Sulfated Glycoaminoglycans and Proteoglycan Syndecan-4 Are Involved in Membrane Fixation of LL-37 and Its Pro-Migratory Effect in Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Peptides, Inhibitors and Antibodies Used in this Study
2.3. Cell Migration Assay
2.4. Measurements of Intracellular Cα2+ Variation
2.5. Immunofluorescence Labeling
2.6. RNA Interference and Expression Analysis by Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Statistic Analysis
3. Results
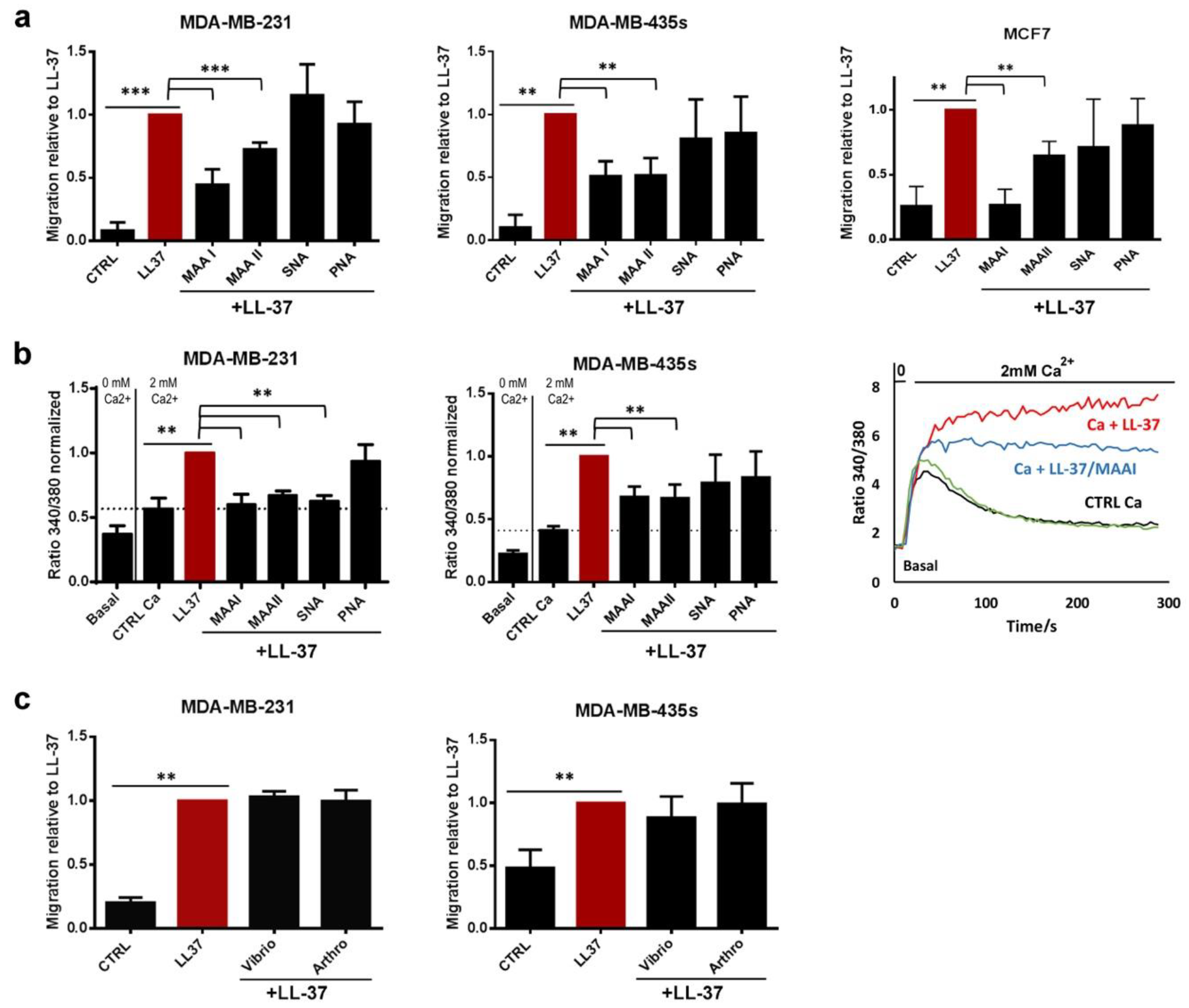
3.1. The Activities of LL-37 Are Blocked by Lectins but Do Not Require α2–3- or α2–6-Linked Sialic Acids
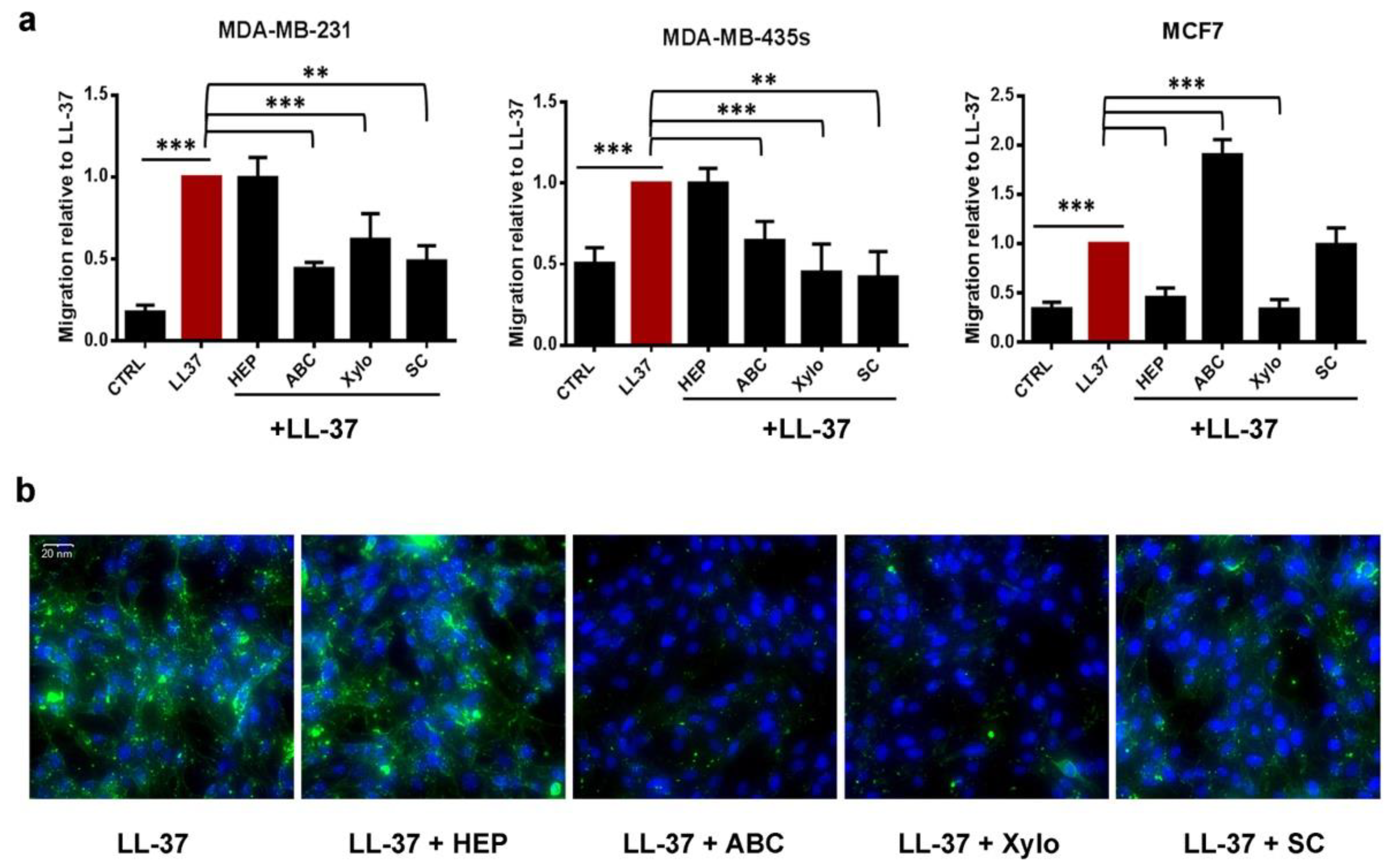
3.2. Membrane Chondroitin Sulfate and/or Heparin Are Involved in Membrane Binding of LL-37 and Are Needed for Its Activities
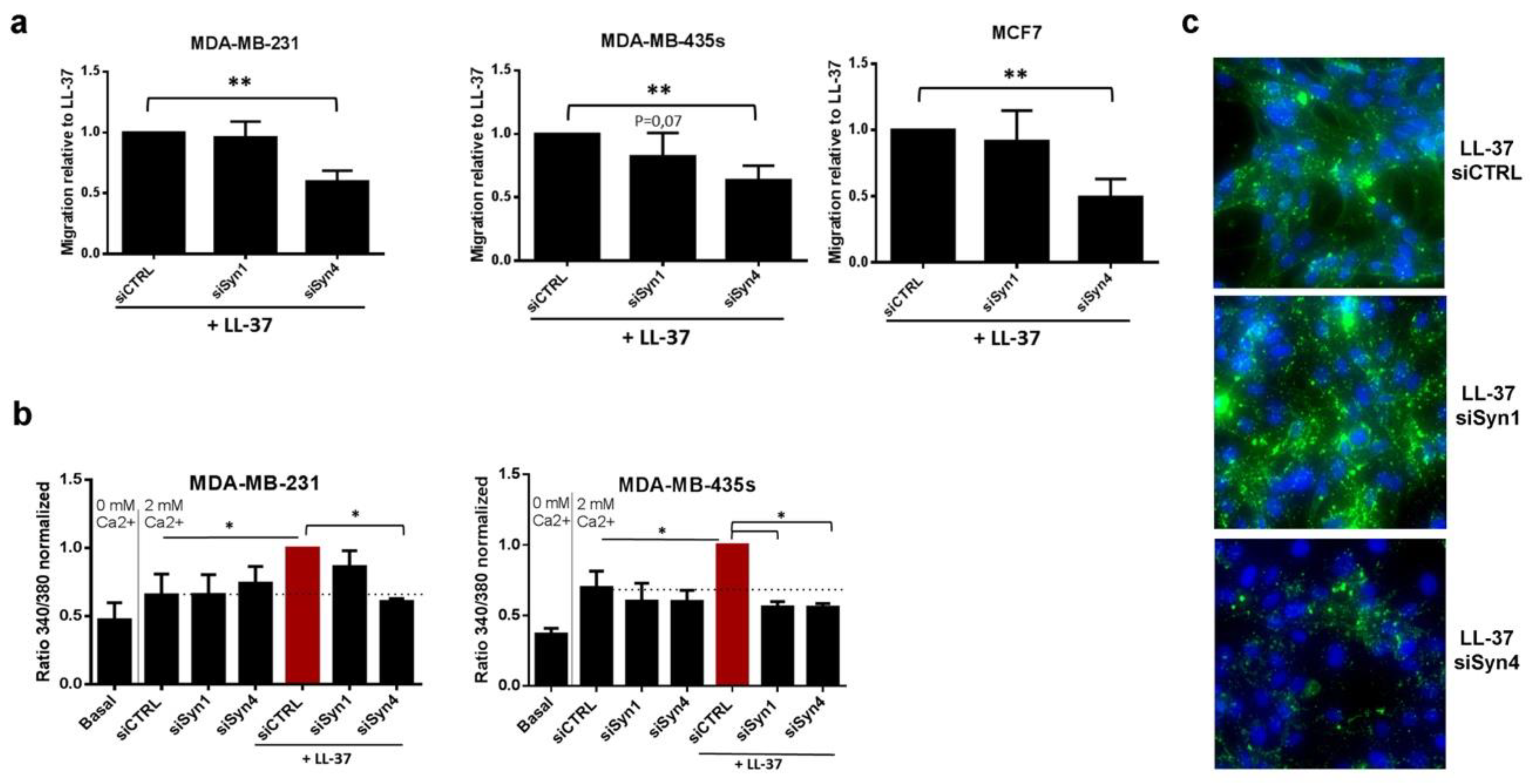
3.3. Identification of Syndecan-4 as Proteoglycan Implicated in Membrane Fixation and LL-37-Induced Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xhindoli, D.; Pacor, S.; Benincasa, M.; Scocchi, M.; Gennaro, R.; Tossi, A. The human cathelicidin LL-37—A pore-forming antibacterial peptide and host-cell modulator. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 546–566. [Google Scholar] [CrossRef] [PubMed]
- Fabisiak, A.; Murawska, N.; Fichna, J. LL-37: Cathelicidin-related antimicrobial peptide with pleiotropic activity. Pharmacol. Rep. 2016, 68, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Piktel, E.; Niemirowicz, K.; Wnorowska, U.; Watek, M.; Wollny, T.; Gluszek, K.; Gozdz, S.; Levental, I.; Bucki, R. The Role of Cathelicidin LL-37 in Cancer Development. Arch. Immunol. Ther. Exp. 2016, 64, 33–46. [Google Scholar] [CrossRef]
- Chen, X.; Zou, X.; Qi, G.; Tang, Y.; Guo, Y.; Si, J.; Liang, L. Roles and Mechanisms of Human Cathelicidin LL-37 in Cancer. Cell. Physiol. Biochem. 2018, 47, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Verjans, E.-T.; Zels, S.; Luyten, W.; Landuyt, B.; Schoofs, L. Molecular mechanisms of LL-37-induced receptor activation: An overview. Peptides 2016, 85, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Von Haussen, J.; Koczulla, R.; Shaykhiev, R.; Herr, C.; Pinkenburg, O.; Reimer, D.; Wiewrodt, R.; Biesterfeld, S.; Aigner, A.; Czubayko, F.; et al. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer 2008, 59, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Tomasinsig, L.; Pizzirani, C.; Skerlavaj, B.; Pellegatti, P.; Gulinelli, S.; Tossi, A.; Di Virgilio, F.; Zanetti, M. The Human Cathelicidin LL-37 Modulates the Activities of the P2 × 7 Receptor in a Structure-dependent Manner. J. Boil. Chem. 2008, 283, 30471–30481. [Google Scholar] [CrossRef] [PubMed]
- Gambade, A.; Zreika, S.; Gueguinou, M.; Chourpa, I.; Fromont, G.; Bouchet, A.M.; Burlaud-Gaillard, J.; Potier-Cartereau, M.; Roger, S.; Aucagne, V.; et al. Activation of TRPV2 and BKCa channels by the LL-37 enantiomers stimulates calcium entry and migration of cancer cells. Oncotarget 2016, 7, 23785–23800. [Google Scholar] [CrossRef] [Green Version]
- Di Nardo, A.; Braff, M.H.; Taylor, K.R.; Na, C.; Granstein, R.D.; McInturff, J.E.; Krutzik, S.; Modlin, R.L.; Gallo, R.L. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J. Immunol. 2007, 178, 1829–1834. [Google Scholar] [CrossRef]
- Sancho-Vaello, E.; François, P.; Bonetti, E.-J.; Lilie, H.; Finger, S.; Gil-Ortiz, F.; Gil-Carton, D.; Zeth, K. Structural remodeling and oligomerization of human cathelicidin on membranes suggest fibril-like structures as active species. Sci. Rep. 2017, 7, 15371. [Google Scholar] [CrossRef]
- Wildman, K.A.H.; Lee, D.-K.; Ramamoorthy, A. Mechanism of Lipid Bilayer Disruption by the Human Antimicrobial Peptide, LL-37. Biochemistry 2003, 42, 6545–6558. [Google Scholar] [CrossRef]
- Henzler-Wildman, K.A.; Martinez, G.V.; Brown, M.F.; Ramamoorthy, A. Perturbation of the Hydrophobic Core of Lipid Bilayers by the Human Antimicrobial Peptide LL-37. Biochemistry 2004, 43, 8459–8469. [Google Scholar] [CrossRef]
- Sood, R.; Domanov, Y.; Pietiäinen, M.; Kontinen, V.P.; Kinnunen, P.K. Binding of LL-37 to model biomembranes: Insight into target vs. host cell recognition. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 983–996. [Google Scholar] [CrossRef]
