Biofortification of Pulse Crops: Status and Future Perspectives
Abstract
1. Introduction
2. Key Micronutrients
2.1. Iron
2.2. Zinc
2.3. Selenium
2.4. Iodine
2.5. Carotenoids
2.6. Folates
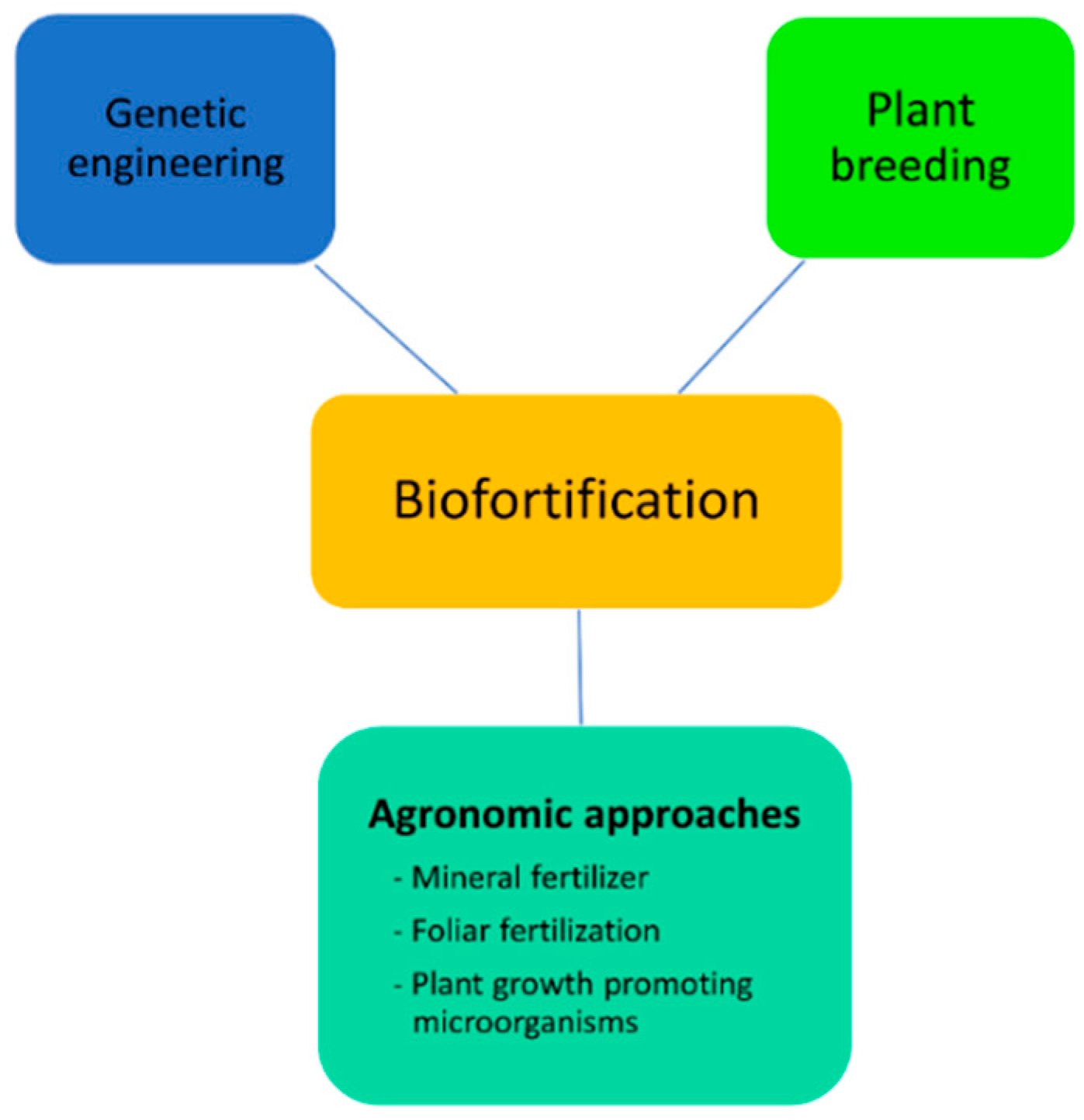
3. Approaches for Improvement of Nutritional Profile
3.1. Dietary Diversification
3.2. Food Supplements
3.3. Food Fortification
3.4. Biofortification
3.4.1. Agronomic Approaches
Mineral Fertilizer
Foliar Fertilization
Plant Growth Promoting Microorganisms
3.4.2. Genetic Engineering
3.4.3. Plant Breeding
4. Recent Research Advances for Biofortification of Pulse Crops
4.1. Iron
4.2. Zinc
4.3. Selenium
4.4. Iodine
4.5. Carotenoids
4.6. Folates
5. Status of Biofortification
6. Challenges and Future Strategies for Biofortification
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waters, B.M.; Grusak, M.A. Quantitative trait locus mapping for seed mineral concentrations in two Arabidopsis thaliana recombinant inbred populations. New Phytol. 2008, 179, 1033–1047. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of Food and Agriculture; Food and Agriculture Organization: Rome, Italy, 2013. [Google Scholar]
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66, 2233. [Google Scholar] [CrossRef] [PubMed]
- Tulchinsky, T.H. Micronutrient deficiency conditions: Global health issues. Public Health Rev. 2010, 32, 243. [Google Scholar] [CrossRef]
- Darnton-Hill, I.; Bloem, M.; Chopra, M. Achieving the millennium development goals through mainstreaming nutrition: Speaking with one voice. Public Health Nutr. 2006, 9, 537–539. [Google Scholar] [CrossRef]
- Ahmed, T.; Hossain, M.; Sanin, K.I. Global burden of maternal and child undernutrition and micronutrient deficiencies. Ann. Nutr. Metab. 2012, 61, 8–17. [Google Scholar] [CrossRef]
- Welch, R.M. Breeding strategies for biofortified staple plant foods to reduce micronutrient malnutrition globally. J. Nutr. 2002, 132, 495S–499S. [Google Scholar] [CrossRef]
- Duranti, M. Grain legume proteins and nutraceutical properties. Fitoterapia 2006, 77, 67–82. [Google Scholar] [CrossRef]
- Patterson, C.A.; Maskus, H.; Dupasquier, C. Pulse crops for health. Cereals Food World 2009, 54, 108–113. [Google Scholar] [CrossRef]
- Roy, F.; Boye, J.I.; Simpson, B.K. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Res. Int. 2010, 43, 432–442. [Google Scholar] [CrossRef]
- Ray, H.; Bett, K.E.; Tar’an, B.; Vandenberg, A.; Thavarajah, D.; Warkentin, T. Mineral micronutrient content of cultivars of field pea, chickpea, common bean, and lentil grown in Saskatchewan, Canada. Crop Sci. 2014, 54, 1698–1708. [Google Scholar] [CrossRef]
- Diapari, M.; Sindhu, A.; Bett, K.; Deokar, A.; Warkentin, T.D.; Tar’an, B. Genetic diversity and association mapping of iron and zinc concentrations in chickpea (Cicer arietinum L.). Genome 2014, 57, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Diapari, M.; Sindhu, A.; Warkentin, T.D.; Bett, K.; Tar’an, B. Population structure and marker-trait association studies of iron, zinc and selenium concentrations in seed of field pea (Pisum sativum L.). Mol. Breed. 2015, 35, 30. [Google Scholar] [CrossRef]
- Jha, A.B.; Tar’an, B.; Diapari, M.; Warkentin, T.D. SNP variation within genes associated with amylose, total starch and crude protein concentration in field pea. Euphytica 2015, 206, 459–471. [Google Scholar] [CrossRef]
- Jha, A.B.; Ashokkumar, K.; Diapari, M.; Ambrose, S.J.; Zhang, H.; Tar’an, B.; Bett, K.E.; Vandenberg, A.; Warkentin, T.D.; Purves, R.W. Genetic diversity of folate profiles in seeds of common bean, lentil, chickpea and pea. J. Food Compos. Anal. 2015, 42, 134–140. [Google Scholar] [CrossRef]
- Messina, M.J. Legumes and soybeans: Overview of their nutritional profiles and health effects. Am. J. Clin. Nutr. 1999, 70, 439S–450S. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Hasim Reja, M.; Nalia, A.; Kanthal, S.; Maji, S.; Venugopalan, V.; Nath, R. Micronutrient biofortification in pulses: An agricultural approach. CJAST 2019, 35, 1–12. [Google Scholar] [CrossRef]
- Curran, J. The nutritional value and health benefits of pulses in relation to obesity, diabetes, heart disease and cancer. Br. J. Nutr. 2012, 108, 1–2. [Google Scholar] [CrossRef]
- Bouis, H.E. Plant breeding: A new tool for fighting micronutrient malnutrition. J. Nutr. 2002, 132, 491S–494S. [Google Scholar] [CrossRef]
- Nestel, P.; Bouis, H.E.; Meenakshi, J.V.; Pfeiffer, W. Biofortification of staple food crops. J. Nutr. 2006, 136, 1064–1067. [Google Scholar] [CrossRef]
- Bouis, H.E.; Hotz, C.; McClafferty, B.; Meenakshi, J.V.; Pfeiffer, W.H. Biofortification: A new tool to reduce micronutrient malnutrition. Food Nutr. Bull. 2011, 32, S31–S40. [Google Scholar] [CrossRef]
- Saltzman, A.; Birol, E.; Bouis, H.E.; Boy, E.; De Moura, F.F.; Islam, Y.; Pfeiffer, W.H. Biofortification: Progress toward a more nourishing future. Glob. Food Secur. 2013, 2, 9–17. [Google Scholar] [CrossRef]
- Upadhyaya, H.D.; Bajaj, D.; Das, S.; Kumar, V.; Gowda, C.L.L.; Sharma, S.; Tyagi, A.K.; Swarup, K.P. Genetic dissection of seed-iron and zinc concentrations in chickpea. Sci. Rep. 2016, 6, 24050. [Google Scholar] [CrossRef] [PubMed]
- Aldemir, S.; Ateş, D.; Temel, H.Y.; Yağmur, B.; Alsaleh, A.; Kahriman, A.; Özkan, H.; Vandenberg, A.; Tanyolaç, M.B. QTLs for iron concentration in seeds of the cultivated lentil (Lens culinaris Medic.) via genotyping by sequencing. Turk. J. Agric. For. 2017, 41, 243–255. [Google Scholar] [CrossRef]
- Khazaei, H.; Podder, R.; Caron, C.T.; Kundu, S.S.; Diapari, M.; Vandenberg, A.; Bett, K.E. Marker-trait association analysis of iron and zinc concentration in lentil (Lens culinaris Medik.) seeds. Plant Genome 2017, 10, 2. [Google Scholar] [CrossRef]
- Ma, Y.; Coyne, C.J.; Grusak, M.A.; Mazourek, M.; Cheng, P.; Main, D.; McGee, R.J. Genome-wide SNP identification, linkage map construction and QTL mapping for seed mineral concentrations and contents in pea (Pisum sativum L.). BMC Plant Biol. 2017, 17, 43. [Google Scholar] [CrossRef]
- Gali, K.K.; Liu, Y.; Sindhu, A.; Diapari, M.; Shunmugam, A.S.K.; Arganosa, G.; Daba, K.; Caron, C.; Lachagari, R.V.B.; Tar’an, B.; et al. Construction of high-density linkage maps for mapping quantitative trait loci for multiple traits in field pea (Pisum sativum L.). BMC Plant Biol. 2018, 18, 172. [Google Scholar] [CrossRef]
- Vandemark, G.J.; Grusak, M.A.; McGee, R.J. Mineral concentrations of chickpea and lentil cultivars and breeding lines grown in the US Pacific Northwest. Crop J. 2018, 6, 253–262. [Google Scholar] [CrossRef]
- Dissanayaka, D. Genome Wide Association Study to Identify Single Nucleotide Polymorphism Markers for Fe, Zn, and Se Concentration in Field Pea Seeds. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, March 2019. [Google Scholar]
- Jha, A.B.; Gali, K.K.; Zhang, H.; Purves, R.W.; Vandenberg, A.; Warkentin, T.D. Folate profile diversity and associated SNPs using genome wide association study in pea. Euphytica 2020. accepted. [Google Scholar]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Hurrell, R.F. Bioavailability of iron. Eur. J. Clin. Nutr. 1997, 51, S4–S8. [Google Scholar]
