Abstract
Health professionals have been one of the groups most affected by the SARS-CoV-2 virus. Currently, there is little scientific evidence on the similarities and differences between COVID-19 infection and the development of long COVID in primary care (PC) workers. Therefore, it is necessary to analyse their clinical and epidemiological profiles in depth. This study was observational and descriptive, including PC professionals who were divided into three comparison groups based on the diagnostic test for acute SARS-CoV-2 infection. The responses were analysed using descriptive and bivariate analysis to examinate the relationship between independent variables and the presence or not of long COVID. Binary logistic regression analysis was also conducted, with each symptom as the dependent variable and each group as the independent variable. The results describe the sociodemographic characteristics of these population groups, revealing that women in the health sector are the most affected by long COVID and that being in this group is associated with its development. Furthermore, individuals with long COVID exhibited the highest number of symptoms and pathologies. Certain symptoms were found to be associated with long COVID development in this population, including an altered sense of smell, pneumonia, fever, and sore throat, among others. Similarly, altered senses of smell and taste, chest tightness, and joint pain, among others, were found to be associated with acute COVID-19 infection. Additionally, patients with pre-existing overweight or obesity were more likely to experience acute COVID-19 and develop long COVID. The data obtained can be crucial for improving the detection, diagnosis, and treatment of long COVID patients, ultimately leading to an enhancement in their quality of life.
Keywords:
long COVID; persistent COVID; post COVID-19; COVID-19; symptoms; pathology; health professional 1. Introduction
The symptomatology associated with COVID-19 is a topic of public health interest due to its rapid transmission and the emergence of new virus variants on all continents. The appearance of these new variants poses an even greater challenge to public health, representing a significant danger to the total control of the pandemic [1,2]. While their definitive origin is still unknown, the most probable theory, according to the World Health Organization (WHO), is that of zoonotic origin [3]. The rapid transmission of the virus occurs through the inhalation of respiratory droplets or aerosols emitted by an infected person [4,5].
COVID-19 infection can manifest as asymptomatic or with mild, moderate or severe symptoms. The range of symptoms associated with the infection is quite broad and can affect various organs and systems [6]. The clinical features of COVID-19 have been extensively studied in both Spanish [7,8] and international studies [9,10,11]. The most common symptoms include fever, dry cough, tiredness or fatigue, dyspnoea, pharyngeal pain, chills and diarrhoea. These symptoms described during the acute phase of the disease can persist over time, leading to persistent COVID-19 or long COVID [12,13].
The absence of a standardized and globally agreed-upon definition makes it challenging to categorize the epidemiology of long COVID and develop potential treatments. To address this issue, the WHO has defined post-COVID condition as an illness that occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, typically occurring 3 months after the onset of symptoms and lasting for at least 2 months, with symptoms that cannot be explained by an alternative diagnosis [14].
The Spanish Society of General Medicine conducted a survey describing the general characteristics of patients with persistent COVID-19 diagnosed during the first wave of the pandemic in the first year [15]. The survey revealed factors such as age (50% of participants aged 36–50 years), sex (more frequent in women), and common symptoms (asthenia, malaise, headache, or mood disturbance) associated with persistent COVID-19 symptoms. The estimated prevalence of persistent COVID-19 in this study was approximately 15% and was increasing. During the early stage of the pandemic, a significant portion of the population faced changes in accessing diagnostic tests, and there was a lack of research on population groups at higher risk of virus exposure.
Many aspects of persistent COVID are still poorly understood, and some studies suggest that this phenomenon may actually encompass multiple different syndromes [16,17]. Specifically, it is unknown which individuals will fully recover and which will continue to experience persistent symptoms [16]. Identifying high-risk individuals would enable the development of strategies to mitigate or prevent symptom persistence [18,19].
It is worth mentioning that vaccination has proven to be an important preventive strategy for long COVID, as it is highly effective and efficient in preventing severe infection and hospitalizations due to COVID-19 [20,21]. Additionally, there are medications available for severe forms of COVID-19 that help reduce the risk of hospitalization or death among high-risk individuals [22]. However, the Ministry of Health in Spain concluded that the potential benefits of vaccination or these medications on the symptoms associated with long COVID are still unclear, although they do not appear to worsen the course of the disease [21].
Due to the mechanism of virus transmission, healthcare professionals are particularly susceptible to infection and transmission. A study utilizing data from the UK Biobank [23] investigated the differences in severe COVID-19 manifestations among various worker groups. The data revealed that healthcare professionals, education and social workers, and other essential workers were at higher risk of developing severe COVID-19 compared to non-essential workers. An international review of coronavirus infection epidemiology and risk factors among healthcare workers in 2020 indicated that healthcare professionals represented a significant proportion of individuals infected with the coronavirus, with a particularly high incidence of infection following unprotected exposures [24].
Preliminary results of the “Factors of COVID-19 Diffusion” project in Spain have demonstrated that the percentage of infected healthcare professionals was a relevant factor contributing to the spread of the pandemic during its upswing [25]. Data from the Spanish seroprevalence study show a higher prevalence of antibodies among health workers compared to other population groups [26], accounting for approximately 10% of cases. Currently, there is a lack of research specifically explaining persistent COVID-19 among primary care (PC) professionals in Spain, who provide initial care to COVID-19 patients. It is important to better understand the factors affecting this population in order to guide public health measures, protect healthcare workers and their families, maintain a functioning healthcare system, and control secondary transmission rates within the community [27,28,29].
