Abstract
Nowadays, there is a need for reliable fluid biomarkers to improve differential diagnosis, prognosis, and the prediction of treatment response, particularly in the management of neurogenerative diseases that display an extreme variability in clinical phenotypes. In recent years, Tau protein has been progressively recognized as a valuable neuronal biomarker in several neurological conditions, not only Alzheimer’s disease (AD). Cerebrospinal fluid and serum Tau have been extensively investigated in several neurodegenerative disorders, from classically defined proteinopathy, e.g., amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), and Parkinson’s disease (PD), but also in inflammatory conditions such as multiple sclerosis (MS), as a marker of axonal damage. In MS, total Tau (t-Tau) may represent, along with other proteins, a marker with diagnostic and prognostic value. In ALS, t-Tau and, mainly, the phosphorylated-Tau/t-Tau ratio alone or integrated with transactive DNA binding protein of ~43 kDa (TDP-43), may represent a tool for both diagnosis and differential diagnosis of other motoneuron diseases or tauopathies. Evidence indicated the crucial role of the Tau protein in the pathogenesis of PD and other parkinsonian disorders. This narrative review summarizes current knowledge regarding non-AD neurodegenerative diseases and the Tau protein.
1. Introduction
In neurological disease management, there is a considerable demand for reliable fluid biomarkers to improve differential diagnosis and for prognostic purposes and the prediction of treatment response. Additionally, the presence of neurodegenerative processes in neurological diseases could be determined or rejected by specific fluid biomarkers and, therefore, helpful for subsequent clinical management. The Tau protein, along with beta-amyloid (Aβ), represents a milestone in Alzheimer’s disease (AD) diagnosis [1]. However, with Tau being a microtubular protein that reflects axonal loss, in recent years, evidence has been collected, particularly in cerebrospinal fluid (CSF) of multiple sclerosis (MS) subjects, to examine its role as a diagnostic and prognostic biomarker. Moreover, a pathological hyperphosphorylated form of the Tau protein (p-Tau) may be released during neurodegenerative processes, leading to a high volume of evidence supporting that total Tau (t-Tau), p-Tau, and its ratio, may be useful in amyotrophic lateral sclerosis (ALS) and frontotemporal spectrum disorder (FTSD) differential diagnosis. Finally, a crucial role of Tau in the pathogenesis of Parkinson’s disease (PD) and other parkinsonian disorders has been unveiled, leading to exciting future perspectives. This narrative review focused on summarizing the Tau protein’s role as a biomarker beyond AD disease. We searched studies published in worldwide, well established scientific databases, mainly PubMed/Medline.
2. Structure, Function, and Measurement of the Tau Protein
Tau, a protein belonging to the family of microtubule associated proteins (MAPs), is involved in cellular structure and localized primarily on a neuron’s axonal tracts and, at lower levels, in glia, either oligodendrocytes or astrocytes (Figure 1a) [2]. We can account for several physiological functions for the Tau protein, mainly including microtubular assembly and axonal stabilization; Tau may also support synaptic plasticity [3]. However, the exact mechanism of microtubule stabilization and assembly remains demanding to evaluate [4]. The alternative splicing of the microtubule associated protein Tau (MAPT) gene is responsible for six different isoforms of the Tau protein [4]. Tau function also depend on phosphorylation, which decreases Tau affinity in the microtubules, ensuring a balance between assembly and disassembly in healthy neurons. However, hyper-phosphorylation may occur in neurodegenerative disease, leading to an evident neuronal loss (Figure 1b) [4]. Furthermore, upon neuronal disruption from any physiological or pathological injury, t-Tau and p-Tau can be released in the extracellular milieu and CSF (Figure 1c). Therefore, Tau can be detected in the CSF of healthy subjects as a reflection of physiological aging with different values depending on the individual’s age, but, more importantly, as a marker of central nervous system (CNS) pathology in patients with neurodegenerative diseases [5,6], representing a biomarker of axonal loss in several neurological conditions. Tau probably experiences a spontaneous clearance from the CSF to serum [7]. Thus, in the same individual, its concentrations will be higher in CSF than in serum or plasma [8] (Figure 1c).
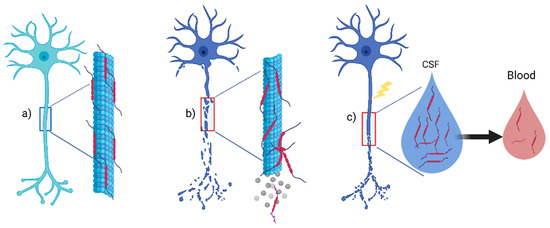
Figure 1.
Tau protein in the central nervous system. (a) Tau protein is a microtubule associated protein (MAP) that contributes with others MAP to axonal stabilization in healthy neurons; (b) phosphorylation of Tau will reduce affinity for microtubule, and in many neurodegenerative diseases, hyperphosphorylated-Tau will induce neuronal death; (c) upon any axonal damage from aging or pathological damage such as inflammation, t-Tau, and p-Tau will be released in CSF. Lower concentrations can also be found in peripheral blood. Abbreviations: CSF: cerebrospinal fluid. Created with Biorender.com.
Tau concentration is obtained mainly with commercially available immunoassays, such as enzyme linked immunosorbent assay (ELISA), electrochemiluminescence (ECL) [9], or Western blot [10]. Recently, a novel technology, high sensitive single molecule assay (SIMOA), has been introduced [11,12]. Given its higher sensitivity than conventional ELISA, SIMOA can measure CSF proteins outside CNS [12].
3. Multiple Sclerosis
MS is a chronic disease of the CNS. The disease pathology is heavily based on inflammation and demyelination. However, in the last decades, axonal and neuronal loss have been recognized from early disease stages [13,14,15], and an increasing number of studies have focused on investigating neurodegeneration and axonal damage, which appear only partially due to inflammatory processes. Therefore, MS can be considered an inflammatory neurodegenerative disease characterized by inflammation bursts resulting in acute axonal damage and a progressive chronic neuronal loss that increases over the years. MS is highly heterogeneous, with clinical manifestations ranging from sensory or motor dysfunction to fatigue and cognitive impairment [16,17]. The core MS phenotypes are relapsing and progressive diseases, further categorized into relapsing–remitting MS (RRMS), secondary progressive MS (SPMS), and primary progressive MS (PPMS) by the rating of disease activity (clinical relapses or magnetic resonance imaging worsening) and disease progression (increased neurologic disability) [18]. Consequently, the introduction of reliable diagnostic, prognostic, and treatment response biomarkers would be essential in clinical practice. To date, diagnostic biomarkers solely rely on identifying intrathecal IgG synthesis [19,20], whereas several CSF and serum molecules have been investigated as prognostic and treatment response biomarkers [21,22], but none have yet been translated in clinical practice. Over recent years, many studies have examined total Tau (t-Tau) and p-Tau in the MS population, particularly exploring their concentration in CSF and association with clinical and radiological parameters, and only a few investigated other biological fluids. As we will analyze in detail in the following subsections, results were not always concordant, possibly based on differences in the patient populations and methodologies, which could have affected the results.
3.1. CSF Tau in MS: Role in the Diagnosis
Studies comparing MS patients and healthy controls (HC) often exposed discrepant results. CSF t-Tau was found increased in most studies [23,24,25], whereas few reported normal or decreased levels in small sample sizes [26,27,28,29,30]. Nonetheless, a recent meta-analysis of 17 studies confirmed that t-Tau increased in MS patients [31]. To note, several of those studies included MS and control groups without age and sex-matching [31]. Most studies were focused on evaluating t-Tau alone or combined with other biomarkers, such as Aβ, neurofilaments light chain (NfL), S100, and GFAP, thus comparing differences in MS population between neuronal and astrocytic proteins [23,25,28,32,33]. Some groups also included the analysis of p-Tau and t-Tau [7,32,33], and some detected an increased immunoreactivity of phosphorylated epitopes in progressive MS patients compared to HC. They suggested that, in the CSF of progressive MS, detection of increased p-Tau in the absence of increased t-Tau may be a highly sensitive marker of axonal damage, helpful in differential diagnosis with relapsing MS [32]. In contrast, Jaworsky et al. found similar p-Tau concentrations in MS and HC, while increased t-Tau were detected in MS patients [7]. To our knowledge, in MS, no studies have evaluated the p-Tau/t-Tau ratio. Given the possible impact of age, sex, and relapses [33], it would be crucial that comparison groups are matched for potential confounding factors in future studies. In addition, most studies were conducted with European patients, such as Pietroboni et al. [28], and these analyses must be extended to different populations [31].
3.2. CSF Tau in MS: A Marker of Phenotypic Variability?
Considering the extreme clinical variability of MS phenotypes, a helpful biomarker might differentiate particularly progressive and inflammatory phenotypes, which have different prognosis and treatment options. Highly heterogeneous results were obtained when categorizing patients based on phenotypes. The group of Kapaci detected high CSF t-Tau levels in progressive MS and ascribed these results to higher neurodegeneration in these forms than in relapsing MS [24]. By contrast, Jaworsky et al. observed lower CSF t-Tau levels in 14 SP patients than in 34 RR patients [7], while Terzi et al. found similar values [34]. The Jaworsky group hypothesized that SP subjects experience a decrease in neuronal volume and axonal quantity, resulting in the loss of Tau resources [7]. The limitation in stratifying people with MS is the small numbers of progressive phenotypes, especially PP, typically included in observational studies. Moreover, some studies may have considered RRMS during relapses and some during remission, possibly affecting the comparison with progressive MS [31].
Among relapsing phenotypes, no significant differences were observed between clinically isolated syndrome (CIS) and RR [31], but we need to consider the evolution of MS diagnostic criteria, with some CIS now being classified as RRMS [35]. Another interesting point would be to assess differences in the demographic features of progressive MS, such as age, since a linear age associated increase in CSF t-Tau has been observed in both HC and AD patients [6].
3.3. CSF Tau in MS: Role in the Prognosis
Regarding biomarkers of disease activity, NfL has been consistently associated with the presence and the number of gadolinium-enhancing lesions [31], whereas both Mori et al. and Virgilio et al. did not observe differences for t-Tau [36,37]. A correlation between magnetic resonance imaging (MRI) lesion load and t-Tau concentrations has been reported by several studies [28,38,39]. Conversely, others observed differences in t-Tau and p-Tau levels in patients with relapses, compared with stable patients [33]. An exciting possibility is that CSF Tau may be a prognostic factor for disability accumulation over time. In particular, based on their results, Frederiksen et al. proposed CSF t-Tau as a predictor of progression from CIS to defined MS [40]. Evidence pointed out that CSF Tau at diagnosis marks chronic neurodegeneration, clinical disability, and poor prognosis [2,7,34,40,41]. Most authors used the expanded disability status scale (EDSS) or, rarely, EDSS plus multiple sclerosis severity score (MSSS) as measures of disability, whereas Virgilio et al. also used age related MSSS (ARMSS) in a cohort of 100 Italian MS patients [41]. However, Edwards et al. performed three or four repeated lumbar punctures over 28 weeks in 16 SPMS patients under dimethyl fumarate (a DMT available worldwide for CIS and RRMS, but also approved for active SPMS in the US) to characterize the pharmacokinetics and CSF penetration of monomethyl fumarate—the drug active metabolite—and evaluate axonal damage biomarkers using the SIMOA assay at Quanterix (Lexington, MA) [42]. CSF t-Tau levels remained stable over the treatment, and the authors found no correlation with EDSS or MRI activity, unlike NfLs [42]. Even though published data are pretty heterogeneous in terms of disease characteristics and treatments, CSF t-Tau at diagnosis seems to correlate with disease duration and disability scores, while results on disease activity seem to be less coherent. We might speculate that t-Tau may reflect a chronic persistent axonal loss, rather than express an axonal damage caused by acute inflammation.
3.4. Tau and Cognitive Impairment
Cognitive impairment (CI) is frequently seen as a disabling symptom in MS patients [43], even in early disease stages [44]. Although its exact physiopathology is unknown, axonal loss from early disease stages may be partially responsible for its development [16]. Characterization of CI and the study of brain atrophy in MS in the last decades represent hot topics. Still, no specific soluble biomarkers are available for CI in MS, unlike other neurodegenerative diseases. Few studies focused on axonal damage and CI biomarkers in MS, mainly NfL, with conflicting results [16,45,46]; only one study described a correlation between CSF Aβ levels and CI [37]. In contrast, recently, Virgilio et al. observed, in 62 patients, a correlation between CSF t-Tau and information processing speed and global cognition, whereas NfL and Aβ could not discriminate CI patients [36]. Moreover, baseline t-Tau and NfL were predictors of brain atrophy after three years of follow up [47]. These preliminary results need confirmation in future studies, opening new possible uses of CSF t-Tau to evaluate CI in MS patients [48].
