17 frequencies and serum IL-22 levels in patients with acute generalized exanthematous pustulosis. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 485–488. [Google Scholar] [CrossRef]
- Jee, A.; Sernoskie, S.C.; Uetrecht, J. Idiosyncratic Drug-Induced Liver Injury: Mechanistic and Clinical Challenges. Int. J. Mol. Sci. 2021, 22, 2954. [Google Scholar] [CrossRef]
- Andrade, R.J.; Chalasani, N.; Bjornsson, E.S.; Suzuki, A.; Kullak-Ublick, G.A.; Watkins, P.B.; Devarbhavi, H.; Merz, M.; Lucena, M.I.; Kaplowitz, N.; et al. Drug-induced liver injury. Nat. Rev. Dis. Primers 2019, 5, 58. [Google Scholar] [CrossRef]
- Foureau, D.M.; Walling, T.L.; Maddukuri, V.; Anderson, W.; Culbreath, K.; Kleiner, D.E.; Ahrens, W.A.; Jacobs, C.; Watkins, P.B.; Fontana, R.J.; et al. Comparative analysis of portal hepatic infiltrating leucocytes in acute drug-induced liver injury, idiopathic autoimmune and viral hepatitis. Clin. Exp. Immunol. 2015, 180, 40–51. [Google Scholar] [CrossRef]
- Bjornsson, E.; Kalaitzakis, E.; Olsson, R. The impact of eosinophilia and hepatic necrosis on prognosis in patients with drug-induced liver injury. Aliment. Pharmacol. Ther. 2007, 25, 1411–1421. [Google Scholar] [CrossRef]
- Snyder, P.W. Chapter 5—Diseases of Immunity. In Pathologic Basis of Veterinary Disease, 6th ed.; Zachary, J.F., Ed.; Mosby: St. Louis, MI, USA, 2017; pp. 242–285.e245. [Google Scholar]
- Kataoka, T.; Nagai, K. Molecular dissection of cytotoxic functions mediated by T cells. In Progress in Biotechnology; Endo, I., Ed.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 13–23. [Google Scholar]
- Chung, W.H.; Hung, S.I.; Yang, J.Y.; Su, S.C.; Huang, S.P.; Wei, C.Y.; Chin, S.W.; Chiou, C.C.; Chu, S.C.; Ho, H.C.; et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat. Med. 2008, 14, 1343–1350. [Google Scholar] [CrossRef]
- Yamada, A.; Arakaki, R.; Saito, M.; Kudo, Y.; Ishimaru, N. Dual Role of Fas/FasL-Mediated Signal in Peripheral Immune Tolerance. Front. Immunol. 2017, 8, 00403. [Google Scholar] [CrossRef]
- Gronich, N.; Maman, D.; Stein, N.; Saliba, W. Culprit Medications and Risk Factors Associated with Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Population-Based Nested Case-Control Study. Am. J. Clin. Dermatol. 2022, 23, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Walch, M.; Dotiwala, F.; Mulik, S.; Thiery, J.; Kirchhausen, T.; Clayberger, C.; Krensky, A.M.; Martinvalet, D.; Lieberman, J. Cytotoxic cells kill intracellular bacteria through granulysin-mediated delivery of granzymes. Cell 2014, 157, 1309–1323. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.V.; Wilson, C.; Finn, M.W.; Wang, T.; Krensky, A.M.; Clayberger, C. Granulysin delivered by cytotoxic cells damages endoplasmic reticulum and activates caspase-7 in target cells. J. Immunol. 2011, 186, 3497–3504. [Google Scholar] [CrossRef]
- Abe, R.; Yoshioka, N.; Murata, J.; Fujita, Y.; Shimizu, H. Granulysin as a marker for early diagnosis of the Stevens-Johnson syndrome. Ann. Intern. Med. 2009, 151, 514–515. [Google Scholar] [CrossRef]
- Weinborn, M.; Barbaud, A.; Truchetet, F.; Beurey, P.; Germain, L.; Cribier, B. Histopathological study of six types of adverse cutaneous drug reactions using granulysin expression. Int. J. Dermatol. 2016, 55, 1225–1233. [Google Scholar] [CrossRef]
- Su, S.C.; Mockenhaupt, M.; Wolkenstein, P.; Dunant, A.; Le Gouvello, S.; Chen, C.B.; Chosidow, O.; Valeyrie-Allanore, L.; Bellon, T.; Sekula, P.; et al. Interleukin-15 Is Associated with Severity and Mortality in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. J. Investig. Dermatol. 2017, 137, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J. Cytotoxic Lymphocytes. In Encyclopedia of Immunobiology; Ratcliffe, M.J.H., Ed.; Academic Press: Oxford, UK, 2016; pp. 363–373. [Google Scholar]
- Sutton, V.R.; Davis, J.E.; Cancilla, M.; Johnstone, R.W.; Ruefli, A.A.; Sedelies, K.; Browne, K.A.; Trapani, J.A. Initiation of apoptosis by granzyme B requires direct cleavage of bid, but not direct granzyme B-mediated caspase activation. J. Exp. Med. 2000, 192, 1403–1414. [Google Scholar] [CrossRef]
- Trapani, J.A. Granzymes: A family of lymphocyte granule serine proteases. Genome Biol. 2001, 2, 3014. [Google Scholar] [CrossRef]
