Symbionts as Filters of Plant Colonization of Islands: Tests of Expected Patterns and Environmental Consequences in the Galapagos
Abstract
1. Introduction
2. Results
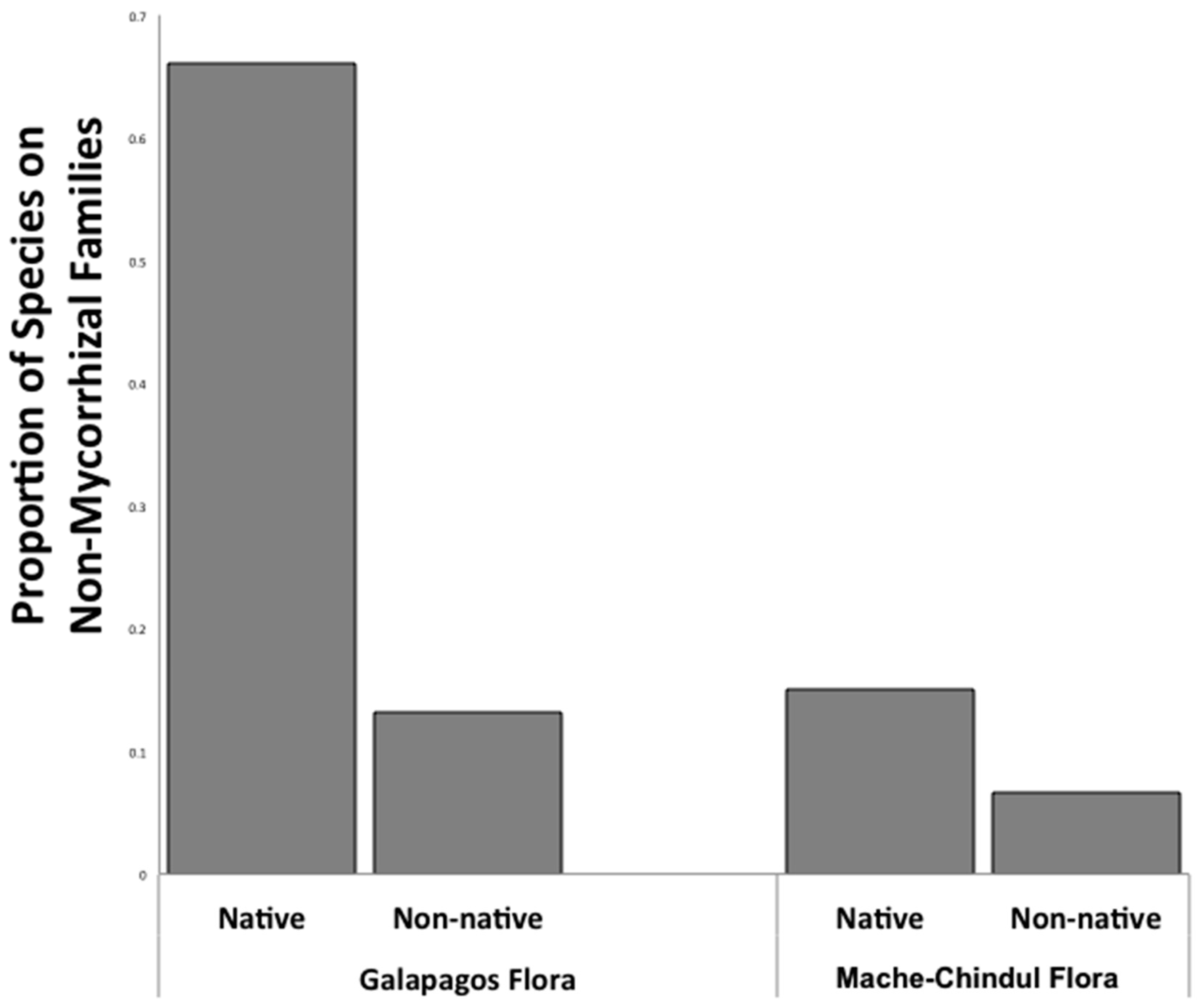
2.1. Flora Mycorrhizal Status Analyses
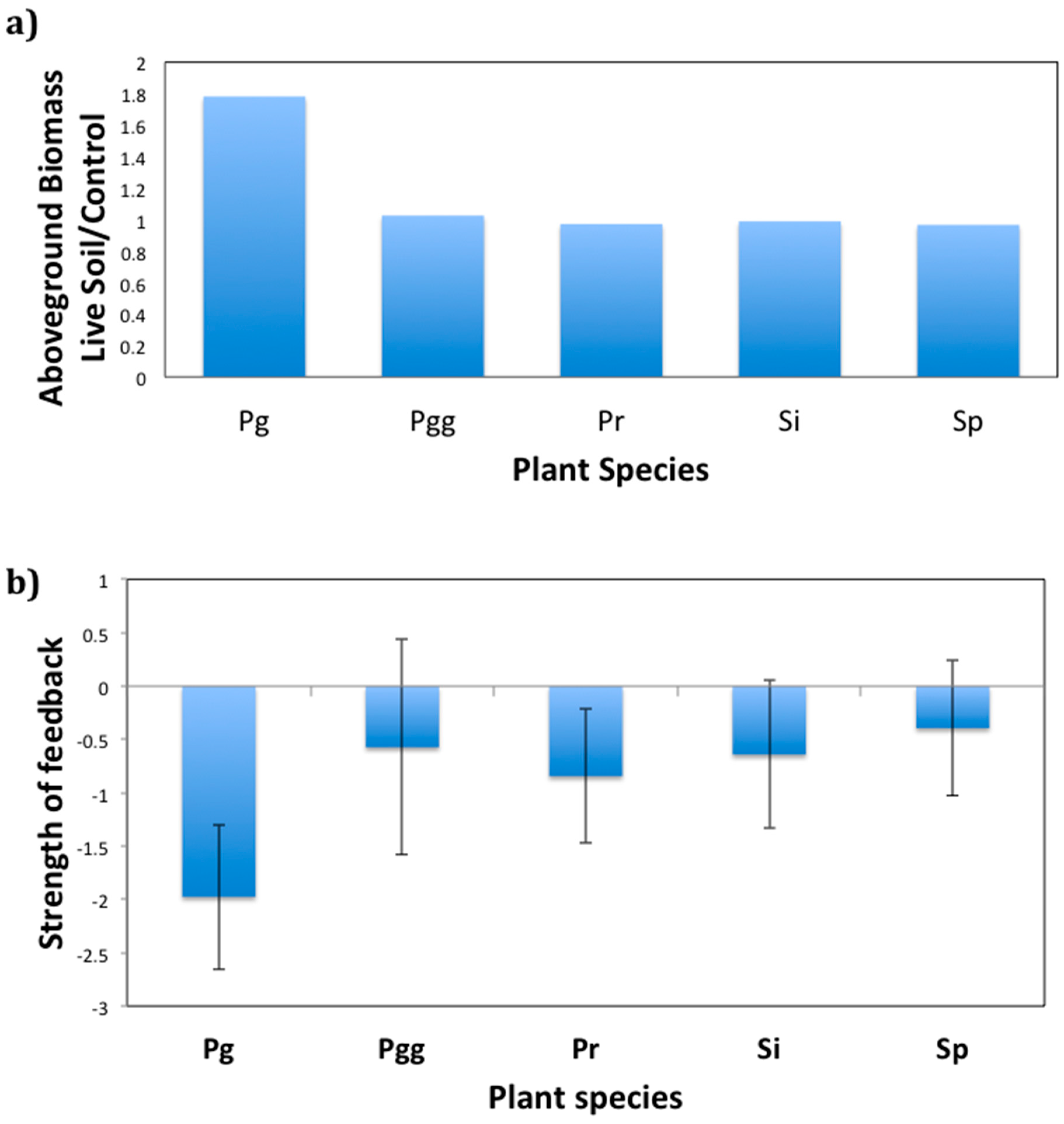
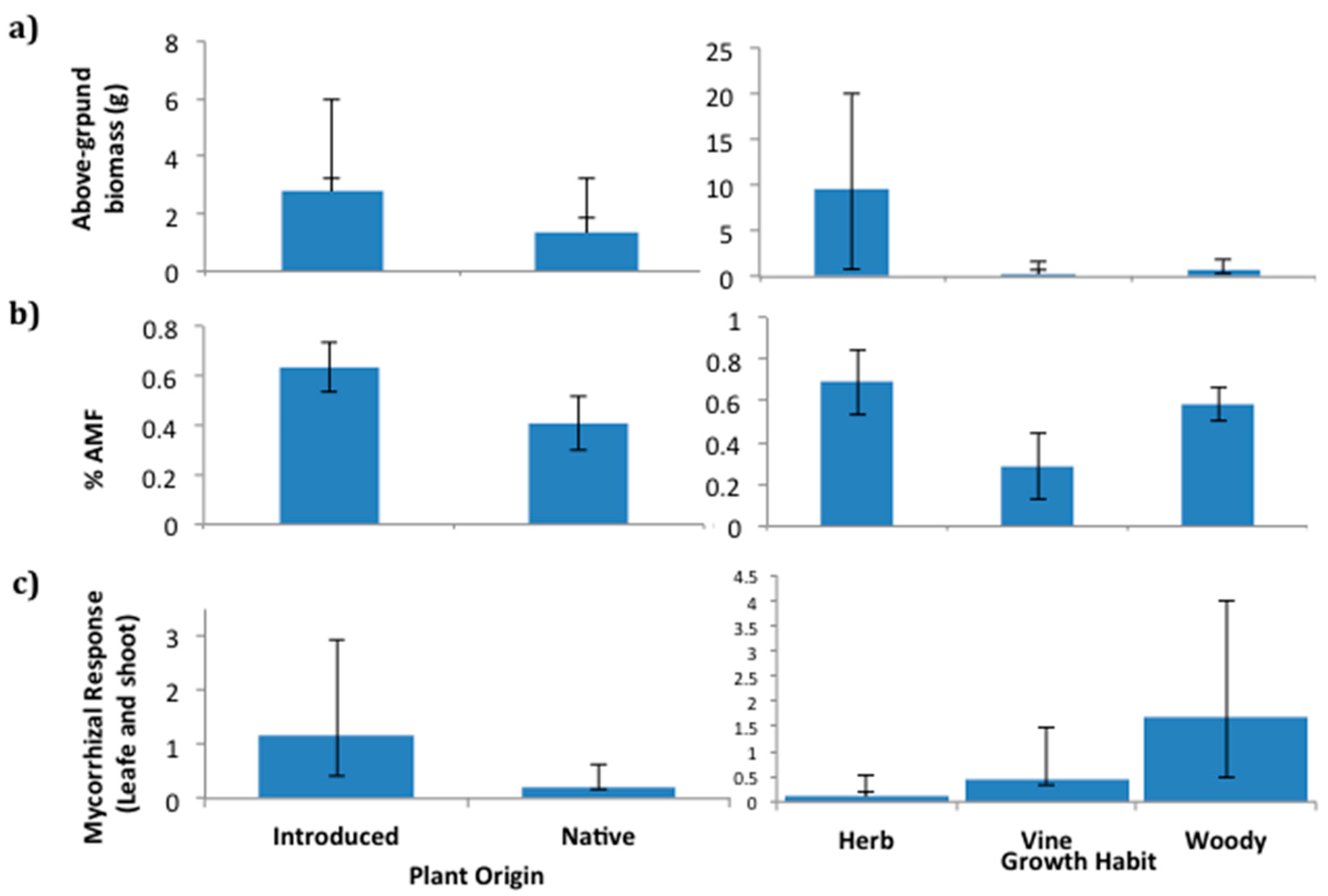
2.2. Plant Growth Response to Soil Fungi
2.3. Plant–Soil Feedback Experiment
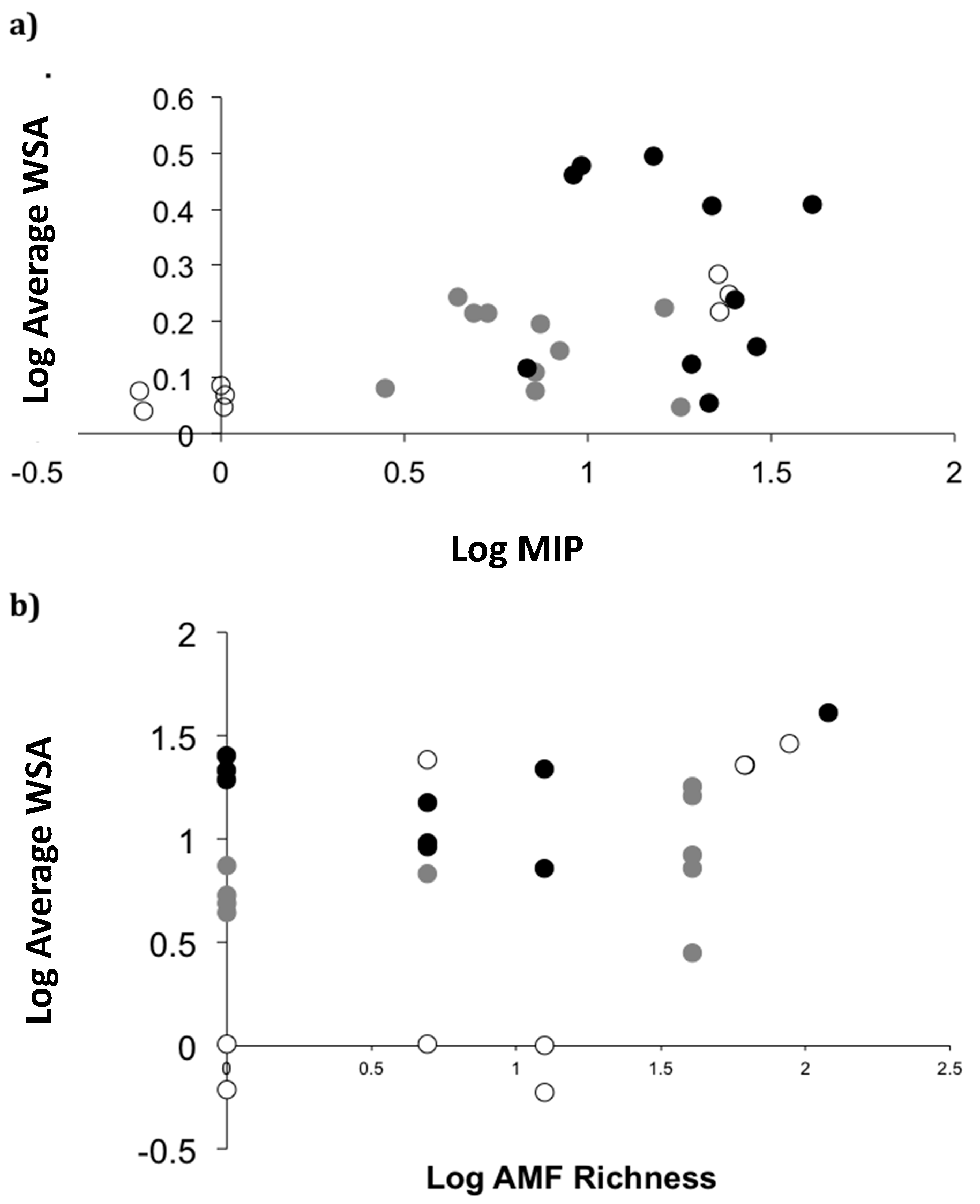
2.4. Field Patterns
3. Discussion
4. Materials and Methods
4.1. Study System
4.2. Experiment 1. Plant Growth Mycorrhizal Response
4.3. Experiment 2. Plant–Soil Feedback
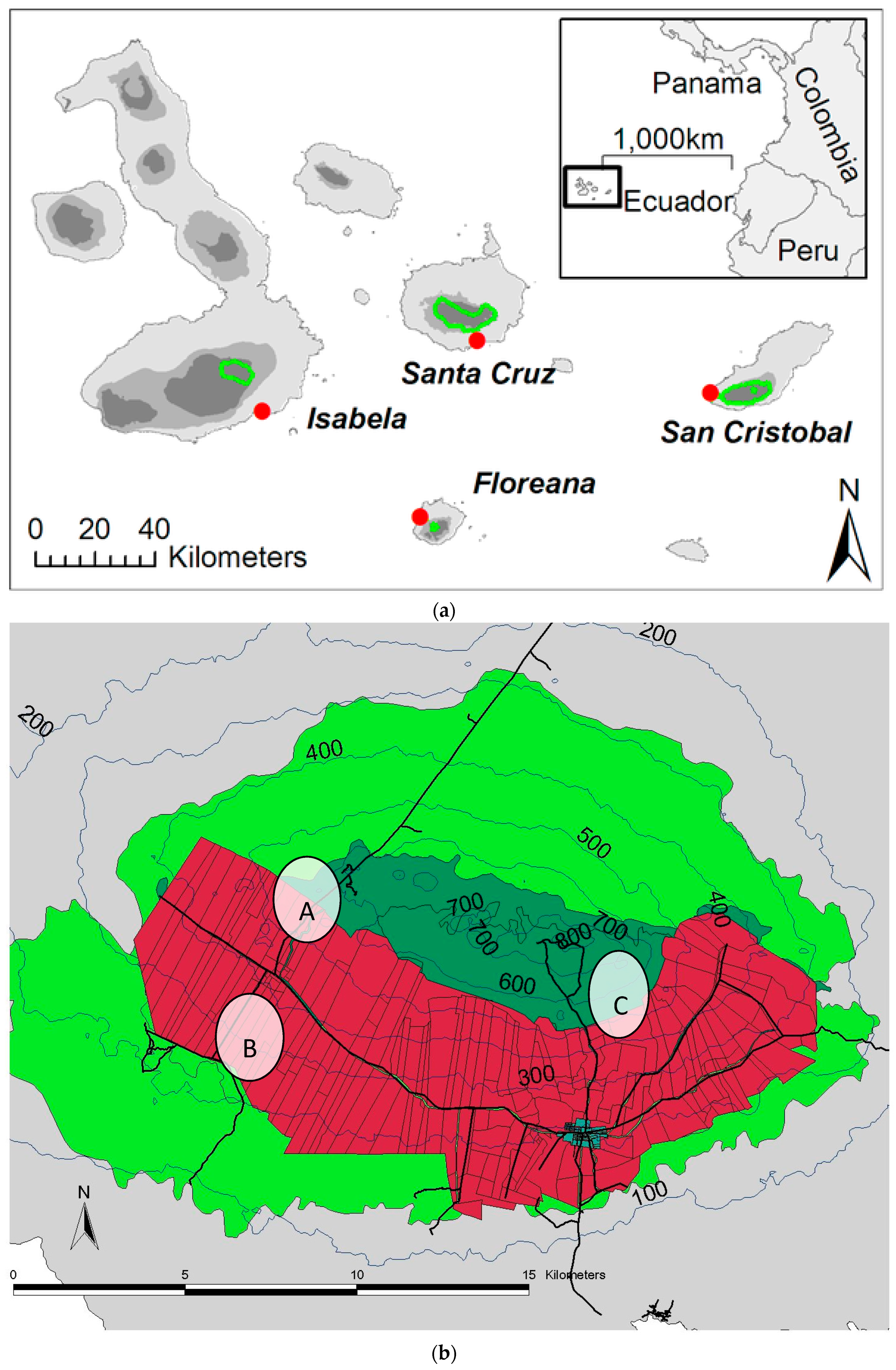
4.4. Field Sites and Sampling
4.5. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 2001; Volume 1. [Google Scholar]
- Thornton, I. Island Colonization: The Origin and Development of Island Communities; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Gillespie, R.G.; Clague, D.A. Encyclopedia of Islands; University of California Press: Berkeley, CA, USA, 2009. [Google Scholar]
- Patiño, J.; Whittaker, R.J.; Borges, P.A.; Fernández-Palacios, J.M.; Ah-Peng, C.; Araújo, M.B.; Ávila, S.P.; Cardoso, P.; Cornuault, J.; de Boer, E.J. A Roadmap for Island Biology: 50 Fundamental Questions after 50 Years of The Theory of Island Biogeography. J. Biogeogr. 2017, 44, 963–983. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: New York, NY, USA, 2010. [Google Scholar]
