Abstract
The response of cocoa (Theobroma cacao L.) to low oxygen availability in the soil and the possibility of recovery after stress relief are associated with the plasticity capacity of each genotype; however, studies evaluating the influence of rootstock on stress response are scarce. Thus, in the northern region of the state of Espírito Santo, municipality of São Mateus, the physiological, biochemical, and anatomical responses and recovery capacity of cocoa PS-1319 grafted onto the rootstocks TSH-1188, Cepec-2002, Pará, Esfip-02, and SJ-02 were evaluated under flooded conditions. The plants were subjected to flooding for 60 days, and their recovery capacity was assessed after this period. The gas exchange, relative chlorophyll content, stem and leaf anatomy, photosynthetic pigments, and carbohydrates were evaluated. All genotypes showed reductions in net photosynthetic assimilation, stomatal conductance, and transpiration rate in the flooded environment compared to the non-flooded environment. All pigments were degraded, with average values of Chl a, Chl b, total Chl, and total carotenoids of 9.33, 10.418, 19.75, and 590.75 μg.mL−1 in the non-flooded environment and 6.43, 7.69, 14.12, and 500.33 μg.mL−1 in the flooded environment. The rootstocks Cepec-2002 and Esfip-02 showed the highest carotenoid accumulation, with 585.78 and 650.47 μg.mL−1, respectively, when compared to SJ-02 (474.03 μg.mL−1), Pará (491.58 μg.mL−1), and TSH-1188 (525.86 491.58 μg.mL−1). The Pará rootstock did not show differences in stomatal density between environments, with values of 32.25 in flooding, 34.83 in non-flooding, and 31.61 in recovery. During flooding, lenticels formed in all rootstocks. After recovery, all rootstocks normalized their gas exchange, carbohydrate levels, and anatomy, showing that the root system was able to re-establish its functions, making these rootstocks suitable for areas at risk of flooding.
1. Introduction
The cocoa (Theobroma cacao L.) is one of the most important tropical fruit trees, mainly due to the commercial value of its almonds, which are the raw material for the chocolate industry [1]. In 2023, the world produced approximately 5,600,100.42 t of almonds, with emphasis on the Ivory Coast (2,377,442.05 t), Ghana (653,700 t), Indonesia (641,741.02 t), Ecuador (375,719.14 t), and Brazil (296,145 t). Together, these countries are responsible for more than 77% of the world’s production of cocoa almonds [2].
One of the most limiting factors in the production of cocoa crops is water availability. For crops to develop well, annual rainfall of 1500 to 2000 mm is required, and must be well distributed [3]. Traditionally, the main commercial cocoa-producing plantations are located in regions where water demand is satisfactorily met by the crop [4]. However, climate change has been one of the obstacles to agricultural production [1]. One of the changes observed is the rainfall regime, which, in excess, can reduce the soil’s drainage capacity [5], in addition to causing flooding in riverside regions, causing waterlogging of cultivated soils.
Soil flooding can result in a reduction in gas concentration, resulting in the low availability (hypoxia) or complete lack (anoxia) of oxygen [6], causing metabolic and physiological disorders in plants [7], such as the production of free radicals and oxidative stress [8], leading to cell lysis, in addition to a reduction in the synthesis of photosynthetic pigments [9], decreased gas exchange [10], a drop in the photosynthetic rate [11], stomatal closure [12], and loss in the translocation of carbohydrates from leaves to roots, thus producing an accumulation of photosynthates in the leaves [13]. All of these factors can limit plant development and growth, as well as reduce productivity.
It is important to emphasize that the ability of plants to develop normally after periods of flooding is fundamental to ensuring their survival in the field; however, the responses of different species and genotypes to these conditions are still limited [8]. Thus, the evaluation of different cocoa genotypes during periods of flooding and after stress plays an important role in understanding the ability of plants to restore their functions after flooding, which can be useful in decision-making, guiding farmers in cultural practices and correct crop management [14].
Although scarce, some studies have investigated the recovery of cocoa plants under post-flooding conditions. Sena Gomes and Kozlowski [15] verified the partial recovery of the Catongo genotype 11 days after 30 days of flooding, with the main recovery observed in the root system. Almeida et al. [16] found that cocoa hybrids flooded for 35 days had a recovery of between 78 and 100% of their photosynthetic capacity 10 days after the stressful conditions. Pinto et al. [14] found that cocoa plants had compromised photosynthetic apparatus during flooding; however, after the humidity conditions were normalized, the plants showed signs of recovery.
It is worth noting that a large part of commercial cocoa cultivation is established with seedlings from grafting, where the effects of scion and rootstock combinations are not known. The cocoa genotypes Pará, TSH-1188, Esfip-02, Cepec-2002, and SJ-02 are commonly used as rootstocks due to their ability to help the grafted plant overcome soil and disease challenges. These genotypes are selected for crucial characteristics such as vegetative vigor, which ensures rapid initial seedling growth, and resistance to pathogens like witches’ broom (Moniliophthora perniciosa) and Ceratocystis wilt (Ceratocystis cacaofunesta), which pose significant threats to cocoa cultivation. However, despite the notable importance of these genotypes in seedling production, providing robustness and quality to the plants, they are not characterized in terms of tolerance to flooded environments. Thus, understanding the effects of flooding on the development of the rootstocks TSH-1188, Cepec-2002, Pará, Esfip-02, and SJ-02, which are used in cocoa production and are of global importance in the chocolate manufacturing industry, is essential to obtain information and identify rootstocks that are more tolerant to flooding, ensuring a longer lifespan and greater sustainability for cocoa plantations in flooded areas [14].
Therefore, studies that seek to understand the effects of flooding on different plant species are important to understand the best management methods, in addition to selecting genotypes that can serve as an alternative to minimize the damage caused by stress. Thus, the objective of this study was to evaluate the physiological, biochemical, and anatomical changes in response to flooding and the recovery capacity of T. cacao L. rootstocks.
2. Materials and Methods
2.1. Experiment Conduction and Seedling Production
The study was conducted for 90 days, during the months of May to July, at the Capixaba Institute for Research, Technical Assistance and Rural Extension (INCAPER), located in the municipality of Linhares, Espírito Santo, at the geographic coordinates: 19°25′00.1″ S and 40°04′35.3″ W. The climate data during the experimental period were monitored through an automatic meteorological station (Figure 1).
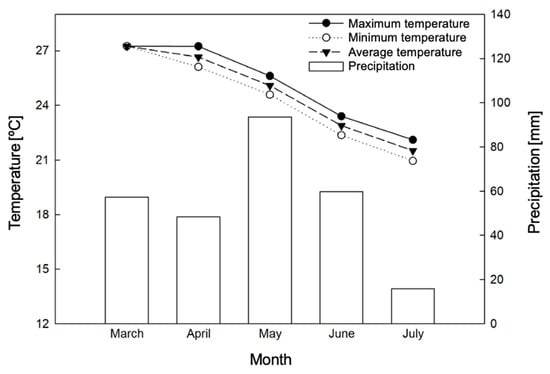
Figure 1.
Total precipitation [mm], relative humidity [%], and maximum [°C], average [°C], and minimum temperatures [°C] recorded at the Linhares weather station (ES), during the acclimatization process and evaluation of the experiment.