3.5. The Role of Tau Imaging in MS
Unlike other neurodegenerative diseases, evaluation of in-vivo Tau brain pathology with Tau radioligands such as [18F]AV-1451 has been poorly explored in MS. Only Zeidan et al. [48] included 12 patients with MS and 60 matched HC (for age, sex, and APOE ε4 status). Cognition was checked with four neuropsychological tests, and, as expected, MS patients displayed statistically significant differences in executive functions and language. No significant differences were observed; however, a trend for higher regional cortical AV-1451 standard uptake value ratios (SUVrs) was observed in MS patients compared to HC, and patients with longer disease duration displayed grater AV-1451 SUVrs.
3.6. Peripheral Tau in MS: New Evidence
Few data on different biological fluids other than CSF in the MS population are available. CSF is less accessible and repeatable than blood, saliva, or tears. However, compared with CSF, most kits used in blood or saliva have not yet been standardized and validated for clinical uses [49]. In 2011, Bartosik et al. evaluated serum t-Tau over 24 months from mitoxantrone administration in 54 MS patients [23]. They observed a decrease in biomarker concentrations, indicating that depletive anti-inflammatory, immunosuppressive DMTs also reduce axonal loss in RR and SP patients. However, both Bartosik and Jaworsky et al. demonstrated that several patients displayed serum levels of t-Tau under the detection limit using a sandwich ELISA, even though MS patients showed high mean t-Tau serum concentrations compared to HC (Innotest hTAU-AG, Innogenetics, Ghent, Belgium) [7,23]. Therefore, as for other molecules (i.e., NfL), it is advisable to use a highly sensitive array to evaluate serum Tau concentration over time after treatment with DMTs. By contrast, Mirzaii-Dizgah et al., using another ELISA (BioAssay Technology Laboratory, Shanghai, China), detected lower Tau protein levels in the serum, but not in the saliva, of 30 MS patients compared to HC. Patients also displayed a negative correlation with EDSS [8]. Finally, Islas-Hernandez et al. used Western blotting (DCTM Protein Assay Kit, BioRad) to comply with Bartosik-Psujek and described that SP patients tend to display reduced levels of t-Tau in serum [10], in line with the results reported years before by Jaworsky et al. [7].
4. Amyotrophic Lateral Sclerosis
ALS is a chronic, rapidly progressive neurodegenerative disease characterized by a motoneurons’ degeneration in selected areas, such as the motor cortex, brainstem, and spinal cord, with a disease duration variable from 3 to 5 years, usually related to the worsening of muscular weakness and respiratory failure [50]. Most of the cases (roughly 85–90%) are sporadic (sALS), while a positive family history for ALS is reported in a minority of patients (fALS) (10–15%) [51]. The clinical phenotype of the disease is heterogeneous at onset and progression [52], and half of the patients display CI, ranging from mild CI to FTD [53,54].
Most ALS patients (>95%) have transactive DNA binding protein of ~43 kDa (TDP-43) inclusions in postmortem studies, whereas Tau is not noteworthy [55] (except for Guam ALS/parkinsonism, which is mainly a tauopathy [56]). No specific diagnostic tests are available for ALS, and the diagnosis is primarily reached by excluding secondary/acquired forms [57]. Indeed, there are no precise prognostic markers, including fluid biomarkers, available for ALS, and, frequently, all of the proposed biological markers give confounding and conflicting results. Only NfLs have been recently proven to mark neurodegeneration and clinical progression in ALS, with the highest levels in ALS rather than FTD [58,59].
4.1. CSF Tau in ALS: Role in the Diagnosis
In recent years, several cytoskeletal proteins have emerged as candidate ALS biomarkers. Among them, the role of the Tau protein in motoneuron diseases has been investigated in a few studies, without achieving univocal results.
Overall, some studies showed a significant difference in CSF t-Tau and p-Tau levels between ALS patients and HC, both with a diagnostic and prognostic role, while other works fail to demonstrate a correlation between the protein and the disease. In a small preliminary study, high Tau levels were shown in 70% of patients compared to controls, with the highest Tau levels in the early disease stage [60]. Similar results were confirmed by the same group years later, showing increased levels of the Tau protein both in comparison with HC and other MND forms [61]. After analyzing p-Tau and t-Tau in CSF separately, p-Tau was not significantly reduced in ALS patients compared with HC. However, t-Tau was increased considerably, with a consequent reduction of the p-Tau/t-Tau ratio [62]. Integrating the ratio with the TDP-43 level in a combined formula, this score resulted in a specific and sensible index for diagnosis [63]. In line with these findings, a recent Italian study observed that ALS patients showed significantly higher CSF t-Tau and a lower p-Tau/t-Tau ratio than controls (p-value < 0.001). However, no differences in p-Tau levels were detected [64]. In another cohort, ALS patients had higher levels of t-Tau and lower p-Tau/t-Tau ratio than ALS mimics and other not neurodegenerative diseases, although without ligh levels of sensibility and specificity [65,66].
4.2. CSF Tau in ALS: Role in the Prognosis
ALS progression and survival are highly variable among patients, without clear markers able to predict it. Can Tau help in this regard? The Grossman group published a consistent paper in 2010, showing deficient CSF levels of p-Tau in ALS and revealing that the p-Tau/t-Tau ratio could distinguish individuals with ALS from HC and individuals with tauopathies. Additionally, in the same study, low p-Tau levels and p-Tau/t-Tau ratio correlated with clinical measures of disease such as the ALS functional rating scale-revised (ALSFRS-R) score and mini-mental state examination (MMSE), and with MRI measures of reduced white matter fractional anisotropy in the corticospinal tract and prefrontal cortex in ALS subgroups [67]. In terms of correlation with upper motor neuron involvement, these results were further confirmed with a study that showed that a low p-Tau/t-Tau ratio was associated with global grey matter brain atrophy and diffuse white matter integrity loss, highlighting that this index can be a marker of central motor degeneration [68]. A recent Italian study [64] showed, by multivariate analysis, that t-Tau acts as an independent negative predictor of overall survival, and high levels of this biomarker are associated with a fast rate of ALSFRS-R score progression. In another above cited cohort, the authors demonstrated that CSF t-Tau correlated with progression rate and muscle strength indexes (e.g., the sniff nasal inspiratory pressure). On the contrary, CSF p-Tau was not related to any ALS clinical feature [65]. In addition, the group of Blasco correlated the Tau level with functional score and disease progression, with possible prognostic meaning, and multivariate analysis revealed that ALSFRS-R at baseline was associated with the p-Tau/t-Tau ratio. Both measurements independently correlated negatively with ALSFRS-R variation over the disease course; consequently, the p-Tau/t-Tau ratio correlated positively with ALSFRS-R changes [69]. Finally, the role of t-Tau as a marker of rapid disease progression was recently confirmed, especially integrating this marker with NfL [70].
These studies, albeit heterogenous in terms of results, methods, and sample size, indicate that p-Tau has poor sensitivity and specificity, but t-Tau, and especially the p-Tau/t-Tau ratio, have moderate sensitivity and specificity for both the diagnosis and prognosis of motoneuron diseases. Tau levels may help to confirm ALS diagnosis and establish the prognosis despite the lack of high specificity, especially when integrated with other fluid biomarkers. However, further studies are mandatory to explore the pathophysiological and neuropathological mechanisms associated with these findings and confirm the clinical value.
5. Frontotemporal Spectrum Disorder
FTSD is an insidious neurodegenerative syndrome resulting from progressive language, behavioral and executive deficits. The disorder is the most frequent early onset dementia and, globally, the third most common form of dementia across all age groups after AD and Lewy bodies dementia (DLB) [71]. Clinically, FTSD is classified into three clinical variants, one behavioral and two with language alterations. The first one is associated with early behavioral and executive deficits (bvFTD); the other two include the nonfluent variant (PNFA), with progressive deficits in speech and grammar, and semantic dementia (SD), which is characterized by a progressive deficit of semantic knowledge and naming. Progressively over the disease course, the symptoms of the three variants can converge and overlap as the damage spreads in the frontal and temporal lobes, showing severe cognitive impairment, parkinsonism, and motoneuron disorders. The disease duration varies from 8 to 10 years, and death is mainly due to cachexia and infections [72]. FTSD patients have multiple neuropathological phenotypes, including the microtubule associated protein tau (MAPT), the TDP-43, or the fused in sarcoma (FUS) protein. In frontotemporal lobar degeneration (FTLD), the commonest subtypes of Tau related disease are Pick’s disease (30%), corticobasal syndrome (CBS) (40%), and progressive supranuclear palsy (PSP) (30%) [73]. For FTSD, an extended clinical assessment associated with language, socio-emotional functioning, cognition, and neuroimaging evaluation (brain magnetic resonance to evaluate the atrophy specific patterns and positron emission tomography for the brain metabolism) can support the diagnosis [74].
5.1. CSF Tau in FTLD: Is a Pathological Role Possible?
Pathologically, it has been shown that FTSD has inclusions mainly containing either Tau or TDP-43. However, a clinicopathological correlation is not easy, and diagnosing the correct proteinopathy during life (without autoptic data) is hard. To date, this is possible only when a genetic cause is present (e.g., mutations in MAPT and Tau pathology, in GRN and C9orf72 and TDP-43 pathology) or for specific phenotypes (e.g., the ALS-FTSD spectrum is primarily associated with TDP-43 pathology).
Overall, the CSF p-Tau/t-Tau ratio has high accuracy in discriminating FTLD patients compared to HC and AD, and it is driven by elevated t-Tau levels in patients [75]. However, it seems that FTSD CSF Tau levels are not different in FTLD patients with underlying Tau pathology (e.g., with MAPT mutations) compared with Tau negative or sporadic FTLD. The first published study in this regard, in 2003, showed only a mild increase in t-Tau levels in FTSD patients compared with nondemented controls and lower levels in the subgroup with Tau mutations compared with AD patients. Moreover, p-Tau was not significantly different in FTSD patients based on genetic status, compared with HC [76]. Similarly, Rohrer and colleagues demonstrated that CSF t-Tau concentrations were considerably higher in the FTLD-TDP-43 and FTLD-Tau groups than in controls, without differences between the patient groups. Similar results were obtained for the p-Tau/t-Tau ratio, which was significantly lower in the FTLD-TDP-43 and FTLD-Tau groups than in controls, but without any difference between the two patient groups [77]. Despite this, the capacity of the ratio to distinguish the pathological phenotypes is debated, and other groups found a significantly reduced CSF p-Tau/t-Tau ratio in FTLD-TDP-43 compared to FTLD-Tau, with a decent predictive value [78,79,80]. The decreased ratio in FTLD-TDP-43 seems to be driven by low levels of p-Tau in FTLD-Tau, while t-Tau levels were similar [75], but a possible copresence of MND in the FTLD-TDP-43 forms must be considered.
5.2. CSF Tau in FTLD: A Marker of Phenotypic Variability?
Considering the extreme clinical variability of FTSD phenotypes, a helpful biomarker would allow to differentiate particularly bvFTD and PNFA and SD, which can have different prognosis and experimental and symptomatic treatment options. For example, regarding differences between phenotypes, results are highly heterogeneous. The group of Bittner described higher t-Tau levels in SD than HC and CBS/PSP patients. Additionally, p-Tau was higher in all FTSD than CBS/PSP subjects but still within the normal range. Furthermore, unremarkable results were detected by comparing p-Tau and t-Tau in PNFA and non-PNFA patients [81]. By contrast, Meeter et al. observed that the p-Tau/t-Tau ratio can discriminate FTSD from controls, but not the clinical subtypes, except for cases with concomitant MND involvement [75]. Although not easy because of the rarity of neurodegenerative language disorders, this investigation certainly deserves to be developed, especially to distinguish the different forms of aphasia and the AD phenotype.