- Duchicela, J.; Vogelsang, K.M.; Schultz, P.A.; Kaonongbua, W.; Middleton, E.L.; Bever, J.D. Non-native Plants and Soil Microbes: Potential Contributors to the Consistent Reduction in Soil Aggregate Stability Caused by the Disturbance of North American Grasslands. New Phytol. 2012, 196, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Rúa, M.A.; Antoninka, A.; Antunes, P.M.; Chaudhary, V.B.; Gehring, C.; Lamit, L.J.; Piculell, B.J.; Bever, J.D.; Zabinski, C.; Meadow, J.F. Home-Field Advantage? Evidence of Local Adaptation among Plants, Soil, and Arbuscular Mycorrhizal Fungi through Meta-Analysis. BMC Evol. Biol. 2016, 16, 122. [Google Scholar] [CrossRef] [PubMed]
- Redecker, D.; Morton, J.B.; Bruns, T.D. Ancestral Lineages of Arbuscular Mycorrhizal Fungi (Glomales). Mol. Phylogenet. Evol. 2000, 14, 276–284. [Google Scholar] [CrossRef]
- Elias, S.A. The Problem of Conifer Species Migration Lag in the Pacific Northwest Region since the Last Glaciation. Quat. Sci. Rev. 2013, 77, 55–69. [Google Scholar] [CrossRef]
- Bueno, C.G.; Moora, M. How Do Arbuscular Mycorrhizal Fungi Travel? New Phytol. 2019, 222, 645–647. [Google Scholar] [CrossRef]
- Abbott, K.C.; Karst, J.; Biederman, L.A.; Borrett, S.R.; Hastings, A.; Walsh, V.; Bever, J.D. Spatial Heterogeneity in Soil Microbes Alters Outcomes of Plant Competition. PLoS ONE 2015, 10, e0125788. [Google Scholar] [CrossRef]
- Maherali, H.; Oberle, B.; Stevens, P.F.; Cornwell, W.K.; McGlinn, D.J. Mutualism Persistence and Abandonment during the Evolution of the Mycorrhizal Symbiosis. Am. Nat. 2016, 188, E113–E125. [Google Scholar] [CrossRef]
- Peay, K.G.; Garbelotto, M.; Bruns, T.D. Evidence of Dispersal Limitation in Soil Microorganisms: Isolation Reduces Species Richness on Mycorrhizal Tree Islands. Ecology 2010, 91, 3631–3640. [Google Scholar] [CrossRef]