The cocoa (Theobroma cacao L.) seedlings used were produced at the Filogônio Peixoto Experimental Station, belonging to the Cocoa Research Center, a research body of the Executive Committee of the Cocoa Cultivar Plan (CEPLAC), located in the municipality of Linhares/ES. The genotypes used were PS-1319 scions, with the rootstock genotypes TSH-1188, Cepec-2002, Pará, Esfip-02, and SJ-02. The seedlings were produced from propagules obtained from mother plants supplied by the CEPLAC Active Germplasm Bank. The genealogy and agronomic descriptors of the six cocoa genotypes used in the experiment are described in Table 1. The cleft grafting technique was used, performed 5 months after sowing the rootstock [17]. Two months after grafting, the seedlings were transplanted into polyethylene bags (25 × 35 cm), one plant per bag, containing a sandy loam substrate with the following chemical characteristics: 0.008 kg m−3 of phosphorus, 0.024 kg m−3 of potassium, 0.007 kg m−3 of sulfur, 0.222 kg m−3 of iron, 0.0026 kg m−3 of zinc, 0.0006 kg m−3 of copper, 0.015 kg m−3 of manganese, 0.00024 kg m−3 of boron, 0.005 kg m−3 of sodium, 0.0486 kg m−3 of magnesium, 0.44088 kg m−3 of calcium, and 9 kg m−3 of organic matter.

Table 1.
Genealogy and agronomic descriptors containing the origin, parents, ancestry, type of pollination, and fruit color and shape of the cocoa (Theobroma cacao L.) genotypes Pará, TSH-1188, Esfip-02, Cepec-2002, SJ-02, and PS-1319.
The plants were transferred to masonry tanks lined with white tarpaulin to prevent water infiltration (Figure 2). A shade cloth was installed 3 m high to block 50% of incident radiation, where the seedlings were acclimatized for 60 days before being subjected to treatments. During acclimatization, the seedlings were irrigated daily with a sprinkler system for 45 min every 4 h. Foliar nitrogen fertilization and pest control were carried out when necessary.

Figure 2.
General view of the cocoa seedling flooding experiment. Masonry tanks lined with white tarpaulin to prevent water infiltration, used in conducting the experiment.
At nine months of age, the plants were subjected to flooding for 60 days, maintaining the water level up to the base of the stem. Water was replenished as needed to prevent column contraction. The dissolved oxygen content was maintained at approximately 0.00898 kg m−3, these values being measured by averaging three readings taken after 3 days without water replenishment. After 60 days of flooding, the tank was emptied, and the plants were irrigated for 30 days under the same conditions as the control, to evaluate the recovery capacity of the evaluated genotypes.
2.2. Gas Exchanges and Chlorophyll Relative Content
Gas exchange and relative chlorophyll content (SPAD) were assessed at 5, 7, 9, 11, 13, 17, 19, 24, 29, 41, 48, and 55 days after flooding, and during recovery at 7, 14, 21, and 29 days after flooding was suspended. Two plants per replicate were used between 7:00 and 10:00 a.m., in fully expanded leaves in the median portion of the plant, at the third node of one leaf per plant. Gas exchange was performed using an infrared gas analyzer (IRGA), model LI-6400 (LI-COR, Lincoln, NE, USA), with a saturating irradiance of 1000 [μmol(photon) m−2 s−1] photons, a temperature of 25 °C, and a CO2 of 400 ppm. From these data, the following were calculated: the ratio between intra/extracellular CO2 concentrations (Ci/Ca); net photosynthetic rate (PN) [μmol(CO2) m−2 s−1]; stomatal conductance (gs) [mmol(H2O) m−2 s−1]; transpiration rate (E) [mmol(H2O) m−2 s−1]; and intrinsic water-use efficiency (WUE) [mol(CO2) mol(H2O)−1]. A portable chlorophyll meter (SPAD-502, Minolta®, Kantō-chihō, Tokyo, Japan) was used to determine the relative chlorophyll content.
2.3. Stem and Leaf Anatomical Evaluations
For anatomical evaluations, one plant per block was used. Lenticel formation was qualitatively assessed by visual evaluation. Stem fixation was performed in FAA solution (formalin (5%), acetic acid (5%), and ethyl alcohol (90%) in 100 mL) at 70% for fifteen days, and then transferred to 70% alcohol, and leaves were fixed directly in 70% alcohol. Histological sections according to [18] were obtained freehand with the aid of a stainless steel blade. Stem and leaf cross sections were subjected to clarification with sodium hypochlorite solution and then washed in distilled water until excess hypochlorite was removed. Sections were stained with safrablau (safranin + Astra blue) and kept in distilled water; paradermal sections of leaves were made using the epidermal impression technique. The temporary slides were observed under a bright-field microscope (Euromex, Gelderland, Duiven, The Netherlands). The images were captured with a microcamera (CMEX 5, Euromex, Gelderland, Duiven, The Netherlands), and the biometric measurements of the tissues were performed with the ImageFocus 4 software. The parameters evaluated for the leaf were stomatal density [mm2], polar diameter/equatorial diameter (PD/DE) ratio, and for the stem, the lenticel length [mm] and lenticel height [mm].
2.4. Photosynthetic Pigments
Pigment extraction was performed 60 days after flooding. Chlorophyll and carotenoid contents were determined using 1–2 cm fragments from the median region of the leaf blade, discarding the central vein, from completely expanded and fresh leaves, from the third node below the apex of the plants, chosen at random. The extraction and quantification of chlorophyll a, b, and total were performed according to the methodology of Arnon [19]. The extraction and quantification of carotenoids were performed according to the methodology described by Duke and Kenyon [20], using the molar absorptivity coefficients of Sandmann et al. [21].
2.5. Carbohydrate Extraction and Quantification
After 60 days of flooding and 30 days after the stress was suspended, leaves and roots of the plants were collected to quantify the levels of total soluble sugars (TSS), reducing sugars (RS), and starch. The plant material was dried in an air circulation and renewal oven (TE-394/3, Tecnal, Piracicaba, São Paulo, Brazil) at 70 °C until constant weight, ground in a Willye-type mill (TE-650, Tecnal and brand) with a 0.6 mm mesh 30 sieve (TE-650C, Tecnal, Piracicaba, São Paulo, Brazil), and stored in a freezer at −18 °C (CHA22, Consul, Santa Catarina, Brazil) until extracts were obtained. The extracts were obtained according to [22], using 0.2 g of leaves and 0.3 g of roots. For the quantification of total soluble sugars (TSS) and starch, the Anthrone method [23] was used, with modifications, using 2 mL of Anthrone solution at 0.19% in sulfuric acid at 93.33%, in a reaction volume of 3 mL, subjected to 100 °C for 3 min. Reducing sugars (RS) were quantified according to the protocol described by [24], through the Dinitrosalicylic Acid (DNS) method.
2.6. Statistical Analysis
The treatments were arranged in a randomized block experimental design, in a factorial scheme (5 × 2). The first factor consisted of five different cocoa rootstocks (TSH-1188, Cepec-2002, Pará, Esfip-02, and SJ-02). The second factor consisted of two conditions, namely flooded and non-flooded. Four blocks were evaluated, each plot consisting of ten plants. Two plants per block were used to measure gas exchange and relative chlorophyll content (SPAD), photosynthetic pigments, and carbohydrate content. For anatomical evaluations of the stem and leaf, one plant per block was used.
The data were subjected to analysis of variance by the F-test at 5% probability. Subsequently, the means were compared by the Scott–Knott clustering test at a significance level of 5% and by the Dunnett test. Statistical analyses were performed using the SISVAR [25] and R programs [26].
