Emerging Novel Psychoactive Substances (2020–2025): GC-MS Approaches for Separation, Detection, and Characterization
Abstract
1. Introduction
2. Analytical Challenges in GC-MS Analysis of NPS
3. Synthetic and Semi-Synthetic Cannabinoids
3.1. Synthetic Cannabinoids in Powder and Herbal Mixtures
3.2. Synthetic Cannabinoids in Infused Paper and Vapes
3.3. Synthetic Cannabinoids in Biological Samples
3.4. Semi-Synthetic Cannabinoids
| Compound | Matrix/Sample Type | Notes/Key Findings | Reference |
|---|---|---|---|
| AB-FUBINACA, AB-CHMINACA, XLR-11 | Seized herbal blends | First profiling of the Jordanian market; wide concentration variability. | Al-Eitan et al., 2020 [87] |
| AMB-FUBINACA, MMB-CHMICA, others | E-liquids, powders, blotting papers, and prison letters | Highlighted new formulations (e-liquids, impregnated papers) used for prison smuggling. | Apirakkan et al., 2020 [62] |
| JWH-122, JWH-210, UR-144 | Oral fluid of consumers | Validated oral fluid method; first disposition data after controlled administration. | La Maida et al., 2020 [101] |
| Δ8-THC, THC-O acetates, CBD-di-O-A | Commercial edibles & herbal products | First report of THC-O acetates and CBD-di-O A in consumer products. | Holt et al., 2022 [133] |
| CH-PIACA (indole-3-acetamide SC) | Seized powders | First identification of CH-PIACA in Europe, linked to class-wide legislative bans. | Pasin et al., 2022 [88] |
| 5F-MDMB-PICA | “American grass” is a herbal drug | Avg. content 2.2% w/w in seized samples; method fully ICH-validated. | Nguyen et al., 2023 [99] |
| 5F-MDMB-PICA, MMB-4en-PICA, 4F-MDMB-BUTINACA, MDMB-4en-PINACA | Prison mail (impregnated paper) | Library updates required for prison screening systems. | Abbott et al., 2023 [97] |
| SCs (not confirmed in samples) | Prison air (fixed + personal samplers) | First attempt to detect airborne SCs; no direct confirmation, but showed feasibility. | Paul et al., 2021 [98] |
| 5F-MDMB-PICA | Seven disposable illicit vapes | All vapes mis-sold as cannabis contained only SCs, highlighting high user risk. | Craft et al., 2024 [61] |
| Semi-synthetic cannabinoids (Δ8-THC, HHC, H4-CBD, HHC-O-Ac, HHCP, THCP) | Seized cannabis products | Reprocessed historical GC-MS data to quantify emergence: 15% (n = 216) of products positive for SSCs; HHC 10% most frequent. | Jørgensen et al., 2024 [129] |
| 21 synthetic cannabinoids (reference set) | NPS bought online (powders) and laced plant material | Built an NMR + GC-MS reference dataset; shows why GC-MS alone can mis-ID some SCs and how pairing with NMR resolves ambiguities. | Hübner et al., 2025 [83] |
| JWH-122, JWH 210, UR-144 and their hydroxylated metabolites | Urine samples of consumers | The hydroxylated metabolites and parent molecules were determined in sub-ng/mL concentrations. The samples underwent initial hydrolysis with β-glucuronidase. | Pellegrini et al., 2020 [41] |
| 2F-QMPSB and precursor 2F-MPSBA | Herbal samples | The new compounds, along with their precursors, were determined in the samples. | Tsochatzis et al., 2021 [89] |
| 50 cannabinoids (synthetic and phytocannabinoids) | Reference standard panel | Targeted GC-MS method differentiated 50 cannabinoids by retention time and spectral dissimilarity; supports systematic classification. | Sisco et al., 2021 [100] |
| 5F-Cumyl-PEGACLONE | Femoral blood | The femoral blood concentrations of 5F-Cumyl-PEGACLONE were used to determine the cause of death. | Giorgetti et al., 2020 [116] |
| 10 indole/indazole carboxamide SCs | Herbal blends | For quantifying multi-component mixtures, GC-MS was preferred when reference materials were unavailable; when they were available, NMR proved more useful. | Liu et al., 2021 [91] |
| 29 cannabinoids and their metabolites | Blood and urine samples | Ultrasound-assisted DLLME was a highly efficient microextraction technique for the quantification of 29 compounds in blood and urine samples. | Mercieca et al., 2020 [40] |
| 5F-ADB-PINACA and AB-FUBINACA | Human plasma samples | Magnetic solid-phase microextraction coupled with DLLME showed enrichment factors of 213 and 437. | Rad et al., 2020 [65] |
| ∆9 -Tetrahydrocannabiphorol | THCP vape pen | GC-MS analysis enabled discrimination between natural and synthetic THCP sources by identifying chemical impurities. | Schirmer et al., 2024 [67] |
| Synthetic cannabinoids | Herbal mixtures | GC-MS and NMR were employed to determine the active compounds together with adulterants and preservatives (vitamin E, vitamin E acetate, oleamide). | Alves et al., 2021 [92] |
| ADB-4en-PINACA | Human hair sample | The human hair sample underwent extraction, but derivatization was not required. | Wang et al., 2023 [111] |
4. Synthetic Cathinones
4.1. Separation of Structural Isomers
4.2. Synthetic Cathinones with a 3,4-Methylenedioxy Moiety
4.3. α-Pyrrolidinophenone Derivatives
4.4. Samples with Several Cathinones
4.5. Synthetic Cathinones in Biological and Environmental Samples
| Compound | Matrix/Sample Type | Notes/Key Findings | Reference |
|---|---|---|---|
| N-cyclohexyl butylone | Pills and crystal samples | Newly detected compound with MS spectra that did not match the libraries. | Mata-Pesquera et al., 2023 [161] |
| 3′,4′-methylenedioxy-2,2-dibromobutyrophenone | Powder | This precursor was identified through MS fragmentation in a sample that was sold as ketamine on the Internet. | Armenta et al., 2020 [34] |
| R and S-isomers of cathinone | Different parts of the Catha edulis plant | Derivatization with menthyl chloroformate and subsequent GC-MS analysis was successful for the quantification of different isomers in various parts of the plant. | Dhabbah, 2020 [57] |
| Five isomers of N-butyl pentylone | Pure compounds | The MS fragmentation pattern (the ratio of ion abundances at 128 and 72 m/z) can be used to distinguish isomers. | Liliedahl et al., 2023 [158] |
| Several amphetamine-like stimulants, including synthetic cathinones | Suspended particulate matter in wastewater | GC-MS technique with the derivatization agent efficiently determined concentrations of amphetamine isomers and other stimulants. | Langa et al., 2024 [53] |
| N-cyclohexyl pentylone | Pale brown powder | GC-MS fragmentation and NMR analysis were used to solve the structure of the newly encountered NPS | Mayer et al., 2024 [29] |
| Methcathinone and ephedrine | Plasma and brain tissues | The GC-MS method was developed for the simultaneous determination of both compounds with a high linearity range (5 to 1000 ng/mg). | Bin Jardan et al., 2021 [47] |
| cathinone, methcathinone, mephedrone, methylone, and ethylone | Wastewater | The selective sorption was done on dummy molecularly imprinted polymers; GC-MS analysis had LOD values between 0.002 and 0.1 ng/mL. | Han et al., 2022 [48] |
| 22 α-pyrrolidinophenone | Pure compounds | Fragmentation pathways were examined using different instruments: GC-EI-MS, LS-ESI-MS/MS, and DART-MS. GC-EI-MS fragmentation mechanism was not dependent on the substituents present. | Davidson et al., 2020 [167] |
| 5-PPDI and three regioisomers | Solution | The order of elution was the same for the three columns used, and compounds were differentiated by the second-generation product ion spectra in ESI-LIT-MS. | Ishii et al., 2022 [169] |
| putylone | Seized tablets | The proposed GC-MS method successfully quantified putylone in seized tablets. | Dixon et al., 2023 [156] |
| 4-CEC, α-PVP, 4-Cl-PVP, and MDPV | Whole blood samples | SPE and derivatization with MSTFA and TMCS, linearity range 5–800 ng/mL, extraction efficiency > 85%. | Antunes et al., 2021 [174] |
| 4-Fluoro-3-methyl-α-PVP | Femoral and heart blood, vitreous humor | Post-mortem analysis of body fluids by GC-MS helped identify this compound. | Hobbs et al., 2022 [178] |
| α-PVT, α-PVP, and MDPV | Oral fluid | BAµE-µLD/GC-MS of three components, with average recoveries between 43.1 and 52.3%, and good selectivity. | Segurado et al., 2022 [170] |
5. Semi-Synthetic and Synthetic Opioids
5.1. Semi-Synthetic Opioids
5.2. Fentanyl and Its Analogues
5.3. Non-Fentanyl Compounds
5.4. Benzodiazepines
| Compound | Matrix/Sample Type | Notes/Key Findings | Reference |
|---|---|---|---|
| Fentanyl and eight analogues | Oral liquids | FPSE microextraction followed by GC-MS provided good recovery, linearity, and selectivity in ng/mL concentrations. | Ares-Fuentes et al., 2022 [196] |
| Fentanyl and acetofentanyl | Soil | Extraction with ethyl acetate, followed by mixing with Troc-Cl and subsequent GC-MS analysis, allowed identification of metabolites. | Valdez et al., 2023 [212] |
| 11 fentanyl analogues | Methanolic solution | The targeted GC-MS technique developed for fentanyl analogues was 25 times more sensitive and had a two-fold increase in retention time difference, compared to standard methods. | Sisco et al., 2021 [210] |
| Fentanyl and butyryl fentanyl | Oral fluid | Developed GC-MS procedure with LOQ of 1 ng/mL. | Camedda et al., 2024 [209] |
| 18 fentanyl-derived opioids | Solution | Presumptive tests were used to detect compounds, which GC-MS further confirmed within 20 min. | Gilbert et al., 2020 [211] |
| U-48800 | White powder | GC-MS was unable to differentiate between closely related isomers; NMR and molecular dynamics provided definitive structural information. | Pereira et al., 2025 [227] |
| Dipyanone, a methadone-related compound | White powder | GC-MS identification was possible based on the peaks at 355 (molecular ion) and 98 (pyrrolidine ring). | Vandeputte et al., 2023 [194] |
| Nine fentanyl compounds | Hair sample | GC-MS analysis was done on the extracts after the sonication of the samples. | Wei and Su, 2022 [208] |
| Methyl-substituted fentanyl derivatives | Pure compounds | GC-MS retention times and fragmentation patterns depend on the position of substituents and allow their differentiation with high precision. | Pollard and Davison, 2023 [70] |
| Brorphine | Powder | Reported structural examination for the first time. | Verougstraete et al., 2020 [230] |
| Oxycodone pills and other prescription drugs | Pills | GC-MS and GC-FID were used to examine counterfeit pills with a physical appearance similar to genuine drugs. In most of them, fentanyl and other opioids were detected. | Nguyen et al., 2022 [188] |
| Fentanyl, desalkylgidazepam, and bromazolam | Blood and urine | GC-MS was applied for the quantification of three opioids; derivatization with MTBSTFA was needed for desalkylgidazepam. | Ballotari et al., 2024 [243] |
6. Hallucinogens, Dissociatives, and Entactogens/Empathogens
6.1. LSD and Its Analogues
6.2. Tryptamines
6.3. Phenylalkylamines
6.4. Ketamine
6.5. Nitrous Oxide
6.6. Hallucinogenic Compounds from Natural Sources
| Compound | Matrix/Sample Type | Notes/Key Findings | Reference |
|---|---|---|---|
| Ketamine | Hair samples | GC-MS provided untargeted analysis, whereas UHPLC-HRMS/MS yielded lower LODs and LOQs. | Matey et al., 2021 [281] |
| 1-Acetyl-LSD | Blotter paper | Identified by GC-MS for the first time in Brazil. | Neves Junior et al., 2022 [253] |
| N2O | Blood | The GC-MS method allowed the determination of blood concentration between 0.1 and 48 mL N2O/L blood. | Lindholm et al., 2024 [289] |
| N2O | Blood | N2O was separated from CO2 using a sodium hydroxide solution before the GC-MS analysis, with n-pentane added as an internal standard. | Li et al., 2024 [288] |
| Safrole, myristicin, and elemicin | Human serum | MonoSpin® extraction kit and GC-MS were used for the extraction of three hallucinogenic compounds from nutmeg. | Usui et al., 2023 [292] |
| N-(bromodimethoxybenzyl)-2-, 3-, and 4-methoxyphenethylamines | Powder | The inverse analogues of the 25B-NBO drug were examined, and their elution times and fragmentation were determined based on the relative positions of substituents. | Almalki et al., 2020 [278] |
| Methylenedioxybenzyl analogues of 25X-NBOMe drugs | Powder | Methylenedioxybenzyl analogues were proactively synthesized and examined by GC-MS, with separation on a Rxi®-17Sil column, and the fragmentation pathways were explained. | Almalki et al., 2020 [275] |
| Methoxetamine (MXE) | Solution | The LC-MS technique was more appropriate for the examination of MXE’s metabolites from in vitro studies than GC-MS, due to their volatility. | Alshamaileh et al., 2020 [284] |
| 5-MeO-MiPT | Urine samples | The GC-MS methodology had LOD and LOQ values of 5 and 18 ng/mL, respectively, with linearity over 25–500 ng/mL. | Emen et al., 2023 [260] |
| 2C-B | Urine sample | Before GC-MS, derivatization was performed using MBTFA following SPE preparation. | Lukić et al., 2022 [272] |
| DTM, harmine, and harmaline | Sweat | GC-MS quantification of the constituents of Ayahuasca from sweat samples of the participants in the religious ritual. | Tavares et al., 2020 [257] |
7. Polydrug Mixtures from Different Classes
| Compounds | Matrix/Sample Type | Notes/Key Findings | Reference |
|---|---|---|---|
| Nine amphetamines, seven synthetic cathinones, five phenethylamines | Solution, blood, and urine | SPE followed by derivatization by pentafluoropropionic anhydride, separation in 11 min, with low LOQ and LOD. | Bouzoukas et al., 2025 [295] |
| Ketamine, methamphetamine, MDMA | Urine | HS-SPME-GC-MS analysis was suitable for the simultaneous detection of compounds and their metabolites in urine, allowing discussion of the pharmacokinetics. | Sim et al., 2025 [36] |
| Compounds from several groups | Powder | A short column and a rapid (10 s °C/s) temperature program allowed the separation of psychoactive compounds, diluents, and adulterants in less than 1 min. | Bloom et al., 2023 [301] |
| Anabolic-androgenic steroids + other NPS | e-cigarettes | GC-MS analysis without derivatization was an appropriate technique for verifying the presence of AAS and NPS. | Harries et al., 2024 [299] |
| AP-237, MDMB-4enPINACA, 5F-ADB | Infused paper | Screening by GC-MS and semi-quantitative analysis by LC-MS | Giorgetti et al., 2022 [300] |
| Eleven different NPS | Infused chocolate | Extraction by methanol, sonication, and centrifugation before GC-MS, with limits of detection between 0.05 and 0.1 mg/mL. | Song et al., 2023 [63] |
| β-U10 and heroin, xylitol, and degradation products of heroin | discarded drug paraphernalia | GC-MS confirmatory technique, although higher amounts were required, and not all compounds were detectable compared to DART-MS. | West et al., 2022 [306] |
| Nineteen psychoactive compounds | In vitro and post-mortem whole blood samples. | SPE-GC-MS method with derivatization mixture (BSTFA and TMCS), three working concentrations for therapeutic, toxic, and lethal concentrations. | Ferreira et al., 2021 [296] |
| Fentanyl, 5F-ADB, 5F-MDMB-P7AICA, and other SC | Bucket bong water, kidney, liver, urine, and hair | LLE followed by GC-MS for initial screening of compounds, quantification by GC-MS (THC and metabolites), and LC-MS/MS for other substances. | Neukamm et al., 2024 [304] |
8. Portable GC-MS Instruments and Machine Learning Advancements of GC-MS
9. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-FDCK | 2-Fluorodeschloroketamine |
| 2-FEA | 2-Fluoroethamphetamine |
| 3-FEA | 3-Fluoroethamphetamine |
| 3-MMC | 3-Methylmethcathinone |
| 4-APB | 4-(2-Aminopropyl)benzofuran |
| 4-CEC | 4-Chloroethcathinone |
| 4-Cl-PVP | 4-Chloro-α-pyrrolidinovalerophenone |
| 4-HO-MiPT | 4-Hydroxy-N-methyl-N-isopropyltryptamine |
| 4-MeO-α-PVP | 4-Methoxy-α-pyrrolidinovalerophenone |
| 5-MeO-MiPT | 5-Methoxy-N-methyl-N-isopropyltryptamine |
| 5-PPDI | 1-(2,3-Dihydro-1H-inden-5-yl)-2-(pyrrolidin-1-yl)butan-1-one |
| 5F-ADB | Methyl 2-{[1-(5-fluoropentyl)-1H-indazole-3-carbonyl]amino}-3,3-dimethylbutanoate |
| 5F-MDMB-P7AICA | Methyl [2-(1-(5-fluoropentyl)-1H-pyrrolo[3,2-b]pyridine-3-carboxamido)-3,3-dimethylbutanoate] |
| 6-APB | 6-(2-Aminopropyl)benzofuran |
| ADB-4en-PINACA | N-[1-amino-3,3-dimethyl-1-oxobutan-2-yl]-1-(pent-4-en-1-yl)-1H-indazole-3-carboxamide |
| ADB-BUTINACA | N-[(2S)-1-amino-3,3-dimethyl-1-oxobutan-2-yl]-1-butyl-1H-indazole-3-carboxamide |
| ADMB-3TMS-PrINACA | N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(3-(trimethylsilyl)propyl)-1H-indazole-3-carboxamide |
| AH-7941 | N-(Cyclohexylmethyl)-N′-(3,4-dichlorobenzyl)benzamide |
| ALD-52 | 1-Acetyl-N,N-diethyllysergamide |
| AP-237 | 1-[4-(3-phenyl-2-propen-1-yl)-1-piperazinyl]-1-butanone |
| Brorphine | 3-{1-[1-(4-bromophenyl)ethyl]piperidin-4-yl}-1H-benzimidazol-2-one |
| CBD | Cannabidiol |
| CH-PIACA | Cyclohexyl indole-3-acetamide-type synthetic cannabinoid |
| Cumyl-3TMS-PrINACA | N-(2-phenylpropan-2-yl)-1-(3-(trimethylsilyl)propyl)-1H-indazole-3-carboxamide |
| Isotonitazene | N,N-Diethyl-2-[5-nitro-2-({4-[(propan-2-yl)oxy]phenyl}methyl)-1H-benzimidazol-1-yl]ethan-1-amine |
| LLE | Liquid-liquid Extraction |
| LOD | Limit of Detection |
| LOQ/(L)LOQ | Limit of Quantification/(Lower) Limit of Quantification |
| MDMB-4en-PINACA | 2-{[1-(pent-4-en-1-yl)-1H-indazole-3-carbonyl]amino}-3,3-dimethylbutanoate |
| MDPV | 3,4-Methylenedioxypyrovalerone |
| Methorphan | 3-Methoxy-N-methylmorphinan |
| MSPE | Magnetic Solid-Phase Extraction |
| MT-45 | 1-Cyclohexyl-4-(1,2-diphenylethyl)piperazine |
| MXE | Methoxetamine; 2-(ethylamino)-2-(3-methoxyphenyl)cyclohexanone |
| NB | Novel Benzodiazepines |
| NPS | Novel Psychoactive Substances |
| PCA | Principal Component Analysis |
| Putylone | 1-(1,3-Benzodioxol-5-yl)-2-(propylamino)butan-1-one |
| SC | Synthetic Cannabinoid |
| SO | Synthetic Opioids |
| SPE | Solid-Phase Extraction |
| SSC | Semi-synthetic Cannabinoid |
| THC | Δ9-Tetrahydrocannabinol |
| THCP | Δ9-Tetrahydrocannabiphorol |
| U-47700 | trans-3,4-Dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide |
| U-50488 | trans-(±)-3,4-Dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]benzamide |
| U-51754 | N-[2-(Dimethylamino)cyclohexyl]-N-methyl-4-methylbenzamide |
| U-69593 | trans-(±)-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]benzamide |
| α-PVP | α-Pyrrolidinovalerophenone |
| β-U10 | N-[2-(Dimethylamino)cyclohexyl]-N-methylnaphthalene-1-carboxamide |
References
- Bade, R.; Rousis, N.; Adhikari, S.; Baduel, C.; Bijlsma, L.; Bizani, E.; Boogaerts, T.; Burgard, D.A.; Castiglioni, S.; Chappell, A.; et al. Three Years of Wastewater Surveillance for New Psychoactive Substances from 16 Countries. Water Res. X 2023, 19, 100179. [Google Scholar] [CrossRef]
- Vincenti, F.; Gregori, A.; Flammini, M.; Di Rosa, F.; Salomone, A. Seizures of New Psychoactive Substances on the Italian Territory during the COVID-19 Pandemic. Forensic Sci. Int. 2021, 326, 110904. [Google Scholar] [CrossRef]
- Papa, P.; Valli, A.; Di Tuccio, M.; Buscaglia, E.; Brambilla, E.; Scaravaggi, G.; Gallo, M.; Locatelli, C.A. Prevalence of Stimulant, Hallucinogen, and Dissociative Substances Detected in Biological Samples of NPS-Intoxicated Patients in Italy. J. Psychoact. Drugs 2021, 53, 247–255. [Google Scholar] [CrossRef]
- Baumann, M.H.; Volkow, N.D. Abuse of New Psychoactive Substances: Threats and Solutions. Neuropsychopharmacology 2016, 41, 663–665. [Google Scholar] [CrossRef]
- Das, P.; Horton, R. The Global Drug Problem: Change but Not Progression. Lancet 2019, 394, 1488–1490. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A.; Bruno, R.; Gisev, N.; Degenhardt, L.; Hall, W.; Sedefov, R.; White, J.; Thomas, K.V.; Farrell, M.; Griffiths, P. New Psychoactive Substances: Challenges for Drug Surveillance, Control, and Public Health Responses. Lancet 2019, 394, 1668–1684. [Google Scholar] [CrossRef] [PubMed]
- New Psychoactive Substances: Global Markets, Glocal Threats and the COVID-19 Pandemic: An Update from the EU Early Warning System; European Monitoring Centre for Drugs and Drug Addiction, Ed.; European Monitoring Centre for Drugs and Drug Addiction: Lisbon Portugal, 2020; ISBN 978-92-9497-557-7. [Google Scholar]
- Van Buskirk, J.; Griffiths, P.; Farrell, M.; Degenhardt, L. Trends in New Psychoactive Substances from Surface and “Dark” Net Monitoring. Lancet Psychiatry 2017, 4, 16–18. [Google Scholar] [CrossRef]
- Tomsone, L.E.; Neilands, R.; Kokina, K.; Bartkevics, V.; Pugajeva, I. Pharmaceutical and Recreational Drug Usage Patterns during and Post COVID-19 Determined by Wastewater-Based Epidemiology. Int. J. Environ. Res. Public Health 2024, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Browne, T.; Gold, M.S.; Martin, D.M. The Rapidly Changing Composition of the Global Street Drug Supply and Its Effects on High-Risk Groups for COVID-19. CPSP 2021, 10, 138–154. [Google Scholar] [CrossRef]
- Dei Cas, M.; Casagni, E.; Arnoldi, S.; Gambaro, V.; Roda, G. Screening of New Psychoactive Substances (NPS) by Gas-Chromatography/Time of Flight Mass Spectrometry (GC/MS-TOF) and Application to 63 Cases of Judicial Seizure. Forensic Sci. Int. Synerg. 2019, 1, 71–78. [Google Scholar] [CrossRef]
- UNODC (United Nations Office on Drugs and Crime) Data from the UNODC Early Warning Advisory on New Psychoactive Substances. Available online: https://www.unodc.org/LSS/Page/NPS/DataVisualisations (accessed on 18 October 2025).