- Koske, R.E.; Gemma, J.N. Arbuscular Mycorrhizal Fungi in Hawaiian Sand Dunes: Island of Kaua’i; University of Hawaii Press: Honolulu, HI, USA, 1996. [Google Scholar]
- Delavaux, C.S.; Weigelt, P.; Dawson, W.; Duchicela, J.; Essl, F.; van Kleunen, M.; König, C.; Pergl, J.; Pyšek, P.; Stein, A. Mycorrhizal Fungi Influence Global Plant Biogeography. Nat. Ecol. Evol. 2019, 3, 424. [Google Scholar] [CrossRef] [PubMed]
- Hooker, J.D.X. On the Vegetation of the Galapagos Archipelago, as Compared with That of Some Other Tropical Islands and of the Continent of America. Trans. Linn. Soc. Lond. 1847, 20, 235–262. [Google Scholar] [CrossRef]
- Guzmán, B.; Heleno, R.; Nogales, M.; Simbaña, W.; Traveset, A.; Vargas, P. Evolutionary History of the Endangered Shrub Snapdragon (G Alvezia Leucantha) of the Galápagos Islands. Divers. Distrib. 2017, 23, 247–260. [Google Scholar] [CrossRef]
- Porter, D.M. Vascular Plants of the Galapagos: Origins and Dispersal. In Patterns of Evolution in Galapagos Organisms, Proceedings of a Meeting of the American Association for the Advancement of Science, Pacific Division; San Francisco State University: San Francisco, CA, USA; California Academy of Sciences: San Francisco, CA, USA, 1983; pp. 33–96. [Google Scholar]
- Trusty, J.L.; Tye, A.; Collins, T.M.; Michelangeli, F.A.; Madriz, P.; Francisco-Ortega, J. Galápagos and Cocos Islands: Geographically Close, Botanically Distant. Int. J. Plant Sci. 2012, 173, 36–53. [Google Scholar] [CrossRef]