- McDowell, L.R. Minerals in Animal and Human Nutrition, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2003; p. 660. [Google Scholar]
- World Health Organization. Iron Deficiency Anaemia: Assessment, Prevention and Control, a Guide for Programme Managers; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- World Health Organization. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia; de Benoist, B., McLean, E., Egli, I., Cogswell, M., Eds.; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Allen, L.H. Anemia and iron deficiency: Effects on pregnancy outcome. Am. J. Clin. Nutr. 2000, 71, 1280S–1284S. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Clark, K.M.; Jing, Y.; Armony-Sivan, R.; Angelilli, M.L.; Jacobson, S.W. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J. Pediatr. 2008, 152, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc deficiency in women, infants, and children. J. Am. Coll. Nutr. 1996, 15, 113–120. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [CrossRef]
- Rostan, E.F.; DeBuys, H.V.; Madey, D.L.; Pinnell, S.R. Evidence supporting zinc as an important antioxidant for skin. Int. J. Dermatol. 2002, 41, 606–611. [Google Scholar] [CrossRef]
- Prasad, A.S.; Bao, B.; Beck, F.W.; Kucuk, O.; Sarkar, F.H. Antioxidant effect of zinc in humans. Free Radic. Biol. Med. 2004, 37, 1182–1190. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. Decreased zinc in the development and progression of malignancy: An important common relationship and potential for prevention and treatment of carcinomas. Expert Opin. Ther. Targets 2017, 21, 51–66. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Jansson, B. The role of selenium as a cancer-protecting trace element. Met. Ions Biol. Syst. 1980, 10, 281–311. [Google Scholar]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Combs, G.F. Selenium as an anticancer nutrient: Roles in cell proliferation and tumor cell invasion. J. Nutr. Biochem. 2008, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Coppinger, R.J.; Diamond, A.M. Selenium deficiency and human disease. In Selenium; Hatfield, D.L., Ed.; Springer: Boston, MA, USA, 2001; pp. 219–233. [Google Scholar]
- Delange, F. The disorders induced by iodine deficiency. Thyroid 1994, 4, 107–128. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Iodine Deficiency in Europe: A Continuing Public Health Problem; Andersson, M., de Benoist, B., Darnton-Hill, I., Eds.; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- World Health Organization; United Nations Children’s Fund; International Council for the Control of Iodine Deficiency Disorders. Assessment of iodine deficiency disorders and monitoring their elimination. In A Guide for Programme Managers, 3rd ed.; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Skeaff, S.A. Iodine deficiency in pregnancy: The effect on neurodevelopment in the child. Nutrients 2011, 3, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N.; Lazarus, J.H.; Moreno-Reyes, R.; Zimmermann, M.B. Consequences of iodine deficiency and excess in pregnant women: An overview of current knowns and unknowns. Am. J. Clin. Nutr. 2016, 104, 918S–923S. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N.; Andersson, M.; Zimmermann, M.B. Global iodine nutrition: Where do we stand in 2013? Thyroid 2013, 23, 523–528. [Google Scholar] [CrossRef]
- Cakmak, I.; Prom-u-thai, C.; Guilherme, L.R.G.; Rashid, A.; Hora, K.; Yazici, A.; Savasli, E.; Kalayci, M.; Tutus, Y.; Phuphong, P.; et al. Iodine biofortification of wheat, rice and maize through fertilizer strategy. Plant Soil 2017, 418, 319–335. [Google Scholar] [CrossRef]
- Gonzali, S.; Kiferle, C.; Perata, P. Iodine biofortification of crops: Agronomic biofortification, metabolic engineering and iodine bioavailability. Curr. Opin. Biotechnol. 2017, 44, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla, B.; Granado, F.; Blanco, I.; Vaquero, M.; Cajigal, C. Lutein in patients with cataracts and age-related macular degeneration: A long-term supplementation study. J. Sci. Food Agric. 2001, 81, 904–909. [Google Scholar] [CrossRef]
- Moeller, S.M.; Jacques, P.F.; Blumberg, J.B. The potential role of dietary xanthophylls in cataract and age-related macular degeneration. J. Am. Coll. Nutr. 2000, 19, 522S–527S. [Google Scholar] [CrossRef]
- Alves-Rodrigues, A.; Shao, A. The science behind lutein. Toxicol. Lett. 2004, 150, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.; Jackson, P.L.; Gutierrez, Y. Subclinical vitamin A deficiency: A potentially unrecognized problem in the United States. Pediatr. Nurs. 1996, 22, 377–389. [Google Scholar] [PubMed]
- Yamaguchi, M.; Uchiyama, S. beta-Cryptoxanthin stimulates bone formation and inhibits bone resorption in tissue culture in vitro. Mol. Cell. Biochem. 2004, 258, 137–144. [Google Scholar] [CrossRef]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef] [PubMed]
- Iannone, A.; Rota, C.; Bergamini, S.; Tomasi, A.; Canfield, L.M. Antioxidant activity of carotenoids: An electron-spin resonance study on beta-carotene and lutein interaction with free radicals generated in a chemical system. J. Biochem. Mol. Toxicol. 1998, 12, 299–304. [Google Scholar] [CrossRef]
- Sujak, A.; Gabrielska, J.; Grudzinski, W.; Borc, R.; Mazurek, P.; Gruszecki, W.I. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: The structural aspects. Arch. Biochem. Biophys. 1999, 371, 301–307. [Google Scholar] [CrossRef]
- Garcia-Casal, N.; Layrisse, M.; Solano, L.; Baron, M.A.; Arguello, F.; Liovera, D.; Ramírez, J.; Leets, I.; Tropper, E. Vitamin A and beta carotene can improve nonheme iron absorption from rice, wheat and corn by humans. J. Nutr. 1998, 128, 646–650. [Google Scholar] [CrossRef]
- Garcia-Casal, N.; Leets, I.; Layrisse, M. Beta carotene and inhibitors of iron absorption modify iron uptake by Caco-2 cells. J. Nutr. 2000, 130, 5–9. [Google Scholar] [CrossRef]
- Bailey, L.B.; Gregory, J.F. Folate metabolism and requirements. J. Nutr. 1999, 129, 779–782. [Google Scholar] [CrossRef]
- Scott, J.; R’ebeill’e, F.; Fletcher, J. Folic acid and folate: The feasibility for nutritional enhancement in plant foods. J. Sci. Food Agric. 2000, 80, 795–824. [Google Scholar] [CrossRef]
- Hanson, A.D.; Roje, S. One-carbon metabolism in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 119–137. [Google Scholar] [CrossRef]
- Basset, G.J.C.; Quinlivan, E.P.; Gregory, J.F., III; Hanson, A.D. Folate synthesis and metabolism in plants and prospects for biofortification. Crop Sci. 2005, 45, 449–453. [Google Scholar] [CrossRef]
- Geisel, J. Folic acid and neural tube defects in pregnancy—A review. J. Perinat. Neonat. Nurs. 2003, 17, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.I.; Allen, L.H.; Mungas, D.M.; Jagust, W.J.; Haan, M.N.; Green, R.; Miller, J.W. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am. J. Clin. Nutr. 2005, 82, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.F.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef]
