Abstract
Public health reports contain limited information regarding the psychological and neurological symptoms of tick-borne diseases (TBDs). Employing a mixed-method approach, this analysis triangulates three sources of symptomology and provides a comparison of official public health information, case reports, medical literature, and the self-reported symptoms of patients with Lyme disease and other TBDs. Out of the fifteen neuropsychiatric symptoms reported in the medical literature for common TBDs, headaches and fatigue and/or malaise are the only two symptoms fully recognized by public health officials. Of TBDs, Lyme disease is the least recognized by public health officials for presenting with neuropsychiatric symptoms; only headaches and fatigue are recognized as overlapping symptoms of Lyme disease. Comparisons from a patient symptoms survey indicate that self-reports of TBDs and the associated symptoms align with medical and case reports. Anxiety, depression, panic attacks, hallucinations, delusions, and pain—ranging from headaches to neck stiffness and arthritis—are common among patients who report a TBD diagnosis. Given the multitude of non-specific patient symptoms, and the number and range of neuropsychiatric presentations that do not align with public health guidance, this study indicates the need for a revised approach to TBD diagnosis and for improved communication from official public health sources regarding the wide range of associated symptoms.
1. Introduction
Patients who are diagnosed with Lyme disease (LD) or other tick-borne diseases often develop chronic or long-term symptoms, leading to debates over the use of terms such as “chronic Lyme” and “post-treatment Lyme disease syndrome” (PTLDS). According to the Centers for Disease Control and Prevention (CDC) in the United States, when Lyme disease and other tick-borne diseases are not treated early, PTLDS may occur from autoimmune phenomena, with or without persistent infection, resulting in pain, brain fog, and fatigue more than six months after antibiotic treatment [1]. However, it is also known that 10–20% of patients who are treated in a timely fashion will also develop PTLDS [2]. Numerous studies report multisystem and severe symptoms that substantially impede a patient’s quality of life [3,4,5]. The psychological distress of chronic illness in patients with Lyme disease in particular can be bi-directional, resulting in further diagnostic burdens for patients and medical providers [3,4]. In other words, patients who are ill, as with any chronic or serious condition, could become depressed from the burden of the illness alone, in addition to a reaction from the pathogen itself.
- This research explores the medical literature related to tick-borne disease (TBD) symptomology, in comparison to official public health reports and to survey respondents who reported a diagnosis of one or more TBD. These comparisons contribute to the study of TBDs by analyzing the symptoms noted by patients and medical reports but are not recognized by public health officials.
- This study presents patient self-reports regarding their diagnosis and quality of life, specifically regarding psychological distress and concomitant neurological symptoms.
1.1. Burden of Diagnosis: Controversial Pathogenic Insults
Tick-borne diseases (TBDs) in the United States account for 77 percent of all vector-borne diseases, as reported by the National Notifiable Diseases System (NNDS), 2004–2016 [6]. Tick-borne diseases represent a varied set of infections that can be caused by bacteria, viruses, and protozoa [7]. Tick-borne infections are “under-appreciated causes of central nervous system (CNS) infection”, as a result of under-diagnosing and under-reporting [7]. LD or Lyme Borreliosis (LB), a tick-borne pathogen causing multisystem symptoms [8,9] in humans, is estimated to account for 476,000 cases per year [10], suggesting that the actual number of TBD cases may be substantially higher, indeed, more than ten times higher than stated in the official reports [7]. Other TBDs have expanded geographically, beyond regions considered endemic, resulting in increased disease cases, ranging from spotted fever rickettsiosis to anaplasmosis and babesiosis [6]. Global public health, as a field, is recognizing the burden of TBDs, which are increasing as a result of both abiotic and biotic factors [11]. Clinical presentations of tick-borne diseases vary, but not uncommonly, TBDs clinical presentations involve CNS manifestations, which include palsy, confusion, meningoencephalitis, encephalitis, meningitis, and encephalopathy [7].
In addition to hard-bodied ticks that spread LD and other TBDs, recent attention is also being paid to tick-borne relapsing fevers (TBRFs), known to be caused by the soft-bodied or Argasidae tick vectors. However, TBRFs can also be transmitted by hard ticks or Ixodidae vectors [12]. Relapsing fever borreliae, such as LD, are spirochaetes. Spirochetal illnesses, such as syphilis and LD have been referred to as “great imitators,” given their symptom similarities with other diseases. For example, often multisystem and nonspecific, syphilis and LD can both result in numerous neuropsychiatric symptoms. In fact, syphilis patients accounted for twenty-five percent of the psychiatric beds at the beginning of the 20th century [13].
1.2. Testing for TBDs, EM Rash, and Diagnostic Struggles
Testing for Lyme disease is particularly controversial given false negative and false positive occurrences. False positives may occur with other spirochetal illnesses, such as syphilis and periodontal disease, as well as infection with the Epstein–Barr virus (EBV) [13,14,15]. Serological testing faces numerous challenges, including the time it takes for a person to generate antibodies, a lack of sensitivity following antibiotic treatment, and the test’s inability to distinguish an active infection from mere past exposure [6,13].
The CDC guidelines are based on serology, but it is common for a patient not to produce antibodies. The resulting test will, therefore, produce a false negative in cases where the infection is acute and the test is performed too soon. False negatives may also occur when a patient is either immune compromised or had received treatment early after contracting a TBD [16,17]. For those with late-stage LD or other TBDs, a false negative is notably relevant to neuropsychiatric sequelae.
Additionally, tick saliva injected into a human host not only carries molecules to reduce histamine, but also carries pain-reducing molecules, meaning that the host may not realize that they have been bitten. In addition, it also introduces molecules that can elude white blood cells, effectively undermining the host’s immune response [18]. A lack of antibody production by some human hosts is problematic in current testing [19], leading some patients to seek out specialty labs, which many physicians and the CDC consider to be unconventional testing. Serologic testing is often negative in early-stage Lyme disease as well, which suggests that treatment should be administered if an EM rash is present [7].
The diagnostic struggles for patients with a TBD, or in whom a TBD is suspected, can cause a significant economic burden, present difficulties for patients in search of a diagnosis, and can lead to long-term impacts on patients’ quality of life. At least 3.4 million Lyme disease tests are performed by specialty laboratories in the United States each year [20,21]. Physicians often fail to test for TBDs, other than Lyme disease, as it is the most recognized. One study found that 75.6% of family practitioners underestimated the incidence rates of erythema migrans (EM) rashes, and approximately one-fourth were aware that the presence of an EM rash, alone, was diagnostic for LD [22]. In endemic areas, EM rashes alone often are used in clinical diagnoses [23]. These findings support a more recent study on long-term patients, which concludes as follows: “Although physical exams and clinical laboratory tests showed few objective abnormalities, standardized symptom questionnaires revealed that patients with PTLDS are highly and clinically significantly symptomatic, with poor health-related quality of life” [5].
Adding to the diagnostic burden, EM rashes are not always present, nor do all patients remember receiving a tick bite [6]. Additionally, the classic “bull’s-eye” EM rash does not appear in many cases [24] and, even when an EM rash is present, clinicians may not recognize it as a symptom of LD. In children, clinicians missed 12% of LD cases that presented with an EM rash; furthermore, they also believed 31% to have LD when they actually did not have the disease [25]. Misunderstandings about the necessity of a bull’s-eye rash, in addition to intermittent symptoms, can also delay diagnosis [26].
Many TBDs other than Lyme disease do not present with the bull’s-eye rash [27]. TBD diagnosis is challenging, especially in areas perceived to be non-endemic where medical practitioners may not be alert to related symptoms [28]. Patients who are left untreated may develop severe neuropsychiatric symptoms and decreased quality of life. In comparison to patients with other chronic illnesses, LD patients present particularly poor quality of life outcomes [3,29,30]. The International Lyme and Associated Diseases Society supports the use of clinical diagnoses, considering the diagnostic challenges [31]. However, case studies of patients being misdiagnosed with LD are not uncommon, such as reports of patients with neoplasms, whose medical treatment was delayed as a result of an LD diagnosis [23].
Late-disseminated LD and other TBDs can cause neurologic, psychiatric, and influenza-like symptoms, accompanied by numerous other multisystem and often debilitating symptoms, resulting in an extremely poor quality of life [3]. Misdiagnoses are more common in urgent care and emergency room settings, and occur as a result of symptom misattribution, symptoms that are intermittent, and of some physicians’ belief that a bull’s-eye rash must be present [26]. The controversial and ongoing debate regarding the clinical diagnosis of Lyme disease begets further diagnostic struggles, as clinicians may fail to test for tick-borne related co-infections, such as rickettsiae or babesiosis, as well as other diseases, such as Chlamydia pneumoniae, with similar clinical presentations [32]. In a large US study, some LD patients waited 10 years for the correct diagnosis and many reported having to travel more than 50 miles for treatment [29].
2. Materials and Methods
2.1. Tickborne Diseases of the United States: A Reference Manual for Health Care Providers
The complexity of diagnosis originates from patients presenting with non-specific and multisystem symptoms, with potential misattribution of symptoms by practitioners, regarding psychiatric and associated neurological problems. Using a mixed- methods approach, the combination of methodological approaches included a systematic review of the literature on psychiatric and neurological symptoms, in addition to a comparison of the case reports and the medical literature, with the Centers for Disease Control and Prevention’s manual: Tickborne Diseases of the United States: A Reference Manual for Health Care Providers [27]. The articles were pulled from English language journals found in PubMed. Only those which presented case reports or research related to TBD in the United States were included; case reports from Europe and other countries were excluded as tick-borne pathogens may cause different symptoms.
2.2. CDC Guidance, Medical Literature, and Patient Self-Reports
These findings are further detailed through comprehensive comparison tables that collect all CDC-reported symptoms for all tick-borne diseases, as documented and shared with health providers. This listing entails a wide range of reported symptoms from a variety of commonly acquired tick-borne diseases, as reported by the CDC. A second table includes all CDC-reported psychological and neurological symptoms, comparing the CDC guidelines for health care providers to the literature found via PubMed. To date, less information is available on the psychiatric and neurological symptoms that extend beyond the CDC-recognized symptoms of “headache” or “altered mental state”. Through a comprehensive search via PubMed, the symptoms are identified and summarized to assess differing and dispersed knowledge regarding possible presentations of Lyme disease and other tick-borne diseases.
Through a mixed-methods approach, 148 patients who self-reported having Lyme disease or another TBD were surveyed using a convenience sample. The patient symptom survey (PSS) was created using Qualtrics, an online survey platform. An anonymous survey link was provided via Facebook and was distributed widely via numerous “shares” among individuals and tick-borne disease organizations. The survey was designed to reach potential respondents who had a diagnosis of LD or another TBD, as they are most likely to frequent health and LD-related organizations online. The survey reached respondents who voluntarily completed questions regarding their self-reported diagnosis and resulting symptoms. Frequencies of psychiatric and neurological symptoms by disease type have been reported here to further assess differing public health and case reports and to inform public health practitioners about the possibility of unusual presentations of Lyme disease and other TBDs. Although there are limitations to using patient self-reports, with limited data regarding the incidence of certain symptoms, survey reports are becoming more commonplace [3,28]. Including patient responses in the literature review was intended to serve as an exploratory approach to determine self-reported symptoms. Given the online nature of the survey, respondents were not asked to verify their diagnosis through laboratory results.
The overall framework was holistic and exploratory to look across outcomes of symptomology using a triangulation approach in comparing differing methods, including the official guidance, case reports, and medical literature, and patient self-reports from a national sample. The patient symptom survey was approved by the Ethics Committee under the Declaration of Helsinki Institutional Review Board Guidelines. Informed consent was obtained from all subjects involved in the study. The authors declare no conflict of interest.
3. Results
3.1. Neurological Symptoms and Sequalae of LD and TBDs
“Lyme neuroborreliosis (LNB) is the neurological manifestation of the systemic infection caused by the spirochete Borrelia burgdorferi (BB) sensu lato” [33]. Lyme borreliosis encompasses all areas of the nervous system, with as many as 20% developing neurological symptoms [34,35]. Below, a review of the key symptoms is presented. The last row for each symptom provides information from the Centers for Disease Control to provide a comparative reference to the scientific literature. Specifically, the CDCs Tick-borne Diseases of the United States: A Reference Manual for Healthcare Providers is referenced for each symptom [27]. The importance of symptom recognition in TBDs cannot be overstated, as they are essential for proper testing and diagnosis. When discrepancies between the scholarly literature and official sources exist, the diagnostic burden for those with TBDs increases (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10 and Table 11).