5.3. Tau in FTLD: Role in the Prognosis
Few data are accessible on the prognostic role of CSF Tau in FTLD patients. In 2014, an Italian group demonstrated that FTSD patients with high CSF Tau levels (≥400 pg/mL) had shorter survival than those with low CSF Tau levels [82]. In 2018, Ljubenkov et al. showed that high baseline levels of CSF t-Tau and p-Tau predicted a fast rate of worsening only in bvFTD patients. Additionally, p-Tau had a similar predictive value as NfL in bvFTD, while t-Tau had predictive value only in PNFA subjects, likely correlating specific differences in Tau production, post-translational changes, or degradation due to the different FTLD subtype [83]. By contrast, moving to plasma, it was demonstrated that p-Tau levels were not correlated with neuropsychological, behavioral, and functional measures and did not help to monitor disease severity or predict prognosis throughout the FTLD spectrum [84]. Similarly, the group of Rojas demonstrated that high baseline plasma t-Tau concentrations were associated with a fast decline in the bvFTD and PSP subgroups of FTLD, but introducing the plasma t-Tau in a Cox model for survival did not change the event probability [85]. Since the p-Tau/t-Tau ratio is a nonspecific marker of neuronal loss, it is not surprising that it has a limited role in subtyping different FTSD phenotypes, across bvFTD, PNFA, and SD in several series. Nevertheless, despite other markers, many reports indicate that the p-Tau/t-Tau ratio is relatively specific in differentiating TDP-43 from Tau pathology, helping the choice of disease-modifying drugs that can target the specific underlying pathological mechanism. At this point, it is clear that further studies may improve understanding of the essential pathophysiological role of plasma Tau levels, but, to date, the extensive overlap with those of HC limits the diagnostic utility.
5.4. Peripheral Tau in FTLD: New Evidence
Current CSF biomarkers, including dosage of TDP-43 and Tau, cannot accurately identify the underlying phenotype and proteinopathy in vivo in FTSD, and, therefore, novel measures should be identified. Hence, the need to investigate plasma biomarkers, including the Tau assay. Plasma Tau has been measured in several studies, observing an increment in plasma Tau levels in patients with CI, but independently from AD or FTSD. In 2018, Rohrer measured plasma Tau concentrations in a large group of FTSD patients with an ultrasensitive detection method, and showed that bvFTD and PNFA displayed higher plasma Tau concentrations than controls. In addition, upon stratifying for genetic data, only the MAPT group had significantly increased concentrations of plasma Tau. On the contrary, there were no correlations of Tau levels with brain volumes, serum NfL concentrations, or disease duration [86]. Another work, including patients with different FTSD variants, showed increased plasma Tau levels in all clinical FTSD subgroups, but—in terms of genotype—only in MAPT mutations [87].
5.5. The Role of Tau Imaging in FLTD
Regarding Tau-PET with [18F]AV-1451, recent clinical trials aspired to diminish pathological protein aggregates in neurodegenerative diseases. Similar to the role of Aβ PET in clinical trials for AD, an imaging marker able to quantify Tau can help develop anti-Tau drugs, supporting participant selection, early intervention, and assessment of proper goals.
To date, in vitro studies with the AV-1451 tracer in non-AD patients obtained contrasting results [88,89]. However, the number of in vivo studies has been growing recently: one of the most consistent studies, published in 2019 by the group of Rabinovici, described the [18F]AV-1451 PET findings of FTSD patients with different clinical and genetic features. A single subject analysis reported a low level pattern of [18F]AV-1451 binding able to coordinate Tau pathology’s expected anatomical distribution and frequency. For example, nfvPPA patients showed increased binding in the left inferior frontal gyrus, CBS patients in frontal white matter, and 50% of bvFTD in frontotemporal areas. However, compared with controls, they frequently did not observe regions with significant retention or a modest overlap between patients and HC [90]. Concerning MAPT mutation, the same group observed an increased [18F]AV-1451 retention in bilateral temporal lobes [90], confirming the results of previous studies [91,92]. In addition, a new promising tracer, the 18F-MK-6240, was recently used in an elegant work to bind Tau in vivo in genetic FTSD. This group reported a mild but significant binding of tracer in amyloid negative MAPT mutation patients, highlighting that a positive [18F]MK-6240 tau-PET does not imply with certainly an AD diagnosis [93], and pointing towards a potential use of Tau tracers as a biomarker in tauopathies beyond AD, even with some limitations due to the modest affinity.
6. Parkinsonian Syndromes
Parkinsonian syndromes, particularly Parkinson’s disease (PD), are prevalent neurodegenerative disorders. Clinically, PD presents bradykinesia, resting tremor, rigidity, postural instability, and nonmotor symptoms [94]. The pathological hallmark of the disease is the progressive depauperation of dopaminergic neurons in the substantia nigra pars compacta (SNc) and the formation of Lewy bodies (LBs) in the residual neurons. LBs consist of aggregated forms of the α-synuclein protein (α-syn), which contribute to the pathogenesis of PD [95]. Even though exact mechanisms underlying the α-syn aggregation are not fully understood, both active and passive immunization strategies targeting this protein have been developed. Unfortunately, immunization with α-syn was effective in animal PD models [96,97], but phase 2 clinical trials failed to meet primary endpoints. These findings questioned the role of α-syn aggregates in cell death and suggested that protein aggregation may be the consequence of damage, rather than being the primary cause of neurodegeneration, and stimulated researchers to find other contributors to PD pathology. In this context, an underappreciated component is the Tau protein, which shares some properties with α-syn, as they are both brain proteins with prion like characteristics. Furthermore, genome wide association studies indicated that single-nucleotide polymorphisms (SNPs) in MAPT and SNCA genes are common risk factors for PD [98,99].
6.1. The Role of the Tau Protein: Evidence from Animal Models and Neuropathology
In neurodegenerative disorders, interactions between α-syn and Tau can promote protein fibrillization and stimulate the creation of pathological inclusions [100]. Evidence confirmed that α-syn contributes to Tau phosphorylation, mainly via the glycogen synthase kinase 3beta (GSK-3β) in the PD animal model [101] and α-synuclein-overexpressing transgenic mice overexpressed α-syn, p-Tau, and p-GSK-3β. Moreover, these proteins are co-localized in large inclusion bodies, similar to LBs [102]. Those results were also replicated in human models: Arima et al. found the co-localization of Tau and α-syn in PD brains [103], while Compta et al. reported a combination of Lewy and AD type inclusions as dementia’s pathological correlates [104], corroborating Tau’s involvement in PD. Furthermore, Tau pathology was explored in PD patients receiving fetal neural allografts as cell replacement therapy: Cisbani et al. found that hyperphosphorylated Tau can be detected in grafted tissue 16 years post-transplantation [105]. Similarly, Ornelas et al. showed neuronal perikaryal inclusions of phosphorylated α-syn and Tau in the graft tissue [106], suggesting that both α-syn and Tau pathology can spread from the host to the graft.
6.2. Tau Protein in PD: Role in the Diagnosis and Prognosis
Published literature has widely investigated CSF Tau levels as biomarkers for PD diagnosis and progression. Higher CSF t-Tau levels were reported in nondemented PD patients compared with HC [99,107] and mainly in the subgroup of patients with short disease duration, implying that the initial PD stages are crucial for neurodegenerative changes. However, 20 patients (62.5%) were treated with levodopa in the study, which opens the question of possible treatment interference. In this regard, it was proved that CSF α-syn levels at 12 months were lower in PD patients treated with dopamine replacement therapy, especially dopamine agonists, but no significant relationships were found with t-Tau and p-Tau levels [108]. A total of 109 newly diagnosed, drug naive, and cognitively spared PD patients displayed significantly reduced CSF Aβ but not t-Tau or p-Tau, compared to controls [109]. Another Italian study found that PD patients showed similar CSF t-Tau and p-Tau as controls, but lower levels compared to dementia with Lewy bodies (DLB), AD, and FTSD subjects [110]. The same research group subsequently found that greater diagnostic accuracy in detecting PD patients could be achieved by combining oligo/total α-syn and Aβ/tau ratios, further confirming the role of Tau in disease assessment [111]. How these biomarkers varied across different disease time points was evaluated by Mollenhauer et al. by sampling CSF α-syn, t-Tau, p-Tau, and Aβ42 levels at baseline and after 6 and 12 months in a large cohort of PD patients and matched HC. Results showed that t-Tau remained stable, and there was a slight increase in p-Tau in PD patients, but there was no correlation with motor scores or dopamine imaging [108]. Conversely, another study showed that p-Tau levels were lower in 112 nondemented PD patients than in HC at baseline and increased significantly after one year, whereas t-Tau levels did not show significant longitudinal changes after the same follow up time [112].
6.3. Tau Protein in PD: A Marker of Phenotypic Variability?
6.3.1. Tau Protein and Motor Symptoms in PD
The relationship between Tau levels and PD motor and nonmotor characteristics has been assessed by several studies. CSF Tau levels helped to detect tremor dominant (TD) PD since non-TD patients displayed higher levels of t-Tau and an increased Tau/Aβ42 index [113,114]. In the context of the Parkinson’s progression marker initiative (PPMI), others found decreased CSF t-Tau levels and a correlation with increased motor severity. Furthermore, low CSF Aβ42 and p-Tau levels were associated with the postural instability gait disturbance (PIGD) dominant phenotype [115]. A subsequent PPMI study involving 660 PD patients partially confirmed these findings [116]. Intriguingly, genetic background might also be relevant, since Vilas et al. [117] described that, in the subgroup of patients with a mutation in the leucine rich repeat kinase 2 (LRRK2) gene, CSF t-Tau levels were higher in PIGD-PD than TD-PD, whereas no differences were detected when all patients (with or without LRRK2 mutation) were analyzed together. Another study aimed to identify distinct subgroups via cluster analysis and found that patients with diffuse malignant PD had the lowest level of Aβ and Aβ/t-Tau ratio in CSF. However, similar t-Tau and p-Tau levels were detected in the mild motor predominant and intermediate phenotypes [118]. In a longitudinal analysis of 403 PD patients, the DATATOP Investigation Group reported that the rate of change in CSF t-Tau levels significantly correlated with the rate of motor unified Parkinson’s disease rating scale (UPDRS) change. Similar findings were observed between CSF t-tau/Aβ42 variations and modifications in total and motor UPDRS [119]. In summary, distinct PD phenotypes and the severity of motor symptoms may underlie specific biomarker dynamics, but the role of Tau has not been unequivocally established.
6.3.2. Tau Protein and Nonmotor Symptoms in PD
Regarding nonmotor symptoms, an increased CSF t-Tau/Aβ42 ratio was described in PD patients with REM sleep behavior disorder [120]. The role of Tau has been primarily explored in the context of CI and PD dementia (PDD) [121]. Several studies showed increased CSF levels of t-Tau and p-Tau in PDD subjects compared with HC [122,123]: PD patients with a high p-Tau and p-Tau/Aβ42 ratio developed subsequent decline in cognitive tasks, particularly memory and executive functions [124]. Similarly, CSF t-Tau/Aβ ratio was associated with Montreal cognitive assessment (MoCA) score at two years in 390 PD patients [125], and t-Tau/Aβ42, t-Tau/α-syn, t-Tau/Aβ42+α-syn, and Aβ42/t-Tau ratios showed a significant association with the risk of progression to dementia over a 3-year follow up [126]. Nonetheless, heterogeneous results have been described, since another study reported that CSF p-Tau concentrations were 20% lower in cognitively normal-PD and CI-PD without dementia than in age matched HC, but levels of t-Tau were not changed in PDD patients [127]. Similarly, Bibl et al. did not find significant differences in t-Tau CSF levels between PDD and controls [128], and there were no correlations between Tau levels and cognitive measures [109]. A longitudinal study [129] evaluating CSF biomarkers in 415 PD patients with ten years of follow up failed to find any significant association between t-Tau, p-tau, and MoCA scores. These findings might support the hypothesis that PD cognitive dysfunction is associated with an AD like CSF biomarker profile [130,131], but caution should be taken when considering CSF Tau measurements in PD.
6.4. Tau Protein in Atypical Parkinsonian Syndromes: Role in Diagnosis
Tau protein evaluation might also be relevant in the differential diagnosis between PD and atypical parkinsonism disorders (APD). APDs are a group of heterogeneous neurodegenerative diseases such as multiple system atrophy (MSA), progressive supranuclear palsy (PSP), corticobasal syndrome (CBS), and DLB. Among α-synucleinopathies, MSA is a progressive neurodegenerative disease characterized by autonomic disturbances, pyramidal, parkinsonian or cerebellar features [132]. Regarding DLB, the diagnosis is suggested by the appearance of parkinsonian symptoms, dementia, hallucinations, and delusions, frequently fluctuating during the day. Clinical diagnostic criteria have been delineated for both DLB and PDD, and the two diseases are differentiated based on the 1-year rule of cognitive and motor symptoms’ occurrence [133]. PSP is a tauopathy characterized by atrophy of the dorsal midbrain and a rapidly evolving parkinsonism with unsteadiness of gait, falls, and alteration of vertical eye movements, even though multiple clinical phenotypes have been included in the diagnosis [134]. CBS consists of various asymmetrical parkinsonian features, dystonia, myoclonus, and different underlying pathological substrates such as corticobasal degeneration (CBD), AD, PSP, and FTSD-TDP-43. Over the years, the terms CBS and CBD have been used interchangeably, but the latter refers to the specific pathological entity of a 4-repeat tauopathy [135]. Due to possible clinical overlap in the initial stages of the disease, the distinction between PD and other parkinsonian syndromes can be challenging, thus prompting researchers to identify reliable biomarkers.