- Sood, R.; Kinnunen, P.K. Cholesterol, lanosterol, and ergosterol attenuate the membrane association of LL-37(W27F) and temporin L. Biochim. Biophys. Acta 2008, 1778, 1460–1466. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Boil. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Pike, L.J. The challenge of lipid rafts. J. Lipid Res. 2009, 50, S323–S328. [Google Scholar] [CrossRef] [Green Version]
- Gueguinou, M.; Gambade, A.; Felix, R.; Chantome, A.; Fourbon, Y.; Bougnoux, P.; Weber, G.; Potier-Cartereau, M.; Vandier, C. Lipid rafts, KCa/ClCa/Ca2+ channel complexes and EGFR signaling: Novel targets to reduce tumor development by lipids? Biochim. Biophys. Acta 2015, 1848, 2603–2620. [Google Scholar] [CrossRef]
- Felgentreff, K.; Beisswenger, C.; Griese, M.; Gulder, T.; Bringmann, G.; Bals, R. The antimicrobial peptide cathelicidin interacts with airway mucus. Peptides 2006, 27, 3100–3106. [Google Scholar] [CrossRef]
- Thomas, A.J.; Pulsipher, A.; Davis, B.M.; Alt, J.A. LL-37 causes cell death of human nasal epithelial cells, which is inhibited with a synthetic glycosaminoglycan. PLoS ONE 2017, 12, e0183542. [Google Scholar] [CrossRef]
- Sandgren, S.; Wittrup, A.; Cheng, F.; Jönsson, M.; Eklund, E.; Busch, S.; Belting, M. The Human Antimicrobial Peptide LL-37 Transfers Extracellular DNA Plasmid to the Nuclear Compartment of Mammalian Cells via Lipid Rafts and Proteoglycan-dependent Endocytosis. J. Boil. Chem. 2004, 279, 17951–17956. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Murakami, T.; Hu, Z.; Tamura, H.; Kuwahara-Arai, K.; Iba, T.; Nagaoka, I. Human Host Defense Cathelicidin Peptide LL-37 Enhances the Lipopolysaccharide Uptake by Liver Sinusoidal Endothelial Cells without Cell Activation. J. Immunol. 2016, 196, 1338–1347. [Google Scholar] [CrossRef] [Green Version]
- Yoshio, H.; Tollin, M.; Gudmundsson, G.H.; Lagercrantz, H.; Jörnvall, H.; Marchini, A.G.; Agerberth, B. Antimicrobial Polypeptides of Human Vernix Caseosa and Amniotic Fluid: Implications for Newborn Innate Defense. Pediatr. Res. 2003, 53, 211–216. [Google Scholar] [CrossRef]
