Abstract: Background
To investigate associations and predictive factors between macular neovascularization (MNV) lesion variants and drusen types in patients with treatment-naïve neovascular age-related macular degeneration (AMD). Methods: Multimodal imaging was retrospectively reviewed for druse type (soft drusen, subretinal drusenoid deposits (SDDs) or mixed) and MNV type (MNV 1, MNV 2, MNV 1/2 or MNV 3). The Consensus on Neovascular AMD Nomenclature (CONAN) classification was used for characterizing MNV at baseline. Results: One eye of each eligible patient was included (n = 191). Patients with predominant SDDs had an increased adjusted odds ratio (aOR) for MNV 2 (23.4453, p = 0.0025) and any type of MNV 3 (8.7374, p < 0.0001). Patients with MNV 1/2 had an aOR for predominant SDDs (0.3284, p = 0.0084). Patients with MNV1 showed an aOR for SDDs (0.0357, p < 0.0001). Eyes with SDDs only without other drusen types showed an aOR for MNV 2 (9.2945, p < 0.0001). Conclusions: SDDs represent a common phenotypic characteristic in AMD eyes with treatment-naïve MNV. The aOR for eyes with predominant SDDs to develop MNV 2 and MNV 3 was much higher, possibly due to their location in the subretinal space. The predominant druse type may help to predict which type of MNV will develop during the course of AMD.
1. Introduction
With the availability of high-resolution retinal imaging, the classification of macular neovascularization (MNV) and drusen has evolved. Based on anatomical localization and multimodal imaging, including fluorescein angiography and spectral domain optical coherence tomography (SD-OCT), a revised classification scheme was proposed by Freund et al. [1]. This classification system built upon Grossniklaus’ and Gass’ original observations from histopathologic slides of neovascular age-related macular degeneration (AMD) differentiating between vessels confined to the sub-retinal pigment epithelium space, described as type 1 MNV, and vessels proliferating above the retinal pigment epithelium in the subneurosensory, subretinal space, described as type 2 MNV [2]. Gass had recognized that a distinction between type 1 and type 2 MNV based on biomicroscopic and fluorescein angiographic findings “is not always easy and in some cases impossible” [3]. Incorporating findings from fluorescein angiography and OCT for grading neovascular AMD, higher incidences of intraretinal neovascularization, described as type 3 MNV, and mixed types 1 and 2 were found compared with those reported in prior studies using fluorescein alone [4]. A new nomenclature was proposed by the Consensus on Neovascular Age-Related Macular Degeneration Nomenclature (CONAN) Study Group in 2020 [5].
Optical coherence tomography has improved our understanding of not only MNV types, but has also expanded and refined distinctions between drusen types. The clinical appearance of reticular pseudodrusen, first described in 1990 [6], could be linked to aggregations containing typical drusen-associated material located in the subretinal space, which were termed subretinal drusenoid deposits [7]. Subretinal drusenoid deposits have a high prevalence in AMD, which has been underestimated prior to the SD-OCT era [7]. Subretinal drusenoid deposits have been recognized as an additional feature of early and both forms of late AMD, called geographic atrophy/complete retinal pigment epithelium and outer retinal (cRORA) and neovascular AMD [8,9,10]. Late AMD is associated with vision loss [11]. The latest report of the Age-Related Eye Diseases Study 2 concluded that subretinal drusenoid deposits significantly contribute to the development of late AMD stages, especially in patients who already present subretinal drusenoid deposits at an early, low-disease-severity stage [11]. Subretinal drusenoid deposits mainly seemed to contribute to the development of geographic atrophy [11]. However, soft drusen and subretinal drusenoid deposits were also found to be risk factors for MNV [12]. There are published data suggesting that patients with subretinal drusenoid deposits are more likely to develop macular type 3 MNV [13,14].
The purpose of this study was to assess the predictive odds ratios between drusen type and MNV lesion variants in patients with treatment-naïve neovascular AMD [5].
2. Materials and Methods
2.1. Ethics
Ethics Committee approval was obtained from the Local Ethics Committee of the Canton of Zurich (approval number: PB_2016-00264). This study adheres to the tenets of the 1964 Declaration of Helsinki and its later amendments.
2.2. Study Design
This is a single-center, retrospective, observational study conducted at the Department of Ophthalmology of the University Hospital of Zurich (USZ), Switzerland.
