Abstract
The intervertebral disc (IVD) is the largest avascular organ of the human body and plays a fundamental role in providing the spine with its unique structural and biomechanical functions. The inner part of the IVD contains the nucleus pulposus (NP), a gel-like tissue characterized by a high content of type II collagen and proteoglycans, which is crucial for the disc’s load-bearing and shock-absorbing properties. With aging and IVD degeneration (IDD), the NP gradually loses its physiological characteristics, leading to low back pain and additional sequelae. In contrast to surrounding spinal tissues, the NP presents a distinctive embryonic development since it directly derives from the notochord. This review aims to explore the embryology of the NP, emphasizing the pivotal roles of key transcription factors, which guide the differentiation and maintenance of the NP cellular components from the notochord and surrounding sclerotome. Through an understanding of NP development, we sought to investigate the implications of the critical developmental aspects in IVD-related pathologies, such as IDD and the rare malignant chordomas. Moreover, this review discusses the therapeutic strategies targeting these pathways, including the novel regenerative approaches leveraging insights from NP development and embryology to potentially guide future treatments.
1. Introduction
The nucleus pulposus (NP) is an avascular gel-like tissue enveloped by the collagen-rich annulus fibrosus (AF) and bordered by thin superior and inferior hyaline cartilaginous endplates (CEPs), collectively comprising the three main tissues composing the intervertebral disc (IVD), a fibrocartilaginous structure situated between the vertebral bodies [1]. The extracellular matrix (ECM) plays a vital role in the biological and biomechanical functions of the NP. It primarily consists of proteoglycans (mainly aggrecan), type II collagen, and sulphated glycosaminoglycans (GAGs), such as chondroitin sulfate (Figure 1) [2].
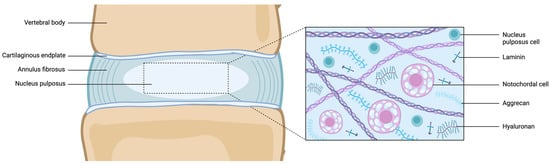
Figure 1.
Schematic representation of the juvenile healthy IVD (left) and microscopic structure of the NP. Abbreviations: IVD = intervertebral disc; NP = nucleus pulposus. Created with BioRender.com.
Due to its high-water content constituting approximately 80% of its wet weight, and its composition rich in collagen, the NP, located at the innermost part of the IVD, provides load-bearing and shock-absorbing properties [3]. Unlike articular cartilage, the human NP matrix can be distinguished by its unique GAG/hydroxyproline ratio, which remains significantly higher across all ages compared to juvenile cartilage [4]. Furthermore, NP cells (NPCs) express matrix-binding proteins, particularly integrins, and exhibit a high level of integrin-laminin binding, suggesting the crucial role of laminin in cell–matrix interactions [5]. It has been suggested that distinctive markers including an aggrecan/type II collagen expression ratio >20, and the stabilized expression of hypoxia-inducible factor 1-α (HIF-1α), glucose transporter 1 (GLUT-1), sonic hedgehog (SHH), Brachyury (TBXT), keratin (KRT)-18/19, carbonic anhydrase (CA)-XII, and cluster of differentiation (CD)-24 define the phenotype of young healthy NPCs [4].
In humans, from development until early postnatal life [6], the NP is characterized by large, vacuolated notochordal cells (NCs) (Figure 2).
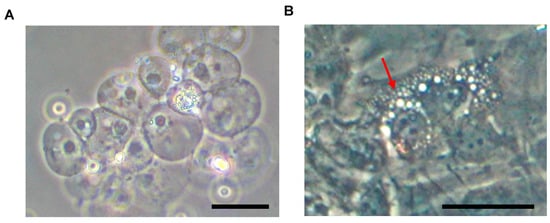
Figure 2.
Murine NC cluster appearance following NP aspiration under microscopic observation (A). After plastic adherence (B), NCs display a characteristic vacuolated morphology (red arrow). Abbreviations: NC = notochordal cell; NP = nucleus pulposus. Scale bars = 50 μm.
However, as early as soon after birth, NCs are gradually replaced by mature NPCs (formerly referred to as small chondrocyte-like cells [CLCs]). By late adolescence (around 17 years of age), only a few sparse NCs can be found, with mature NPCs accumulating due to the onset and advancement of intervertebral disc degeneration (IDD) [7,8]. It has been suggested that the morphological differences between NCs and mature NPCs represent different stages of cellular activity or differentiation, rather than two different lineages. In this context, all the cells residing within the NP may be considered to have differentiated along the notochordal lineage, with the morphological differences representing different physiological or pathological stages of cell aging and IDD [4].
The IVD’s avascular nature required notochordal-derived NPCs to develop distinctive characteristics in order to adapt to an acidic, hypoxic, and hyperosmotic environment [1]. In particular, NCs are involved in the constitution of an anti-angiogenic niche, through the secretion of an anti-angiogenic ECM and the release of anti-angiogenic factors [9]. The degree of GAG glycosylation has been shown to inhibit endothelial migration in vitro by providing the IVD with water-imbibing properties and high magnitudes of pressurization, which may hinder blood vessel ingrowth [10]. The ability of aggrecan to inhibit endothelial cell adhesion/migration and nerve outgrowth depends on the degree of glycosylation of GAGs, suggesting that chondroitin sulfate is largely responsible for proteoglycans’ anti-angiogenic effects [11]. Together with the chondroitin sulfate, the Noggin (Nog) factor inhibits endothelial cell invasion and tubular formation [12]. Fas ligand (FasL), expressed by normal NPCs, induces apoptosis in vascular endothelial cells, thereby inhibiting blood vessel infiltration [13]. Moreover, FasL also plays a key role in limiting the recruitment of immunogenic cells, including macrophages and lymphocytes which, combined with limited vascularization, make the healthy IVD one of the few largely immune-privileged tissues [14]. The thin CEPs anchor the IVD to the adjacent vertebral bones, serving as a selectively permeable barrier. This structure enables nutrient uptake into the IVD mainly through diffusion via the CEPs or from the restricted blood supply in the outer layers of the AF [9]. The avascular nature of the NP persists throughout life but may be compromised as part of the IDD cascade [9].
The embryonic development of the human NP provides invaluable clues to understanding its complex physiology and pathology. Unlike other species such as rodents, which retain NCs for up to half of their lifespan, human NCs start to be depleted early during fetal life and become undetectable by late childhood. The early loss of the NC population has been associated with the onset of IDD changes, thus suggesting the fundamental role of these cells in maintaining healthy NP homeostasis [15]. For this reason, it has also been speculated that NCs may serve as a promising cell source for IVD regeneration [16]. This review aims to investigate the complex process leading to the development of the NP from the embryonic notochord. The interplay among the plethora of transcription factors involved and the cellular changes occurring at different embryonic stages will be illustrated. Furthermore, the relevance of NP development to pathological changes, including both IDD and the aberrant development of malignant chordoma from notochordal remnants, will be discussed.
2. Embryonic Development of the NP
During embryogenesis, the IVD develops through the maturation of two key structures: the notochord and sclerotome (Figure 3). These components give rise to the NP and the surrounding tissues (i.e., AF, CEPs, vertebrae, and other vertebral soft tissues), respectively [17]. The notochord is a rod-like, transient structure that forms in the midline of chordates and vertebrates. In the third week of human gestation (equivalent to embryonic day [E]6.0 in mice), the initially single-layered blastocyst undergoes reorganization into a three-layered gastrula composed of endoderm, ectoderm, and mesoderm [18]. During this pivotal process, the cells of the dorsal organizer, situated at the midline of the gastrula, form a distinct structure from the adjacent mesoderm, known as the chordamesoderm (equivalent to E7.5 in mice) [19]. Through mediolateral intercalation and convergence towards the dorsal midline (i.e., convergent extension, occurring approximately between E8.5–10.5 in mice), chordamesoderm cells become vacuolated and arrange themselves in an elongated pile surrounded by a dense ECM rich in collagens and sulfated proteoglycans, forming the notochordal sheath. Importantly, the pressure within the amniotic cavity plays a critical role in orchestrating convergent extension and ensuring proper embryo development along the anterior–posterior axis [20].
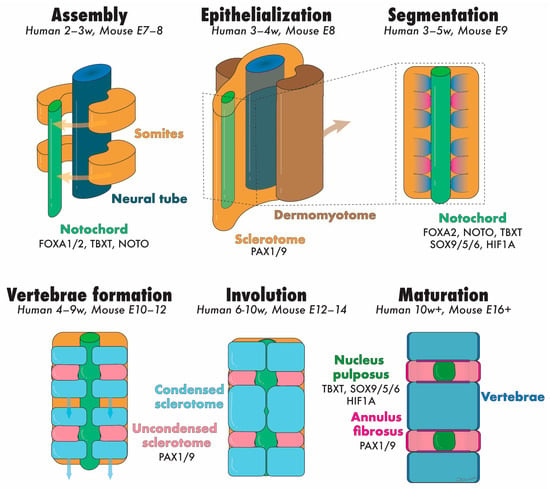
Figure 3.
Schematic representation of the developmental stages of the NP and annotation of the transcription factors speculated to be involved. Abbreviation: E = embryonic day, NP = nucleus pulposus, and w = embryonic week. Colored text matches the tissues illustrated with identical colors. Created with Adobe Illustrator.
Mechanical forces acting on the developing notochord at this stage are crucial for subsequent embryo elongation and locomotion development in various chordate organisms [18]. The osmotic pressure exerted within the vacuoles against the notochordal sheath confers upon the notochord its characteristic rod-like appearance [17]. The notochordal sheath is essential to contain the hydrostatic pressure within the notochord and guide NP development. Indeed, gene mutations affecting the development of the notochordal sheath, such as SOX5/6 and SHH, may result in the complete failure of NP patterning in mice [21]. Similarly, smoothened (SMO) deletion from SHH-expressing murine cells prevents the notochordal sheath from being formed and the NP from developing within the IVD region [22]. However, SMO deletion after the formation of the notochordal sheath did not hinder NP development in a mouse model [23].
During gastrulation (equivalent to E8.0 in mice), the paraxial mesoderm, located at either side of the neural tube, gradually thickens to form the somitomeres, eventually undergoing segmentation and assembling into single units termed somites. This process is finely regulated temporally and spatially, proceeding in an anterior to posterior fashion along the embryo axis [17]. Soon after formation, in a process termed ephithelialization, each somite differentiates into two distinct tissues: the dermomyotome (which forms the skin of the back area and the underlying paraspinal muscles) and the sclerotome (which forms the AF, CEP, vertebrae, and other connective tissues of the spine) [24]. These events occur during the fourth week of human gestation [25]. A more detailed discussion of somite development is out of the scope of this review and has been extensively described elsewhere [17].