- Salomone, A.; Palamar, J.J.; Vincenti, M. Should NPS Be Included in Workplace Drug Testing? Drug Test. Anal. 2020, 12, 191–194. [Google Scholar] [CrossRef]
- Catalani, V.; Arillotta, D.; Corkery, J.M.; Guirguis, A.; Vento, A.; Schifano, F. Identifying New/Emerging Psychoactive Substances at the Time of COVID-19; A Web-Based Approach. Front. Psychiatry 2021, 11, 632405. [Google Scholar] [CrossRef] [PubMed]
- Guirguis, A.; Moosa, I.; Gittins, R.; Schifano, F. What About Drug Checking? Systematic Review and Netnographic Analysis of Social Media. CN 2020, 18, 906–917. [Google Scholar] [CrossRef]
- Arillotta, D.; Schifano, F.; Napoletano, F.; Zangani, C.; Gilgar, L.; Guirguis, A.; Corkery, J.M.; Aguglia, E.; Vento, A. Novel Opioids: Systematic Web Crawling Within the e-Psychonauts’ Scenario. Front. Neurosci. 2020, 14, 149. [Google Scholar] [CrossRef]
- Feltmann, K.; Elgán, T.H.; Böttcher, M.; Lierheimer, S.; Hermansson, S.; Beck, O.; Gripenberg, J. Feasibility of Using Breath Sampling of Non-Volatiles to Estimate the Prevalence of Illicit Drug Use among Nightlife Attendees. Sci. Rep. 2022, 12, 20283. [Google Scholar] [CrossRef]
- Gripenberg-Abdon, J.; Elgán, T.H.; Wallin, E.; Shaafati, M.; Beck, O.; Andréasson, S. Measuring Substance Use in the Club Setting: A Feasibility Study Using Biochemical Markers. Subst. Abus. Treat. Prev. Policy 2012, 7, 7. [Google Scholar] [CrossRef]
- Papaseit, E.; Pérez-Mañá, C.; Pérez-Acevedo, A.P.; Hladun, O.; Torres-Moreno, M.C.; Muga, R.; Torrens, M.; Farré, M. Cannabinoids: From Pot to Lab. Int. J. Med. Sci. 2018, 15, 1286–1295. [Google Scholar] [CrossRef]
- Patocka, J.; Zhao, B.; Wu, W.; Klimova, B.; Valis, M.; Nepovimova, E.; Kuca, K. Flakka: New Dangerous Synthetic Cathinone on the Drug Scene. Int. J. Mol. Sci. 2020, 21, 8185. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, X.; Ming, Z.; Jiang, L.; Zhou, Y. Simultaneous Determination of 16 Kinds of Synthetic Cathinones in Human Urine Using a Magnetic Nanoparticle Solid-Phase Extraction Combined with Gas Chromatography-Mass Spectrometry. Separations 2021, 9, 3. [Google Scholar] [CrossRef]
- Cunha, R.L.; Oliveira, C.D.S.L.; De Oliveira, A.L.; Maldaner, A.O.; Do Desterro Cunha, S.; Pereira, P.A.P. An Overview of New Psychoactive Substances (NPS) in Northeast Brazil: NMR-Based Identification and Analysis of Ecstasy Tablets by GC-MS. Forensic Sci. Int. 2023, 344, 111597. [Google Scholar] [CrossRef]
- Lo Faro, A.; Berardinelli, D.; Cassano, T.; Dendramis, G.; Montanari, E.; Montana, A.; Berretta, P.; Zaami, S.; Busardò, F.; Huestis, M. New Psychoactive Substances Intoxications and Fatalities during the COVID-19 Epidemic. Biology 2023, 12, 273. [Google Scholar] [CrossRef]
- Yanini, A.; Esteve-Turrillas, F.A.; De La Guardia, M.; Armenta, S. Ion Mobility Spectrometry and High Resolution Mass-Spectrometry as Methodologies for Rapid Identification of the Last Generation of New Psychoactive Substances. J. Chromatogr. A 2018, 1574, 91–100. [Google Scholar] [CrossRef]
- Logan, B.K.; Reinhold, L.E.; Xu, A.; Diamond, F.X. Identification of Synthetic Cannabinoids in Herbal Incense Blends in the United States. J. Forensic Sci. 2012, 57, 1168–1180. [Google Scholar] [CrossRef]
- Pasin, D.; Cawley, A.; Bidny, S.; Fu, S. Current Applications of High-Resolution Mass Spectrometry for the Analysis of New Psychoactive Substances: A Critical Review. Anal. Bioanal. Chem. 2017, 409, 5821–5836. [Google Scholar] [CrossRef]
- Marginean, I.; Rowe, W.F.; Lurie, I.S. The Role of Ultra High Performance Liquid Chromatography with Time of Flight Detection for the Identification of Synthetic Cannabinoids in Seized Drugs. Forensic Sci. Int. 2015, 249, 83–91. [Google Scholar] [CrossRef]
- Muhamadali, H.; Watt, A.; Xu, Y.; Chisanga, M.; Subaihi, A.; Jones, C.; Ellis, D.I.; Sutcliffe, O.B.; Goodacre, R. Rapid Detection and Quantification of Novel Psychoactive Substances (NPS) Using Raman Spectroscopy and Surface-Enhanced Raman Scattering. Front. Chem. 2019, 7, 412. [Google Scholar] [CrossRef]
- Mayer, A.; Black, C.; Copp, B.R. Identification and Characterisation of the Recently Detected Cathinone N-Cyclohexyl Pentylone. Forensic Sci. Int. 2025, 367, 112387. [Google Scholar] [CrossRef]
- Liliedahl, R.E.; Davidson, J.T. The Differentiation of Synthetic Cathinone Isomers Using GC-EI-MS and Multivariate Analysis. Forensic Chem. 2021, 26, 100349. [Google Scholar] [CrossRef]
- Davidson, J.T.; Sasiene, Z.J.; Jackson, G.P. Fragmentation Pathways of Odd- and Even-Electron N-Alkylated Synthetic Cathinones. Int. J. Mass. Spectrom. 2020, 453, 116354. [Google Scholar] [CrossRef]
- Graziano, S.; Anzillotti, L.; Mannocchi, G.; Pichini, S.; Busardò, F.P. Screening Methods for Rapid Determination of New Psychoactive Substances (NPS) in Conventional and Non-Conventional Biological Matrices. J. Pharm. Biomed. Anal. 2019, 163, 170–179. [Google Scholar] [CrossRef]
- Scientific Working Group for the Analysis of Seized Drugs (SWGDRUG) MS Library, Version 3.14. Available online: https://www.swgdrug.org/ms.htm (accessed on 18 October 2025).
- Armenta, S.; Gil, C.; Ventura, M.; Esteve-Turrillas, F.A. Unexpected Identification and Characterization of a Cathinone Precursor in the New Psychoactive Substance Market: 3′,4′-Methylenedioxy-2,2-Dibromobutyrophenone. Forensic Sci. Int. 2020, 306, 110043. [Google Scholar] [CrossRef]
- Żubrycka, A.; Kwaśnica, A.; Haczkiewicz, M.; Sipa, K.; Rudnicki, K.; Skrzypek, S.; Poltorak, L. Illicit Drugs Street Samples and Their Cutting Agents. The Result of the GC-MS Based Profiling Define the Guidelines for Sensors Development. Talanta 2022, 237, 122904. [Google Scholar] [CrossRef]
- Sim, J.; Kyung, S.Y.; Jo, J.; Choi, H.; Jeong, S.; Lee, N.; Kim, S. Simultaneous Determination of Methamphetamine, MDMA, and Ketamine and Their Metabolites in Urine Using a Rapid and Simple HS-SPME-GC–MS Method: A Forensic Study on Drug Abuse Patterns in South Korea. J. Chromatogr. B 2025, 1264, 124720. [Google Scholar] [CrossRef]
- Peters, F.T.; Wissenbach, D.K.; Busardo, F.P.; Marchei, E.; Pichini, S. Method Development in Forensic Toxicology. Curr. Pharm. Des. 2017, 23, 5455–5467. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, A.L.; Mohr, A.L.A.; Chan-Hosokawa, A.; Harper, C.; Huestis, M.A.; Limoges, J.F.; Miles, A.K.; Scarneo, C.E.; Kerrigan, S.; Liddicoat, L.J.; et al. Recommendations for Toxicological Investigation of Drug-Impaired Driving and Motor Vehicle Fatalities—2021 Update. J. Anal. Toxicol. 2021, 45, 529–536. [Google Scholar] [CrossRef]
- Grapp, M.; Kaufmann, C.; Schwelm, H.M.; Neukamm, M.A.; Blaschke, S.; Eidizadeh, A. Intoxication Cases Associated with the Novel Designer Drug 3′,4′-methylenedioxy-α-pyrrolidinohexanophenone and Studies on Its Human Metabolism Using High-resolution Mass Spectrometry. Drug Test. Anal. 2020, 12, 1320–1335. [Google Scholar] [CrossRef]
- Mercieca, G.; Odoardi, S.; Mestria, S.; Cassar, M.; Strano-Rossi, S. Application of Ultrasound-assisted Liquid–Liquid Microextraction Coupled with Gas Chromatography and Mass Spectrometry for the Rapid Determination of Synthetic Cannabinoids and Metabolites in Biological Samples. J. Sep. Sci. 2020, 43, 2858–2868. [Google Scholar] [CrossRef]
- Pellegrini, M.; Marchei, E.; Papaseit, E.; Farré, M.; Zaami, S. UHPLC-HRMS and GC-MS Screening of a Selection of Synthetic Cannabinoids and Metabolites in Urine of Consumers. Medicina 2020, 56, 408. [Google Scholar] [CrossRef]
- Verhoeven, M.; Bonetti, J.; Kranenburg, R.; Van Asten, A. Chemical Identification and Differentiation of Positional Isomers of Novel Psychoactive Substances–A Comprehensive Review. TrAC Trends Anal. Chem. 2023, 166, 117157. [Google Scholar] [CrossRef]
- Lee, H.Z.S.; Ng, J.Y.J.; Ong, M.C.; Lim, J.L.W.; Yap, T.W.A. Technical Note: Unequivocal Identification of 5-Methoxy-DiPT with NOESY NMR and GC-IRD. Forensic Sci. Int. 2020, 316, 110537. [Google Scholar] [CrossRef]
- Sim, S.B.D.; Lee, H.Z.S.; Ong, M.C.; Zhang, S.; Lim, K.A.; Lim, J.L.W.; Yap, T.W.A. Synthesis, Characterization and Differentiation of the Structural Isomers of Valine and Tert -leucine Derived Synthetic Cannabinoids. Drug Test. Anal. 2024, 16, 420–434. [Google Scholar] [CrossRef]
- Di Trana, A.; Carlier, J.; Berretta, P.; Zaami, S.; Ricci, G. Consequences of COVID-19 Lockdown on the Misuse and Marketing of Addictive Substances and New Psychoactive Substances. Front. Psychiatry 2020, 11, 584462. [Google Scholar] [CrossRef] [PubMed]
- Scherbaum, N.; Bonnet, U.; Hafermann, H.; Schifano, F.; Bender, S.; Grigoleit, T.; Kuhn, J.; Nyhuis, P.; Preuss, U.W.; Reymann, G.; et al. Availability of Illegal Drugs During the COVID-19 Pandemic in Western Germany. Front. Psychiatry 2021, 12, 648273. [Google Scholar] [CrossRef]
- Bin Jardan, Y.A.; Mohamed, K.; Abbas, N.; El-Gendy, M.; Alsaif, N.; Alanazi, M.; Mohammed, M.; Abounassif, M.; Hefnawy, M. Development and Validation of GC–MS Method for Determination of Methcathinone and Its Main Metabolite in Mice Plasma and Brain Tissue after SPE: Pharmacokinetic and Distribution Study. J. Pharm. Biomed. Anal. 2021, 194, 113798. [Google Scholar] [CrossRef]
- Han, C.; Tan, D.; Wang, Y.; Yu, Z.; Sun, X.; Wang, D. Selective Extraction of Synthetic Cathinones New Psychoactive Substances from Wastewater, Urine and Cocktail Using Dummy Molecularly Imprinted Polymers. J. Pharm. Biomed. Anal. 2022, 215, 114765. [Google Scholar] [CrossRef]
- Chen, X.; Wu, X.; Luan, T.; Jiang, R.; Ouyang, G. Sample Preparation and Instrumental Methods for Illicit Drugs in Environmental and Biological Samples: A Review. J. Chromatogr. A 2021, 1640, 461961. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.-H.; Hsieh, C.-H.; Chaou, C.-H.; Chen, C.-K.; Yen, T.-H.; Liao, S.-C.; Seak, C.-J.; Chen, H.-Y. Synthetic Cathinone Poisoning from Ingestion of Drug-Laced “Instant Coffee Packets” in Taiwan. Hum. Exp. Toxicol. 2021, 40, 1403–1412. [Google Scholar] [CrossRef]
- Gould, O.; Nguyen, N.; Honeychurch, K.C. New Applications of Gas Chromatography and Gas Chromatography-Mass Spectrometry for Novel Sample Matrices in the Forensic Sciences: A Literature Review. Chemosensors 2023, 11, 527. [Google Scholar] [CrossRef]
- Bolcato, V.; Carelli, C.; Radogna, A.; Freni, F.; Moretti, M.; Morini, L. New Synthetic Cathinones and Phenylethylamine Derivatives Analysis in Hair: A Review. Molecules 2021, 26, 6143. [Google Scholar] [CrossRef]
- Langa, I.M.; Lado Ribeiro, A.R.; Ratola, N.; Gonçalves, V.M.F.; Tiritan, M.E.; Ribeiro, C. Amphetamine-like Substances and Synthetic Cathinones in Portuguese Wastewater Influents: Enantiomeric Profiling and Role of Suspended Particulate Matter. Forensic Sci. Int. 2024, 361, 112128. [Google Scholar] [CrossRef]
- Kerrigan, S.; Savage, M.; Cavazos, C.; Bella, P. Thermal Degradation of Synthetic Cathinones: Implications for Forensic Toxicology. J. Anal. Toxicol. 2016, 40, 1–11. [Google Scholar] [CrossRef]
- Tsujikawa, K.; Kuwayama, K.; Kanamori, T.; Iwata, Y.T.; Inoue, H. Thermal Degradation of α-Pyrrolidinopentiophenone during Injection in Gas Chromatography/Mass Spectrometry. Forensic Sci. Int. 2013, 231, 296–299. [Google Scholar] [CrossRef]
- Cheng, W.-C.; Wong, W.-C. Forensic Drug Analysis of Chloro-N,N-Dimethylcathinone (CDC) and Chloroethcathinone (CEC): Identification of 4-CDC and 4-CEC in Drug Seizures and Differentiation from Their Ring-Substituted Positional Isomers. Forensic Sci. Int. 2019, 298, 268–277. [Google Scholar] [CrossRef]
- Dhabbah, A.M. Determination of Chiral Cathinone in Fresh Samples of Catha Edulis. Forensic Sci. Int. 2020, 307, 110105. [Google Scholar] [CrossRef]
- Tyler Davidson, J.; Piacentino, E.L.; Sasiene, Z.J.; Abiedalla, Y.; DeRuiter, J.; Clark, C.R.; Berden, G.; Oomens, J.; Ryzhov, V.; Jackson, G.P. Identification of Novel Fragmentation Pathways and Fragment Ion Structures in the Tandem Mass Spectra of Protonated Synthetic Cathinones. Forensic Chem. 2020, 19, 100245. [Google Scholar] [CrossRef]
- Stuhmer, E.L.; McGuffin, V.L.; Waddell Smith, R. Discrimination of Seized Drug Positional Isomers Based on Statistical Comparison of Electron-Ionization Mass Spectra. Forensic Chem. 2020, 20, 100261. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, D.; Hua, Z.; Xu, P.; Wang, Y.; Di, B.; Liao, J.; Su, M. Machine Learning-Assisted Rapid Screening of Four Types of New Psychoactive Substances in Drug Seizures. J. Chem. Inf. Model. 2023, 63, 815–825. [Google Scholar] [CrossRef]
- Craft, S.; Sunderland, P.; Millea, M.F.; Pudney, C.R.; Sutcliffe, O.B.; Freeman, T.P. Detection and Quantification of Synthetic Cannabinoids in Seven Illicitly Sourced Disposable Vapes Submitted by an Individual Presenting to a UK Drug and Alcohol Service. Addiction 2025, 120, 549–554. [Google Scholar] [CrossRef]
- Apirakkan, O.; Frinculescu, A.; Denton, H.; Shine, T.; Cowan, D.; Abbate, V.; Frascione, N. Isolation, Detection and Identification of Synthetic Cannabinoids in Alternative Formulations or Dosage Forms. Forensic Chem. 2020, 18, 100227. [Google Scholar] [CrossRef]
- Song, C.; Jia, W.; Liu, C.; Hua, Z.; Meng, X.; Zhao, Y.; Li, T.; Cai, L.; Zhao, X. New Trends of New Psychoactive Substances (NPS)-Infused Chocolate: Identification and Quantification of Trace Level of NPS in Complex Matrix by GC-MS and NMR. Talanta 2023, 255, 124257. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kwon, E.; Oh, S.J.; Choi, M.R.; Lee, S.-R.; Jung, B.H.; Lee, W.; Hong, J. Thermal Transformation of CBD, CBDA, and Δ9-THC during e-Cigarette Vaping: Identification of Conversion Products by GC–MS. J. Chromatogr. A 2025, 1749, 465909. [Google Scholar] [CrossRef] [PubMed]
- Shokoohi Rad, S.; Dalali, N.; Baheri, T. Combination of Magnetic Solid-phase Extraction with Dispersive Liquid–Liquid Microextraction Followed by GC-MS for Trace Analysis of Synthetic Cannabinoids in Plasma Samples. Micro Nano Lett. 2020, 15, 545–549. [Google Scholar] [CrossRef]
- Kranenburg, R.F.; Verduin, J.; Stuyver, L.I.; De Ridder, R.; Van Beek, A.; Colmsee, E.; Van Asten, A.C. Benefits of Derivatization in GC–MS-Based Identification of New Psychoactive Substances. Forensic Chem. 2020, 20, 100273. [Google Scholar] [CrossRef]
- Schirmer, W.; Schürch, S.; Weinmann, W. The Identification of Synthetic Impurities in a Vape Pen Containing Δ9-Tetrahydrocannabiphorol Using Gas Chromatography Coupled with Mass Spectrometry. Psychoactives 2024, 3, 491–500. [Google Scholar] [CrossRef]
- Abiedalla, Y.; Almalki, A.J.; DeRuiter, J.; Clark, C.R. GC–MS and GC–IR Analysis of Methylenedioxyphenylalkylamine Analogues of the Psychoactive 25X-NBOMe Drugs. Forensic Chem. 2021, 23, 100314. [Google Scholar] [CrossRef]