Given that healthcare professionals have been one of the most affected groups, the aim of this study was to conduct a more comprehensive analysis of their clinical and epidemiological profiles. Additionally, it was important to describe the clinical and epidemiological profiles of patients with long COVID treated in PC and compare them with the profiles of patients with acute COVID-19 infection and those without COVID-19 infection. On the other hand, it was also considered important to study the symptoms that turned out to be associated with a higher prevalence of being infected with COVID-19 or developing long COVID when experiencing them.
2. Materials and Methods
2.1. Study Design
We conducted an observational, descriptive, multicentric study with three comparison groups, classified according to the results of the acute infection diagnostic test (ADT: antigen detection test and/or polymerase chain reaction (PCR)), by SARS-CoV-2, and the existence or not of long COVID-19 or post-COVID-19 syndrome, following the WHO definition [30].
The groups were: (1) “Cases”: patients with acute infection by SARS-CoV-2 (positive ADT) and long COVID; (2) “Controls 1”: patients with acute infection by SARS-CoV-2 (positive ADT) and absence of criteria for long COVID; (3) “Controls 2”: individuals without acute infection by SARS-CoV-2, for whom an ADT had been requested for presenting an acute clinical picture due to the presence of COVID-19 or for being a close contact of a confirmed case of COVID-19.
2.2. Study Population
The study included PC professionals of the Spanish National Health System (NHS).
Selection criteria were as follows: (1) being a health professional of the NHS; (2) meeting clinical or epidemiological criteria for suspected COVID-19 disease and/or long COVID (Suspected COVID-19 disease was defined as the presence of acute respiratory symptoms consistent with sudden onset in the last 10 days, including cough, dyspnoea, sore throat or rhinitis, with or without fever. Other symptoms such as anosmia, ageusia, diarrhoea, chest pain or headache, among others, were also considered symptoms of suspected SARS-CoV-2 infection. From the epidemiological perspective, suspected COVID-19 disease was also considered if the healthcare professional had been in close proximity, less than 2 m, to a confirmed case for a cumulative time of more than 15 min in a 24 h period without adequate protection measures. Long COVID was defined as a symptomatic condition that persisted in patients who had had COVID-19 and continued to experience symptoms after the acute phase of the disease, lasting for at least 4 weeks from the onset of symptoms [14,30]); and (3) undergoing an ADT and consenting to participate in the study. In the questionnaire, patients indicated whether the diagnosis of COVID was made by antigen test or real-time polymerase chain reaction (RT-PCR, on a nasal/pharyngeal swab). Exclusion criteria was as follows: refusal to participate in the research study.
The Office for National Statistics of the United Kingdom has estimated that one in 10 patients diagnosed with COVID-19 have symptoms beyond 12 weeks after diagnosis and, therefore, a long COVID prevalence of 10% was assumed [31]. Therefore, a sample of 138 individuals would be sufficient to achieve the objective proposed for this study, for a confidence level of 95% and a precision of ±5 percentage points (calculations made with the Granmo programme: https://www.imim.es/ofertadeserveis/software-public/granmo/ (accessed on 13 February 2021)).
2.3. Procedure
The enrolment procedure was initiated to deepen the clinical-epidemiological analysis of patients with persistent COVID-19, which led to the initiation of the EPICOVID-AP21 investigation. The information from study participants was obtained through an online questionnaire created using the Google Forms tool. The questionnaire was sent to members of the Spanish Society of Family and Community Medicine and the Spanish Society of General Practitioners. Each participant received an invitation and a link to access the online questionnaire. Interested participants completed the questionnaire online before providing informed consent.
The research project received authorization from the management of the Cordoba and Guadalquivir Health District and the Cordoba South Health Management Area. Additionally, it obtained approval from the Clinical Research Ethics Committee of the Reina Sofia Hospital in Cordoba (Ethical certificate number: 5033). Informed consent was obtained from the patients participating in the study, ensuring voluntariness. Data processing was conducted in compliance with the European Data Protection Regulation and Organic Law 3/2018 on Personal Data Protection and guarantee of digital rights.
2.4. Statistical Analysis
The questionnaire data were stored in a survey created with Google Forms in Google Drive. These data were exported to an Excel spreadsheet and analysed with the statistical software SPSS version 17.0 (IBM Inc., Chicago, IL, USA). A descriptive and comparative analysis of the groups was conducted. Confidence intervals of 95% (95% CIs) were estimated for the main parameters of the study.
Bivariate analysis was performed to determine whether there was any relationship between groups (healthcare professionals and general population) and to test the relationship between the independent variables and the presence or absence of persistent COVID-19 disease. For this analysis, the Chi-square test was used, as well as mean comparison test for independent samples such as Student’s t-test or ANOVA (after testing normality using the Kolmogorov–Smirnov test). If the data did not meet the assumption of normality, non-parametric tests such as the Kruskal–Wallis test were used. Bilateral contrasts were used, and a significance level of p ≤ 0.05 was applied.
A binary logistic regression analysis was performed to test which variables were associated with the prevalence of having acute COVID-19 and long COVID. The dependent variables were: acute COVID-19 (yes vs. no) and long COVID (yes vs. no). The independent variables included in the regression model were: sociodemographic variables (age—treated as a dummy variable, sex, location, and clinical pathologies (only those pathologies with a frequency of >10 were included in the model)). Odds ratio (OR) values and their respective 95% confidence intervals (95% CIs) were found for each variable. Statistical analysis was performed using the IBM Statistic SPSS 20 program with a significance level of 95% (p = 0.05).
3. Results
Of the 1490 primary care professionals included in the study, 61.6% were doctors, 8.4% were nurses, 6% were nursing assistants, and the rest belonged to other categories such as physiotherapists, occupational therapists, speech therapists, nutritionists, pharmacists, psychologists, etc. Furthermore, 74.9% lived in urban areas (>20,000 inhabitants), and 25.1% lived in rural areas, with no significant differences between comparison groups.