- Vargas, P.; Heleno, R.; Traveset, A.; Nogales, M. Colonization of the Galápagos Islands by Plants with No Specific Syndromes for Long-distance Dispersal: A New Perspective. Ecography 2012, 35, 33–43. [Google Scholar] [CrossRef]
- Carvajal-Endara, S.; Hendry, A.P.; Emery, N.C.; Davies, T.J. Habitat Filtering Not Dispersal Limitation Shapes Oceanic Island Floras: Species Assembly of the Galápagos Archipelago. Ecol. Lett. 2017, 20, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Bever, J.D.; Schultz, P.A.; Pringle, A.; Morton, J.B. Arbuscular Mycorrhizal Fungi: More Diverse than Meets the Eye, and the Ecological Tale of Why. Bioscience 2001, 51, 923–931. [Google Scholar] [CrossRef]
- Koske, R.E.; Gemma, J.N.; Flynn, T. Mycorrhizae in Hawaiian Angiosperms: A Survey with Implications for the Origin of the Native Flora. Am. J. Bot. 1992, 79, 853–862. [Google Scholar] [CrossRef]
- Gemma, J.N.; Koske, R.E. Mycorrhizae in Recent Volcanic Substrates in Hawaii. Am. J. Bot. 1990, 77, 1193–1200. [Google Scholar] [CrossRef]
- Crawford, K.M.; Bauer, J.T.; Comita, L.S.; Eppinga, M.B.; Johnson, D.J.; Mangan, S.A.; Queenborough, S.A.; Strand, A.E.; Suding, K.N.; Umbanhowar, J.; et al. When and Where Plant-Soil Feedback May Promote Plant Coexistence: A Meta-Analysis. Ecol. Lett. 2019, 22, 1274–1284. [Google Scholar] [CrossRef]
- Koziol, L.; Bever, J.D. Mycorrhizal Response Trades off with Plant Growth Rate and Increases with Plant Successional Status. Ecology 2015, 96, 1768–1774. [Google Scholar] [CrossRef]
- Bauer, J.T.; Koziol, L.; Bever, J.D. Ecology of Floristic Quality Assessment: Testing for Correlations between Coefficients of Conservatism, Species Traits and Mycorrhizal Responsiveness. AoB Plants 2017, 10, plx073. [Google Scholar] [CrossRef]