3. Results
3.1. The Recovery of Gas Exchanges and SPAD
All genotypes cocoa (Theobroma cacao L.) showed decreases in net photosynthetic rate (PN) (Figure 3), stomatal conductance (gs) (Figure 3), transpiration rate (E) (Figure 4), intrinsic water-use efficiency (WUE) (Figure 4), and relative chlorophyll content (SPAD unit) in leaves (Figure 5). However, there was an increase in the ratio of intra/extracellular CO2 concentrations (Ci/Ca) (Figure 4) under flooding compared to the non-flooded environment. The Esfip-02 genotype showed more sensitive behavior at the beginning of flooding (Figure 3 and Figure 6), while SJ-02 was more stable, since its behavior in the flooded environment did not differ from the behavior during the non-flooded environment when compared to the other genotypes studied. All genotypes, after 40 days of flooding, showed stability in the parameters, maintaining photosynthesis below 2 [μmol(CO2) m−2 s−1], conductance below 0.1 [(H2O) m−2 s−1], and transpiration below 1.0 [(H2O) m−2 s−1] (Figure 3).
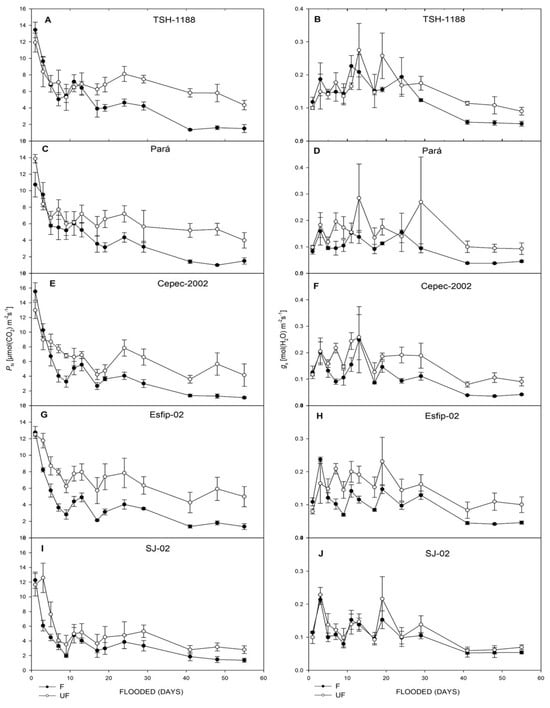
Figure 3.
Net photosynthetic rate (PN) (A,C,E,G,I), and gs stomatal conductance (B,D,F,H,J), of unflooded (UF) and flooded (F) cocoa genotypes TSH-1188, Pará, Cepec-2002, Esfip-02, and SJ-02, evaluated over 55 days of flooding.
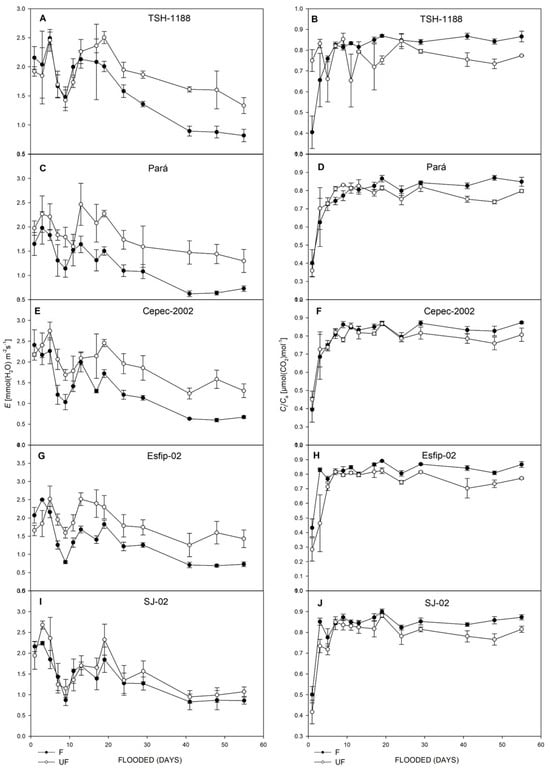
Figure 4.
Transpiration (E) (A,C,E,G,I), and ratio of intra/extracellular CO2 concentrations (Ci/Ca), (B,D,F,H,J), of unflooded (UF) and flooded (F) cocoa genotypes TSH-1188, Pará, Cepec-2002, Esfip-02, and SJ-02, evaluated over 55 days of flooding.
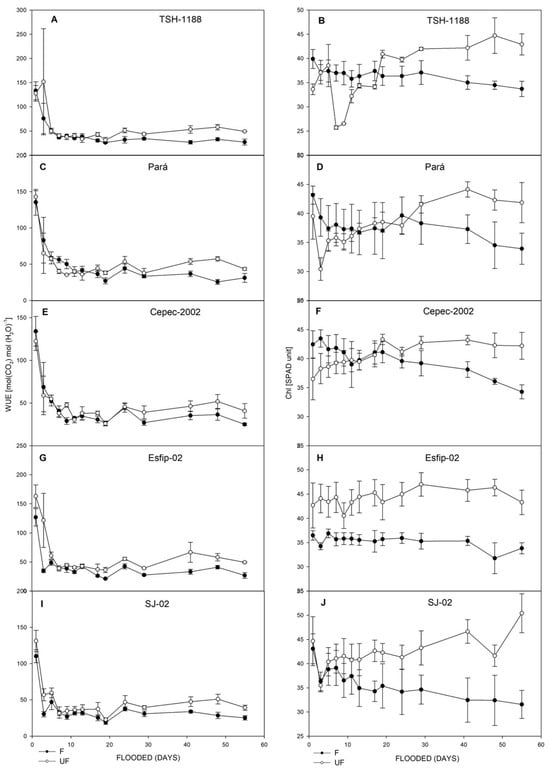
Figure 5.
Water-use efficiency (WUE) (A,C,E,G,I), and Chl (SPAD unit) (B,D,F,H,J), of unflooded (UF) and flooded (F) cocoa genotypes TSH-1188, Pará, Cepec-2002, Esfip-02, and SJ-02, evaluated over 55 days of flooding.
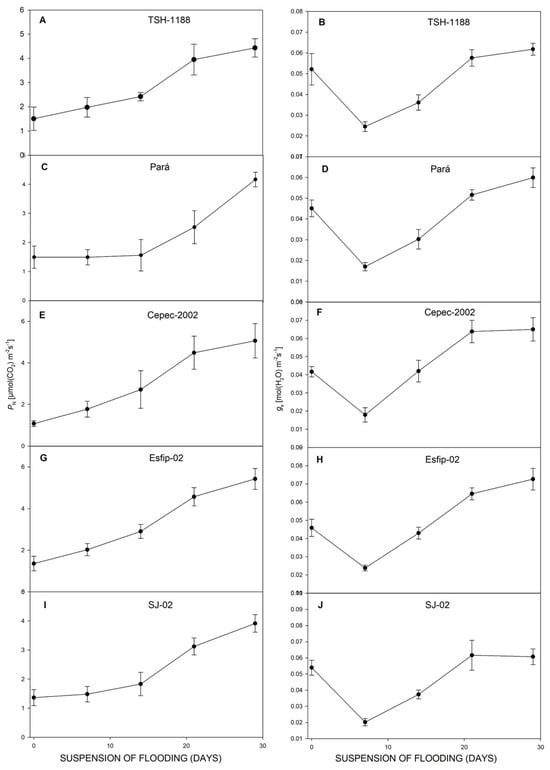
Figure 6.
Net photosynthetic rate (PN) (A,C,E,G,I), and gs stomatal conductance (B,D,F,H,J), of cocoa genotypes TSH-1188, Pará, Cepec-2002, Esfip-02, and SJ-02 during recovery after the flooding interruption. The starting point represents the last assessment (55 days of flooding).
After recovery, all genotypes restored their photosynthesis (Figure 6), transpiration (Figure 6), carbon concentration ratio (Figure 7), and WUE (Figure 8). However, at 29 days of recovery, the genotypes Cepec-2002, Esfip-02, and SJ-02 reduced internal carbon (Figure 7). The genotypes TSH-1188 and Pará were unable to return stomatal conductance to the non-flooded condition level after only 30 days of recovery (Figure 6), while the genotypes Pará and Cepec-2002 were unable to recover their SPAD (Figure 8).