Regarding COVID status, 73% of the participants did not have COVID-19 (negative ADT results), 16.8% had acute COVID infection, and 10.2% of the patients had the clinical picture of long COVID. The patients with long COVID had a mean duration of 35.23 ± 20.70 weeks (95% CI: 31.89–38.57; range = 8–114 weeks). The mean age of the entire sample was 46 ± 11.37 years (95% CI: 46.53–47; range = 14–94 years), with no statistically significant differences between the comparison groups (Table 1). Significant differences were observed in the diagnosis of long COVID based on sex, with 84.9% (CI 95%: 79.2–90.6%) identified in women and 15.1% (CI 95%: 14.6–27.5) identified in men (p = 0.007).

Table 1.
Sociodemographic characteristics according to comparison group.
Of the patients in the study, 81.2% were symptomatic. The mean number of clinical signs or symptoms experienced by the patients was 4.44 ± 6.05 (95% CI: 4.18–4.74; median = 2; range: 0–35). Significant differences were observed by group, with an average number of symptoms in patients without COVID-19 of 2.64 ± 4 (95% CI: 2.42–2.86; range = 0–24), in patients with acute COVID-19 infection of 6.38 ± 5.26 (95% CI: 5.81–6.96; range = 0–24), and in those with long COVID of 14.60 ± 8.78 (CI 95%: 13.25–15.94; range = 1–35) (Kruskal–Wallis; p < 0.001).
Table 2 shows the results of the binary regression model considering sociodemographic characteristics of patients with acute COVID-19 and long COVID. In those with long COVID, significant results were obtained according to sex (higher prevalence of long COVID in women; OR: 1.90; 95% CI: 1.32–2.74; p = 0.001).

Table 2.
Sociodemographic characteristics of patients with acute COVID-19 and long COVID: logistic regression final models.
Table 3 present the results for the symptoms, signs and clinical presentation studied, categorized according to the comparison group. Patients with long COVID-19 exhibited a higher prevalence of fatigue (78.9%; p < 0.001), headache (69.7%; p < 0.001), muscle or joint pain (63.2%; p < 0.001) and dyspnoea (61.2%; p < 0.010) compared to those with acute COVID-19 or individuals without COVID-19.

Table 3.
Symptoms, signs reported by patients according to comparison group.
Table 4 and Figure 1 present the signs and symptoms observed in patients with acute COVID-19 and long COVID, as determined by the binary logistic regression model. Significant findings were obtained for patients with acute COVID-19 who experienced an altered sense of smell (OR: 11.39; 95% CI: 5.98–31.74; p < 0.001), altered taste (OR: 2.28; 95% CI: 1.17–4.45; p = 0.016), chest tightness (OR: 4.37; 95% CI: 1.38–13.86; p = 0.012), pneumonia (p < 0.001), loss of appetite (p < 0.001), joint pain (p = 0.035), abdominal pain (p = 0.005), or eye discomfort (p = 0.009).

Table 4.
Signs and symptoms in patients with acute COVID-19 and long COVID: logistic regression model.
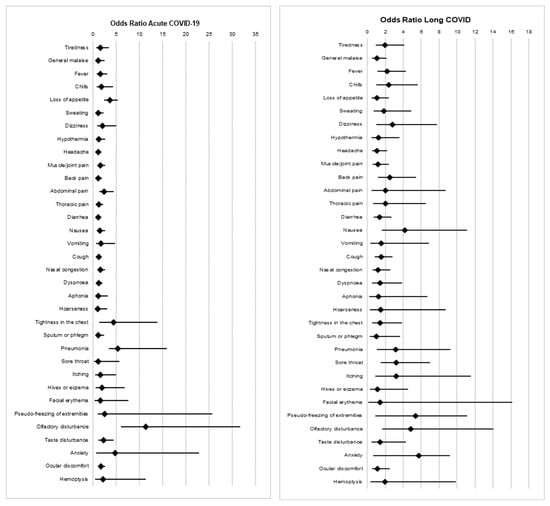
Figure 1.
Signs and symptoms in patients with acute COVID-19 and long COVID: forest plot and logistic regression model.
Similarly, significant findings were also obtained for patients who developed long COVID and presented with an altered sense of smell (OR: 4.83; 95% CI: 1.66–14.04; p = 0.004), pneumonia (OR: 3.18; 95% CI: 1.09–9.29; p = 0.035), fever (OR: 2.20; 95% CI: 1.14–4.27; p = 0.019), chills (OR: 2.38; 95% CI: 1.01–5.60; p = 0.047), sore throat (OR: 3.20; 95% CI: 1.47–6.99; p = 0.004), dizziness (OR: 2.80; 95% CI: 1.01–7.75; p = 0.048), nausea (OR: 4.20; 95% CI: 1.58–11.13; p = 0.004), or back pain (OR: 2.53; 95% CI: 1.182–5.43; p = 0.017).
The average number of pathologies reported by the patients was 0.80 ± 1.04 (95% CI: 0.74–0.86; range: 0–9), with significant differences observed among the groups (Kruskal–Wallis; p < 0.001). The group of patients with long COVID (1.40 ± 1.50; 95% CI: 1.17–1.63; range = 0–9) had a higher average number of pathologies compared to those with acute COVID-19 (0.82 ± 1.01; 95% CI: 0.70–0.93; range = 0–4) or without COVID-19 (0.71 ± 0.96; 95% CI: 0.66–0.77; range = 0–6). Table 5 presents the results for the prevalence of pathologies reported by patients, according to the comparison group. Among 20 pathologies studied, 11 were more frequently reported in patients with long COVID. Notably, overweight or obesity (18.3%; p < 0.001), asthma (9.4%; p = 0.007), hyperlipemia (8.4; p = 0.018), and anxiety syndrome (7.4%; p < 0.001) had a higher prevalence among patients with long COVID compared to those without.