- McCully, K.S. Homocysteine, vitamins, and vascular disease prevention. Am. J. Clin. Nutr. 2007, 86, 1563S–1568S. [Google Scholar] [CrossRef]
- Choi, S.W.; Friso, S. Interactions between folate and aging for carcinogenesis. Clin. Chem. Lab. Med. 2005, 43, 1151–1157. [Google Scholar] [CrossRef]
- Pitkin, R.M. Folate and neural tube defects. Am. J. Clin. Nutr. 2007, 85, 285S–288S. [Google Scholar] [CrossRef]
- Scholl, T.O.; Johnson, W.G. Folic acid: Influence on the outcome of pregnancy. Am. J. Clin. Nutr. 2000, 71, 1295S–1303S. [Google Scholar] [CrossRef]
- Wallock, L.M.; Tamura, T.; Mayr, C.A.; Johnston, K.E.; Ames, B.N.; Jacob, R.A. Low seminal plasma folate concentrations are associated with low sperm density and count in male smokers and nonsmokers. Fertil. Steril. 2001, 75, 252–259. [Google Scholar] [CrossRef]
- Tamura, T.; Picciano, M.F. Folate and human reproduction. Am. J. Clin. Nutr. 2006, 83, 993–1016. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S.; Hotz, C. Dietary diversification/modification strategies to enhance micronutrient content and bioavailability of diets in developing countries. Br. J. Nutr. 2001, 85, S159–S166. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R. How to ensure adequate iron absorption from iron-fortified food. Nutr. Rev. 2002, 60, S7–S15. [Google Scholar] [CrossRef]
- World Health Organization and Food and Agriculture Organization. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Cook, J.D. Diagnosis and management of iron-deficiency anaemia. Best Pract. Res. Clin. Haematol. 2005, 18, 319–332. [Google Scholar] [CrossRef]
- Blancquaert, D.; Storozhenko, S.; Van Daele, J.; Stove, C.; Visser, R.; Lambert, W.; Van Der Straeten, D. Enhancing pterin and paraaminobenzoate content is not sufficient to successfully biofortify potato tubers and Arabidopsis thaliana plants with folate. J. Exp. Bot. 2013, 64, 3899–3909. [Google Scholar] [CrossRef]
- Shohag, M.J.I.; Wei, Y.; Yang, X. Changes of folate and other potential health promoting phytochemicals in legume seeds as affected by germination. J. Agric. Food Chem. 2012, 60, 9137–9143. [Google Scholar] [CrossRef]
- Hefni, M.; Öhrvik, V.; Tabekha, M.M.; Witthöft, C. Folate content in foods commonly consumed in Egypt. Food Chem. 2010, 121, 540–545. [Google Scholar] [CrossRef]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; de Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Wiltgren, A.R.; Booth, A.O.; Kaur, G.; Cicerale, S.; Lacy, K.E.; Thorpe, M.G.; Keast, R.S.; Riddell, L.J. Micronutrient supplement use and diet quality in university students. Nutrients 2015, 7, 1094–1107. [Google Scholar] [CrossRef]
- Stoltzfus, R.J. Iron interventions for women and children in low-income countries. J. Nutr. 2011, 141, 756S–762S. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization and Food and Agriculture Organization of the United Nations. Guidelines on Food Fortification with Micronutrients; Allen, L., de Benoist, B., Dary, O., Hurrell, R., Eds.; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Gómez-Galera, S.; Rojas, E.; Sudhakar, D.; Zhu, C.; Pelacho, A.M.; Capell, T.; Christou, P. Critical evaluation of strategies for mineral fortification of staple food crops. Transgenic Res. 2010, 19, 165–180. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets-iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.L.; Zhu, Y.G.; Zhang, M.; Huang, M.Z. Selecting iodine-enriched vegetables and the residual effect of iodate application to soil. Biol. Trace Elem. Res. 2004, 101, 265–276. [Google Scholar] [CrossRef]
- Hartikainen, H. Biogeochemistry of selenium and its impact on food chain quality and human health. J. Trace Elem. Med. Biol. 2005, 18, 309–318. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortifying crops with essential mineral elements. Trends Plant Sci. 2005, 10, 586–593. [Google Scholar] [CrossRef]
- Eurola, M.; Ekholm, P.; Ylinen, M.; Koivistoinen, P.; Varo, P. Effects of selenium fertilization on the selenium content of selected Finnish fruits and vegetables. Acta Agric. Scand. 1989, 39, 345–350. [Google Scholar] [CrossRef]
- Eurola, M.H.; Ekholm, P.I.; Ylinen, M.E.; Koivistoinen, P.E.; Varo, P.T. Selenium in Finnish foods after beginning the use of selenate-supplemented fertilisers. J. Sci. Food Agric. 1991, 56, 57–70. [Google Scholar] [CrossRef]
- Alfthan, G.; Eurola, M.; Ekholm, P. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 42–147. [Google Scholar] [CrossRef]
- Winkler, J.T. Biofortification: Improving the nutritional quality of staple crops. In Access Not Excess; Pasternak, C., Ed.; Smith-Gordon Publishing: St Ives, UK, 2011; pp. 100–112. [Google Scholar]
- Grusak, M.A.; DellaPenna, D. Improving the nutrient composition of plants to enhance human nutrition and health. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 133–161. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; Rengel, Z. Soil and foliar zinc biofortification in field pea (Pisum sativum L.). Grain accumulation and bioavailability in raw and cooked grains. Food Chem. 2016, 212, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Hirschi, K.D. Nutrient biofortification of food crops. Annu. Rev. Nutr. 2009, 29, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Frossard, E.; Bucher, M.; Machler, F.; Mozafar, A.; Hurrell, R. Potential for increasing the content and bioavailability of Fe, Zn and Ca in plants for human nutrition. J. Sci. Food Agric. 2000, 80, 861–879. [Google Scholar] [CrossRef]
- Ismail, A.M.; Heuer, S.; Thomson, M.J.; Wissuwa, M. Genetic and genomic approaches to develop rice germplasm for problem soils. Plant Mol. Biol. 2007, 65, 547–570. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Quiroz, C.; De-la-Cruz-Lázaro, E.; Osorio-Osorio, R.; Sánchez-Chávez, E. Biofortification of cowpea beans with iron: Iron’s influence on mineral content and yield. J. Soil Sci. Plant Nutr. 2015, 15, 839–847. [Google Scholar] [CrossRef]
- Ali, B.; Ali, A.; Tahir, M.; Ali, S. Growth, Seed yield and quality of mungbean as influenced by foliar application of iron sulfate. Pak. J. Life Soc. Sci. 2014, 12, 20–25. [Google Scholar]
- Salih, H.O. Effect of foliar fertilization of Fe, B and Zn on nutrient concentration and seed protein of Cowpea “Vigna unguiculata”. IOSR J. Aric. Vet. Sci. 2013, 6, 42–46. [Google Scholar] [CrossRef]
- Nandan, B.; Sharma, B.C.; Chand, G.; Bazgalia, K.; Kumar, R.; Banotra, M. Agronomic fortification of Zn and Fe in chickpea an emerging tool for nutritional security—A global perspective. Acta Sci. Nutr. Health 2018, 2, 12–19. [Google Scholar]
- Shivay, Y.S.; Prasad, R.; Pal, M. Effects of source and method of zinc application on yield, zinc biofortification of grain, and Zn uptake and use efficiency in chickpea (Cicer arietinum L.). Commun. Soil Sci. Plant Anal. 2015, 46, 2191–2200. [Google Scholar] [CrossRef]
- Hidoto, L.; Worku, W.; Mohammed, H.; Taran, B. Effects of zinc application strategy on zinc content and productivity of chickpea grown under zinc deficient soils. J. Soil Sci. Plant Nutr. 2017, 17, 112–126. [Google Scholar] [CrossRef][Green Version]