- Beauvais, D.M.; Burbach, B.J.; Rapraeger, A.C. The syndecan-1 ectodomain regulates alphavbeta3 integrin activity in human mammary carcinoma cells. J. Cell Biol. 2004, 167, 171–181. [Google Scholar] [CrossRef]
- Rauch, B.H.; Millette, E.; Kenagy, R.D.; Daum, G.; Fischer, J.W.; Clowes, A.W. Syndecan-4 Is Required for Thrombin-induced Migration and Proliferation in Human Vascular Smooth Muscle Cells. J. Boil. Chem. 2005, 280, 17507–17511. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Christiansen, M.N.; Chik, J.; Lee, L.Y.; Anugraham, M.; Abrahams, J.L.; Packer, N.H. Cell surface protein glycosylation in cancer. Proteomics 2014, 14, 525–546. [Google Scholar] [CrossRef]
- Guo, H.; Abbott, K.L. Functional Impact of Tumor-Specific N-Linked Glycan Changes in Breast and Ovarian Cancers. Adv. Cancer Res. 2015, 126, 281–303. [Google Scholar]
- Okolicsanyi, R.K.; Van Wijnen, A.J.; Cool, S.M.; Stein, G.S.; Griffiths, L.R.; Haupt, L.M. Heparan Sulfate Proteoglycans and Human Breast Cancer Epithelial Cell Tumorigenicity. J. Cell. Biochem. 2014, 115, 967–976. [Google Scholar] [CrossRef]
- Geisler, C.; Jarvis, D.L. Effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology 2011, 21, 988–993. [Google Scholar] [CrossRef]
- Kim, S.; Oh, D.-B.; Kang, H.A.; Kwon, O. Features and applications of bacterial sialidases. Appl. Microbiol. Biotechnol. 2011, 91, 1–15. [Google Scholar] [CrossRef]
- Lishko, V.K.; Moreno, B.; Podolnikova, N.P.; Ugarova, T.P. Identification of Human Cathelicidin Peptide LL-37 as a Ligand for Macrophage Integrin αMβ2 (Mac-1, CD11b/CD18) that Promotes Phagocytosis by Opsonizing Bacteria. Res. Rep. Biochem. 2016, 2016, 39–55. [Google Scholar]
- Lohmander, L.S.; Hascall, V.C.; Caplan, A.I. Effects of 4-methylumbelliferyl-beta-D-xylopyranoside on chondrogenesis and proteoglycan synthesis in chick limb bud mesenchymal cell cultures. J. Boil. Chem. 1979, 254, 10551–10561. [Google Scholar]