Table 5.
Pathologies reported by patients according to comparison group.
Table 6 presents the results of the binary regression model analysing the pathologies in patients with acute COVID-19 and long COVID. Significant results were observed in patients with acute COVID-19, indicating a higher prevalence of the disease in people who were overweight or obese (higher prevalence of acute COVID-19 in individuals with overweight or obesity; OR: 1.48; 95% CI: 1.13–1.93; p = 0.004). In patients with long COVID, significant results were obtained for overweight or obesity (OR: 1.95; 95% CI: 1.39–2.74; p < 0.001), asthma (OR: 1.54; 95% CI: 1.01–2.39; p = 0.049), anxiety (OR: 3.15; 95% CI: 1.99–4.98; p < 0.001), and coagulation disorders (OR: 2.87; 95% CI: 1.95–3.84; p < 0.001), indicating a higher prevalence of long COVID in patients with these conditions.

Table 6.
Clinical pathologies of patients with acute COVID-19 and long COVID: logistic regression final models.
4. Discussion
The objective of this study was to conduct a more comprehensive analysis of the clinical and epidemiological profiles of primary care professionals in Spain. Additionally, it was considered important to describe them in greater detail and compare them with the profiles of patients with acute COVID-19 infection and those who did not have it. On the other hand, it was also considered important to study the symptoms that turned out to be associated with a higher prevalence of being infected with COVID-19 or developing long COVID when experiencing them.
Our results indicated statistically significant differences in the presence of long COVID between men and women, with a higher prevalence among women. Women were also been shown to be more likely to develop the condition. The analysis of COVID-19 cases in healthcare workers reported to the Red Nacional de Vigilancia Epidemiológica (RENAVE) in 2020 confirmed a significantly higher prevalence of cases in women compared to men [32]. Findings from the literature also support the higher prevalence of long COVID among women [33,34,35,36]. This could be explained by the role of hormones in the acute phase of infection and even after recovery from the disease [33]. Additionally, it has been found that women exhibit a higher production of IgG antibodies, which may translate into a beneficial effect, leading to more favourable outcomes for women [5,37,38].
Likewise, statistically significant differences were observed in relation to the symptoms caused by COVID-19 infection. Symptoms were more pronounced in the group that had long COVID than in the sample that only had acute COVID-19, and even more pronounced than in those who were not infected. However, it is also noteworthy that the highest percentage of the population included in the study experienced symptoms resulting from COVID-19. For example, in the study by Carfi et al. [39], only 12.6% of patients were totally asymptomatic. The most common first symptom in both the acute COVID-19 and long COVID groups was tiredness or fatigue, followed by headache, muscle or joint pain, and dyspnoea. Similar results were reported in other studies, where 32% of the sample reported persistent fatigue [40], 87% fever and 91% malaise or general malaise [41]. It was also found that a large percentage of the population exhibited signs of dyspnoea, muscle weakness and headache, even weeks after testing negative for COVID-19 [13,42,43]. Similarly, a systematic review by Wong and Weitzer [44], which included 21 studies of COVID, suggests that fatigue was a predominant symptom in patients with long COVID in 12 of the studies.
Our results also demonstrated that certain symptoms can be associated with a higher prevalence of experiencing an acute COVID-19 infection or developing long COVID. Symptoms such as altered taste and smell, chest tightness, pneumonia, loss of appetite, joint pain, abdominal pain and/or eye discomfort were found to be associated with acute COVID-19. Similarly, in this population, there were symptoms associated with long COVID, including altered sense of smell, pneumonia, fever, chills, sore throat, dizziness, nausea, and/or back pain.
Other authors also emphasize the importance of establishing an association between symptoms and COVID-19 to facilitate the diagnosis of the disease [45]. Several studies have highlighted the high prevalence of patients experiencing alterations in taste and smell following the infection [46,47,48]. However, none of them analysed these as symptoms potentially associated with COVID-19 infection, nor did they examine the associations with the rest of the symptomatology. Regarding the symptoms associated with long COVID, Arjun et al. emphasized their importance in helping prioritize the at-risk population and designing new intervention plans, although contrary to our study, they found that gender was not associated with long COVID [49]. Similarly, there are no studies that examine the symptoms associated with long COVID. At this stage, it is important to highlight the multisystem effects of COVID-19, which can lead to complications when the person is infected or during the course of the disease. Although the epidemiology and pathophysiology of these symptoms are currently not well understood [10], the complications can affect the respiratory system [50] and the cardiovascular [51] renal [52], thromboembolic [53], neurological [54,55] and autoimmune systems [56]. The results of this study emphasized the presence of statistically significant differences in the average number of pathologies between people with long COVID and those who only had acute COVID or were COVID-negative; long COVID individuals showed a higher average number of pathologies, particularly overweight or obesity, asthma, hyperlipidaemia and anxiety syndrome. Similarly, both overweight and obesity have been shown to be associated with the increased prevalence of both COVID-19 infection and the development of long COVID, as well as asthma, anxiety and coagulation disorders. These results are consistent with other studies that suggest that people who are overweight or obese are more likely to have prolonged symptoms following COVID infection [57,58,59]. However, other studies claim that factors such as hypertension, chronic kidney disease, and immunocompromised states are related [60].