Table 1.
Headache is a common feature of many TBDs. It often presents with other neurological symptoms. Case reports and scholarly literature indicate headache occurs in LD, Borrelia miyamotoi disease (BMD), and Powasan Virus, among others.

Table 2.
Confusion/Altered Mental Status. Altered mental status is found in patients with ehrlichiosis, LD, TBRF, and Powassan, and anaplasmosis, among others. Numerous clinical reports indicated confusion and altered mental status as symptoms of TBDs.

Table 3.
Pain may present as rheumatological, muscle-skeletal, or neurological. The present study asked survey respondents about pain severity in general. Below, pain is covered with a focus on neurological and also in general, including arthritis, joint, and muscle pain, which tend to present as common symptoms with other frequently occurring symptoms, such as headache and fatigue.

Table 4.
Scholarship related to LD or tick-borne disease and seizures appears in individual case reports more frequently than population studies. Official recognition by the CDC of seizures in tick-borne disease are noted only in Powassan virus disease and tick-borne encephalitis virus (TBE).

Table 5.
Vertigo and/or dizziness may not be fully recognized as a TBD symptom. Medical literature and case reports demonstrate that vertigo is present in cases of LD and TBRF.

Table 6.
Tingling and numbness tend to occur in the extremities and are associated with LD in case reports.

Table 7.
Cognitive function (concentration, word finding, and memory difficulty)–limited literature is available regarding cognitive function among TBD patients in the United States. Concentration tends to be related to Tick-borne encephalitis, which is endemic in Europe and Asia. The following studies are reported from the US.

Table 8.
Paralysis: difficulty swallowing (dysphagia) and Bell’s palsy. This exploratory study was designed to capture different sources of information and assess the possible disconnect among them, with respect to patient symptoms. Dysphagia is one symptom with which medical providers may not be familiar, but self-reports or case reports in the medical literature might suggest that the symptom may require further exploration. Unlike dysphagia, Bell’s palsy is recognized by official public health sources and is suggested as a recognized symptom of LD in approximately eight percent of cases [81].

Table 9.
Difficulty with, or slurred speech (dysarthria). Dysarthria is a neurological manifestation of a number of tick-borne diseases. Numerous case reports link difficulty with speech or slurred speech to anaplasmosis, LD, and PVD. The CDC does not report dysarthria as a symptom of any TBD, indicating more research is needed with this neurological manifestation.

Table 10.
Low blood pressure (hypotension). Hypotension is associated with autonomic nervous system dysfunction and is demonstrated primarily with ehrlichiosis in the case reports. The National Organization for Rare Diseases (NORD) recognizes hypotension with ehrlichiosis and RMSF, but the CDC does not link this symptom to any TBD.

Table 11.
Fainting (syncope). Syncope has been reported with Lyme disease and other tick-borne diseases. Although syncope, generally, may be associated with neurological conditions, it may have cardiovascular etiologies. In the TBD case reports, fainting tended to be associated with Lyme carditis, rather than neurological origins.
3.2. TBD and Psychiatric Symptoms and Sequelae
Psychiatric manifestations are reported among patients with LD and co-infections or other TBDs (Maxwell, 2020 [3]). Hájek et al. (2002) [111], for example found the following: “Higher prevalence of antibodies to Borrelia burgdorferi in psychiatric patients than in healthy subjects”. Bransfield (2018) [67] noted the following: “There is increasing evidence and recognition that Lyme borreliosis, and other associated tick-borne diseases (LB/TBD) cause mental symptoms” (p. 1). Fallon et al. (2021) provided insights on patients diagnosed with LB in hospital settings in Denmark, noting the increased risk of suicide and suicide attempts, and other mental disorders, in a Danish cohort study [112]. However, the literature is generally scant in disaggregating specific psychiatric manifestations. Searches for “depression” and “tick-borne”, for example result in canine studies or those related to Tick-borne encephalitis, a disease primarily found in Europe and Asia. Overall, the psychiatric symptoms related to TBDs have been largely ignored. The literature review below includes psychiatric manifestations beyond depression and anxiety and extends to hallucinations and delusions. Fatigue and malaise are included in this section, as they are common symptoms of TBDs, generally, but are also linked bi-directionally to patients with Lyme disease and other TBDs (Table 12, Table 13, Table 14 and Table 15).

Table 12.
Depression is found in many patients with LD and is noted by a state-level public health report as a clinical feature of babesiosis.

Table 13.
Anxiety is not well studied in the medical literature as a symptom of TBDs, but is shown to be present with LD. TBRF is also associated with anxiety.

Table 14.
Fatigue and malaise. Fatigue (i.e., extreme tiredness) or malaise (i.e., lack of wellbeing) are often reported in the literature as primarily clinical features of Lyme and other tick-borne diseases, particularly in cases of human monocytic ehrlichiosis (HME). Although ample evidence supports fatigue and malaise as symptoms of LD, scholarly literature is burdened with debates regarding chronic LD or post-treatment Lyme disease syndrome (PTLDS). The findings, below, focus on the earlier stages of LD and other tick-borne diseases.

Table 15.
Mania, panic attacks, delusions, or hallucinations. Tick-borne illnesses are noted by patients and advocacy organizations to cause psychiatric symptoms similar to bi-polar and schizophrenia, reportedly leading to unnecessary institutionalization and/or misdiagnosis. Official sources of information are lacking on these psychiatric manifestations, which may delay diagnosis, as medical providers are not informed via the public health system. Mania, panic attacks, hallucinations, and delusions have been combined in this section to cover the overlapping presence of the three clinical manifestations in the scholarly literature.
The clinical outcomes for patients with tick-borne diseases are largely unrecognized, even though Lyme disease is the most common vector-borne disease in the United States [62]. Lyme disease is a catch-all term used by many LD organizations to characterize a host of tick-borne illnesses. As a result, patients may request Lyme disease testing, which may not be conclusive or may result in negative laboratory findings if the patient is infected with a different TBD. “Patients with LD are more likely to report nonspecific long-term sequelae, especially those experiencing persistent symptoms posttreatment” [62]. Given the varying and non-specific clinical presentations and the long-term outcomes of tick-borne diseases, clinicians referring to the CDC guidelines may miss additional symptoms, especially those with neuropsychiatric involvement. Below, the full range of symptoms for every TBD reported by the CDC is summarized in Table 16.