Hansson et al. [136] found increased CSF t-Tau levels in MSA and CBS patients compared with PD, and lower t-Tau levels in PSP than MSA and HC, whereas CSF p-Tau levels were significantly lower in MSA and PSP than in controls, but no differences were detected compared with PD patients. In the validation cohort of the same study [136], however, there were no differences between groups in t-Tau levels, and significantly lower levels of p-Tau were observed just in PSP patients compared with HC and PD. Furthermore, in the first cohort, high levels of NfL were associated with high levels of CSF t-Tau, but this was not confirmed in the validation cohort. The discrepancies of these results have challenged these biomarkers’ diagnostic utility, as highlighted in other studies. Sussmuth et al. observed higher CSF t-Tau in PSP-parkinsonism (PSP-P), MSA-parkinsonism (MSA-P), and MSA-cerebellar (MSA-C) when compared with PD. Patients with PSP-Richardson’s syndrome (PSP-RS) had normal t-Tau levels. By contrast, PSP-P patients displayed significantly higher levels than PSP-RS, PD, and HC. CSF p-Tau was not informative in differentiating APD. However, P-tau/T-tau ratios were lower in PSP and MSA when compared with PD [137]. Similarly, several studies confirmed higher CSF t-Tau in MSA than PD [138,139,140,141]. In contrast, CSF t-Tau and p-Tau did not help to discriminate between MSA and PD in other cohorts [142,143]. Nonetheless, the good clinical accuracy of Tau/α-syn ratio in discriminating DLB patients was reported [143], in line with the increase in t-Tau and p-Tau found in DLB [144,145]. Evidence suggested that CSF Tau levels are higher in DLB than in PSP and CBD [145] and compared with other synucleinopathies [123,143], but significantly lower than in AD [146]. Furthermore, recent studies reported substantially lower plasmatic p-Tau levels in DLB than in AD, and an association with the progression of cognitive decline [58]. Even though Tau level could be affected by age [6,104,142], it might still be helpful as a prognostic factor.
6.5. The Role of Tau Imaging in Parkinsonian Syndromes
The contribution of brain Tau aggregates can be evaluated in vivo using specific radioligands, such as [18F]AV-1451, also known as [18F]T807 [147,148]. This radiotracer has been extensively used in parkinsonian disorders: a reduction in PD patients of its volume of distribution compared with controls reflected the loss of pigmented neurons in the SN [149]. The relationship between Tau imaging and CI has also been widely investigated, and Tau aggregates in PD correlate with the severity of CI in both DLB and PDD [150,151], with a more significant Tau burden in DLB than PDD tissue [152]. Nonetheless, Tau binding of [18F]AV-1451 was lower than binding predicted from pathological studies: for instance, Winer et al. reported no differences in the patterns of Tau deposition in 15 cognitively normal PD, 14 cognitively impaired PD, and 49 cognitively normal HC [153]. Similarly, it was suggested that Tau pathology, evaluated through [18F]AV-1451, is uncommon in PD with mild CI and no significant correlation with cognitive dysfunction [154], at baseline as well as after a 2-year follow up [155], was observed. However, cortical Tau aggregates were found in DLB and CI PD, suggesting that patients with DLB display a spectrum of Tau pathology [156]. Regarding other APDs, several studies observed in PSP-RS elevated radioligand uptake mostly in subcortical structures, including midbrain, dentate nucleus, thalamus, subthalamic nucleus, globus pallidus, and striatum, compared to controls [157,158]. A multicenter study involving 33 PSP, 26 PD, and 46 HC patients found that [18F]AV-1451 uptake in the globus pallidus had the highest accuracy in PSP differential diagnosis [159]. Moreover, different degrees and patterns of Tau uptake may reflect the presence or absence of AD pathology in CBS patients [160]. In summary, Tau imaging may be valuable in parkinsonian disorders, even though some limitations due to potential off target binding should be considered when interpreting the results [161].
6.6. Future Perspectives: Tau Based Therapies
The involvement of Tau in several neurodegenerative diseases makes it a suitable therapeutic target in the evolving scenario of potential disease-modifying treatments. Currently developed strategies include active Tau vaccines, monoclonal antibodies, the development of microtubule-stabilizing agents, and post-translational modifications [162]. BIIB092 (Gosuranemab) lowered CSF Tau levels in PSP [134] and showed a favorable safety profile in a phase 1 trial [163], but it did not meet the primary and secondary endpoints in the phase 2 PASSPORT study [164]. Davunetide, a neuropeptide with microtubule-stabilizing properties, failed to confirm the efficacy reported in animal models [165]. Another approach using Tideglusib, an inhibitor of GSK-3β, was unable to show significant clinical differences in a multicenter, randomized, double blind, placebo controlled trial [166]. In the context of Tau based therapies in PD, the inhibitors of GSK-3β (L803-mt and AR-A014418) reduced Tau phosphorylation and spared dopaminergic neurons from cell death in mesencephalic cultures [167,168].
7. Final Remarks and Conclusions
We present the current principle knowledge on Tau protein in different non-AD neurodegenerative diseases. Table 1 and Figure 2 summarize the main findings for all the discussed neurodegenerative diseases.

Table 1.
Overview of the principal findings regarding Tau protein in non-AD neurodegenerative diseases.
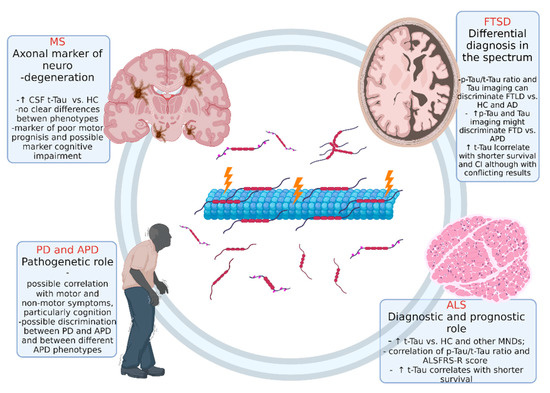
Figure 2.
Overview of the role of Tau protein in different non-AD neurodegenerative disease. Abbreviations: ALS: amyotrophic lateral sclerosis, FTSD: frontotemporal spectrum disorder; MS: multiple sclerosis; PD: Parkinson’s disease. Figure created with Biorender.com.
The existing expertise ranges from conditions where Tau plays neuropathological roles (FTD and certain APD) to newly recognized neurodegenerative diseases (i.e., MS) where the Tau protein represents an interesting axonal damage biomarker that will be further investigated in the future. Several lines of evidence support the crucial role of Tau in the pathogenesis of PD and other parkinsonian disorders. Nonetheless, multiple challenges still have to be overcome to obtain reliable imaging and biofluid markers. Developing trackers of early diagnosis and disease progression will provide, indeed, invaluable help for the research of novel therapeutic strategies. ALS Tau levels may help in the diagnostic processes and define patients’ prognosis, particularly when associated with other markers. However, further studies are mandatory to explore the pathophysiological and neuropathological mechanisms related to the exposed findings. The most exciting role of Tau and ratios in FTD could be the differentiation between the subtype of the spectrum, particularly TDP-43 patients from Tau pathology, leading to the development of specific and distinct DMTs. However, several limitations to the presented literature need to be pointed out. Potential confounders of the studies include preanalytical and analytical variables, different criteria for control selection, clinical heterogeneity of patient cohorts, and the different sample size of the study groups. Nonetheless, highly standardized procedures could help reduce the variability in CSF and, more importantly, in serum Tau measurement, thus effectively defining the usefulness of this biomarker.
Author Contributions
Conceptualization, data curation, research and writing—original draft preparation: E.V., F.D.M. and E.C.; writing—review and editing, U.D., R.C., L.M. and C.C.; funding: R.C., L.M. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the AGING Project for the Department of Excellence at the Department of Translational Medicine (DIMET), Università del Piemonte Orientale, Novara, Italy.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AD | Alzheimer’s disease |
| ALS | amyotrophic lateral sclerosis |
| APD | atypical parkinsonism disorders |
| ARMSS | Age related multiple sclerosis severity score |
| Aβ | amyloid-beta |
| bvFTD | behavioral variant of frontotemporal dementia |
| CBD | corticobasal degeneration |
| CIS | clinically isolated syndrome |
| CSF | cerebrospinal fluid |
| DMT | disease-modifying treatment |
| DLB | Lewy bodies dementia |
| ECL | electrochemiluminescence |
| EDSS | expanded disability status scale |
| ELISA | enzyme linked immunosorbent assay |
| fALS | familial ALS |
| FTSD | frontotemporal spectrum disorder |
| FTLD | frontotemporal lobar degeneration |
| GSK-3β | glycogen synthase kinase 3beta |
| HC | healthy controls |
| LB | Lewy body |
| LRRK2 | leucine-rich repeat kinase 2 |
| MMSE | Mini mental state examination |
| MND | motorneuron disease |
| MOCA | Montreal cognitive assessment |
| MS | multiple sclerosis |
| MSA | multiple system atrophy |
| MSSS | multiple sclerosis severity score |
| NfL | neurofilaments light chain |
| PDD | Parkinson’s disease dementia |
| PIGD | postural instability gait disturbance |
| PNFA | progressive nonfluent aphasia |
| PP | primary progressive |
| p-Tau | phosphorylated Tau |
| RR | relapsing–remitting |
| sALS | sporadic ALS |
| SD | semantic dementia |
| SIMOA | high sensitive single molecule assay |
| SN | substantia nigra |
| SP | secondary progressive |
| TD | tremor dominant |
| TDP-43 | transactive DNA binding protein of ~43 kDa |
| t-Tau | total Tau |
| UPDRS | Unified Parkinson’s disease rating scale |
| α-syn | α-synuclein |
References
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Gentile, A.; Mori, F.; Bernardini, S.; Centonze, D. Role of amyloid-β CSF levels in cognitive deficit in MS. Clin. Chim. Acta 2015, 449, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Didonna, A. Tau at the interface between neurodegeneration and neuroinflammation. Genes Immun. 2020, 21, 288–300. [Google Scholar] [CrossRef]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Paternicò, D.; Galluzzi, S.; Drago, V.; Bocchio-Chiavetto, L.; Zanardini, R.; Pedrini, L.; Baronio, M.; Amicucci, G.; Frisoni, G.B. Cerebrospinal fluid markers for Alzheimer’s disease in a cognitively healthy cohort of young and old adults. Alzheimer’s Dement. 2012, 8, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, M.; Vanderstichele, H.; Agren, H.; Zachrisson, O.; Edsbagge, M.; Wikkelsø, C.; Skoog, I.; Wallin, A.; Wahlund, L.O.; Marcusson, J.; et al. Tau and Abeta42 in cerebrospinal fluid from healthy adults 21–93 years of age: Establishment of reference values. Clin. Chem. 2001, 47, 1776–1781. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, J.; Psujek, M.; Janczarek, M.; Szczerbo-Trojanowska, M.; Bartosik-Psujek, H. Total-tau in cerebrospinal fluid of patients with multiple sclerosis decreases in secondary progressive stage of disease and reflects degree of brain atrophy. Upsala J. Med. Sci. 2012, 117, 284–292. [Google Scholar] [CrossRef][Green Version]