There was also a high prevalence of pathologies such as stroke, depression, coagulation disorders, renal failure and/or anaemia. Other studies also indicated an apparent association of COVID-19 with stroke, likely due to shared factors between them [55]. Similarly, regarding health complications and long-term effects of long COVID, mental health symptoms such as depression, anxiety or anxious syndrome, stress, psychiatric disorders, insomnia, etc., have been observed [61]. Likewise, Sonnweber et al. [62] demonstrated that disturbances in iron homeostasis and anaemia were common issues following COVID-19 infection and gradually decreased during their follow-up. A significant proportion of the population exhibited iron deficiency or persistent anaemia after COVID-19, which may contribute to a higher burden of persistent symptoms in these people. Although there are limited studies related on this topic, it has also been shown that persistent COVID can lead to loss of renal function months after infection (even in patients who did not require hospital admission). This represents an important clinical condition that should be given special attention in patients who have had COVID [63].
Our results also presented statistically significant data on the patients who had asthma when infected with the COVID-19 virus and subsequently developed persistent COVID. However, no significant data were obtained for people with chronic obstructive pulmonary disease (COPD), despite the fact that people who have asthma and/or COPD are at an increased risk of COVID-19 infection and developing more severe symptoms. It appears that these diseases are quite rare as comorbidities with COVID [64,65].
However, before concluding, it is worth mentioning the importance of these results for early diagnosis. The obtained data describe both the sociodemographic and clinical profiles of the healthcare population in Spain and their relationships with infection and the development of long COVID. This comes at a time when, despite the availability of reliable methods such as RT-PCR and serological tests as diagnostic tests, access to these tests is limited in other regions with low socio-economic levels, mostly developing countries. In addition, it is important to highlight the major health-related issues regarding the lack of services and shortages of ICU beds that have been and continue to be experienced in many areas [66,67].
This study had a number of limitations. The type of survey used (self-administered) can introduce an information selection bias.
In addition, the limited evidence on the clinical characteristics of COVID and long COVID in healthcare professionals made it difficult to compare with the results of our study. Similarly, the scarcity of studies analysing the associations of COVID-19 and long COVID symptoms made it difficult to compare our results, emphasizing the lack of studies examining associated symptoms of these conditions in healthcare professionals. Due to these biases, external validation of the study may be limited. However, it is important to consider that the results obtained in the study represent valuable data for the investigation of symptoms and/or complications associated with or derived from COVID-19 and long COVID in healthcare professionals. They hold great importance for future research on this disease, as well as for the design of studies and research protocols on persistent COVID. Additionally, a larger sample size of health professionals presenting COVID-19 and long COVID could address the differences between different professional categories.
Some strengths of this study include the significant number of participants who collaborated and the importance of the results associated with COVID-19 infection and long COVID development, given the limited existence of studies examining these aspects. It is of great significance to obtain a population profile and contribute to early diagnosis, as well as establish early treatment and intervention measures to prevent symptom persistence and/or severity.
5. Conclusions
In conclusion, our findings described the sociodemographic and clinical characteristics of healthcare workers of the SNH, who developed COVID-19, whether acute or long-term syndrome.
Thus, this study provided relevant information on the differences in symptomatology between healthcare workers who were infected with COVID-19, those who developed long COVID and those who were not infected. This information can be significant for the early detection of symptoms and for the diagnosis of the disease.
Regarding the previous pathologies of health workers, it is important to highlight the diseases that developed from the infection, such as nervous system disorders, and those previous pathologies that led to the development of long COVID. This will facilitate the implementation of multidisciplinary prevention and/or treatment programs aimed at improving the quality of life and well-being of this population group.
Finally, the highlights of this study include the following:
- The prevalence of long COVID was found to be higher in females, in addition to the greater probability of its development.
- The study revealed a high prevalence of fatigue in this population. However, there are other symptoms associated with long COVID, such as an altered sense of smell, pre-existing pneumonia and fever, sore throat, dizziness, nausea, and/or back pain.
- Certain symptoms associated with acute COVID-19 were also identified, such as an altered sense of taste and smell, chest tightness, and pneumonia.
Overweight or obese individuals are at a higher risk of contracting an acute COVID-19 infection or developing long COVID later on, as are those with asthma, anxiety, and coagulation disorders.
Author Contributions
Conceptualization, E.R.-R. and L.Á.P.-d.-T.; methodology, E.R.-R. and L.Á.P.-d.-T.; software, L.Á.P.-d.-T.; validation, L.S.-V., E.R.-R. and L.Á.P.-d.-T.; formal analysis, E.R.-R. and L.Á.P.-d.-T.; investigation, E.R.-R., J.G.-L., J.F.-S. and L.Á.P.-d.-T.; resources, J.J.G.-B., L.S.-V. and L.Á.P.-d.-T.; data curation, L.Á.P.-d.-T.; writing—original draft preparation, E.R.-R., J.G.-L., J.F.-S. and L.Á.P.-d.-T.; writing—review and editing, E.R.-R., J.G.-L., J.F.-S., R.V.-S., R.Á.C.-J., C.J.-G. and L.Á.P.-d.-T.; visualization, E.R.-R., J.J.G.-B., J.G.-L., J.F.-S., R.V.-S., M.S.-P. and L.Á.P.-d.-T.; supervision, E.R.-R., J.G.-L., J.G.-S., J.F.-S., R.V.-S., J.J.G.-B. and L.Á.P.-d.-T.; project administration, L.Á.P.-d.-T.; funding acquisition, E.R.-R. and L.Á.P.-d.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the call for research and innovation projects in the field of primary care, regional hospitals, and high-resolution hospital centres of the Public Health System of Andalusia in 2021 by the Progreso y Salud Foundation of the Ministry of Health and Families of the Junta de Andalucía, with EXP. No. AP-0184-2021-C2-F2.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Burgos (UBU 032/2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rahimi, F.; Talebi Bezmin Abadi, A. Implications of the Emergence of a New Variant of SARS-CoV-2, VUI-202012/01. Arch. Med. Res. 2021, 52, 569. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Nadeau, S.; Yared, M.; Voinov, P.; Xie, N.; Roemer, C.; Stadler, T. CoV-Spectrum: Analysis of globally shared SARS-CoV-2 data to identify and characterize new variants. Bioinformatics 2022, 38, 1735. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO-Convened Global Study of Origins of SARS-CoV-2: China Part [Internet]. 2021 [Cited 14 April 2023]. Available online: https://www.who.int/publications/i/item/who-convened-global-study-of-origins-of-sars-cov-2-china-part (accessed on 14 April 2023).