Table 16.
CDC Tickborne Diseases of the United States: A Reference Manual for Healthcare Providers (2018). Summary of all TBD Symptoms.
Using the systematic literature review and the CDC summary table, Table 16 offers a symptom comparison for tick-borne diseases that are more common in the US and within the medical literature. Specifically, the focus includes the following: LD, BVD, babesiosis, ehrlichiosis, tularemia, anaplasmosis, RMSF, and PVD. Table 17 summarizes the neuropsychic symptoms used in the present study’s survey of LD patients, who reported tick bites and subsequent TBDs.

Table 17.
Symptom comparison from medical literature and the public health reference manual for common TBDs.
The comparison summary in Table 17 indicates that out of the fifteen neurological symptoms reported in the medical literature, headaches and fatigue, and/or malaise are the only two symptoms that the CDC fully recognizes in accordance with the scholarly studies and case reports. Of those fifteen more common neuropsychiatric symptoms, Lyme disease is the least recognized TBD by the CDC for presenting neuropsychiatric symptoms, and the CDC does not acknowledge eleven symptoms found in the literature. Only headaches, fatigue, and pain were reported by the CDC as over-lapping symptoms of LD. The neurological symptoms that were least recognized by the CDC but were reported in scientific studies included the following: difficulty swallowing (dysphagia), speech impairment (dysarthria), low blood pressure (hypotension), fainting, vertigo and dizziness, and seizures.
3.3. Patient Symptom Survey Results
A total of 239 respondents completed the online survey before the predetermined cut-off date of 31 March 2021. Only respondents who reported at least one tick-bite encounter in a United States county after 1 January 2000 were included in the final analytical cohort. The final analytical cohort consisted of 148 respondents (61.92% of all respondents), spread over 144 US counties. Of these 148, 81 (54.73%) reported a diagnosis of anaplasmosis, babesiosis, ehrlichiosis, Lyme disease, and/or Rocky Mountain spotted fever. The analytical cohort included a wide range of ages and an approximately equal number of males and females, although these varied within each diagnostic cohort. Notably, ~83% of all respondents who reported a TBD diagnosis were female, despite making up only ~46% of the analytical cohort (Table 18).