- Janos, D.P. Mycorrhizae Influence Tropical Succession. Biotropica 1980, 12, 56–64. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C. A Meta-analysis of Context-dependency in Plant Response to Inoculation with Mycorrhizal Fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef]
- Schmidt, S.K.; Scow, K.M. Mycorrhizal Fungi on the Galapagos Islands. Biotropica 1986, 18, 236–240. [Google Scholar] [CrossRef]
- Pringle, A.; Bever, J.D.; Gardes, M.; Parrent, J.L.; Rillig, M.C.; Klironomos, J.N. Mycorrhizal Symbioses and Plant Invasions. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 699–715. [Google Scholar] [CrossRef]
- Vogelsang, K.M.; Bever, J.D. Mycorrhizal Densities Decline in Association with Nonnative Plants and Contribute to Plant Invasion. Ecology 2009, 90, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Barto, E.K.; Antunes, P.M.; Stinson, K.; Koch, A.M.; Klironomos, J.N.; Cipollini, D. Differences in Arbuscular Mycorrhizal Fungal Communities Associated with Sugar Maple Seedlings in and Outside of Invaded Garlic Mustard Forest Patches. Biol. Invasions 2011, 13, 2755–2762. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Lekberg, Y.; Klironomos, J.; Maherali, H. Does Responsiveness to Arbuscular Mycorrhizal Fungi Depend on Plant Invasive Status? Ecol. Evol. 2017, 7, 6482–6492. [Google Scholar] [CrossRef]
- Duchicela, J.; Sullivan, T.S.; Bontti, E.; Bever, J.D. Soil Aggregate Stability Increase Is Strongly Related to Fungal Community Succession along an Abandoned Agricultural Field Chronosequence in the B Olivian A Ltiplano. J. Appl. Ecol. 2013, 50, 1266–1273. [Google Scholar] [CrossRef]