Around E13.5 in mice, the notochord initiates segmentation along the anterior–posterior axis, showing early signs of expansion into the IVD anlagen. The transition from the rod-like notochord and sclerotome to the respective NPs and vertebrae occurs simultaneously on E14.5 [26]. The notochord indeed plays a prominent role in this complex process. Recent evidence has suggested that notochord ablation impeded both vertebral and IVD segmentation in chick embryos. Conversely, sclerotome ablation prevented notochord segmentation and the further development of surrounding vertebral structures. These findings demonstrated that the notochord itself is not intrinsically segmented, and that the sclerotome orchestrates the correct development of NPs from the notochord, and the physiological assembly of the IVD and vertebral tissues from the sclerotome [27]. Intriguingly, regions where mesenchymal cells develop into vertebral bodies lack NCs, though seemingly not due to increased NC death [28].
Several models have been proposed to elucidate the transformation of the notochord into the NPs. According to the “pressure” model, NCs are “squeezed” away from the vertebrae towards the region where the NPs form. The osmotic swelling and resistance related to type II collagen fibrils within the middle region of the vertebral body may induce the migration of the notochord towards the IVD regions [29]. It has been previously shown that mutant mice lacking the notochordal sheath still presented normal vertebrae, although the notochord retained a rod-like structure and NCs were dispersed throughout the vertebrae [22]. Structural alterations of the notochordal sheath may also result in the failed segregation of the notochord from the vertebral bodies. Type II collagen is one of the major components of the notochordal sheath and is directly deposited by NCs into the surrounding ECM. In COL2A1-null mice, the notochord was not removed from the vertebral bodies and the IVDs did not develop, probably because of the inability of the notochordal sheath to withstand osmotic pressure. Furthermore, these animals showed enlarged vertebral bodies and abnormal collagen fibril deposition [29].
According to the “repulsion/attraction” model, the regions where NPs are supposed to develop may express attractant molecules that specifically recruit NCs to aggregate in NP-forming areas. Alternatively, repulsive signals may be expressed in vertebrae-forming regions, driving NCs away. Putative pathways involved in this mechanism include Eph/ephrin and Robo/Slit. The former has been described to set and maintain tissue borders and promote selective cell segregation in several areas of the developing embryo. Indeed, the EphA4 receptor has been localized in the notochord whereas ephrins have been identified in the surrounding mesenchyme. Therefore, these mediators may be essential to retain NCs where the NPs will eventually develop. Conversely, when Slit glycoproteins bind to Robo receptors, NCs tend to be repelled. Since Robo is expressed by mesenchymal cells and Slit by NCs, this may suggest an additional mechanism contributing to the segregation of the two cell types towards their respective tissues [26].
After week 10 (by E15.5 in mice), distinct NPs containing NCs and large vacuolated cells are identifiable [28]. Several different mechanisms have been identified to play a key role in the notochord-to-NP transition, including mechanical forces. In a recent study, the authors postulated that, at the early stage of NP development, the segmented notochord bulges exerting a bi-axial strain on the surrounding sclerotome, which may confer to the AF its typical cross-aligned collagen fiber orientation. Curiously, as notochord bulging does not occur in avians, these animals lack NPs and present a retained notochord surrounded by concentrically aligned collagen fibers [30].
3. Transcription Factors in NP Development
Transcription factors play a pivotal role in orchestrating the intricate developmental cascade during the embryogenesis of tissues and prospective organs [31]. These specialized proteins regulate gene expression by binding to specific DNA sequences, thereby modulating the rate of expression of target genes as a mean to guide cellular differentiation, proliferation, and tissue morphogenesis [31]. Through their precise control of gene transcription, transcription factors exert profound influence over the multifaceted pathways driving the embryogenic development of tissues, including the NP [32]. While the intricate regulatory mechanisms governing the NP remain elusive and warrant comprehensive further exploration, it is worthwhile to delve into the key transcription factors crucial for NP tissue development, as evidenced by genetic mouse studies.
FOXA1 and FOXA2, which are expressed early in development, are intricately involved in regulating the spatiotemporal organization of the notochord. Specifically, they regulate the expression of other key transcription factors, i.e., TBXT, and Homeobox Gene Not (NOTO), and are thereby pivotal in controlling the morphogenesis of notochord formation and defining NC types, as evidenced by severe IVD deformities in respective knockout models [33]. Moreover, FOXA1 and FOXA2 are involved in the transition of NCs to NPCs. The aptly named factor NOTO is a highly conserved factor involved in left–right patterning and specifically expressed in the posterior notochord during gastrulation. It works downstream of other factors such as TBXT and FOXA2, though their specific functions remain obscure. Similarly, TBXT, under control of Shh, is crucial for notochordal development as well as other axial structures, and the deletion of TBXT during development prevents spinal formation [34,35,36]. Moreover, TBXT expression following development is involved in ECM production [37] and has been thereby identified as a pivotal marker for NPCs [4]. Both NOTO and TBXT have shown the capacity, when their expression is enhanced, to upregulate key NP ECM production genes [38,39].
SOX5, SOX6, and SOX9 collectively contribute to NP and inner AF formation, with SOX9 regarded as a master regulator in chondrogenesis, and directly promote type II collagen and aggrecan production. The absence of SOX9 results in complete cartilage absence, highlighting its indispensability in tissue development. SOX5 and SOX6 enhance SOX9 function and are expressed in both the sclerotome and notochord during the critical developmental stages [40]. Alternatively, PAX1 and PAX9 can interfere with the activation of the aggrecan gene by SOX9 and SOX5/6 during development [41,42]. Through this mechanism, PAX1/PAX9 with SOX9, SOX5, and SOX6, are pivotal in delineating the inner AF and outer AF regions of the disc [43,44]. This is achieved through a complex regulatory network that involves intricate positive and negative feedback loops, as well as their interconnected regulation with bone morphogenetic protein (BMP) and transforming growth factor (TGF)-β pathways. Finally, as the NP constitutes the largest avascular tissue in the human body [9], resident cells are subjected to oxygen and nutrient paucity, demanding metabolic alterations to support NPC survival [45,46]. A key transcription factor regulator to support this adaptation is HIF-1α, a regulator of glycolytic metabolism and suppressor of hypoxia-induced apoptosis [47]. The repression of HIF1A during development leads to severe cell death in the notochord and the complete disappearance of the NP, underlining its pivotal role in supporting cell survival in the hypoxic disc environment [48].
Understanding the regulatory mechanisms of these transcription factors provides invaluable insights into the complex developmental processes underpinning NP formation and maintenance. Notably, most observations are derived from murine embryos, as human samples are limited due to ethical concerns, and direct translation requires caution. Nevertheless, harnessing this knowledge may pave the way for novel therapeutic interventions targeting IVD-related pathologies and degenerative disorders. Moreover, due to their critical role in the development and maintenance of the NPC phenotype, these transcription factors are key markers to be used for assessments of healthy and diseased NPCs [4,49]. The main transcription factors involved in NP development are summarized in Table 1.

Table 1.
Summary of the main transcription factors involved in orchestrating NP development.
4. Cellular Composition of Notochord to NP
For humans, as development progresses, NCs gradually transition into mature NPCs. However, a clear consensus on the terminology for these different cell types is still lacking [15,60,61]. Mature NPCs share similarities with the chondrocytes of hyaline cartilage but exhibit distinct collagen-to-proteoglycan ratios, gene expression profiles, and biological responses [15,62,63,64], tailored to their unique roles in the IVD. Interestingly, inherent repair mechanisms involving local stem or progenitor cells have been documented, which participate in the maintenance and potential regeneration of the IVD. Understanding these transitions and the regulatory mechanisms of the cellular population transitions may not only shed light on developmental biology but also open avenues for therapeutic strategies in treating IVD-related diseases.
Lineage and phenotypical characterization studies have confirmed that mature NPCs and NCs are of the same descent [55,65], though others have hypothesized origins from inner AF or CEP tissues [66]. Nevertheless, due to their evident differences in cell function and morphology, it is valuable to differentiate between NPCs and NCs. Bach et al. recommended distinguishing embryonic NCs (eNCs), NCs, and NPCs; both eNCs and NCs present as vacuolated cells, but eNCs refer to the NCs populating the embryonic notochord, while NCs are the specific vacuolated cells of the NP within the developed IVD [67,68]. Moreover, eNCs have been suggested to present a single large fluid-filled vacuole, while more mature NCs exhibit fragmented vacuolation [60]. Both eNCs and NCs are highly positive for KRT-18/19, CD24, TBXT, CD55, and CDH2, among other key markers [4,60]. The NCs begin their transition towards mature NPCs prior to birth and fully transition by around ten years of age in humans [60,69]. This differs from species such as mice, rats, and non-chondrodystrophic dogs, which maintain their NCs into adulthood, and from horses, goats, or sheep, in which NCs have mostly receded at birth [60,69]. These developmental aspects are critical for selecting the appropriate animal models to assess IVD-related diseases or therapies, particularly considering the relatively high regenerative potential of NCs [60,70,71,72].
The transition from NCs to NPCs is poorly understood, yet it is speculated to potentially involve, in part, the transition of TIE2-expressing NP progenitor cells [73]. Induced pluripotent stem cell (iPSC) differentiation experimental work has highlighted the transition of NCs to mature NPCs involving a high rate of TIE2-expressing cell populations [74], and recent investigations have demonstrated that TIE2-enriched NPC populations exhibit cells with fragmented vacuolation [75]. These results suggest that TIE2-expressing NP progenitor cells function as a potential transitional cell population, although these observations have not been made in vivo. Moreover, TIE2+ NPCs have been observed in both human and canine NP fetal tissues [73]. Conversely, contradicting reports are available in mice, where cultured fetal mouse NPCs have shown high positivity for TIE2 [76]; however, developing mice transcriptomic studies failed to observe any TIE2+ cells within the NP [77]. Moreover, in humans, isolated TIE2+ NPCs are only detected up to early adolescence and show a sharp decline after 20 years of age. Additional stem-like or progenitor-like cells displaying properties similar to mesenchymal stromal cells have been identified in various species within the NP; however, these generally remain poorly defined [73]. Moreover, their role or place in the transition from NCs to NPCs remains unknown.