Table 18.
Respondent Characteristics by Diagnostic Category. Summary statistics for the number of respondents, and their age and sex, who reported a tick-borne disease are provided for each disease (i.e., by diagnostic category).
Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5 provide the results from the patient symptom survey, comprising 148 respondents from across the United States who reported a diagnosis of a tick-borne disease. As is evidenced from Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5, there is a wide diversity of noted tick-borne disease outcomes, which impact the physiological and psychological reported symptomology. Among all of the respondents, symptoms were evident across all categories of reported conditions, with the least-reported in this sample being facial palsy, seizures, fainting, and fever. For each TBD, the rates of the symptoms reported in this survey demonstrated that the most often reported diseases were Lyme disease, followed by Rocky Mountain spotted fever, babesiosis, ehrlichiosis, and anaplasmosis, with specific occurrences of underlying symptomology, respective to each TBD. The charts, below, provide the percentages of respondents who indicated that they experience each symptom, organized by disease diagnosis.
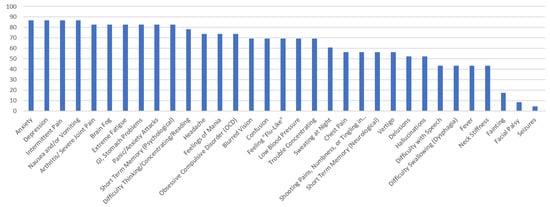
Figure 1.
Percentage of Respondents with Ehrlichiosis Reporting Specific Symptoms.
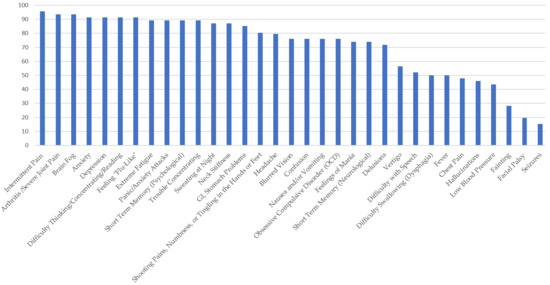
Figure 2.
Percentage of Respondents with Babesiosis Reporting Specific Symptoms.
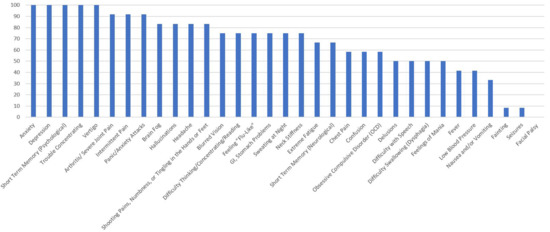
Figure 3.
Percentage of Respondents with Anaplasmosis Reporting Specific Symptoms.
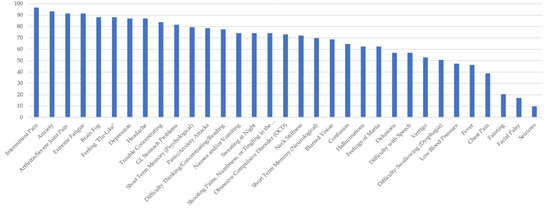
Figure 4.
Percentage of Respondents with Lyme disease Reporting Specific Symptoms.
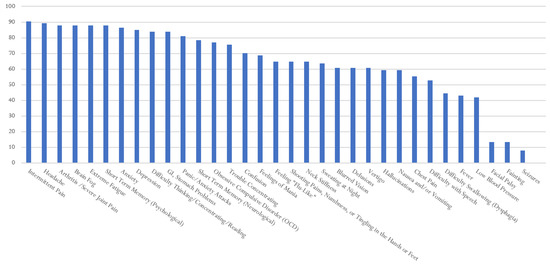
Figure 5.
Percentage of Respondents with RMSF Reporting Specific Symptoms.
The survey respondents who reported a diagnosis of ehrlichiosis point to very high levels of neuropsychiatric symptoms. The most prominent (over 80% of the reports) were as follows: arthritis, with severe joint pain and swelling (particularly the knees and other large joints); brain fog, headache, and short-term memory. The following symptoms accounted for over 90% of the reports: anxiety; depression; GI/stomach problems; intermittent pain in tendons, muscles, joints, and bones; nausea and/or vomiting; and panic attacks. The most common symptoms involved pain, GI, and psychological symptoms, specifically those related to anxiety and depression. Only one respondent reported Bell’s palsy or seizures, and only two reported fainting.
In Figure 2: Survey respondents who reported a diagnosis of babesiosis experienced symptoms similar to ehrlichiosis, but at higher frequencies. More than 90 percent of those surveyed reported the following: arthritis, with severe joint pain and swelling; intermittent pain; anxiety; depression; brain fog; headaches; feeling “flu-like”; trouble concentrating; and extreme fatigue. Over 80% reported shooting pains, numbness or tingling in the hands or feet, hallucinations, obsessive compulsive disorder (OCD), nausea and/or vomiting, sweating at night, difficulty thinking/concentrating/reading, and GI (stomach) problems. Facial/Bell’s palsy and fainting were the least reported symptoms among those who self-reported a diagnosis of babesiosis.
Figure 3 shows the percentage of PSS respondents with anaplasmosis, who reported neurological, psychiatric, and pain-related symptoms. One hundred percent of those with a self-reported diagnosis of anaplasmosis reported anxiety, depression, short-term memory and concentration difficulties, and panic attacks. Over 85% also reported the following: hallucinations, obsessive compulsive disorder (OCD), arthritis, brain fog, headaches, extreme fatigue, and intermittent pain.
Figure 4 shows the survey respondents who reported a diagnosis of Lyme disease. LD respondents experienced similar symptoms to all TBDs, at frequencies of over 90 percent for intermittent pain, anxiety, and extreme fatigue. Arthritis and joint swelling, feeling flu-like, brain fog, depression, and headaches were also reported frequently (~87%). Patients with LD in this study reported numerous neuropsychiatric issues. For Lyme disease respondents, only a few symptoms were reported by under 50% of the respondents, they were as follows: low blood pressure, fever, chest pain, fainting, facial palsy (Bell’s palsy), dysphagia, and seizures. Of note, those with LD also reported experiencing hallucinations, mania, OCD, short-term memory problems, confusion, and tingling in the hands or feet.
In Figure 5, ~90 percent of survey respondents who reported a diagnosis of Rocky Mountain spotted fever experienced intermittent pain, anxiety, headache, brain fog, depression and short-term memory problems. All the other reported symptoms were below 90 percent. The following symptoms were reported with a frequency above 80 percent: headaches; arthritis, with severe joint pain and swelling; brain fog; extreme fatigue; depression; difficulty thinking, concentrating, and/or reading; GI/stomach problems; OCD; and panic/anxiety attacks. Of note, respondents also experienced hallucinations (68%), delusions (70%), neck stiffness, mania, and confusion. Over 40% of the respondents reported dysphagia, or difficulty swallowing. Similar to other TBDs, the following were reported by fewer respondents: facial palsy, fainting, and seizures.
PSS respondents reported a large variety of pain, neurological, and psychiatric symptoms. In addition to their symptoms, they also reported delayed and frustrating diagnostic experiences. Over 80% responded with negative sentiments when asked their opinion about their experiences with medical providers. When asked to detail their experiences in writing, the top negative sentiment score was associated with the following terms used by PSS respondents: doctors, believe, symptoms, listen, diagnose, and understand. These negative sentiments are associated with terms that equate to the burden of diagnosis and frustration with the medical community.
Given the number of available symptoms from which respondents could select, the most frequent were generally extreme fatigue, short-term memory issues, intermittent pain, headaches, depression, panic attacks, GI/stomach problems, and brain fog. Of note, more than half of the respondents reported unusual symptoms, such as hallucinations and delusions. Other frequently reported symptoms included nausea and vomiting, and neck stiffness. Surprisingly, approximately one-quarter of the respondents reported Bell’s palsy, which is often considered a classic sign of LD. Figure 6 details Bell’s palsy by TBD. Although bartonellosis was not included in the overall reporting, it is included below, given the large number of respondents who reported Bell’s palsy as a related symptom. Bartonellosis, LD, and babesiosis were the three most-reported TBDs, with Bell’s palsy at 21–26%. Bartonellosis was not included in the other charts or the results in this study due to the debate regarding tick transmission. The CDC does not include bartonellosis as a TBD in its official guidance to healthcare providers.
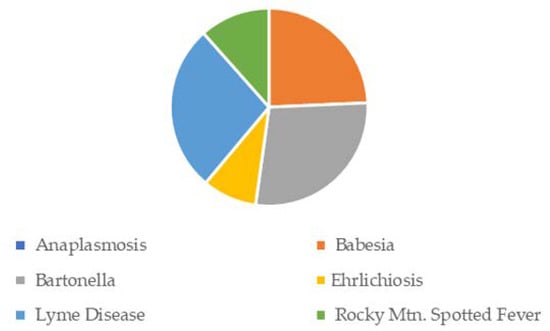
Figure 6.
Facial/Bell’s Palsy Symptom Frequency, by TBD.
4. Discussion
4.1. Case Reports, Medical Literature, and Official Public Health Guidance
Through an extensive literature review, we explored the neuropsychiatric consequences of tick-borne diseases in the United States, using the symptoms and sequelae reported in the medical literature. The search revealed numerous neuropsychiatric case reports and documentation of symptoms not commonly reported by the Centers for Disease Control and Prevention in the reference manual for medical providers. Especially noteworthy is the disconnect between the scholarly literature regarding the psychiatric manifestations of LD and the lack of inclusion by the CDC of psychiatric symptoms, other than “altered mental status” in some TBD’s. The lack of concordance between the scholarly literature and the official guidance may add to the burden of diagnosis for patients presenting with psychiatric symptoms, specifically. These results suggest algorithms for specific TBDs could be developed for appropriate clinical diagnoses. Additionally, the clinical case definition needs to be expanded or broadened to reflect the growing understanding of TBDs, as reflected in the accumulating scientific, peer-reviewed, published literature.