- Mirzaii-Dizgah, M.-H.; Mirzaii-Dizgah, M.-R.; Mirzaii-Dizgah, I. Serum and saliva total tau protein as a marker for relapsing-remitting multiple sclerosis. Med. Hypotheses 2020, 135, 109476. [Google Scholar] [CrossRef]
- Jalili, R.; Chenaghlou, S.; Khataee, A.; Khalilzadeh, B.; Rashidi, M.-R. An Electrochemiluminescence Biosensor for the Detection of Alzheimer’s Tau Protein Based on Gold Nanostar Decorated Carbon Nitride Nanosheets. Molecules 2022, 27, 431. [Google Scholar] [CrossRef] [PubMed]
- Islas-Hernandez, A.; Aguilar-Talamantes, H.S.; Bertado-Cortes, B.; Mejia-Delcastillo, G.D.J.; Carrera-Pineda, R.; Cuevas-Garcia, C.F.; Garcia-Delatorre, P. BDNF and Tau as biomarkers of severity in multiple sclerosis. Biomark. Med. 2018, 12, 717–726. [Google Scholar] [CrossRef]
- Chouliaras, L.; Thomas, A.; Malpetti, M.; Donaghy, P.; Kane, J.; Mak, E.; Savulich, G.; Prats-Sedano, M.A.; Heslegrave, A.J.; Zetterberg, H.; et al. Differential levels of plasma biomarkers of neurodegeneration in Lewy body dementia, Alzheimer’s disease, frontotemporal dementia and progressive supranuclear palsy. J. Neurol. Neurosurg. Psychiatry 2022. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.H.; Rissin, D.M.; Kan, C.W.; Fournier, D.R.; Piech, T.; Campbell, T.G.; Meyer, R.E.; Fishburn, M.W.; Cabrera, C.; Patel, P.P.; et al. The Simoa HD-1 Analyzer: A Novel Fully Automated Digital Immunoassay Analyzer with Single-Molecule Sensitivity and Multiplexing. J. Lab. Autom. 2016, 21, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Frischer, J.M.; Bramow, S.; Dal-Bianco, A.; Lucchinetti, C.F.; Rauschka, H.; Schmidbauer, M.; Laursen, H.; Sorensen, P.S.; Lassmann, H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009, 132, 1175–1189. [Google Scholar] [CrossRef]
- Filippi, M.; Rocca, M.A. Classifying silent progression in relapsing–remitting MS. Nat. Rev. Neurol. 2019, 15, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Cree, B.A.C.; Hollenbach, J.A.; Bove, R.; Kirkish, G.; Sacco, S.; Caverzasi, E.; Bischof, A.; Gundel, T.; Zhu, A.H.; Papinutto, N.; et al. Silent progression in disease activity–free relapsing multiple sclerosis. Ann. Neurol. 2019, 85, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, M.; Gaetani, L.; Salvadori, N.; Chipi, E.; Gentili, L.; Borrelli, A.; Parnetti, L. Cognitive impairment in multiple sclerosis: Lessons from cerebrospinal fluid biomarkers. Neural Regen. Res. 2021, 16, 36–42. [Google Scholar] [CrossRef]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Lublin, F.D. New Multiple Sclerosis Phenotypic Classification. Eur. Neurol. 2014, 72, 1–5. [Google Scholar] [CrossRef]
- Crespi, I.; Vecchio, D.; Serino, R.; Saliva, E.; Virgilio, E.; Sulas, M.G.; Bellomo, G.; Dianzani, U.; Cantello, R.; Comi, C. K Index is a Reliable Marker of Intrathecal Synthesis, and an Alternative to IgG Index in Multiple Sclerosis Diagnostic Work-Up. J. Clin. Med. 2019, 8, 446. [Google Scholar] [CrossRef]
- Vecchio, D.; Bellomo, G.; Serino, R.; Virgilio, E.; Lamonaca, M.; Dianzani, U.; Cantello, R.; Comi, C.; Crespi, I. Intrathecal kappa free light chains as markers for multiple sclerosis. Sci. Rep. 2020, 10, 20329. [Google Scholar] [CrossRef]
- Thebault, S.; Booth, R.A.; Freedman, M.S. Blood Neurofilament Light Chain: The Neurologist’s Troponin? Biomedicines 2020, 8, 523. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, T.; Akgün, K.; Brück, W. Molecular biomarkers in multiple sclerosis. J. Neuroinflamm. 2019, 16, 272. [Google Scholar] [CrossRef] [PubMed]
- Bartosik-Psujek, H.; Psujek, M.; Jaworski, J.; Stelmasiak, Z. Total tau and S100b proteins in different types of multiple sclerosis and during immunosuppressive treatment with mitoxantrone. Acta Neurol. Scand. 2011, 123, 252–256. [Google Scholar] [CrossRef]
- Kapaki, E.; Paraskevas, G.P.; Michalopoulou, M.; Kilidireas, K. Increased cerebrospinal fluid tau protein in multiple sclerosis. Eur. Neurol. 2000, 43, 228–232. [Google Scholar] [CrossRef]
- Novakova, L.; Axelsson, M.; Khademi, M.; Zetterberg, H.; Blennow, K.; Malmeström, C.; Piehl, F.; Olsson, T.; Lycke, J. Cerebrospinal fluid biomarkers as a measure of disease activity and treatment efficacy in relapsing-remitting multiple sclerosis. J. Neurochem. 2016, 141, 296–304. [Google Scholar] [CrossRef]
- Hein Nee Maier, K.; Kohler, A.; Diem, R.; Sättler, M.B.; Demmer, I.; Lange, P.; Bähr, M.; Otto, M. Biological markers for axonal degeneration in CSF and blood of patients with the first event indicative for multiple sclerosis. Neurosci. Lett. 2008, 436, 72–76. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; Zurdo, J.M.; Hernanz, A.; Medina-Acebron, S.; De Bustos, F.; Barcenilla, B.; Sayed, Y.; Ayuso-Peralta, L. Tau protein concentrations in cerebrospinal fluid of patients with multiple sclerosis. Acta Neurol. Scand. 2002, 106, 351–354. [Google Scholar] [CrossRef]
- Pietroboni, A.M.; Di Cola, F.S.; Scarioni, M.; Fenoglio, C.; Spanò, B.; Arighi, A.; Cioffi, S.M.; Oldoni, E.; De Riz, M.A.; Basilico, P.; et al. CSF β-amyloid as a putative biomarker of disease progression in multiple sclerosis. Mult. Scler. J. 2016, 23, 1085–1091. [Google Scholar] [CrossRef]
- Valis, M.; Talab, R.; Stourac, P.; Andrys, C.; Masopust, J. Tau protein, phosphorylated tau protein and beta-amyloid42 in the cerebrospinal fluid of multiple sclerosis patients. Neuro Endocrinol. Lett. 2008, 29, 971–976. [Google Scholar] [PubMed]
- Guimarães, J.; Cardoso, M.J.; Sá, M.J. Tau protein seems not to be a useful routine clinical marker of axonal damage in multiple sclerosis. Mult. Scler. J. 2006, 12, 354–356. [Google Scholar] [CrossRef]
- Momtazmanesh, S.; Shobeiri, P.; Saghazadeh, A.; Teunissen, C.E.; Burman, J.; Szalardy, L.; Klivenyi, P.; Bartos, A.; Fernandes, A.; Rezaei, N. Neuronal and glial CSF biomarkers in multiple sclerosis: A systematic review and meta-analysis. Rev. Neurosci. 2021, 32, 573–595. [Google Scholar] [CrossRef] [PubMed]
- Kalatha, T.; Hatzifilippou, E.; Arnaoutoglou, M.; Balogiannis, S.; Koutsouraki, E. Glial and neuroaxonal biomarkers in a mul-tiple sclerosis (MS) cohort. Hell. J. Nucl. Med. 2019, 22 (Suppl. 2), 113–121. [Google Scholar] [PubMed]
- Martínez, M.A.M.; Olsson, B.; Bau, L.; Matas, E.; Calvo, C.; Andreasson, U.; Blennow, K.; Romero-Pinel, L.; Martínez-Yélamos, S.; Zetterberg, H. Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult. Scler. J. 2015, 21, 550–561. [Google Scholar] [CrossRef]
- Terzi, M.; Birinci, A.; Cetinkaya, E.; Onar, M.K. Cerebrospinal fluid total tau protein levels in patients with multiple sclerosis. Acta Neurol. Scand. 2007, 115, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Virgilio, E.; Vecchio, D.; Crespi, I.; Puricelli, C.; Barbero, P.; Galli, G.; Cantello, R.; Dianzani, U.; Comi, C. Cerebrospinal fluid biomarkers and cognitive functions at multiple sclerosis diagnosis. J. Neurol. 2022, 1–9. [Google Scholar] [CrossRef]
- Mori, F.; Rossi, S.; Sancesario, G.; Codecà, C.; Mataluni, G.; Monteleone, F.; Buttari, F.; Kusayanagi, H.; Castelli, M.; Motta, C.; et al. Cognitive and Cortical Plasticity Deficits Correlate with Altered Amyloid-β CSF Levels in Multiple Sclerosis. Neuropsychopharmacology 2010, 36, 559–568. [Google Scholar] [CrossRef]
- Brettschneider, J.; Petzold, A.; Junker, A.; Tumani, H. Axonal damage markers in the cerebrospinal fluid of patients with clinically isolated syndrome improve predicting conversion to definite multiple sclerosis. Mult. Scler. J. 2006, 12, 143–148. [Google Scholar] [CrossRef]
- Kosehasanogullari, G.; Ozakbas, S.; Idiman, E. Tau protein levels in the cerebrospinal fluid of the patients with multiple sclerosis in an attack period: Low levels of tau protein may have significance, too. Clin. Neurol. Neurosurg. 2015, 136, 107–109. [Google Scholar] [CrossRef]
- Frederiksen, J.; Kristensen, K.; Bahl, J.; Christiansen, M. Tau protein: A possible prognostic factor in optic neuritis and multiple sclerosis. Mult. Scler. J. 2011, 18, 592–599. [Google Scholar] [CrossRef]
- Virgilio, E.; Vecchio, D.; Crespi, I.; Serino, R.; Cantello, R.; Dianzani, U.; Comi, C. Cerebrospinal Tau levels as a predictor of early disability in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 56, 103231. [Google Scholar] [CrossRef]
- Edwards, K.R.; Kamath, A.; Button, J.; Kamath, V.; Mendoza, J.P.; Zhu, B.; Plavina, T.; Woodward, C.; Penner, N. A pharmacokinetic and biomarker study of delayed-release dimethyl fumarate in subjects with secondary progressive multiple sclerosis: Evaluation of cerebrospinal fluid penetration and the effects on exploratory biomarkers. Mult. Scler. Relat. Disord. 2021, 51, 102861. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef]
- Virgilio, E.; Vecchio, D.; Crespi, I.; Barbero, P.; Caloni, B.; Naldi, P.; Cantello, R.; Dianzani, U.; Comi, C. Serum Vitamin D as a Marker of Impaired Information Processing Speed and Early Disability in Multiple Sclerosis Patients. Brain Sci. 2021, 11, 1521. [Google Scholar] [CrossRef] [PubMed]
- Aktas, O.; Renner, A.; Huss, A.; Filser, M.; Baetge, S.; Stute, N.; Gasis, M.; Lepka, K.; Goebels, N.; Senel, M.; et al. Serum neurofilament light chain: No clear relation to cognition and neuropsychiatric symptoms in stable MS. Neurol. Neuroimmunol. Neuroinflammation 2020, 7, e885. [Google Scholar] [CrossRef]
- Friedova, L.; Motyl, J.; Srpova, B.; Oechtering, J.; Barro, C.; Vodehnalova, K.; Andelova, M.; Noskova, L.; Fialová, L.; Havrdova, E.K.; et al. The weak association between neurofilament levels at multiple sclerosis onset and cognitive performance after 9 years. Mult. Scler. Relat. Disord. 2020, 46, 102534. [Google Scholar] [CrossRef]
- Mellergård, J.; Tisell, A.; Blystad, I.; Grönqvist, A.; Blennow, K.; Olsson, B.; Dahle, C.; Vrethem, M.; Lundberg, P.; Ernerudh, J. Cerebrospinal fluid levels of neurofilament and tau correlate with brain atrophy in natalizumab-treated multiple sclerosis. Eur. J. Neurol. 2017, 24, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Zeydan, B.; Lowe, V.J.; Reichard, R.R.; Przybelski, S.A.; Lesnick, T.G.; Schwarz, C.G.; Senjem, M.L.; Gunter, J.L.; Parisi, J.E.; Machulda, M.M.; et al. Imaging Biomarkers of Alzheimer Disease in Multiple Sclerosis. Ann. Neurol. 2020, 87, 556–567. [Google Scholar] [CrossRef]