- Posadas, S.J.; Padial, A.; Torres, M.J.; Mayorga, C.; Leyva, L.; Sanchez, E.; Alvarez, J.; Romano, A.; Juarez, C.; Blanca, M. Delayed reactions to drugs show levels of perforin, granzyme B, and Fas-L to be related to disease severity. J. Allergy Clin. Immunol. 2002, 109, 155–161. [Google Scholar] [CrossRef]
- Viard, I.; Wehrli, P.; Bullani, R.; Schneider, P.; Holler, N.; Salomon, D.; Hunziker, T.; Saurat, J.H.; Tschopp, J.; French, L.E. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science 1998, 282, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Palermo, B.; Lenig, D.; Miettinen, M.; Matikainen, S.; Julkunen, I.; Forster, R.; Burgstahler, R.; Lipp, M.; Lanzavecchia, A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur. J. Immunol. 1999, 29, 1617–1625. [Google Scholar] [CrossRef]
- Campbell, J.J.; Haraldsen, G.; Pan, J.; Rottman, J.; Qin, S.; Ponath, P.; Andrew, D.P.; Warnke, R.; Ruffing, N.; Kassam, N.; et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 1999, 400, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Nagira, M.; Takagi, S.; Kakizaki, M.; Nishimura, M.; Wang, J.; Gray, P.W.; Matsushima, K.; Yoshie, O. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int. Immunol. 1999, 11, 81–88. [Google Scholar] [CrossRef]
- Ogawa, K.; Morito, H.; Hasegawa, A.; Miyagawa, F.; Kobayashi, N.; Watanabe, H.; Sueki, H.; Tohyama, M.; Hashimoto, K.; Kano, Y.; et al. Elevated serum thymus and activation-regulated chemokine (TARC/CCL17) relates to reactivation of human herpesvirus 6 in drug reaction with eosinophilia and systemic symptoms (DRESS)/drug-induced hypersensitivity syndrome (DIHS). Br. J. Dermatol. 2014, 171, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Komatsu-Fujii, T.; Kaneko, S.; Chinuki, Y.; Suyama, Y.; Ohta, M.; Niihara, H.; Morita, E. Serum TARC levels are strongly correlated with blood eosinophil count in patients with drug eruptions. Allergol. Int. 2017, 66, 116–122. [Google Scholar] [CrossRef]
- Ogawa, K.; Morito, H.; Hasegawa, A.; Daikoku, N.; Miyagawa, F.; Okazaki, A.; Fukumoto, T.; Kobayashi, N.; Kasai, T.; Watanabe, H.; et al. Identification of thymus and activation-regulated chemokine (TARC/CCL17) as a potential marker for early indication of disease and prediction of disease activity in drug-induced hypersensitivity syndrome (DIHS)/drug rash with eosinophilia and systemic symptoms (DRESS). J. Dermatol. Sci. 2013, 69, 38–43. [Google Scholar] [CrossRef]
- Komatsu-Fujii, T.; Chinuki, Y.; Niihara, H.; Hayashida, K.; Ohta, M.; Okazaki, R.; Kaneko, S.; Morita, E. The thymus and activation-regulated chemokine (TARC) level in serum at an early stage of a drug eruption is a prognostic biomarker of severity of systemic inflammation. Allergol. Int. 2018, 67, 90–95. [Google Scholar] [CrossRef]
- Choquet-Kastylevsky, G.; Intrator, L.; Chenal, C.; Bocquet, H.; Revuz, J.; Roujeau, J.C. Increased levels of interleukin 5 are associated with the generation of eosinophilia in drug-induced hypersensitivity syndrome. Br. J. Dermatol. 1998, 139, 1026–1032. [Google Scholar] [CrossRef]
- Miyagawa, F.; Hasegawa, A.; Imoto, K.; Ogawa, K.; Kobayashi, N.; Ito, K.; Fujita, H.; Aihara, M.; Watanabe, H.; Sueki, H.; et al. Differential expression profile of Th1/Th2-associated chemokines characterizes Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) and drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/DRESS) as distinct entities. Eur. J. Dermatol. 2015, 25, 87–89. [Google Scholar] [CrossRef]
- Teraki, Y.; Fukuda, T. Skin-Homing IL-13-Producing T Cells Expand in the Circulation of Patients with Drug Rash with Eosinophilia and Systemic Symptoms. Dermatology 2017, 233, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Kano, Y.; Yamazaki, Y.; Kimishima, M.; Mizukawa, Y.; Shiohara, T. Defective regulatory T cells in patients with severe drug eruptions: Timing of the dysfunction is associated with the pathological phenotype and outcome. J. Immunol. 2009, 182, 8071–8079. [Google Scholar] [CrossRef] [PubMed]
- Ushigome, Y.; Mizukawa, Y.; Kimishima, M.; Yamazaki, Y.; Takahashi, R.; Kano, Y.; Shiohara, T. Monocytes are involved in the balance between regulatory T cells and Th17 cells in severe drug eruptions. Clin. Exp. Allergy 2018, 48, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).