The maturation of NCs to NPCs is marked by the expression of GD2 and CD24 [4], and is characterized by a considerably smaller cell diameter. NPCs are usually present as single cells within the NP matrix [60], until IDD and aging promote these cells to form clusters. As part of the avascular and enriched water-binding proteoglycans in the NP, NPCs form a highly specialized population able to cope with harsh osmotic, hypoxic, low-glucose, and acidic environments. Key markers such as HIF-1α, tonicity-responsive enhancer binding protein (TonEBP), and GLUT-1 form key adaptations for this cell type to survive and allow for their active involvement in the continuous remodeling of the proteoglycan and collagen-rich NP [49,78,79]. Nevertheless, as part of aging, IDD, and wear and tear, a decline in the number of NPCs is observed, and a switch from active NPCs to senescent cells may occur [80], which may actively promote IDD and inflammation [81,82]. Moreover, the progression of IDD may in turn attract new immunogenic [14,83,84] and other homing cells [85,86] to integrate into the IVDs, thereby drastically changing the overall cellular composition of the NP.
Finally, though the general implication of NCs to mature NPCs suggests a straightforward transition, recent evidence indicates that this representation may be an oversimplification. Specific cell profiling assays, particularly single-cell RNA sequencing studies, have highlighted separate NPC populations present within the disc. Moreover, further distinctions between the types of NPCs and their differentiation pathways have been found to be linked to certain pathologies and symptoms [87,88,89]. For example, Jiang et al. [90] identified a specific MMP3+, SLC7A2+, and TM4SF1+ NPC clusters associated with symptomatic low back pain. In contrast, Han et al. [91] revealed up to seven diverse NPC populations. These included two specific populations, i.e., inflammatory NPCs, overexpressing chemokines such as CXCL2 and CXCL8, and hypertrophic NPCs strongly expressing MMPs, which were both found to be linked to IDD progression. Moreover, these IDD-associated populations appeared to play key roles in metal-ion transport and redox alterations, highlighting the role of oxidative stress in IDD. Complementary findings by Li et al. [92] identified five distinct NPC populations, with four of these populations showing an increased presence in IDD samples. They referred to these as calcification-inhibitory, calcification, inflammatory, and fibrogenic NPC populations, each characterized by unique markers. Overall, these studies illustrate that NPCs can likely be classified into distinct populations and have the ability to differentiate within the fully developed IVD. This process is further associated with the progression of IDD and low back pain. Notably, there are clear distinctions among the populations identified between different studies [87,88,89,90,91,92,93]. This warrants further careful investigations and calls for an evidence-based consensus within the spine community, providing a valuable basis for a future review.
5. The Impact of Aging and Degeneration on the NP
IDD is recognized as a major contributor to low back pain, a condition affecting millions and acknowledged as the primary cause of disability worldwide [94]. Yet, despite its staggering prevalence and socioeconomic impact, available therapies remain merely palliative. IDD involves complex biochemical and structural changes within the disc, leading to the reduced function of the IVD, thereby compromising the spine as a functional unit, which may promote pain and disability [1]. Observing the continuous development and changes, particularly the transformation of the NP cell population from NCs into mature NPCs and the impact of aging on this process, is crucial for comprehending the pathophysiology of IDD [95]. As previously detailed, the large vacuolated NCs are the primary cellular component of the embryonic NP, and represent a highly metabolically active cell type that produces copious amounts of proteoglycans [4]. The transition of NCs present at birth to post-natal mature NPCs coincides with a period of high cell death within the IVD [6]. Moreover, as part of the aging process, mature NPCs are worn down by several factors, e.g., high biomechanical forces [96], the accumulation of reactive oxygen species and oxidative stress [75], and (epi)genetic modifications [97], all of which can be further exacerbated by lifestyle factors and genetic predispositions [98,99,100], resulting in a gradual decline in the number and activity of NPCs [101,102]. This age-related decline is accompanied by significant alterations in the ECM, including a reduction in the proteoglycan content and an increase in collagen cross-linking. These changes lead to a decrease in the resilience and mechanical properties of the NP, making it more susceptible to IDD [103].
The proteoglycan depletion occurring with aging and IDD has been attributed to a combination of reduced synthesis by NPCs and increased degradation by proteolytic enzymes [104]. Specifically, aggrecanundergoes fragmentation and depletion, contributing to decreased hydration and altered biomechanical properties [1]. The collagen composition in the NP also changes. There is an increase in collagen cross-linking, particularly of type I collagen, which replaces type II collagen. This change in collagen composition contributes to the fibrosis and loss of elasticity observed in degenerative IVDs [29]. Matrix metalloproteinases (MMPs), particularly MMP-1, -3, -7, -13, and -14, are upregulated in IDD [105], and degrade various ECM components, including collagens and proteoglycans, thus compromising the NP structural integrity. A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) enzymes, notably ADAMTS-4 and -5, are also implicated in aggrecan degradation. Their increased activity induces aggrecan fragmentation, contributing to proteoglycan loss and NP degeneration [106]. Pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, IL-17, tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ) are elevated in the degenerative NP. These cytokines stimulate MMP and ADAMTS expression, promoting ECM degradation and inflammation. Additionally, chemokines, including monocyte chemoattractant protein-1 (MCP-1), can attract immune cells to the otherwise immune-privileged NP, exacerbating inflammation and tissue damage [84].
Mature NPCs in the adult NP are less metabolically active than their embryonic counterparts and produce fewer proteoglycans. This results in a decrease in the hydration and gel-like properties of the NP, leading to a more fibrous and cartilaginous structure [63]. Further cellular changes can also occur as NPCs undergo senescence with aging and IDD, characterized by irreversible growth arrest and altered gene expression. Indeed, senescent NPCs secrete pro-inflammatory cytokines, MMPs, and reactive oxygen species, contributing to tissue inflammation and degradation [95]. Furthermore, the increased expression of apoptotic markers, such as cleaved caspase-3 and BAX, indicates apoptotic cell death in the aging NP, particularly in response to oxidative stress and pro-inflammatory cytokines [104]. Advanced glycation end products (AGEs) accumulate in the NP with age and IDD, leading to the increased cross-linking of matrix proteins. This cross-linking stiffens the ECM, impairing NP hydration and elasticity [107]. Finally, biomarkers of angiogenesis, including vascular endothelial growth factor (VEGF) and CD31, are also elevated in the aging NP. Increased neoangiogenesis correlates with IDD and vascular infiltration, exacerbating inflammation and matrix degradation. Neurotrophic factors like nerve growth factor (NGF) and substance P reflect neoinnervation of the aging NP. This nerve ingrowth is speculated to contribute to discogenic pain and inflammation [108]. These changes are depicted in Figure 4.
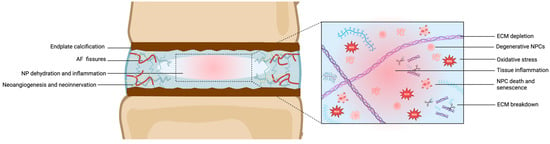
Figure 4.
Schematic representation of IDD (left) and microscopic structure of the degenerative NP. Abbreviations: AF = annulus fibrosus; ECM = extracellular matrix; IDD = intervertebral disc degeneration; NP = nucleus pulposus; NPC = nucleus pulposus cells. ROS = reactive oxygen species. Created with BioRender.com.
In short, the impact of aging on the NP structure and physiology is a complex process involving the developmental transitions and age-related changes in cellular activity and matrix composition. Understanding these processes is essential for the development of effective therapeutic strategies to prevent or treat age-related IDD and associated low back pain.
6. The Pathogenesis of Chordoma
Throughout IVD maturation, most of the notochord regresses, leaving behind the NP as its primary remnant in the adult spine. However, in some instances, NCs can persist in the distinct sites of the spine and proliferate abnormally, potentially leading to the development of chordoma. Chordoma is a rare and slow-growing bone tumor predominantly affecting the axial skeleton, with the sacrum and skull base being the most common sites. Specifically, approximately 50% of chordomas arise in the sacral region, 30% in the skull base, and 20% in other areas of the spine [109,110]. The preference for specific anatomical locations is believed to be linked to the persistence of notochordal remnants in these regions [111], predisposing individuals to chordoma development later in life [65]. Additionally, several studies have shown that TBXT is consistently expressed in chordomas, regardless of their anatomical location [36]. Therefore, increased TBXT expression serves as a hallmark of chordomas and is often used as a diagnostic marker for these specific tumors [112]. Although the pathogenesis of chordoma is not fully understood, TBXT expression in chordomas supports the theory that these tumors arise from remnant NCs that have undergone malignant transformation. Furthermore, the NC origin of chordoma cancer cells engenders specific cellular adaptations that complicate the treatment of chordoma. As NCs are resistant to hypoxia-related stress (in part due to high HIF-1α expression) and present metabolic adaptations to strive under harsh nutrient conditions, chordomas are inherently resistant. Markers such as HIF-1α and factors involved with metabolic regulation (e.g., GLUT-1) have been suggested as prognostic markers for overall chordoma survival [113,114]. Following its onset, chordoma typically exhibits slow growth, but may evolve into a locally aggressive tumor, infiltrating adjacent tissues and structures, including the spine, nearby nerves, and blood vessels [109]. Managing chordomas in regions such as the skull base and sacral area poses considerable challenges due to the complex anatomy and proximity to vital structures. Consequently, timely detection and intervention play a pivotal role in enhancing patient outcomes in chordoma cases. Effective treatment often necessitates a multidisciplinary approach, incorporating surgery, radiation therapy, and occasionally chemotherapy [109].
Achieving local control through surgical resection with adequate margins is a critical prognostic factor. However, as many tumors are located near vital structures, complete resection may be challenging and increases the difficulty of the procedure [111]. Despite the low radiosensitivity of chordomas, radiotherapy may be applied as an alternative or adjunctive treatment for tumors that are unresectable or challenging to resect completely [115]. Notably, carbon ion radiotherapy has been identified as an effective treatment option for unresectable chordomas in recent years [116]. Generally, chordomas are considered to be resistant to conventional chemotherapy, and no standard treatment regimen has been established. Currently, molecularly targeted therapies and immune checkpoint inhibitors are subjects of clinical research [111]. Previous studies report that the mean overall survival for patients with chordoma is 6.29 years, with survival rates of 67.6% at 5 years, 39.9% at 10 years, and 13.1% at 20 years. Aggressive modern surgical techniques have led to 10-year overall survival rates reaching 95% for skull base chordomas and ranging from 58% to 100% for chordomas of the mobile spine and sacrum [117]. Carbon ion radiotherapy achieves local control rates of 96%, 76%, and 54% at 1, 5, and 10 years, respectively. The overall survival rates for these intervals are 99%, 85%, and 69%, respectively [118]. Local recurrence and metastasis may occur during the prolonged course of the disease, necessitating careful and long-term follow-up [111].