- Safaiyan, F.; Kolset, S.O.; Prydz, K.; Gottfridsson, E.; Lindahl, U.; Salmivirta, M. Selective Effects of Sodium Chlorate Treatment on the Sulfation of Heparan Sulfate. J. Boil. Chem. 1999, 274, 36267–36273. [Google Scholar] [CrossRef] [Green Version]
- Nikitovic, D.; Kouvidi, K.; Voudouri, K.; Berdiaki, A.; Karousou, E.; Passi, A.; Tzanakakis, G.N. The Motile Breast Cancer Phenotype Roles of Proteoglycans/Glycosaminoglycans. BioMed Res. Int. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lendorf, M.E.; Manon-Jensen, T.; Kronqvist, P.; Multhaupt, H.A.B.; Couchman, J.R. Syndecan-1 and Syndecan-4 Are Independent Indicators in Breast Carcinoma. J. Histochem. Cytochem. 2011, 59, 615–629. [Google Scholar] [CrossRef] [Green Version]
- Okolicsanyi, R.K.; Buffiere, A.; Jacinto, J.M.; Chacon-Cortes, D.; Chambers, S.K.; Youl, P.H.; Haupt, L.M.; Griffiths, L.R. Association of heparan sulfate proteoglycans SDC1 and SDC4 polymorphisms with breast cancer in an Australian Caucasian population. Tumor Biol. 2015, 36, 1731–1738. [Google Scholar] [CrossRef]
- Vuoriluoto, K.; Högnäs, G.; Meller, P.; Lehti, K.; Ivaska, J. Syndecan-1 and -4 differentially regulate oncogenic K-ras dependent cell invasion into collagen through α2β1 integrin and MT1-MMP. Matrix Boil. 2011, 30, 207–217. [Google Scholar] [CrossRef]
- Hassan, H.; Greve, B.; Pavão, M.S.G.; Kiesel, L.; Ibrahim, S.A.; Götte, M. Syndecan-1 modulates β-integrin-dependent and interleukin-6-dependent functions in breast cancer cell adhesion, migration, and resistance to irradiation. FEBS J. 2013, 280, 2216–2227. [Google Scholar] [CrossRef]
- Barlow, P.G.; Beaumont, P.E.; Cosseau, C.; Mackellar, A.; Wilkinson, T.S.; Hancock, R.E.W.; Haslett, C.; Govan, J.R.W.; Simpson, A.J.; Davidson, D.J. The Human Cathelicidin LL-37 Preferentially Promotes Apoptosis of Infected Airway Epithelium. Am. J. Respir. Cell Mol. Boil. 2010, 43, 692–702. [Google Scholar] [CrossRef]
- Malm, J.; Sorensen, O.E.; Persson, T.; Frohm-Nilsson, M.; Johansson, B.; Bjartell, A.; Lilja, H.; Ståhle-Bäckdahl, M.; Borregaard, N.; Egesten, A. The Human Cationic Antimicrobial Protein (hCAP-18) Is Expressed in the Epithelium of Human Epididymis, Is Present in Seminal Plasma at High Concentrations, and Is Attached to Spermatozoa. Infect. Immun. 2000, 68, 4297–4302. [Google Scholar] [CrossRef] [Green Version]