- Abiedalla, Y.; Almalki, A.J.; DeRuiter, J.; Clark, C.R. GC–MS and GC–IR Analysis of Substituted N-Benzyl 4-Bromo-2,5-Dimethoxyphenylisopropylamines. Forensic Chem. 2021, 24, 100326. [Google Scholar] [CrossRef]
- Pollard, A.; Davidson, J.T. Investigating the Effect of Substitution Location on Fentanyl Analog Identification for Methyl-Substituted Fentanyl Analogs Using GC-EI-MS. Forensic Chem. 2023, 36, 100534. [Google Scholar] [CrossRef]
- Gonçalves, J.L.; Alves, V.L.; Aguiar, J.; Caldeira, M.J.; Teixeira, H.M.; Câmara, J.S. Structure Assignment of Seized Products Containing Cathinone Derivatives Using High Resolution Analytical Techniques. Metabolites 2021, 11, 144. [Google Scholar] [CrossRef]
- Pulver, B.; Riedel, J.; Westphal, F.; Luhn, S.; Schönberger, T.; Schäper, J.; Auwärter, V.; Luf, A.; Pütz, M. A New Synthetic Cathinone: 3,4-EtPV or 3,4-Pr-PipVP? An Unsuccessful Attempt to Circumvent the German Legislation on New Psychoactive Substances. Drug Test. Anal. 2023, 15, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, M.; Alder, R.; Clancy, L.; Fu, S. Portable Testing Techniques for the Analysis of Drug Materials. WIREs Forensic Sci. 2022, 4, e1461. [Google Scholar] [CrossRef]
- Alves, V.L.; Gonçalves, J.L.; Aguiar, J.; Teixeira, H.M.; Câmara, J.S. The Synthetic Cannabinoids Phenomenon: From Structure to Toxicological Properties. A Review. Crit. Rev. Toxicol. 2020, 50, 359–382. [Google Scholar] [CrossRef]
- Chakravarti, B.; Ravi, J.; Ganju, R.K. Cannabinoids as Therapeutic Agents in Cancer: Current Status and Future Implications. Oncotarget 2014, 5, 5852–5872. [Google Scholar] [CrossRef]
- Lafaye, G.; Karila, L.; Blecha, L.; Benyamina, A. Cannabis, Cannabinoids, and Health. Dialogues Clin. Neurosci. 2017, 19, 309–316. [Google Scholar] [CrossRef]
- European Union Drugs Agency. European Drug Report 2024; European drug report ... (Online); Publications Office: Luxembourg, 2024. [Google Scholar]
- Roque-Bravo, R.; Silva, R.S.; Malheiro, R.F.; Carmo, H.; Carvalho, F.; Da Silva, D.D.; Silva, J.P. Synthetic Cannabinoids: A Pharmacological and Toxicological Overview. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 187–209. [Google Scholar] [CrossRef]
- Lobo Vicente, J.; Chassaigne, H.; Holland, M.V.; Reniero, F.; Kolář, K.; Tirendi, S.; Vandecasteele, I.; Vinckier, I.; Guillou, C. Systematic Analytical Characterization of New Psychoactive Substances: A Case Study. Forensic Sci. Int. 2016, 265, 107–115. [Google Scholar] [CrossRef]
- Mazzarino, M.; Torre, X.D.L.; Botrè, F. A Liquid Chromatography–Mass Spectrometry Method Based on Class Characteristic Fragmentation Pathways to Detect the Class of Indole-Derivative Synthetic Cannabinoids in Biological Samples. Anal. Chim. Acta 2014, 837, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Potts, A.J.; Cano, C.; Thomas, S.H.L.; Hill, S.L. Synthetic Cannabinoid Receptor Agonists: Classification and Nomenclature. Clin. Toxicol. 2020, 58, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Cooper, Z.D. Adverse Effects of Synthetic Cannabinoids: Management of Acute Toxicity and Withdrawal. Curr. Psychiatry Rep. 2016, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Hubner, E.; Schmid, M.G.; Manojlovic, V.; Gattringer, D.; Pferschy-Wenzig, E.; Kunert, O. NMR Spectroscopic Reference Data of Synthetic Cannabinoids Sold on the Internet. Magn. Reson. Chem. 2025, 63, 241–255. [Google Scholar] [CrossRef]
- Cozier, G.E.; Andrews, R.C.; Frinculescu, A.; Kumar, R.; May, B.; Tooth, T.; Collins, P.; Costello, A.; Haines, T.S.F.; Freeman, T.P.; et al. Instant Detection of Synthetic Cannabinoids on Physical Matrices, Implemented on a Low-Cost, Ultraportable Device. Anal. Chem. 2023, 95, 13829–13837. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. MDMB-4en-PINACA: EMCDDA Initial Report on the New Psychoactive Substance Methyl 3,3 Dimethyl 2 (1 (Pent 4 En 1 Yl) 1H Indazole 3 Carboxamido)Butanoate (MDMB 4en PINACA): In Accordance with Article 5b of Regulation (EC) No 1920/2006 (as Amended); Publications Office: Luxembourg, 2020. [Google Scholar]
- Oomen, P.E.; Schori, D.; Tögel-Lins, K.; Acreman, D.; Chenorhokian, S.; Luf, A.; Karden, A.; Paulos, C.; Fornero, E.; Gerace, E.; et al. Cannabis Adulterated with the Synthetic Cannabinoid Receptor Agonist MDMB-4en-PINACA and the Role of European Drug Checking Services. Int. J. Drug Policy 2022, 100, 103493. [Google Scholar] [CrossRef]
- AL-Eitan, L.N.; Asa’ad, A.S.; Battah, A.H.; Aljamal, H.A. Application of Gas Chromatography–Mass Spectrometry for the Identification and Quantitation of Three Common Synthetic Cannabinoids in Seized Materials from the Jordanian Market. ACS Omega 2020, 5, 4172–4180. [Google Scholar] [CrossRef]
- Pasin, D.; Nedahl, M.; Mollerup, C.B.; Tortzen, C.; Reitzel, L.A.; Dalsgaard, P.W. Identification of the Synthetic Cannabinoid-type New Psychoactive Substance, CH-PIACA, in Seized Material. Drug Test. Anal. 2022, 14, 1645–1651. [Google Scholar] [CrossRef]
- Tsochatzis, E.D.; Alberto Lopes, J.; Holland, M.V.; Reniero, F.; Palmieri, G.; Guillou, C. Identification and Analytical Characterization of a Novel Synthetic Cannabinoid-Type Substance in Herbal Material in Europe. Molecules 2021, 26, 793. [Google Scholar] [CrossRef]
- Al-Matrouk, A.; Orabi, K.Y. Identification and Chemical Structure Elucidation of Synthetic Cannabinoids Samples Seized in Kuwait during 2019–2023 Using GC–MS and NMR Spectroscopy. Forensic Sci. Res. 2025, 10, owae026. [Google Scholar] [CrossRef]
- Liu, C.; Jia, W.; Meng, X.; Hua, Z. Identification and Quantification of 10 Indole/Indazole Carboxamide Synthetic Cannabinoids in 36 Herbal Blends by Gas Chromatography-mass Spectrometry and Nuclear Magnetic Resonance Spectroscopy. J. Forensic Sci. 2021, 66, 2156–2166. [Google Scholar] [CrossRef]
- Alves, V.L.; Gonçalves, J.L.; Aguiar, J.; Caldeira, M.J.; Teixeira, H.M.; Câmara, J.S. Highly Sensitive Screening and Analytical Characterization of Synthetic Cannabinoids in Nine Different Herbal Mixtures. Anal. Bioanal. Chem. 2021, 413, 2257–2273. [Google Scholar] [CrossRef]
- Cole, C.; Jones, L.; McVeigh, J.; Kicman, A.; Syed, Q.; Bellis, M. Adulterants in Illicit Drugs: A Review of Empirical Evidence. Drug Test. Anal. 2011, 3, 89–96. [Google Scholar] [CrossRef]
- Grobério, T.S.; Zacca, J.J.; Botelho, É.D.; Talhavini, M.; Braga, J.W.B. Discrimination and Quantification of Cocaine and Adulterants in Seized Drug Samples by Infrared Spectroscopy and PLSR. Forensic Sci. Int. 2015, 257, 297–306. [Google Scholar] [CrossRef]
- Adamowicz, P.; Meissner, E.; Maślanka, M. Fatal Intoxication with New Synthetic Cannabinoids AMB-FUBINACA and EMB-FUBINACA. Clin. Toxicol. 2019, 57, 1103–1108. [Google Scholar] [CrossRef]
- Lobato-Freitas, C.; Brito-da-Costa, A.M.; Dinis-Oliveira, R.J.; Carmo, H.; Carvalho, F.; Silva, J.P.; Dias-da-Silva, D. Overview of Synthetic Cannabinoids ADB-FUBINACA and AMB-FUBINACA: Clinical, Analytical, and Forensic Implications. Pharmaceuticals 2021, 14, 186. [Google Scholar] [CrossRef] [PubMed]
- Abbott, M.J.; Dunnett, J.; Wheeler, J.; Davidson, A. The Identification of Synthetic Cannabinoids in English Prisons. Forensic Sci. Int. 2023, 348, 111613. [Google Scholar] [CrossRef]
- Paul, R.; Smith, S.; Gent, L.; Sutherill, R. Air Monitoring for Synthetic Cannabinoids in a UK Prison: Application of Personal Air Sampling and Fixed Sequential Sampling with Thermal Desorption Two-dimensional Gas Chromatography Coupled to Time-of-flight Mass Spectrometry. Drug Test. Anal. 2021, 13, 1678–1685. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Nguyen, H.C.; Nguyen, D.T.; Huynh, Q.C.; Le, M.H.; Huynh, H.T.; Pham, D.T. Into the Synthetic Cannabinoid 5-Fluoro-MDMB-PICA in “American Grass” Illicit Drug: Extraction, Isolation, Structure Elucidation, and GC/MS Determination. Talanta Open 2023, 8, 100246. [Google Scholar] [CrossRef]
- Sisco, E.; Burns, A.; Moorthy, A.S. A Framework for the Development of Targeted Gas Chromatography Mass Spectrometry (GC-MS) Methods: Synthetic Cannabinoids. J. Forensic Sci. 2021, 66, 1908–1918. [Google Scholar] [CrossRef]
- La Maida, N.; Pellegrini, M.; Papaseit, E.; Pérez-Mañá, C.; Poyatos, L.; Ventura, M.; Galindo, L.; Busardò, F.P.; Pichini, S.; Farré, M.; et al. Determination of the Synthetic Cannabinoids JWH-122, JWH-210, UR-144 in Oral Fluid of Consumers by GC-MS and Quantification of Parent Compounds and Metabolites by UHPLC-MS/MS. Int. J. Mol. Sci. 2020, 21, 9414. [Google Scholar] [CrossRef]
- Minakata, K.; Yamagishi, I.; Nozawa, H.; Hasegawa, K.; Suzuki, M.; Gonmori, K.; Suzuki, O.; Watanabe, K. Sensitive Identification and Quantitation of Parent Forms of Six Synthetic Cannabinoids in Urine Samples of Human Cadavers by Liquid Chromatography–Tandem Mass Spectrometry. Forensic Toxicol. 2017, 35, 275–283. [Google Scholar] [CrossRef]
- Sorribes-Soriano, A.; Verdeguer, J.; Pastor, A.; Armenta, S.; Esteve-Turrillas, F.A. Determination of Third-Generation Synthetic Cannabinoids in Oral Fluids. J. Anal. Toxicol. 2021, 45, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Scheidweiler, K.B.; Huestis, M.A. Simultaneous Quantification of 20 Synthetic Cannabinoids and 21 Metabolites, and Semi-Quantification of 12 Alkyl Hydroxy Metabolites in Human Urine by Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2014, 1327, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.S.; Silva, I.; Ajenjo, A.C.; Dias, M.J. Validation and Application of an UPLC–MS/MS Method for the Quantification of Synthetic Cannabinoids in Urine Samples and Analysis of Seized Materials from the Portuguese Market. Forensic Sci. Int. 2014, 243, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Scheidweiler, K.B.; Jarvis, M.J.Y.; Huestis, M.A. Nontargeted SWATH Acquisition for Identifying 47 Synthetic Cannabinoid Metabolites in Human Urine by Liquid Chromatography-High-Resolution Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2015, 407, 883–897. [Google Scholar] [CrossRef]
- Kong, T.Y.; Kim, J.-H.; Kim, D.K.; Lee, H.S. Synthetic Cannabinoids Are Substrates and Inhibitors of Multiple Drug-Metabolizing Enzymes. Arch. Pharm. Res. 2018, 41, 691–710. [Google Scholar] [CrossRef]
- Abbate, V.; Schwenk, M.; Presley, B.C.; Uchiyama, N. The Ongoing Challenge of Novel Psychoactive Drugs of Abuse. Part I. Synthetic Cannabinoids (IUPAC Technical Report). Pure Appl. Chem. 2018, 90, 1255–1282. [Google Scholar] [CrossRef]
- Rouxinol, D.; Dias Da Silva, D.; Silva, J.P.; Carvalho, F.; Bastos, M.D.L.; Carmo, H. Biodistribution and Metabolic Profile of 3,4-Dimethylmethcathinone (3,4-DMMC) in Wistar Rats through Gas Chromatography–Mass Spectrometry (GC–MS) Analysis. Toxicol. Lett. 2020, 320, 113–123. [Google Scholar] [CrossRef]
- Hasegawa, K.; Wurita, A.; Minakata, K.; Gonmori, K.; Nozawa, H.; Yamagishi, I.; Watanabe, K.; Suzuki, O. Postmortem Distribution of AB-CHMINACA, 5-Fluoro-AMB, and Diphenidine in Body Fluids and Solid Tissues in a Fatal Poisoning Case: Usefulness of Adipose Tissue for Detection of the Drugs in Unchanged Forms. Forensic Toxicol. 2015, 33, 45–53. [Google Scholar] [CrossRef]
- Wang, Y.; Han, L.; Yi, L.; Liu, J.; Qiu, S.; Gu, J.; Bai, H.; Li, J.; Wurita, A.; Hasegawa, K. Newly Emerging Synthetic Cannabinoid ADB-4en-PINACA: Its Identification and Quantification in an Authentic Human Hair Sample by GC–MS/MS. Forensic Toxicol. 2023, 41, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, Y.; Yang, H.; Liu, J.; Wurita, A.; Hasegawa, K. Quantification of MDMB-4en-PINACA and ADB-BUTINACA in Human Hair by Gas Chromatography–Tandem Mass Spectrometry. Forensic Toxicol. 2022, 40, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Xiang, P.; Shen, M. Current Status of Hair Analysis in Forensic Toxicology in China. Forensic Sci. Res. 2021, 6, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Kintz, P. Hair Analysis in Forensic Toxicology: An Updated Review with a Special Focus on Pitfalls. Curr. Pharm. Des. 2017, 23, 5480–5486. [Google Scholar] [CrossRef]
- Tsanaclis, L.; Andraus, M.; Wicks, J. Hair Analysis When External Contamination Is in Question: A Review of Practical Approach for the Interpretation of Results. Forensic Sci. Int. 2018, 285, 105–110. [Google Scholar] [CrossRef]
- Giorgetti, A.; Mogler, L.; Halter, S.; Haschimi, B.; Alt, A.; Rentsch, D.; Schmidt, B.; Thoma, V.; Vogt, S.; Auwärter, V. Four Cases of Death Involving the Novel Synthetic Cannabinoid 5F-Cumyl-PEGACLONE. Forensic Toxicol. 2020, 38, 314–326. [Google Scholar] [CrossRef]
- Elliott, S.; Sedefov, R.; Evans-Brown, M. Assessing the Toxicological Significance of New Psychoactive Substances in Fatalities. Drug Test. Anal. 2018, 10, 120–126. [Google Scholar] [CrossRef]
- Kokosa, J.M. Advances in Solvent-Microextraction Techniques. TrAC Trends Anal. Chem. 2013, 43, 2–13. [Google Scholar] [CrossRef]
- Fotouhi, M.; Seidi, S.; Shanehsaz, M.; Naseri, M.T. Magnetically Assisted Matrix Solid Phase Dispersion for Extraction of Parabens from Breast Milks. J. Chromatogr. A 2017, 1504, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Latifeh, F.; Yamini, Y.; Seidi, S. Ionic Liquid-Modified Silica-Coated Magnetic Nanoparticles: Promising Adsorbents for Ultra-Fast Extraction of Paraquat from Aqueous Solution. Environ. Sci. Pollut. Res. 2016, 23, 4411–4421. [Google Scholar] [CrossRef]
- Dalali, N.; Habibizadeh, M.; Rostamizadeh, K.; Nakisa, S. Synthesis of Magnetite Multi-walled Carbon Nanotubes Composite and Its Application for Removal of Basic Dyes from Aqueous Solutions. Asia-Pac. J. Chem. Eng. 2014, 9, 552–561. [Google Scholar] [CrossRef]
- Pereira, J.A.M.; Gonçalves, J.; Porto-Figueira, P.; Figueira, J.A.; Alves, V.; Perestrelo, R.; Medina, S.; Câmara, J.S. Current Trends on Microextraction by Packed Sorbent–Fundamentals, Application Fields, Innovative Improvements and Future Applications. Analyst 2019, 144, 5048–5074. [Google Scholar] [CrossRef]
- Zschiesche, A.; Carlier, J.; Pietsch, J.; Scheu, M.; Seibt, J.; Busardò, F.P.; Auwärter, V.; Huppertz, L.M. Synthetic cannabinoid receptor agonists containing silicon: Exploring the metabolic pathways of ADMB- and Cumyl-3TMS-PrINACA in human urine specimens and post mortem material compared to In Vitro and in silico data. Arch. Toxicol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Sun, Y.; Wu, M.; Zhang, Z.; Zhang, T.; Wang, H.; Li, F.; Yang, L.; Xu, Y.; Liu, Z.-J.; et al. Carbon-Silicon Switch Led to the Discovery of Novel Synthetic Cannabinoids with Therapeutic Effects in a Mouse Model of Multiple Sclerosis. Eur. J. Med. Chem. 2021, 226, 113878. [Google Scholar] [CrossRef]
