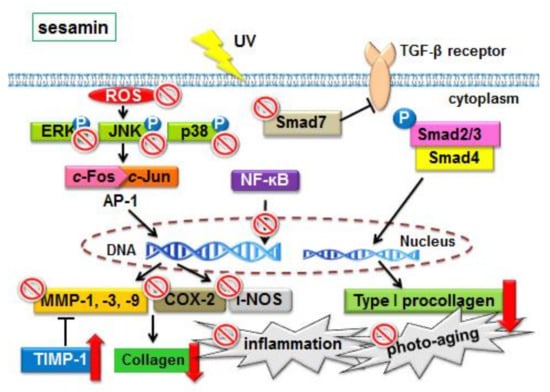
Protective Effects of Sesamin against UVB-Induced Skin Inflammation and Photodamage In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and UV Irradiation
2.3. Detection of ROS Production
2.4. Western Blot Analysis
2.5. Measuring Total Collagen Synthesis in Fibroblasts
2.6. Immunofluorescence Staining
2.7. Effect of Sesamin Treatment on Photodamage in Hairless Mice
2.7.1. Animals
2.7.2. Experimental Design
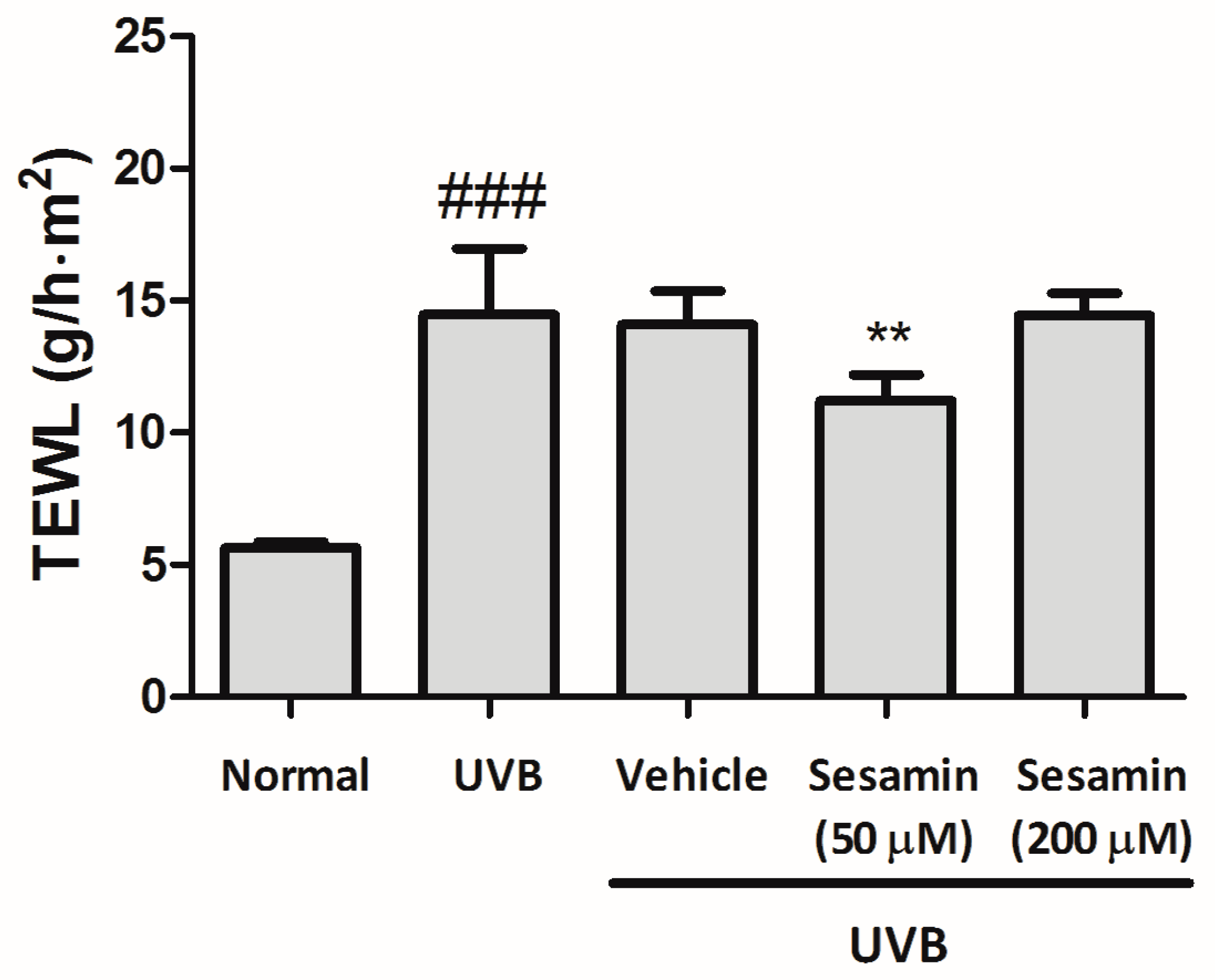
2.7.3. Detection of Erythema (a* Value) and Transepidermal Water Loss of Mice Skin
2.7.4. Immunohistological Analysis
2.8. Statistical Analysis
3. Results
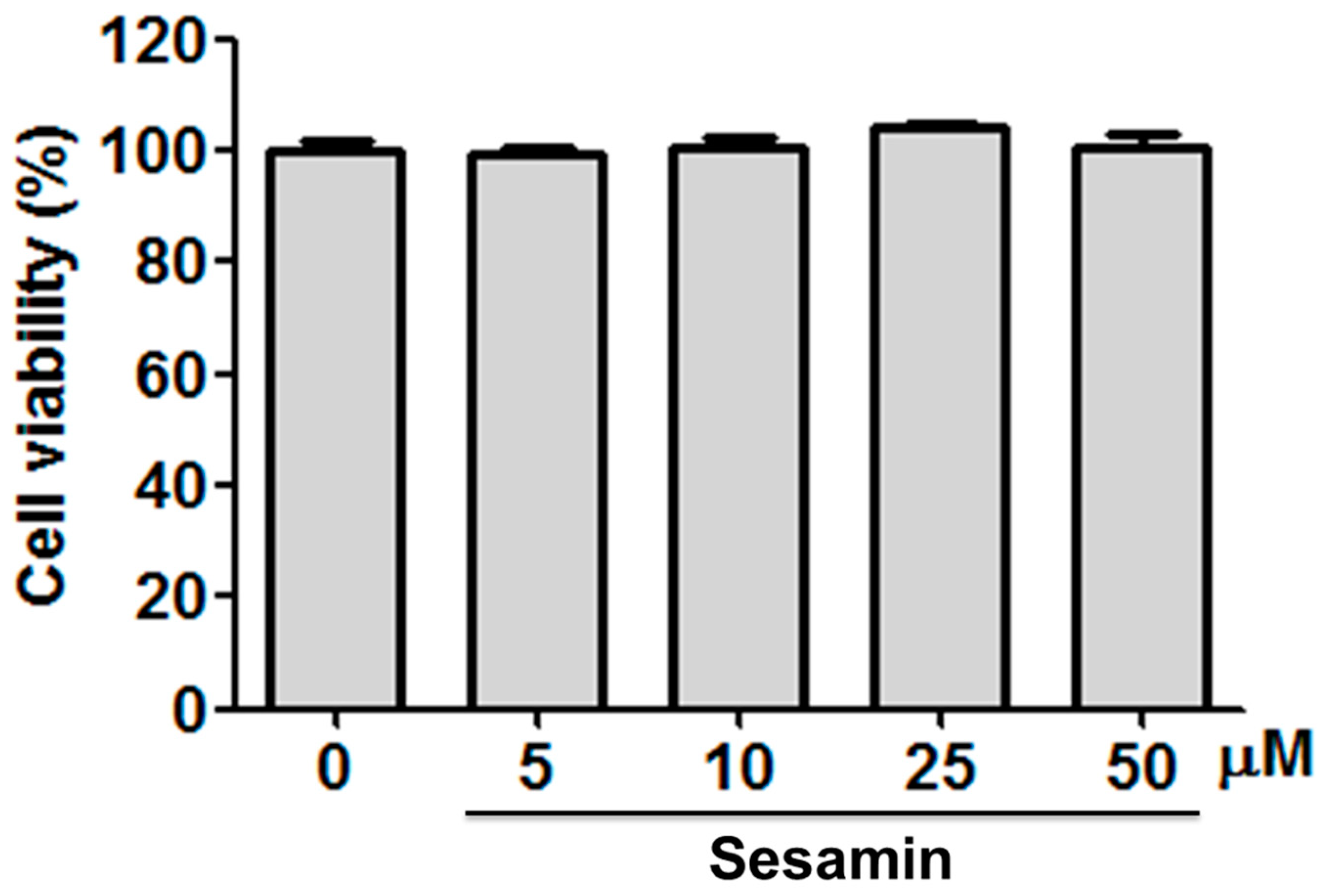
3.1. Sesamin Did Not Cause Cytotoxicity in Hs68 Cells
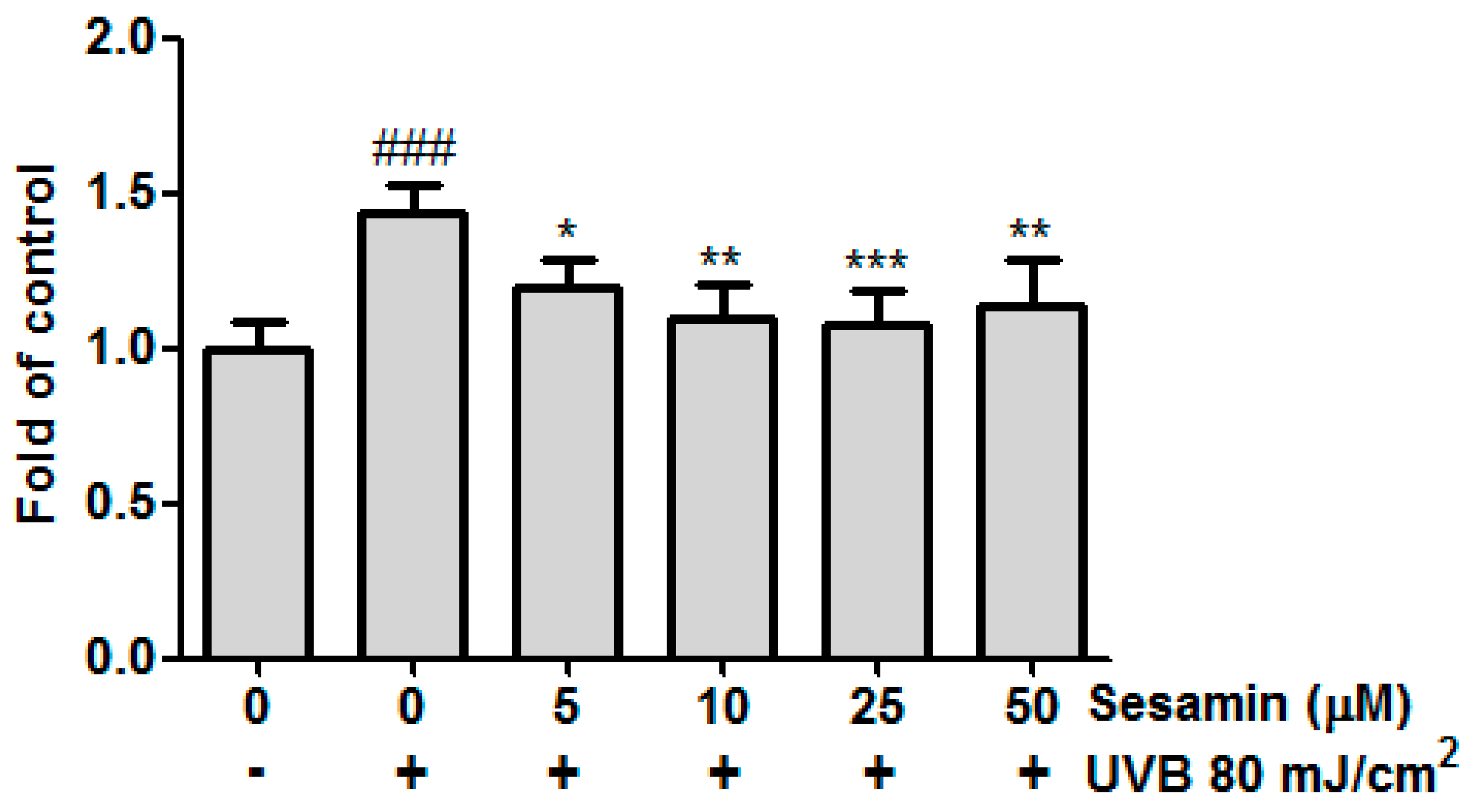
3.2. Sesamin Inhibited Intracellular ROS Formation in Hs68 Cells
3.3. Effects and Mechanisms of Sesamin on Skin Photodamage
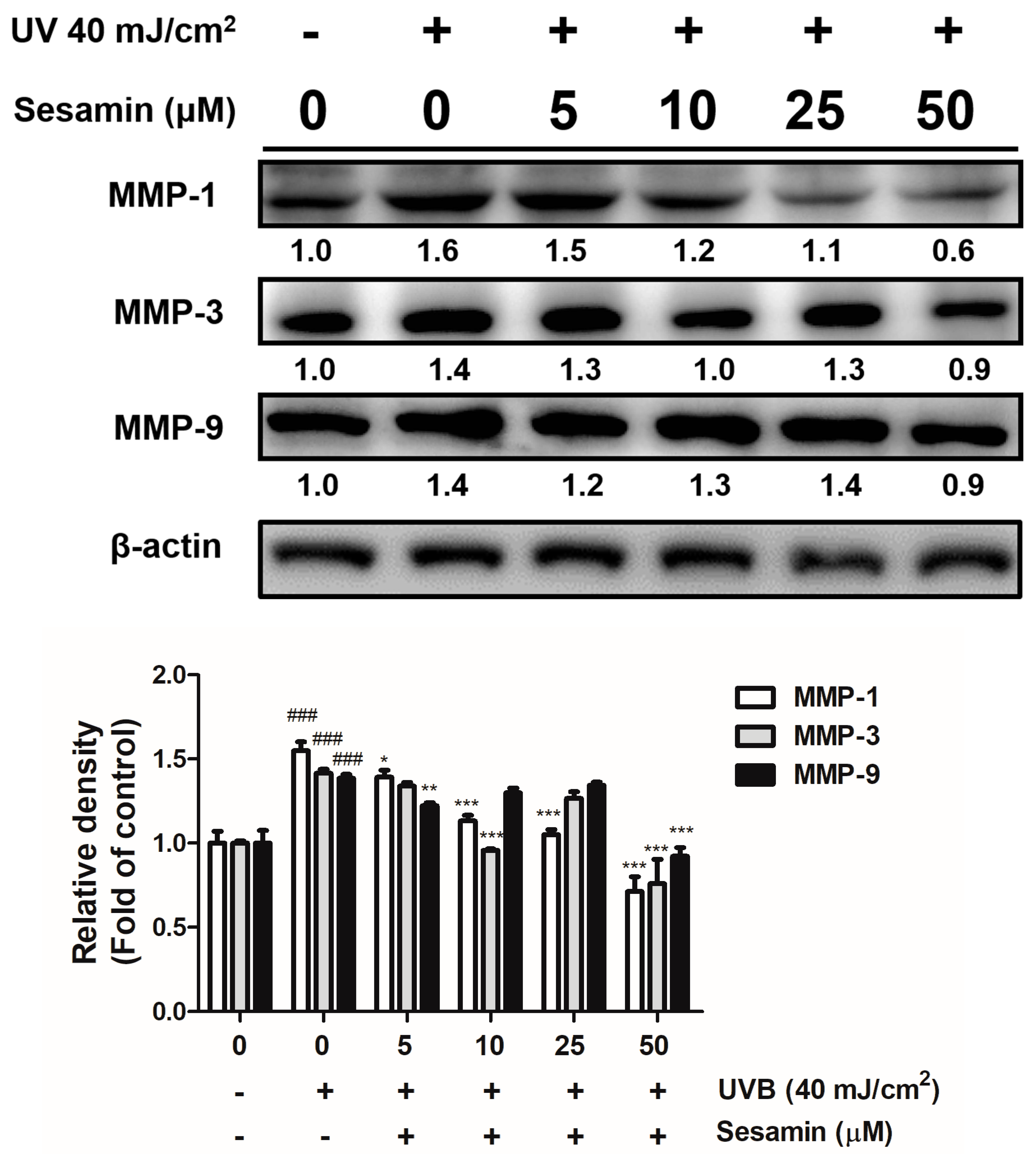
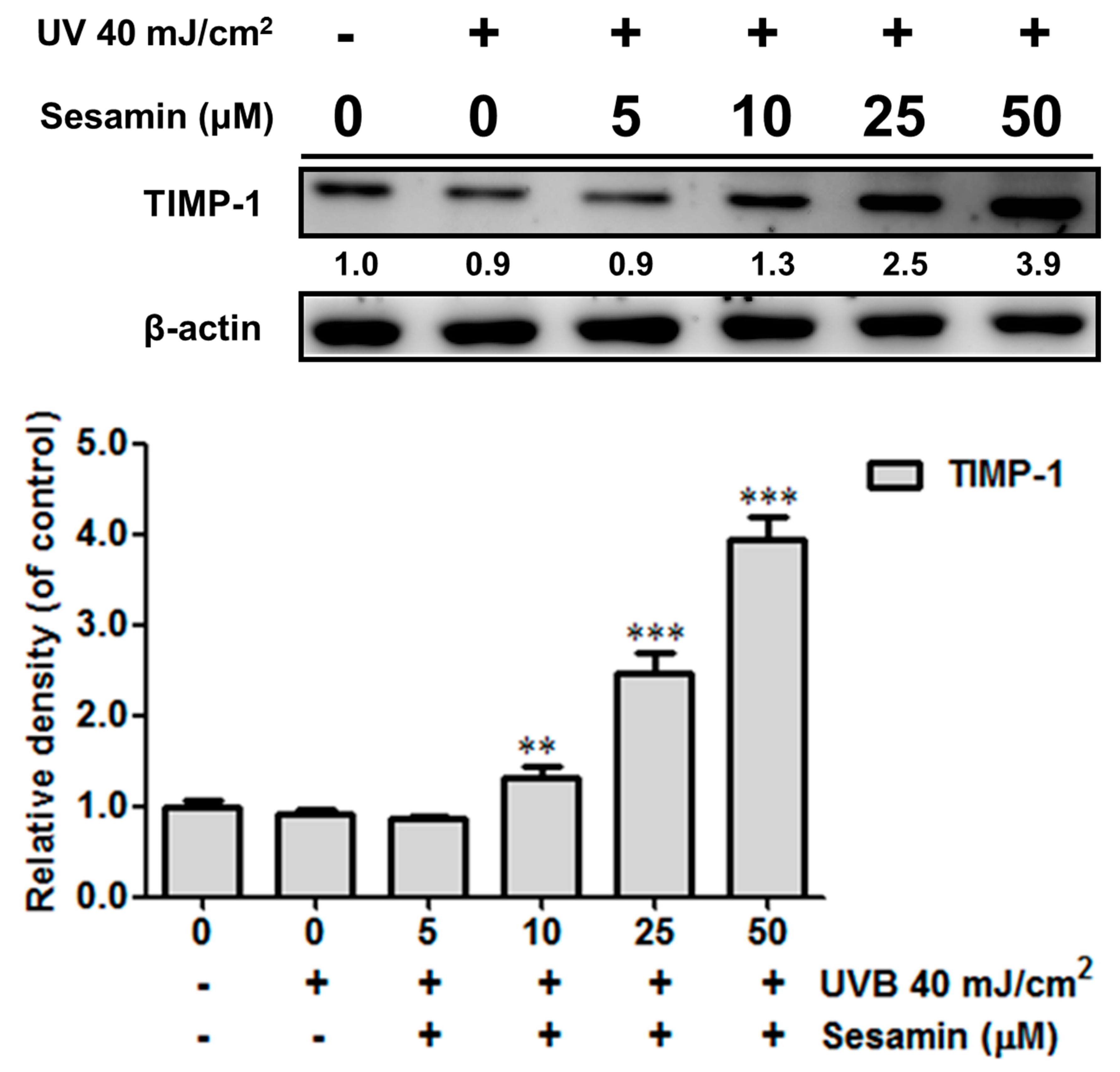
3.3.1. Sesamin Inhibited UVB-Induced Overexpression of MMPs and Increased TIMP Expression
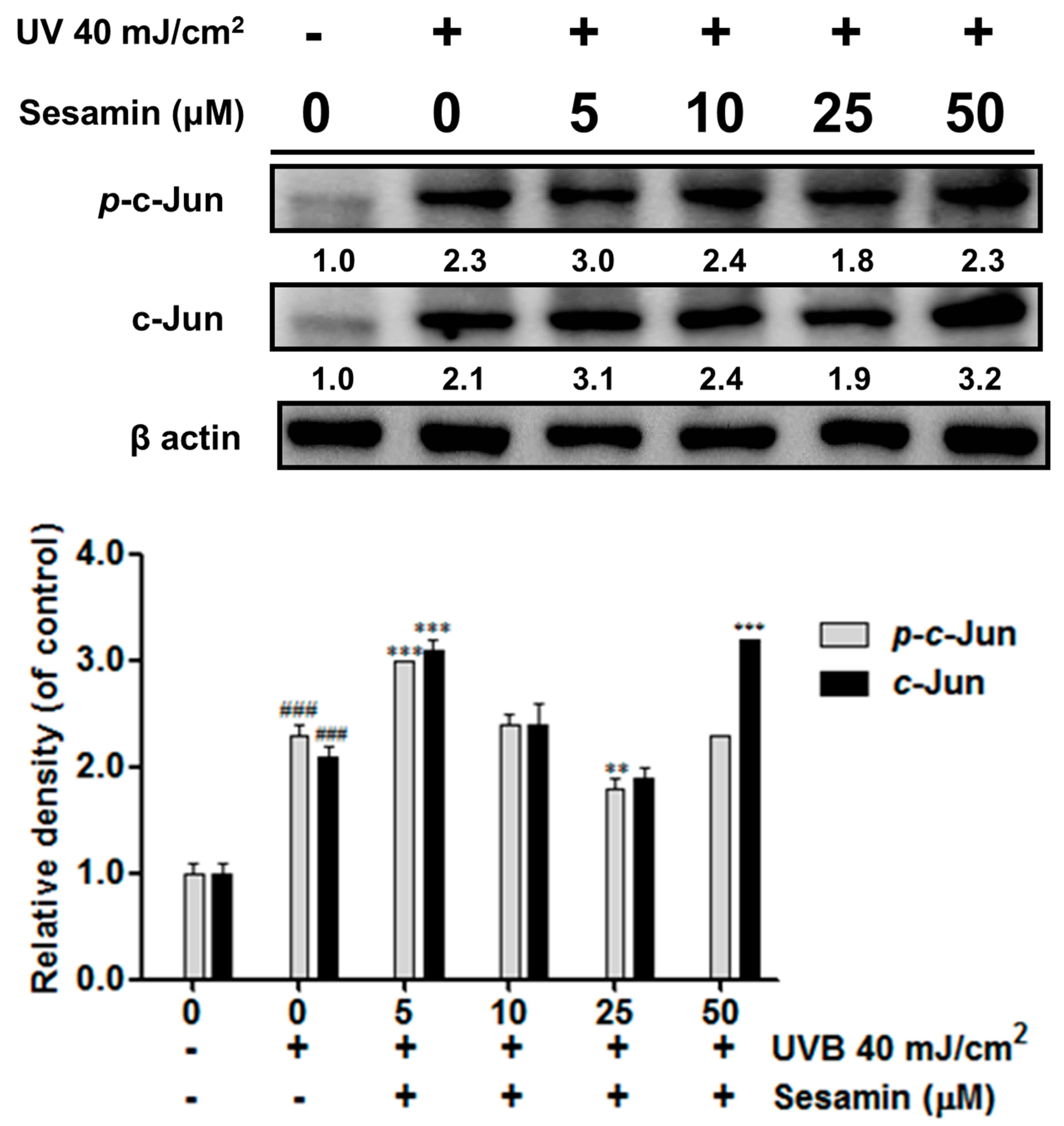
3.3.2. Sesamin Inhibited UVB-Induced Overexpression of c-Jun/p-c-Jun
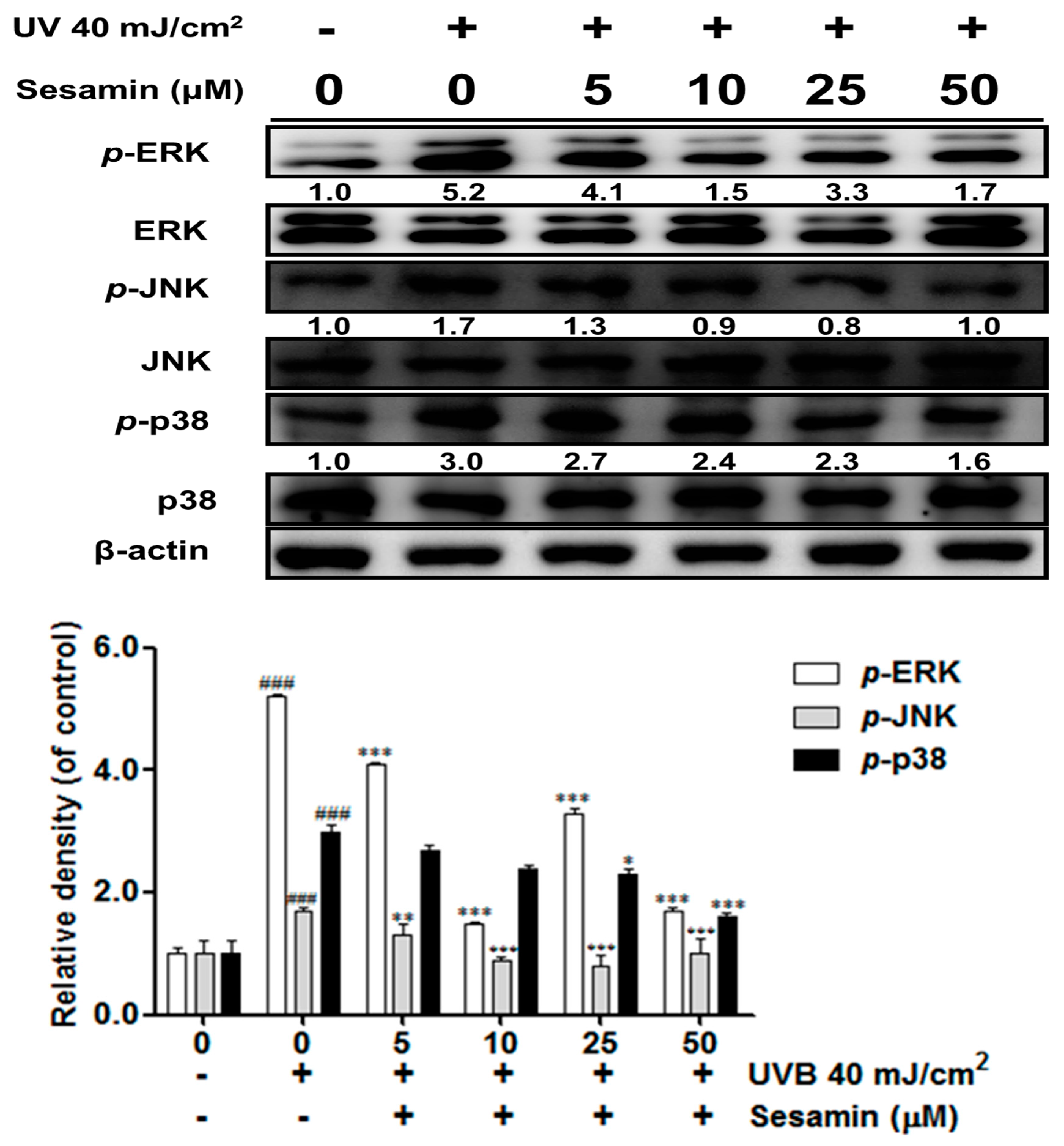
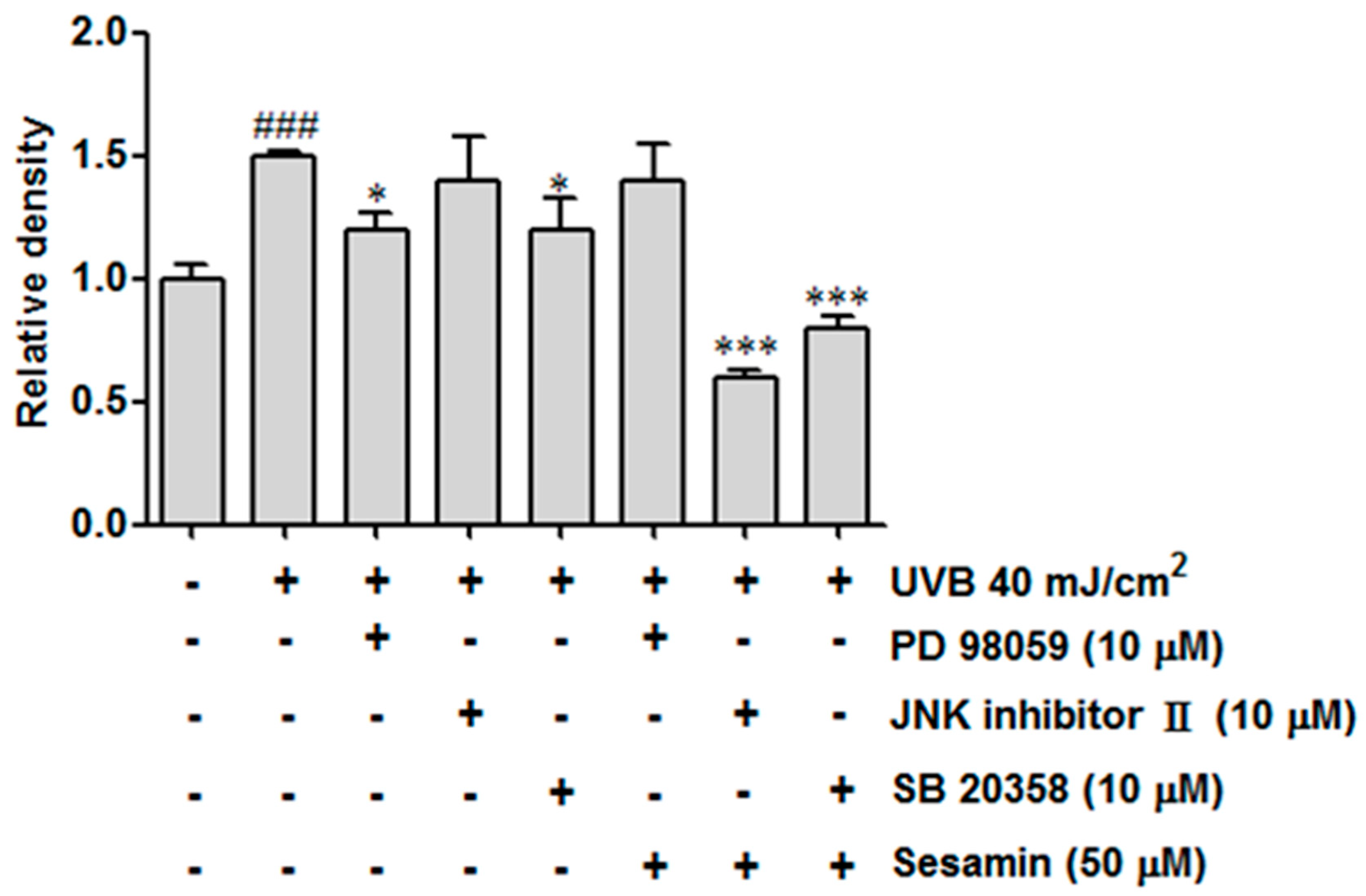
3.3.3. Sesamin Inhibited the Upregulation of MAP Kinases Induced by UVB Irradiation
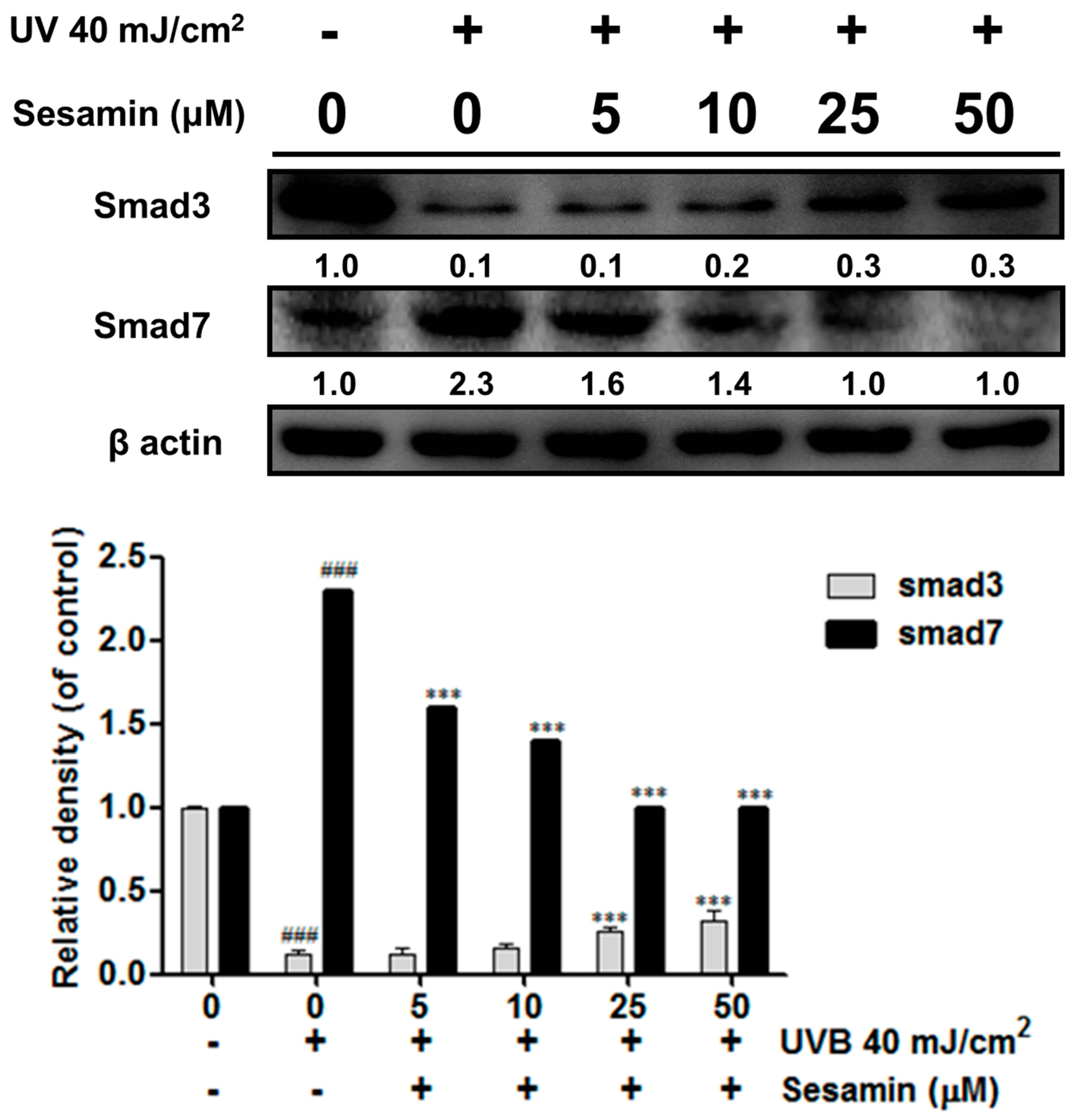
3.3.4. Sesamin Modulated the Expressions of Smad3 and Smad7
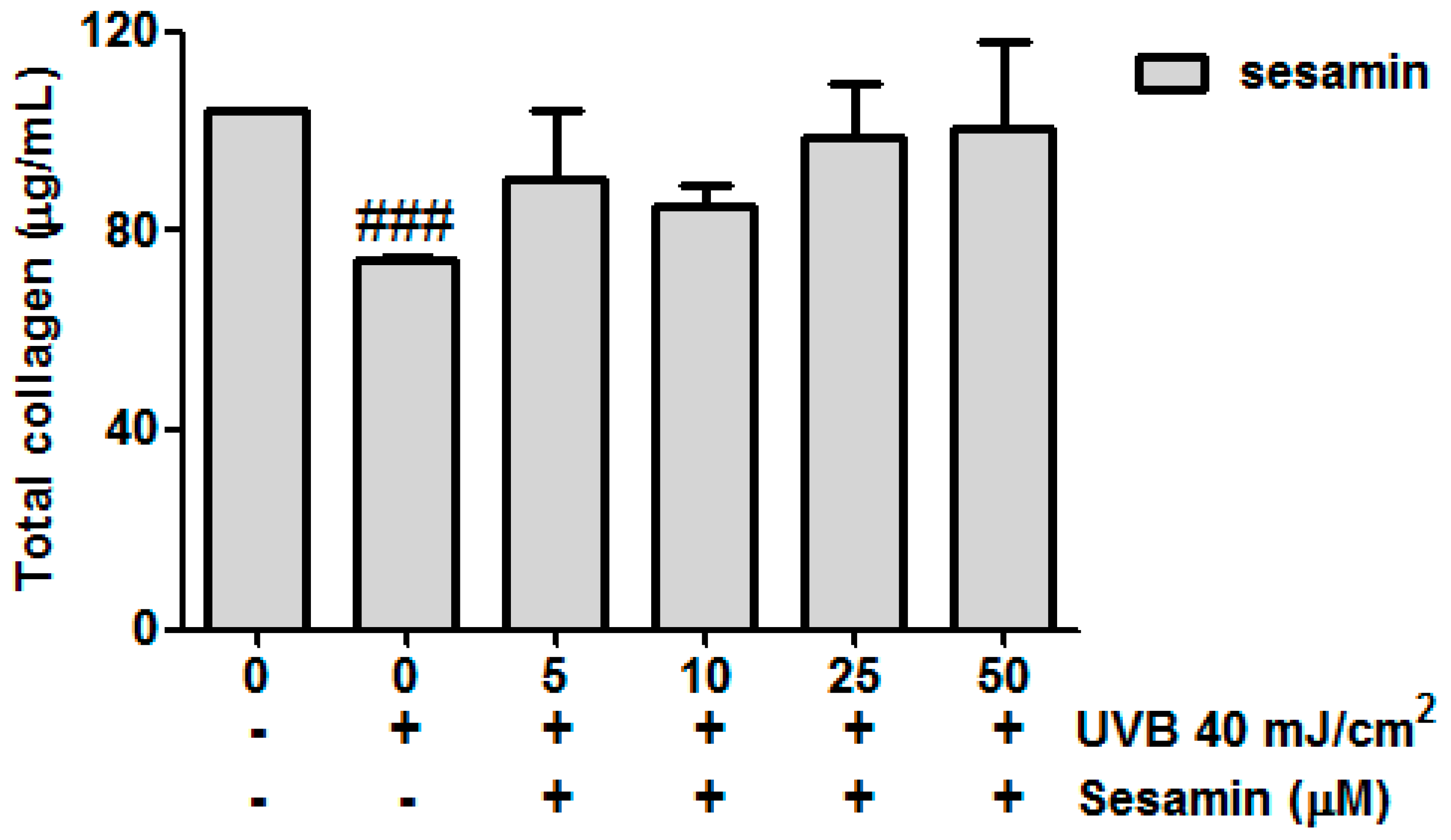
3.3.5. Sesamin Attenuated UVB-Inhibited Total Collagen Biosynthesis
3.4. Anti-Inflammatory Effect of Sesamin
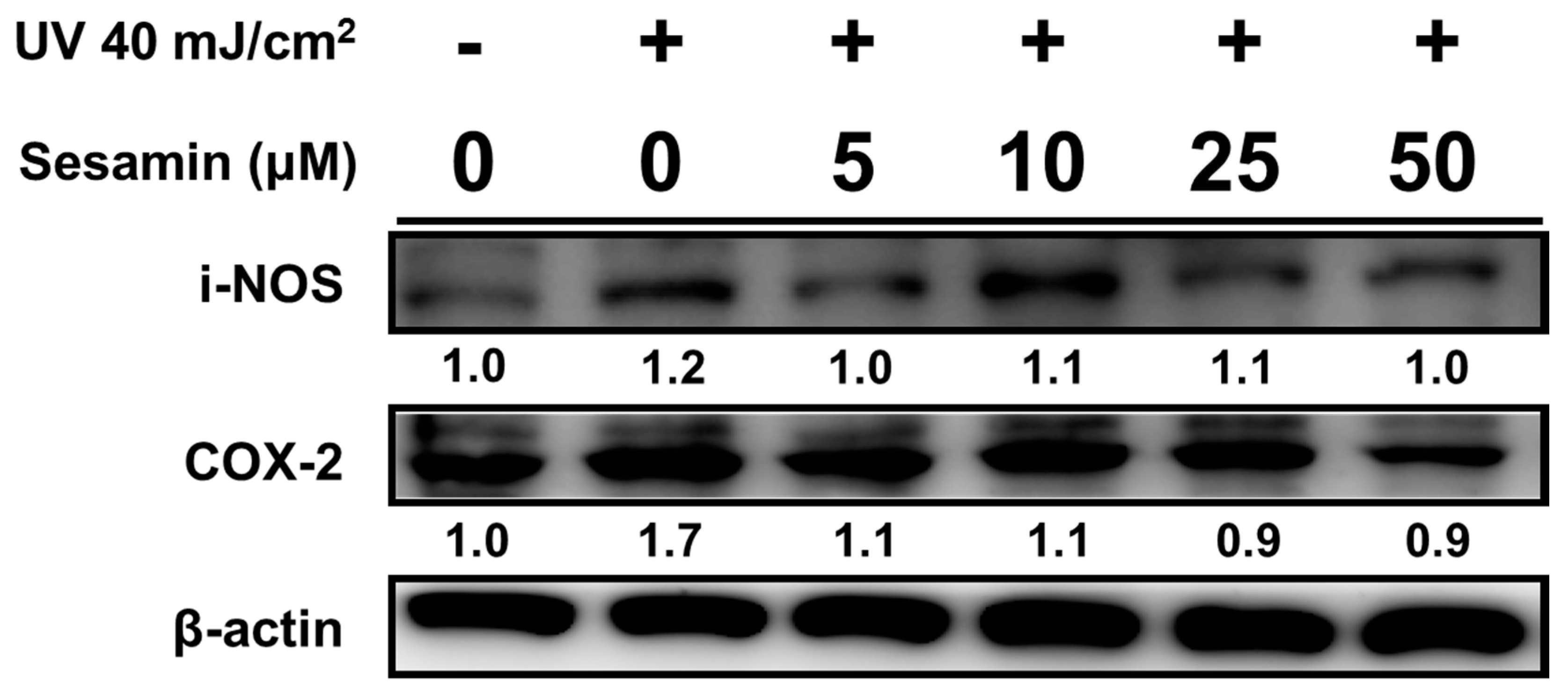
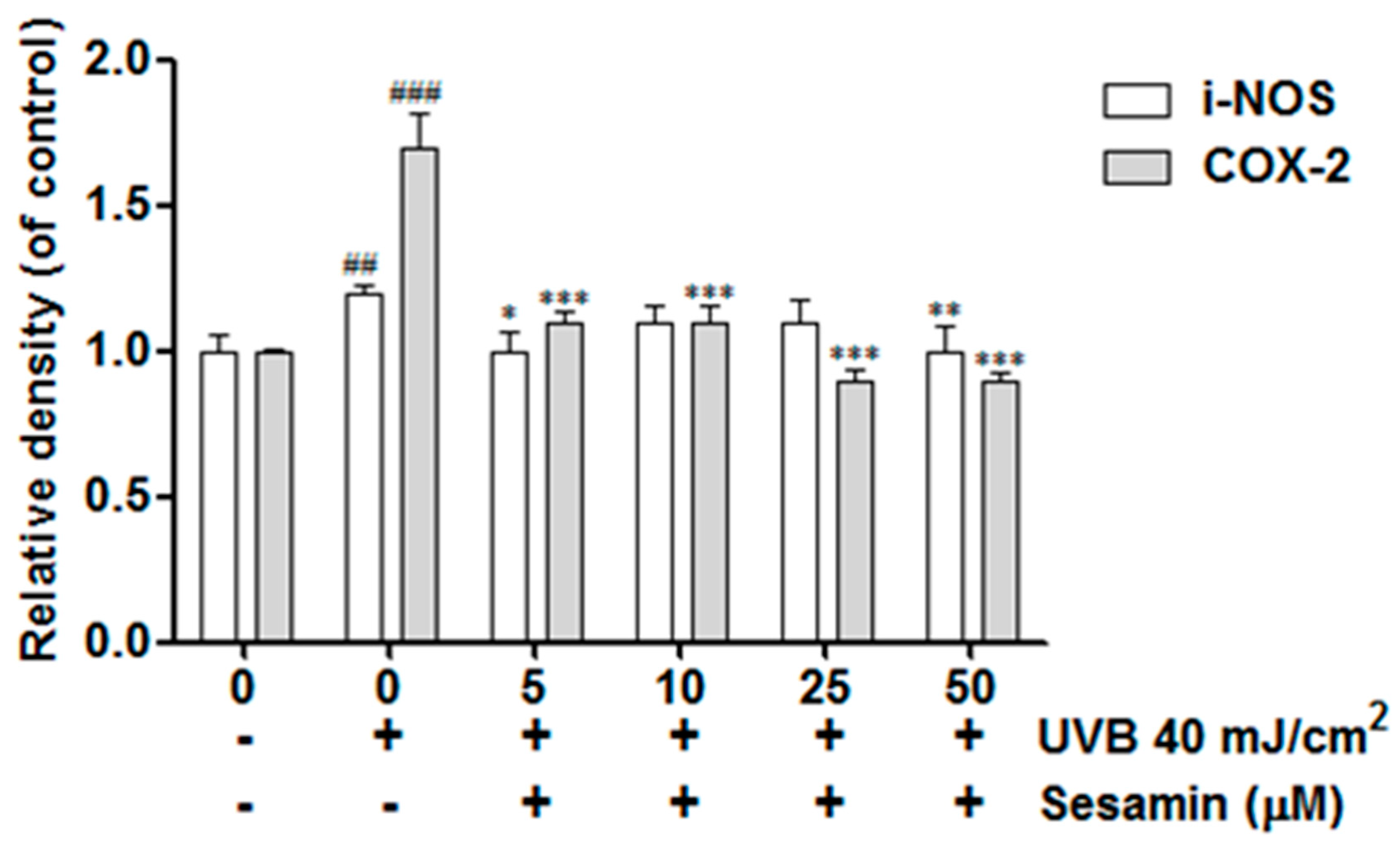
3.4.1. Sesamin Ameliorated UVB-Induced Overexpression of i-NOS and COX-2
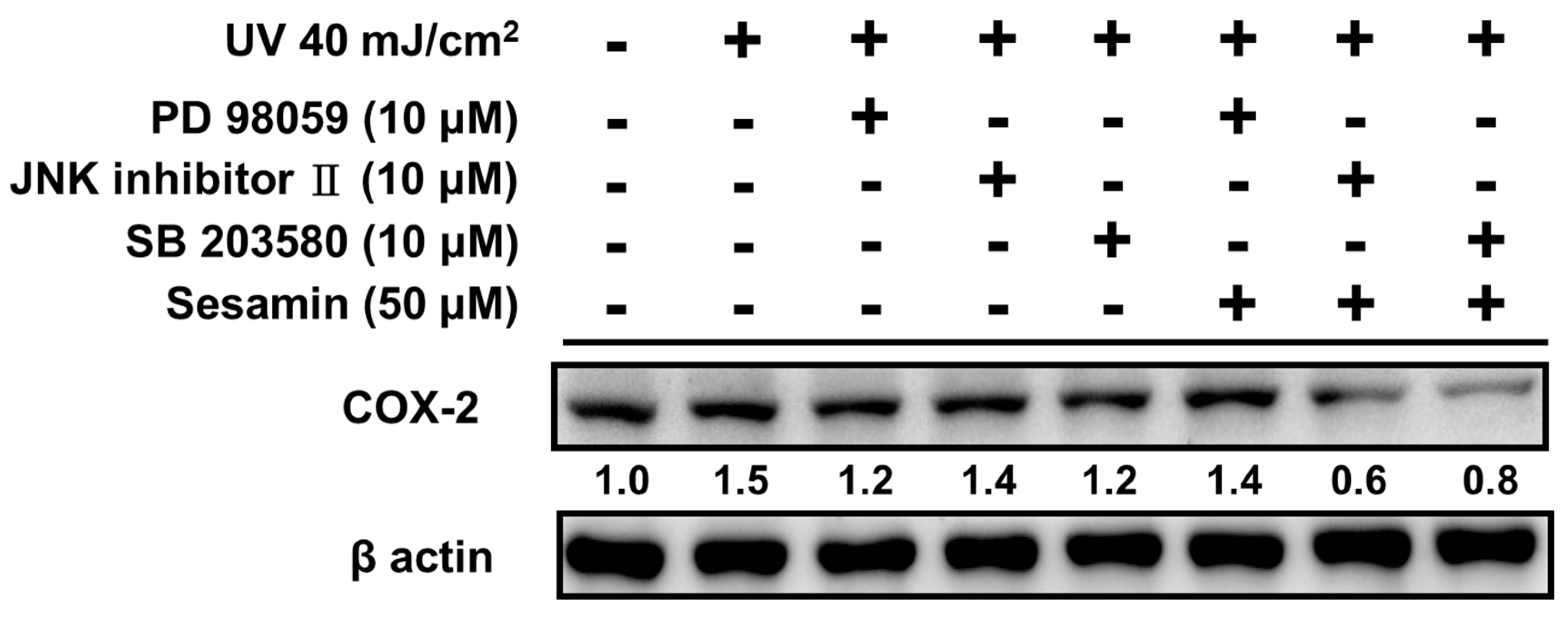
3.4.2. Sesamin Reduced COX-2 Expression by Inhibiting MAP Kinase Expression