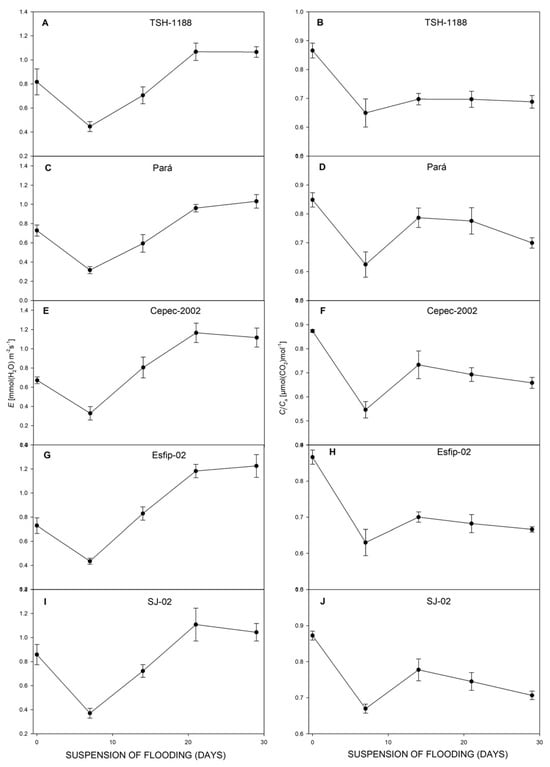
Figure 7.
Transpiration (E) (A,C,E,G,I), and ratio of intra/extracellular CO2 concentrations (Ci/Ca), (B,D,F,H,J), of cocoa genotypes TSH-1188, Pará, Cepec-2002, Esfip-02, and SJ-02 during recovery after the flooding interruption. The starting point represents the last assessment (55 days of flooding).
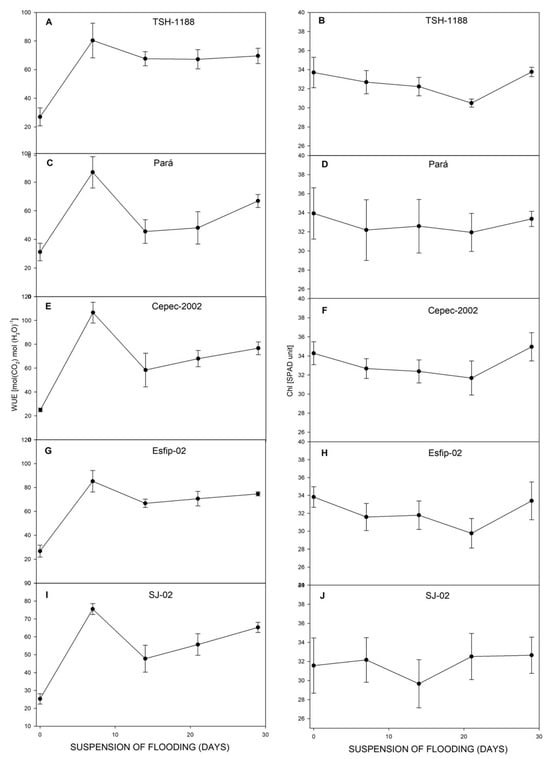
Figure 8.
Water-use efficiency (WUE) (A,C,E,G,I), and Chl (SPAD unit) (B,D,F,H,J), of cocoa genotypes TSH-1188, Pará, Cepec-2002, Esfip-02, and SJ-02 during recovery after the flooding interruption. The starting point represents the last assessment (55 days of flooding).
3.2. Stem and Leaf Anatomy
From the ninth day of flooding, all plants presented hypertrophied lenticels, which remained until the end of the experimental period (Figure 9), and after the flooding was removed, the stem began to reduce the openings. The length of the lenticels (Figure 10) was statistically equal in the genotypes under flooding, and only SJ-02 and Pará did not reduce the length of the lenticels after recovery (Table 2), but there was a reduction in the height of the lenticels after the suspension of the stress (Table 2). The stomatal density increased for SJ-02 and Esfip-02 under flooding, while only SJ-02 reduced the DP/DE ratio in flooding when compared to the other environments (Table 2).

Figure 9.
Formation of hypertrophied lenticels in the stem during flooding stress of Theobroma cacao L. seedlings: (A) non-flooded stem, (B) flooded stem, and (C) stem after recovery.
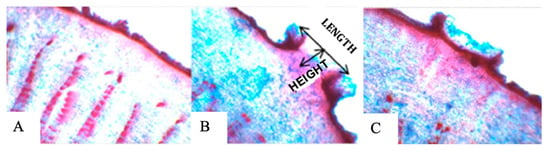
Figure 10.
Anatomical sections of hypertrophied lenticels on the stem during flooding stress of Theobroma cacao L. seedlings: (A) non-flooded stem, (B) flooded stem, and (C) stem after recovery.

Table 2.
Leaf and stem anatomical characteristics (stomatal density, polar diameter/equatorial diameter ratio (DP/DE ratio), lenticel length, and lenticel height), evaluated in flooded and non-flooded plants after 60 days of flooding and recovered after 30 days of flooding suspension in the five assessed cocoa genotypes.
3.3. Flooding on Pigment Contents
There was no statistical difference between the genotypes for chlorophyll content, which was only affected by the environment. Flooding caused a reduction in the content of photosynthetic pigments (Table 3). Carotenoid content differed among the genotypes studied, with Esfip-02 and Cepec-2002 presenting the highest values among the five rootstocks evaluated (Table 3). Among the environments, carotenoid content was degraded by flooding (Table 3).

Table 3.
Analysis of Chlorophyll (Chl a, Chl b, and Chl total) [μg mL−1] and Total carotenoids [μg mL−1] in flooded and non-flooded environments evaluated after 60 days of flooding.
3.4. Effect of Flooding and Recovery on the Carbohydrates
All genotypes showed an accumulation of reducing sugar and total soluble sugar in the leaves during flooding. After flooding was suspended, only SJ-02 did not reduce the accumulation of reducing sugar in the leaves. Except for Pará, soluble sugar in the leaves was reduced during flooding suspension for the other genotypes (Table 4). During flooding, TSH-1188 accumulated more reducing sugar and less soluble sugar; on the other hand, Esfip-02 and Cepec-2002 showed the opposite behavior (Table 4). In the root system, the accumulation of total soluble sugar increased with flooding, while starch content increased during recovery, and the genotypes Esfip-02, SJ-02, and TSH-1188 accumulated greater amounts of starch in the roots. In the leaves, flooding caused starch accumulation, which reduced after recovery (Table 4).

Table 4.
Quantification of carbohydrates reducing sugar (RS) [mg g−1] DM of leaf, total soluble sugar (TSS) [mg g−1] DM of leaf, and root of five genotypes of non-flooded, flooded, and recovered cocoa.
4. Discussion
4.1. Gas Exchange and SPAD
Cocoa tree (Theobroma cacao L.) plants had distinct behaviors in relation to the different genotypes and flooded, non-flooded, and control environments to which they were subjected. When kept under flooding, the plants showed negative behavior in relation to gas exchange (Figure 3, Figure 4 and Figure 5). The reduction in net photosynthetic rate under flooding may be related to stomatal closure by photoinhibition [27] or to photochemical impairment, causing limitation in leaf gas exchange [28]. The rootstock SJ-02 showed low photosynthetic rates in the control plants. However, due to the low photosynthetic rates in relation to the other rootstocks, the effect of flooding was not very pronounced in SJ-02. This finding is consistent with Bertolde et al. [29], who observed a reduction in PN after 45 days of flooding in cocoa genotypes, except for SJ-02, noting that the longer the plant stress period, the greater the reduction in PN as a consequence of the inhibition of photosynthesis.