- Panayides, J.-L.; Riley, D.L.; Hasenmaile, F.; Van Otterlo, W.A.L. The Role of Silicon in Drug Discovery: A Review. RSC Med. Chem. 2024, 15, 3286–3344. [Google Scholar] [CrossRef]
- Tanaka, R.; Kawamura, M.; Ito, M.; Kikura-Hanajiri, R. Identification of Two Lysergic Acid Diethylamide Analogs, 1-(3-(Trimethylsilyl) Propionyl) Lysergic Acid Diethylamide (1S-LSD) and 1-(2-Thienoyl)-6-Allyl-nor-d-Lysergic Acid Diethylamide (1T-AL-LAD), in Paper Sheet Products Distributed on the Internet. Forensic Toxicol. 2025, 43, 370–376. [Google Scholar] [CrossRef]
- Ujváry, I. Hexahydrocannabinol and Closely Related Semi-synthetic Cannabinoids: A Comprehensive Review. Drug Test. Anal. 2024, 16, 127–161. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. Hexahydrocannabinol (HHC) and Related Substances: Technical Report; Publications Office: Luxembourg, 2023. [Google Scholar]
- Jørgensen, C.F.; Rasmussen, B.S.; Linnet, K.; Thomsen, R. Emergence of Semi-synthetic Cannabinoids in Cannabis Products Seized in Eastern Denmark over a 6-year Period. J. Forensic Sci. 2024, 69, 2009–2017. [Google Scholar] [CrossRef]
- Citti, C.; Linciano, P.; Russo, F.; Luongo, L.; Iannotta, M.; Maione, S.; Laganà, A.; Capriotti, A.L.; Forni, F.; Vandelli, M.A.; et al. A Novel Phytocannabinoid Isolated from Cannabis Sativa L. with an in Vivo Cannabimimetic Activity Higher than Δ9-Tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Sci. Rep. 2019, 9, 20335. [Google Scholar] [CrossRef]
- Caprari, C.; Ferri, E.; Vandelli, M.A.; Citti, C.; Cannazza, G. An Emerging Trend in Novel Psychoactive Substances (NPSs): Designer THC. J. Cannabis Res. 2024, 6, 21. [Google Scholar] [CrossRef]
- Watanabe, S.; Murakami, T.; Muratsu, S.; Fujiwara, H.; Nakanishi, T.; Seto, Y. Discrepancies between the Stated Contents and Analytical Findings for Electronic Cigarette Liquid Products: Identification of the New Cannabinoid, Δ9 -tetrahydrocannabihexol Acetate. Drug Test. Anal. 2025, 17, 694–700. [Google Scholar] [CrossRef]
- Holt, A.K.; Poklis, J.L.; Peace, M.R. ∆8-THC, THC-O Acetates and CBD-Di-O Acetate: Emerging Synthetic Cannabinoids Found in Commercially Sold Plant Material and Gummy Edibles. J. Anal. Toxicol. 2022, 46, 940–948. [Google Scholar] [CrossRef]
- Valente, M.J.; Guedes De Pinho, P.; De Lourdes Bastos, M.; Carvalho, F.; Carvalho, M. Khat and Synthetic Cathinones: A Review. Arch. Toxicol. 2014, 88, 15–45. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.P. Cathinone Derivatives: A Review of Their Chemistry, Pharmacology and Toxicology. Drug Test. Anal. 2011, 3, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, M.; Celiński, R.; Kuś, P.; Kowalska, T.; Sajewicz, M. The Newest Cathinone Derivatives as Designer Drugs: An Analytical and Toxicological Review. Forensic Toxicol. 2018, 36, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Apirakkan, O.; Frinculescu, A.; Shine, T.; Parkin, M.C.; Cilibrizzi, A.; Frascione, N.; Abbate, V. Analytical Characterization of Three Cathinone Derivatives, 4-MPD, 4F–PHP and bk-EPDP, Purchased as Bulk Powder from Online Vendors. Drug Test. Anal. 2018, 10, 372–378. [Google Scholar] [CrossRef]
- Frański, R.; Gierczyk, B.; Zdrojewska, A.; Kasperkowiak, M. Comments on the Paper Entitled Rapid Tentative Identification of Synthetic Cathinones in Seized Products Taking Advantage of the Full Capabilities of Triple Quadrupole Analyzer. Forensic Toxicol. 2019, 37, 504–506. [Google Scholar] [CrossRef]
- Nadal-Gratacós, N.; Pazos, M.D.; Pubill, D.; Camarasa, J.; Escubedo, E.; Berzosa, X.; López-Arnau, R. Structure–Activity Relationship of Synthetic Cathinones: An Updated Review. ACS Pharmacol. Transl. Sci. 2024, 7, 2588–2603. [Google Scholar] [CrossRef]
- Fabregat-Safont, D.; Barneo-Muñoz, M.; Carbón, X.; Hernández, F.; Martinez-Garcia, F.; Ventura, M.; Stove, C.P.; Sancho, J.V.; Ibáñez, M. Understanding the Pharmacokinetics of Synthetic Cathinones: Evaluation of the Blood–Brain Barrier Permeability of 13 Related Compounds in Rats. Addict. Biol. 2021, 26, e12979. [Google Scholar] [CrossRef] [PubMed]
- Barratt, M.J.; Ferris, J.A.; Winstock, A.R. Safer Scoring? Cryptomarkets, Social Supply and Drug Market Violence. Int. J. Drug Policy 2016, 35, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.-P.; Lan, Y.-S.; Lee, Y.-H.; Lee, Y.-C.; Chou, Y.-C.; Lee, H.-H.; Chang, M.-Y.; Liang, S.-S.; Lin, Y.-C. Optimizing Analytical Precision in the Identification of Synthetic Cathinones and Isomers: A Comparative Assessment of Diverse GC–MS Operating Parameters. Anal. Sci. 2024, 40, 1397–1407. [Google Scholar] [CrossRef]
- Kranenburg, R.F.; Peroni, D.; Affourtit, S.; Westerhuis, J.A.; Smilde, A.K.; Van Asten, A.C. Revealing Hidden Information in GC–MS Spectra from Isomeric Drugs: Chemometrics Based Identification from 15 eV and 70 eV EI Mass Spectra. Forensic Chem. 2020, 18, 100225. [Google Scholar] [CrossRef]
- Gilbert, N.; Mewis, R.E.; Sutcliffe, O.B. Classification of Fentanyl Analogues through Principal Component Analysis (PCA) and Hierarchical Clustering of GC–MS Data. Forensic Chem. 2020, 21, 100287. [Google Scholar] [CrossRef]
- Nuñez-Montero, M.; Lombroni, C.; Maida, N.; Rotolo, M.; Pichini, S.; Papaseit, E.; Hladun, O.; Ventura, M.; Poyatos, L.; Pérez-Mañá, C.; et al. GC–MS/MS Determination of Synthetic Cathinones: 4-Chloromethcathinone, N-Ethyl Pentedrone, and N-Ethyl Hexedrone in Oral Fluid and Sweat of Consumers under Controlled Administration: Pilot Study. Int. J. Mol. Sci. 2023, 24, 9387. [Google Scholar] [CrossRef]
- De Campos, E.G.; Da Costa, B.R.B.; Dos Santos, F.S.; Monedeiro, F.; Alves, M.N.R.; Santos Junior, W.J.R.; De Martinis, B.S. Alternative Matrices in Forensic Toxicology: A Critical Review. Forensic Toxicol. 2022, 40, 1–18. [Google Scholar] [CrossRef]
- Núñez-Montero, M.; Pérez-Mañá, C.; Hladun, O.; Poyatos, L.; Caicedo, D.A.; De La Rosa, G.; Argote, M.C.; Martín, S.; Ventura, M.; Maida, N.L.; et al. Acute Pharmacological Effects of Two Synthetic Cathinones in Humans: An Observational Study of N-Ethylhexedrone and N-Ethyl-nor-Pentedrone. Pharmaceuticals 2025, 18, 721. [Google Scholar] [CrossRef]
- Papaseit, E.; Pérez-Mañá, C.; De Sousa Fernandes Perna, E.B.; Olesti, E.; Mateus, J.; Kuypers, K.P.; Theunissen, E.L.; Fonseca, F.; Torrens, M.; Ramaekers, J.G.; et al. Mephedrone and Alcohol Interactions in Humans. Front. Pharmacol. 2020, 10, 1588. [Google Scholar] [CrossRef]
- Papaseit, E.; Olesti, E.; Pérez-Mañá, C.; Torrens, M.; Fonseca, F.; Grifell, M.; Ventura, M.; De La Torre, R.; Farré, M. Acute Pharmacological Effects of Oral and Intranasal Mephedrone: An Observational Study in Humans. Pharmaceuticals 2021, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Poyatos, L.; Papaseit, E.; Olesti, E.; Pérez-Mañá, C.; Ventura, M.; Carbón, X.; Grifell, M.; Fonseca, F.; Torrens, M.; De La Torre, R.; et al. A Comparison of Acute Pharmacological Effects of Methylone and MDMA Administration in Humans and Oral Fluid Concentrations as Biomarkers of Exposure. Biology 2021, 10, 788. [Google Scholar] [CrossRef] [PubMed]
- Poyatos, L.; Lo Faro, A.F.; Berardinelli, D.; Sprega, G.; Malaca, S.; Pichini, S.; Huestis, M.A.; Papaseit, E.; Pérez-Mañá, C.; Busardò, F.P.; et al. Methylone and MDMA Pharmacokinetics Following Controlled Administration in Humans. Int. J. Mol. Sci. 2022, 23, 14636. [Google Scholar] [CrossRef]
- Poyatos, L.; Pérez-Mañá, C.; Hladun, O.; Núñez-Montero, M.; De La Rosa, G.; Martín, S.; Barriocanal, A.M.; Carabias, L.; Kelmendi, B.; Taoussi, O.; et al. Pharmacological Effects of Methylone and MDMA in Humans. Front. Pharmacol. 2023, 14, 1122861. [Google Scholar] [CrossRef] [PubMed]
- Di Trana, A.; La Maida, N.; De La Rosa, G.; Di Giorgi, A.; Graziano, S.; Aldhaehri, K.; Papaseit, E.; Hladun, O.; Farré, M.; Pérez, C.; et al. Early and Mid-Term Disposition of α-PVP and Its Unknown Metabolites in Urine and Oral Fluid Through a Multi-Analytical Hyphenated Approach Following a Single Non-Controlled Administration to Healthy Volunteers. AAPS J. 2025, 27, 25. [Google Scholar] [CrossRef]
- Zawilska, J.B. (Ed.) Synthetic Cathinones; Current Topics in Neurotoxicity; Springer International Publishing: Cham, Switzerland, 2018; Volume 12, ISBN 978-3-319-78706-0. [Google Scholar]
- Soares, J.; Costa, V.M.; Bastos, M.D.L.; Carvalho, F.; Capela, J.P. An Updated Review on Synthetic Cathinones. Arch. Toxicol. 2021, 95, 2895–2940. [Google Scholar] [CrossRef]
- Dixon, D.I.; Millea, M.F.; Wilcock, A.T.M.; Costello, A.; Ellison, J.R.; Lord, S.; O’Brian, K.A.; Mewis, R.E.; Sutcliffe, O.B. Synthesis, Characterisation and Quantification of the New Psychoactive Substance 1-(1,3-Benzodioxol-5-Yl)-2-(Propylamino)Butan-1-One (Bk-PBDB, Putylone). Forensic Chem. 2023, 35, 100523. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Y.; Li, Z.; Zhao, J.; Wang, X.; Wang, K.; Su, M.; Xiang, P. Separation and Identification of the Synthetic Cathinone Isomers Dipentylone and N-Ethylpentylone Using Chromatographic and Mass Spectral Characteristics. Forensic Chem. 2024, 37, 100551. [Google Scholar] [CrossRef]
- Liliedahl, R.E.; Hutzell, E.; Haley, M.; Predecki, D.P.; Davidson, J.T. The Differentiation of N-Butyl Pentylone Isomers Using GC-EI-MS and NMR. Forensic Sci. Int. 2023, 351, 111815. [Google Scholar] [CrossRef]
- Lee, H.Z.S.; Ong, M.C.; Lim, J.L.W.; Yap, T.W.A. Technical Note: N-Isopropylbutylone Unveiled–Differentiating the New Synthetic Cathinone in Ecstasy from Its Close Analogues with GC-MS and NMR Spectroscopy. Forensic Sci. Int. 2025, 370, 112448. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.D.; Freeman, S.; Sumnall, H.R.; Measham, F.; Cole, J. Analysis of NRG ‘Legal Highs’ in the UK: Identification and Formation of Novel Cathinones. Drug Test. Anal. 2011, 3, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Mata-Pesquera, M.; Fabregat-Safont, D.; Gil, C.; Ventura, M.; Steinmetz, F.P.; Ibáñez, M. Characterization of the Recently Detected Cathinone N-Cyclohexyl Butylone: From Structure Elucidation to in Silico Supported Pharmacological/Toxicological Considerations. Microchem. J. 2023, 190, 108577. [Google Scholar] [CrossRef]
- Paškan, M.; Dobšíková, K.; Kuchař, M.; Setnička, V.; Kohout, M. Synthesis and Absolute Configuration of Cyclic Synthetic Cathinones Derived from A-tetralone. Chirality 2024, 36, e23646. [Google Scholar] [CrossRef]
- Zaitsu, K.; Katagi, M.; Tsuchihashi, H.; Ishii, A. Recently Abused Synthetic Cathinones, α-Pyrrolidinophenone Derivatives: A Review of Their Pharmacology, Acute Toxicity, and Metabolism. Forensic Toxicol. 2014, 32, 1–8. [Google Scholar] [CrossRef]
- Kavanagh, P.; Gofenberg, M.; Shevyrin, V.; Dvorskaya, O.; Dowling, G.; Grigoryev, A. Tentative Identification of the Phase I and II Metabolites of Two Synthetic Cathinones, MDPHP and α-PBP, in Human Urine. Drug Test. Anal. 2020, 12, 1442–1451. [Google Scholar] [CrossRef]
- Simmler, L.; Buser, T.; Donzelli, M.; Schramm, Y.; Dieu, L.; Huwyler, J.; Chaboz, S.; Hoener, M.; Liechti, M. Pharmacological Characterization of Designer Cathinones In Vitro. Br. J. Pharmacol. 2013, 168, 458–470. [Google Scholar] [CrossRef]
- Coppola, M.; Mondola, R. 3,4-Methylenedioxypyrovalerone (MDPV): Chemistry, Pharmacology and Toxicology of a New Designer Drug of Abuse Marketed Online. Toxicol. Lett. 2012, 208, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.T.; Sasiene, Z.J.; Abiedalla, Y.; DeRuiter, J.; Clark, C.R.; Jackson, G.P. Fragmentation Pathways of α-Pyrrolidinophenone Synthetic Cathinones and Their Application to the Identification of Emerging Synthetic Cathinone Derivatives. Int. J. Mass. Spectrom. 2020, 453, 116343. [Google Scholar] [CrossRef]
- Namera, A.; Kawamura, M.; Nakamoto, A.; Saito, T.; Nagao, M. Comprehensive Review of the Detection Methods for Synthetic Cannabinoids and Cathinones. Forensic Toxicol. 2015, 33, 175–194. [Google Scholar] [CrossRef]
- Ishii, H.; Yokoyama, A.; Saito, K.; Kataoka, H. Synthesis and Analytical Differentiation of a Novel Synthetic Cathinone 1-(2,3-Dihydro-1H-Inden-5-Yl)-2-(Pyrrolidin-1-Yl)Butan-1-One (5-PPDI) and Its Regioisomers. Forensic Chem. 2022, 27, 100393. [Google Scholar] [CrossRef]
- Segurado, A.M.; Ahmad, S.M.; Neng, N.R.; Maniés-Sequeira, M.M.; Gaspar, H.; Nogueira, J.M.F. Simple Analytical Strategy for Screening Three Synthetic Cathinones (α-PVT, α-PVP, and MDPV) in Oral Fluids. Analytica 2022, 3, 14–23. [Google Scholar] [CrossRef]
- Machado, F.; Franco, J.; Vieira, D.N.; Margalho, C. Development and Validation of a GC–MS-EI Method to Determine α-PHP in Blood: Application to Samples Collected during Medico-Legal Autopsies. J. Anal. Toxicol. 2023, 47, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Sisco, E.; Burns, A.; Moorthy, A.S. Development and Evaluation of a Synthetic Cathinone Targeted Gas Chromatography Mass Spectrometry (GC-MS) Method. J. Forensic Sci. 2021, 66, 1919–1928. [Google Scholar] [CrossRef]
- Woźniak, M.K.; Banaszkiewicz, L.; Wiergowski, M.; Tomczak, E.; Kata, M.; Szpiech, B.; Namieśnik, J.; Biziuk, M. Development and Validation of a GC–MS/MS Method for the Determination of 11 Amphetamines and 34 Synthetic Cathinones in Whole Blood. Forensic Toxicol. 2020, 38, 42–58. [Google Scholar] [CrossRef]
- Antunes, M.; Sequeira, M.; De Caires Pereira, M.; Caldeira, M.J.; Santos, S.; Franco, J.; Barroso, M.; Gaspar, H. Determination of Selected Cathinones in Blood by Solid-Phase Extraction and GC–MS. J. Anal. Toxicol. 2021, 45, 233–242. [Google Scholar] [CrossRef]
- Cláudia, M.; Pedro, A.; Tiago, R.; Francisco, C.R.; Eugenia, G. Determination of New Psychoactive Substances in Whole Blood Using Microwave Fast Derivatization and Gas Chromatography/Mass Spectrometry. J. Anal. Toxicol. 2020, 44, 92–102. [Google Scholar] [CrossRef]
- Júlio, S.; Ferro, R.A.; Santos, S.; Alexandre, A.; Caldeira, M.J.; Franco, J.; Barroso, M.; Gaspar, H. Synthesis of Emerging Cathinones and Validation of a SPE GC–MS Method for Their Simultaneous Quantification in Blood. Anal. Bioanal. Chem. 2023, 415, 571–589. [Google Scholar] [CrossRef]
- Synowiec, K.; Rojek, S.; Maciów-Głąb, M.; Kula, K.; Romańczuk, A.; Kłys, M. The Role of GC-EI-MS and Derivatization in the Detection of New Psychoactive Substances Exemplified by 49 Synthetic Cathinones. J. Anal. Chem. 2022, 77, 1315–1324. [Google Scholar] [CrossRef]
- Hobbs, J.M.; DeRienz, R.T.; Baker, D.D.; Shuttleworth, M.R.; Pandey, M. Fatal Intoxication by the Novel Cathinone 4-Fluoro-3-Methyl-α-PVP. J. Anal. Toxicol. 2022, 46, e101–e104. [Google Scholar] [CrossRef]