- Ibrahim, E.A.; Ramadan, W.A. Effect of zinc foliar spray alone and combined with humic acid or/and chitosan on growth, nutrient elements content and yield of dry bean (Phaseolus vulgaris L.) plants sown at different dates. Sci. Hortic. 2015, 184, 101–115. [Google Scholar] [CrossRef]
- Ram, H.; Rashid, A.; Zhang, W.; Duarte, A.P.; Phattarakul, N.; Simunji, S.; Kalayci, M.; Freitas, R.; Rerkasem, B.; Bal, R.S.; et al. Biofortification of wheat, rice and common bean by applying foliar zinc fertilizer along with pesticides in seven countries. Plant Soil 2016, 403, 389–401. [Google Scholar] [CrossRef]
- Sida-Arreola, J.P.; Sánchez, E.; Ojeda-Barrios, D.L.; Ávila-uezada, G.D.; Flores-Córdova, M.A.; Márquez-Quiroz, C.; Preciado-Rangel, P. Can biofortification of zinc improve the antioxidant capacity and nutritional quality of beans? Emir. J. Food Agric. 2017, 29, 237–241. [Google Scholar]
- Smrkolj, P.; Germ, M.; Kreft, I.; Stibilj, V. Respiratory potential and Se compounds in pea (Pisum sativum L.) plants grown from Se-enriched seeds. J. Exp. Bot. 2006, 57, 3595–3600. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; Rodrigo, S.; Santamaria, O.; Chen, Y.; McGrath, S.P. Selenium accumulation and speciation in biofortified chickpea (Cicer arietinum L.) under Mediterranean conditions. J. Sci. Food Agric. 2014, 94, 1101–1106. [Google Scholar] [CrossRef]
- Smrkolj, P.; Osvald, M.; Osvald, J.; Stibilj, V. Selenium uptake and species distribution in selenium-enriched bean (Phaseolus vulgaris L.) seeds obtained by two different cultivations. Eur. Food Res. Technol. 2007, 225, 233–237. [Google Scholar] [CrossRef]
- Rahman, M.M.; Erskine, W.; Materne, M.A.; McMurray, L.M.; Thavarajah, P.; Thavarajah, D.; Siddique, K.H.M. Enhancing selenium concentration in lentil (Lens culinaris subsp. culinaris) through foliar application. J. Agric. Sci. 2015, 153, 656–665. [Google Scholar]
- FAO. The Plant Production and Protection Division (AGP)—Soil Biological Management with Beneficial Microorganisms; FAO: Rome, Italy, 2019. [Google Scholar]
- Mahaffee, W.F.; Kloepper, J.W. Applications of plant growth-promoting rhizobacteria in sustainable agriculture. In Soil Biota: Management in Sustainable Farming Systems; Pankhurst, C.E., Doube, B.M., Gupta, V.V.S.R., Grace, P.R., Eds.; CSIRO: Melbourne, Australia, 1994; pp. 23–31. [Google Scholar]
- Panhwar, Q.A.; Othman, R.; Rahman, Z.A.; Meon, S.; Ismail, M.R. Isolation and characterization of phosphate-solubilizing bacteria from aerobic rice. Afr. J. Biotechnol. 2012, 11, 2711–2719. [Google Scholar]
- Sreevidya, M.; Gopalakrishnan, S.; Kudapa, H.; Varshney, R.K. Exploring PGP actinomycetes from vermicompost and rhizosphere soil for yield enhancement in chickpea. Braz. J. Microbiol. 2016, 47, 85–95. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Vadlamudi, S.; Samineni, S.; Sameer Kumar, C.V. Plant growth-promotion and biofortification of chickpea and pigeonpea through inoculation of biocontrol potential bacteria, isolated from organic soils. Springerplus 2016, 5, 1882. [Google Scholar] [CrossRef] [PubMed]
- Rengel, Z.; Batten, G.D.; Crowley, D.D. Agronomic approaches for improving the micronutrient density in edible portions of field crops. Field Crop. Res. 1999, 60, 27–40. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Elsevier: London, UK, 2007. [Google Scholar]
- Cavagnaro, T.R. The role of arbuscular mycorrhizas in improving plant zinc nutrition under low soil zinc concentrations: A review. Plant Soil 2008, 304, 315–325. [Google Scholar] [CrossRef]
- Tokala, R.K.; Strap, J.L.; Jung, C.M.; Crawford, D.L.; Salove, M.H.; Deobald, L.A.; Bailey, J.F.; Morra, M.J. Novel plant-microbe rhizosphere interaction involving Streptomyces lydicus WYEC108 and the pea plant (Pisum sativum). Appl. Environ. Microbiol. 2002, 68, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.; Burgos, A.; Fiscella, T.; Rivas, R.; Velazquez, E.; Rodrıguez-Barrueco, C.; Cervantes, E.; Chamber, M.; Igual, J.M. Differential effects of co-inoculations with Pseudomonas jessenii PS06 (a phosphate-solubilizing bacterium) and Mesorhizobium ciceri C-2/2 strains on the growth and seed yield of chickpea under greenhouse and field conditions. Plant Soil 2006, 287, 43–50. [Google Scholar] [CrossRef]
- Minorsky, P.V. On the inside. Plant Physiol. 2008, 146, 323–324. [Google Scholar] [CrossRef]
- Soe, K.M.; Bhromsiri, A.; Karladee, D. Effects of selected endophytic actinomycetes (Streptomyces sp.) and Bradyrhizobia from Myanmar on growth, nodulation, nitrogen fixation and yield of different soybean varieties. CMU J. Nat. Sci. 2010, 9, 95–109. [Google Scholar]
- Gopalakrishnan, S.; Srinivas, V.; Prakash, B.; Sathya, A.; Vijayabharathi, R. Plant growth-promoting traits of Pseudomonas geniculata isolated from chickpea nodules. 3 Biotech 2015, 5, 653–661. [Google Scholar] [CrossRef]
- Sathya, A.; Vijayabharati, R.; Srinivas, V.; Gopalakrishnan, S. Plant growth-promoting action-bacteria on chickpea seed mineral density: An upcoming complementary tool for sustainable biofortification strategy. 3 Biotech 2013, 6, 138. [Google Scholar] [CrossRef]
- Pellegrino, E.; Bedini, S. Enhancing ecosystem services in sustainable agriculture: Biofertilization and biofortification of chickpea (Cicer arietinum L.) by arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2014, 68, 429–439. [Google Scholar] [CrossRef]
- Khalid, S.; Asghar, H.N.; Akhtar, M.J.; Aslam, A.; Zahir, Z.A. Biofortification of iron in chickpea by plant growth promoting rhizobacteria. Pak. J. Bot. 2015, 47, 1191–1194. [Google Scholar]
- Mayer, J.E.; Pfeiffer, W.H.; Bouis, P. Biofortified crops to alleviate micronutrient malnutrition. Curr. Opin. Plant Biol. 2008, 11, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Perez-Massot, E.; Banakar, R.; Gomez-Galera, S.; Zorrilla-Lopez, U.; Sanahuja, G.; Arjo, G.; Miralpeix, B.; Vamvaka, E.; Farré, G.; Rivera, S.M.; et al. The contribution of transgenic plants to better health through improved nutrition: Opportunities and constraints. Genes Nutr. 2013, 8, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.M.P.; Vasconcelos, M.W. Producing more with less: Strategies and novel technologies for plant-based food biofortification. Food Res. Int. 2013, 54, 961–971. [Google Scholar] [CrossRef]
- Christou, P.; Twyman, R.M. The potential of genetically enhanced plants to address food insecurity. Nutr. Res. Rev. 2004, 17, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Newell-McGloughlin, M. Nutritionally improved agricultural crops. Plant Physiol. 2008, 147, 939–953. [Google Scholar] [CrossRef]
- Hefferon, K.L. Can biofortified crops help attain food security? Curr. Mol. Biol. Rep. 2016, 2, 180–185. [Google Scholar] [CrossRef]
- Goto, F.; Yoshihara, T.; Saiki, H. Iron accumulation and enhanced growth in transgenic lettuce plants expressing the iron-binding protein ferritin. Theor. Appl. Genet. 2000, 100, 658–664. [Google Scholar] [CrossRef]