7. Potential Therapeutic Strategies: Taking Inspiration from NP Development
As discussed, the development of the IVD involves a regulatory pathway with transcription factors, cell adjustments, and ECM changes (Figure 2). After birth, aging and stress lead to accumulating damage, compromising cellular integrity and ECM composition, which may lead IVD tissues to no longer be able to cope with biomechanical limits and potentially cause pain and disability [81,82]. Researchers are currently seeking novel therapies by leveraging the developmental processes of the IVD [60,119], as NCs are speculated to have notable regenerative potential [15,61,120]. Efforts target the integration of these cells and their associated matrisome into variable strategies for regenerating the IVD and tempering lower back and neck pain.
Cell therapy aims to either repopulate the NP with active ECM-producing cells or to rely on paracrine signaling from the injected cells to reactivate or home endemic cells to support IVD repair [16,85]. Among the various cell types under investigation for IVD regeneration [16], NCs have garnered scientific interest due to their unique regenerative properties [120]. These cells display high potency to instruct cells to take on active chondrogenic phenotypes resembling NPCs, temper inflammatory environments, and limit angiogenesis and nerve ingrowth [121,122,123]. However, challenges still exist as NCs are notoriously difficult to culture ex vivo with limited sources available and ethical considerations abound. However, advancements in reprogramming techniques present promising avenues, as elaborated upon below [124,125,126]. Alternatively, investigations are exploring NP progenitor cell transplantation for discogenic pain alleviation. These TIE2-expressing progenitor cells display stem cell-like traits, including enhanced proliferation, differentiation, paracrine activity, and ECM production [127,128,129]. Although these cells also face challenges regarding their maintenance and expansion, recent advancements in processing methods have supported their mass production with satisfactory phenotype retention [74,127,129,130,131]. The re-introduction of NP progenitor cells offers a compelling transplantation product, as they may theoretically constitute an unlimited source of regenerative cells within the NP environment [120].
Alternative strategies aim to employ cell-free methods to utilize the signaling factors produced by regenerative cell populations [132,133,134], particularly their extracellular vesicles (EVs) [135,136,137,138]. Research demonstrates that EVs derived from NCs contain potent bioactive molecules capable of modulating cellular behavior and are potentially able to promote tissue repair [139,140]. Furthermore, recent work has highlighted the therapeutic potential of EVs derived from TIE2-enhanced NPCs to reverse the induced IDD in a rat model [141]. These EVs outperformed those obtained from mesenchymal stromal cells as well as standard NPCs, indicating the potential benefits of drawing inspiration from embryonic sources.
Gene therapy and cell reprogramming techniques represent promising strategies for intervention in IDD [142,143]. Harnessing the regulatory power of notochordal and NP-related transcription factors, researchers have attempted to steer cellular differentiation towards phenotypes conducive to IVD regeneration. For example, the overexpression of NOTO, TBXT, or a combination of NOTO with SOX9, SOX5, and SOX6 has been utilized to promote human iPSCs to take on an NC or NPC phenotype. These studies were able to show an increase in key NC/NPC markers and the production capacity of IVD-related ECM proteins [74,124,144]. In a recent in vivo study, iPSCs were induced to differentiate towards mesendoderm progenitor cells (MEPCs), precursors of NCs. Three different MEPCs doses were injected intradiscally 5 weeks after nucleotomy in a sheep model. One month after transplantation, MEPCs were shown to be viable, actively replicating, and expressing TBXT, KRT-8, -18, -19, and FOXA2 [145]. Alternatively, gene therapy strategies have used IVD development-related transcription factors to drive catabolic/senescent NPCs towards a more active and regenerative phenotype [146,147]. For example, the transfection of TBXT promoted an anabolic NPC phenotype in NPCs originally compromised by natural IDD [38]. These approaches highlight the anabolic potential of embryonically involved transcription factors to support the potential regenerative products against IDD (Table 1).
Finally, ongoing research explores the potential of notochordal tissue as a scaffold for IVD regeneration. This NC-derived matrix (NCM) is rich in bioactive factors and ECM, which shows promise as an instructive matrix to induce tissue repair and has demonstrated efficacy in treating IDD in preclinical models [148]. Studies indicate that NCM enhances ECM production, cell proliferation, and the expression of NPC markers [11,149]. While challenges in optimizing decellularization methods persist, decellularized NCM could potentially be used as a cell-free therapeutic agent or as a carrier [150,151] for cell-based therapies to support NP tissue repair [60].
In short, by capitalizing on the developmental mechanisms inherent to the NP, these diverse regenerative approaches hold significant promise for revolutionizing the treatment of IDD and associated pathologies. Through a multifaceted approach encompassing gene therapy, cell-based interventions, and biomimetic scaffolds, researchers aim to unlock the full regenerative potential, offering hope for improved outcomes and the quality of life for affected individuals. Nevertheless, these strategies are still in the early stages of development and will still have to overcome significant scientific, regulatory, and scalability hurdles before their widespread application in clinical settings [152,153,154].
Author Contributions
Conceptualization, L.A. and J.S., Figure production: L.A. and J.S.; writing—original draft preparation, L.A., J.S., C.R.-F., S.T., K.J., A.N. and E.d.R.; writing—review and editing, L.A., J.S., C.R.-F., S.T., K.J., A.N., E.d.R., D.S. and G.V.; supervision, D.S., R.P., G.V. and V.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The support from the iPSspine (European Union’s Horizon 2020 Research and Innovation Programme under grant agreement no. 825925) project is gratefully acknowledged. Also, we would acknowledge the Japanese Agency for Medical Research and Development (AMED) for their support under grant number JP23ym0126124.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vo, N.V.; Hartman, R.A.; Patil, P.R.; Risbud, M.V.; Kletsas, D.; Iatridis, J.C.; Hoyland, J.A.; Le Maitre, C.L.; Sowa, G.A.; Kang, J.D. Molecular mechanisms of biological aging in intervertebral discs. J. Orthop. Res. 2016, 34, 1289–1306. [Google Scholar] [CrossRef]
- Nerurkar, N.L.; Elliott, D.M.; Mauck, R.L. Mechanical design criteria for intervertebral disc tissue engineering. J. Biomech. 2010, 43, 1017–1030. [Google Scholar] [CrossRef]
- Tilotta, V.; Vadalà, G.; Ambrosio, L.; Cicione, C.; Di Giacomo, G.; Russo, F.; Papalia, R.; Denaro, V. Mesenchymal stem cell-derived secretome enhances nucleus pulposus cell metabolism and modulates extracellular matrix gene expression in vitro. Front. Bioeng. Biotechnol. 2023, 11, 1152207. [Google Scholar] [CrossRef]
- Risbud, M.V.; Schoepflin, Z.R.; Mwale, F.; Kandel, R.A.; Grad, S.; Iatridis, J.C.; Sakai, D.; Hoyland, J.A. Defining the phenotype of young healthy nucleus pulposus cells: Recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J. Orthop. Res. 2015, 33, 283–293. [Google Scholar] [CrossRef]
- Soma, H.; Sakai, D.; Nakamura, Y.; Tamagawa, S.; Warita, T.; Schol, J.; Matsushita, E.; Naiki, M.; Sato, M.; Watanabe, M. Recombinant Laminin-511 Fragment (iMatrix-511) Coating Supports Maintenance of Human Nucleus Pulposus Progenitor Cells In Vitro. Int. J. Mol. Sci. 2023, 24, 16713. [Google Scholar] [CrossRef]
- Trout, J.J.; Buckwalter, J.A.; Moore, K.C.; Landas, S.K. Ultrastructureofthe human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell 1982, 14, 359–369. [Google Scholar] [CrossRef]
- Matta, A.; Karim, M.Z.; Isenman, D.E.; Erwin, W.M. Molecular Therapy for Degenerative Disc Disease: Clues from Secretome Analysis of the Notochordal Cell-Rich Nucleus Pulposus. Sci. Rep. 2017, 7, srep45623. [Google Scholar] [CrossRef]
- Ambrosio, L.; Mazzuca, G.; Maguolo, A.; Russo, F.; Cannata, F.; Vadalà, G.; Maffeis, C.; Papalia, R.; Denaro, V. The burden of low back pain in children and adolescents with overweight and obesity: From pathophysiology to prevention and treatment strategies. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720X231188831. [Google Scholar] [CrossRef]
- Fournier, D.E.; Kiser, P.K.; Shoemaker, J.K.; Battié, M.C.; Séguin, C.A. Vascularization of the human intervertebral disc: A scoping review. Jor Spine 2020, 3, e1123. [Google Scholar] [CrossRef]