- Benedicte, L.; Camille, R.; Audrey, C.; Deborah, I.; Morgan, B.; Marie, D.; David, B.; Delphine, A.; Severine, F.; Guillaume, D.; et al. Case Report on Two-Cathinones Abuse: MPHP and N-Ethyl-4′methylnorpentedrone, with a Fatal Outcome. Forensic Toxicol. 2020, 38, 243–254. [Google Scholar] [CrossRef]
- Daziani, G.; Taoussi, O.; Berardinelli, D.; Bambagiotti, G.; Huestis, M.A.; Busardò, F.P.; Carlier, J. Comparison of Authentic Urine N-Ethylpentedrone Metabolites to Predicted in Silico and In Vitro Human Hepatocyte Metabolism. J. Pharm. Biomed. Anal. 2025, 267, 117170. [Google Scholar] [CrossRef]
- Langa, I.; Gonçalves, R.; Tiritan, M.E.; Ribeiro, C. Wastewater Analysis of Psychoactive Drugs: Non-Enantioselective vs Enantioselective Methods for Estimation of Consumption. Forensic Sci. Int. 2021, 325, 110873. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Castrignanò, E.; Kasprzyk-Hordern, B.; Wolfaardt, G.M. Wastewater-Based Epidemiology and Enantiomeric Profiling for Drugs of Abuse in South African Wastewaters. Sci. Total Environ. 2018, 625, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.-T.; Chang, H.-H.; Wang, Y.-T.; Chen, W.-T.; Lin, T.-C.; Chyueh, S.-C.; Chen, C.-F. Screening Synthetic Cathinones Mixed with Instant Coffee and Fruit Juice Powder Using Hydrophobic Carbon Dot Probes. Sens. Actuators B Chem. 2025, 442, 138127. [Google Scholar] [CrossRef]
- Carelli, C.; Radogna, A.; Bolcato, V.; Moretti, M.; Vignali, C.; Merli, D.; Morini, L. Old and New Synthetic and Semi-Synthetic Opioids Analysis in Hair: A Review. Talanta Open 2022, 5, 100108. [Google Scholar] [CrossRef]
- Garneau, B.; Desharnais, B.; Beauchamp-Doré, A.; Lavallée, C.; Mireault, P.; Lajeunesse, A. Challenges Related to Three Cases of Fatal Intoxication to Multiple Novel Synthetic Opioids. J. Anal. Toxicol. 2020, 44, 86–91. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2021: Trends and Developments; Publications Office: Luxembourg, 2021. [Google Scholar]
- Bonner, L. DEA Issues Safety Alert on Counterfeit Meds. Pharm. Today 2021, 27, 21. [Google Scholar] [CrossRef]
- Nguyen, L.; Evans, A.; Frank, G.; Levitas, M.; Mennella, A.; Short, L.C. Genuine and Counterfeit Prescription Pill Surveillance in Washington, D.C. Forensic Sci. Int. 2022, 339, 111414. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Baig, Y.; Nardiello, D.; Quinto, M. How New Nanotechnologies Are Changing the Opioid Analysis Scenery? A Comparison with Classical Analytical Methods. Forensic Sci. Res. 2024, 9, owae001. [Google Scholar] [CrossRef]
- Koo, C.; Cox, M.; Klass, G.; Johnston, M. Stereochemical Analysis of Methorphan Using (−)-Menthyl Chloroformate. J. Forensic Sci. 2012, 57, 1549–1555. [Google Scholar] [CrossRef]
- Salerno, T.M.G.; Zamengo, L.; Coppolino, C.; Cucinotta, L.; Donato, P.; Trovato, E.; Vento, F.; Zancanaro, F.; Frison, G.; Mondello, L. Investigation of Adulterated Seized Heroin Samples and Direct Stereochemical Analysis of Methorphan by Chiral HPLC–MS/MS. Anal. Bioanal. Chem. 2025, 417, 5187–5198. [Google Scholar] [CrossRef]
- Janssen, P.A.J.; Jageneau, A.H. A New Series of Potent Analgesics. J. Pharm. Pharmacol. 1957, 9, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.C.; Sitkowski, K.; Heneghan, C.; Aronson, J.K. The Oxford Catalogue of Opioids: A Systematic Synthesis of Opioid Drug Names and Their Pharmacology. Brit J. Clin. Pharma 2021, 87, 3790–3812. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, M.M.; Walton, S.E.; Shuda, S.A.; Papsun, D.M.; Krotulski, A.J.; Stove, C.P. Detection, Chemical Analysis, and Pharmacological Characterization of Dipyanone and Other New Synthetic Opioids Related to Prescription Drugs. Anal. Bioanal. Chem. 2023, 415, 5165–5180. [Google Scholar] [CrossRef]
- Wilson, N.; Kariisa, M.; Seth, P.; Smith, H.; Davis, N.L. Drug and Opioid-Involved Overdose Deaths—United States, 2017–2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Ares-Fuentes, A.M.; Lorenzo, R.A.; Fernández, P.; Fernández, A.M.; Furton, K.G.; Kabir, A.; Carro, A.M. Determination of Synthetic Opioids in Oral Fluid Samples Using Fabric Phase Sorptive Extraction and Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2022, 1663, 462768. [Google Scholar] [CrossRef]
- Valdez, C.A. Gas Chromatography-Mass Spectrometry Analysis of Synthetic Opioids Belonging to the Fentanyl Class: A Review. Crit. Rev. Anal. Chem. 2022, 52, 1938–1968. [Google Scholar] [CrossRef]
- Payne, R.; Mathias, S.D.; Pasta, D.J.; Wanke, L.A.; Williams, R.; Mahmoud, R. Quality of Life and Cancer Pain: Satisfaction and Side Effects with Transdermal Fentanyl versus Oral Morphine. JCO 1998, 16, 1588–1593. [Google Scholar] [CrossRef]
- Donner, B.; Zenz, M.; Strumpf, M.; Raber, M. Long-Term Treatment of Cancer Pain With Transdermal Fentanyl. J. Pain. Symptom Manag. 1998, 15, 168–175. [Google Scholar] [CrossRef]
- Cannaert, A.; Ambach, L.; Blanckaert, P.; Stove, C.P. Activity-Based Detection and Bioanalytical Confirmation of a Fatal Carfentanil Intoxication. Front. Pharmacol. 2018, 9, 486. [Google Scholar] [CrossRef]
- Saloner, B.; McGinty, E.E.; Beletsky, L.; Bluthenthal, R.; Beyrer, C.; Botticelli, M.; Sherman, S.G. A Public Health Strategy for the Opioid Crisis. Public Health Rep. 2018, 133, 24S–34S. [Google Scholar] [CrossRef]
- Valdez, C.A.; Leif, R.N.; Mayer, B.P. An Efficient, Optimized Synthesis of Fentanyl and Related Analogs. PLoS ONE 2014, 9, e108250. [Google Scholar] [CrossRef]
- Stogner, J.M. The Potential Threat of Acetyl Fentanyl: Legal Issues, Contaminated Heroin, and Acetyl Fentanyl “Disguised” as Other Opioids. Ann. Emerg. Med. 2014, 64, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.G.; Pollack, H.A. Addressing the Fentanyl Threat to Public Health. N. Engl. J. Med. 2017, 376, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Dybowski, M.P.; Dawidowicz, A.L. Furanylfentanyl in Whole Blood Measured by GC–MS/MS after QuEChERS Extraction in a Fatal Case. Forensic Toxicol. 2020, 38, 496–504. [Google Scholar] [CrossRef]
- Qin, N.; Shen, M.; Xiang, P.; Wen, D.; Shen, B.; Deng, H.; Qiang, H.; Song, F.; Shi, Y. Determination of 37 Fentanyl Analogues and Novel Synthetic Opioids in Hair by UHPLC-MS/MS and Its Application to Authentic Cases. Sci. Rep. 2020, 10, 11569. [Google Scholar] [CrossRef] [PubMed]
- Strayer, K.E.; Antonides, H.M.; Juhascik, M.P.; Daniulaityte, R.; Sizemore, I.E. LC-MS/MS-Based Method for the Multiplex Detection of 24 Fentanyl Analogues and Metabolites in Whole Blood at Sub Ng mL−1 Concentrations. ACS Omega 2018, 3, 514–523. [Google Scholar] [CrossRef]
- Wei, Q.; Su, F.H. Determination of Nine Fentanyl Drugs in Hair Samples by GC-MS/MS and LC-MS/MS. ACS Omega 2022, 7, 19176–19182. [Google Scholar] [CrossRef]
- Camedda, N.; Dagoli, S.; Anzillotti, L.; Cecchi, R. Development and Validation of a Gas Chromatography-Mass Spectrometry Method for the Determination of Fentanyl and Butyryl Fentanyl in Oral Fluid. Anal. Sci. Adv. 2025, 6, e202400038. [Google Scholar] [CrossRef]
- Sisco, E.; Burns, A.; Moorthy, A.S. Development and Evaluation of a Synthetic Opioid Targeted Gas Chromatography Mass Spectrometry (GC-MS) Method. J. Forensic Sci. 2021, 66, 2369–2380. [Google Scholar] [CrossRef]
- Gilbert, N.; Antonides, L.H.; Schofield, C.J.; Costello, A.; Kilkelly, B.; Cain, A.R.; Dalziel, P.R.V.; Horner, K.; Mewis, R.E.; Sutcliffe, O.B. Hitting the Jackpot–Development of Gas Chromatography–Mass Spectrometry (GC–MS) and Other Rapid Screening Methods for the Analysis of 18 Fentanyl-derived Synthetic Opioids. Drug Test. Anal. 2020, 12, 798–811. [Google Scholar] [CrossRef]
- Valdez, C.A.; Rosales, J.A.; Leif, R.N. Determination of Fentanyl and Acetylfentanyl in Soil in Their Intact Form and Orthogonal Corroboration of Their Presence by EI-GC-MS Using Chloroformate Chemistry. Forensic Chem. 2023, 34, 100504. [Google Scholar] [CrossRef]
- Desage, M.; Guilluy, R.; Brazier, J.L.; Chaudron, H.; Girard, J.; Cherpin, H.; Jumeau, J. Gas Chromatography with Mass Spectrometry or Isotope-Ratio Mass Spectrometry in Studying the Geographical Origin of Heroin. Anal. Chim. Acta 1991, 247, 249–254. [Google Scholar] [CrossRef]
- Valdez, C.A.; Rosales, J.A.; Vu, A.K.; Leif, R.N. Detection and Confirmation of Fentanyls in High Clay-content Soil by Electron Ionization Gas Chromatography-mass Spectrometry. J. Forensic Sci. 2023, 68, 2138–2152. [Google Scholar] [CrossRef] [PubMed]
- Valdez, C.A.; Leif, R.N.; Corzett, T.H.; Dreyer, M.L. Analysis, Identification and Confirmation of Synthetic Opioids Using Chloroformate Chemistry: Retrospective Detection of Fentanyl and Acetylfentanyl in Urine and Plasma Samples by EI-GC-MS and HR-LC-MS. PLoS ONE 2022, 17, e0275931. [Google Scholar] [CrossRef]
- Valdez, C.A.; Leif, R.N.; Sanner, R.D.; Corzett, T.H.; Dreyer, M.L.; Mason, K.E. Structural Modification of Fentanyls for Their Retrospective Identification by Gas Chromatographic Analysis Using Chloroformate Chemistry. Sci. Rep. 2021, 11, 22489. [Google Scholar] [CrossRef] [PubMed]
- Nan, Q.; Hejian, W.; Ping, X.; Baohua, S.; Junbo, Z.; Hongxiao, D.; Huosheng, Q.; Fenyun, S.; Yan, S. Investigation of Fragmentation Pathways of Fentanyl Analogues and Novel Synthetic Opioids by Electron Ionization High-Resolution Mass Spectrometry and Electrospray Ionization High-Resolution Tandem Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2020, 31, 277–291. [Google Scholar] [CrossRef]
- Leary, P.E.; Kizzire, K.L.; Chan Chao, R.; Niedziejko, M.; Martineau, N.; Kammrath, B.W. Evaluation of Portable Gas Chromatography–Mass Spectrometry (GC–MS) for the Analysis of Fentanyl, Fentanyl Analogs, and Other Synthetic Opioids. J. Forensic Sci. 2023, 68, 1601–1614. [Google Scholar] [CrossRef]
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. Synthetic Opioids: A Review and Clinical Update. Ther. Adv. Psychopharmacol. 2022, 12, 20451253221139616. [Google Scholar] [CrossRef]
- Richeval, C.; Gaulier, J.-M.; Romeuf, L.; Allorge, D.; Gaillard, Y. Case Report: Relevance of Metabolite Identification to Detect New Synthetic Opioid Intoxications Illustrated by U-47700. Int. J. Leg. Med. 2019, 133, 133–142. [Google Scholar] [CrossRef]
- Baumann, M.H.; Tocco, G.; Papsun, D.M.; Mohr, A.L.; Fogarty, M.F.; Krotulski, A.J. U-47700 and Its Analogs: Non-Fentanyl Synthetic Opioids Impacting the Recreational Drug Market. Brain Sci. 2020, 10, 895. [Google Scholar] [CrossRef] [PubMed]
- Kyei-Baffour, K.; Lindsley, C.W. DARK Classics in Chemical Neuroscience: U-47700. ACS Chem. Neurosci. 2020, 11, 3928–3936. [Google Scholar] [CrossRef]
- Solimini, R.; Pichini, S.; Pacifici, R.; Busardò, F.P.; Giorgetti, R. Pharmacotoxicology of Non-Fentanyl Derived New Synthetic Opioids. Front. Pharmacol. 2018, 9, 654. [Google Scholar] [CrossRef]
- Popławska, M.; Bednarek, E.; Naumczuk, B.; Kozerski, L.; Błażewicz, A. Identification and Structure Characterization of Five Synthetic Opioids: 3,4-Methylenedioxy-U-47700, o-Methyl-Acetylfentanyl, 2-Thiophenefentanyl, Benzoylfentanyl and Benzoylbenzylfentanyl. Forensic Toxicol. 2021, 39, 45–58. [Google Scholar] [CrossRef]
- Natsuka, K.; Nakamura, H.; Nishikawa, Y.; Negoro, T.; Uno, H.; Nishimura, H. Synthesis and Structure-Activity Relationships of 1-Substituted 4-(1,2-Diphenylethyl)Piperazine Derivatives Having Narcotic Agonist and Antagonist Activity. J. Med. Chem. 1987, 30, 1779–1787. [Google Scholar] [CrossRef]
- Kadem, S.N.; Arslan, Z.; Turkmen, Z. A New Synthetic Opioid Threat: A Comprehensive Review on MT-45. Forensic Sci. Int. 2025, 371, 112479. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.B.; Família, C.; Martins, D.; Cunha, M.; Dias, M.; Neng, N.R.; Gaspar, H.; Quintas, A. Drug-Checking and Monitoring New Psychoactive Substances: Identification of the U-48800 Synthetic Opioid Using Mass Spectrometry, Nuclear Magnetic Resonance Spectroscopy, and Bioinformatic Tools. Int. J. Mol. Sci. 2025, 26, 2219. [Google Scholar] [CrossRef]
- Uchiyama, N.; Matsuda, S.; Kawamura, M.; Kikura-Hanajiri, R.; Goda, Y. Identification of Two New-Type Designer Drugs, Piperazine Derivative MT-45 (I-C6) and Synthetic Peptide Noopept (GVS-111), with Synthetic Cannabinoid A-834735, Cathinone Derivative 4-Methoxy-α-PVP, and Phenethylamine Derivative 4-Methylbuphedrine from Illegal Products. Forensic Toxicol. 2014, 32, 9–18. [Google Scholar] [CrossRef]
- Krotulski, A.J.; Papsun, D.M.; Walton, S.E.; Logan, B.K. Metonitazene in the United States—Forensic Toxicology Assessment of a Potent New Synthetic Opioid Using Liquid Chromatography Mass Spectrometry. Drug Test. Anal. 2021, 13, 1697–1711. [Google Scholar] [CrossRef]
- Verougstraete, N.; Vandeputte, M.M.; Lyphout, C.; Cannaert, A.; Hulpia, F.; Van Calenbergh, S.; Verstraete, A.G.; Stove, C. First Report on Brorphine: The Next Opioid on the Deadly New Psychoactive Substance Horizon? J. Anal. Toxicol. 2021, 44, 937–946. [Google Scholar] [CrossRef]
- Blanckaert, P.; Cannaert, A.; Van Uytfanghe, K.; Hulpia, F.; Deconinck, E.; Van Calenbergh, S.; Stove, C. Report on a Novel Emerging Class of Highly Potent Benzimidazole NPS Opioids: Chemical and In Vitro Functional Characterization of Isotonitazene. Drug Test. Anal. 2020, 12, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Bromig, G. Über neue starkwirkende Analgetika und ihre klinische Erprobung. Klin. Wochenschr. 1958, 36, 960–963. [Google Scholar] [CrossRef]
- Kanamori, T.; Okada, Y.; Segawa, H.; Yamamuro, T.; Kuwayama, K.; Tsujikawa, K.; Iwata, Y.T. Analysis of Highly Potent Synthetic Opioid Nitazene Analogs and Their Positional Isomers. Drug Test. Anal. 2023, 15, 449–457. [Google Scholar] [CrossRef]
- Batlle, E.; Lizano, E.; Viñas, M.; Dolors Pujol, M. 1,4-Benzodiazepines and New Derivatives: Description, Analysis, and Organic Synthesis. In Medicinal Chemistry; Vašková, J., Vaško, L., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-173-1. [Google Scholar]
- Griffin, C.E.; Kaye, A.M.; Bueno, F.R.; Kaye, A.D. Benzodiazepine Pharmacology and Central Nervous System–Mediated Effects. Ochsner J. 2013, 13, 214–223. [Google Scholar]
- Zawilska, J.B.; Wojcieszak, J. An Expanding World of New Psychoactive Substances—Designer Benzodiazepines. NeuroToxicology 2019, 73, 8–16. [Google Scholar] [CrossRef]
- Hikin, L.J.; Coombes, G.; Rice-Davies, K.; Couchman, L.; Smith, P.; Morley, S. Post Mortem Blood Bromazolam Concentrations and Co-Findings in 96 Coronial Cases within England and Wales. Forensic Sci. Int. 2024, 354, 111891. [Google Scholar] [CrossRef] [PubMed]
- Drug-Related Deaths in Scotland in 2021; National Records of Scotland: Edinburgh, UK, 2022.