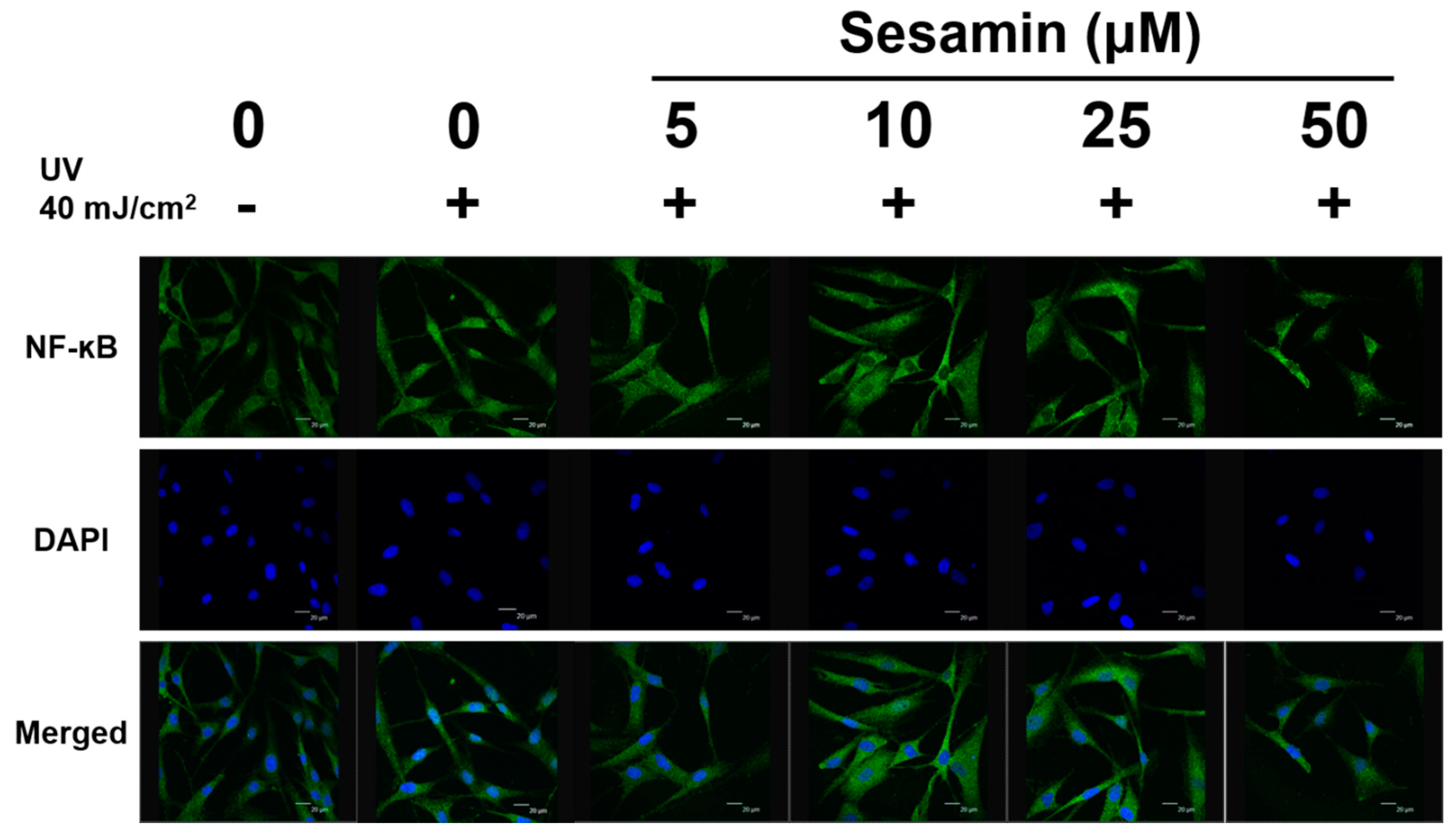
3.4.3. Sesamin Inhibited UVB-Induced NF-κB Activation
3.5. Sesamin Protected Mouse Skin from UVB-Irradiation-Induced Damage
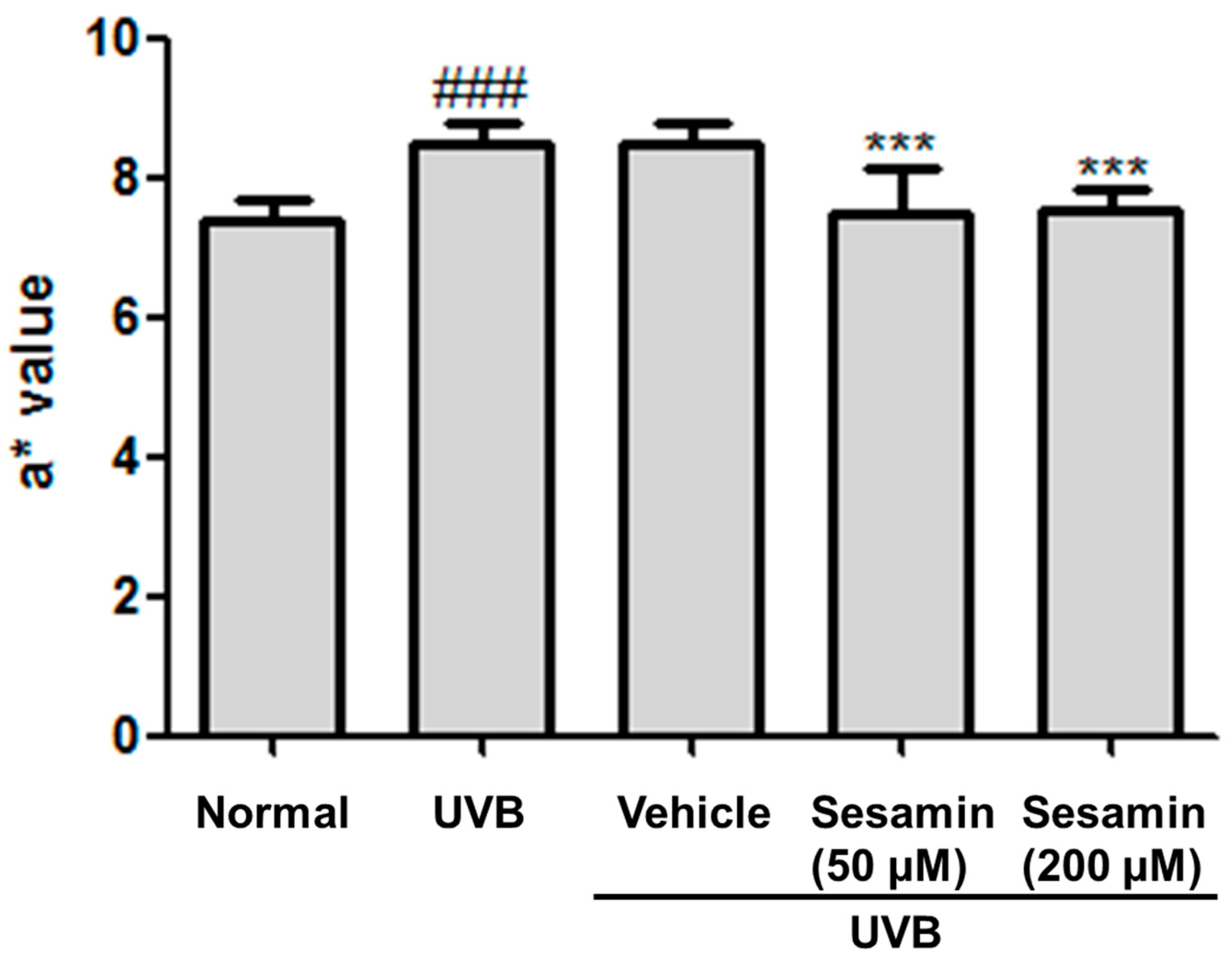
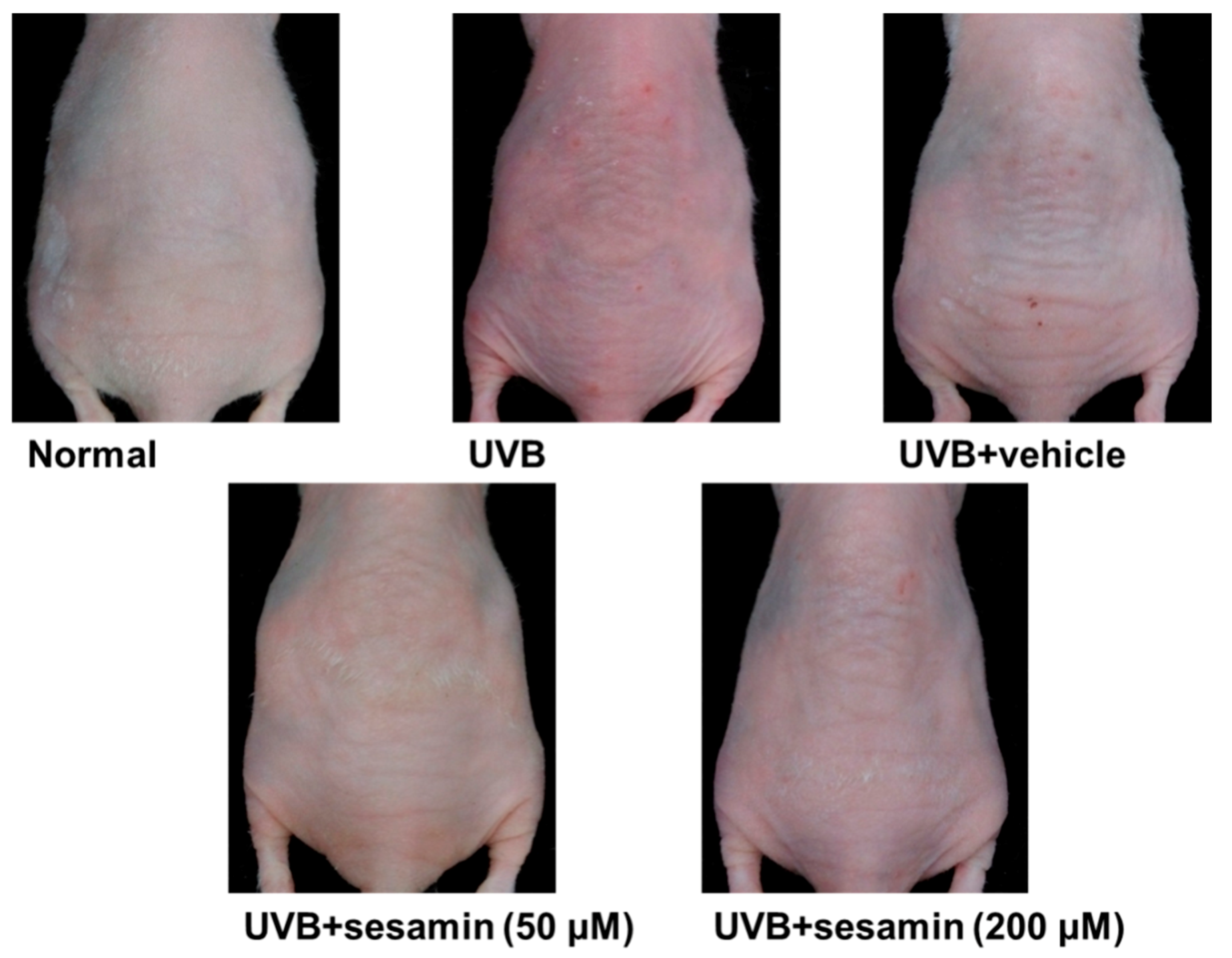
3.5.1. Sesamin Reduced UVB-Induced Skin Erythema and Damage
3.5.2. Sesamin Reduced UVB-Induced Wrinkle Formation
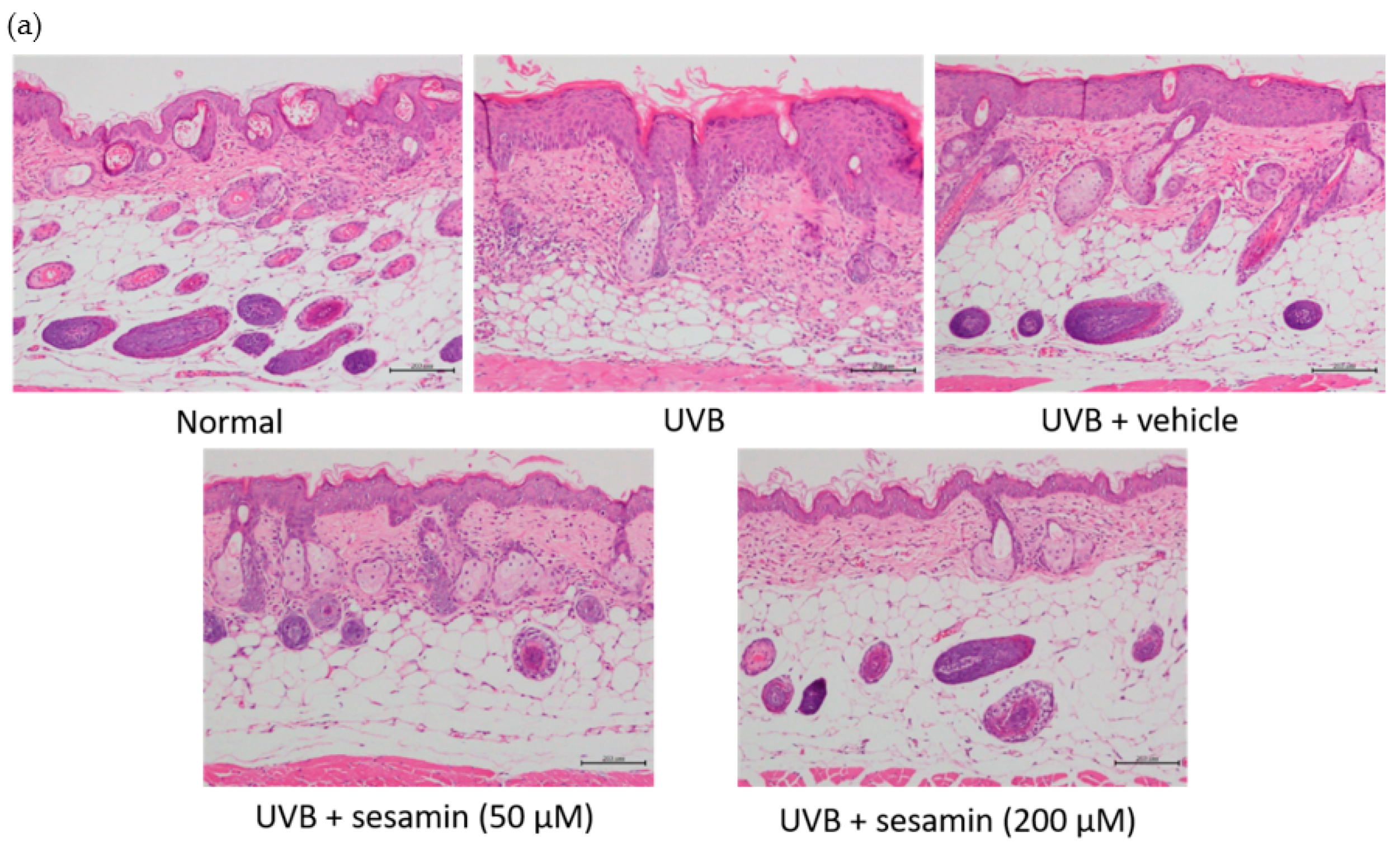
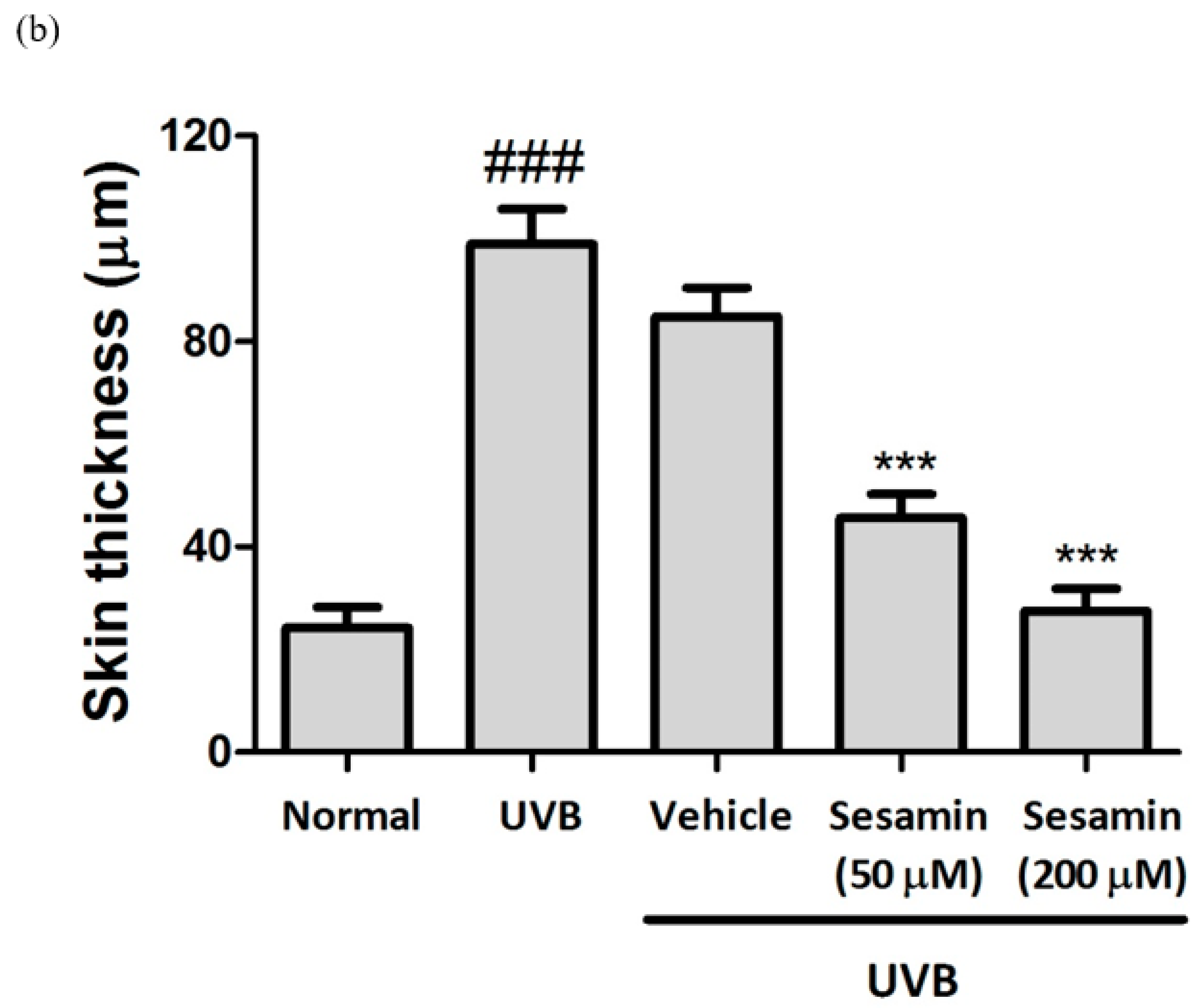
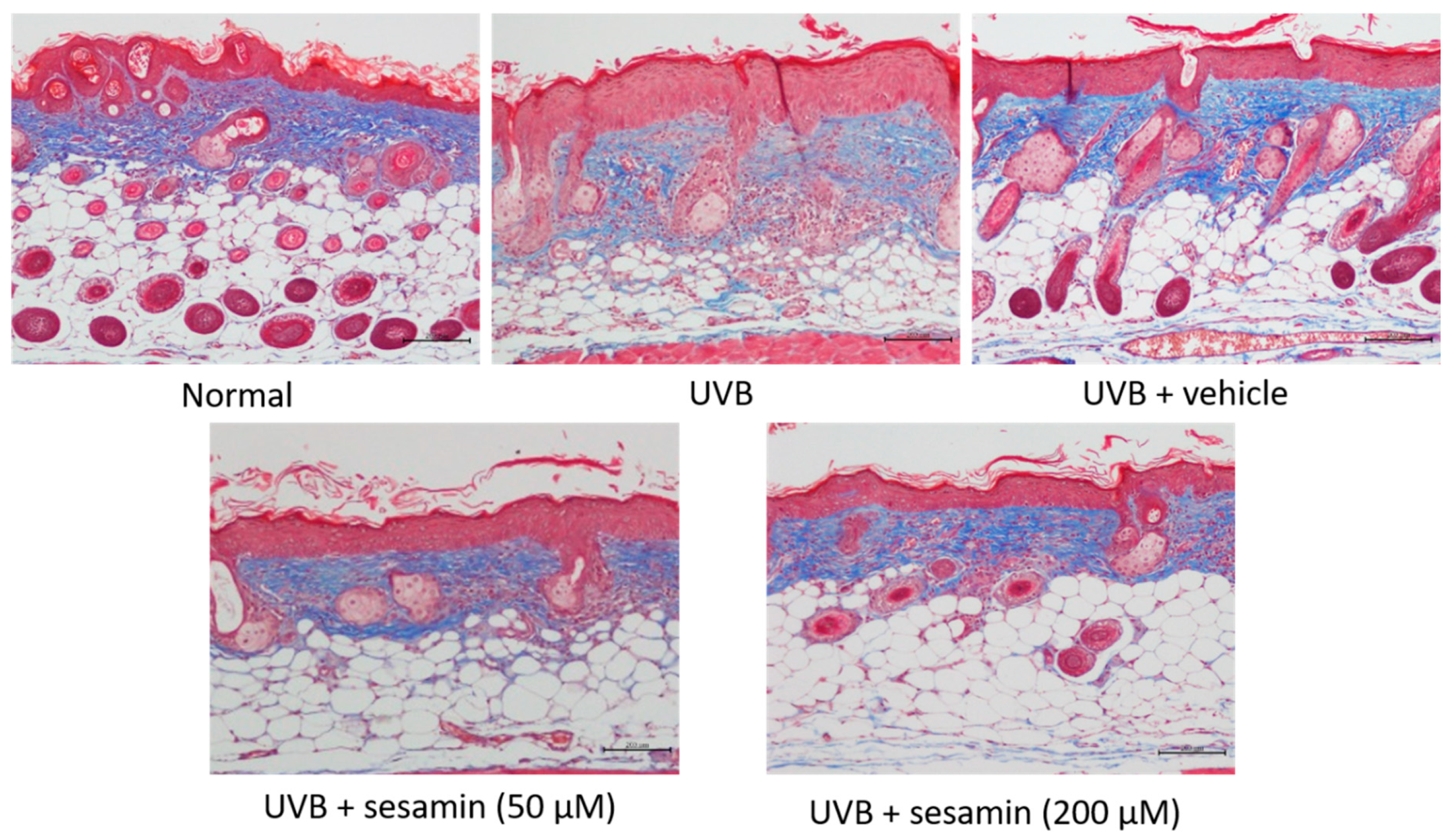
3.5.3. Sesamin Reduced UVB-Induced Epidermal Hyperplasia and Restored Collagen Content
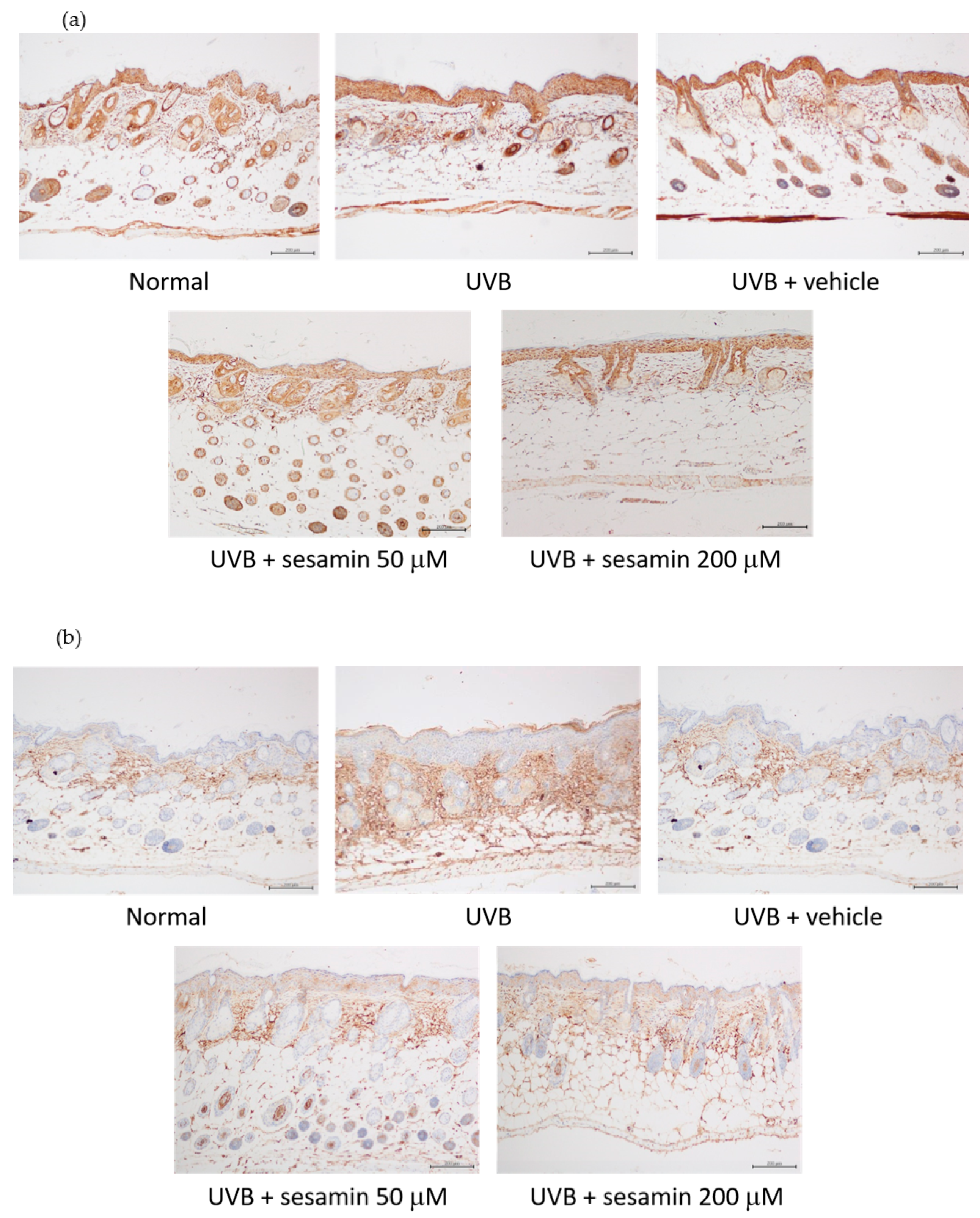
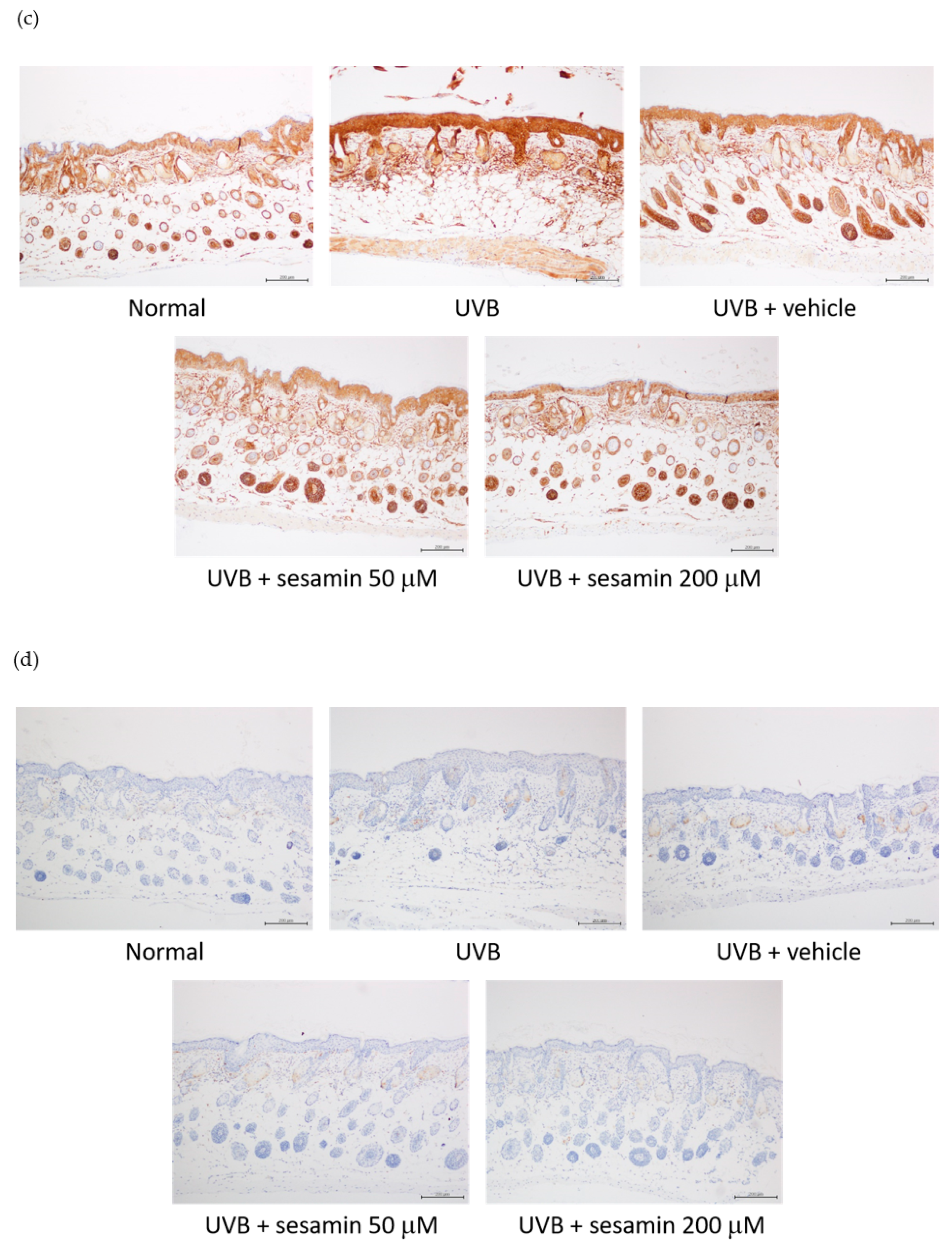
3.5.4. Sesamin Inhibited Photodamage-Related Protein Levels in UVB-Irradiated Mouse Skin
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, M.K.; Kim, E.J.; Cheng, Y.; Shin, M.H.; Oh, J.H.; Lee, D.H.; Chung, J.H. Inhibition of DNA methylation in the COL1A2 promoter by anacardic acid prevents UV-induced decrease of type I procollagen expression. J. Investig. Dermatol. 2017, 137, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J. The role of elastin and collagen in cutaneous aging: Intrinsic aging versus photoexposure. J. Drugs Dermatol. 2008, 7, s12–s16. [Google Scholar] [PubMed]
- Zhao, X.; Qi, Y.; Yi, R.; Park, K.Y. Anti-ageing skin effects of Korean bamboo salt on SKH1 hairless mice. Int. J. Biochem. Cell Biol. 2018, 103, 1–13. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Quan, T.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Oxidative exposure impairs TGF-β pathway via reduction of type II receptor and SMAD3 in human skin fibroblasts. Age 2014, 36, 9623. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.J.; Bowden, G.T. Ultraviolet B regulation of transcription factor families: Roles of nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr. Cancer Drug Targets 2007, 7, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Moon, K.M.; Chung, K.W.; Jeong, J.W.; Park, D.; Kim, D.H.; Yu, B.P.; Chung, H.Y. The underlying mechanism of proinflammatory NF-kappaB activation by the mTORC2/Akt/IKKα pathway during skin aging. Oncotarget 2016, 7, 52685–52694. [Google Scholar] [CrossRef] [PubMed]
- Kuanpradit, C.; Jaisin, Y.; Jungudomjaroen, S.; Akter Mitu, S.; Puttikamonkul, S.; Sobhon, P.; Cummins, S.F. Attenuation of UV-B exposure-induced inflammation by abalone hypobranchial gland and gill extracts. Int. J. Mol. Med. 2017, 39, 1083–1090. [Google Scholar] [CrossRef]
- Kanny, G.; De Hauteclocque, C.; Moneret-Vautrin, D.A. Sesame seed and sesame seed oil contain masked allergens of growing importance. Allergy 1996, 51, 952–957. [Google Scholar] [CrossRef]
- Zanardi, I.; Travagli, V.; Gabbrielli, A.; Chiasserini, L.; Bocci, V. Physico-chemical characterization of sesame oil derivatives. Lipids 2008, 43, 877–886. [Google Scholar] [CrossRef]
- Liu, C.M.; Zheng, G.H.; Ming, Q.L.; Chao, C.; Sun, J.M. Sesamin protects mouse liver against nickel-induced oxidative DNA damage and apoptosis by the PI3K-Akt pathway. J. Agric. Food Chem. 2013, 61, 1146–1154. [Google Scholar] [CrossRef]
- Narasimhulu, C.A.; Selvarajan, K.; Litvinov, D.; Parthasarathy, S. Anti-atherosclerotic and anti-inflammatory actions of sesame oil. J. Med. Food 2015, 18, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Selvarajan, K.; Narasimhulu, C.A.; Bapputty, R.; Parthasarathy, S. Anti-inflammatory and antioxidant activities of the nonlipid (aqueous) components of sesame oil: Potential use in atherosclerosis. J. Med. Food 2015, 18, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.Y.; You, Y.J.; Liu, Y.J.; Hou, C.W.; Wu, C.S.; Wen, K.C.; Lin, C.Y.; Chiang, H.M. Sesamol inhibited melanogenesis by regulating melanin-related signal transduction in B16F10 cells. Int. J. Mol. Sci. 2018, 19, 1108. [Google Scholar] [CrossRef] [PubMed]