2.3. Data Collection
All fluoresceine angiographies (FA) performed between 2011 and 2013 at the University Hospital of Zurich were screened for the presence of treatment-naïve macular neovascularization (MNV). Inclusion criteria for this study were the presence of treatment-naïve MNV secondary to AMD as evidenced by multimodal imaging, including FA, spectral-domain optical coherence tomography (SD-OCT), fundus autofluorescence (FAF) imaging and by clinical examination in patients aged ≥50 years. All neovascular lesion types, including polypoidal choroidal vasculopathy (PCV), were evaluated. Eyes with PCV were included only when structural signs of AMD were also present. Eyes with central serous chorioretinopathy (CSC) with or without PCV were excluded. In patients with no available baseline images of the study eye prior to the development of MNV, druse type was evaluated in the fellow eye provided there was no evidence of MNV. Only one eye per participant was selected and included in the study. If both eyes of the same patient were eligible, one eye was chosen randomly.
Exclusion criteria included patients with bilateral MNV at baseline, eyes with predominantly fibrotic lesions and those with such poor image quality that reliable classification of MNV and drusen was not possible.
Patients with bilateral MNV at baseline were excluded from the study as there would not be any pre-conversion images or a non-neovascular fellow eye to allow the grading of drusen type.
Neovascularizations that were confined to the space under the retinal pigment epithelium (RPE) were defined as MNV 1. PCV was considered an aneurysmal variant of MNV 1 and therefore subsumed under MNV 1. Neovascularizations proliferating above the RPE, in the subneurosensory, subretinal space, were defined as MNV 2. Cases with mixed type 1 and 2 lesions were recorded as a separate subgroup termed MNV 1/2. Cases with multiple lesion types including any type 3 lesion (MNV 1/3 and MNV 2/3) were summarized under MNV 3.
Descriptive statistics regarding the participant demographics are listed in Table 1.

Table 1.
Demographic data.
FA images were obtained with either a fundus camera system (Carl Zeiss AG, Oberkochen, Germany) or the Heidelberg Viewing Module (version 6.0.9.0) included in our Spectralis SD-OCT device (version 1.9.10.0; Heidelberg Engineering GmbH, Heidelberg, Germany). Indocyanine green angiography (ICGA) and FAF images were obtained from all patients using the confocal scanning laser ophthalmoscope (CSLO) (Heidelberg Retina Angiograph, HRA2, Heidelberg Engineering, Heidelberg, Germany).
2.4. Image Grading and Analysis
Neovascular lesions were subtyped according to CONAN criteria [1,5]. See Figure 1 and Figure 2 for examples [1,4].
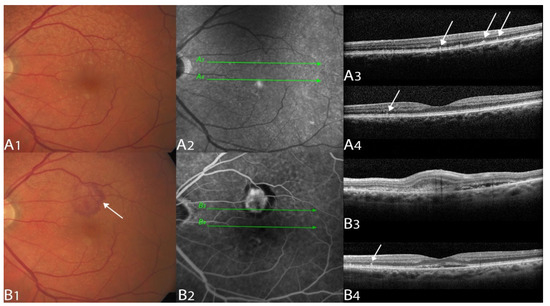
Figure 1.
Multimodal imaging example of a type 2 macular neovascularization.
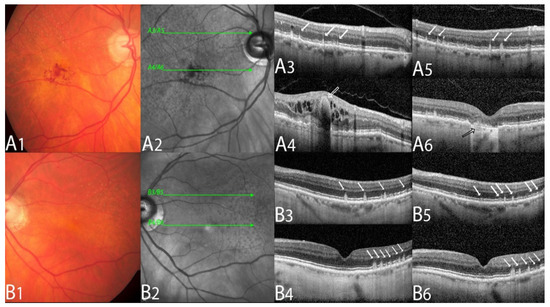
Figure 2.
Multimodal imaging example of a type 3 macular neovascularization.
Drusen types were categorized into soft drusen and SDDs. SDDs were considered present when there was OCT evidence of ≥5 definite SDDs above the RPE in >1 B-scan, with consistent changes in either the near-infrared imaging or the blue light channel, as previously described [7]. Soft drusen were determined from color fundus photographs and confirmed by SD-OCT. If both types of drusen were present, the predominance of either soft drusen or SDDs was determined by two experienced graders; in the case of discordance among the readers, a senior retina specialist was consulted (SAZ) (see Table 2).

Table 2.
Descriptive statistics of drusen types and MNV types.
The druse type that occupied the greater number of subfields in the Early Treatment Diabetic Retinopathy Study (ETDRS) macular grid was determined as the predominant druse type.