- Wilson, G.W.; Hartnett, D.C. Interspecific Variation in Plant Responses to Mycorrhizal Colonization in Tallgrass Prairie. Am. J. Bot. 1998, 85, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.L.; Richardson, S.; Koziol, L.; Palmer, C.E.; Yermakov, Z.; Henning, J.A.; Schultz, P.A.; Bever, J.D. Locally Adapted Arbuscular Mycorrhizal Fungi Improve Vigor and Resistance to Herbivory of Native Prairie Plant Species. Ecosphere 2015, 6, 1–16. [Google Scholar] [CrossRef]
- Koziol, L.; Schultz, P.A.; House, G.L.; Bauer, J.T.; Middleton, E.L.; Bever, J.D. The Plant Microbiome and Native Plant Restoration: The Example of Native Mycorrhizal Fungi. BioScience 2018, 68, 996–1006. [Google Scholar] [CrossRef]
- Ali, J.R.; Aitchison, J.C. Exploring the Combined Role of Eustasy and Oceanic Island Thermal Subsidence in Shaping Biodiversity on the Galápagos. J. Biogeogr. 2014, 41, 1227–1241. [Google Scholar] [CrossRef]
- Geist, D.J.; Snell, H.; Snell, H.; Goddard, C.; Kurz, M.D. A Paleogeographic Model of the Galápagos Islands and Biogeographical and Evolutionary Implications. The Galápagos: A Natural Laboratory for the Earth Sciences; American Geophysical Union: Washington DC, USA, 2014; pp. 145–166. [Google Scholar]
- Triantis, K.; Whittaker, R.J.; Fernández-Palacios, J.M.; Geist, D.J. Oceanic Archipelagos: A Perspective on the Geodynamics and Biogeography of the World’s smallest Biotic Provinces. Issue Front. Biogeogr. 2016, 8, 1–9. [Google Scholar]