- Menchén, D.A.; Vázquez, J.B.; Allende, J.M.B.; García, G.H. Viral pneumonia. COVID-19 pneumonia. Medicine 2022, 13, 3224–3234. [Google Scholar] [PubMed]
- Kissler, S.M.; Tedijanto, C.; Goldstein, E.; Grad, Y.H.; Lipsitch, M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 2020, 368, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Sanidad; Centro de Coordinación de Alertas y Emergencias Sanitarias. Información Científica-técnica. Enfermedad Por Coronavirus, COVID-19. 2021. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/ITCoronavirus/home.htm (accessed on 14 April 2023).
- Martínez, I.P.; de Torres, L.A.P.; Lama, J.G.; García, C.J.; Montero, R.S.; Garrido, F.R. Características clínico-epidemiológicas de la infección por el virus SARS-CoV-2 en médicos de familia: Un estudio de casos y controles. Aten. Primaria 2021, 53, 101956. [Google Scholar] [CrossRef] [PubMed]
- Pérula de Torres, L.Á.; González-Lama, J.; Jiménez García, C.; Sánchez Montero, R.; Rider Garrido, F.; Ortega López, Y.; Pajares Conde, D.; Ramírez Baena, M.; Párraga Martínez, I.; Romero-Rodríguez, E. Frequency and predictive validity of olfactory and taste dysfunction in patients with SARS-CoV-2 infection. Med. Clin. 2021, 156, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid—Mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef]
- Aiyegbusi, O.L.; Hughes, S.E.; Turner, G.; Rivera, S.C.; McMullan, C.; Chandan, J.S.; Haroon, S.; Price, G.; Davies, E.H.; Nirantharakumar, K. Symptoms, complications and management of long COVID: A review. J. R. Soc. Med. 2021, 114, 428–442. [Google Scholar] [CrossRef]
- van Kessel, S.A.M.; Olde Hartman, T.C.; Lucassen, P.L.B.J.; van Jaarsveld, C.H.M. Post-acute and long-COVID-19 symptoms in patients with mild diseases: A systematic review. Fam. Pract. 2022, 39, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Centros Para el Control y la Prevención de Enfermedades. Afecciones Persistentes al COVID-19 y Afecciones Posteriores al COVID-19 [Internet]. 2022. Available online: https://espanol.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 14 April 2023).
- Orrù, G.; Bertelloni, D.; Diolaiuti, F.; Mucci, F.; Di Giuseppe, M.; Biella, M.; Gemignani, A.; Ciacchini, R.; Conversano, C. Long-COVID Syndrome? A Study on the Persistence of Neurological, Psychological and Physiological Symptoms. Healthcare 2021, 9, 575. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet. Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Sociedad Española de Médicos Generales y de Familia Encuesta COVID-19 Persistente. Encuesta COVID-19 Persistente. Available online: https://www.colfisioaragon.org/ficheros/ResultadosENCUESTA_2020.pdf (accessed on 14 April 2023).
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Honigsbaum, M.; Krishnan, L. Taking pandemic sequelae seriously: From the Russian influenza to COVID-19 long-haulers. Lancet 2020, 396, 1389–1391. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Exellence (NICE). COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19; National Institute for Health and Care Exellence: London, UK, 2020; p. 188. [Google Scholar]
- Meeting the challenge of long COVID. Nat. Med. 2020, 26, 1803. [CrossRef] [PubMed]
- Bernal, E.; García-Villalba, E.; Pons, E.; Vicente, M.R.; Tomás, C.; Minguela, A.; Hernández, M.D.; Puche, G.; Carter, P.; Martinez, M.; et al. Role of vaccination and anti-SARS-CoV-2 antibodies in the clinical outcome of hospitalized COVID-19 patients. Med. Clin. 2023, 160, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Marco, J.J.G.; Pasquín, M.J.Á.; Martín, S.M.; Miranda, A.P.J. Papel protector de las actuales vacunas para las variantes del virus SARS-CoV-2 y la COVID persistente. FMC—Form. Médica Contin. Atención Primaria 2022, 29, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Centros Para el Control y la Prevención de Enfermedades (CDC). Tratamientos y Medicamentos Para el COVID-19 [Internet]. [Cited 25 May 2023]. Available online: https://espanol.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html (accessed on 1 June 2023).
- Mutambudzi, M.; Niedwiedz, C.; Macdonald, E.B.; Leyland, A.; Mair, F.; Anderson, J.; Celis-Morales, C.; Cleland, J.; Forbes, J.; Gill, J.; et al. Occupation and risk of severe COVID-19: Prospective cohort study of 120,075 UK Biobank participants. Occup. Environ. Med. 2020, 78, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Dana, T.; Buckley, D.I.; Selph, S.; Fu, R.; Totten, A.M. Epidemiology of and Risk Factors for Coronavirus Infection in Health Care Workers: A Living Rapid Review. Ann. Intern. Med. 2020, 173, 120–136. [Google Scholar] [CrossRef]
- Centro Nacional de Epidemiología (CNE); CIBER de Epidemiología y Salud Pública (CIBERESP) Proyecto Factores de Difusión COVID-19 en España. 2020. Available online: https://repisalud.isciii.es/handle/20.500.12105/10817 (accessed on 14 April 2023).