Flooding conditions caused a reduction in gs in all rootstocks (Figure 3). However, SJ-02 did not show any difference in its behavior between environments when compared to the other genotypes (Figure 3). Reduced gs values can be associated with degradation of the root system, since the roots are unable to maintain water absorption in the anoxic environment [5]. Thus, the lower the stomatal conductance generated by the increased stress time, the greater the loss in photosynthesis, since the plants are unable to produce ATP and NADPH2 and begin to dissipate the absorbed light energy, increasing the chances of photoinhibition [30].
It is noted that in our study, there was a reduction in transpiration after the flooded environment compared to the control. Bertolde et al. [13] found an 80% reduction in transpiration compared to the control after 20 days of flooding, corroborating the data found in this work. However, it should be highlighted that the SJ-02 genotype maintained transpiration levels in the flooded environment similar to the control, suggesting greater plasticity to this environment.
When stomatal limitations occur, there is consequently a reduction in CO2 capture, and, thus, a reduction in the Ci/Ca ratio, this reduction being related to the reduction in gs. However, it should be noted that all rootstocks presented Ci/Ca values close to or greater than 0.7 (Figure 4), which, according to [13], is indicative that stomatal restriction was not sufficient to limit the Ci/Ca ratio [31].
The stress generated by flooding conditions induced a reduction in water-use efficiency in all genotypes, with the most pronounced reductions in the TSH-1188 and Esfip-02 genotypes (Figure 5). Unlike the data observed in our study, for flooded water conditions, when plants present high values of WUE, it is indicative of greater physiological plasticity, given the stressful conditions of the environment [32,33]. Thus, the increase in water-use efficiency during flooding indicates that the plant is using more water in this environment [34]. Therefore, it is possible to verify with the data presented in our study that the cocoa plants had a reduced water absorption capacity by the roots.
In addition to stomatal closure, chlorophyll degradation and the accumulation of soluble sugar in the leaves during flooding contributed to the reduction in photosynthesis [35]. High values of the SPAD index indicate that the FSII is not compromised [5], but this did not occur with the rootstocks studied, since all showed a decline in their green index (Figure 5). Possibly associated with the increase in acetaldehyde and acetic acid and the action of ethylene [36] in the leaves through chlorophyll degradation [37].
After stress suspension, all rootstocks reestablished PN (Figure 6), E (Figure 7), and WUE (Figure 8). This is an indication that the plants managed to reestablish water absorption and reactivate the photosynthesis process. Corroborating the results presented in this study, Sena Gomes and Kozlowski [15] observed the recovery of stomatal conductance and transpiration to control levels after the suspension of flooding stress in the Catongo genotype. Almeida et al. [16] found that cocoa plants flooded for 35 days recovered 78 to 100% of their photosynthetic capacity 10 days after the flooding was lifted. Pinto et al. [14] reported impaired photosynthetic function in the genotypes TSH-1188, Esfip-02, Cepec-2002, SJ-02, and Pará during flooding, with normal function restored after moisture normalization. Possibly, the recovery in gas exchange can be associated with better management of reactive oxygen compounds, improving photosynthetic acclimation after flooding [8].
4.2. Adaptations of the Anatomy of the Stem and Leaf
All rootstocks showed lenticel formation (Table 2, Figure 9), with no differences between them under flooding conditions. The appearance of lenticels is quite common in cocoa genotypes as a form of adaptation to stress; in our study, all rootstocks showed lenticel formation nine days after flooding, with no differences between them under flooding conditions. In different studies, the appearance of lenticels on the stem of cocoa has been observed during flooding, after 3, 8, 10, and 12 days of the plants being under this condition [3,13,38,39]. In our study, all rootstocks showed the formation of lenticels after nine days of flooding, with no differences between them in the flooded condition. The length of the lenticels remained the same during recovery, except for the SJ-02 and Pará genotypes, and their height regressed when the plants were removed from the flooding and placed to recover (Table 2).
Lenticels are structures that facilitate the transport of oxygen to the roots and protect the tissues from toxic substances arising from fermentation [40]. The presence of hypertrophied lenticels promotes the release of toxic compounds that are formed by the roots [41], such as the production of lactate, acetaldehyde, and ethanol [42]. Among these compounds, the most harmful during flooding is ethanol. When the plant is unable to eliminate all the ethanol produced, it causes darkening of the root system and consequent death, impairing the growth of new roots [5].
Based on the data shown, it can be seen that the length of the lenticels remained the same during recovery, except for the SJ-02 and Pará genotypes, and their height decreased when the plants were removed from the flooded area and placed to recover (Table 2). Kolb et al. [43], working with flooded plants, observed that when comparing the height and length of the lenticels with those of plants grown in drained soils, there were significant differences between the environments. The presence of lenticels indicates that oxygen is not completely absent, allowing plants to survive in this type of environment [44].
The increase in stomatal density in the SJ-02, Pará, and Esfip-02 genotypes during flooding contributed to the conservation of CO2 assimilation, being an essential strategy to increase photosynthesis and tolerate stress conditions [45], as observed for these genotypes during the recovery process. On the other hand, the TSH-1188 and Cepec-2002 rootstocks showed a reduction in stomatal density during flooding, associated with stomatal closure. This response can be considered to be a form of protection by plants to avoid excessive water loss [46], becoming a survival mechanism against water saturation of the root system [30].
The return of stomatal density similar to the non-flooded environment of the genotypes after the suspension of flooding stress confirms that changes in the density and size of stomata in leaves developed in flooded environments are a sign of the plasticity of the species to adapt to the environment [43]. The Pará rootstock did not present statistical differences between the three environments (Table 2), showing that it was the only one that did not present plasticity.
4.3. Pigment Contents
Flooding negatively influenced pigments, reducing the plants’ light absorption capacity. Chlorophyll and carotenoids are directly linked to the photosynthetic capacity of plants, as they have functions in the absorption and transfer of light energy to the PSII [47], and are essential for plant development [48]. The stressful condition generated by excess water and reduced oxygen concentration in the roots limits the presence of nitrogen and magnesium in the leaves, which are constituents of the pigments [49].
The reduction in chlorophyll is due to an increase in the chlorophyllase enzyme, which degrades its molecules. This is because stress inhibits the chlorophyll precursor molecule 5-aminolevulinic acid [50]. Bertold et al. [13], studying cocoa clones, found a reduction in chlorophyll a and b 30 days after flooding, a response similar to that found in the present work, where, after 60 days of flooding, chlorophyll degradation caused by flooding had occurred (Table 3). Chlorophyll b is responsible for capturing light by transferring it to chlorophyll a and thus participating in photochemical reactions [50]. With the reduction in this chlorophyll in the flooded environment (Table 3), it can be said that there was a reduction in light capture. Flooding favored a reduction in the a/b ratio (Table 3), indicating a disruption in the functioning of the photochemical phase. Furthermore, reductions in chlorophyll levels favor the appearance of reactive oxygen species that oxidize the pigments [51].
Analyzing the environments, it was found that flooding degraded carotenoids. The Cepec-2002 and Esfip-02 genotypes showed higher carotenoid values compared to the Pará and TSH-1188 genotypes (Table 3). Carotenoids are responsible for protecting photosystems against photodegradation and photooxidation [52]. The reduction in this pigment confirms that the cocoa plants suffered photodegradation caused by the long period of flooding and consequent photosynthetic decline [53].