- Heilborn, J.D.; Nilsson, M.F.; Jimenez, C.I.; Sandstedt, B.; Borregaard, N.; Tham, E.; Sorensen, O.E.; Weber, G.; Stahle, M. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int. J. Cancer 2005, 114, 713–719. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Tomchuck, S.L.; Zwezdaryk, K.J.; Danka, E.S.; Scandurro, A.B. Leucine Leucine-37 Uses Formyl Peptide Receptor–Like 1 to Activate Signal Transduction Pathways, Stimulate Oncogenic Gene Expression, and Enhance the Invasiveness of Ovarian Cancer Cells. Mol. Cancer Res. 2009, 7, 907–915. [Google Scholar] [CrossRef]
- Weghuber, J.; Aichinger, M.C.; Brameshuber, M.; Wieser, S.; Ruprecht, V.; Plochberger, B.; Madl, J.; Horner, A.; Reipert, S.; Lohner, K.; et al. Cationic amphipathic peptides accumulate sialylated proteins and lipids in the plasma membrane of eukaryotic host cells. Biochim. Biophys. Acta (BBA)-Biomembr. 2011, 1808, 2581–2590. [Google Scholar] [CrossRef] [Green Version]
- Tkachenko, E.; Elfenbein, A.; Tirziu, D.; Simons, M. Syndecan-4 Clustering Induces Cell Migration in a PDZ-Dependent Manner. Circ. Res. 2006, 98, 1398–1404. [Google Scholar] [CrossRef] [Green Version]
- Tkachenko, E. Fibroblast growth factor 2 endocytosis in endothelial cells proceed via syndecan-4-dependent activation of Rac1 and a Cdc42-dependent macropinocytic pathway. J. Cell Sci. 2004, 117, 3189–3199. [Google Scholar] [CrossRef] [Green Version]
- Elfenbein, A.; Simons, M. Syndecan-4 signaling at a glance. J. Cell Sci. 2013, 126, 3799–3804. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Jin, H.; Rapraeger, A.C. Syndecan-1 and Syndecan-4 Capture Epidermal Growth Factor Receptor Family Members and the α3β1 Integrin via Binding Sites in Their Ectodomains. J. Boil. Chem. 2015, 290, 26103–26113. [Google Scholar] [CrossRef]