- Ministerio de Sanidad; Consejo Interterritorial Sistema Nacional de Salud; Instituto de Salud Carlos III. Estudio ENE-COVID: Informe Final. Estudio Nacional de Sero-Epidemiología de la Infección por SARS-CoV-2 en España. 2020. Available online: https://www.sanidad.gob.es/ciudadanos/ene-covid/home.htm (accessed on 14 April 2023).
- Menges, D.; Ballouz, T.; Anagnostopoulos, A.; Aschmann, H.E.; Domenghino, A.; Fehr, J.S.; Puhan, M.A. Estimating the burden of post-COVID-19 syndrome in a population-based cohort study of SARS-CoV-2 infected individuals: Implications for healthcare service planning. medRxiv 2021. [Google Scholar] [CrossRef]
- Dzinamarira, T.; Mhango, M.; Dzobo, M.; Ngara, B.; Chitungo, I.; Makanda, P.; Atwine, J.; Nkambule, S.J.; Musuka, G. Risk factors for COVID-19 among healthcare workers. A protocol for a systematic review and meta-analysis. PLoS ONE 2021, 16, e0250958. [Google Scholar] [CrossRef] [PubMed]
- Parkin, A.; Davison, J.; Tarrant, R.; Ross, D.; Halpin, S.; Simms, A.; Salman, R.; Sivan, M. A Multidisciplinary NHS COVID-19 Service to Manage Post-COVID-19 Syndrome in the Community. J. Prim. Care Community Health 2021, 12, 21501327211010994. [Google Scholar] [CrossRef]
- Ministerio de Sanidad; Instituto de Salud Carlos III. Estrategia de Vigilancia y Control Frente a COVID-19 Tras la Fase Aguda de la Pandemia; Madrid, Spain, 2022. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Nueva_estrategia_vigilancia_y_control.pdf (accessed on 14 April 2023).
- The Prevalence of Long COVID Symptoms and COVID-19 Complications [Internet]. Office for National Statistics. [Cited 17 January 2023]. Available online: https://www.ons.gov.uk/news/statementsandletters/theprevalenceoflongcovidsymptomsandcovid19complications (accessed on 14 April 2023).
- Instituto de Salud Carlos III; Red Nacional de Vigilancia Epidemiológica. Análisis de los Casos de COVID-19 en Personal Sanitario Notificados a la RENAVE Hasta el 10 de Mayo en España. Informe a 29 de Mayo de 2020. Equipo COVID-19. RENAVE. CNE. CNM (ISCIII). 2020. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes%20COVID-19/COVID-19%20en%20personal%20sanitario%2029%20de%20mayo%20de%202020.pdf (accessed on 14 April 2023).
- Bai, F.; Tomasoni, D.; Falcinella, C.; Barbanotti, D.; Castoldi, R.; Mulè, G.; Augello, M.; Mondatore, D.; Allegrini, M.; Cona, A.; et al. Female gender is associated with long COVID syndrome: A prospective cohort study. Clin. Microbiol. Infect. 2022, 28, 611.e9–611.e16. [Google Scholar] [CrossRef] [PubMed]
- Ortona, E.; Malorni, W. Long COVID: To investigate immunological mechanisms and sex/gender related aspects as fundamental steps for tailored therapy. Eur. Respir. J. 2022, 59, 2102245. [Google Scholar] [CrossRef] [PubMed]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Perlis, R.H.; Santillana, M.; Ognyanova, K.; Safarpour, A.; Lunz Trujillo, K.; Simonson, M.D.; Green, J.; Quintana, A.; Druckman, J.; Baum, M.A.; et al. Prevalence and Correlates of Long COVID Symptoms Among US Adults. JAMA Netw. Open 2022, 5, e2238804. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Dai, C.; Cai, P.; Wang, J.; Xu, L.; Li, J.; Hu, G.; Wang, Z.; Zheng, F.; Wang, L. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: A possible reason underlying different outcome between sex. J. Med. Virol. 2020, 92, 2050. [Google Scholar] [CrossRef] [PubMed]
- Akkız, H. The Biological Functions and Clinical Significance of SARS-CoV-2 Variants of Corcern. Front. Med. 2022, 9, 849217. [Google Scholar] [CrossRef] [PubMed]
- Saloner, B.; Parish, K.; Julie Ward, M.A.; Grace DiLaura, R.; Sharon Dolovich, J. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar]
- De Pablos-Florido, V.; Córdoba-Peláez, P.; Jiménez-Gutiérrez, P.M. Dolor Persistente Como Secuela de la COVID-19: Una Revisión Sistemática. Arch. Med. Univ. 2021, 3, 80–85. [Google Scholar]
- Mauricio Trelles, P.B.; Gutierrez Cadillo, D.N. Caracterización clínica epidemiológica de las secuelas Covid-19 en adultos recuperados de un hospital de Huancayo. Rev. Peru. Cienc. La Salud 2022, 4, e364. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Barnett, J.; Brill, S.E.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, T.E.; Jacob, J.; Jarvis, H.C.; et al. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021, 76, 396–398. [Google Scholar] [CrossRef]