2.5. Statistical Analysis
Data were organized in Microsoft Excel (Microsoft Corp., Redmond, WA, USA) and statistically analyzed using SPSS software version 23 (SPSS Inc., Chicago, IL, USA) and R.app v4.1.0 GUI 1.76 for MacOS (The R Foundation for Statistical Computing c/o Institute for Statistics and Mathematics, 1020 Vienna, Austria). Descriptive statistics, such as the mean and standard deviation (SD), were computed. The number of neovascular AMD lesions as identified by the anatomic classification system was recorded.
Adjusted odds ratios (aORs), as a predictor between druse type and MNV type, were calculated in R using binary logistic regression. As the characteristic structural changes in AMD are age-related, age will not be normally distributed in our dataset. The statistical feature “age” will additionally contribute to the odds ratios (ORs). As we wanted to evaluate the effect of drusen on MNV development alone, we adjusted the ORs for age. The statistical significance level (α) was defined as 0.05. Statistical analysis results with a p-value less than 0.05 were interpreted as statistically significant.
3. Results
A total of 3980 eyes with treatment-naïve neovascular AMD were screened. Among these, 536 eyes met the eligibility criteria. Of those, 154 eyes were excluded due to advanced fibrovascular scarring and/or poor image quality precluding a reliable classification of MNV and drusen type. Eventually, 382 eyes of 191 patients were eligible. Only 1 eye per patient was included, resulting in 191 study eyes (n = 191) (Table 1). If both eyes were eligible, one eye was chosen randomly. Patients with the predominant druse type of SDD had higher aORs for MNV 2 and MNV 3 (Table 3). Patients with MNV 1 were less likely to have predominant SDDs (Table 3). No clear predictor for any druse type was found for mixed MNV 1/2 lesions. Patients with soft drusen showed the highest overall aOR with MNV 1 lesion (Table 3).

Table 3.
Predictors (aOR adjusted for age) between druse and MNV type.
History of diabetes, arterial hypertension and glaucoma did not show statistically significant correlations (Phi/Cramer-v) with neither MNV type nor druse type (Table 4).

Table 4.
Correlations (Phi/Cramer-V) between MNV type and druse type with systemic factors.
4. Discussion
The subretinal space is an unusual location for the extracellular deposition of material, and not only changes in the sub-RPE location, but also changes anterior to the RPE cells, may play an important role in AMD pathogenesis. The presence of SDDs seems to be associated with thin choroidal thickness [15,16]. Eyes with sub-RPE predominant soft drusen show the highest overall odds ratio for developing neovascular AMD [17]. We observed the same trend in our data (Table 3). However, subretinal SDDs clearly seem to be associated with late AMD, including complete outer retinal atrophy (cRORA) as well as MNV in AMD [11,18,19,20]. There seemed to be debate as to whether SDDs could be linked to an AMD MNV subtype [21]. Cohen et al. were the first to observe a correlation between SDD and the MNV 3 subtype [13]. Marsiglia et al. demonstrated that patients with MNV 1 were less likely to have SDDs and that patients with MNV 3 were more likely to have SDDs in their non-neovascular fellow eye [14]. They did not observe an association between SDDs and MNV 2, which might be due to the low number of only 10 eyes with MNV 2 in their series [14]. Applying the CONAN Study Group criteria for MNV classification to this dataset, we could demonstrate that, in AMD patients with treatment-naïve MNV, SDDs were more likely to be a risk factor for MNV 2 and MNV 3 as compared with soft drusen (Table 3). MNV 2 occurs in the subretinal space above the RPE. MNV 3 originates intraretinally (supra-RPE) and penetrates the RPE only in later stages. Therefore, SDDs seem to be a plausible risk factor for the development of both the MNV 2 and MNV 3 subtypes. This association is supported by findings from Spaide et al., Rabiolo et al. and Lee et al., who demonstrated that SDDs would preferably progress to MNV 2 and MNV 3 [16,19,20,22]. We could confirm this finding with MNV 2 and with MNV 3 for predominant SDDs. Interestingly, we did not find a statistically significant odds ratio for SDDs only with any type of MNV 3. This might be due to our classification of the presence of any MNV 3 under one group. Hence, the MNV 3 group in our series also included mixed lesions, such as MNV 1/3 and MNV 2/3, making it difficult to find a statistical predictor.
Ahmed et al. frequently discovered MNV 2 without the presence of extracellular deposits [23]. This highlights that SDDs may be difficult to reliably detect. Rabiolo et al. also recommended using multimodal imaging with at least two different modalities to reliably detect SDDs [22].
The limitations of this study include its retrospective nature and the fact that graders were not masked to the original diagnosis of neovascular AMD. The non-neovascular fellow eye was used to identify the type of drusen in patients with no available baseline images of the study eye prior to the development of MNV; however, this is supported by prior studies showing intraindividual symmetry in eyes with AMD [24,25,26,27].