- Tsonis, A.I.; Afratis, N.; Gialeli, C.; Piperigkou, Z.; Skandalis, S.S.; Theocharis, A.D.; Tzanakakis, G.N.; Karamanos, N.K.; Ellina, M.-I.; Ellina, M. Evaluation of the coordinated actions of estrogen receptors with epidermal growth factor receptor and insulin-like growth factor receptor in the expression of cell surface heparan sulfate proteoglycans and cell motility in breast cancer cells. FEBS J. 2013, 280, 2248–2259. [Google Scholar] [CrossRef]
- Montrose, K.; Yang, Y.; Sun, X.; Wiles, S.; Krissansen, G.W. Xentry, a new class of cell-penetrating peptide uniquely equipped for delivery of drugs. Sci. Rep. 2013, 3, 3. [Google Scholar] [CrossRef]
- Maenuma, K.; Yim, M.; Komatsu, K.; Hoshino, M.; Takahashi, Y.; Bovin, N.; Irimura, T. Use of a library of mutated Maackia amurensis hemagglutinin for profiling the cell lineage and differentiation. Proteomics 2008, 8, 3274–3283. [Google Scholar] [CrossRef]
- Cooney, C.A.; Jousheghany, F.; Yao-Borengasser, A.; Phanavanh, B.; Gomes, T.; Kieber-Emmons, A.M.; Siegel, E.R.; Suva, L.J.; Ferrone, S.; Kieber-Emmons, T.; et al. Chondroitin sulfates play a major role in breast cancer metastasis: A role for CSPG4 and CHST11 gene expression in forming surface P-selectin ligands in aggressive breast cancer cells. Breast Cancer Res. 2011, 13, R58. [Google Scholar] [CrossRef]
- Fernandez-Vega, I.; García, O.; Crespo, A.; Castañón, S.; Menéndez, P.; Astudillo, A.; Quirós, L.M. Specific genes involved in synthesis and editing of heparan sulfate proteoglycans show altered expression patterns in breast cancer. BMC Cancer 2013, 13, 24. [Google Scholar] [CrossRef]
- Kaneider, N.C.; Djanani, A.; Wiedermann, C.J. Heparan Sulfate Proteoglycan–Involving Immunomodulation by Cathelicidin Antimicrobial Peptides LL-37 and PR-39. Sci. World J. 2007, 7, 1832–1838. [Google Scholar] [CrossRef]
- Nakase, I.; Tadokoro, A.; Kawabata, N.; Takeuchi, T.; Katoh, H.; Hiramoto, K.; Negishi, M.; Nomizu, M.; Sugiura, Y.; Futaki, S. Interaction of Arginine-Rich Peptides with Membrane-Associated Proteoglycans Is Crucial for Induction of Actin Organization and Macropinocytosis. Biochemistry 2007, 46, 492–501. [Google Scholar] [CrossRef]