- You, Y.J.; Wu, P.Y.; Liu, Y.J.; Hou, C.W.; Wu, C.S.; Wen, K.C.; Lin, C.Y.; Chiang, H.M. Sesamol inhibited ultraviolet radiation-induced hyperpigmentation and damage in C57BL/6 mouse skin. Antioxidants 2019, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Jeng, K.C.; Hou, R.C.; Wang, J.C.; Ping, L.I. Sesamin inhibits lipopolysaccharide-induced cytokine production by suppression of p38 mitogen-activated protein kinase and nuclear factor-κB. Immunol. Lett. 2005, 97, 101–106. [Google Scholar] [CrossRef]
- Hou, R.C.; Chen, H.L.; Tzen, J.T.; Jeng, K.C. Effect of sesame antioxidants on LPS-induced NO production by BV2 microglial cells. Neuroreport 2003, 14, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, E.M.; Chibli, L.A.; Yamamoto, C.H.; Pereira, M.C.; Vilela, F.M.; Rodarte, M.P.; Pinto, M.A.; do Amaral Mda, P.; Silverio, M.S.; Araujo, A.L.; et al. Antinociceptive and anti-inflammatory activities of the sesame oil and sesamin. Nutrients 2014, 6, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.M.; Chang, H.; Yao, P.W.; Chen, Y.S.; Jeng, K.C.; Wang, J.S.; Hou, C.W. Sesamin reduces acute hepatic injury induced by lead coupled with lipopolysaccharide. J. Chin. Med Assoc. 2014, 77, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y.; Obayashi, M.; Ishikawa, T.; Kiso, Y.; Ono, Y.; Yamashita, K. Dietary tocotrienol reduces UVB-induced skin damage and sesamin enhances tocotrienol effects in hairless mice. J. Nutr. Sci. Vitaminol. 2008, 54, 117–123. [Google Scholar] [CrossRef]
- Wu, P.Y.; Huang, C.C.; Chu, Y.; Huang, Y.H.; Lin, P.; Liu, Y.H.; Wen, K.C.; Lin, C.Y.; Hsu, M.C.; Chiang, H.M. Alleviation of ultraviolet B-induced photodamage by Coffea arabica extract in human skin fibroblasts and hairless mouse skin. Int. J. Mol. Sci. 2017, 18, 782. [Google Scholar] [CrossRef]