- Cornejo, M.C.; Cho, S.K.; Giannarelli, C.; Iatridis, J.C.; Purmessur, D. Soluble factors from the notochordal-rich intervertebral disc inhibit endothelial cell invasion and vessel formation in the presence and absence of pro-inflammatory cytokines. Osteoarthr. Cartil. 2015, 23, 487–496. [Google Scholar] [CrossRef]
- de Vries, S.; Doeselaar, M.V.; Meij, B.; Tryfonidou, M.; Ito, K. Notochordal Cell Matrix As a Therapeutic Agent for Intervertebral Disc Regeneration. Tissue Eng. Part A 2019, 25, 830–841. [Google Scholar] [CrossRef]
- Reese, D.E.; Hall, C.E.; Mikawa, T. Negative Regulation of Midline Vascular Development by the Notochord. Dev. Cell 2004, 6, 699–708. [Google Scholar] [CrossRef]
- Chen, S.; Fu, P.; Wu, H.; Pei, M. Meniscus, articular cartilage and nucleus pulposus: A comparative review of cartilage-like tissues in anatomy, development and function. Cell Tissue Res. 2017, 370, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Lyu, F.J.; Wang, H.; Zheng, Z. The involvement of immune system in intervertebral disc herniation and degeneration. JOR Spine 2022, 5, e1196. [Google Scholar] [CrossRef]
- Williams, R.J.; Laagland, L.T.; Bach, F.C.; Ward, L.; Chan, W.; Tam, V.; Medzikovic, A.; Basatvat, S.; Paillat, L.; Vedrenne, N.; et al. Recommendations for intervertebral disc notochordal cell investigation: From isolation to characterization. JOR Spine 2023, 6, e1272. [Google Scholar] [CrossRef]
- Schol, J.; Sakai, D. Comprehensive narrative review on the analysis of outcomes from cell transplantation clinical trials for discogenic low back pain. N. Am. Spine Soc. J. 2023, 13, 100195. [Google Scholar] [CrossRef]
- Alkhatib, B.; Ban, G.I.; Williams, S.; Serra, R. IVD Development: Nucleus Pulposus Development and Sclerotome Specification. Curr. Mol. Biol. Rep. 2018, 4, 132–141. [Google Scholar] [CrossRef]
- Stemple, D.L. Structure and function of the notochord: An essential organ for chordate development. Development 2005, 132, 2503–2512. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Tamplin, O.J.; Beckers, A.; Gossler, A.; Rossant, J. Live Imaging and Genetic Analysis of Mouse Notochord Formation Reveals Regional Morphogenetic Mechanisms. Dev. Cell 2007, 13, 884–896. [Google Scholar] [CrossRef]
- Imuta, Y.; Koyama, H.; Shi, D.; Eiraku, M.; Fujimori, T.; Sasaki, H. Mechanical control of notochord morphogenesis by extra-embryonic tissues in mouse embryos. Mech. Dev. 2014, 132, 44–58. [Google Scholar] [CrossRef]
- Smits, P.; Li, P.; Mandel, J.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B.; Lefebvre, V. The Transcription Factors L-Sox5 and Sox6 Are Essential for Cartilage Formation. Dev. Cell 2001, 1, 277–290. [Google Scholar] [CrossRef]
- Choi, K.-S.; Harfe, B.D. Hedgehog signaling is required for formation of the notochord sheath and patterning of nuclei pulposi within the intervertebral discs. Proc. Natl. Acad. Sci. USA 2011, 108, 9484–9489. [Google Scholar] [CrossRef]
- Williams, S.; Alkhatib, B.; Serra, R. Development of the axial skeleton and intervertebral disc. In Vertebrate Skeletal Development; Elsevier: Amsterdam, The Netherlands, 2019; pp. 49–90. [Google Scholar] [CrossRef]
- Kalcheim, C.; Ben-Yair, R. Cell rearrangements during development of the somite and its derivatives. Curr. Opin. Genet. Dev. 2005, 15, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; O’Rahilly, R. The Primitive Streak, the Caudal Eminence and Related Structures in Staged Human Embryos. Cells Tissues Organs 2004, 177, 2–20. [Google Scholar] [CrossRef]
- Lawson, L.; Harfe, B.D. Notochord to Nucleus Pulposus Transition. Curr. Osteoporos. Rep. 2015, 13, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.; Pang, A.S.W.; Evans, S.E.; Stern, C.D. The role of the notochord in amniote vertebral column segmentation. Dev. Biol. 2018, 439, 3–18. [Google Scholar] [CrossRef]
- Sivakamasundari, V.; Lufkin, T. Bridging the Gap: Understanding Embryonic Intervertebral Disc Development. Cell Dev. Biol. 2012, 1, 103. [Google Scholar] [CrossRef]
- Aszódi, A.; Chan, D.; Hunziker, E.; Bateman, J.F.; Fässler, R. Collagen II Is Essential for the Removal of the Notochord and the Formation of Intervertebral Discs. J. Cell Biol. 1998, 143, 1399–1412. [Google Scholar] [CrossRef]
- Ghazanfari, S.; Werner, A.; Ghazanfari, S.; Weaver, J.C.; Smit, T.H. Morphogenesis of aligned collagen fibers in the annulus fibrosus: Mammals versus avians. Biochem. Biophys. Res. Commun. 2018, 503, 1168–1173. [Google Scholar] [CrossRef]
- Spitz, F.; Furlong, E.E. Transcription factors: From enhancer binding to developmental control. Nat. Rev. Genet. 2012, 13, 613–626. [Google Scholar] [CrossRef]
- Nakamichi, R.; Asahara, H. The transcription factors regulating intervertebral disc development. JOR Spine 2020, 3, e1081. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Lo, Y.; Harfe, B.D. Foxa1 and Foxa2 are required for formation of the intervertebral discs. PLoS ONE 2013, 8, e55528. [Google Scholar] [CrossRef]
- Pennimpede, T.; Proske, J.; Konig, A.; Vidigal, J.A.; Morkel, M.; Bramsen, J.B.; Herrmann, B.G.; Wittler, L. In vivo knockdown of Brachyury results in skeletal defects and urorectal malformations resembling caudal regression syndrome. Dev. Biol. 2012, 372, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Kwan, K.M.; Mackem, S. Putative oncogene Brachyury (T) is essential to specify cell fate but dispensable for notochord progenitor proliferation and EMT. Proc. Natl. Acad. Sci. USA 2016, 113, 3820–3825. [Google Scholar] [CrossRef] [PubMed]
- Vujovic, S.; Henderson, S.; Presneau, N.; Odell, E.; Jacques, T.S.; Tirabosco, R.; Boshoff, C.; Flanagan, A.M. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J. Pathol. 2006, 209, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xia, Y.; Yue, C.; Xin, T.; Wang, Q.; Zhang, H.; Shen, C.; Shen, M.; Gu, Y.; Shen, J. Brachyury positively regulates extracellular matrix synthesis via directly promoting aggrecan transcription in nucleus pulposus. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2023, 37, e22976. [Google Scholar] [CrossRef]
- Tang, S.; Richards, J.; Khan, S.; Hoyland, J.; Gallego-Perez, D.; Higuita-Castro, N.; Walter, B.; Purmessur, D. Nonviral Transfection with Brachyury Reprograms Human Intervertebral Disc Cells to a Pro-Anabolic Anti-Catabolic/Inflammatory Phenotype: A Proof of Concept Study. J. Orthop. Res. 2019, 37, 2389–2400. [Google Scholar] [CrossRef]
- Colombier, P.; Halgand, B.; Chedeville, C.; Chariau, C.; Francois-Campion, V.; Kilens, S.; Vedrenne, N.; Clouet, J.; David, L.; Guicheux, J.; et al. NOTO Transcription Factor Directs Human Induced Pluripotent Stem Cell-Derived Mesendoderm Progenitors to a Notochordal Fate. Cells 2020, 9, 509. [Google Scholar] [CrossRef]
- Barrionuevo, F.; Taketo, M.M.; Scherer, G.; Kispert, A. Sox9 is required for notochord maintenance in mice. Dev. Biol. 2006, 295, 128–140. [Google Scholar] [CrossRef]
- Sivakamasundari, V.; Kraus, P.; Sun, W.; Hu, X.; Lim, S.L.; Prabhakar, S.; Lufkin, T. A developmental transcriptomic analysis of Pax1 and Pax9 in embryonic intervertebral disc development. Biol. Open 2017, 6, 187–199. [Google Scholar] [CrossRef]
- Takimoto, A.; Kokubu, C.; Watanabe, H.; Sakuma, T.; Yamamoto, T.; Kondoh, G.; Hiraki, Y.; Shukunami, C. Differential transactivation of the upstream aggrecan enhancer regulated by PAX1/9 depends on SOX9-driven transactivation. Sci. Rep. 2019, 9, 4605. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, I.; Hill, R.E.; Balling, R.; Munsterberg, A.; Imai, K. Pax1 and Pax9 activate Bapx1 to induce chondrogenic differentiation in the sclerotome. Development 2003, 130, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.; Wilm, B.; Sakai, N.; Imai, K.; Maas, R.; Balling, R. Pax1 and Pax9 synergistically regulate vertebral column development. Development 1999, 126, 5399–5408. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, E.E.; Wilson, N.; Barcellona, M.N.; Ni Neill, T.; Bagnall, J.; Brama, P.A.J.; Cunniffe, G.M.; Darwish, S.L.; Butler, J.S.; Buckley, C.T. Preclinical to clinical translation for intervertebral disc repair: Effects of species-specific scale, metabolism, and matrix synthesis rates on cell-based regeneration. JOR Spine 2023, 6, e1279. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, E.E.; Buckley, C.T. Consolidating and re-evaluating the human disc nutrient microenvironment. JOR Spine 2022, 5, e1192. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Schipani, E.; Shapiro, I.M. Hypoxic regulation of nucleus pulposus cell survival: From niche to notch. Am. J. Pathol. 2010, 176, 1577–1583. [Google Scholar] [CrossRef]
- Merceron, C.; Mangiavini, L.; Robling, A.; Wilson, T.L.; Giaccia, A.J.; Shapiro, I.M.; Schipani, E.; Risbud, M.V. Loss of HIF-1alpha in the notochord results in cell death and complete disappearance of the nucleus pulposus. PLoS ONE 2014, 9, e110768. [Google Scholar] [CrossRef]
- Thorpe, A.A.; Binch, A.L.; Creemers, L.B.; Sammon, C.; Le Maitre, C.L. Nucleus pulposus phenotypic markers to determine stem cell differentiation: Fact or fiction? Oncotarget 2016, 7, 2189–2200. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Chen, P.B.; Xu, Q.Y.; Li, B.; Jiang, S.D.; Jiang, L.S.; Zheng, X.F. Constitutive and conditional gene knockout mice for the study of intervertebral disc degeneration: Current status, decision considerations, and future possibilities. JOR Spine 2023, 6, e1242. [Google Scholar] [CrossRef]