4.4. The Carbohydrates
Flooding stress on the root system generates changes in plants due to the lack of oxygen in the roots, leading the plant to adapt, such as through the accumulation of metabolites in the aerial part [28], since the plant tends to save energy to ensure its survival, thus tending to maintain a balance in the distribution of photoassimilates until the flooding is suspended [54]. This fact explains the accumulation of reducing sugar (RS) in the leaves of plants kept under flooding, with emphasis on the TSH-1188 genotype (Table 4). It is also known that plants under flooding store carbohydrates as a way to prevent oxidative stress [55].
The data obtained in this study also indicate that there was an accumulation of total soluble sugar (TSS) in the leaves of the genotypes in the flooded environment, with higher TSS in the leaves of the Esfip-02 and Cepec-2002 genotypes (Table 4). The increase in soluble sugars in the leaves contributed to the osmoregulation of the plants. This accumulation in the leaves probably reduces A rates and contributes to a satisfactory pressure potential and maintains the water content inside the cells, thus avoiding and reducing the damage caused by stress [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. It should be noted that the TSS produced tends to accumulate in the aerial part of the plants, as there is less translocation from the leaf to the roots due to the compromise of the phloem, which can cause damage to photosynthetic rates [57].
The allocation of carbohydrates in the root system showed differences between environments, where flooding favored an increase in TSS in all rootstocks (Table 4). The increased accumulation of carbohydrates in the TSS root system observed in plants under flooding, as presented in our study, is reported as a survival mechanism in environments with flooding stress [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]. Thus, under this type of stress, plants were unable to utilize carbohydrates, which is a sign of low tolerance to flooding [35]. Thus, this adaptation allows the plant to modify its gene expression, responsible for conferring tolerance to stressful conditions [59].
Under flooding conditions, the lower rate of phloem transport to the roots results in starch accumulation, as observed in the leaves and roots of plants when compared to the non-flooded environment, while the Cepec-2002 and Pará rootstocks showed lower starch accumulations (Table 4). This occurs because the root system under anoxia requires large amounts of sugars for anaerobic respiration [29]. The maintenance of glycolysis and the induction of fermentation during flooding are potential characteristics that confer tolerance to flooding [28]. Since carbohydrate formation depends on the efficient capture of light energy and oxygen supplies, the reduction of oxygen caused by flooding hinders photosynthesis and respiration, consequently hindering carbohydrate formation [60].
The data obtained demonstrate that, during recovery from stress, there was a reduction in RS, TSS, and starch in the leaves, with no differences between the rootstocks (Table 4). The root system during the recovery period generated an increase in starch and a reduction in TSS (Table 4). This fact is related to the increased biosynthesis through the use of stored carbohydrates; thus, the plants suffered less stress during recovery [61]. It should be noted that the plant recovery phase after flooding can be equally stressful, being related to plant performance during this period [8]. Thus, plant survival after flooding may be related to the ability to maintain carbohydrate levels [62].
It is worth noting that the adaptation of cocoa plants to the flooded environment is related to a series of factors that allow the plants to survive in stressful and non-ideal conditions for their development. Furthermore, the morphophysiological modifications of the plants, such as the appearance of lenticels, stomatal density, and carbohydrate and photosynthetic pigment contents, are essential for the adaptive success of the species.
Thus, we highlight that the use of the genotypes TSH-1188, Cepec-2002, Pará, Esfip-02, and SJ-02 in our study is due to the need to characterize these genotypes in flooded environments. These genotypes are commonly used as rootstocks in cocoa production; however, there are few studies characterizing these genotypes regarding their tolerance to flooding and the recovery of plants after suffering stress. Furthermore, the differences observed in the genotypes are possibly due to their original and parental characteristics. However, as demonstrated, although the plants suffered alterations in photosynthesis during flooding, all genotypes resumed their normal functions after recovering from the stress, demonstrating that they may be suitable for planting in locations subject to flooding. This finding is a milestone for the production of seedlings through grafting, as it allows farmers to plan crop management and assists in decision-making regarding the genotypes that should be planted in their area.
Furthermore, since cocoa cultivation is a dynamic activity requiring periodic crop renewal, studies characterizing other genotypes with potential for planting in areas with biotic and abiotic limitations, such as tolerance to pests and diseases and thermal, light, water, and saline stress, are fundamental, potentially ensuring greater sustainability for the crop.
5. Conclusions
The exposure time of the cocoa (Theobroma cacao L.) rootstocks TSH-1188, Cepec-2002, Pará, Esfip-02, and SJ-02 to flooding caused limitations in gas exchange. At 55 days after flooding, all genotypes showed reductions in net photosynthetic assimilation, stomatal conductance, and transpiration rate in the flooded environment compared to the non-flooded environment.
The photosynthetic pigments of the cocoa rootstocks TSH-1188, Cepec-2002, Pará, Esfip-02, and SJ-02 were degraded during flooding, with average values of Chl a, Chl b, total Chl, and total carotenoids of 9.33, 10.418, 19.75, and 590.75 μg.mL−1 in the non-flooded environment and 6.43, 7.69, 14.12, and 500.33 μg.mL−1 in the flooded environment. The rootstocks Cepec-2002 and Esfip-02 showed higher carotenoid accumulation with 585.78 and 650.47 μg.mL−1, respectively, when compared with SJ-02 (474.03 μg.mL−1), Pará (491.58 μg.mL−1), and TSH-1188 (525.86, 491.58 μg.mL−1).
The Pará rootstock did not show differences in stomatal density between environments, with values of 32.25 in flooding, 34.83 in non-flooding, and 31.61 in recovery.
After nine days of flooding, the cocoa genotypes TSH-1188, Cepec-2002, Pará, Esfip-02, and SJ-02 showed morphological changes through the appearance of hypertrophied lenticels on the stem, suggesting adaptation to stress.
After recovery, the rootstocks TSH-1188, Cepec-2002, Pará, Esfip-02, and SJ-02 showed the ability to normalize their gas exchange, carbohydrate exchange, and anatomical processes, demonstrating that the root system managed to re-establish its functions and indicating tolerance of these genotypes to areas at risk of flooding.
Author Contributions
Conceptualization, E.N.d.S., S.D.-A., K.T.K. and C.A.S.S.; Methodology, S.D.-A., M.L.P.B.P. and V.d.S.O.; Software, V.d.S.O., J.C. and C.d.S.D.; Validation, A.R.F., T.C.d.S., B.C.N., E.N.d.S. and S.D.-A.; Formal analysis, V.d.S.O. and L.d.O.A.; Investigation, M.L.P.B.P., T.R.F., B.C.N. and J.C.; Resources, E.N.d.S. and S.D.-A.; Data curation, V.d.S.O. and M.L.P.B.P.; Writing—original draft preparation, V.d.S.O. and M.L.P.B.P.; Writing—review and editing, V.d.S.O. and S.D.-A.; Visualization, V.d.S.O. and J.C.; Supervision, V.d.S.O., J.A.M.F. and S.D.-A.; Project administration, E.N.d.S. and S.D.-A.; Funding acquisition, E.N.d.S. and S.D.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Secretaria da Agricultura, Abastecimento, Aquicultura e Pesca (SEAG) e Fundação de Amparo a Pesquisa e Inovação do Espirito Santo—FAPES, grant number DI 004/2022 SEAG/FAPES—Banco de projetos Fase III.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Ci/Ca | ratio between intra/extracellular CO2 concentrations |
| Chl a | chlorophyll a |
| Chl b | chlorophyll b |
| DNS | dinitrosalicylic acid method |
| E | transpiration |
| FAA 70% | formaldehyde, acetic acid, and 70% ethanol |
| gs | stomatal conductance |
| INCAPER | Capixaba Institute for Research, Technical Assistance and Rural Extension |
| PD/DE | polar diameter/equatorial diameter ratio |
| PN | net photosynthetic rate |
| RS | reducing sugars |
| SPAD | Soil–Plant Analysis Development |
| TSS | total soluble sugars |
| WUE | water-use efficiency |
References
- Lahive, F.; Hadley, P.; Daymond, A.J. The physiological responses of cacao to the environment and the implications for climate change resilience. A review. Agron. Sustain. Dev. 2019, 39, 5. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations—FAO: Faostat. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 21 June 2025).