- Porter, D.M.; Wiggins, I.L. Flora of the Galápagos Islands; Stanford University Press: Stanford, CA, USA, 1971. [Google Scholar]
- Itow, S. Altitudinal Change in Plant Endemism, Species Turnover, and Diversity on Isla Santa Cruz, the Galapagos Islands; University of Hawai’i Press: Honolulu, HI, USA, 1992. [Google Scholar]
- Trueman, M.; Hobbs, R.J.; Van Niel, K. Interdisciplinary Historical Vegetation Mapping for Ecological Restoration in Galapagos. Landsc. Ecol. 2013, 28, 519–532. [Google Scholar] [CrossRef]
- Restrepo, A.; Colinvaux, P.; Bush, M.; Correa-Metrio, A.; Conroy, J.; Gardener, M.R.; Jaramillo, P.; Steinitz-Kannan, M.; Overpeck, J. Impacts of Climate Variability and Human Colonization on the Vegetation of the Galápagos Islands. Ecology 2012, 93, 1853–1866. [Google Scholar] [CrossRef]
- Watson, J.; Trueman, M.; Tufet, M.; Henderson, S.; Atkinson, R. Mapping Terrestrial Anthropogenic Degradation on the Inhabited Islands of the Galapagos Archipelago. Oryx 2010, 44, 79–82. [Google Scholar] [CrossRef]
- Mauchamp, A. Threats from Alien Plant Species in the Galápagos Islands. Conserv. Biol. 1997, 11, 260–263. [Google Scholar] [CrossRef]
- Guézou, A.; Trueman, M.; Buddenhagen, C.E.; Chamorro, S.; Guerrero, A.M.; Pozo, P.; Atkinson, R. An Extensive Alien Plant Inventory from the Inhabited Areas of Galapagos. PLoS ONE 2010, 5, e10276. [Google Scholar] [CrossRef]
- Jaramillo, P.; Guézou, A. Charles Darwin Foundation Galápagos Speczies Checklist. Puerto Ayora: Charles Darwin Foundation. Available online: http://www.darwinfoundation.org/datazone/checklists/vascular-plants/ (accessed on 23 July 2012).