- Ionescu, A.; Kozhemyakina, E.; Nicolae, C.; Kaestner, K.H.; Olsen, B.R.; Lassar, A.B. FoxA family members are crucial regulators of the hypertrophic chondrocyte differentiation program. Dev. Cell 2012, 22, 927–939. [Google Scholar] [CrossRef]
- Weinstein, D.C.; Ruiz i Altaba, A.; Chen, W.S.; Hoodless, P.; Prezioso, V.R.; Jessell, T.M.; Darnell, J.E., Jr. The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell 1994, 78, 575–588. [Google Scholar] [CrossRef]
- Beckers, A.; Alten, L.; Viebahn, C.; Andre, P.; Gossler, A. The mouse homeobox gene Noto regulates node morphogenesis, notochordal ciliogenesis, and left right patterning. Proc. Natl. Acad. Sci. USA 2007, 104, 15765–15770. [Google Scholar] [CrossRef]
- Abdelkhalek, H.B.; Beckers, A.; Schuster-Gossler, K.; Pavlova, M.N.; Burkhardt, H.; Lickert, H.; Rossant, J.; Reinhardt, R.; Schalkwyk, L.C.; Muller, I.; et al. The mouse homeobox gene Not is required for caudal notochord development and affected by the truncate mutation. Genes Dev. 2004, 18, 1725–1736. [Google Scholar] [CrossRef]
- McCann, M.R.; Tamplin, O.J.; Rossant, J.; Seguin, C.A. Tracing notochord-derived cells using a Noto-cre mouse: Implications for intervertebral disc development. Dis. Models Mech. 2012, 5, 73–82. [Google Scholar] [CrossRef]
- Tsingas, M.; Ottone, O.K.; Haseeb, A.; Barve, R.A.; Shapiro, I.M.; Lefebvre, V.; Risbud, M.V. Sox9 deletion causes severe intervertebral disc degeneration characterized by apoptosis, matrix remodeling, and compartment-specific transcriptomic changes. Matrix Biol. J. Int. Soc. Matrix Biol. 2020, 94, 110–133. [Google Scholar] [CrossRef]
- Smits, P.; Lefebvre, V. Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs. Development 2003, 130, 1135–1148. [Google Scholar] [CrossRef]
- Adham, I.M.; Gille, M.; Gamel, A.J.; Reis, A.; Dressel, R.; Steding, G.; Brand-Saberi, B.; Engel, W. The scoliosis (sco) mouse: A new allele of Pax1. Cytogenet. Genome Res. 2005, 111, 16–26. [Google Scholar] [CrossRef]
- Wu, W.J.; Zhang, X.K.; Zheng, X.F.; Yang, Y.H.; Jiang, S.D.; Jiang, L.S. SHH-dependent knockout of HIF-1 alpha accelerates the degenerative process in mouse intervertebral disc. Int. J. Immunopathol. Pharmacol. 2013, 26, 601–609. [Google Scholar] [CrossRef]
- Bach, F.C.; Poramba-Liyanage, D.W.; Riemers, F.M.; Guicheux, J.; Camus, A.; Iatridis, J.C.; Chan, D.; Ito, K.; Le Maitre, C.L.; Tryfonidou, M.A. Notochordal Cell-Based Treatment Strategies and Their Potential in Intervertebral Disc Regeneration. Front. Cell Dev. Biol. 2021, 9, 780749. [Google Scholar] [CrossRef]
- Basatvat, S.; Bach, F.C.; Barcellona, M.N.; Binch, A.L.; Buckley, C.T.; Bueno, B.; Chahine, N.O.; Chee, A.; Creemers, L.B.; Dudli, S.; et al. Harmonization and standardization of nucleus pulposus cell extraction and culture methods. JOR Spine 2023, 6, e1238. [Google Scholar] [CrossRef]
- Mwale, F.; Roughley, P.; Antoniou, J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: A requisite for tissue engineering of intervertebral disc. Eur. Cells Mater. 2004, 8, 58–63, discussion 63–54. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Ludwinski, F.E.; Gnanalingham, K.K.; Atkinson, R.A.; Freemont, A.J.; Hoyland, J.A. Notochordal and nucleus pulposus marker expression is maintained by sub-populations of adult human nucleus pulposus cells through aging and degeneration. Sci. Rep. 2017, 7, 1501. [Google Scholar] [CrossRef] [PubMed]
- Volleman, T.N.E.; Schol, J.; Morita, K.; Sakai, D.; Watanabe, M. Wnt3a and wnt5a as Potential Chondrogenic Stimulators for Nucleus Pulposus Cell Induction: A Comprehensive Review. Neurospine 2020, 17, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Cohn, M.J.; Harfe, B.D. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: Implications for disk degeneration and chordoma formation. Dev. Dyn. 2008, 237, 3953–3958. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Lim, T.H.; Kim, J.G.; Jeong, S.T.; Masuda, K.; An, H.S. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine (Phila Pa 1976) 2003, 28, 982–990. [Google Scholar] [CrossRef]
- Rodrigues-Pinto, R.; Berry, A.; Piper-Hanley, K.; Hanley, N.; Richardson, S.M.; Hoyland, J.A. Spatiotemporal analysis of putative notochordal cell markers reveals CD24 and keratins 8, 18, and 19 as notochord-specific markers during early human intervertebral disc development. J. Orthop. Res. 2016, 34, 1327–1340. [Google Scholar] [CrossRef]
- Bagwell, J.; Norman, J.; Ellis, K.; Peskin, B.; Hwang, J.; Ge, X.; Nguyen, S.V.; McMenamin, S.K.; Stainier, D.Y.; Bagnat, M. Notochord vacuoles absorb compressive bone growth during zebrafish spine formation. Elife 2020, 9, e51221. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.C.; de Vries, S.A.; Krouwels, A.; Creemers, L.B.; Ito, K.; Meij, B.P.; Tryfonidou, M.A. The species-specific regenerative effects of notochordal cell-conditioned medium on chondrocyte-like cells derived from degenerated human intervertebral discs. Eur. Cells Mater. 2015, 30, 132–146, discussion 146-137. [Google Scholar] [CrossRef] [PubMed]
- Alini, M.; Diwan, A.D.; Erwin, W.M.; Little, C.B.; Melrose, J. An update on animal models of intervertebral disc degeneration and low back pain: Exploring the potential of artificial intelligence to improve research analysis and development of prospective therapeutics. JOR Spine 2023, 6, e1230. [Google Scholar] [CrossRef]
- Alini, M.; Eisenstein, S.M.; Ito, K.; Little, C.; Kettler, A.A.; Masuda, K.; Melrose, J.; Ralphs, J.; Stokes, I.; Wilke, H.J. Are animal models useful for studying human disc disorders/degeneration? Eur. Spine J. 2008, 17, 2–19. [Google Scholar] [CrossRef]
- Thompson, K.; Moore, S.; Tang, S.; Wiet, M.; Purmessur, D. The chondrodystrophic dog: A clinically relevant intermediate-sized animal model for the study of intervertebral disc-associated spinal pain. JOR Spine 2018, 1, e1011. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Schol, J.; Bach, F.C.; Tekari, A.; Sagawa, N.; Nakamura, Y.; Chan, S.C.W.; Nakai, T.; Creemers, L.B.; Frauchiger, D.A.; et al. Successful fishing for nucleus pulposus progenitor cells of the intervertebral disc across species. JOR Spine 2018, 1, e1018. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Chen, P.; Ma, C.Y.; Li, C.; Au, T.Y.K.; Tam, V.; Peng, Y.; Wu, R.; Cheung, K.M.C.; et al. Directed Differentiation of Notochord-like and Nucleus Pulposus-like Cells Using Human Pluripotent Stem Cells. Cell Rep. 2020, 30, 2791–2806.e5. [Google Scholar] [CrossRef]
- Tamagawa, S.; Sakai, D.; Nojiri, H.; Nakamura, Y.; Warita, T.; Matsushita, E.; Schol, J.; Soma, H.; Ogasawara, S.; Munesada, D.; et al. SOD2 orchestrates redox homeostasis in intervertebral discs: A novel insight into oxidative stress-mediated degeneration and therapeutic potential. Redox Biol. 2024, 71, 103091. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.S.; Li, D.D.; Wang, C.G.; Ying, L.W.; Wang, J.K.; Yang, B.; Shu, J.W.; Huang, X.P.; Zhang, Y.A.; Yu, C.; et al. An esterase-responsive ibuprofen nano-micelle pre-modified embryo derived nucleus pulposus progenitor cells promote the regeneration of intervertebral disc degeneration. Bioact. Mater. 2023, 21, 69–85. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Shi, X.; Han, J.; Chen, J.; Zhang, X.; Xie, D.; Li, Z.; Niu, X.; Chen, L.; et al. Characterization of the Nucleus Pulposus Progenitor Cells via Spatial Transcriptomics. Adv. Sci. 2024, 11, e2303752. [Google Scholar] [CrossRef] [PubMed]
- Gogate, S.S.; Fujita, N.; Skubutyte, R.; Shapiro, I.M.; Risbud, M.V. Tonicity enhancer binding protein (TonEBP) and hypoxia-inducible factor (HIF) coordinate heat shock protein 70 (Hsp70) expression in hypoxic nucleus pulposus cells: Role of Hsp70 in HIF-1alpha degradation. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2012, 27, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Johnson, Z.I.; Risbud, M.V. Understanding nucleus pulposus cell phenotype: A prerequisite for stem cell based therapies to treat intervertebral disc degeneration. Curr. Stem Cell Res. Ther. 2015, 10, 307–316. [Google Scholar] [CrossRef]
- Kelsey, R. Targeting NP cell senescence in IVDD. Nat. Rev. Rheumatol. 2024, 20, 197. [Google Scholar] [CrossRef]
- Diwan, A.D.; Melrose, J. Intervertebral disc degeneration and how it leads to low back pain. JOR Spine 2023, 6, e1231. [Google Scholar] [CrossRef]
- Oichi, T.; Taniguchi, Y.; Oshima, Y.; Tanaka, S.; Saito, T. Pathomechanism of intervertebral disc degeneration. JOR Spine 2020, 3, e1076. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Cai, W.; Liu, F.; Cheng, K.; Guo, D.; Liu, Z. An in-depth analysis of the immunomodulatory mechanisms of intervertebral disc degeneration. JOR Spine 2022, 5, e1233. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Schol, J.; Sakai, D.; Warita, T.; Nukaga, T.; Sako, K.; Wangler, S.; Tamagawa, S.; Zeiter, S.; Alini, M.; Grad, S. Homing of vertebral-delivered mesenchymal stromal cells for degenerative intervertebral discs repair—An in vivo proof-of-concept study. JOR Spine 2023, 6, e1228. [Google Scholar] [CrossRef]