- Schepici, G.; Silvestro, S.; Trubiani, O.; Bramanti, P.; Mazzon, E. Salivary Biomarkers: Future Approaches for Early Diagnosis of Neurodegenerative Diseases. Brain Sci. 2020, 10, 245. [Google Scholar] [CrossRef]
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef]
- Zou, Z.-Y.; Zhou, Z.-R.; Che, C.-H.; Liu, C.-Y.; He, R.-L.; Huang, H.-P. Genetic epidemiology of amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, B.; Robberecht, W. The phenotypic variability of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2014, 10, 661–670. [Google Scholar] [CrossRef]
- De Marchi, F.; Carrarini, C.; De Martino, A.; Diamanti, L.; Fasano, A.; Lupica, A.; Russo, M.; Salemme, S.; Spinelli, E.G.; Bombaci, A. Cognitive dysfunction in amyotrophic lateral sclerosis: Can we predict it? Neurol. Sci. 2021, 42, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Strong, M.J.; Abrahams, S.; Goldstein, L.H.; Woolley, S.; Mclaughlin, P.; Snowden, J.; Mioshi, E.; Roberts-South, A.; Benatar, M.; Hortobágyi, T.; et al. Amyotrophic lateral sclerosis—Frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 153–174. [Google Scholar] [CrossRef]
- Couratier, P.; Corcia, P.; Lautrette, G.; Nicol, M.; Marin, B. ALS and frontotemporal dementia belong to a common disease spectrum. Rev. Neurol. 2017, 173, 273–279. [Google Scholar] [CrossRef]
- Geser, F.; Winton, M.J.; Kwong, L.K.; Xu, Y.; Xie, S.X.; Igaz, L.M.; Garruto, R.M.; Perl, D.P.; Galasko, D.; Lee, V.M.-Y.; et al. Pathological TDP-43 in parkinsonism–dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol. 2008, 115, 133–145. [Google Scholar] [CrossRef]
- Verber, N.; Shaw, P.J. Biomarkers in amyotrophic lateral sclerosis: A review of new developments. Curr. Opin. Neurol. 2020, 33, 662–668. [Google Scholar] [CrossRef]
- Delaby, C.; Alcolea, D.; Carmona-Iragui, M.; Illán-Gala, I.; Morenas-Rodríguez, E.; Barroeta, I.; Altuna-Azkargorta, M.; Estellés, T.; Santos-Santos, M.; Turon-Sans, J.; et al. Differential levels of Neurofilament Light protein in cerebrospinal fluid in patients with a wide range of neurodegenerative disorders. Sci. Rep. 2020, 10, 9161. [Google Scholar] [CrossRef]
- Verde, F.; Otto, M.; Silani, V. Neurofilament Light Chain as Biomarker for Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Front. Neurosci. 2021, 15, 679199. [Google Scholar] [CrossRef] [PubMed]
- Süssmuth, S.D.; Tumani, H.; Ecker, D.; Ludolph, A.C. Amyotrophic lateral sclerosis: Disease stage related changes of tau protein and S100 beta in cerebrospinal fluid and creatine kinase in serum. Neurosci. Lett. 2003, 353, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Sussmuth, S.D.; Sperfeld, A.D.; Hinz, A.; Brettschneider, J.; Endruhn, S.; Ludolph, A.C.; Tumani, H. CSF glial markers correlate with survival in amyotrophic lateral sclerosis. Neurology 2010, 74, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Wilke, C.; Deuschle, C.; Rattay, T.W.; Maetzler, W.; Synofzik, M. Total tau is increased, but phosphorylated tau not decreased, in cerebrospinal fluid in amyotrophic lateral sclerosis. Neurobiol. Aging 2015, 36, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Bourbouli, M.; Rentzos, M.; Bougea, A.; Zouvelou, V.; Constantinides, V.C.; Zaganas, I.; Evdokimidis, I.; Kapaki, E.; Paraskevas, G.P. Cerebrospinal Fluid TAR DNA-Binding Protein 43 Combined with Tau Proteins as a Candidate Biomarker for Amyotrophic Lateral Sclerosis and Frontotemporal Dementia Spectrum Disorders. Dement. Geriatr. Cogn. Disord. 2017, 44, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Agnello, L.; Colletti, T.; Sasso, B.L.; Vidali, M.; Spataro, R.; Gambino, C.M.; Giglio, R.V.; Piccoli, T.; Bivona, G.; La Bella, V.; et al. Tau protein as a diagnostic and prognostic biomarker in amyotrophic lateral sclerosis. Eur. J. Neurol. 2021, 28, 1868–1875. [Google Scholar] [CrossRef]
- Scarafino, A.; D’Errico, E.; Introna, A.; Fraddosio, A.; Distaso, E.; Tempesta, I.; Morea, A.; Mastronardi, A.; Leante, R.; Ruggieri, M.; et al. Diagnostic and prognostic power of CSF Tau in amyotrophic lateral sclerosis. J. Neurol. 2018, 265, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rumeileh, S.; Vacchiano, V.; Zenesini, C.; Polischi, B.; De Pasqua, S.; Fileccia, E.; Mammana, A.; Di Stasi, V.; Capellari, S.; Salvi, F.; et al. Diagnostic-prognostic value and electrophysiological correlates of CSF biomarkers of neurodegeneration and neuroinflammation in amyotrophic lateral sclerosis. J. Neurol. 2020, 267, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Grossman, M.; Elman, L.; McCluskey, L.; McMillan, C.T.; Boller, A.; Powers, J.; Rascovsky, K.; Hu, W.; Shaw, L.; Irwin, D.J.; et al. Phosphorylated tau as a candidate biomarker for amyotrophic lateral sclerosis. JAMA Neurol. 2014, 71, 442–448. [Google Scholar] [CrossRef]
- Schreiber, S.; Spotorno, N.; Schreiber, F.; Acosta-Cabronero, J.; Kaufmann, J.; Machts, J.; Debska-Vielhaber, G.; Garz, C.; Bittner, D.; Hensiek, N.; et al. Significance of CSF NfL and tau in ALS. J. Neurol. 2018, 265, 2633–2645. [Google Scholar] [CrossRef]
- Lanznaster, D.; Hergesheimer, R.C.; Bakkouche, S.E.; Beltran, S.; Vourc’H, P.; Andres, C.R.; Dufour-Rainfray, D.; Corcia, P.; Blasco, H. Aβ1-42 and Tau as Potential Biomarkers for Diagnosis and Prognosis of Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020, 21, 2911. [Google Scholar] [CrossRef]
- Kojima, Y.; Kasai, T.; Noto, Y.-I.; Ohmichi, T.; Tatebe, H.; Kitaoji, T.; Tsuji, Y.; Kitani-Morii, F.; Shinomoto, M.; Allsop, D.; et al. Amyotrophic lateral sclerosis: Correlations between fluid biomarkers of NfL, TDP-43, and tau, and clinical characteristics. PLoS ONE 2021, 16, e0260323. [Google Scholar] [CrossRef]
- Bang, J.; Spina, S.; Miller, B.L. Frontotemporal dementia. Lancet 2015, 386, 1672–1682. [Google Scholar] [CrossRef]
- Finger, E.C. Frontotemporal Dementias. Contin. Lifelong Learn. Neurol. 2016, 22, 464–489. [Google Scholar] [CrossRef]
- Mann, D.M.; Snowden, J.S. Frontotemporal lobar degeneration: Pathogenesis, pathology and pathways to phenotype. Brain Pathol. 2017, 27, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Swift, I.J.; Sogorb-Esteve, A.; Heller, C.; Synofzik, M.; Otto, M.; Graff, C.; Galimberti, D.; Todd, E.; Heslegrave, A.J.; Van Der Ende, E.L.; et al. Fluid biomarkers in frontotemporal dementia: Past, present and future. J. Neurol. Neurosurg. Psychiatry 2021, 92, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Meeter, L.H.; Vijverberg, E.G.; Del Campo, M.; Rozemuller, A.J.; Kaat, L.D.; de Jong, F.J.; van der Flier, W.M.; Teunissen, C.E.; van Swieten, J.C.; Pijnenburg, Y.A. Clinical value of neurofilament and phospho-tau/tau ratio in the frontotemporal dementia spectrum. Neurology 2018, 90, e1231–e1239. [Google Scholar] [CrossRef] [PubMed]
- Rosso, S.M.; van Herpen, E.; Pijnenburg, Y.A.L.; Schoonenboom, N.S.M.; Scheltens, P.; Heutink, P.; van Swieten, J.C. Total tau and Phosphorylated tau 181 Levels in the Cerebrospinal Fluid of Patients With Frontotemporal Dementia Due to P301L and G272V tau Mutations. Arch. Neurol. 2003, 60, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Foiani, M.S.; Cicognola, C.; Ermann, N.; Woollacott, I.O.C.; Heller, C.; Heslegrave, A.J.; Keshavan, A.; Paterson, R.W.; Ye, K.; Kornhuber, J.; et al. Searching for novel cerebrospinal fluid biomarkers of tau pathology in frontotemporal dementia: An elusive quest. J. Neurol. Neurosurg. Psychiatry 2019, 90, 740–746. [Google Scholar] [CrossRef]
- Borroni, B.; Benussi, A.; Archetti, S.; Galimberti, D.; Parnetti, L.; Nacmias, B.; Sorbi, S.; Scarpini, E.; Padovani, A. Csf p-tau181/tau ratio as biomarker for TDP pathology in frontotemporal dementia. Amyotroph. Lateral Scler. Front. Degener. 2014, 16, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.T.; Watts, K.; Grossman, M.; Glass, J.; Lah, J.J.; Hales, C.; Shelnutt, M.; Van Deerlin, V.; Trojanowski, J.Q.; Levey, A.I. Reduced CSF p-Tau181 to Tau ratio is a biomarker for FTLD-TDP. Neurology 2013, 81, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rumeileh, S.; Mometto, N.; Bartoletti-Stella, A.; Polischi, B.; Oppi, F.; Poda, R.; Stanzani-Maserati, M.; Cortelli, P.; Liguori, R.; Capellari, S.; et al. Cerebrospinal Fluid Biomarkers in Patients with Frontotemporal Dementia Spectrum: A Single-Center Study. J. Alzheimer’s Dis. 2018, 66, 551–563. [Google Scholar] [CrossRef]
- Körtvelyessy, P.; Heinze, H.J.; Prudlo, J.; Bittner, D. CSF Biomarkers of Neurodegeneration in Progressive Non-fluent Aphasia and Other Forms of Frontotemporal Dementia: Clues for Pathomechanisms? Front. Neurol. 2018, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Borroni, B.; Benussi, A.; Cosseddu, M.; Archetti, S.; Padovani, A. Cerebrospinal Fluid Tau Levels Predict Prognosis in Non-Inherited Frontotemporal Dementia. Neurodegener. Dis. 2014, 13, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Ljubenkov, P.A.; Staffaroni, A.M.; Rojas, J.C.; Allen, I.E.; Wang, P.; Heuer, H.; Karydas, A.; Kornak, J.; Cobigo, Y.; Seeley, W.W.; et al. Cerebrospinal fluid biomarkers predict frontotemporal dementia trajectory. Ann. Clin. Transl. Neurol. 2018, 5, 1250–1263. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Karikari, T.; Ashton, N.; Gazzina, S.; Premi, E.; Benussi, L.; Ghidoni, R.; Rodriguez, J.L.; Emeršič, A.; Simrén, J.; et al. Diagnostic and prognostic value of serum NfL and p-Tau(181) in frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry 2020, 91, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Illán-Gala, I.; Lleo, A.; Karydas, A.; Staffaroni, A.M.; Zetterberg, H.; Sivasankaran, R.; Grinberg, L.T.; Spina, S.; Kramer, J.H.; Ramos, E.M.; et al. Plasma Tau and Neurofilament Light in Frontotemporal Lobar Degeneration and Alzheimer Disease. Neurology 2021, 96, e671–e683. [Google Scholar] [CrossRef] [PubMed]
- Foiani, M.; Woollacott, I.; Heller, C.; Bocchetta, M.; Heslegrave, A.; Dick, K.M.; Russell, L.L.; Marshall, C.; Mead, S.; Schott, J.; et al. Plasma tau is increased in frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 2018, 89, 804–807. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H.; et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020, 26, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Marquié, M.; Normandin, M.D.; Vanderburg, C.R.; Costantino, I.M.; Bien, E.A.; Rycyna, L.G.; Klunk, W.E.; Mathis, C.A.; Ikonomovic, M.D.; Debnath, M.L.; et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann. Neurol. 2015, 78, 787–800. [Google Scholar] [CrossRef]