Diagnostic delay for patients with a developing or lingering illness is costly and frustrating for patients and caregivers; burdens healthcare systems and resources when patients continue to seek a diagnosis; and incurs unnecessary costs for insurers. The present study surveyed TBD respondents to provide additional insight on the complex nature of diagnostic struggles, symptoms, and symptom severity found in the medical literature. In light of the number of neurological, pain, and psychiatric symptoms among patients with TBDs, which have been discovered in the scientific and case reports and which have limited overlapping symptom reports from official public health sources for healthcare providers, further study regarding neuropsychiatric symptoms and pain in TBDs is needed.
4.2. TBDs and Disease Progression, and Importance of Early Detection
Lyme and other TBD sequelae can present in all systems of the human body [138]. Early stages of Lyme disease occur between 1 and 30 days and are accompanied by viral-like illness, such as fatigue, fever, chills, myalgia (or joint and muscle pain), and headaches [139]. As the disease progresses to the early-disseminated period (1–4 months), symptoms worsen and arthritis (e.g., monoarticular and oligoarticular), fatigue, vision changes, and other problems develop for a patient. Late-stage disseminated Lyme disease can occur months and years after the initial tick bite and also after initial treatment and can include a host of debilitating multisystem symptoms [140]. Lyme and tick-borne disease patients may also develop encephalomyelitis or peripheral neuropathy [139]. Evidence from recent scholarship supports patient accounts of mental health challenges as a result of a TBD. Neuropsychiatric Lyme disease, caused by the pathogen Lyme borreliosis, presents as follows:
significant comorbidity which may include developmental disorders, autism spectrum disorders, schizoaffective disorders, bipolar disorder, depression, anxiety disorders (panic disorder, social anxiety disorder, generalized anxiety disorder, posttraumatic stress disorder, intrusive symptoms), eating disorders, decreased libido, sleep disorders, addiction, opioid addiction, cognitive impairments, dementia, seizure disorders, suicide, violence, anhedonia, depersonalization, dissociative episodes, derealization and other impairments.[67]
Diagnostic burdens become a further challenge in late-disseminated LD and other TBDs. Maxwell [3] surveyed patients who had been diagnosed with LD and reported that not one was diagnosed by a psychiatrist, even though the vast majority reported psychiatric-related symptoms. Horowitz and Freeman noted the following: “Neuropsychiatric symptoms may result from and/or worsen when Lyme disease and associated coinfections, such as Bartonella spp. and Babesiosis spp., are present” [114].
As Lyme disease and other tick-borne diseases progress, physicians may overlook seemingly unrelated symptoms and sequalae, and may implicate purely psychiatric causes, rather than recognizing that neurologic and psychiatric symptoms are two prominent presenting characteristics of the progressing disease.
4.3. Patient Symptom Survey
The PSS supports the findings in the literature and medical case reports that suggest that neurological, psychiatric, and pain symptoms accompany all TBDs, but that are not acknowledged by official public health sources. Arthritic and neurological-related pain were prominent symptoms and included a wide-ranging list of unusual presentations. Difficulty swallowing was one neurological manifestation that was reported by a large number of patients who self-reported a TBD. Vertigo was also noted in significant numbers among the PSS respondents. However, pain and psychiatric symptoms were dominant presentations across all TBD diagnoses, with a focus on anxiety, depression, and panic attacks. Additional reported symptoms also included symptoms not acknowledged by public health, including delusions, hallucinations, and OCD. Bell’s palsy, often noted as a classic sign of LD, was reported less frequently by the respondents than anticipated, given the official CDC guidance.
5. Conclusions
Medical practitioners in the U.S. are not adequately alerted by official sources to the myriad—and often inconsistent—range of symptoms that may accompany many tick-transmissible illnesses. The literature and survey respondent data unite in offering an encompassing descriptive approach to TBDs that can augment public health knowledge, and the picture that it paints of patients with TBDs points to possible painful and poor quality of life health outcomes. The comprehensive literature review, combined with focused observational data, suggests that patients suffer at much higher rates than indicated in official public health reports.
Left untreated, TBDs can become chronic. Without a change in approach, this picture also conveys a bleak outlook for early and proper diagnosis. Negative patient sentiment related to the PSS respondents’ medical experiences demonstrates frustration with the burden of obtaining a diagnosis. Given inadequate testing, the multitude of non-specific patient symptoms, and the number and range of neuropsychiatric presentations that do not align with public health guidance, this study suggests the need for a revised approach to TBD diagnosis and improved communication from official public health sources regarding the broad symptomology associated with TBDs. Neuropsychiatric symptoms, depending on the context, should alert practitioners that there might be an infectious etiology.
The limitations of this study include possible inaccuracies regarding self-reported symptoms based on patient recall, especially among those who are ill; independent confirmation of patient self-reports were not included in the study. Survey respondents could have other conditions or illnesses that contribute to symptoms. This study was exploratory and designed to prompt further discussions regarding patient symptoms. Future research should include confirmation to rule out other illnesses or possible misrepresentation by the survey respondents. However, comparison of patient reports with an in-depth review of the neuropsychiatric TBD literature suggests that patient self-reports align with case reports and medical studies.
In summary, an expanded or broadened case surveillance definition, including neuropsychiatric and neurologic features—although they may not be present in every patient—would help to alert practitioners to the broader range of tick-borne disease manifestations.
Author Contributions
Conceptualization, S.P.M. and C.L.M.; methodology, S.P.M., C.B. and K.C.T.; formal analysis, S.P.M. and C.B.; investigation, S.P.M. and C.L.M.; resources, S.P.M.; data curation, S.P.M., C.B., C.L.M. and K.C.T.; writing—original draft preparation, S.P.M. and C.B.; writing—review and editing, S.P.M., C.L.M. and K.C.T.; visualization, S.P.M. and C.B.; project administration, S.P.M., C.L.M. and K.C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The patient symptom survey was approved by the Ethics Committee under the Declaration of Helsinki Institutional Review Board Guidelines. Informed consent was obtained from all subjects involved in the study. The authors declare no conflict of interest.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Centers for Disease Control. Post-Treatment Lyme Disease Syndrome. Available online: https://www.cdc.gov/lyme/postlds/index.html (accessed on 21 September 2021).
- Morrissette, M.; Pitt, N.; González, A.; Strandwitz, P.; Caboni, M.; Rebman, A.W.; Knight, R.; D’Onofrio, A.; Aucott, J.N.; Soloski, M.J.; et al. A Distinct Microbiome Signature in Posttreatment Lyme Disease Patients. mBio 2020, 11, e02310-20. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.P. The Elusive Understanding of Lyme Disease in Non-Endemic Geographic Areas: An Exploratory Survey of Patients With Chronic Symptoms in Texas. J. Patient Exp. 2020, 7, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Katon, W.J. Epidemiology and Treatment of Depression in Patients with Chronic Medical Illness. Dialogues Clin. Neurosci. 2011, 13, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Rebman, A.W.; Aucott, J.N.; Weinstein, E.R.; Bechtold, K.T.; Smith, K.C.; Leonard, L. Living in Limbo: Contested Narratives of Patients with Chronic Symptoms Following Lyme Disease. Qual. Health Res. 2017, 27, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Bobe, J.R.; Jutras, B.L.; Horn, E.J.; Embers, M.E.; Bailey, A.; Moritz, R.L.; Zhang, Y.; Soloski, M.J.; Ostfeld, R.S.; Marconi, R.T.; et al. Recent Progress in Lyme Disease and Remaining Challenges. Front. Med. 2021, 8, 1276. [Google Scholar] [CrossRef]
- Bradshaw, M.J.; Bloch, K.C. Tick-Borne Infections of the Central Nervous System. In Neurological Complications of Infectious Disease; Hasbun, R., Bloch, K., Bhimraj, A., Eds.; Springer Publications: New York, NY, USA, 2021. [Google Scholar]
- Jares, T.M.; Mathiason, M.A.; Kowalski, T.J. Functional Outcomes in Patients with Borrelia Burgdorferi Reinfection. Ticks Tick Borne Dis. 2014, 5, 58–62. [Google Scholar] [CrossRef]
- Feder, H.M.; Johnson, B.J.B.; O’Connell, S.; Shapiro, E.D.; Steere, A.C.; Wormser, G.P. A Critical Appraisal of “Chronic Lyme Disease”. N. Engl. J. Med. 2007, 357, 1422–1430. [Google Scholar] [CrossRef]