- Oliveira, V.S.; Pinheiro, A.P.B.; Cerri Neto, B.; Arantes, S.D.; Silva, C.A.; Crasque, J.; Pinto, M.L.P.B.; Santos, G.P.; Pagoto, A.L.R.; Nascimento, A.L.; et al. Effect of Flooding Under the Gas Exchange of Cocoa Seedlings. J. Agric. Sci. 2019, 11, 233. [Google Scholar] [CrossRef]
- Souza, C.A.S.; Aguilar, M.A.G.; Dias, L.A.S.; Siqueira, P.R. Relações Hídrica e Irrigação. In Cacau: Do Plantio à Colheita; Souza, C.A.S., Dias, L.A.S., Aguilar, M.A.G., Borém, A., Eds.; UFV: Viçosa, Brazil, 2016; p. 287. [Google Scholar]
- Vidal, D.B.; Andrade, I.L.M.M.; Dalmolin, Â.; Mielke, M. Photosynthesis and growth of copaiba seedlings subjected to soil flooding. Floresta Ambiente 2019, 26, e20160596. [Google Scholar] [CrossRef]
- Sairam, R.K.; Kumutha, D.; Ezhilmathi, K.; Deshmukh, P.S.; Srivastava, G.C. Physiology and biochemistry of waterlogging tolerance in plants. Biol. Plant. 2008, 52, 401–412. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Aclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef]
- Yeung, E.; Bailey-Serres, J.; Sasidharan, R. After The Deluge: Plant Revival Post-Flooding. Trends Plant Sci. 2019, 24, 443–454. [Google Scholar] [CrossRef]
- Rosa, D.B.C.J.; Scalon, S.D.P.Q.; Cremon, T.; Dresch, D.M. Gas exchanges and antioxidant activity in Copaifera langsdorffii desf. seedlings after flooding. Am. J. Plant Sci. 2018, 9, 979–994. [Google Scholar] [CrossRef]
- Jackson, M.B.; Colmer, T.D. Response and adaptation by plants to flooding stress. Ann. Bot. 2005, 96, 501–505. [Google Scholar] [CrossRef]
- Chen, X.; Visser, E.J.; De Kroon, H.; Pierik, R. Fitness consequences of natural variation in flooding induced shoot elongation in Rumex palustris. New Phytol. 2011, 190, 409–420. [Google Scholar] [CrossRef]
- Herrera, A.; Tezara, W.; Marin, O.; Rengifo, E. Estomatal and non estomatal limitations of photosynthesis in trees of a tropical seasonally flooded forest. Physiol. Plant. 2008, 134, 41–48. [Google Scholar] [CrossRef]
- Bertolde, F.Z.; Almeida, A.A.F.; Pirovani, C.P.; Gomes, F.P.; Ahnert, D.; Baligar, V.C.; Valle, R.R. Physiological and biochemical responses of Theobroma cacao L. genotypes to flooding. Photosynthetica 2012, 50, 447–457. [Google Scholar] [CrossRef]
- Pinto, M.L.P.B.; Crasque, J.; Cerri Neto, B.; Ferreira, T.R.; Souza, C.A.S.; Falqueto, A.R.; Souza, T.C.; Machado Filho, J.A.; Arantes, L.O.; Dousseau-Arantes, S. Morphophysiological responses of Theobroma cacao L. rootstocks to flooding and post-flooding conditions. Photosynthetica 2023, 61, 377–389. [Google Scholar] [CrossRef]
- Sena Gomes, A.R.; Kozlowski, T.T. The effects of flooding on water relations and growth of Theobroma cacao var. catongo seedlings. J. Hortic. Sci. 1986, 61, 265–276. [Google Scholar] [CrossRef]
- Almeida, A.-A.F.; Valle, R.R. Ecophysiology of the cacao tree. Braz. J. Plant Physiol. 2007, 19, 425–448. [Google Scholar] [CrossRef]
- Sodré, G.A. Formação de Mudas de Cacaueiro, Onde Nasce a Boa Cacauicultura; Boletim Técnico; CEPLAC/CEPEC: Itabuna, Bazil, 2013. [Google Scholar]
- Dousseau, S.; Alvarenga, A.A.; Castro, E.M.; Soares, R.P.; Emrich, E.B.; Melo, L.A. Anatomia foliar de Tabebuia serratifolia (Vahl) Nich. (Bignoniaceae) propagadas in vitro, in vivo e durante a aclimatização. Cienc. Agrotecnol. 2008, 32, 1694–1700. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolates choroplasts. Polyphenoloxidade in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Duke, S.O.; Kenyon, W.H. Effects of dimethazone (FMC 57020) on chloroplast development II. Pigment synthesis and photosynthetic function in cowpea (Vigna unguiculata L.) primary leaves. Pestic. Biochem. Physiol. 1986, 25, 11–18. [Google Scholar] [CrossRef]
- Sandmann, G.; Böger, P. Comporison of the bleaching activity of Norflurazon and Oxyfluorfen. Weed Sci. 1983, 31, 338–341. [Google Scholar] [CrossRef]
- Zanandrea, I.; Alves, J.D.; Deuner, S.; Goulart, P.F.P.; Henrique, P.D.C.; Silveira, N.M. Tolerance of Sesbania virgata plants to flooding. Aust. J. Bot. 2010, 57, 661–669. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.I. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. J. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar 4.3 Sistema de Análises Estatísticas; UFLA: Lavras, Brazil, 1999. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 21 June 2025).