- Lowe, V.J.; Curran, G.; Fang, P.; Liesinger, A.M.; Josephs, K.A.; Parisi, J.E.; Kantarci, K.; Boeve, B.F.; Pandey, M.; Bruinsma, T.; et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol. Commun. 2016, 4, 13. [Google Scholar] [CrossRef]
- Tsai, R.M.; Bejanin, A.; Lesman-Segev, O.; Lajoie, R.; Visani, A.; Bourakova, V.; O’Neil, J.P.; Janabi, M.; Baker, S.; Lee, S.E.; et al. (18)F-flortaucipir (AV-1451) tau PET in frontotemporal dementia syndromes. Alzheimer’s Res. Ther. 2019, 11, 1–18. [Google Scholar] [CrossRef]
- Jones, W.R.B.; Cope, T.E.; Passamonti, L.; Fryer, T.D.; Hong, Y.T.; Aigbirhio, F.; Kril, J.J.; Forrest, S.L.; Allinson, K.; Coles, J.P.; et al. [(18)F]AV-1451 PET in behavioral variant frontotemporal dementia due to MAPT mutation. Ann. Clin. Transl. Neurol. 2016, 3, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Puschmann, A.; Schöll, M.; Ohlsson, T.; van Swieten, J.; Honer, M.; Englund, E.; Hansson, O. 18F-AV-1451 tau PET imaging correlates strongly with tau neuropathology in MAPT mutation carriers. Brain 2016, 139, 2372–2379. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.P.; Bezgin, G.; Savard, M.; Pascoal, T.A.; Finger, E.; Laforce, R.; Sonnen, J.A.; Soucy, J.-P.; Gauthier, S.; Rosa-Neto, P.; et al. 18F-MK-6240 tau-PET in genetic frontotemporal dementia. Brain 2021. [Google Scholar] [CrossRef] [PubMed]
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Guajardo, V.; Annibali, A.; Jensen, P.H.; Romero-Ramos, M. α-Synuclein Vaccination Prevents the Accumulation of Parkinson Disease-Like Pathologic Inclusions in Striatum in Association With Regulatory T Cell Recruitment in a Rat Model. J. Neuropathol. Exp. Neurol. 2013, 72, 624–645. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, J.R.; Olesen, M.N.; Otzen, D.E.; Romero-Ramos, M.; Sanchez-Guajardo, V. α-Synuclein vaccination modulates regulatory T cell activation and microglia in the absence of brain pathology. J. Neuroinflamm. 2016, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, A.; Ross, O.A.; Ioannidis, J.P.A.; Soto-Ortolaza, A.I.; Moisan, F.; Aasly, J.; Annesi, G.; Bozi, M.; Brighina, L.; Chartier-Harlin, M.-C.; et al. Independent and joint effects of the MAPT and SNCA genes in Parkinson disease. Ann. Neurol. 2011, 69, 778–792. [Google Scholar] [CrossRef]
- Nalls, M.A.; Pankratz, N.; Lill, C.M.; Do, C.B.; Hernandez, D.G.; Saad, M.; DeStefano, A.L.; Kara, E.; Bras, J.; Sharma, M.; et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 2014, 46, 989–993. [Google Scholar] [CrossRef]
- Giasson, B.I.; Forman, M.S.; Higuchi, M.; Golbe, L.I.; Graves, C.L.; Kotzbauer, P.T.; Trojanowski, J.Q.; Lee, V.M.-Y. Initiation and Synergistic Fibrillization of Tau and Alpha-Synuclein. Science 2003, 300, 636–640. [Google Scholar] [CrossRef]
- Duka, T.; Duka, V.; Joyce, J.N.; Sidhu, A. α-Synuclein contributes to GSK-3β-catalyzed Tau phosphorylation in Parkinson’s disease models. FASEB J. 2009, 23, 2820–2830. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, T.; Credle, J.; Rodriguez, O.; Wills, J.; Oaks, A.W.; Masliah, E.; Sidhu, A. Hyperphosphorylated Tau in an α-synuclein-overexpressing transgenic model of Parkinson’s disease. Eur. J. Neurosci. 2011, 33, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Hirai, S.; Sunohara, N.; Aoto, K.; Izumiyama, Y.; Uéda, K.; Ikeda, K.; Kawai, M. Cellular co-localization of phosphorylated tau- and NACP/α-synuclein-epitopes in Lewy bodies in sporadic Parkinson’s disease and in dementia with Lewy bodies. Brain Res. 1999, 843, 53–61. [Google Scholar] [CrossRef]
- Compta, Y.; Parkkinen, L.; O’Sullivan, S.S.; Vandrovcova, J.; Holton, J.L.; Collins, C.; Lashley, T.; Kallis, C.; Williams, D.R.; de Silva, R.; et al. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: Which is more important? Brain 2011, 134, 1493–1505. [Google Scholar] [CrossRef]
- Cisbani, G.; Maxan, A.; Kordower, J.H.; Planel, E.; Freeman, T.B.; Cicchetti, F. Presence of tau pathology within foetal neural allografts in patients with Huntington’s and Parkinson’s disease. Brain 2017, 140, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, A.S.; Adler, C.H.; Serrano, G.E.; Curry, J.R.; Shill, H.A.; Kopyov, O.; Beach, T.G. Co-Existence of tau and α-synuclein pathology in fetal graft tissue at autopsy: A case report. Park. Relat. Disord. 2020, 71, 36–39. [Google Scholar] [CrossRef]
- Vranová, H.P.; Mares, J.; Nevrlý, M.; Stejskal, D.; Zapletalová, J.; Hluštík, P.; Kaňovský, P. CSF markers of neurodegeneration in Parkinson’s disease. J. Neural Transm. 2010, 117, 1177–1181. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Caspell-Garcia, C.J.; Coffey, C.S.; Taylor, P.; Shaw, L.M.; Trojanowski, J.Q.; Singleton, A.; Frasier, M.; Marek, K.; Galasko, D.; et al. Longitudinal CSF biomarkers in patients with early Parkinson disease and healthy controls. Neurology 2017, 89, 1959–1969. [Google Scholar] [CrossRef]
- Alves, G.; Brønnick, K.; Aarsland, D.; Blennow, K.; Zetterberg, H.; Ballard, C.; Kurz, M.W.; Andreasson, U.; Tysnes, O.-B.; Larsen, J.P.; et al. CSF amyloid-β and tau proteins, and cognitive performance, in early and untreated Parkinson’s Disease: The Norwegian ParkWest study. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1080–1086. [Google Scholar] [CrossRef]
- Parnetti, L.; Chiasserini, D.; Bellomo, G.; Giannandrea, D.; De Carlo, C.; Qureshi, M.M.; Ardah, M.T.; Varghese, S.; Bonanni, L.; Borroni, B.; et al. Cerebrospinal fluid Tau/α-synuclein ratio in Parkinson’s disease and degenerative dementias. Mov. Disord. 2011, 26, 1428–1435. [Google Scholar] [CrossRef]
- Parnetti, L.; Farotti, L.; Eusebi, P.; Chiasserini, D.; De Carlo, C.; Giannandrea, D.; Salvadori, N.; Lisetti, V.; Tambasco, N.; Rossi, A.; et al. Differential role of CSF alpha-synuclein species, tau, and Aβ42 in Parkinson’s Disease. Front. Aging Neurosci. 2014, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Dolatshahi, M.; Pourmirbabaei, S.; Kamalian, A.; Ashraf-Ganjouei, A.; Yaseri, M.; Aarabi, M.H. Longitudinal Alterations of Alpha-Synuclein, Amyloid Beta, Total, and Phosphorylated Tau in Cerebrospinal Fluid and Correlations Between Their Changes in Parkinson’s Disease. Front. Neurol. 2018, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. CSF biomarkers in different phenotypes of Parkinson disease. J. Neural Transm. 2011, 119, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Vranová, H.P.; Mareš, J.; Hluštík, P.; Nevrlý, M.; Stejskal, D.; Zapletalová, J.; Obereigneru, R.; Kaňovský, P. Tau protein and beta-amyloid(1-42) CSF levels in different phenotypes of Parkinson’s disease. J. Neural Transm. 2012, 119, 353–362. [Google Scholar] [CrossRef]
- Kang, J.-H.; Irwin, D.J.; Chen-Plotkin, A.S.; Siderowf, A.; Caspell, C.; Coffey, C.S.; Waligorska, T.; Taylor, P.; Pan, S.; Frasier, M.; et al. Association of cerebrospinal fluid β-amyloid 1-42, T-tau, P-tau181, and α-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol. 2013, 70, 1277–1287. [Google Scholar] [CrossRef]
- Kang, J.H.; Mollenhauer, B.; Coffey, C.S.; Toledo, J.B.; Weintraub, D.; Galasko, D.R.; Irwin, D.J.; Van Deerlin, V.; Chen-Plotkin, A.S.; Caspell-Garcia, C.; et al. CSF biomarkers associated with disease heterogeneity in early Parkinson’s disease: The Parkinson’s Progression Markers Initiative study. Acta Neuropathol. 2016, 131, 935–949. [Google Scholar] [CrossRef]
- Vilas, D.; Shaw, L.M.; Taylor, P.; Berg, D.; Brockmann, K.; Aasly, J.; Marras, C.; Pont-Sunyer, C.; Ríos, J.; Marek, K.; et al. Cerebrospinal fluid biomarkers and clinical features in leucine-rich repeat kinase 2 (LRRK2) mutation carriers. Mov. Disord. 2016, 31, 906–914. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.-M.; Zeighami, Y.; Dagher, A.; Postuma, R.B. Clinical criteria for subtyping Parkinson’s disease: Biomarkers and longitudinal progression. Brain 2017, 140, 1959–1976. [Google Scholar] [CrossRef]
- Zhang, J.; Mattison, H.A.; Liu, C.-Q.; Ginghina, C.; Auinger, P.; McDermott, M.P.; Stewart, T.; Kang, U.J.; Parkinson Study Group DATATOP Investigators; Cain, K.C.; et al. Longitudinal assessment of tau and amyloid beta in cerebrospinal fluid of Parkinson disease. Acta Neuropathol. 2013, 126, 671–682. [Google Scholar] [CrossRef]
- Pagano, G.; De Micco, R.; Yousaf, T.; Wilson, H.; Chandra, A.; Politis, M. REM behavior disorder predicts motor progression and cognitive decline in Parkinson disease. Neurology 2018, 91, e894–e905. [Google Scholar] [CrossRef]
- Irwin, D.; White, M.; Toledo, J.; Xie, S.X.; Robinson, J.L.; Van Deerlin, V.; Lee, V.M.-Y.; Leverenz, J.; Montine, T.J.; Duda, J.E.; et al. Neuropathologic substrates of Parkinson disease dementia. Ann. Neurol. 2012, 72, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Compta, Y.; Martí, M.J.; Ibarretxe-Bilbao, N.; Junqué, C.; Valldeoriola, F.; Muñoz, E.; Ezquerra, M.; Ríos, J.; Tolosa, E. Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson’s disease. Mov. Disord. 2009, 24, 2203–2210. [Google Scholar] [CrossRef] [PubMed]
- Gmitterová, K.; Gawinecka, J.; Llorens, F.; Varges, D.; Valkovič, P.; Zerr, I. Cerebrospinal fluid markers analysis in the differential diagnosis of dementia with Lewy bodies and Parkinson’s disease dementia. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cholerton, B.; Shi, M.; Ginghina, C.; Cain, K.C.; Auinger, P.; Zhang, J. CSF tau and tau/Aβ(42) predict cognitive decline in Parkinson’s disease. Park. Relat. Disord. 2015, 21, 271–276. [Google Scholar] [CrossRef]
- Schrag, A.; Siddiqui, U.F.; Anastasiou, Z.; Weintraub, D.; Schott, J. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson’s disease: A cohort study. Lancet Neurol. 2017, 16, 66–75. [Google Scholar] [CrossRef]
- Delgado-Alvarado, M.; Aguayo, R.D.; Navalpotro-Gómez, I.; Gago, B.; Gorostidi, A.; Jiménez-Urbieta, H.; Quiroga-Varela, A.; Ruiz-Martínez, J.; Bergareche, A.; Rodríguez-Oroz, M.C. Ratios of proteins in cerebrospinal fluid in Parkinson’s disease cognitive decline: Prospective study. Mov. Disord. 2018, 33, 1809–1813. [Google Scholar] [CrossRef]
- Montine, T.J.; Shi, M.; Quinn, J.; Peskind, E.R.; Craft, S.; Ginghina, C.; Chung, K.A.; Kim, H.; Galasko, D.R.; Jankovic, J.; et al. CSF Aβ(42) and tau in Parkinson’s disease with cognitive impairment. Mov. Disord. 2010, 25, 2682–2685. [Google Scholar] [CrossRef] [PubMed]
- Bibl, M.; Esselmann, H.; Lewczuk, P.; Trenkwalder, C.; Otto, M.; Kornhuber, J.; Wiltfang, J.; Mollenhauer, B. Combined Analysis of CSF Tau, Aβ42, Aβ1–42% and Aβ1–40% in Alzheimer’s Disease, Dementia with Lewy Bodies and Parkinson’s Disease Dementia. Int. J. Alzheimer’s Dis. 2010, 2010, 1–7. [Google Scholar] [CrossRef]