- Katselou, M.; Papoutsis, I.; Nikolaou, P.; Spiliopoulou, C.; Athanaselis, S. Metabolites Replace the Parent Drug in the Drug Arena. The Cases of Fonazepam and Nifoxipam. Forensic Toxicol. 2017, 35, 1–10. [Google Scholar] [CrossRef]
- Maskell, P.D.; Wilson, G.; Manchester, K.R. Designer Benzodiazepines Gidazepam and Desalkygidazepam (Bromonordiazepam): What Do We Know? J. Anal. Toxicol. 2023, 47, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, P.F.; Deitche, A.; Wise, L.M.; Patrick, S.L.; Holloway-Beth, A.; Ellison, R.; Trecki, J.; Gerona, R.; Wahl, M.S. Notes from the Field: Seizures, Hyperthermia, and Myocardial Injury in Three Young Adults Who Consumed Bromazolam Disguised as Alprazolam—Chicago, Illinois, February 2023. MMWR Morb. Mortal. Wkly. Rep. 2024, 72, 1392–1393. [Google Scholar] [CrossRef]
- Wagmann, L.; Manier, S.K.; Felske, C.; Gampfer, T.M.; Richter, M.J.; Eckstein, N.; Meyer, M.R. Flubromazolam-Derived Designer Benzodiazepines: Toxicokinetics and Analytical Toxicology of Clobromazolam and Bromazolam. J. Anal. Toxicol. 2021, 45, 1014–1027. [Google Scholar] [CrossRef]
- Ballotari, M.; Truver, M.T.; Dhoble, L.R.; Kinsey, A.M.; Hoyer, J.L.; Chronister, C.W.; Goldberger, B.A. A Postmortem Case Report Involving Fentanyl, Desalkylgidazepam, and Bromazolam. J. Anal. Toxicol. 2024, 48, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, A.; Sommer, M.J.; Wilde, M.; Perdekamp, M.G.; Auwärter, V. A Case of Fatal Multidrug Intoxication Involving Flualprazolam: Distribution in Body Fluids and Solid Tissues. Forensic Toxicol. 2022, 40, 180–188. [Google Scholar] [CrossRef]
- Marland, V.; Reid, R.; Brandon, A.M.; Hill, K.; Cruickshanks, F.; McKenzie, C.; Norman, C.; Nic Daéid, N.; Menard, H. Changing Trends in Novel Benzodiazepine Use within Scottish Prisons: Detection, Quantitation, Prevalence, and Modes of Use. Drug Test. Anal. 2024, 16, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; Chatha, M.; Klein, A.K.; McCorvy, J.D.; Meyer, M.R.; Wagmann, L.; Stratford, A.; Brandt, S.D. Pharmacological and Biotransformation Studies of 1-Acyl-Substituted Derivatives of -Lysergic Acid Diethylamide (LSD). Neuropharmacology 2020, 172, 107856. [Google Scholar] [CrossRef]
- Wagmann, L.; Richter, L.H.J.; Kehl, T.; Wack, F.; Bergstrand, M.P.; Brandt, S.D.; Stratford, A.; Maurer, H.H.; Meyer, M.R. In Vitro Metabolic Fate of Nine LSD-Based New Psychoactive Substances and Their Analytical Detectability in Different Urinary Screening Procedures. Anal. Bioanal. Chem. 2019, 411, 4751–4763. [Google Scholar] [CrossRef]
- Brandt, S.D.; Kavanagh, P.V.; Westphal, F.; Stratford, A.; Odland, A.U.; Klein, A.K.; Dowling, G.; Dempster, N.M.; Wallach, J.; Passie, T.; et al. Return of the Lysergamides. Part VI: Analytical and Behavioural Characterization of 1-cyclopropanoyl-d-lysergic Acid Diethylamide (1CP-LSD). Drug Test. Anal. 2020, 12, 812–826. [Google Scholar] [CrossRef]
- Brandt, S.D.; Kavanagh, P.V.; Westphal, F.; Pulver, B.; Morton, K.; Stratford, A.; Dowling, G.; Halberstadt, A.L. Return of the Lysergamides. Part VII: Analytical and Behavioural Characterization of 1-valeroyl-d-lysergic Acid Diethylamide (1V-LSD). Drug Test. Anal. 2022, 14, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.D.; Kavanagh, P.V.; Westphal, F.; Stratford, A.; Elliott, S.P.; Hoang, K.; Wallach, J.; Halberstadt, A.L. Return of the Lysergamides. Part I: Analytical and Behavioural Characterization of 1-propionyl-d-lysergic Acid Diethylamide (1P-LSD). Drug Test. Anal. 2016, 8, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.D.; Kavanagh, P.V.; Westphal, F.; Stratford, A.; Elliott, S.P.; Dowling, G.; Wallach, J.; Halberstadt, A.L. Return of the Lysergamides. Part V: Analytical and Behavioural Characterization of 1-butanoyl-d-lysergic Acid Diethylamide (1B-LSD). Drug Test. Anal. 2019, 11, 1122–1133. [Google Scholar] [CrossRef]
- Kavanagh, P.V.; Westphal, F.; Pulver, B.; Elliott, S.P.; Stratford, A.; Halberstadt, A.L.; Brandt, S.D. Analytical and Behavioral Characterization of 1-dodecanoyl-LSD (1DD-LSD). Drug Test. Anal. 2025, 17, 101–109. [Google Scholar] [CrossRef]
- Junior, L.F.N.; Fabris, A.L.; Barbosa, I.L.; De Carvalho Ponce, J.; Martins, A.F.; Costa, J.L.; Yonamine, M. Lucy Is Back in Brazil with a New Dress. Forensic Sci. Int. 2022, 341, 111497. [Google Scholar] [CrossRef]
- Tusiewicz, K.; Wachełko, O.; Zawadzki, M.; Szpot, P. Forensic Aspects of Designer LSD Analogs Identification by GC–MS (EI) and UV Spectroscopy. Molecules 2024, 29, 5717. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiang, P.; Wen, D.; Shen, B.; Wang, X.; Li, L.; Deng, H.; Chen, H.; Yan, H.; Shen, M.; et al. Sensitive Quantitative Analysis of Psilocin and Psilocybin in Hair Samples from Suspected Users and Their Distribution in Seized Hallucinogenic Mushrooms. Forensic Toxicol. 2021, 39, 464–473. [Google Scholar] [CrossRef]
- Pires, A.P.S.; De Oliveira, C.D.R.; Moura, S.; Dörr, F.A.; Silva, W.A.E.; Yonamine, M. Gas Chromatographic Analysis of Dimethyltryptamine and β-carboline Alkaloids in Ayahuasca, an Amazonian Psychoactive Plant Beverage. Phytochem. Anal. 2009, 20, 149–153. [Google Scholar] [CrossRef]
- Tavares, L.; Monedeiro, F.; Bordin, D.M.; De Martinis, B.S. Investigation of Ayahuasca β-Carboline Alkaloids and Tryptamine in Sweat Samples from Religious Community Participants by GC-MS. J. Anal. Toxicol. 2020, 44, 601–609. [Google Scholar] [CrossRef]
- De Martinis, B. Sweat as an Alternative Matrix for Amphetamines and MethylenEdioxy Derivatives Analysis. CPA 2008, 4, 274–278. [Google Scholar] [CrossRef]
- Neukamm, M.A.; Pollak, S.; Thoma, V.; Vogt, S.; Huppertz, L.M.; Auwärter, V. A Fatal Case of Aspiration Due to Consumption of the Hallucinogenic Tryptamine Derivative Dipropyltryptamine (DPT). J. Pharm. Biomed. Anal. 2024, 240, 115959. [Google Scholar] [CrossRef] [PubMed]
- Emen, E.; Aslan, R.; Aydoğdu, M.; Ertaş, H.; Annette Akgür, S. Development of a Chemometric Method in Urine Sample for Newly Designed Hallucinating Psychoactive Substances: 5-MeO-MiPT. Ege Tıp Derg. 2023, 62, 355–363. [Google Scholar] [CrossRef]
- Göl, E.; Çok, I. New Psychoactive Substances in Turkey: Narcotics Cases Assessed by the Council of Forensic Medicine between 2016 and 2017 in Ankara, Turkey. Forensic Sci. Int. 2019, 294, 113–123. [Google Scholar] [CrossRef]
- Nikolaou, P.; Papoutsis, I.; Stefanidou, M.; Spiliopoulou, C.; Athanaselis, S. 2C-I-NBOMe, an “N-Bomb” That Kills with “Smiles”. Toxicological and Legislative Aspects. Drug Chem. Toxicol. 2015, 38, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; Geyer, M.A. Effects of the Hallucinogen 2,5-Dimethoxy-4-Iodophenethylamine (2C-I) and Superpotent N-Benzyl Derivatives on the Head Twitch Response. Neuropharmacology 2014, 77, 200–207. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Andrzejczak, D. Next Generation of Novel Psychoactive Substances on the Horizon–A Complex Problem to Face. Drug Alcohol. Depend. 2015, 157, 1–17. [Google Scholar] [CrossRef]
- Giulietti, M.; Vivenzio, V.; Piva, F.; Principato, G.; Bellantuono, C.; Nardi, B. How Much Do We Know about the Coupling of G-Proteins to Serotonin Receptors? Mol. Brain 2014, 7, 49. [Google Scholar] [CrossRef]