- Dalmolin, A.C.; Dalmagro, H.J.; Lobo, F.; Antunes, M.Z.; Ortíz, C.E.R.; Vourlitis, G.L. Photosynthetic light and carbon dioxide response of the invasive tree Vochysia divergens Pohl to experimental flooding and shading. Photosynthetica 2013, 51, 379–386. [Google Scholar] [CrossRef]
- Branco, M.C.S.; Almeida, A.A.F.; Dalmolin, Â.C.; Ahnert, D.; Baligar, V.C. Influence of low light intensity and soil flooding on cacao physiology. Sci. Hortic. 2017, 217, 243–257. [Google Scholar] [CrossRef]
- Bertolde, F.Z.; Almeida, A.A.F.; Corrêa, R.X.; Gomes, F.P.; Gaiotto, F.A.; Baligar, V.C.; Loguércio, L.L. Molecular, physiological and morphological analysis of waterlogging tolerance in clonal genotypes of Theobroma cacao L. Tree Physiol. 2010, 30, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Klumb, E.K.; Braga, E.J.B.; Bianchi, V.J. Differential expression of genes involved in the response of Prunus spp. rootstocks under soil flooding. Sci. Hortic. 2019, 261, 109038. [Google Scholar] [CrossRef]
- Mielke, M.S.; De Almeida, A.A.F.; Gomes, F.P.; Aguilar, M.A.G.; Mangabeira, P.A.O. Leaf gas exchange, chlorophyll fluorescence and growth responses of Genipa americana seedlings to soil flooding. Environ. Exp. Bot. 2003, 50, 221–231. [Google Scholar] [CrossRef]
- Li, F.; Bao, W.; Wu, N.; You, C. Growth, biomass partitioning, and water-use efficiency of a leguminous shrub (Bauhinia faberi var. Microphylla) in response to various water availabilities. New For. 2008, 36, 53–65. [Google Scholar] [CrossRef]
- Silva, C.E.M.; Gonçalves, J.F.C.; Feldpausch, T.R. Water-use efficiency of tree species following calcium and phosphorus application on an abandoned pasture, central amazonia, Brazil. Environ. Exp. Bot. 2008, 64, 189–195. [Google Scholar] [CrossRef]
- Mielke, M.S.; De Almeida, A.A.F.; Gomes, F.P.; Mangabeira, P.A.O.; Silva, D.D.C. Effects of soil flooding on leaf gas exchange and growth of two neotropical pioneer tree species. New For. 2005, 29, 161–168. [Google Scholar] [CrossRef]
- Camison, Á.; Martín, M.Á.; Dorado, F.J.; Moreno, G.; Solla, A. Changes in carbohydrates induced by drought and waterlogging in Castanea sativa. Trees 2019, 34, 579–591. [Google Scholar] [CrossRef]
- Lourenço, L.F.G.; Guerreiro, R.G.O.; Romagnolo, M.B.; Pastorini, L.; Souza, L.A. Efeito do alagamento e da seca sobre o crescimento e fisiologia de Peltophorum dubium (Fabaceae). Rev. Agronegocio Ambiente 2024, 17, e12664. [Google Scholar] [CrossRef]
- Ouyang, W.; Struik, P.C.; Yin, X.; Yang, J. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 2017, 68, 5191–5205. [Google Scholar] [CrossRef] [PubMed]
- Rehem, B.C.; De Almeida, A.A.F.; Mielke, M.S.; Gomes, F.P.; Valle, R.R. Photosynthetic and growth responses of Theobroma cacao L. clones to waterlogging. J. Trop. Agric. 2010, 48, 17–22. [Google Scholar]
- Almeida, J.; Tezara, W.; Herrera, A. Physiological responses to drought and experimental water deficit and waterlogging of four clones of cacao (Theobroma cacao L.) selected for cultivation in Venezuela. Agric. Water Manag. 2016, 171, 80–88. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Rennenberg, H. Molecular and physiological responses of trees to waterlogging stress. Plant Cell Environ. 2014, 37, 2245–2259. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhao, C.Y.; Li, J.; Li, J.Y.; Peng, G. Morphological, physiological, and biochemical responses of Populus euphratica to soil flooding. Photosynthetica 2015, 53, 110–117. [Google Scholar] [CrossRef]
- Voesenek, L.A.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Kolb, R.M.; Medri, M.E.; Bianchini, E.; Pimenta, J.A.; Giloni, P.C.; Correa, G.T. Anatomia ecológica de Sebastiania commersoniana (Baillon) Smith & Downs (Euphorbiaceae) submetida ao alagamento. Braz. J. Bot. 1998, 21, 3. [Google Scholar] [CrossRef]
- Melo, L.A.; Magalhães, P.C. Influência da aplicação de cálcio e alagamento do solo sobre características anatômicas das folhas de milho (Zea mays L.) “Saracura” BRS-4154. Ver. Bras. Milho Sorgo. 2010, 3, 333–342. [Google Scholar] [CrossRef]
- Melo, L.A.D.; Melo, H.C.D.; Davide, A.C.; Castro, E.M.D.; Santos, J.D.P.D. Estaquia e efeito da deficiência hídrica ou inundação sobre características morfoanatômicas de Cestrum axillare Vell. Cienc. Florest. 2017, 27, 325–337. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Nogueira, N.O.; Martins, L.D.; Tomaz, M.A.; Andrade, F.V.; Passos, R.R. Teor de nitrogênio, clorofila e relação clorofila-carotenoide em café arábica em solo submetido a diferentes corretivos de acidez. Rev. Bras. Ciênc. Agrárias 2013, 8, 390–395. [Google Scholar] [CrossRef]
- Nascimento, R.; Deuner, S.; Ferreira, L.S.; Badinelli, P.G.; Kerber, R.S. Crescimento e teores de clorofila e carotenóides em três cultivares de soja em função da adubação com magnésio. Rev. Ceres 2009, 56, 3. [Google Scholar]
- Zaidi, P.H.; Maniselvan, P.; Yadav, P.; Singh, A.K.; Sultana, R.; Dureja, P.; Singh, R.P.; Srinivasan, G. Stress-adaptive changes in tropical maize (Zea mays L.) under excessive soil moisture estress. Maydica 2007, 52, 159–171. [Google Scholar]
- de Oliveira Freire, J.L.; Cavalcante, L.F.; do Nascimento, R.; Rebequi, A.M. Teores de clorofila e composição mineral foliar do maracujazeiro irrigado com águas salinas e biofertilizante. Rev. Ciênc. Agrárias 2013, 36, 57–70. [Google Scholar] [CrossRef]
- Carvalho, L.M.; Casali, V.W.D.; Souza, M.A.; Cecon, P.R. Disponibilidade de água no solo e crescimento de Artemísia. Hortic. Bras. 2003, 21, 726–730. [Google Scholar] [CrossRef]
- Streit, N.M.; Canterle, L.P.; Canto, M.W.; Hecktheuer, L.H.H. As clorofilas. Ciênc. Rural 2005, 35, 748–755. [Google Scholar] [CrossRef]
- Pezeshki, S.R. Wetland plant responses to soil flooding. Environ. Exp. Bot. 2001, 46, 299–312. [Google Scholar] [CrossRef]
- Colmer, T.D.; Voesenek, L.A.C.J. Flooding tolerance: Suites of plants traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef]
- Tamang, G.; Fukao, T. Plant adaptation to multiple stresses during submergence and following desubmergence. Int. J. Mol. Sci. 2015, 16, 30164–30180. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Hammami, M. Effects of drought stress on phenolic accumulation in greenhouse-grown olive trees (Olea europaea). Biochem. Syst. Ecol. 2020, 92, 104112. [Google Scholar] [CrossRef]
- Ferner, E.; Rennenberg, H.; Kreuzwieser, J. Effect of flooding on C metabolism of flood-tolerant (Quercus robur) and non-tolerant (Fagus sylvatica) tree species. Tree Physiol. 2012, 32, 135–145. [Google Scholar] [CrossRef]
- Qin, X.; Li, F.; Chen, X.; Xie, Y. Growth responses and non-structural carbohydrates in three wetland macrophyte species following submergence and de-submergence. Acta Physiol. Plant. 2013, 35, 2069–2074. [Google Scholar] [CrossRef]
- Gangola, M.P.; Ramadoss, B.R. Sugars play a critical role in abiotic stress tolerance in plants. In Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants; Wani, S.H., Ed.; Academic Press: London, UK, 2018; pp. 17–38. [Google Scholar] [CrossRef]
- Sasidharan, R.; Hartman, S.; Liu, Z.; Martopawiro, S.; Sajeev, N.; Van Veen, H.; Voesenek, L.A. Signal dynamics and interactions during flooding stress. Plant Physiol. 2018, 176, 2069–2074. [Google Scholar] [CrossRef]
- Ye, X.Q.; Meng, J.L.; Zeng, B.; Wu, M. Improved flooding tolerance and carbohydrate status of flood-tolerant plant Arundinella anomala at lower water temperature. PLoS ONE 2018, 13, e0192608. [Google Scholar] [CrossRef] [PubMed]
- Winkel, A.; Visser, E.J.; Colmer, T.D.; Brodersen, K.P.; Voesenek, L.A.; Sand-Jensen, K.; Pedersen, O. Leaf gas films, underwater photosynthesis and plant species distributions in a flood gradient. Plant Cell Environ. 2016, 39, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.