- Kugeler, K.J.; Schwartz, A.M.; Delorey, M.J.; Mead, P.S.; Hinckley, A.F. Estimating the Frequency of Lyme Disease Diagnoses—United States, 2010–2018. Emerg. Infect. Dis. 2021, 27, 616–619. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X. Ecology, Epidemiology and Global Public Health Burden of Tick-Borne Diseases. In Transmission Dynamics of Tick-Borne Diseases with Co-Feeding, Developmental and Behavioural Diapause; Springer Publishing: New York, NY, USA, 2020. [Google Scholar]
- Talagrand-Reboul, E.; Boyer, P.H.; Bergström, S.; Vial, L.; Boulanger, N. Relapsing Fevers: Neglected Tick-Borne Diseases. Front. Cell. Infect. Microbiol. 2018, 8, 95. [Google Scholar] [CrossRef]
- Fallon, B.A. Neuropsychiatric Lyme Disease: The New ‘Great Imitator’. Psychiatr. Times 2004, 21, 7. [Google Scholar]
- Pavletic, A.J.; Marques, A.R. Early Disseminated Lyme Disease Causing False-Positive Serology for Primary Epstein-Barr Virus Infection: Report of 2 Cases. Clin. Infect. Dis. 2017, 65, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Tumminello, R.; Glaspey, L.; Bhamidipati, A.; Sheehan, P.; Patel, S. Early Disseminated Lyme Disease Masquerading as Mononucleosis: A Case Report. J. Emerg. Med. 2017, 53, e133–e135. [Google Scholar] [CrossRef] [PubMed]
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging Tick-Borne Diseases. Clin. Microbiol. Rev. 2020, 33, e00083-18. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; McBride, J.W. Tick-Borne Emerging Infections. Clin. Lab. Med. 2017, 37, 317–340. [Google Scholar] [CrossRef]
- Blisnick, A.; Šimo, L.; Grillon, C.; Fasani, F.; Brûlé, S.; Le Bonniec, B.; Prina, E.; Marsot, M.; Relmy, A.; Blaise-Boisseau, S.; et al. The Immunomodulatory Effect of IrSPI, a Tick Salivary Gland Serine Protease Inhibitor Involved in Ixodes Ricinus Tick Feeding. Vaccines 2019, 7, 148. [Google Scholar] [CrossRef]
- Dattwyler, R.J.; Volkman, D.J.; Luft, B.J.; Halperin, J.J.; Thomas, J.; Golightly, M.G. Seronegative Lyme Disease. N. Engl. J. Med. 1988, 319, 1441–1446. [Google Scholar] [CrossRef]
- Conant, J.L.; Powers, J.; Sharp, G.; Mead, P.S.; Nelson, C.A. Lyme Disease Testing in a High-Incidence State. Am. J. Clin. Pathol. 2018, 149, 234–240. [Google Scholar] [CrossRef]
- Pritt, B.S.; Respicio-Kingry, L.B.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; Bjork, J.; Liu, G.; Kingry, L.C.; Mead, P.S.; Neitzel, D.F.; et al. Borrelia Mayonii Sp. Nov., a Member of the Borrelia Burgdorferi Sensu Lato Complex, Detected in Patients and Ticks in the Upper Midwestern United States. Int. J. Syst. Evol. Microbiol. 2016, 66, 4878–4880. [Google Scholar] [CrossRef]
- Henry, B.; Crabtree, A.; Roth, D.; Blackman, D.; Morshed, M. Lyme Disease. Can. Fam. Physician 2012, 58, E289–E295. [Google Scholar]
- Nelson, C.; Elmendorf, S.; Mead, P. Neoplasms Misdiagnosed as “Chronic Lyme Disease”. JAMA Intern. Med. 2015, 175, 132. [Google Scholar] [CrossRef]
- Aucott, J.N.; Seifter, A. Misdiagnosis of Early Lyme Disease as the Summer Flu. Orthop. Rev. 2011, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Nigrovic, L.E.; Thompson, A.D.; Fine, A.M.; Kimia, A. Clinical Predictors of Lyme Disease among Children with a Peripheral Facial Palsy at an Emergency Department in a Lyme Disease-Endemic Area. Pediatrics 2008, 122, e1080–e1085. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.G.; Herman, R.J.; Rebman, A.; Moon, K.A.; Aucott, J.; Heaney, C.; Schwartz, B.S. Obstacles to Diagnosis and Treatment of Lyme Disease in the USA: A Qualitative Study. BMJ Open 2018, 8, e021367. [Google Scholar] [CrossRef] [PubMed]
- National Center for Emerging and Zoonotic Infectious Diseases (USA). Division of Vector-Borne Diseases. In Tickborne Diseases of the United States: A Reference Manual for Health Care Providers, 5th ed.; Centers for Disease Control and Prevention, 2018. Available online: https://stacks.cdc.gov/view/cdc/55837 (accessed on 15 September 2021).
- Maxwell, S.P.; McNeely, C.L.; Thomas, K.; Brooks, C. Tick-Borne Surveillance Patterns in Perceived Non-Endemic Geographic Areas: Human Tick Encounters and Disease Outcomes. Healthcare 2021, 9, 771. [Google Scholar] [CrossRef]
- Johnson, L.; Aylward, A.; Stricker, R.B. Healthcare Access and Burden of Care for Patients with Lyme Disease: A Large United States Survey. Health Policy 2011, 102, 64–71. [Google Scholar] [CrossRef]
- Johnson, L.; Wilcox, S.; Mankoff, J.; Stricker, R.B. Severity of Chronic Lyme Disease Compared to Other Chronic Conditions: A Quality of Life Survey. PeerJ 2014, 2, e322. [Google Scholar] [CrossRef]
- Cameron, D.J.; Johnson, L.B.; Maloney, E.L. Evidence Assessments and Guideline Recommendations in Lyme Disease: The Clinical Management of Known Tick Bites, Erythema Migrans Rashes and Persistent Disease. Expert Rev. Anti-Infect. Ther. 2014, 12, 1103–1135. [Google Scholar] [CrossRef]
- Van Hout, M.C. The Controversies, Challenges and Complexities of Lyme Disease: A Narrative Review. J. Pharm. Pharm. Sci. 2018, 21, 429–436. [Google Scholar] [CrossRef]
- Djukic, M.; Larsen, J.; Lingor, P.; Nau, R. Unilateral Phrenic Nerve Lesion in Lyme Neuroborreliosis. BMC Pulm. Med. 2013, 13, 4. [Google Scholar] [CrossRef]
- Logigian, E.L.; Kaplan, R.F.; Steere, A.C. Chronic Neurologic Manifestations of Lyme Disease. N. Engl. J. Med. 1990, 323, 1438–1444. [Google Scholar] [CrossRef]
- Reik, L.; Steere, A.C.; Bartenhagen, N.H.; Shope, R.E.; Malawista, S.E. Neurologic Abnormalities of Lyme Disease. Medicine 1979, 58, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Maloney, E.L. Evidence-Based, Patient-Centered Treatment of Erythema Migrans in the United States. Antibiotics 2021, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.S.; Shapiro, E.D. Lyme Disease. Clin. Lab. Med. 2010, 30, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Garro, A.; Dash, M.; VanBuren, J.M.; Nigrovic, L.E. Development of a Pediatric Lyme Meningitis Symptom Measurement Instrument Using a Delphi Technique. Ticks Tick Borne Dis. 2020, 11, 101418. [Google Scholar] [CrossRef]
- Christen, H.-J.; Hanefeld, F.; Eiffert, H.; Thomssen, R. Epidemiology and Clinical Manifestations of Lyme Borreliosis in Childhood.: A Prospective Multicentre Study with Special Regard to Neuroborreliosis. Acta Paediatrica 1993, 82, 1–76. [Google Scholar] [CrossRef]
- Sharan, K.P.; Krause, P.J.; Sikand, V.; Telford, S.; Baltimore, R.; Persing, D.; Christianson, D.; Brassard, P.; Devarajan, P.; Lepore, T.; et al. Babesiosis and Babesiosis/Lyme Disease Coinfection in Children and Adults† 910. Pediatr. Res. 1998, 43, 157. [Google Scholar] [CrossRef][Green Version]
- Maggi, R.G.; Mozayeni, B.R.; Pultorak, E.L.; Hegarty, B.C.; Bradley, J.M.; Correa, M.; Breitschwerdt, E.B. Bartonella Spp. Bacteremia and Rheumatic Symptoms in Patients from Lyme Disease–Endemic Region. Emerg. Infect. Dis. 2012, 18, 783–791. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B.; Maggi, R.G.; Duncan, A.W.; Nicholson, W.L.; Hegarty, B.C.; Woods, C.W. Bartonella Species in Blood of Immunocompetent Persons with Animal and Arthropod Contact. Emerg. Infect. Dis. 2007, 13, 938–941. [Google Scholar] [CrossRef]
- Chowdri, H.R.; Gugliotta, J.L.; Berardi, V.P.; Goethert, H.K.; Molloy, P.J.; Sterling, S.L.; Telford, S.R. Borrelia Miyamotoi Infection Presenting as Human Granulocytic Anaplasmosis: A Case Report. Ann. Intern. Med. 2013, 159, 21. [Google Scholar] [CrossRef]
- Saito, T.; Walker, D. Ehrlichioses: An Important One Health Opportunity. Vet. Sci. 2016, 3, 20. [Google Scholar] [CrossRef]
- Fishbein, D.B.; Dawson, J.E.; Robinson, L.E. Human Ehrlichiosis in the United States, 1985 to 1990. Ann. Intern. Med. 1994, 120, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.B. Hidden Dangers: Non-Lyme Tick-Borne Diseases. Nursing 2013, 43, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Bakken, J.S.; Dumler, J.S. Human Granulocytic Anaplasmosis. Infect. Dis. Clin. N. Am. 2015, 29, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Raval, M.; Singhal, M.; Guerrero, D.; Alonto, A. Powassan Virus Infection: Case Series and Literature Review from a Single Institution. BMC Res. Notes 2012, 5, 594. [Google Scholar] [CrossRef] [PubMed]
- Geier, C.; Davis, J.; Siegel, M. Severe Human Monocytic Ehrlichiosis Presenting with Altered Mental Status and Seizures. BMJ Case Rep. 2016, 2016, bcr2016215967. [Google Scholar] [CrossRef]
- McCall, C.L.; Curns, A.T.; Rotz, L.D.; Singleton, J.A.; Treadwell, T.A.; Comer, J.A.; Nicholson, W.L.; Olson, J.G.; Childs, J.E. Fort Chaffee Revisited: The Epidemiology of Tick-Borne Rickettsial and Ehrlichial Diseases at a Natural Focus. Vector Borne Zoonotic Dis. 2001, 1, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Chabria, S.B.; Lawrason, J. Altered Mental Status, an Unusual Manifestation of Early Disseminated Lyme Disease: A Case Report. J. Med. Case Rep. 2007, 1, 62. [Google Scholar] [CrossRef]