- Glennon, R.A.; Raghupathi, R.; Bartyzel, P.; Teitler, M.; Leonhardt, S. Binding of Phenylalkylamine Derivatives at 5-HT1C and 5-HT2 Serotonin Receptors: Evidence for a Lack of Selectivity. J. Med. Chem. 1992, 35, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, K.; Świt, P.; Malek, K. 2-(4-Iodo-2,5-Dimethoxyphenyl)- N -[(2-Methoxyphenyl)Methyl]Ethanamine (25I-NBOME): A Harmful Hallucinogen Review. J. Anal. Toxicol. 2021, 44, 947–956. [Google Scholar] [CrossRef]
- Kamińska, K.; Świt, P.; Malek, K. 25C-NBOMe Short Characterisation. Forensic Toxicol. 2020, 38, 490–495. [Google Scholar] [CrossRef]
- Kyriakou, C.; Marinelli, E.; Frati, P.; Santurro, A.; Afxentiou, M.; Zaami, S.; Busardo, F.P. NBOMe: New Potent Hallucinogens--Pharmacology, Analytical Methods, Toxicities, Fatalities: A Review. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3270–3281. [Google Scholar]
- Hill, S.L.; Doris, T.; Gurung, S.; Katebe, S.; Lomas, A.; Dunn, M.; Blain, P.; Thomas, S.H.L. Severe Clinical Toxicity Associated with Analytically Confirmed Recreational Use of 25I–NBOMe: Case Series. Clin. Toxicol. 2013, 51, 487–492. [Google Scholar] [CrossRef]
- Poklis, J.L.; Dempsey, S.K.; Liu, K.; Ritter, J.K.; Wolf, C.; Zhang, S.; Poklis, A. Identification of Metabolite Biomarkers of the Designer Hallucinogen 25I-NBOMe in Mouse Hepatic Microsomal Preparations and Human Urine Samples Associated with Clinical Intoxication. J. Anal. Toxicol. 2015, 39, 607–616. [Google Scholar] [CrossRef]
- Lukić, V.; Micić, R.; Radosavljević, Ž. Veridentification and Determination of 2,5-Dimetoxy-4-Bromophenethylamine (2C-B) in Real Sample by GC-MS Methods and Derivatization after SPE Preparation. Bull. Nat. Sci. Res. 2022, 12, 1–4. [Google Scholar] [CrossRef]
- Coelho Neto, J.; Andrade, A.F.B.; Lordeiro, R.A.; Machado, Y.; Elie, M.; Ferrari Júnior, E.; Arantes, L.C. Preventing Misidentification of 25I-NBOH as 2C-I on Routine GC–MS Analyses. Forensic Toxicol. 2017, 35, 415–420. [Google Scholar] [CrossRef]
- Lopes, K.P.S.; Lucena, M.C.C.; Vidal, L.M.T.; Ayala, A.P.; Alencar, F.A.M.; Ricardo, N.M.P.S. Study of 25R-NBOHs (R = Br, Cl, Et, I): Thermal Stability Investigation and Presumptive Identification by SCXRD and PXRD Data. Forensic Chem. 2024, 40, 100588. [Google Scholar] [CrossRef]
- Almalki, A.J.; Clark, C.R.; Abiedalla, Y.; DeRuiter, J. GC–MS Analysis of Methylenedioxybenzyl Analogues of the Serotonin Receptor Agonists 25X-NBOMe Drugs. Forensic Chem. 2020, 21, 100284. [Google Scholar] [CrossRef]
- Cottler, L.B.; Womack, S.B.; Compton, W.M.; Ben-Abdallah, A. Ecstasy Abuse and Dependence among Adolescents and Young Adults: Applicability and Reliability of DSM-IV Criteria. Hum. Psychopharmacol. 2001, 16, 599–606. [Google Scholar] [CrossRef]
- Baumann, M.H.; Partilla, J.S.; Lehner, K.R.; Thorndike, E.B.; Hoffman, A.F.; Holy, M.; Rothman, R.B.; Goldberg, S.R.; Lupica, C.R.; Sitte, H.H.; et al. Powerful Cocaine-Like Actions of 3,4-Methylenedioxypyrovalerone (MDPV), a Principal Constituent of Psychoactive ‘Bath Salts’ Products. Neuropsychopharmacology 2013, 38, 552–562. [Google Scholar] [CrossRef]
- Almalki, A.J.; Clark, C.R.; Abiedalla, Y.; DeRuiter, J. GC–MS Analysis of N-(Bromodimethoxybenzyl)-2-, 3-, and 4-Methoxyphenethylamines: Inverse Analogues of the Psychoactive 25B-NBOMe Drug. Forensic Chem. 2020, 21, 100277. [Google Scholar] [CrossRef]
- Domino, E.F.; Chodoff, P.; Corssen, G. Pharmacologic Effects of CI-581, a New Dissociative Anesthetic, in Man. Clin. Pharma Ther. 1965, 6, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Dalgarno, P.J.; Shewan, D. Illicit Use of Ketamine in Scotland. J. Psychoact. Drugs 1996, 28, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Matey, J.M.; Montalvo, G.; García-Ruiz, C.; Zapata, F.; López-Fernández, A.; Martínez, M.A. Prevalence Study of Drugs and New Psychoactive Substances in Hair of Ketamine Consumers Using a Methanolic Direct Extraction Prior to High-Resolution Mass Spectrometry. Forensic Sci. Int. 2021, 329, 111080. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, J.B. Methoxetamine–a Novel Recreational Drug with Potent Hallucinogenic Properties. Toxicol. Lett. 2014, 230, 402–407. [Google Scholar] [CrossRef]
- Morris, H.; Wallach, J. From PCP to MXE: A Comprehensive Review of the Non-medical Use of Dissociative Drugs. Drug Test. Anal. 2014, 6, 614–632. [Google Scholar] [CrossRef]
- Alshamaileh, M.; Hussain, I.; Baron, M.; Croxton, R.; Vetter, M.; Gonzalez-Rodriguez, J. A Study of In Vitro Metabolism and Cytotoxicity of Mephedrone and Methoxetamine in Human and Pig Liver Models Using GC/MS and LC/MS Analyses. Open Chem. 2020, 18, 1507–1522. [Google Scholar] [CrossRef]
- Joncquel Chevalier-Curt, M.; Grzych, G.; Tard, C.; Lannoy, J.; Deheul, S.; Hanafi, R.; Douillard, C.; Vamecq, J. Nitrous Oxide Abuse in the Emergency Practice, and Review of Toxicity Mechanisms and Potential Markers. Food Chem. Toxicol. 2022, 162, 112894. [Google Scholar] [CrossRef]
- Brugnone, F.; Perbellini, L.; Cerpelloni, M.; Soave, C.; Cecco, A.; Giuliari, C. Nitrous Oxide in Blood and Urine of Operating Theatre Personnel and the General Population. Int. Arch. Occup. Environ. Heath 1996, 68, 22–26. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, L.; Ma, X.; Li, S.; Xue, Y.; Yan, P.; Chen, M.; Wu, J. Recreational Nitrous Oxide Abuse: Prevalence, Neurotoxicity, and Treatment. Neurotox. Res. 2021, 39, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Z.; Qiang, H.; Xie, W.; Su, M.; Xiang, P.; Shi, Y. Quantitative Determination of Nitrous Oxide in Human Blood by HS-GC–MS: Forensic Application of Two Fatal Poisoning Cases. Forensic Sci. Int. 2024, 360, 112067. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, A.Ø.; Nielsen, M.K.K.; Kristensen, M.; Rasmussen, B.S. Driving under the Influence of Nitrous Oxide–A Retrospective Study of HS-GC-MS Analysis in Whole Blood. Forensic Sci. Int. 2024, 354, 111904. [Google Scholar] [CrossRef]
- Atherton, R.R. The ‘Nutmeg Challenge’: A Dangerous Social Media Trend. Arch. Dis. Child. 2021, 106, 517–518. [Google Scholar] [CrossRef]
- Reynoard, J.; Torrents, R.; Domange, B.; Glaizal, M.; De Haro, L.; Simon, N. Nutmeg Poisoning: Ten Years (2008–2018) of Experience from the Marseille Poison Control Center. La Presse Médicale 2019, 48, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Usui, K.; Kubota, E.; Kobayashi, H.; Fujita, Y.; Hatanaka, K.; Kamijo, Y.; Funayama, M.; Mimasaka, S. Detection of Major Psychoactive Compounds (Safrole, Myristicin, and Elemicin) of Nutmeg in Human Serum via GC–MS/MS Using MonoSpin® Extraction: Application in a Nutmeg Poisoning Case. J. Pharm. Biomed. Anal. 2023, 234, 115565. [Google Scholar] [CrossRef]
- Siebert, D.J. Localization of Salvinorin A and Related Compounds in Glandular Trichomes of the Psychoactive Sage, Salvia Divinorum. Ann. Bot. 2004, 93, 763–771. [Google Scholar] [CrossRef]
- Willard, M.A.B.; Hurd, J.E.; Smith, R.W.; McGuffin, V.L. Statistical Comparison of Mass Spectra of Salvinorins in Salvia Divinorum and Related Salvia Species. Forensic Chem. 2020, 17, 100192. [Google Scholar] [CrossRef]
- Bouzoukas, C.; Nikolaou, P.; Athanaselis, S.; Dona, A.; Spiliopoulou, C.; Papoutsis, I. Development, Validation and Applications of a GC/MS Method for the Simultaneous Determination of 9 Amphetamines and 12 NPS Analogues in Blood and Urine. Forensic Sci. Int. 2025, 370, 112469. [Google Scholar] [CrossRef]