- Chiang, H.M.; Chen, C.W.; Lin, T.Y.; Kuo, Y.H. N-phenethyl caffeamide and photodamage: Protecting skin by inhibiting type I procollagen degradation and stimulating collagen synthesis. Food Chem. Toxicol. 2014, 72C, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Wu, P.Y.; Chen, C.W.; Lyu, J.L.; Liu, Y.J.; Wen, K.C.; Lin, C.Y.; Kuo, Y.H.; Chiang, H.M. Protective effects and mechanisms of N-phenethyl caffeamide from uva-induced skin damage in human epidermal keratinocytes through Nrf2/HO-1 regulation. Int. J. Mol. Sci. 2019, 20, 164. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.Y.; Lyu, J.L.; Liu, Y.J.; Chien, T.Y.; Hsu, H.C.; Wen, K.C.; Chiang, H.M. Fisetin Regulates Nrf2 Expression and the Inflammation-Related Signaling Pathway to Prevent UVB-Induced Skin Damage in Hairless Mice. Int. J. Mol. Sci. 2017, 18, 2118. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Lin, T.Y.; You, Y.J.; Wen, K.C.; Sung, P.J.; Chiang, H.M. Antiinflammatory and Antiphotodamaging Effects of Ergostatrien-3β-ol, Isolated from Antrodia camphorata, on Hairless Mouse Skin. Molecules 2016, 21, 1213. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Yuan, W.; Mori, Y.; Varga, J. Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-β involves functional cooperation with p300/CBP transcriptional coactivators. Oncogene 2000, 19, 3546–3555. [Google Scholar] [CrossRef]
- Kim, Y.G.; Sumiyoshi, M.; Sakanaka, M.; Kimura, Y. Effects of ginseng saponins isolated from red ginseng on ultraviolet B-induced skin aging in hairless mice. Eur. J. Pharmacol. 2009, 602, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Godic, A.; Poljsak, B.; Adamic, M.; Dahmane, R. The role of antioxidants in skin cancer prevention and treatment. Oxidative Med. Cell. Longev. 