- Fallon, B.A.; Kochevar, J.M.; Gaito, A.; Nields, J.A. The Underdiagnosis of Neuropsychiatric Lyme disease in Children and Adults. Clin. N. Am. 1998, 21, 693–703. [Google Scholar] [CrossRef]
- Ratnasamy, N.; Everett, E.D.; Roland, W.E.; McDonald, G.; Caldwell, C.W. Central Nervous System Manifestations of Human Ehrlichiosis. Clin. Infect. Dis. 1996, 23, 314–319. [Google Scholar] [CrossRef]
- Hamburg, B.J.; Storch, G.A.; Micek, S.T.; Kollef, M.H. The Importance of Early Treatment with Doxycycline in Human Ehrlichiosis. Medicine 2008, 87, 53–60. [Google Scholar] [CrossRef]
- Khan, A.M.; Shahzad, S.R.; Ashraf, M.F.; Naseer, U. Powassan Virus Encephalitis, Severe Babesiosis and Lyme Carditis in a Single Patient. BMJ Case Rep. 2019, 12, e231645. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sharma, A.; Grover, P. Triple Tick Attack. Cureus 2019, 11, e4064. [Google Scholar] [CrossRef] [PubMed]
- Dotevall, L.; Eliasson, T.; Hagberg, L.; Mannheimer, C. Pain as Presenting Symptom in Lyme Neuroborreliosis. Eur. J. Pain 2003, 7, 235–239. [Google Scholar] [CrossRef]
- Kohler, J.; Thoden, U. Schmerzsyndrome bei zeckenübertragenen Borrelieninfektionen des Nervensystems. Schmerz 1987, 1, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Zimering, J.H.; Williams, M.R.; Eiras, M.E.; Fallon, B.A.; Logigian, E.L.; Dworkin, R.H. Acute and Chronic Pain Associated with Lyme Borreliosis: Clinical Characteristics and Pathophysiologic Mechanisms. Pain 2014, 155, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, D.T.; Callister, S.M.; Schell, R.F. Lyme Arthritis: Current Concepts and a Change in Paradigm. Clin. Vaccine Immunol. 2008, 15, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, N.; Boyer, P.; Talagrand-Reboul, E.; Hansmann, Y. Ticks and Tick-Borne Diseases. Médecine Mal. Infect. 2019, 49, 87–97. [Google Scholar] [CrossRef]
- Mac, S.; Bahia, S.; Simbulan, F.; Pullenayegum, E.M.; Evans, G.; Patel, S.N.; Sander, B. Long-Term Sequelae and Health-Related Quality of Life Associated With Lyme Disease: A Systematic Review. Clin. Infect. Dis. 2020, 71, 440–452. [Google Scholar] [CrossRef]
- Fretz, E.A.; Miller, A.; King, M.A. Case 2: Fever and Myalgia in a 16-Year-Old Boy. Pediatr. Rev. 2020, 41, 140–144. [Google Scholar] [CrossRef]
- Bratton, R.L.; Corey, R. Tick-Borne Disease. Am. Fam. Phys. 2005, 71, 2323–2330. [Google Scholar]
- Bransfield, R.C. The Psychoimmunology of Lyme and Associated Diseases. Neurol. Psychiatry Brain Res. 2014, 20, 8. [Google Scholar] [CrossRef]
- Brogan, G.X.; Homan, C.S.; Vicellio, P. The Enlarging Clinical Spectrum of Lyme Disease: Lyme Cerebral Vasculitis, a New Disease Entity. Ann. Emerg. Med. 1990, 19, 572–576. [Google Scholar] [CrossRef]
- Bransfield, R. Neuropsychiatric Lyme Borreliosis: An Overview with a Focus on a Specialty Psychiatrist’s Clinical Practice. Healthcare 2018, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Schober, H.; Simma, B.; Lütschg, J.; Blassnig-Ezeh, A. Central Nervous System Lyme Disease—Presentation of Two Cases. Neuropediatrics 2012, 43, PS17_04. [Google Scholar] [CrossRef]
- Kugeler, K.J.; Griffith, K.S.; Gould, L.H.; Kochanek, K.; DeLorey, M.J.; Biggerstaff, B.J.; Mead, P.S. A Review of Death Certificates Listing Lyme Disease as a Cause of Death in the United States. Clin. Infect. Dis. 2011, 52, 364–367. [Google Scholar] [CrossRef]
- Jozefowicz-Korczynska, M.; Zamyslowska-Szmytke, E.; Piekarska, A.; Rosiak, O. Vertigo and Severe Balance Instability as Symptoms of Lyme Disease—Literature Review and Case Report. Front. Neurol. 2019, 10, 1172. [Google Scholar] [CrossRef]
- Sowula, K.; Szaleniec, J.; Dworak, M.; Przeklasa, M.; Maraj, M.; Ceranowicz, P.; Tomik, J. Vertigo as One of the Symptoms of Lyme Disease. JCM 2021, 10, 2814. [Google Scholar] [CrossRef]
- Moscatello, A.L.; Worden, D.L.; Nadelman, R.B.; Wormser, G.; Lucente, F. Otolaryngologic Aspects of Lyme Disease. Laryngoscope 1991, 101, 592–595. [Google Scholar] [CrossRef]
- Rosenhall, U.; Hanner, P.; Kaijser, B. Borrelia Infection and Vertigo. Acta Oto-Laryngol. 1988, 106, 111–116. [Google Scholar] [CrossRef]
- Bush, L.M.; Vazquez-Pertejo, M.T. Tick Borne Illness—Lyme Disease. Dis. Mon. 2018, 64, 195–212. [Google Scholar] [CrossRef]
- Oleson, C.V.; Sivalingam, J.; O’Neill, B.; Staas, W. Transverse Myelitis Secondary To Coexistent Lyme Disease And Babesiosis. J. Spinal Cord Med. 2003, 26, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Zeller, J.L.; Burke, A.E.; Glass, R.M. Lyme Disease. JAMA 2007, 297, 2664. [Google Scholar] [CrossRef] [PubMed][Green Version]
- University of Chicago, Center for Peripheral Neuropathy. Types of Peripheral Neuropathy—Inflammatory. Available online: http://peripheralneuropathycenter.uchicago.edu/learnaboutpn/typesofpn/inflammatory/lymedisease.shtml (accessed on 30 October 2021).
- Gazendam, N.; Yeung, C.; Farina, J.M.; Saldarriaga, C.; Mendoza, I.; Baranchuk, A.; Heart, L. Neglected Tropical Diseases and Other Infectious Diseases Affecting the Heart; Elsevier: Amsterdam, The Netherlands, 2022; pp. 61–71. ISBN 978-0-323-91122-1. [Google Scholar]
- Patel, K.; Shah, S.; Subedi, D. Clinical Association: Lyme Disease and Guillain-Barre Syndrome. Am. J. Emerg. Med. 2017, 35, 1583-e1. [Google Scholar] [CrossRef] [PubMed]
- Haglund, M.; Günther, G. Tick-Borne Encephalitis—Pathogenesis, Clinical Course and Long-Term Follow-up. Vaccine 2003, 21, S11–S18. [Google Scholar] [CrossRef]
- College of Family Physicians of Canada. New-Onset Bell Palsy and Lyme Disease. Can. Fam. Physician 2017, 63, 941. [Google Scholar]
- Malik, R.; Farrow, B.R.H. Tick Paralysis in North America and Australia. Vet. Clin. N. Am. Small Anim. Pract. 1991, 21, 157–171. [Google Scholar] [CrossRef]
- De Cauwer, H.; Declerck, S.; De Smet, J.; Matthyssen, P.; Pelzers, E.; Eykens, L.; Lagrou, K. Motor Neuron Disease Features In a Patient with Neuroborreliosis and a Cervical Anterior Horn LesION. Acta Clin. Belg. 2009, 64, 225–227. [Google Scholar] [CrossRef]
- Bierman, S.M.; van Kooten, B.; Vermeeren, Y.M.; Bruintjes, T.D.; van Hees, B.C.; Bruinsma, R.A.; Landman, G.W.; van Bemmel, T.; Zomer, T.P. Incidence and Characteristics of Lyme Neuroborreliosis in Adult Patients with Facial Palsy in an Endemic Area in the Netherlands. Epidemiol. Infect. 2019, 147, e160. [Google Scholar] [CrossRef]
- Kim, M.; Kim, W.C.; Park, D.-S. Neurogenic Bladder in Lyme Disease. Int. Neurourol. J. 2012, 16, 201. [Google Scholar] [CrossRef]
- Grant, A.C.; Hunter, S.; Partin, W.C. A Case of Acute Monocytic Ehrlichiosis with Prominent Neurologic Signs. Neurology 1997, 48, 1619–1623. [Google Scholar] [CrossRef]
- Mir, M.A.; Grant, J. Dysarthria and Thrombocytopenia after Tick Bite. Blood 2003, 122, 2538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uminski, K.; Kadkhoda, K.; Houston, B.L.; Lopez, A.; MacKenzie, L.J.; Lindsay, R.; Walkty, A.; Embil, J.; Zarychanski, R. Anaplasmosis: An Emerging Tick-Borne Disease of Importance in Canada. IDCases 2018, 14, e00472. [Google Scholar] [CrossRef] [PubMed]
- Kemenesi, G.; Bányai, K. Tick-Borne Flaviviruses, with a Focus on Powassan Virus. Clin. Microbiol. Rev. 2019, 32, E00106–E00117. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.L.; Banda, B.K.; Burnsides, C.L.; Stuber, A.J. Zoonosis: Update on Existing and Emerging Vector-Borne Illnesses in the USA. Curr. Emerg. Hosp. Med. Rep. 2019, 7, 91–106. [Google Scholar] [CrossRef]
- Rossier, E.; Harrison, R.J.; Lemieux, B. A Case of Powassan Virus Encephalitis. Can. Med. Assoc. J. 1974, 110, 1173–1174. [Google Scholar]
- El Khoury, M.Y.; Camargo, J.F.; White, J.L.; Backenson, B.P.; Dupuis, A.P.; Escuyer, K.L.; Kramer, L.; St. George, K.; Chatterjee, D.; Prusinski, M.; et al. Potential Role of Deer Tick Virus in Powassan Encephalitis Cases in Lyme Disease–Endemic Areas of New York, USA. Emerg. Infect. Dis. 2013, 19, 1926–1933. [Google Scholar] [CrossRef]
- Piantadosi, A.; Rubin, D.B.; McQuillen, D.P.; Hsu, L.; Lederer, P.A.; Ashbaugh, C.D.; Duffalo, C.; Duncan, R.; Thon, J.; Bhattacharyya, S.; et al. Emerging Cases of Powassan Virus Encephalitis in New England: Clinical Presentation, Imaging, and Review of the Literature. Clin. Infect. Dis. 2016, 62, 707–713. [Google Scholar] [CrossRef]
- Gustaw, K.; Mirecka, U. Dysarthria as the Isolated Clinical Symptom of Borreliosis—A Case Report. Ann. Agric. Environ. Med. 2001, 8, 95–97. [Google Scholar]
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P. Reorganization of Genera in the Families Rickettsiaceae and Anaplasmataceae in the Order Rickettsiales: Unification of Some Species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, Descriptions of Six New Species Combinations and Designation of Ehrlichia Equi and ‘HGE Agent’ as Subjective Synonyms of Ehrlichia Phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar]