- Vasconcelos, M.; Datta, K.; Oliva, N.; Khalekuzzaman, M.; Torrizo, L.; Krishnan, S.; Oliveira, M.; Goto, F.; Datta, S.K. Enhanced iron and zinc accumulation in transgenic rice with the ferritin gene. Plant Sci. 2003, 164, 371–378. [Google Scholar] [CrossRef]
- Trijatmiko, K.R.; Dueñas, C.; Tsakirpaloglou, N.; Torrizo, L.; Arines, F.M.; Adeva, C.; Balindong, J.; Oliva, N.; Sapasap, M.V.; Borrero, J.; et al. Biofortified indica rice attains iron and zinc nutrition dietary targets in the field. Sci. Rep. 2016, 6, 19792. [Google Scholar] [CrossRef]
- Paine, J.A.; Shipton, C.A.; Chaggar, S.; Howells, R.M.; Kennedy, M.J.; Vernon, G.; Wright, S.Y.; Hinchliffe, E.; Adams, J.L.; Silverstone, A.L.; et al. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat. Biotechnol. 2005, 23, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Zhu, C.; Farre, G.; Ramessar, K.; Bassie, L.; Breitenbach, J.; Perez Conesa, D.; Ros, G.; Sandmann, G.; Capell, T.; et al. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc. Natl. Acad. Sci. USA 2009, 106, 7762–7767. [Google Scholar] [CrossRef] [PubMed]
- Blancquaert, D.; De Steur, H.; Gellynck, X.; Van Der Straeten, D. Present and future of folate biofortification of crop plants. J. Exp. Bot. 2014, 65, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Storozhenko, S.; De Brouwer, V.; Volckaert, M.; Navarrete, O.; Blancquaert, D.; Zhang, G.F.; Lambert, W.; Van Der Straeten, D. Folate fortification of rice by metabolic engineering. Nat. Biotechnol. 2007, 25, 1277–1279. [Google Scholar] [CrossRef] [PubMed]
- Hossain, T.; Rosenberg, I.; Selhub, J.; Kishore, G.; Beachy, R.; Schubert, K. Enhancement of folate in plants through metabolic engineering. Proc. Natl. Acad. Sci. USA 2004, 101, 5158–5163. [Google Scholar] [CrossRef] [PubMed]
- Aragao, F.J.L.; Barros, L.M.G.; De Sousa, M.V.; Grossi de Sa, M.F.; Almeida, E.R.P.; Gander, E.S.; Rech, E.L. Expression of a methionine-rich storage albumin from the Brazil nut (Bertholletia excelsa H.B.K., Lecythidaceae) in transgenic bean plants (Phaseolus vulgaris L., Fabaceae). Genet. Mol. Biol. 1999, 22, 445–449. [Google Scholar] [CrossRef]
- Molvig, L.; Tabe, L.M.; Eggum, B.O.; Moore, A.E.; Craig, S.; Spencer, D.; Higgins, T.J. Enhanced methionine levels and increased nutritive value of seeds of transgenic lupins (Lupinus angustifolius L.) expressing a sunflower seed albumin gene. Proc. Natl. Acad. Sci. USA 1997, 94, 8393–8398. [Google Scholar] [CrossRef]
- Bortesi, L.; Fischer, R. The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol. Adv. 2015, 33, 41–52. [Google Scholar] [CrossRef]
- Jaganathan, D.; Ramasamy, K.; Sellamuthu, G.; Jayabalan, S.; Venkataraman, G. CRISPR for crop improvement: An update review. Front. Plant Sci. 2018, 9, 985. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Spalding, M.H.; Weeks, D.P.; Yang, B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 2012, 30, 390–392. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Wei, P.; Zhang, B.; Gou, F.; Feng, Z.; Mao, Y.; Yang, L.; Zhang, H.; Xu, N.; et al. The CRISPR/Cas9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnol. J. 2014, 12, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Brooks, C.; Nekrasov, V.; Lippman, Z.B.; Van Eck, J. Efficient gene editing in tomato in the first generation using the clustered regularly interspaced short palindromic repeats/CRISPR-associated9 system. Plant Physiol. 2014, 166, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Curtin, S.J.; Xiong, Y.; Michno, J.M.; Campbell, B.W.; Stec, A.O.; Čermák, T.; Starker, C.; Voytas, D.F.; Eamens, A.L.; Stupar, R.M. CRISPR/Cas9 and TALENs generate heritable mutations for genes involved in small RNA processing of Glycine max and Medicago truncatula. Plant Biotechnol. J. 2018, 16, 1125–1137. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, C.; Sun, Z.; Wang, L.; Duanmu, D.; Fan, Q. Genome editing in cowpea Vigna unguiculata using CRISPR-Cas9. Int. J. Mol. Sci. 2019, 20, 2471. [Google Scholar] [CrossRef]
- Inaba, M.; Macer, D. Policy, regulation and attitudes towards agricultural biotechnology in Japan. J. Int. Biotechnol. Laws 2004, 1, 45–53. [Google Scholar] [CrossRef]
- Watanabe, K.N.; Sassa, Y.; Suda, E.; Chen, C.H.; Inaba, M.; Kikuchi, A. Global political, economic, social and technological issues on transgenic crops-review. Plant Biotechnol. J. 2005, 22, 515–522. [Google Scholar] [CrossRef]
- Wesseler, J.; Zilberman, D. The economic power of the Golden Rice opposition. Environ. Dev. Econ. 2014, 19, 724–742. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Sec. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Welch, R.M.; Graham, R.D. Agriculture: The real nexus for enhancing bioavailable micronutrients in food crops. J. Trace Elem. Med. Biol. 2005, 18, 299–307. [Google Scholar] [CrossRef]
- Pfeiffer, W.H.; McClafferty, B. HarvestPlus: Breeding crops for better nutrition. Crop Sci. 2007, 47, S88–S100. [Google Scholar] [CrossRef]
- Bouis, H.E.; Welch, R.M. Biofortification—A sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci. 2010, 50, S20–S32. [Google Scholar] [CrossRef]
- Bouis, H.E. Micronutrient fortification of plants through plant breeding: Can it improve nutrition in man at low cost? Proc. Nutr. Soc. 2003, 62, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Bouis, H.E. Enrichment of food staples through plant breeding: A new strategy for fighting micronutrient malnutrition. Nutrition 2000, 16, 701–704. [Google Scholar] [CrossRef]
- Beebe, S.; Gonzalez, A.V.; Rengifo, J. Research on trace minerals in the common bean. Food Nutr. Bull. 2000, 21, 387–391. [Google Scholar] [CrossRef]
- Islam, F.M.A.; Basford, K.E.; Jara, C.; Redden, R.J.; Beebe, S.E. Seed compositional and disease resistance differences among gene pools in cultivated common bean. Genet. Resour. Crop Evol. 2002, 49, 285–293. [Google Scholar] [CrossRef]
- Blair, M.W.; Astudillo, C.; Grusak, M.; Graham, R.; Beebe, S. Inheritance of seed iron and zinc content in common bean (Phaseolus vulgaris L.). Mol. Breed. 2009, 23, 197–207. [Google Scholar] [CrossRef]
- Blair, M.W.; Medina, J.I.; Astudillo, C.; Rengifo, J.; Beebe, S.E.; Machado, G.; Graham, R. QTL for seed iron and zinc concentrations in a recombinant inbred line population of Mesoamerican common beans (Phaseolus vulgaris L.). Theor. Appl. Genet. 2010, 121, 1059–1070. [Google Scholar] [CrossRef]
- Blair, M.W.; Astudillo, C.; Rengifo, J.; Beebe, S.E.; Graham, R. QTL for seed iron and zinc concentrations in a recombinant inbred line population of Andean common beans (Phaseolus vulgaris L.). Theor. Appl. Genet. 2011, 122, 511–521. [Google Scholar] [CrossRef]
- Sarker, A.; El-Askhar, F.; Uddin, M.J.; Million, E.; Yadav, N.K.; Dahan, R.; Wolfgang, P. Lentil improvement for nutritional security in the developing world. Presented at the ASA-CSSA-SSSA International Annual Meeting, New Orleans, LA, USA, 4–8 November 2007. [Google Scholar]
- HarvestPlus. Biofortification Progress Briefs: Iron and Zinc Lentils. 2014. Available online: www.HarvestPlus.org (accessed on 15 September 2019).