5. Conclusions
The anatomical classification of MNV, which incorporates FA and SD-OCT findings, seems to provide more accurate information about associations between the type of drusen and type of MNV. Predicting the type of MNV for patients with intermediate AMD might inform more personalized patient care with respect to monitoring and future treatment.
Author Contributions
Conceptualization: S.A.Z.; Methodology: S.A.Z., D.R.M. and K.J.; Software: D.R.M. and K.J.; Validation: S.A.Z., D.R.M., K.J., E.H.S., M.E., K.B.F. and M.D.T.; Formal analysis: S.A.Z., D.R.M., K.J., E.H.S., M.E., K.B.F. and M.D.T.; Investigation: S.A.Z., D.R.M., M.D.T., A.B., R.R., M.M.K. and J.M.G.; Resources: S.A.Z. and D.R.M.; Data curation: S.A.Z., D.R.M., K.J., A.B. and R.R., M.M.K. and J.M.G.; Writing—Original draft preparation: S.A.Z. and D.R.M.; Writing—Review and editing: S.A.Z., D.R.M., E.H.S., M.E. and K.B.F.; Visualization: D.R.M. and K.J.; Supervision: S.A.Z. and D.R.M.; Project administration: S.A.Z.; Funding acquisition: N/A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethics Committee approval was obtained from the Local Ethics Committee of the Canton of Zurich (approval number: PB_2016-00264). This study adheres to the tenets of the 1964 Declaration of Helsinki and its later amendments.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data, aside from the data published in this manuscript, are not publicly available due to privacy restrictions.
Conflicts of Interest
K.B.F.: Consultant—Allergan, Bayer HealthCare, Carl Zeiss Meditec, Genentech, Inc., Heidelberg Engineering, Novartis, Regeneron; Research support—Genentech, Inc., Roche. S.A.Z.: Consultant—Allergan, Bayer HealthCare, Novartis, Roche; Grant Support—Bayer HealthCare, Novartis. None of the disclosures are relevant for this manuscript.
References
- Freund, K.B.; Zweifel, S.A.; Engelbert, M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina 2010, 30, 1333–1349. [Google Scholar] [CrossRef] [PubMed]
- Grossniklaus, H.E.; Gass, J.D. Clinicopathologic correlations of surgically excised type 1 and type 2 submacular choroidal neovascular membranes. Am. J. Ophthalmol. 1998, 126, 59–69. [Google Scholar] [CrossRef]
- Gass, J.D. Biomicroscopic and histopathologic considerations regarding the feasibility of surgical excision of subfoveal neovascular membranes. Am. J. Ophthalmol. 1994, 118, 285–298. [Google Scholar] [CrossRef]
- Jung, J.J.; Chen, C.Y.; Mrejen, S.; Gallego-Pinazo, R.; Xu, L.; Marsiglia, M.; Boddu, S.; Freund, K.B. The incidence of neovascular subtypes in newly diagnosed neovascular age-related macular degeneration. Am. J. Ophthalmol. 2014, 158, 769–779 e762. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef]
- Mimoun, G.; Soubrane, G.; Coscas, G. Macular drusen. J. Fr. Ophtalmol. 1990, 13, 511–530. [Google Scholar]
- Zweifel, S.A.; Spaide, R.F.; Curcio, C.A.; Malek, G.; Imamura, Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology 2010, 117, 303–312 e301. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Alten, F.; Steinberg, J.S.; Jaffe, G.J.; Fleckenstein, M.; Mukesh, B.N.; Hohman, T.C.; Holz, F.G.; Geographic Atrophy Progression (GAP) Study Group. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5009–5015. [Google Scholar] [CrossRef]
- Steinberg, J.S.; Auge, J.; Jaffe, G.J.; Fleckenstein, M.; Holz, F.G.; Schmitz-Valckenberg, S.; Group, G.A.P.S. Longitudinal analysis of reticular drusen associated with geographic atrophy in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4054–4060. [Google Scholar] [CrossRef]
- Zhou, Q.; Daniel, E.; Maguire, M.G.; Grunwald, J.E.; Martin, E.R.; Martin, D.F.; Ying, G.S.; Comparison of Age-Related Macular Degeneration Treatments Trials Research Group. Pseudodrusen and Incidence of Late Age-Related Macular Degeneration in Fellow Eyes in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016, 123, 1530–1540. [Google Scholar] [CrossRef]