- Croft, A.S.; Illien-Junger, S.; Grad, S.; Guerrero, J.; Wangler, S.; Gantenbein, B. The Application of Mesenchymal Stromal Cells and Their Homing Capabilities to Regenerate the Intervertebral Disc. Int. J. Mol. Sci. 2021, 22, 3519. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; Shi, D.; Chen, K.; Zhao, J.; He, S.; Bai, Y.; Shen, P.; Ni, H. Single-cell RNA Seq reveals cellular landscape-specific characteristics and potential etiologies for adolescent idiopathic scoliosis. JOR Spine 2021, 4, e1184. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Glaeser, J.D.; Salehi, K.; Kaneda, G.; Mathkar, P.; Wagner, A.; Ho, R.; Sheyn, D. Single-cell atlas unveils cellular heterogeneity and novel markers in human neonatal and adult intervertebral discs. iScience 2022, 25, 104504. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; He, J.; Zhu, J.; Xu, Z.; Wang, Z.; Yan, J.; Hu, O.; Bai, Z.; Chen, L.; Xie, Y.; et al. Spatially defined single-cell transcriptional profiling characterizes diverse chondrocyte subtypes and nucleus pulposus progenitors in human intervertebral discs. Bone Res. 2021, 9, 37. [Google Scholar] [CrossRef]
- Jiang, W.; Glaeser, J.D.; Kaneda, G.; Sheyn, J.; Wechsler, J.T.; Stephan, S.; Salehi, K.; Chan, J.L.; Tawackoli, W.; Avalos, P.; et al. Intervertebral disc human nucleus pulposus cells associated with back pain trigger neurite outgrowth in vitro and pain behaviors in rats. Sci. Transl. Med. 2023, 15, eadg7020. [Google Scholar] [CrossRef]
- Han, S.; Zhang, Y.; Zhang, X.; Zhang, H.; Meng, S.; Kong, M.; Liu, X.; Ma, X. Single-Cell RNA Sequencing of the Nucleus Pulposus Reveals Chondrocyte Differentiation and Regulation in Intervertebral Disc Degeneration. Front. Cell Dev. Biol. 2022, 10, 824771. [Google Scholar] [CrossRef]
- Li, Z.; Ye, D.; Dai, L.; Xu, Y.; Wu, H.; Luo, W.; Liu, Y.; Yao, X.; Wang, P.; Miao, H.; et al. Single-Cell RNA Sequencing Reveals the Difference in Human Normal and Degenerative Nucleus Pulposus Tissue Profiles and Cellular Interactions. Front. Cell Dev. Biol. 2022, 10, 910626. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Z.; Zhang, H.; Cui, P.; Li, Y.; Chen, X.; Kong, C.; Wang, W.; Lu, S. Single-cell sequencing: New insights for intervertebral disc degeneration. Biomed. Pharmacother. Biomed. Pharmacother. 2023, 165, 115224. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.L.; de Luca, K.; Haile, L.M.; Steinmetz, J.D.; Culbreth, G.T.; Cross, M.; Kopec, J.A.; Ferreira, P.H.; Blyth, F.M.; Buchbinder, R.; et al. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [Google Scholar] [CrossRef] [PubMed]
- Tessier, S.; Risbud, M.V. Understanding embryonic development for cell-based therapies of intervertebral disc degeneration: Toward an effort to treat disc degeneration subphenotypes. Dev. Dyn. 2020, 250, 302–317. [Google Scholar] [CrossRef]
- Walsh, A.J.L.; Lotz, J.C. Biological response of the intervertebral disc to dynamic loading. J. Biomech. 2004, 37, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, L.; Yang, M.; Li, B.; Yi, J.; Ai, X.; Zhang, Y.; Huang, B.; Li, C.; Feng, C.; et al. A positive feedback loop between EZH2 and NOX4 regulates nucleus pulposus cell senescence in age-related intervertebral disc degeneration. Cell Div. 2020, 15, s13008–s13020. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fernández, C.; Francisco, V.; Pino, J.; Mera, A.; González-Gay, M.A.; Gómez, R.; Lago, F.; Gualillo, O. Molecular Relationships among Obesity, Inflammation and Intervertebral Disc Degeneration: Are Adipokines the Common Link? Int. J. Mol. Sci. 2019, 20, 2030. [Google Scholar] [CrossRef] [PubMed]
- Schumann, B.; Bolm-Audorff, U.; Bergmann, A.; Ellegast, R.; Elsner, G.; Grifka, J.; Haerting, J.; Jäger, M.; Michaelis, M.; Seidler, A. Lifestyle factors and lumbar disc disease: Results of a German multi-center case-control study (EPILIFT). Arthritis Res. Ther. 2010, 12, R193. [Google Scholar] [CrossRef]
- Chan, D.; Song, Y.; Sham, P.; Cheung, K.M.C. Genetics of disc degeneration. Eur. Spine J. 2006, 15, 317–325. [Google Scholar] [CrossRef]
- Bonnaire, F.C.; Danalache, M.; Sigwart, V.A.; Breuer, W.; Rolauffs, B.; Hofmann, U.K. The intervertebral disc from embryonic development to disc degeneration: Insights into spatial cellular organization. Spine J. 2021, 21, 1387–1398. [Google Scholar] [CrossRef]
- Peck, S.H.; McKee, K.K.; Tobias, J.W.; Malhotra, N.R.; Harfe, B.D.; Smith, L.J. Whole Transcriptome Analysis of Notochord-Derived Cells during Embryonic Formation of the Nucleus Pulposus. Sci. Rep. 2017, 7, 10504. [Google Scholar] [CrossRef] [PubMed]
- Vergroesen, P.P.; Kingma, I.; Emanuel, K.S.; Hoogendoorn, R.J.; Welting, T.J.; van Royen, B.J.; van Dieen, J.H.; Smit, T.H. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthr. Cartil. 2015, 23, 1057–1070. [Google Scholar] [CrossRef]
- Sampara, P.; Banala, R.R.; Vemuri, S.K.; Av, G.R.; Gpv, S. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: A review. Gene Ther. 2018, 25, 67–82. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Nerlich, A.; Mittermaier, N.; Weiler, C.; Lumenta, C.; Wuertz, K.; Boos, N. Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur. Spine J. 2009, 18, 1573–1586. [Google Scholar] [CrossRef]
- Pockert, A.J.; Richardson, S.M.; Le Maitre, C.L.; Lyon, M.; Deakin, J.A.; Buttle, D.J.; Freemont, A.J.; Hoyland, J.A. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009, 60, 482–491. [Google Scholar] [CrossRef]
- Francisco, V.; Pino, J.; González-Gay, M.Á.; Lago, F.; Karppinen, J.; Tervonen, O.; Mobasheri, A.; Gualillo, O. A new immunometabolic perspective of intervertebral disc degeneration. Nat. Rev. Rheumatol. 2021, 18, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Mohd Isa, I.L.; Teoh, S.L.; Mohd Nor, N.H.; Mokhtar, S.A. Discogenic Low Back Pain: Anatomy, Pathophysiology and Treatments of Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2022, 24, 208. [Google Scholar] [CrossRef] [PubMed]
- Walcott, B.P.; Nahed, B.V.; Mohyeldin, A.; Coumans, J.V.; Kahle, K.T.; Ferreira, M.J. Chordoma: Current concepts, management, and future directions. Lancet Oncol. 2012, 13, e69–e76. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Sommer, J. Building a global consensus approach to chordoma: A position paper from the medical and patient community. Lancet Oncol. 2015, 16, e71–e83. [Google Scholar] [CrossRef]
- Ulici, V.; Hart, J. Chordoma. Arch. Pathol. Lab. Med. 2022, 146, 386–395. [Google Scholar] [CrossRef]
- Tarpey, P.S.; Behjati, S.; Young, M.D.; Martincorena, I.; Alexandrov, L.B.; Farndon, S.J.; Guzzo, C.; Hardy, C.; Latimer, C.; Butler, A.P.; et al. The driver landscape of sporadic chordoma. Nat. Commun. 2017, 8, 890. [Google Scholar] [CrossRef] [PubMed]
- Mammar, H.; Polivka, M.; Belkacemi, Y.; Lot, G.; Froelich, S.; Carpentier, A.; Clemenceau, S.; Gaillard, S.; Paquis, P.; Birtwisle-Peyrottes, I.; et al. Hypoxia and Metabolism Regulation in Chordomas: Correlation Between Biology and Clinical Features for Potential Targeted Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, E89–E90. [Google Scholar] [CrossRef]
- He, G.; Liu, X. Hypoxia-Inducible Factor-1α (HIF-1α) as a Factor to Predict the Prognosis of Spinal Chordoma. Spine (Phila Pa 1976) 2024, 49, 661–669. [Google Scholar] [CrossRef]
- Kabolizadeh, P.; Chen, Y.-L.; Liebsch, N.; Hornicek, F.J.; Schwab, J.H.; Choy, E.; Rosenthal, D.I.; Niemierko, A.; DeLaney, T.F. Updated Outcome and Analysis of Tumor Response in Mobile Spine and Sacral Chordoma Treated With Definitive High-Dose Photon/Proton Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 254–262. [Google Scholar] [CrossRef]
- Williams, D.; Ford, C. Carbon Ion Beam Therapy for Chordoma: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines; CADTH: Ottawa, ON, Canada, 2018. [Google Scholar]
- Barber, S.M.; Sadrameli, S.S.; Lee, J.J.; Fridley, J.S.; Teh, B.S.; Oyelese, A.A.; Telfeian, A.E.; Gokaslan, Z.L. Chordoma—Current Understanding and Modern Treatment Paradigms. J. Clin. Med. 2021, 10, 1054. [Google Scholar] [CrossRef]
- Dong, M.; Liu, R.; Zhang, Q.; Wang, D.; Luo, H.; Wang, Y.; Chen, J.; Ou, Y.; Wang, X. Efficacy and safety of carbon ion radiotherapy for chordomas: A systematic review and meta-analysis. Radiat. Oncol. 2023, 18, s13014–s13023. [Google Scholar] [CrossRef] [PubMed]
- Härtl, R.; Bonassar, L.; Bonassar, L.J. Biological Approaches to Spinal Disc Repair and Regeneration for Clinicians; Thieme Medical Publishers, Incorporated: Leipzig, Germany, 2017. [Google Scholar]
- Williams, R.J.; Tryfonidou, M.A.; Snuggs, J.W.; Le Maitre, C.L. Cell sources proposed for nucleus pulposus regeneration. JOR Spine 2021, 4, e1175. [Google Scholar] [CrossRef]
- Purmessur, D.; Cornejo, M.C.; Cho, S.K.; Hecht, A.C.; Iatridis, J.C. Notochordal cell-derived therapeutic strategies for discogenic back pain. Glob. Spine J. 2013, 3, 201–218. [Google Scholar] [CrossRef]