- Nair, R.M.; Thavarajah, D.; Thavarajah, P.; Giri, R.R.; Ledesma, D.; Yang, R.Y.; Hanson, P.; Easdown, W.; Hughes, J.D.A.; Keatinge, J.D.H. Mineral and phenolic concentrations of mungbean [Vigna radiata (L.) R. Wilczek var. radiata] grown in semi-arid tropical India. J. Food Compos. Anal. 2015, 39, 23–32. [Google Scholar] [CrossRef]
- Ariza-Nieto, M.; Blair, M.W.; Welch, R.M.; Glahn, R.P. Screening of bioavailability patterns in eight bean (Phaseolus vulgaris L.) genotypes using the Caco-2 cell in vitro model. J. Agric. Food Chem. 2007, 55, 7950–7956. [Google Scholar] [CrossRef] [PubMed]
- DellaValle, D.M.; Vandenberg, A.; Glahn, R.P. Seed coat removal improves iron bioavailability in cooked lentils: Studies using an in vitro digestion/Caco-2 cell culture model. J. Agric. Food Chem. 2013, 61, 8084–8089. [Google Scholar] [CrossRef] [PubMed]
- Moraghan, J.T.; Padilla, J.; Etchevers, J.D.; Grafton, K.; Acosta-Gallegos, J.A. Iron accumulation in seed of common bean. Plant Soil 2002, 246, 175–183. [Google Scholar] [CrossRef]
- Thavarajah, D.; Ruszkowski, J.; Vandenberg, A. High potential for selenium biofortification of lentils (Lens culinaris L.). J. Agric. Food Chem. 2008, 56, 10747–10753. [Google Scholar] [CrossRef] [PubMed]
- Thavarajah, D.; Thavarajah, P.; Sarker, A.; Materne, M.; Vandemark, G.; Shrestha, R.; Idrissi, O.; Hacikamiloglu, O.; Bucak, B.; Vandenberg, A. A global survey of effects of genotype and environment on selenium concentration in lentils (Lens culinaris L.): Implications for nutritional fortification strategies. Food Chem. 2011, 125, 72–76. [Google Scholar] [CrossRef]
- Thavarajah, D.; Warkentin, T.; Vandenberg, A. Natural enrichment of selenium in Saskatchewan field peas (Pisum sativum L.). Can. J. Plant Sci. 2010, 90, 383–389. [Google Scholar] [CrossRef]
- Abbo, S.; Molina, C.; Jungmann, R.; Grusak, M.A.; Berkovitch, Z.; Reifen, R.; Kahl, G.; Winter, P.; Reifen, R. QTL governing carotenoid concentration and weight in seeds of chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2005, 111, 185–195. [Google Scholar] [CrossRef]
- Thavarajah, D.; Thavarajah, P. Evaluation of chickpea (Cicer arietinum L.) micronutrient composition: Biofortification opportunities to combat global micronutrient malnutrition. Food Res. Int. 2012, 49, 99–104. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Tar’an, B.; Diapari, M.; Arganosa, G.; Warkentin, T.D. Effect of cultivar and environment on carotenoid profile of pea and chickpea. Crop Sci. 2014, 54, 2225–2235. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Diapari, M.; Jha, A.B.; Tar’an, B.; Arganosa, G.; Warkentin, T.D. Genetic diversity of nutritionally important carotenoids in 94 pea and 121 chickpea accessions. J. Food Compos. Anal. 2015, 43, 49–60. [Google Scholar] [CrossRef]
- Rezaei, M.K.; Deokar, A.; Tar’an, B. Identification and expression analysis of candidate genes involved in carotenoid biosynthesis in chickpea seeds. Front. Plant Sci. 2016, 7, 1867. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.K.; Deokar, A.A.; Arganosa, G.; Roorkiwal, M.; Pandey, S.K.; Warkentin, T.D.; Varshney, R.K.; Tar′an, B. Mapping quantitative trait loci for carotenoid concentration in three F2 populations of chickpea. Plant Genome 2019, 12, 190067. [Google Scholar] [CrossRef]
- Vahteristo, L.; Lehikoinen, K.; Ollilainen, V.; Varo, P. Application of an HPLC assay for the determination of folate derivatives in some vegetables, fruits and berries consumed in Finland. Food Chem. 1997, 59, 589–597. [Google Scholar] [CrossRef]
- Han, J.Y.; Tyler, R.T. Determination of folate concentrations in pulses by a microbiological method employing trienzyme extraction. J. Agric. Food Chem. 2003, 51, 5315–5318. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, M.; Englert, K.; Kapfer, S.; Kirchhoff, E. Folate contents of legumes determined by optimized enzyme treatment and stable isotope dilution assays. J. Food Compos. Anal. 2007, 20, 411–419. [Google Scholar] [CrossRef]
- Sen Gupta, D.; Thavarajah, D.; Thavarajah, P.; McGee, R.; Coyne, C.J.; Kumar, S. Lentils (Lens culinaris L.), a rich source of folates. J. Agric. Food Chem. 2013, 61, 7794–7799. [Google Scholar] [CrossRef]
- Zhang, H.; Jha, A.B.; Warkentin, T.D.; Vandenberg, A.; Purves, R.W. Folate stability and method optimization for folate extraction from seeds of pulse crops using LC-SRM MS. J. Food Compos. Anal. 2018, 71, 44–55. [Google Scholar] [CrossRef]
- Zhang, H.; Jha, A.B.; De Silva, D.; Purves, R.W.; Warkentin, T.D.; Vandenberg, A. Improved folate monoglutamate extraction and application to folate quantification from wild lentil seeds by ultra-performance liquid chromatography-selective reaction monitoring mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1121, 39–47. [Google Scholar] [CrossRef]
- Combs, G.F.; Lü, J. Selenium as a cancer preventive agent. In Selenium; Hatfield, D.L., Ed.; Springer: Boston, MA, USA, 2001; pp. 205–217. [Google Scholar]
- Thavarajah, D.; Vandenberg, A.; George, G.N.; Pickering, I.J. Chemical form of selenium in naturally selenium rich lentils (Lens culinaris L.) from Saskatchewan. J. Agric. Food Chem. 2007, 55, 7337–7341. [Google Scholar] [CrossRef]
- Fuge, R.; Johnson, C. Iodine and human health, the role of environmental geochemistry and diet: A review. Appl. Geochem. 2015, 63, 282–302. [Google Scholar] [CrossRef]
- Mackowiak, C.L.; Grossl, P.R. Iodate and iodide effects on iodine uptake and partitioning in rice (Oryza sativa L.) grown in solution culture. Plant Soil 1999, 212, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Smolen, S.; Sady, W.; Ledwozyw-Smolen, I.; Strzetelski, P.; Liszka-Skoczylas, M.; Rozek, S. Quality of fresh and stored carrots depending on iodine and nitrogen fertilization. Food Chem. 2014, 159, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Macias, J.; Leija-Martinez, P.; Gonzales-Morales, S.; Juarez-Maldonado, A.; Benavides-Mendoza, A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef] [PubMed]
- McCallum, J.; Timmerman-Vaughan, G.; Frew, T.; Russel, A. Biochemical and genetic linkage analysis of green seed colour in field pea. J. Am. Soc. Hortic. Sci. 1997, 122, 218–225. [Google Scholar] [CrossRef]
- Abbo, S.; Bonfil, D.J.; Berkovitch, Z.; Reifen, R. Towards enhancing lutein concentration in chickpea, cultivar and management effects. Plant Breed. 2010, 129, 407–411. [Google Scholar] [CrossRef]
- Holasová, M.; Dostálová, R.; Fiedlerová, V.; Horáček, J. Variability of lutein content in peas (Pisum sativum L.) in relation to the variety, season and chlorophyll content. Czech. J. Food Sci. 2009, 27, S188–S191. [Google Scholar] [CrossRef]
- Marles, M.A.S.; Warkentin, T.D.; Bett, K.E. Genetic abundance of carotenoids and polyphenolics in the hull of field pea (Pisum sativum L.). J. Sci. Food Agric. 2013, 93, 463–470. [Google Scholar] [CrossRef]
- Lu, W.; Haynes, K.; Wiley, E.; Clevidence, B. Carotenoid content and colour in diploid potatoes. J. Am. Soc. Hortic. Sci. 2001, 126, 722–726. [Google Scholar] [CrossRef]
- Beyer, P.; Al-Babili, S.; Ye, X.; Lucca, P.; Schaub, P.; Welsch, R.; Potrykus, I. Golden Rice: Introducing the beta-carotene biosynthesis pathway into rice endosperm by genetic engineering to defeat vitamin A deficiency. J. Nutr. 2002, 132, 506S–510S. [Google Scholar] [CrossRef]
- Amorim, E.P.; Vilarinhos, A.D.; Cohen, K.O.; Amorim, V.B.O.; Santos-Serejo, J.A.D.; Silva, S.O.; Pestana, K.N.; Santos, V.J.D.; Paes, N.S.; Monte, D.C.; et al. Genetic diversity of carotenoid-rich bananas evaluated by Diversity Arrays Technology (DArT). Genet. Mol. Biol. 2009, 32, 96–103. [Google Scholar] [CrossRef]