2014, 2014, 860479. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.B.; Yun, J.G.; Hwang, J.K. Protective effects of standardized Siegesbeckia glabrescens extract and its active compound kirenol against UVB-induced photoaging through inhibition of MAPK/NF-κB pathways. J. Microbiol. Biotechnol. 2017, 27, 242–250. [Google Scholar] [CrossRef]
- Puglia, C.; Offerta, A.; Saija, A.; Trombetta, D.; Venera, C. Protective effect of red orange extract supplementation against UV-induced skin damages: Photoaging and solar lentigines. J. Cosmet. Dermatol. 2014, 13, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.; Garcia, C.C. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Xu, J.; Chen, S.; Yang, F. Antioxidant activity of extracts of black sesame seed (Sesamum indicum L.) by supercritical carbon dioxide extraction. J. Agric. Food Chem. 2004, 52, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.F.; Hou, C.W.; Yao, P.W.; Wu, S.P.; Peng, Y.F.; Shen, M.L.; Lin, C.H.; Chao, Y.Y.; Chang, M.H.; Jeng, K.C. Sesamin ameliorates oxidative stress and mortality in kainic acid-induced status epilepticus by inhibition of MAPK and COX-2 activation. J. Neuroinflammation 2011, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Philips, N.; Suarez-Perez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; Gonzalez, S. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef]
- Rittie, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Willenbrock, F.; Murphy, G. Structure-function relationships in the tissue inhibitors of metalloproteinases. Am. J. Respir. Crit. Care Med. 1994, 150, S165–S170. [Google Scholar] [CrossRef]
- Gilchrest, B.A. Photoaging. J. Investig. Dermatol. 2013, 133, E2–E6. [Google Scholar] [CrossRef] [Green Version]