- Ferreira, A.B.; Lobo Castro, A.; Tarelho, S.; Domingues, P.; Franco, J.M. GC-MS–Still Standing for Clinical and Forensic Analysis: Validation of a Multidrug Method to Detect and Quantify Illicit Drugs. Aust. J. Forensic Sci. 2023, 55, 107–128. [Google Scholar] [CrossRef]
- Menéndez-Quintanal, L.M.; Matey, J.M.; Del Fresno González, V.; Bravo Serrano, B.; Hernández-Díaz, F.J.; Zapata, F.; Montalvo, G.; García-Ruiz, C. The State of the Art in Post-Mortem Redistribution and Stability of New Psychoactive Substances in Fatal Cases: A Review of the Literature. Psychoactives 2024, 3, 525–610. [Google Scholar] [CrossRef]
- Nisbet, L.A.; Wylie, F.M.; Scott, K.S. Applications of Sample Preparation Techniques in the Analysis of New Psychoactive Substances. Separations 2024, 11, 258. [Google Scholar] [CrossRef]
- Harries, R.L.; Norman, C.; Reid, R.; Nic Daéid, N.; Nisbet, L.A. Detection of Anabolic-Androgenic Steroids in e-Cigarettes Seized from Prisons: A Case Study. Forensic Sci. Int. 2024, 356, 111965. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, A.; Brunetti, P.; Pelotti, S.; Auwärter, V. Detection of AP-237 and Synthetic Cannabinoids on an Infused Letter Sent to a German Prisoner. Drug Test. Anal. 2022, 14, 1779–1784. [Google Scholar] [CrossRef]
- Bloom, M.B.; Sisco, E.; Lurie, I.S. Development and Validation of a Rapid GC–MS Method for Seized Drug Screening. Forensic Chem. 2023, 33, 100479. [Google Scholar] [CrossRef]
- Capistran, B.A.; Sisco, E. Validation of a Rapid GC–MS Method for Forensic Seized Drug Screening Applications. Forensic Chem. 2024, 41, 100609. [Google Scholar] [CrossRef] [PubMed]
- Black, C.; Russell, M.; Mayo, E.; Aitcheson, C. Rapid Analysis of Amphetamine-type Substances Using Agilent’s QuickProbe Gas Chromatograph/Mass Spectrometer Technology. J. Mass. Spectrom. 2023, 58, e4976. [Google Scholar] [CrossRef]
- Neukamm, M.A.; Halter, S.; Auwärter, V.; Schmitt, G.; Giorgetti, A.; Bartel, M. Death after Smoking of Fentanyl, 5F-ADB, 5F-MDMB-P7AICA and Other Synthetic Cannabinoids with a Bucket Bong. Forensic Toxicol. 2024, 42, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.L. Analysis of Substituted Cathinones and Fentanyl Analogs by Gas Chromatography-Infrared Spectroscopy (GC-IRD) Using Nitrogen Carrier Gas. Forensic Chem. 2025, 44, 100654. [Google Scholar] [CrossRef]
- West, H.; Fitzgerald, J.L.; Hopkins, K.L.; Leeming, M.G.; DiRago, M.; Gerostamoulos, D.; Clark, N.; Dietze, P.; White, J.M.; Ziogas, J.; et al. Trace Residue Identification, Characterization, and Longitudinal Monitoring of the Novel Synthetic Opioid β-U10, from Discarded Drug Paraphernalia. Drug Test. Anal. 2022, 14, 1576–1586. [Google Scholar] [CrossRef]
- Lefrancois, E.; Belackova, V.; Silins, E.; Latimer, J.; Jauncey, M.; Shimmon, R.; Mozaner Bordin, D.; Augsburger, M.; Esseiva, P.; Roux, C.; et al. Substances Injected at the Sydney Supervised Injecting Facility: A Chemical Analysis of Used Injecting Equipment and Comparison with Self-Reported Drug Type. Drug Alcohol. Depend. 2020, 209, 107909. [Google Scholar] [CrossRef]
- Fiorentin, T.R.; Logan, B.K. Analytical Findings in Used Syringes from a Syringe Exchange Program. Int. J. Drug Policy 2020, 81, 102770. [Google Scholar] [CrossRef]
- Gozdzialski, L.; Aasen, J.; Larnder, A.; Ramsay, M.; Borden, S.A.; Saatchi, A.; Gill, C.G.; Wallace, B.; Hore, D.K. Portable Gas Chromatography–Mass Spectrometry in Drug Checking: Detection of Carfentanil and Etizolam in Expected Opioid Samples. Int. J. Drug Policy 2021, 97, 103409. [Google Scholar] [CrossRef]
- Harper, L.; Powell, J.; Pijl, E.M. An Overview of Forensic Drug Testing Methods and Their Suitability for Harm Reduction Point-of-Care Services. Harm Reduct. J. 2017, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, W.; Li, H.; Xing, Y.; Hou, K.; Li, H. Rapid On-Site Detection of Illegal Drugs in Complex Matrix by Thermal Desorption Acetone-Assisted Photoionization Miniature Ion Trap Mass Spectrometer. Anal. Chem. 2019, 91, 3845–3851. [Google Scholar] [CrossRef]
- Leary, P.E.; Kammrath, B.W.; Lattman, K.J.; Beals, G.L. Deploying Portable Gas Chromatography–Mass Spectrometry (GC-MS) to Military Users for the Identification of Toxic Chemical Agents in Theater. Appl. Spectrosc. 2019, 73, 841–858. [Google Scholar] [CrossRef]
- Leary, P.E.; Dobson, G.S.; Reffner, J.A. Development and Applications of Portable Gas Chromatography–Mass Spectrometry for Emergency Responders, the Military, and Law-Enforcement Organizations. Appl. Spectrosc. 2016, 70, 888–896. [Google Scholar] [CrossRef]
- Ma, Q.; Bai, H.; Li, W.; Wang, C.; Cooks, R.G.; Ouyang, Z. Rapid Analysis of Synthetic Cannabinoids Using a Miniature Mass Spectrometer with Ambient Ionization Capability. Talanta 2015, 142, 190–196. [Google Scholar] [CrossRef]
- Mielczarek, P.; Silberring, J.; Smoluch, M. Miniaturzation in Mass Spectrometry. Mass. Spectrom. Rev. 2020, 39, 453–470. [Google Scholar] [CrossRef]
- Fiorentin, T.R.; Krotulski, A.J.; Martin, D.M.; Browne, T.; Triplett, J.; Conti, T.; Logan, B.K. Detection of Cutting Agents in Drug-Positive Seized Exhibits within the United States. J. Forensic Sci. 2019, 64, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Frinculescu, A.; Shine, T.; Ramsey, J.; Couchman, L.; Frascione, N.; Abbate, V. Analysis of Drugs Seized from Amnesty Bins at Two Major United Kingdom Summer Music Festivals Using Two Portable Gas Chromatography-mass Spectrometry (GC–MS) Instruments. Drug Test. Anal. 2024, 16, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Frinculescu, A.; Mercer, B.; Shine, T.; Ramsey, J.; Couchman, L.; Douce, D.; Frascione, N.; Abbate, V. Assessment of a Single Quadrupole Mass Spectrometer Combined with an Atmospheric Solids Analysis Probe for the On-Site Identification of Amnesty Bin Drugs. J. Am. Soc. Mass. Spectrom. 2024, 35, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
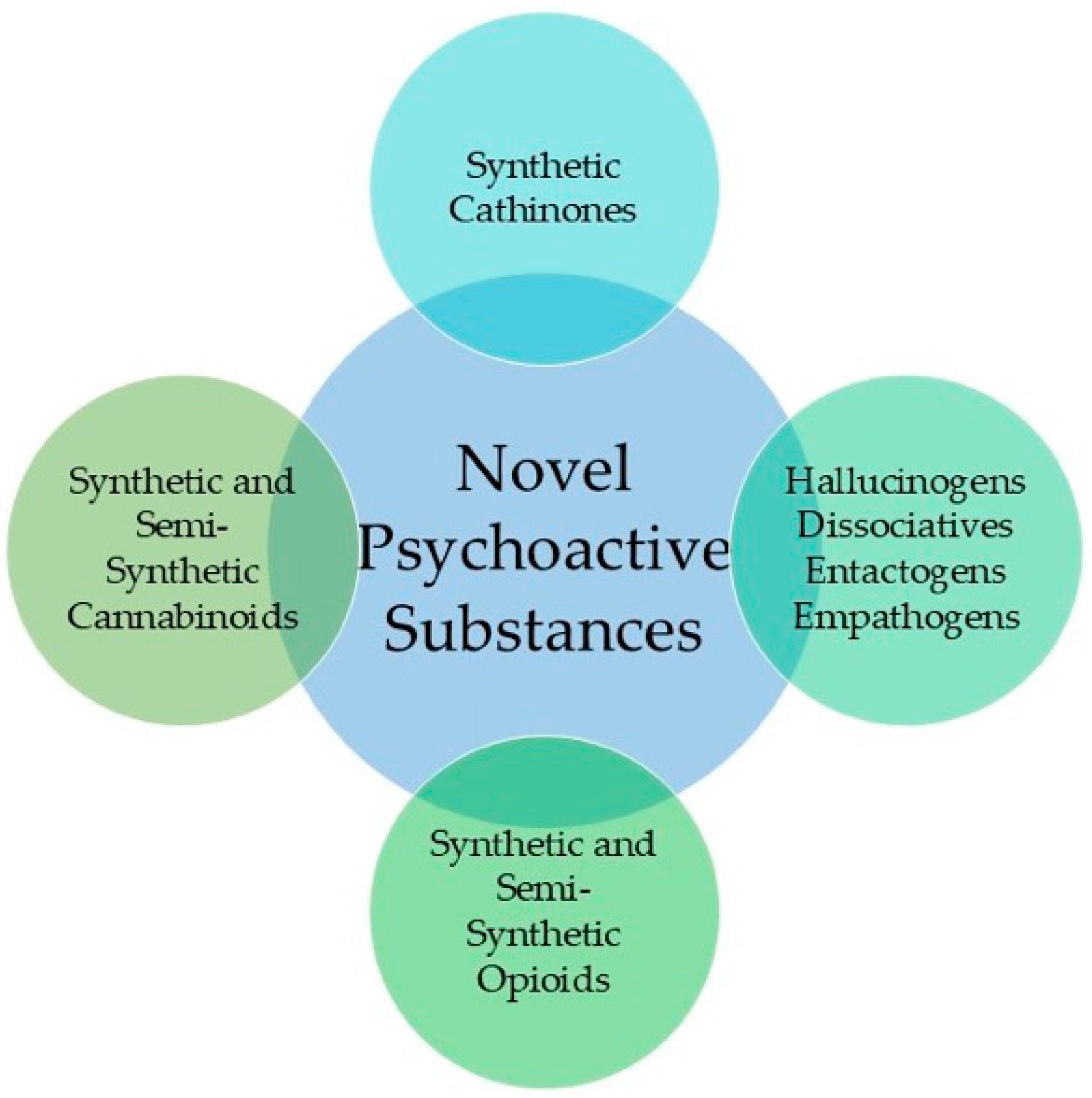

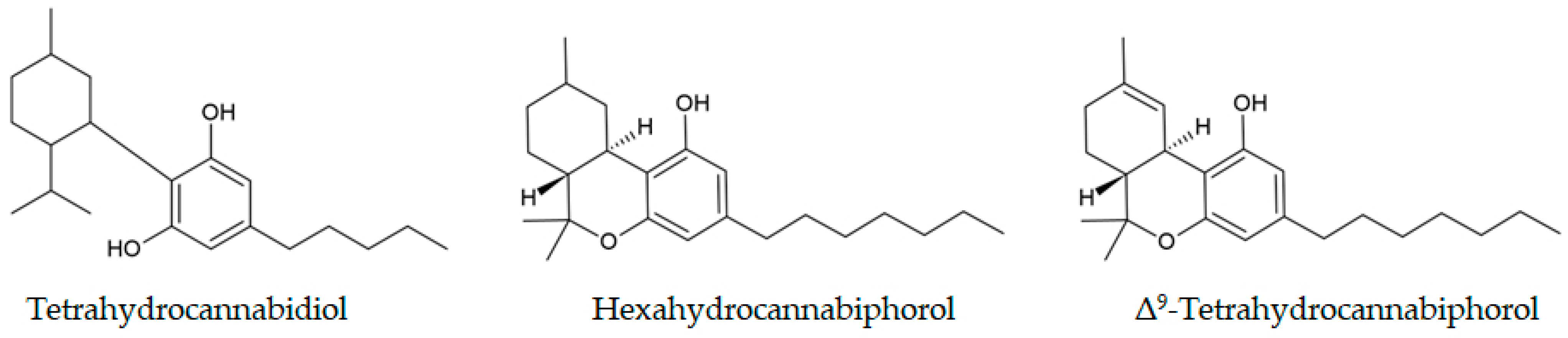
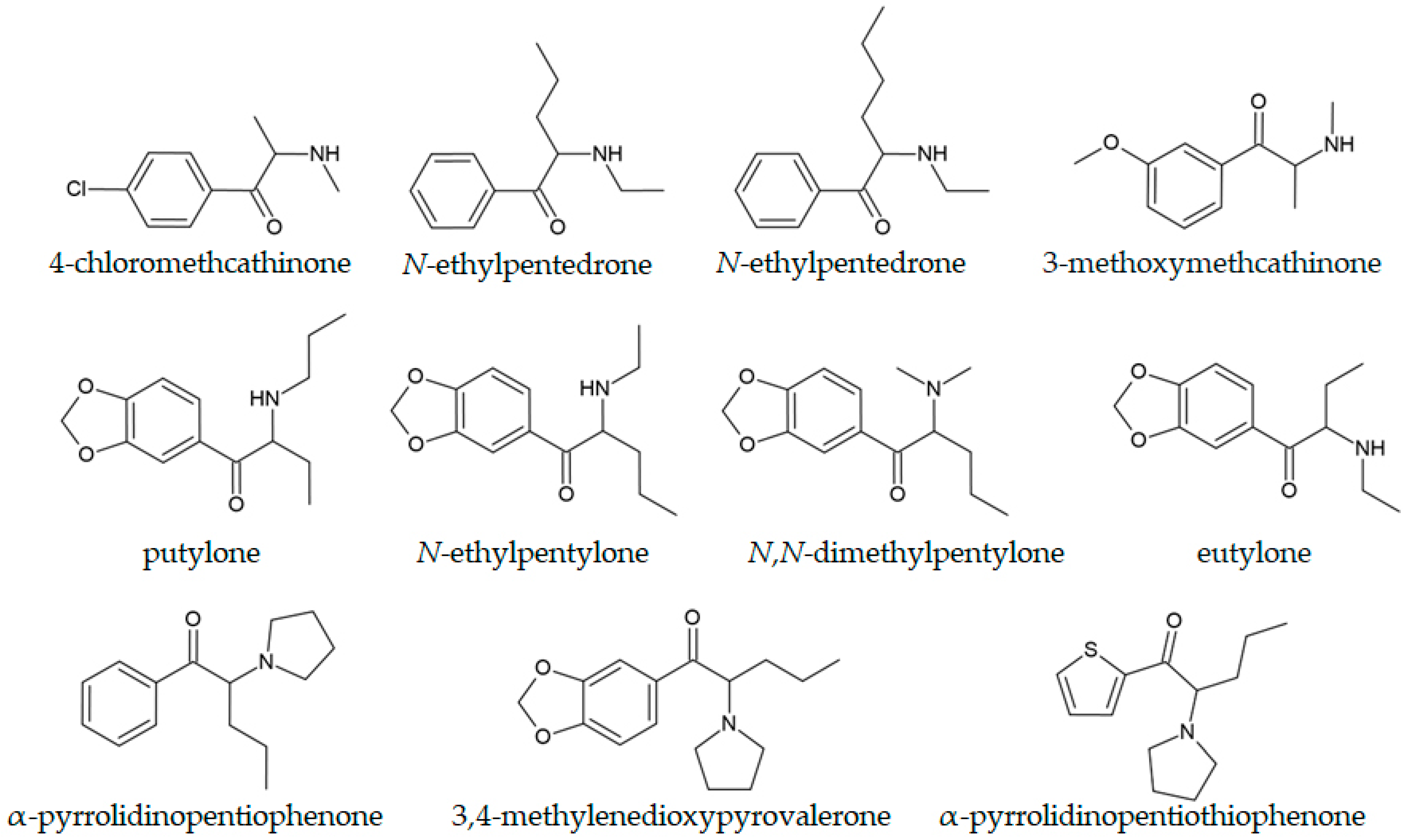
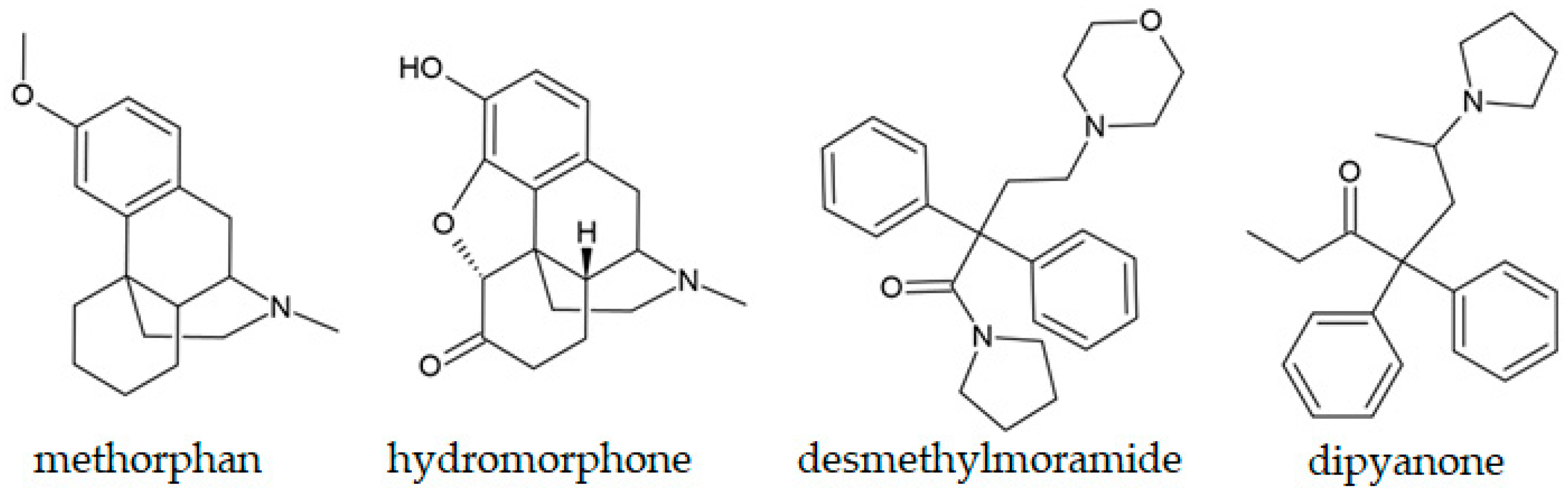
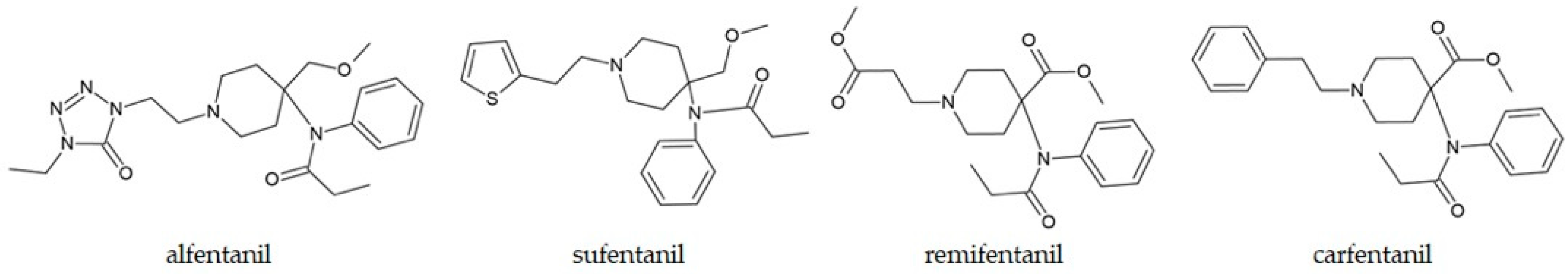
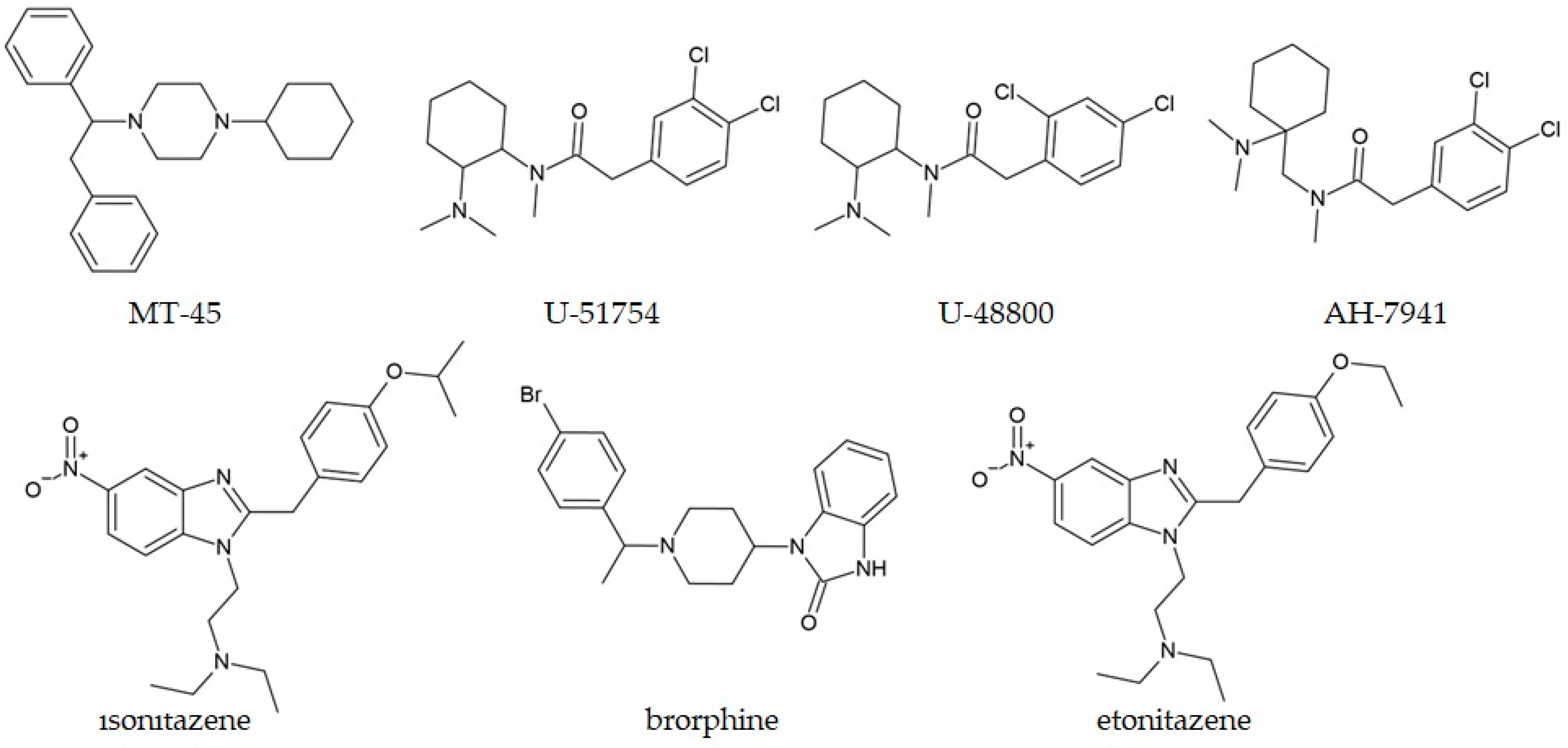
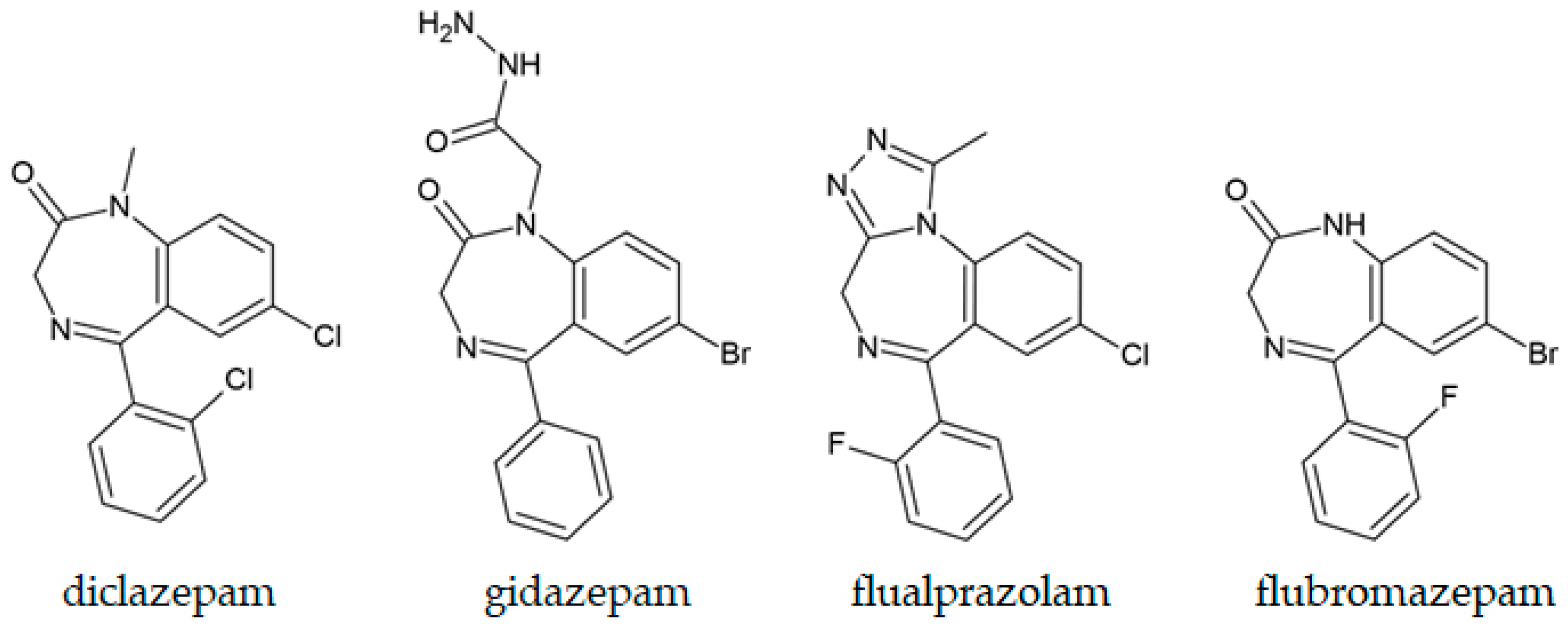
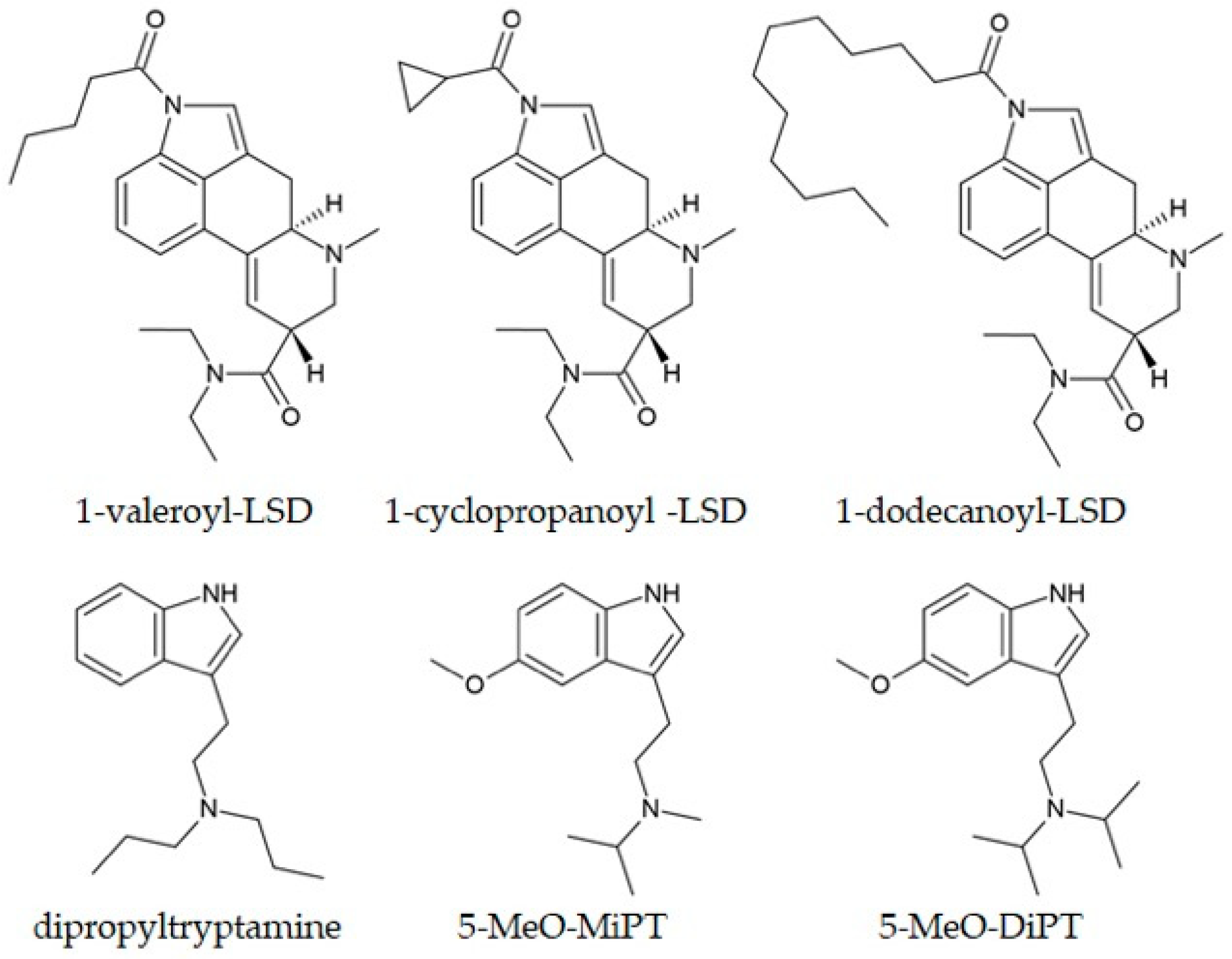
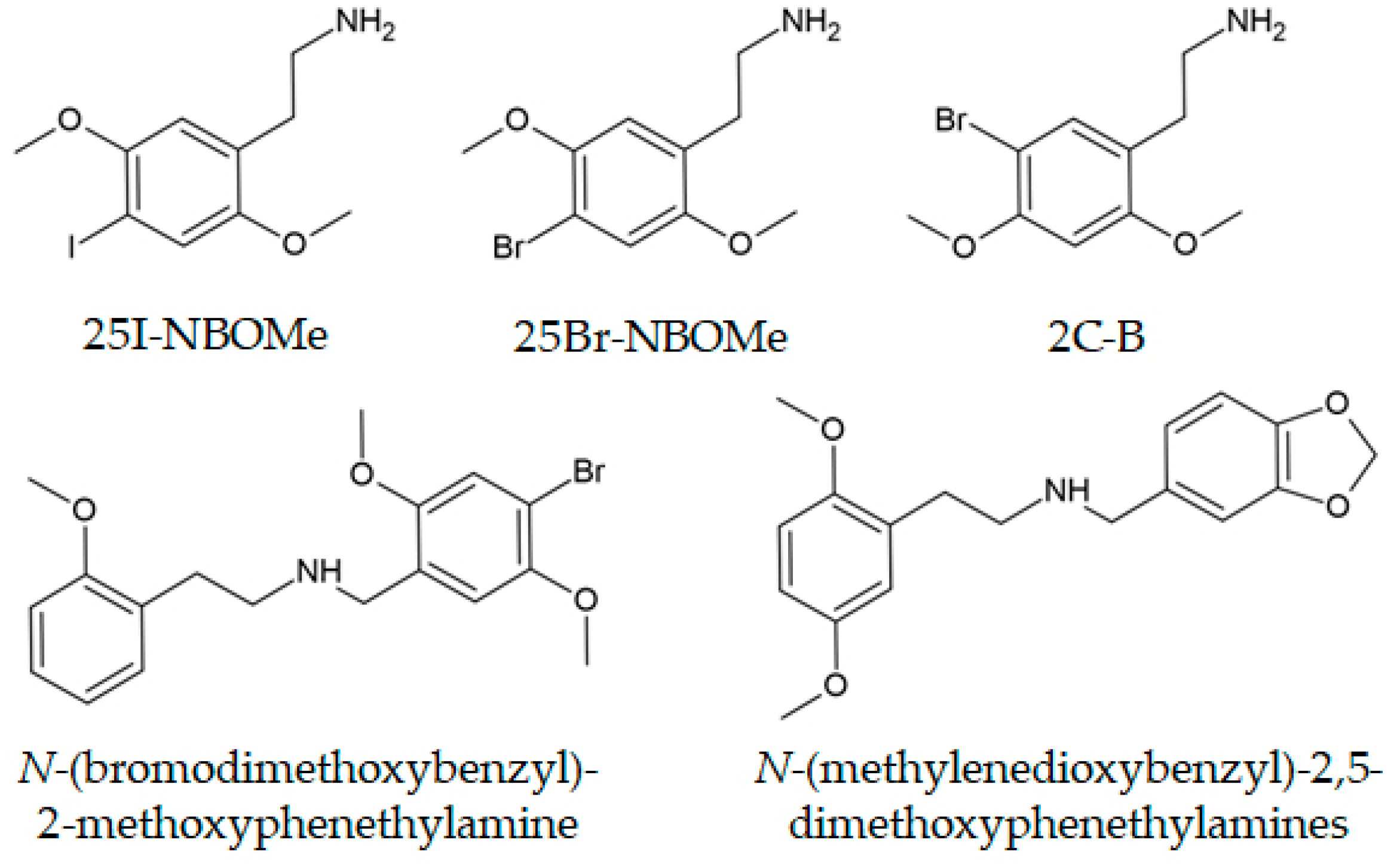
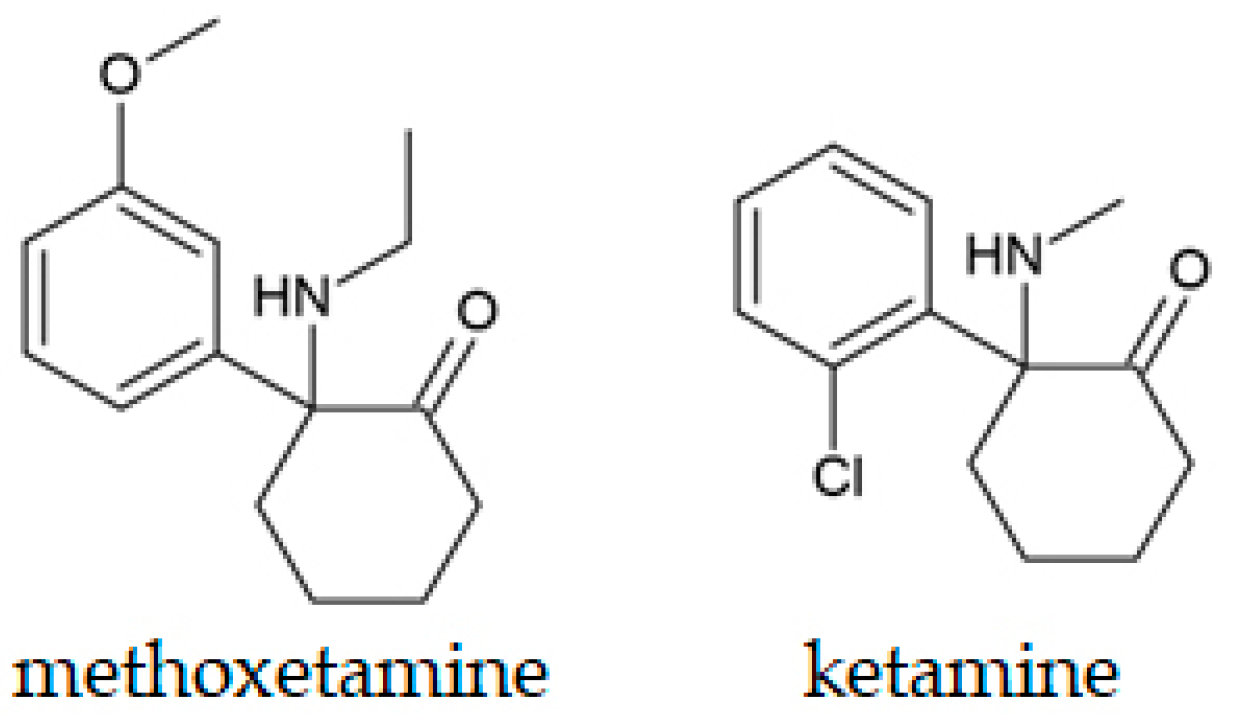
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimić, D. Emerging Novel Psychoactive Substances (2020–2025): GC-MS Approaches for Separation, Detection, and Characterization. Chemosensors 2025, 13, 426. https://doi.org/10.3390/chemosensors13120426
Dimić D. Emerging Novel Psychoactive Substances (2020–2025): GC-MS Approaches for Separation, Detection, and Characterization. Chemosensors. 2025; 13(12):426. https://doi.org/10.3390/chemosensors13120426
Chicago/Turabian StyleDimić, Dušan. 2025. "Emerging Novel Psychoactive Substances (2020–2025): GC-MS Approaches for Separation, Detection, and Characterization" Chemosensors 13, no. 12: 426. https://doi.org/10.3390/chemosensors13120426
APA StyleDimić, D. (2025). Emerging Novel Psychoactive Substances (2020–2025): GC-MS Approaches for Separation, Detection, and Characterization. Chemosensors, 13(12), 426. https://doi.org/10.3390/chemosensors13120426