- Fernandez-Orozco, R.; Gallardo-Guerrero, L.; Hornero-Me’ndez, D. Carotenoid profiling in tubers of different potato (Solanum sp.) cultivars: Accumulation of carotenoids mediated by xanthophyll esterification. Food Chem. 2013, 141, 2864–2872. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.K.; Arcot, J.; Paterson, J. Folate assay of foods by traditional and trienzyme treatments using cryoprotected Lactobacillus casei. Food Chem. 2000, 71, 545–552. [Google Scholar] [CrossRef]
- Chew, S.C.; Loh, S.P.; Khor, G.L. Determination of folate content in commonly consumed Malaysian foods. Int. Food Res. J. 2012, 19, 189–197. [Google Scholar]
- Fajardo, V.; Alonso-Aperte, E.; Varela-Moreiras, G. Total folate content in ready-to eat vegetable meals from the Spanish market. J. Food Compos. Anal. 2017, 64, 223–231. [Google Scholar] [CrossRef]
- De Brouwer, V.; Storozhenko, S.; Van De Steene, J.C.; Wille, S.M.; Stove, C.P.; Van Der Straeten, D.; Lambert, W.E. Optimisation and validation of a liquid chromatography-tandem mass spectrometry method for folates in rice. J. Chromatogr. A 2008, 1215, 125–132. [Google Scholar] [CrossRef]
- De Brouwer, V.; Storozhenko, S.; Stove, C.P.; Van Daele, J.; Van der Straeten, D.; Lambert, W.E. Ultra-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) for the sensitive determination of folates in rice. J. Chromatogr. B 2010, 878, 509–513. [Google Scholar] [CrossRef]
- Camara, J.E.; Lowenthal, M.S.; Phinney, K.W. Determination of fortified and endogenous folates in food-based standard reference materials by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 4561–4568. [Google Scholar] [CrossRef]
- Khanal, S.; Xue, J.; Khanal, R.; Xie, W.; Shi, J.; Pauls, K.P.; Navabi, A. Quantitative trait loci analysis of folate content in dry beans, Phaseolus vulgaris L. Int. J. Agron. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Konings, E.J.; Roomans, H.H.; Dorant, E.; Goldbohm, R.A.; Saris, W.H.; van den Brandt, P.A. Folate intake of the Dutch population according to newly established liquid chromatography data for foods. Am. J. Clin. Nutr. 2001, 73, 765–776. [Google Scholar] [CrossRef]
- Henderson, G.I.; Perez, T.; Schenker, S.; Mackins, J.; Antony, A.C. Maternaltofetal transfer of 5-methyltetrahydrofolate by the perfused human placental cotyledon: Evidence for a concentrative role by placental folate receptors in fetal folate delivery. J. Lab. Clin. Med. 1995, 126, 184–203. [Google Scholar]
- Scaglione, F.; Panzavolta, G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica 2014, 44, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C.; Loechl, C.; de Brauw, A.; Eozenou, P.; Gilligan, D.; Moursi, M.; Munhaua, B.; van Jaarsveld, P.; Carriquiry, A.; Meenakshi, J.V. A large-scale intervention to introduce orange sweet potato in rural Mozambique increases vitamin A intakes among children and women. Br. J. Nutr. 2012, 108, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C.; Loechl, C.; Lubowa, A.; Tumwine, J.K.; Ndeezi, G.; Nandutu Masawi, A.; Baingana, R.; Carriquiry, A.; de Brauw, A.; Meenakshi, J.V.; et al. Introduction of β-carotene-rich orange sweet potato in rural Uganda results in increased vitamin A intakes among children and women and improved vitamin A status among children. J. Nutr. 2012, 142, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Gannon, B.; Kaliwile, C.; Arscott, S.A.; Schmaelzle, S.; Chileshe, J.; Kalungwana, N.; Mosonda, M.; Pixley, K.; Masi, C.; Tanumihardjo, S.A. Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: A community-based, randomized placebo-controlled trial. Am. J. Clin. Nutr. 2014, 100, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.L.; Mehta, S.; Udipi, S.A.; Ghugre, P.S.; Luna, S.V.; Wenger, M.J.; Murray-Kolb, L.E.; Przybyszewski, E.M.; Haas, J.D. A randomized trial of iron-biofortified pearl millet in school children in India. J. Nutr. 2015, 145, 1576–1581. [Google Scholar] [CrossRef]
- Haas, J.; Luna, S.V.; Lung’aho, M.G.; Ngabo, F.; Wenger, M.; Murray-Kolb, L.; Beebe, S.; Gahutu, J.; Egli, I. Consuming iron biofortified beans significantly improved iron status in Rwandan women after 18 weeks. J. Nutr. 2017, 146, 1586–1592. [Google Scholar] [CrossRef]
- Brinch-Pedersen, H.; Borg, S.; Tauris, B.; Holm, P.B. Molecular genetic approaches to increasing mineral availability and vitamin content of cereals. J. Cereal Sci. 2007, 46, 308–326. [Google Scholar] [CrossRef]
- Warkentin, T.D.; Delgerjav, O.; Arganosa, G.; Rehman, A.U.; Bett, K.E.; Anbessa, Y.; Rossnagel, B.; Raboy, V. Development and characterization of low-phytate pea. Crop Sci. 2012, 52, 74–78. [Google Scholar] [CrossRef]
- Liu, X.; Glahn, R.P.; Arganosa, G.C.; Warkentin, T.D. Iron bioavailability in low phytate pea. Crop Sci. 2015, 55, 320–330. [Google Scholar] [CrossRef]
- Shewry, P.R.; Ward, J.L. Exploiting genetic variation to improve wheat composition for the prevention of chronic diseases. Food Energy Secur. 2012, 1, 47–60. [Google Scholar] [CrossRef]
- Shi, J.; Wang, H.; Hazebroek, J.; Ertl, D.S.; Harp, T. The maize low-phytic acid 3 encodes a myo-inositol kinase that plays a role in phytic acid biosynthesis in developing seeds. Plant J. 2005, 42, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Brinch-Pedersen, H.; Sørensen, L.D.; Holm, P.B. Engineering crop plants: Getting a handle on phosphate. Trends Plant Sci. 2002, 7, 118–125. [Google Scholar] [CrossRef]
- Campion, B.; Sparvoli, F.; Doria, E.; Tagliabue, G.; Galasso, I.; Fileppi, M.; Bollini, R.; Nielsen, E. Isolation and characterisation of an lpa (low phytic acid) mutant in common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2009, 118, 1211–1221. [Google Scholar] [CrossRef]
- Panzeri, D.; Cassani, E.; Doria, E.; Tagliabue, G.; Forti, L.; Campion, B.; Bollini, R.; Brearley, C.A.; Pilu, R.; Nielsen, E.; et al. A defective ABC transporter of the MRP family, responsible for the bean lpa1 mutation, affects the regulation of the phytic acid pathway, reduces seed myo-inositol and alters ABA sensitivity. New Phytol. 2011, 191, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Shunmugam, A.S.K.; Bock, C.; Arganosa, G.C.; Georges, F.; Gray, G.R.; Warkentin, T.D. Accumulation of phosphorus-containing compounds in developing seeds of low-phytate pea (Pisum sativum L.) mutants. Plants 2015, 4, 1–26. [Google Scholar] [CrossRef]
- Cominelli, E.; Confalonieri, M.; Carlessi, M.; Cortinovis, G.; Daminati, M.G.; Porch, T.G.; Losa, A.; Sparvoli, F. Phytic acid transport in Phaseolus vulgaris: A new low phytic acid mutant in the PvMRP1 gene and study of the PvMRPs promoters in two different plant systems. Plant Sci. 2018, 270, 1–12. [Google Scholar] [CrossRef]
- Petry, N.; Egli, I.; Campion, B.; Nielsen, E.; Hurrell, R. Genetic reduction of phytate in common bean (Phaseolus vulgaris L.) seeds increases iron absorption in young women. J. Nutr. 2013, 143, 1219–1224. [Google Scholar] [CrossRef]
- Vermeris, W.; Nicholson, R. Phenolic Compound Biochemistry; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Kerala, India, 2006; pp. 23–67. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Remesy, C. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Hart, J.J.; Tako, E.; Kochian, L.V.; Glahn, R.P. Identification of black bean (Phaseolus vulgaris L.) polyphenols that inhibit and promote iron uptake by Caco-2 cells. J. Agric. Food Chem. 2015, 63, 5950–5956. [Google Scholar] [CrossRef]
- Jha, A.B.; Purves, R.W.; Elessawy, F.M.; Zhang, H.; Vandenberg, A.; Warkentin, T.D. Polyphenolic profile of seed components of white and purple flower pea lines. Crop Sci. 2019, 59, 2711–2719. [Google Scholar] [CrossRef]
- Gregorio, G.B.; Senadhira, D.; Htut, H.; Graham, R.D. Breeding for trace mineral density in rice. Food Nutr. Bull. 2000, 21, 382–386. [Google Scholar] [CrossRef]