- Clark, J.L.; Neill, D.A.; Asanza, M. Floristic Checklist of the Mache-Chindul Mountains of Northwestern Ecuador. Contrib. U. S. Natl. Herb. 2006, 54, 1–180. [Google Scholar]
- Brundrett, M.C. Mycorrhizal Associations and Other Means of Nutrition of Vascular Plants: Understanding the Global Diversity of Host Plants by Resolving Conflicting Information and Developing Reliable Means of Diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, Y.-L. Phylogenetic Distribution and Evolution of Mycorrhizas in Land Plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.W.T.; Williamson, M.M. Topsin-M: The New Benomyl for Mycorrhizal-Suppression Experiments. Mycologia 2008, 100, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Kahiluoto, H.; Ketoja, E.; Vestberg, M. Creation of a Non-Mycorrhizal Control for a Bioassay of AM Effectiveness. Mycorrhiza 2000, 9, 241–258. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A New Method Which Gives an Objective Measure of Colonization of Roots by Vesicular—Arbuscular Mycorrhizal Fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Garnier, E.; Shipley, B.; Roumet, C.; Laurent, G. A Standardized Protocol for the Determination of Specific Leaf Area and Leaf Dry Matter Content. Funct. Ecol. 2001, 15, 688–695. [Google Scholar] [CrossRef]
- Bever, J.D. Feeback between Plants and Their Soil Communities in an Old Field Community. Ecology 1994, 75, 1965–1977. [Google Scholar] [CrossRef]
- Stohlgren, T.J.; Falkner, M.B.; Schell, L.D. A Modified-Whittaker Nested Vegetation Sampling Method. Vegetatio 1995, 117, 113–121. [Google Scholar] [CrossRef]
- Kercher, S.M.; Frieswyk, C.B.; Zedler, J.B. Effects of Sampling Teams and Estimation Methods on the Assessment of Plant Cover. J. Veg. Sci. 2003, 14, 899–906. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate Stability and Size Distribution. Agron. Monogr. 1986, 9, 425–442. [Google Scholar]
- Gerdemann, J.W.; Nicolson, T.H. Spores of Mycorrhizal Endogone Species Extracted from Soil by Wet Sieving and Decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duchicela, J.; Bever, J.D.; Schultz, P.A. Symbionts as Filters of Plant Colonization of Islands: Tests of Expected Patterns and Environmental Consequences in the Galapagos. Plants 2020, 9, 74. https://doi.org/10.3390/plants9010074
Duchicela J, Bever JD, Schultz PA. Symbionts as Filters of Plant Colonization of Islands: Tests of Expected Patterns and Environmental Consequences in the Galapagos. Plants. 2020; 9(1):74. https://doi.org/10.3390/plants9010074
Chicago/Turabian StyleDuchicela, Jessica, James D. Bever, and Peggy A. Schultz. 2020. "Symbionts as Filters of Plant Colonization of Islands: Tests of Expected Patterns and Environmental Consequences in the Galapagos" Plants 9, no. 1: 74. https://doi.org/10.3390/plants9010074
APA StyleDuchicela, J., Bever, J. D., & Schultz, P. A. (2020). Symbionts as Filters of Plant Colonization of Islands: Tests of Expected Patterns and Environmental Consequences in the Galapagos. Plants, 9(1), 74. https://doi.org/10.3390/plants9010074