- Lerche, S.; Wurster, I.; Röben, B.; Machetanz, G.; Zimmermann, M.; Bernhard, F.; Stransky, E.; Deuschle, C.; Schulte, C.; Hansson, O.; et al. Parkinson’s disease: Evolution of cognitive impairment and CSF Aβ(1–42) profiles in a prospective longitudinal study. J. Neurol. Neurosurg. Psychiatry 2019, 90, 165–170. [Google Scholar] [CrossRef]
- Siderowf, A.; Xie, S.X.; Hurtig, H.; Weintraub, D.; Duda, J.; Chen-Plotkin, A.; Shaw, L.M.; Van Deerlin, V.; Trojanowski, J.Q.; Clark, C. CSF amyloid β 1-42 predicts cognitive decline in Parkinson disease. Neurology 2010, 75, 1055–1061. [Google Scholar] [CrossRef]
- Alves, G.; Lange, J.; Blennow, K.; Zetterberg, H.; Andreasson, U.; Førland, M.G.; Tysnes, O.-B.; Larsen, J.P.; Pedersen, K.F. CSF Aβ 42 predicts early-onset dementia in Parkinson disease. Neurology 2014, 82, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Fanciulli, A.; Stankovic, I.; Krismer, F.; Seppi, K.; Levin, J.; Wenning, G.K. Multiple system atrophy. Int. Rev. Neurobiol. 2019, 149, 137–192. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.-P.; McKeith, I.G.; Burn, D.J.; Boeve, B.F.; Weintraub, D.; Bamford, C.; Allan, L.; Thomas, A.J.; O’Brien, J.T. New evidence on the management of Lewy body dementia. Lancet Neurol. 2020, 19, 157–169. [Google Scholar] [CrossRef]
- Coughlin, D.G.; Litvan, I. Progressive supranuclear palsy: Advances in diagnosis and management. Park. Relat. Disord. 2020, 73, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, V.C.; Paraskevas, G.P.; Paraskevas, P.G.; Stefanis, L.; Kapaki, E. Corticobasal degeneration and corticobasal syndrome: A review. Clin. Park. Relat. Disord. 2019, 1, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Janelidze, S.; Hall, S.; Magdalinou, N.; Lees, A.J.; Andreasson, U.; Norgren, N.; Linder, J.; Forsgren, L.; Constantinescu, R.; et al. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology 2017, 88, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Süssmuth, S.D.; Uttner, I.; Landwehrmeyer, B.; Pinkhardt, E.H.; Brettschneider, J.; Petzold, A.; Kramer, B.; Schulz, J.B.; Palm, C.; Otto, M.; et al. Differential pattern of brain-specific CSF proteins tau and amyloid-β in Parkinsonian syndromes. Mov. Disord. 2010, 25, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Abdo, W.F.; De Jong, D.; Hendriks, J.C.; Horstink, M.W.; Kremer, B.P.; Bloem, B.R.; Verbeek, M.M. Cerebrospinal fluid analysis differentiates multiple system atrophy from Parkinson’s disease. Mov. Disord. 2004, 19, 571–579. [Google Scholar] [CrossRef]
- Abdo, W.F.; Bloem, B.R.; Van Geel, W.J.; Esselink, R.A.; Verbeek, M.M. CSF neurofilament light chain and tau differentiate multiple system atrophy from Parkinson’s disease. Neurobiol. Aging 2007, 28, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.K.; Eeftens, J.M.; Aerts, M.B.; Esselink, R.A.; Bloem, B.R.; Kuiperij, H.B.; Verbeek, M.M. CSF levels of DJ-1 and tau distinguish MSA patients from PD patients and controls. Parkinsonism Relat. Disord. 2014, 20, 112–115. [Google Scholar] [CrossRef]
- Herbert, M.; Aerts, M.B.; Ebeenes, M.; Enorgren, N.; Esselink, R.A.J.; Bloem, B.R.; Kuiperij, B.; Verbeek, M.M. CSF Neurofilament Light Chain but not FLT3 Ligand Discriminates Parkinsonian Disorders. Front. Neurol. 2015, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Bradner, J.; Hancock, A.M.; Chung, K.A.; Quinn, J.F.; Peskind, E.R.; Galasko, D.; Jankovic, J.; Zabetian, C.P.; Kim, H.M.; et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann. Neurol. 2011, 69, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Llorens, F.; Schmitz, M.; Varges, D.; Kruse, N.; Gotzmann, N.; Gmitterová, K.; Mollenhauer, B.; Zerr, I. Cerebrospinal α-synuclein in α-synuclein aggregation disorders: Tau/α-synuclein ratio as potential biomarker for dementia with Lewy bodies. J. Neurol. 2016, 263, 2271–2277. [Google Scholar] [CrossRef]
- Van Harten, A.C.; Kester, M.I.; Visser, P.-J.; Blankenstein, M.; Pijnenburg, Y.A.; van der Flier, W.; Scheltens, P. Tau and p-tau as CSF biomarkers in dementia: A meta-analysis. Clin. Chem. Lab. Med. (CCLM) 2011, 49, 353–366. [Google Scholar] [CrossRef]
- Schoonenboom, N.S.M.; Reesink, F.E.; Verwey, N.A.; Kester, M.I.; Teunissen, C.E.; van de Ven, P.M.; Pijnenburg, Y.A.L.; Blankenstein, M.A.; Rozemuller, A.J.; Scheltens, P.; et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology 2011, 78, 47–54. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Locascio, J.J.; Schulz-Schaeffer, W.; Sixel-Döring, F.; Trenkwalder, C.; Schlossmacher, M.G. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: A cohort study. Lancet Neurol. 2011, 10, 230–240. [Google Scholar] [CrossRef]
- Chien, D.T.; Bahri, S.; Szardenings, A.K.; Walsh, J.C.; Mu, F.; Su, M.-Y.; Shankle, W.R.; Elizarov, A.; Kolb, H.C. Early Clinical PET Imaging Results with the Novel PHF-Tau Radioligand [F-18]-T. J. Alzheimer’s Dis. 2013, 34, 457–468. [Google Scholar] [CrossRef]
- Xia, C.-F.; Arteaga, J.; Chen, G.; Gangadharmath, U.; Gomez, L.F.; Kasi, D.; Lam, C.; Liang, Q.; Liu, C.; Mocharla, V.P.; et al. [18F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimer’s Dement. 2013, 9, 666–676. [Google Scholar] [CrossRef]
- Hansen, A.K.; Knudsen, K.; Lillethorup, T.P.; Landau, A.M.; Parbo, P.; Fedorova, T.; Audrain, H.; Bender, D.; Østergaard, K.; Brooks, D.; et al. In vivo imaging of neuromelanin in Parkinson’s disease using 18F-AV-1451 PET. Brain 2016, 139, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A.; Attems, J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol. 2008, 115, 427–436. [Google Scholar] [CrossRef]
- Horvath, J.; Herrmann, F.R.; Burkhard, P.R.; Bouras, C.; Kövari, E. Neuropathology of dementia in a large cohort of patients with Parkinson’s disease. Park. Relat. Disord. 2013, 19, 864–868. [Google Scholar] [CrossRef]
- Walker, L.; McAleese, K.E.; Thomas, A.J.; Johnson, M.; Martin-Ruiz, C.; Parker, C.; Colloby, S.J.; Jellinger, K.; Attems, J. Neuropathologically mixed Alzheimer’s and Lewy body disease: Burden of pathological protein aggregates differs between clinical phenotypes. Acta Neuropathol. 2015, 129, 729–748, discussion 864. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.R.; Maass, A.; Pressman, P.; Stiver, J.; Schonhaut, D.R.; Baker, S.L.; Kramer, J.; Rabinovici, G.D.; Jagust, W.J. Associations Between Tau, β-Amyloid, and Cognition in Parkinson Disease. JAMA Neurol. 2018, 75, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.K.; Damholdt, M.F.; Fedorova, T.D.; Knudsen, K.; Parbo, P.; Ismail, R.; Østergaard, K.; Brooks, D.; Borghammer, P. In Vivo cortical tau in Parkinson’s disease using 18F-AV-1451 positron emission tomography. Mov. Disord. 2017, 32, 922–927. [Google Scholar] [CrossRef]
- Hansen, A.K.; Parbo, P.; Ismail, R.; Østergaard, K.; Brooks, D.J.; Borghammer, P. Tau Tangles in Parkinson’s Disease: A 2-Year Follow-Up Flortaucipir PET Study. J. Park. Dis. 2020, 10, 161–171. [Google Scholar] [CrossRef]
- Gomperts, S.N.; Locascio, J.J.; Makaretz, S.J.; Schultz, A.; Caso, C.; Vasdev, N.; Sperling, R.; Growdon, J.H.; Dickerson, B.C.; Johnson, K. Tau Positron Emission Tomographic Imaging in the Lewy Body Diseases. JAMA Neurol. 2016, 73, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, L.; Rodríguez, P.V.; Hong, Y.T.; Allinson, K.S.J.; Williamson, D.; Borchert, R.J.; Sami, S.; Cope, T.; Bevan-Jones, W.R.; Jones, P.S.; et al. 18F-AV-1451 positron emission tomography in Alzheimer’s disease and progressive supranuclear palsy. Brain 2017, 140, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Choi, J.Y.; Hwang, M.S.; Lee, S.H.; Ryu, Y.H.; Lee, M.S.; Lyoo, C.H. Subcortical 18F-AV-1451 binding patterns in progressive supranuclear palsy. Mov. Disord. 2017, 32, 134–140. [Google Scholar] [CrossRef]
- Schonhaut, D.R.; McMillan, C.T.; Spina, S.; Dickerson, B.C.; Siderowf, A.; Devous, M.D., Sr.; Tsai, R.; Winer, J.; Russell, D.S.; Litvan, I.; et al. 18F-flortaucipir tau positron emission tomography distinguishes established progressive supranuclear palsy from controls and Parkinson disease: A multicenter study. Ann. Neurol. 2017, 82, 622–634. [Google Scholar] [CrossRef]
- Smith, R.; Schöll, M.; Widner, H.; van Westen, D.; Svenningsson, P.; Hägerström, D.; Ohlsson, T.; Jögi, J.; Nilsson, C.; Hansson, O. In vivo retention of 18F-AV-1451 in corticobasal syndrome. Neurology 2017, 89, 845–853. [Google Scholar] [CrossRef]
- Whitwell, J.L. Tau Imaging in Parkinsonism: What Have We Learned So Far? Mov. Disord. Clin. Pract. 2018, 5, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.; Avila, J.; Schöll, M.; Kovacs, G.G.; Kövari, E.; Skrabana, R.; Evans, L.D.; Kontsekova, E.; Malawska, B.; De Silva, R.; et al. A walk through tau therapeutic strategies. Acta Neuropathol. Commun. 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Boxer, A.L.; Qureshi, I.; Ahlijanian, M.; Grundman, M.; Golbe, L.I.; Litvan, I.; Honig, L.S.; Tuite, P.; McFarland, N.R.; O’Suilleabhain, P.; et al. Safety of the tau-directed monoclonal antibody BIIB092 in progressive supranuclear palsy: A randomised, placebo-controlled, multiple ascending dose phase 1b trial. Lancet Neurol. 2019, 18, 549–558. [Google Scholar] [CrossRef]
- Dam, T.; Boxer, A.L.; Golbe, L.I.; Höglinger, G.U.; Morris, H.R.; Litvan, I.; Lang, A.E.; Corvol, J.-C.; Aiba, I.; Grundman, M.; et al. Safety and efficacy of anti-tau monoclonal antibody gosuranemab in progressive supranuclear palsy: A phase 2, randomized, placebo-controlled trial. Nat. Med. 2021, 27, 1451–1457. [Google Scholar] [CrossRef]
- Boxer, A.L.; Lang, A.E.; Grossman, M.; Knopman, D.S.; Miller, B.L.; Schneider, L.S.; Doody, R.S.; Lees, A.; Golbe, L.I.; Williams, D.R.; et al. Davunetide in patients with progressive supranuclear palsy: A randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol. 2014, 13, 676–685. [Google Scholar] [CrossRef]
- Tolosa, E.; Litvan, I.; Höglinger, G.U.; Burn, D.; Lees, A.; Andrés, M.V.; Gómez-Carrillo, B.; León, T.; Del Ser, T. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov. Disord. 2014, 29, 470–478. [Google Scholar] [CrossRef]
- Chung, E.S.; Bok, E.; Sohn, S.; Lee, Y.D.; Baik, H.H.; Jin, B.K. GT1b-induced neurotoxicity is mediated by the Akt/GSK-3/tau signaling pathway but not caspase-3 in mesencephalic dopaminergic neurons. BMC Neurosci. 2010, 11, 74. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Ying, C.; Li, W.; Ruan, H.; Zhu, X.; You, Y.; Han, Y.; Chen, R.; Wang, Y.; et al. Inhibition of glycogen synthase kinase-3β protects dopaminergic neurons from MPTP toxicity. Neuropharmacology 2007, 52, 1678–1684. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).