- Purmessur, D.; Schek, R.M.; Abbott, R.D.; Ballif, B.A.; Godburn, K.E.; Iatridis, J.C. Notochordal conditioned media from tissue increases proteoglycan accumulation and promotes a healthy nucleus pulposus phenotype in human mesenchymal stem cells. Arthritis Res. Ther. 2011, 13, R81. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Acevedo, L.; Wang, X.; Karim, M.Z.; Matta, A.; Mehrkens, A.; Schaeren, S.; Feliciano, S.; Jakob, M.; Martin, I.; et al. Notochordal cell conditioned medium (NCCM) regenerates end-stage human osteoarthritic articular chondrocytes and promotes a healthy phenotype. Arthritis Res. Ther. 2016, 18, 125. [Google Scholar] [CrossRef]
- Sheyn, D.; Ben-David, S.; Tawackoli, W.; Zhou, Z.; Salehi, K.; Bez, M.; De Mel, S.; Chan, V.; Roth, J.; Avalos, P.; et al. Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model. Theranostics 2019, 9, 7506–7524. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Jing, L.; Willard, V.P.; Wu, C.L.; Guilak, F.; Chen, J.; Setton, L.A. Differentiation of human induced pluripotent stem cells into nucleus pulposus-like cells. Stem Cell Res. Ther. 2018, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lee, E.J.; Jing, L.; Christoforou, N.; Leong, K.W.; Setton, L.A. Differentiation of mouse induced pluripotent stem cells (iPSCs) into nucleus pulposus-like cells in vitro. PLoS ONE 2013, 8, e75548. [Google Scholar] [CrossRef] [PubMed]
- Sako, K.; Sakai, D.; Nakamura, Y.; Schol, J.; Matsushita, E.; Warita, T.; Horikita, N.; Sato, M.; Watanabe, M. Effect of Whole Tissue Culture and Basic Fibroblast Growth Factor on Maintenance of Tie2 Molecule Expression in Human Nucleus Pulposus Cells. Int. J. Mol. Sci. 2021, 22, 4723. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Sakai, D.; Schol, J.; Nakai, T.; Suyama, K.; Watanabe, M. Sciatic nerve regeneration by transplantation of in vitro differentiated nucleus pulposus progenitor cells. Regen Med. 2017, 12, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.; Hackel, S.; Croft, A.S.; Albers, C.E.; Gantenbein, B. The effects of 3D culture on the expansion and maintenance of nucleus pulposus progenitor cell multipotency. JOR Spine 2021, 4, e1131. [Google Scholar] [CrossRef] [PubMed]
- Sako, K.; Sakai, D.; Nakamura, Y.; Matsushita, E.; Schol, J.; Warita, T.; Horikita, N.; Sato, M.; Watanabe, M. Optimization of Spheroid Colony Culture and Cryopreservation of Nucleus Pulposus Cells for the Development of Intervertebral Disc Regenerative Therapeutics. Appl. Sci. 2021, 11, 3309. [Google Scholar] [CrossRef]
- Zhang, X.; Guerrero, J.; Croft, A.S.; Albers, C.E.; Hackel, S.; Gantenbein, B. Spheroid-Like Cultures for Expanding Angiopoietin Receptor-1 (aka. Tie2) Positive Cells from the Human Intervertebral Disc. Int. J. Mol. Sci. 2020, 21, 9423. [Google Scholar] [CrossRef]
- Mehrkens, A.; Matta, A.; Karim, M.Z.; Kim, S.; Fehlings, M.G.; Schaeren, S.; Mark Erwin, W. Notochordal cell-derived conditioned medium protects human nucleus pulposus cells from stress-induced apoptosis. Spine J. Off. J. North Am. Spine Soc. 2017, 17, 579–588. [Google Scholar] [CrossRef]
- Arkesteijn, I.T.; Smolders, L.A.; Spillekom, S.; Riemers, F.M.; Potier, E.; Meij, B.P.; Ito, K.; Tryfonidou, M.A. Effect of coculturing canine notochordal, nucleus pulposus and mesenchymal stromal cells for intervertebral disc regeneration. Arthritis Res. Ther. 2015, 17, 60. [Google Scholar] [CrossRef]
- Erwin, W.M.; Ashman, K.; O’Donnel, P.; Inman, R.D. Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum. 2006, 54, 3859–3867. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, T.J.; Vaso, K.; Danias, G.; Chionuma, H.N.; Weiser, J.R.; Iatridis, J.C. Extracellular Vesicles as an Emerging Treatment Option for Intervertebral Disc Degeneration: Therapeutic Potential, Translational Pathways, and Regulatory Considerations. Adv. Healthc. Mater. 2022, 11, e2100596. [Google Scholar] [CrossRef] [PubMed]
- Krut, Z.; Pelled, G.; Gazit, D.; Gazit, Z. Stem Cells and Exosomes: New Therapies for Intervertebral Disc Degeneration. Cells 2021, 10, 2241. [Google Scholar] [CrossRef]
- Tilotta, V.; Vadalà, G.; Ambrosio, L.; Di Giacomo, G.; Cicione, C.; Russo, F.; Darinskas, A.; Papalia, R.; Denaro, V. Wharton’s Jelly mesenchymal stromal cell-derived extracellular vesicles promote nucleus pulposus cell anabolism in an in vitro 3D alginate-bead culture model. JOR Spine 2024, 7, e1274. [Google Scholar] [CrossRef] [PubMed]
- Tilotta, V.; Vadalà, G.; Ambrosio, L.; Russo, F.; Cicione, C.; Di Giacomo, G.; Papalia, R.; Denaro, V. Mesenchymal Stem Cell-Derived Exosomes: The New Frontier for the Treatment of Intervertebral Disc Degeneration. Appl. Sci. 2021, 11, 11222. [Google Scholar] [CrossRef]
- Bach, F.; Libregts, S.; Creemers, L.; Meij, B.; Ito, K.; Wauben, M.; Tryfonidou, M. Notochordal-cell derived extracellular vesicles exert regenerative effects on canine and human nucleus pulposus cells. Oncotarget 2017, 8, 88845–88856. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.C.; de Vries, S.A.; Riemers, F.M.; Boere, J.; van Heel, F.W.; van Doeselaar, M.; Goerdaya, S.S.; Nikkels, P.G.; Benz, K.; Creemers, L.B.; et al. Soluble and pelletable factors in porcine, canine and human notochordal cell-conditioned medium: Implications for IVD regeneration. Eur. Cell Mater. 2016, 32, 163–180. [Google Scholar] [CrossRef]
- Ambrosio, L.; Schol, J.; Ruiz-Fernandez, C.; Tamagawa, S.; Soma, H.; Tilotta, V.; Di Giacomo, G.; Cicione, C.; Nakayama, S.; Kamiya, K.; et al. ISSLS PRIZE in Basic Science 2024: Superiority of nucleus pulposus cell-versus mesenchymal stromal cell-derived extracellular vesicles in attenuating disc degeneration and alleviating pain. Eur. Spine J. 2024, 33, 1713–1727. [Google Scholar] [CrossRef]
- Hubert, M.G.; Vadala, G.; Sowa, G.; Studer, R.K.; Kang, J.D. Gene therapy for the treatment of degenerative disk disease. J. Am. Acad. Orthop. Surg. 2008, 16, 312–319. [Google Scholar] [CrossRef]
- Vadala, G.; Sowa, G.A.; Smith, L.; Hubert, M.G.; Levicoff, E.A.; Denaro, V.; Gilbertson, L.G.; Kang, J.D. Regulation of transgene expression using an inducible system for improved safety of intervertebral disc gene therapy. Spine (Phila Pa 1976) 2007, 32, 1381–1387. [Google Scholar] [CrossRef]
- Seki, S.; Iwasaki, M.; Makino, H.; Yahara, Y.; Miyazaki, Y.; Kamei, K.; Futakawa, H.; Nogami, M.; Tran Canh Tung, N.; Hirokawa, T.; et al. Direct Reprogramming and Induction of Human Dermal Fibroblasts to Differentiate into iPS-Derived Nucleus Pulposus-like Cells in 3D Culture. Int. J. Mol. Sci. 2022, 23, 4059. [Google Scholar] [CrossRef]
- Cicione, C.; Tilotta, V.; Giacomo, G.D.; Ambrosio, L.; Russo, F.; Papalia, R.; Vadalà, G.; Denaro, V. Mesendoderm Progenitor Cells Derived from Pluripotent Stem Cells for Disc Regeneration: A Preliminary Study in an Ovine Model. Orthop. Proc. 2024, 106-B, 104. [Google Scholar] [CrossRef]
- Vadala, G.; Sowa, G.A.; Kang, J.D. Gene therapy for disc degeneration. Expert Opin. Biol. Ther. 2007, 7, 185–196. [Google Scholar] [CrossRef]
- Levicoff, E.A.; Kim, J.S.; Sobajima, S.; Wallach, C.J.; Larson, J.W., 3rd; Robbins, P.D.; Xiao, X.; Juan, L.; Vadala, G.; Gilbertson, L.G.; et al. Safety assessment of intradiscal gene therapy II: Effect of dosing and vector choice. Spine (Phila Pa 1976) 2008, 33, 1509–1516, discussion 1517. [Google Scholar] [CrossRef]
- Bach, F.C.; Tellegen, A.R.; Beukers, M.; Miranda-Bedate, A.; Teunissen, M.; de Jong, W.A.M.; de Vries, S.A.H.; Creemers, L.B.; Benz, K.; Meij, B.P.; et al. Biologic canine and human intervertebral disc repair by notochordal cell-derived matrix: From bench towards bedside. Oncotarget 2018, 9, 26507–26526. [Google Scholar] [CrossRef]
- Potier, E.; de Vries, S.; van Doeselaar, M.; Ito, K. Potential application of notochordal cells for intervertebral disc regeneration: An in vitro assessment. Eur. Cells Mater. 2014, 28, 68–80, discussion 80-61. [Google Scholar] [CrossRef]
- Schmitz, T.C.; van Genabeek, B.; Pouderoijen, M.J.; Janssen, H.M.; van Doeselaar, M.; Crispim, J.F.; Tryfonidou, M.A.; Ito, K. Semi-synthetic degradable notochordal cell-derived matrix hydrogel for use in degenerated intervertebral discs: Initial in vitro characterization. J. Biomed. Mater. Res. Part A 2023, 111, 1903–1915. [Google Scholar] [CrossRef]
- Schmitz, T.C.; van Doeselaar, M.; Tryfonidou, M.A.; Ito, K. Detergent-Free Decellularization of Notochordal Cell-Derived Matrix Yields a Regenerative, Injectable, and Swellable Biomaterial. ACS Biomater. Sci. Eng. 2022, 8, 3912–3923. [Google Scholar] [CrossRef]
- Thorpe, A.A.; Bach, F.C.; Tryfonidou, M.A.; Le Maitre, C.L.; Mwale, F.; Diwan, A.D.; Ito, K. Leaping the hurdles in developing regenerative treatments for the intervertebral disc from preclinical to clinical. JOR Spine 2018, 1, e1027. [Google Scholar] [CrossRef]
- Takahashi, T.; Donahue, R.P.; Nordberg, R.C.; Hu, J.C.; Currall, S.C.; Athanasiou, K.A. Commercialization of regenerative-medicine therapies. Nat. Rev. Bioeng. 2023, 1, 906–929. [Google Scholar] [CrossRef]
- Silverman, L.I.; Flanagan, F.; Rodriguez-Granrose, D.; Simpson, K.; Saxon, L.H.; Foley, K.T. Identifying and Managing Sources of Variability in Cell Therapy Manufacturing and Clinical Trials. Regen. Eng. Transl. Med. 2019, 5, 354–361. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).