- Haas, J.D.; Beard, J.L.; Murray-Kolb, L.E.; del Mundo, A.M.; Felix, A.; Gregorio, G.B. Iron-biofortified rice improves the iron stores of non-anemic Filipino women. J. Nutr. 2005, 135, 2823–2830. [Google Scholar] [CrossRef] [PubMed]
- Tsakirpaloglou, N.; Mallikarjuna Swamy, B.P.; Acuin, C.; Slamet-Loedin, I.H. Biofortified Zn and Fe rice: Potential contribution for dietary mineral and human health. In Nutritional Quality Improvement in Plants. Concepts and Strategies in Plant Sciences; Jaiwal, P., Chhillar, A., Chaudhary, D., Jaiwal, R., Eds.; Springer: Cham, Switzerland, 2019; pp. 1–24. [Google Scholar]
- Winger, R.; Konig, J.; House, D. Technological issues associated with iodine fortification of foods. Trends Food Sci. Technol. 2008, 19, 94–101. [Google Scholar] [CrossRef]
- Nayyar, H.; Kaur, S.; Singh, S.; Upadhyaya, H.D. Differential sensitivity of Desi (small-seeded) and Kabuli (large-seeded) chickpea genotypes to water stress during seed filling: Effects on accumulation of seed reserves and yield. J. Sci. Food Agric. 2006, 86, 2076–2082. [Google Scholar] [CrossRef]
- Tar’an, B.; Warkentin, T.D.; Tullu, A.; Vandenberg, A. Genetic mapping of ascochyta blight resistance in chickpea (Cicer arietinum L.) using a simple sequence repeat linkage map. Genome 2007, 50, 26–34. [Google Scholar] [CrossRef]
- Bueckert, R.A.; Wagenhoffer, S.; Hnatowich, G.; Warkentin, T.D. Effect of heat and precipitation on pea yield and reproductive performance in the field. Can. J. Plant Sci. 2015, 95, 629–639. [Google Scholar] [CrossRef]
- Atienza, S.G.; Palomino, C.; Gutiérrez, N.; Alfaro, C.M.; Rubiales, D.; Torres, A.M.; Ávila, C.M. QTLs for ascochyta blight resistance in faba bean (Vicia faba L.): Validation in field and controlled conditions. Crop Pasture Sci. 2016, 67, 216–224. [Google Scholar] [CrossRef]
- Jha, A.B.; Tar’an, B.; Stonehouse, R.; Warkentin, T.D. Identification of QTLs associated with improved resistance to ascochyta blight in an interspecific pea recombinant inbred line population. Crop Sci. 2016, 56, 2926–2939. [Google Scholar] [CrossRef]
- Jha, A.B.; Gali, K.K.; Tar’an, B.; Warkentin, T.D. Fine mapping of QTLs for ascochyta blight resistance in pea using heterogeneous inbred families. Front. Plant Sci. 2017, 8, 765. [Google Scholar] [CrossRef]
| Micronutrient | Pulse Crop | Plant Material | Concentration Range (mg kg−1) | Genomic Region/Marker | Reference |
|---|---|---|---|---|---|
| Iron | Common bean | Genotype | 34–89 | 7 QTLs | [169] |
| Common bean | Genotype | 35–92 | [170] | ||
| Common bean | DOR364 X G19833 | 40–85 | 13 QTLs | [171] | |
| Common bean | G14519 X G4825 | 36–97 | 5 QTLs | [172] | |
| Common bean | G21242 X G21078 | 28–95 | 6 QTLs | [173] | |
| Common bean | Genotype | 30–110 | [175] | ||
| Lentil | Genotype | 41–109 | [174] | ||
| Lentil | ILL 8006 X CDC Milestone | 37–176 | 21 QTLs | [24] | |
| Lentil | Genotype | 41–102 | 9 SNPs | [25] | |
| Lentil | Genotype | 69–86 | [28] | ||
| Chickpea | Genotype | 48–57 | [28] | ||
| Lentil | Genotype | 76–100 | [11] | ||
| Pea | Genotype | 48–58 | [11] | ||
| Common bean | Genotype | 58–81 | [11] | ||
| Chickpea | Genotype | 49–56 | [11] | ||
| Chickpea | Genotype | 36–86 | 4 SNPs | [12] | |
| Chickpea | ICC 4958 X ICC 8261 | 40–67 | 6 QTLs | [23] | |
| Chickpea | Genotype | 40–91 | 10 SNPs | [23] | |
| Mungbean | Genotype | 35–87 | [176] | ||
| Pea | Genotype | 26–94 | 9 SNPs | [13] | |
| Pea | PI 648006 X PI 357292 | 37–62 | 5 QTLs | [26] | |
| Pea | Orb X CDC Striker | 26–49 | 4 QTLs | [27] | |
| Pea | Carerra X CDC Striker | 34–67 | 6 QTLs | [27] | |
| Pea | Genotype | 29–91 | 3 SNPs | [29] | |
| Zinc | Common bean | Genotype | 21–54 | 11 QTLs | [169] |
| Common bean | Genotype | 21–60 | [170] | ||
| Common bean | DOR364 X G19833 | 18–42 | 13 QTLs | [171] | |
| Common bean | G14519 X G4825 | 17–49 | 8 QTLs | [172] | |
| Common bean | G21242 X G21078 | 17–57 | 3 QTLs | [173] | |
| Common bean | Genotype | 25–60 | [175] | ||
| Lentil | Genotype | 22–77 | [174] | ||
| Lentil | Genotype | 23–54 | 12 SNPs | [25] | |
| Lentil | Genotype | 46–55 | [28] | ||
| Chickpea | 35–43 | [28] | |||
| Lentil | Genotype | 37–51 | [11] | ||
| Pea | Genotype | 27–34 | [11] | ||
| Common bean | Genotype | 25–33 | [11] | ||
| Chickpea | Genotype | 21–28 | [11] | ||
| Chickpea | Genotype | 19–62 | 5 SNPs | [12] | |
| Chickpea | ICC 4958 X ICC 8261 | 28–48 | 5 QTLs | [23] | |
| Chickpea | Genotype | 27–62 | 10 SNPs | [23] | |
| Mungbean | Genotype | 21–62 | [176] | ||
| Pea | Genotype | 14–93 | 2 SNPs | [13] | |
| Pea | PI 648006 X PI 357292 | 31–62 | 5 QTLs | [26] | |
| Pea | Orb X CDC Striker | 25–34 | 4 QTLs | [27] | |
| Pea | Carerra X CDC Striker | 17–41 | 6 QTLs | [27] | |
| Pea | Genotype | 13–51 | 7 SNP | [29] | |
| Selenium | Lentil | Genotype | 0.3–2.6 | [180] | |
| Lentil | Genotype | 0.01–0.3 | [181] | ||
| Lentil | Genotype | 0.4–0.5 | [28] | ||
| Chickpea | Genotype | 0.3–0.4 | [28] | ||
| Lentil | Genotype | 0.9–1.6 | [11] | ||
| Pea | Genotype | 0.4–0.5 | [11] | ||
| Common bean | Genotype | 0.4–0.5 | [11] | ||
| Chickpea | Genotype | 0.6–0.9 | [11] | ||
| Mungbean | Genotype | 0.2–0.9 | [176] | ||
| Pea | Genotype | 0.03–1.8 | [182] | ||
| Pea | Genotype | 0.08–5.5 | [13] | ||
| Pea | Orb X CDC Striker | 0.3–2.2 | 3 QTLs | [27] | |
| Pea | Carerra X CDC Striker | 0.1–6.8 | 6 QTLs | [27] | |
| Pea | Genotype | 0.1– 8.7 | 44 SNPs | [29] | |
| Carotenoids | Chickpea | Not available | 5 QTLs | [183] | |
| Chickpea | Genotype | 311–880 | [184] | ||
| Chickpea | Genotype | 11–19 | [185] | ||
| Chickpea | Genotype | 9–31 | [186] | ||
| Chickpea | Genotype | 22–44 | [187] | ||
| Chickpea | CDC Jade X CDC Frontier | 15–58 | 8 QTLs | [188] | |
| Chickpea | Cory X CDC Jade | 2–78 | 5 QTLs | [188] | |
| Chickpea | ICC4475 X CDC Jade | 22–84 | 5 QTLs | [188] | |
| Pea | Genotype | 7–23 | [185] | ||
| Pea | Genotype | 6–27 | [186] | ||
| Folates | Pea | Genotype | 0.6 | [189] | |
| Pea | Genotype | 0.3–0.7 | [190] | ||
| Common bean | Genotype | 1.4–1.6 | [190] | ||
| Lentil | Genotype | 1.5–2.0 | [190] | ||
| Pea | Genotype | 0.5 | [87] | ||
| Lentil | Genotype | 0.7 | [87] | ||
| Faba bean | Genotype | 1.0 | [87] | ||
| Chickpea | Genotype | 1.5 | [87] | ||
| Chickpea | Genotype | 2.7 | [191] | ||
| Common bean | Genotype | 1.1–1.6 | [191] | ||
| Lentil | Genotype | 1.1–1.5 | [191] | ||
| Pea | Genotype | 0.1–0.2 | [191] | ||
| Pea | Genotype | 0.4–2.0 | [192] | ||
| Chickpea | Genotype | 0.4–1.2 | [192] | ||
| Lentil | Genotype | 2.2–2.9 | [192] | ||
| Chickpea | Genotype | 3.5–5.9 | [15] | ||
| Common bean | Genotype | 1.6–2.3 | [15] | ||
| Lentil | Genotype | 1.4–1.8 | [15] | ||
| Pea | Genotype | 0.2–0.3 | [15] | ||
| Chickpea | Genotype | 4.0–4.3 | [193] | ||
| Common bean | Genotype | 2.4–3.0 | [193] | ||
| Lentil | Genotype | 1.2–1.6 | [193] | ||
| Pea | Genotype | 0.1–0.2 | [193] | ||
| Lentil | Genotype | 1.7–5.0 | [194] | ||
| Pea | Genotype | 0.1–0.6 | 31 SNPs | [30] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jha, A.B.; Warkentin, T.D. Biofortification of Pulse Crops: Status and Future Perspectives. Plants 2020, 9, 73. https://doi.org/10.3390/plants9010073
Jha AB, Warkentin TD. Biofortification of Pulse Crops: Status and Future Perspectives. Plants. 2020; 9(1):73. https://doi.org/10.3390/plants9010073
Chicago/Turabian StyleJha, Ambuj B., and Thomas D. Warkentin. 2020. "Biofortification of Pulse Crops: Status and Future Perspectives" Plants 9, no. 1: 73. https://doi.org/10.3390/plants9010073
APA StyleJha, A. B., & Warkentin, T. D. (2020). Biofortification of Pulse Crops: Status and Future Perspectives. Plants, 9(1), 73. https://doi.org/10.3390/plants9010073