- Wong, T.L.; Weitzer, D.J. Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)-A Systemic Review and Comparison of Clinical Presentation and Symptomatology. Medicina 2021, 57, 418. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, N. Taste alteration in COVID-19: Significant geographical differences exist in the prevalence of the symptom. J. Infect. Public Health 2021, 14, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Chiu, N.C.; Peng, C.C.; Lin, C.H.; Tai, Y.L.; Lee, M.D.; Cheng, Y.J.; Tan, B.F.; Lin, C.Y. One-Seventh of Patients with COVID-19 Had Olfactory and Gustatory Abnormalities as Their Initial Symptoms: A Systematic Review and Meta-Analysis. Life 2020, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, N.; Colella, G. Self-reported smell and taste alteration as the sole clinical manifestation of SARS-CoV-2 infection. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, e95–e99. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Perisetti, A.; Lee-Smith, W.M.; Gajendran, M.; Bansal, P.; Goyal, H. Taste Changes (Dysgeusia) in COVID-19: A Systematic Review and Meta-analysis. Gastroenterology 2020, 159, 1132–1133. [Google Scholar] [CrossRef]
- Arjun, M.C.; Singh, A.K.; Pal, D.; Das, K.; Alekhya, G.; Venkateshan, M.; Mishra, B.; Patro, B.K.; Mohapatra, P.R.; Subba, S.H. Characteristics and predictors of Long COVID among diagnosed cases of COVID-19. PLoS ONE 2022, 17, e0278825. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Yamamoto, S.; Nagata, M.; Ogasawara, N.; Hasegawa, S. Long COVID presenting with intermittent fever after COVID-19 pneumonia. Radiol. Case Rep. 2021, 16, 2478–2481. [Google Scholar] [CrossRef] [PubMed]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020, 5, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, X.; Ren, J.; Sun, Y.; Yu, R.; Li, K.; Zheng, L.; Yang, J. Risk factors and prognosis for COVID-19-induced acute kidney injury: A meta-analysis. BMJ Open 2020, 10, e042573. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D.; García-Sanchez, A.; Rali, P.; Muriel, A.; Bikdeli, B.; Ruiz-Artacho, P.; Le Mao, R.; Rodríguez, C.; Hunt, B.J.; Monreal, M. Incidence of VTE and Bleeding Among Hospitalized Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis. Chest 2021, 159, 1182. [Google Scholar] [CrossRef]
- Yamakawa, M.; Kuno, T.; Mikami, T.; Takagi, H.; Gronseth, G. Clinical Characteristics of Stroke with COVID-19: A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2020, 29, 105288. [Google Scholar] [CrossRef] [PubMed]
- Favas, T.T.; Dev, P.; Chaurasia, R.N.; Chakravarty, K.; Mishra, R.; Joshi, D.; Mishra, V.N.; Kumar, A.; Singh, V.K.; Pandey, M.; et al. Neurological manifestations of COVID-19: A systematic review and meta-analysis of proportions. Neurol. Sci. 2020, 41, 3437–3470. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, C.; Bayry, J. Autoimmune and inflammatory diseases following COVID-19. Nat. Rev. Rheumatol. 2020, 16, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, L.; De Maria, L.; Quarato, M.; Caputi, A.; Gesualdo, L.; Migliore, G.; Cavone, D.; Sponselli, S.; Pipoli, A.; Inchingolo, F.; et al. Association between Long COVID and Overweight/Obesity. J. Clin. Med. 2021, 10, 4143. [Google Scholar] [CrossRef]
- Nittas, V.; Gao, M.; West, E.A.; Ballouz, T.; Menges, D.; Wulf Hanson, S.; Puhan, M.A. Long COVID Through a Public Health Lens: An Umbrella Review. Public Health Rev. 2022, 43, 5. [Google Scholar] [CrossRef]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Sathiavageesan, S.; Sundaram, V.; Sundaram, N.; Shanmugam, V.B.; Selvaraj, J.; Vivek, N.; Ravi, G.K.; Velan, M.; Palaniappan, C.; Singaravelu, V.; et al. Fulminant onset COVID-19: Predictors and outcome. Postgrad. Med. J. 2022, 98, 742–749. [Google Scholar] [CrossRef]
- Gómez Conesa, A. How does physiotherapy approach mental health in Long-COVID? Fisioterapia 2022, 44, 1–5. [Google Scholar] [CrossRef]
- Sonnweber, T.; Grubwieser, P.; Sahanic, S.; Böhm, A.K.; Pizzini, A.; Luger, A.; Schwabl, C.; Koppelstätter, S.; Kurz, K.; Puchner, B.; et al. The Impact of Iron Dyshomeostasis and Anaemia on Long-Term Pulmonary Recovery and Persisting Symptom Burden after COVID-19: A Prospective Observational Cohort Study. Metabolites 2022, 12, 546. [Google Scholar] [CrossRef] [PubMed]
- De Francisco, Á.M.; Fresnedo, G.F. Enfermedad renal en la COVID-19 persistente: Un objetivo inmediato para Nefrología. Nefrologia 2023, 43, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Green, I.; Merzon, E.; Vinker, S.; Golan-Cohen, A.; Magen, E. COVID-19 Susceptibility in Bronchial Asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 684–692.e1. [Google Scholar] [CrossRef] [PubMed]
- Halpin, D.M.G.; Faner, R.; Sibila, O.; Badia, J.R.; Agusti, A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet. Respir. Med. 2020, 8, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Roels, N.I.; Estrella, A.; Maldonado-Salcedo, M.; Rapp, R.; Hansen, H.; Hardon, A. Confident futures: Community-based organizations as first responders and agents of change in the face of the COVID-19 pandemic. Soc. Sci. Med. 2022, 294, 114639. [Google Scholar] [CrossRef] [PubMed]
- Perez Perez, G.I.; Talebi Bezmin Abadi, A. Ongoing Challenges Faced in the Global Control of COVID-19 Pandemic. Arch. Med. Res. 2020, 51, 574–576. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).