- Sergeev, A.; Skoromets, A.; Sergeeva, A.; Barantsevich, E.; Matyushkin, N. Rare Manifestations of Lyme Neuroborreliosis. J. Neurol. Sci. 2019, 405, 122. [Google Scholar] [CrossRef]
- Mantovani, E.; Costa, I.P.; Gauditano, G.; Bonoldi, V.L.N.; Higuchi, M.L.; Yoshinari, N.H. Description of Lyme Disease-like Syndrome in Brazil: Is It a New Tick Borne Disease or Lyme Disease Variation? Braz. J. Med. Biol. Res. 2007, 40, 443–456. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dysarthria. Available online: https://www.mayoclinic.org/diseases-conditions/dysarthria/symptoms-causes/syc-20371994 (accessed on 10 October 2021).
- National Organization for Rare Diseases (NORD). Human Monocytic Ehrlichiosis (HME). Available online: https://rarediseases.org/rare-diseases/human-monocytic-ehrlichiosis-hme/ (accessed on 11 June 2021).
- Cheng, D.; Yakobi-Shvlli, R.; Fernandez, J. Life-Threatening Hypotension from Babesiosis Hemolysis. Am. J. Emerg. Med. 2002, 20, 367. [Google Scholar] [CrossRef] [PubMed]
- Sherr, V.T. Human Babesiosis—An Unrecorded Reality: Absence of Formal Registry Undermines Its Detection, Diagnosis and Treatment, Suggesting Need for Immediate Mandatory Reporting. Med. Hypotheses 2004, 63, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Dumler, J.S. Anaplasma and Ehrlichia Infection. Ann. N. Y. Acad. Sci. 2005, 1063, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human Ehrlichiosis and Anaplasmosis. Clin. Lab. Med. 2010, 30, 261–292. [Google Scholar] [CrossRef]
- Chapman, A.S.; Bakken, J.S.; Folk, S.M.; Paddock, C.D.; Bloch, K.C.; Krusell, A.; Sexton, D.J.; Buckingham, S.C.; Marshall, G.S.; A Storch, G.; et al. Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever, Ehrlichioses, and Anaplasmosis—United States: A Practical Guide for Physicians and Other Health-Care and Public Health Professionals. MMWR Recomm. Rep. 2006, 55, 1–27. [Google Scholar]
- Muhammad, S.; Simonelli, R.J. Lyme Carditis: A Case Report and Review of Management. Hosp. Pharm. 2018, 53, 263–265. [Google Scholar] [CrossRef]
- Kostić, T.; Momčilović, S.; Perišić, Z.D.; Apostolović, S.R.; Cvetković, J.; Jovanović, A.; Barać, A.; Šalinger-Martinović, S.; Tasić-Otašević, S. Manifestations of Lyme Carditis. Int. J. Cardiol. 2017, 232, 24–32. [Google Scholar] [CrossRef]
- Manek, M.; Kulkarni, A.; Viera, A. Hint of Lyme, an Uncommon Cause of Syncope. Case Rep. 2014, 2014, bcr2013201547. [Google Scholar] [CrossRef]
- Reese, Z.; Maru, P. Unexplained Recurrent Fevers and the Importance of Inquiring About Occupation: A Case Report. TMF 2016, 17, 11. [Google Scholar] [CrossRef][Green Version]
- Kanjwal, K.; Karabin, B.; Kanjwal, Y.; Grubb, B.P. Postural Orthostatic Tachycardia Syndrome Following Lyme Disease. Cardiol. J. 2011, 18, 63–66. [Google Scholar] [PubMed]
- Vasiljević, Z.; Dmitrović, R.; Naumović, Z.; Ostojić, M.; Radosavljević, M.; Karadzić, A.; Prostran, M.; Colić, M. Common Form of Lyme Borreliosis Carditis—Complete Heart Block with Syncope: Report on 3 Cases. Cardiology 1996, 87, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Hájek, T.; Pasková, B.; Janovská, D.; Bahbouh, R.; Hájek, P.; Libiger, J.; Höschl, C. Higher prevalence of antibodies to Borrelia burgdorferi in psychiatric patients than in healthy subjects. Am. J. Psychiatry 2002, 159, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Fallon, B.A.; Madsen, T.; Erlangsen, A.; Benros, M.E. Lyme Borreliosis and Associations with Mental Disorders and Suicidal Behavior: A Nationwide Danish Cohort Study. Am. J. Psychiatry 2021, 178, 921–931. [Google Scholar] [CrossRef]
- Rebman, A.W.; Bechtold, K.T.; Yang, T.; Mihm, E.A.; Soloski, M.J.; Novak, C.B.; Aucott, J.N. The Clinical, Symptom, and Quality-of-Life Characterization of a Well-Defined Group of Patients with Posttreatment Lyme Disease Syndrome. Front. Med. 2017, 4, 224. [Google Scholar] [CrossRef]
- Horowitz, R.I.; Freeman, P.R. Precision Medicine: The Role of the MSIDS Model in Defining, Diagnosing, and Treating Chronic Lyme Disease/Post Treatment Lyme Disease Syndrome and Other Chronic Illness: Part 2. Healthcare 2018, 6, 129. [Google Scholar] [CrossRef]
- Fallon, B.A.; Neilds, J. Lyme Disease: A Neuropsychiatric Illness. Am. J. Psychiatry 1994, 151, 1571–1583. [Google Scholar] [CrossRef]
- Garakani, A.; Mitton, A.G. New-Onset Panic, Depression with Suicidal Thoughts, and Somatic Symptoms in a Patient with a History of Lyme Disease. Case Rep. Psychiatry 2015, 2015, 457947. [Google Scholar] [CrossRef]
- Tickborne Diseases in Michigan: A Reference for Health Care Providers. Available online: https://www.michigan.gov/documents/emergingdiseases/tick_borne_disease_table_for_clinicians_578830_7.pdf (accessed on 29 December 2021).
- Keilp, J.G.; Corbera, K.; Gorlyn, M.; Oquendo, M.A.; Mann, J.J.; Fallon, B.A. Neurocognition in Post-Treatment Lyme Disease and Major Depressive Disorder. Arch. Clin. Neuropsychol. 2019, 34, 466–480. [Google Scholar] [CrossRef]
- Bransfield, R.C.; Wulfman, J.S.; Harvey, W.T.; Usman, A.I. The Association between Tick-Borne Infections, Lyme Borreliosis and Autism Spectrum Disorders. Med. Hypotheses 2008, 70, 967–974. [Google Scholar] [CrossRef]
- Mayne, P. Clinical Determinants of Lyme Borreliosis, Babesiosis, Bartonellosis, Anaplasmosis, and Ehrlichiosis in an Australian Cohort. Int. J. Gen. Med. 2014, 8, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Bransfield, R. Suicide and Lyme and Associated Diseases. Neuropsychiatr. Dis. Treat. 2017, 13, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- Bacon, R.M.; Kugeler, K.J.; Mead, P.S. Surveillance for Lyme Disease—United States, 1992–2006. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2008, 57, SS-10. [Google Scholar]
- Moon, K.A.; Pollak, J.; Hirsch, A.G.; Aucott, J.N.; Nordberg, C.; Heaney, C.D.; Schwartz, B.S. Epidemiology of Lyme Disease in Pennsylvania 2006–2014 Using Electronic Health Records. Ticks Tick Borne Dis. 2019, 10, 241–250. [Google Scholar] [CrossRef]
- Shapiro, E.D.; Gerber, M.A. Lyme Disease. Clin. Infect. Dis. 2000, 31, 533–542. [Google Scholar] [CrossRef]
- Treib, J.; Grauer, M.T.; Haass, A.; Langenbach, J.; Holzer, G.; Woessner, R. Chronic Fatigue Syndrome in Patients with Lyme Borreliosis. Eur. Neurol. 2000, 43, 107–109. [Google Scholar] [CrossRef]
- Weinstein, R.S. Human Ehrlichiosis. Am. Fam. Physician 1996, 54, 1971–1976. [Google Scholar]
- Wallace, B.J.; Brady, G.; Ackman, D.M.; Wong, S.J.; Jacquette, G.; Lloyd, E.E.; Birkhead, G.S. Human Granulocytic Ehrlichiosis in New York. Arch. Intern. Med. 1998, 158, 769. [Google Scholar] [CrossRef]
- Bhalla, V.; Rodgers, B.; Lin, J. Sudden Sensorineural Hearing Loss in Human Monocytic Ehrlichiosis. Ear. Nose Throat. J. 2017, 96, 328–342. [Google Scholar] [CrossRef]
- Bakken, J.S. Clinical and Laboratory Characteristics of Human Granulocytic Ehrlichiosis. JAMA 1996, 275, 199. [Google Scholar] [CrossRef]
- Russell, A.; Prusinski, M.; Sommer, J.; O’Connor, C.; White, J.; Falco, R.; Kokas, J.; Vinci, V.; Gall, W.; Tober, K.; et al. Epidemiology and Spatial Emergence of Anaplasmosis, New York, USA, 2010–2018. Emerg. Infect. Dis. 2021, 27, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.M.; Kumar, S.; Krause, P.J. Persistence of Babesia Microti Infection in Humans. Pathogens 2019, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Fallon, B.A.; Schwartzberg, M.; Bransfield, R.; Zimmerman, B.; Scotti, A.; Weber, C.A.; Liebowitz, M.R. Late-Stage Neuropsychiatric Lyme Borreliosis. Psychosomatics 1995, 36, 295–300. [Google Scholar] [CrossRef]
- Van Den Bergen, H.A.; Smith, J.P.; van Der Zwan, A. Lyme-Psychose [Lyme Psychosis]. Ned. Tijdschr. Geneeskd. 1993, 137, 2098–2100. [Google Scholar]
- Binalsheikh, I.M.; Griesemer, D.; Wang, S.; Alvarez-Altalef, R. Lyme Neuroborreliosis Presenting as Alice in Wonderland Syndrome. Pediatr. Neurol. 2012, 46, 185–186. [Google Scholar] [CrossRef]
- Stricker, R.B.; Winger, E.E. Musical Hallucinations in Patients with Lyme Disease. South. Med. J. 2003, 96, 711–715. [Google Scholar] [CrossRef]
- Barnett, W.; Sigmund, D.; Roelcke, U.; Mundt, C. Endomorphes Paranoid-Halluzinatorisches Syndrom Durch Borrelienenzephalitis [Endogenous Paranoid-Hallucinatory Syndrome Caused by Borrelia Encephalitis]. Nervenarzt 1991, 62, 445–447. [Google Scholar]
- Sherr, V.T. Panic Attacks May Reveal Previously Unsuspected Chronic Disseminated Lyme Disease. J. Psychiatr. Pract. 2000, 6, 352–356. [Google Scholar] [CrossRef]
- Moniuszko, A.; Czupryna, P.; Zajkowska, J.; Pancewicz, S.A.; Grygorczuk, S.; Kondrusik, M. Zespół Post Lyme Jako Problem Kliniczny [Post Lyme Syndrome as a Clinical Problem]. Pol. Merkur. Lekarski. 2009, 26, 227–230. [Google Scholar]
- Wright, W.F.; Riedel, D.J.; Talwani, R.; Gilliam, B.L. Diagnosis and Management of Lyme Disease. Am. Fam. Physician 2012, 85, 1086–1093. [Google Scholar]
- Global Lyme Alliance. Available online: https://www.globallymealliance.org/about-lyme/diagnosis/stages/ (accessed on 11 June 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).