- Agron, E.; Domalpally, A.; Cukras, C.A.; Clemons, T.E.; Chen, Q.; Lu, Z.; Chew, E.Y.; Keenan, T.D.L.; Areds; Groups, A.R. Reticular Pseudodrusen: The Third Macular Risk Feature for Progression to Late Age-related Macular Degeneration: Age-Related Eye Disease Study 2 Report 30. Ophthalmology, 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, S.A.; Imamura, Y.; Spaide, T.C.; Fujiwara, T.; Spaide, R.F. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology 2010, 117, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.Y.; Dubois, L.; Tadayoni, R.; Delahaye-Mazza, C.; Debibie, C.; Quentel, G. Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularisation. Br. J. Ophthalmol. 2007, 91, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Marsiglia, M.; Boddu, S.; Chen, C.Y.; Jung, J.J.; Mrejen, S.; Gallego-Pinazo, R.; Freund, K.B. Correlation between neovascular lesion type and clinical characteristics of nonneovascular fellow eyes in patients with unilateral, neovascular age-related macular degeneration. Retina 2015, 35, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.G.; Gan, A.; Yanagi, Y.; Wong, T.Y.; Spaide, R. Association between Choroidal Thickness and Drusen Subtypes in Age-Related Macular Degeneration. Ophthalmol. Retina 2018, 2, 1196–1205. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Lee, C.S.; Kim, S.S.; Koh, H.J.; Lee, S.C.; Byeon, S.H. Drusen Subtypes and Choroidal Characteristics in Asian Eyes with Typical Neovascular Age-Related Macular Degeneration. Retina 2020, 40, 490–498. [Google Scholar] [CrossRef]
- Lee, J.; Choi, S.; Lee, C.S.; Kim, M.; Kim, S.S.; Koh, H.J.; Lee, S.C.; Byeon, S.H. Neovascularization in Fellow Eye of Unilateral Neovascular Age-related Macular Degeneration According to Different Drusen Types. Am. J. Ophthalmol. 2019, 208, 103–110. [Google Scholar] [CrossRef]
- Wightman, A.J.; Guymer, R.H. Reticular pseudodrusen: Current understanding. Clin. Exp. Optom. 2019, 102, 455–462. [Google Scholar] [CrossRef]
- Spaide, R.F.; Ooto, S.; Curcio, C.A. Subretinal drusenoid deposits AKA pseudodrusen. Surv. Ophthalmol. 2018, 63, 782–815. [Google Scholar] [CrossRef]
- Spaide, R.F. IMPROVING THE AGE-RELATED MACULAR DEGENERATION CONSTRUCT: A New Classification System. Retina 2018, 38, 891–899. [Google Scholar] [CrossRef]
- Wilde, C.; Patel, M.; Lakshmanan, A.; Morales, M.A.; Dhar-Munshi, S.; Amoaku, W.M. Prevalence of reticular pseudodrusen in eyes with newly presenting neovascular age-related macular degeneration. Eur. J. Ophthalmol. 2016, 26, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Rabiolo, A.; Sacconi, R.; Cicinelli, M.V.; Querques, L.; Bandello, F.; Querques, G. Spotlight on reticular pseudodrusen. Clin. Ophthalmol. 2017, 11, 1707–1718. [Google Scholar] [CrossRef]
- Ahmed, D.; Stattin, M.; Haas, A.M.; Graf, A.; Krepler, K.; Ansari-Shahrezaei, S. Drusen characteristics of type 2 macular neovascularization in age-related macular degeneration. BMC Ophthalmol. 2020, 20, 381. [Google Scholar] [CrossRef]
- Pauleikhoff, D.; Radermacher, M.; Spital, G.; Muller, C.; Brumm, G.; Lommatzsch, A.; Bird, A.C. Visual prognosis of second eyes in patients with unilateral late exudative age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2002, 240, 539–542. [Google Scholar] [CrossRef]
- Lavin, M.J.; Eldem, B.; Gregor, Z.J. Symmetry of disciform scars in bilateral age-related macular degeneration. Br. J. Ophthalmol. 1991, 75, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Yannuzzi, L.A.; Ladas, I.D.; Guyer, D.R.; Slakter, J.S.; Sorenson, J.A. Choroidal neovascularization in second eyes of patients with unilateral exudative age-related macular degeneration. Ophthalmology 1995, 102, 1380–1386. [Google Scholar] [CrossRef]
- Mann, S.S.; Rutishauser-Arnold, Y.; Peto, T.; Jenkins, S.A.; Leung, I.; Xing, W.; Bird, A.C.; Bunce, C.; Webster, A.R. The symmetry of phenotype between eyes of patients with early and late bilateral age-related macular degeneration (AMD). Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 209–214. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).