- Massague, J.; Wotton, D. Transcriptional control by the TGF-β/Smad signaling system. EMBO J. 2000, 19, 1745–1754. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Z.; Chen, Y. Regulation of TGF-β signaling by Smad7. Acta Biochim. Biophys. Sin. 2009, 41, 263–272. [Google Scholar] [CrossRef]
- van der Heide, L.P.; van Dinther, M.; Moustakas, A.; ten Dijke, P. TGFβ activates mitogen- and stress-activated protein kinase-1 (MSK1) to attenuate cell death. J. Biol. Chem. 2011, 286, 5003–5011. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Shao, Y.; He, T.; Voorhees, J.J.; Fisher, G.J. Reduced expression of connective tissue growth factor (CTGF/CCN2) mediates collagen loss in chronologically aged human skin. J. Investig. Dermatol. 2010, 130, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Monda, A.; Takemoto, R.; Matsuoka, Y.; Kitamura, C.; Ohashi, K.; Shibuya, H.; Inoue, A. Sesamin suppresses activation of microglia and p44/42 MAPK pathway, which confers neuroprotection in rat intracerebral hemorrhage. Neuroscience 2013, 232, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, S.E.; Olson, E.R.; Zhang, J.; Cooper, S.J.; Melton, T.; Criswell, P.J.; Casanova, A.; Dong, Z.; Hu, C.; Saboda, K.; et al. p38 MAP kinase plays a functional role in UVB-induced mouse skin carcinogenesis. Mol. Carcinog. 2011, 50, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.L.; Labasi, J.M.; Zhu, Y.; Tang, X.; McClure, K.; Gabel, C.A.; Athar, M.; Bickers, D.R. Role of p38 MAPK in UVB-induced inflammatory responses in the skin of SKH-1 hairless mice. J. Investig. Dermatol. 2005, 124, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yu, D.; Cho, Y.Y.; Bode, A.M.; Ma, W.; Yao, K.; Li, S.; Li, J.; Bowden, G.T.; Dong, Z.; et al. Sunlight UV-induced skin cancer relies upon activation of the p38α signaling pathway. Cancer Res. 2013, 73, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, anti-inflammatory, and anti-aging properties of mycosporine-like amino acids: Molecular and Cellular Mechanisms in the Protection of Skin-aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.R. The stratum corneum: Structure and function in health and disease. Dermatol. Ther. 2004, 17, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.V.; Harding, C.R. Moisturization and skin barrier function. Dermatol. Ther. 2004, 17, 43–48. [Google Scholar] [CrossRef]
- Watson, R.E.; Gibbs, N.K.; Griffiths, C.E.; Sherratt, M.J. Damage to skin extracellular matrix induced by UV exposure. Antioxid. Redox Signal. 2014, 21, 1063–1077. [Google Scholar] [CrossRef]
| Group | Wrinkle Score (10th Week) |
|---|---|
| Normal mice | 0.7 ± 1.2 a |
| UVB-irradiated mice | 4.5 ± 1.9 b |
| UVB-irradiated mice + vehicle | 5.3 ± 1.2 b |
| UVB-irradiated mice + sesamin (50 μM) | 1.3 ± 1.6 a,c |
| UVB-irradiated mice + sesamin (200 μM) | 1.3 ± 1.0 a,c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-Y.; Wu, P.-Y.; Hou, C.-W.; Chien, T.-Y.; Chang, Q.-X.; Wen, K.-C.; Lin, C.-Y.; Chiang, H.-M. Protective Effects of Sesamin against UVB-Induced Skin Inflammation and Photodamage In Vitro and In Vivo. Biomolecules 2019, 9, 479. https://doi.org/10.3390/biom9090479
Lin T-Y, Wu P-Y, Hou C-W, Chien T-Y, Chang Q-X, Wen K-C, Lin C-Y, Chiang H-M. Protective Effects of Sesamin against UVB-Induced Skin Inflammation and Photodamage In Vitro and In Vivo. Biomolecules. 2019; 9(9):479. https://doi.org/10.3390/biom9090479
Chicago/Turabian StyleLin, Tzu-Yu, Po-Yuan Wu, Chien-Wei Hou, Ting-Yi Chien, Qiao-Xin Chang, Kuo-Ching Wen, Chien-Yih Lin, and Hsiu-Mei Chiang. 2019. "Protective Effects of Sesamin against UVB-Induced Skin Inflammation and Photodamage In Vitro and In Vivo" Biomolecules 9, no. 9: 479. https://doi.org/10.3390/biom9090479