- Amoura, M.; Illien, F.; Joliot, A.; Guitot, K.; Offer, J.; Sagan, S.; Burlina, F. Head to tail cyclisation of cell-penetrating peptides: Impact on GAG-dependent internalisation and direct translocation. Chem. Commun. 2019, 55, 4566–4569. [Google Scholar] [CrossRef]
- Letoha, T.; Keller-Pintér, A.; Kúsz, E.; Kolozsi, C.; Bozsó, Z.; Toth, G.; Vizler, C.; Oláh, Z.; Szilák, L. Cell-penetrating peptide exploited syndecans. Biochim. Biophys. Acta (BBA)-Biomembr. 2010, 1798, 2258–2265. [Google Scholar] [CrossRef] [Green Version]
- Theocharis, A.D.; Karamanos, N.K. Proteoglycans remodeling in cancer: Underlying molecular mechanisms. Matrix Biol. 2019, 75–76, 220–259. [Google Scholar] [CrossRef]
- Potapenko, I.O.; Lüders, T.; Russnes, H.G.; Helland, Å.; Sørlie, T.; Kristensen, V.N.; Nord, S.; Lingjærde, O.C.; Børresen-Dale, A.-L.; Haakensen, V.D. Glycan-related gene expression signatures in breast cancer subtypes; relation to survival. Mol. Oncol. 2015, 9, 861–876. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habes, C.; Weber, G.; Goupille, C. Sulfated Glycoaminoglycans and Proteoglycan Syndecan-4 Are Involved in Membrane Fixation of LL-37 and Its Pro-Migratory Effect in Breast Cancer Cells. Biomolecules 2019, 9, 481. https://doi.org/10.3390/biom9090481
Habes C, Weber G, Goupille C. Sulfated Glycoaminoglycans and Proteoglycan Syndecan-4 Are Involved in Membrane Fixation of LL-37 and Its Pro-Migratory Effect in Breast Cancer Cells. Biomolecules. 2019; 9(9):481. https://doi.org/10.3390/biom9090481
Chicago/Turabian StyleHabes, Chahrazed, Günther Weber, and Caroline Goupille. 2019. "Sulfated Glycoaminoglycans and Proteoglycan Syndecan-4 Are Involved in Membrane Fixation of LL-37 and Its Pro-Migratory Effect in Breast Cancer Cells" Biomolecules 9, no. 9: 481. https://doi.org/10.3390/biom9090481
APA StyleHabes, C., Weber, G., & Goupille, C. (2019). Sulfated Glycoaminoglycans and Proteoglycan Syndecan-4 Are Involved in Membrane Fixation of LL-37 and Its Pro-Migratory Effect in Breast Cancer Cells. Biomolecules, 9(9), 481. https://doi.org/10.3390/biom9090481




