Tannin Gels and Their Carbon Derivatives: A Review
Abstract
1. The Chemistry of Tannins
1.1. Definition and Classification
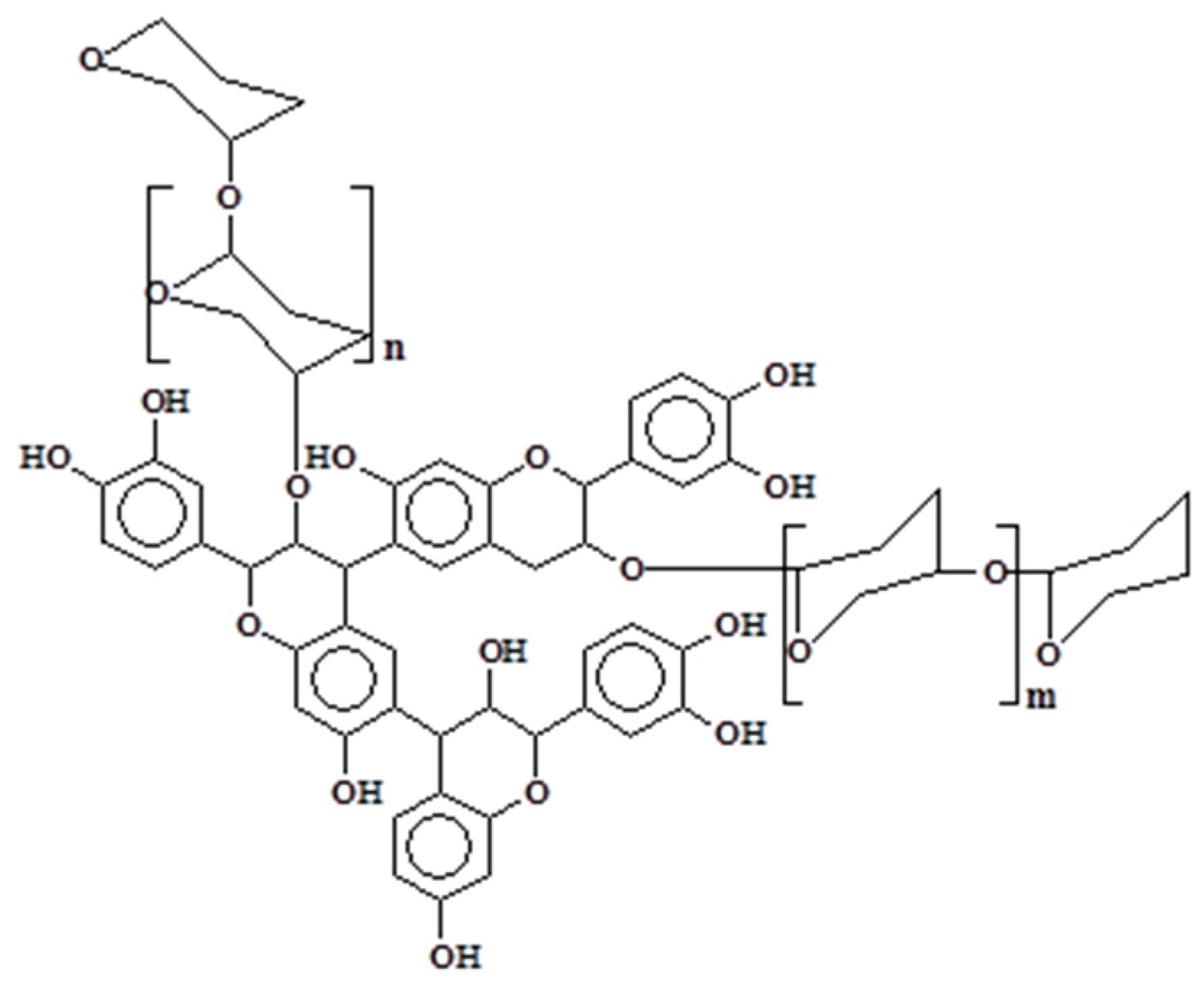
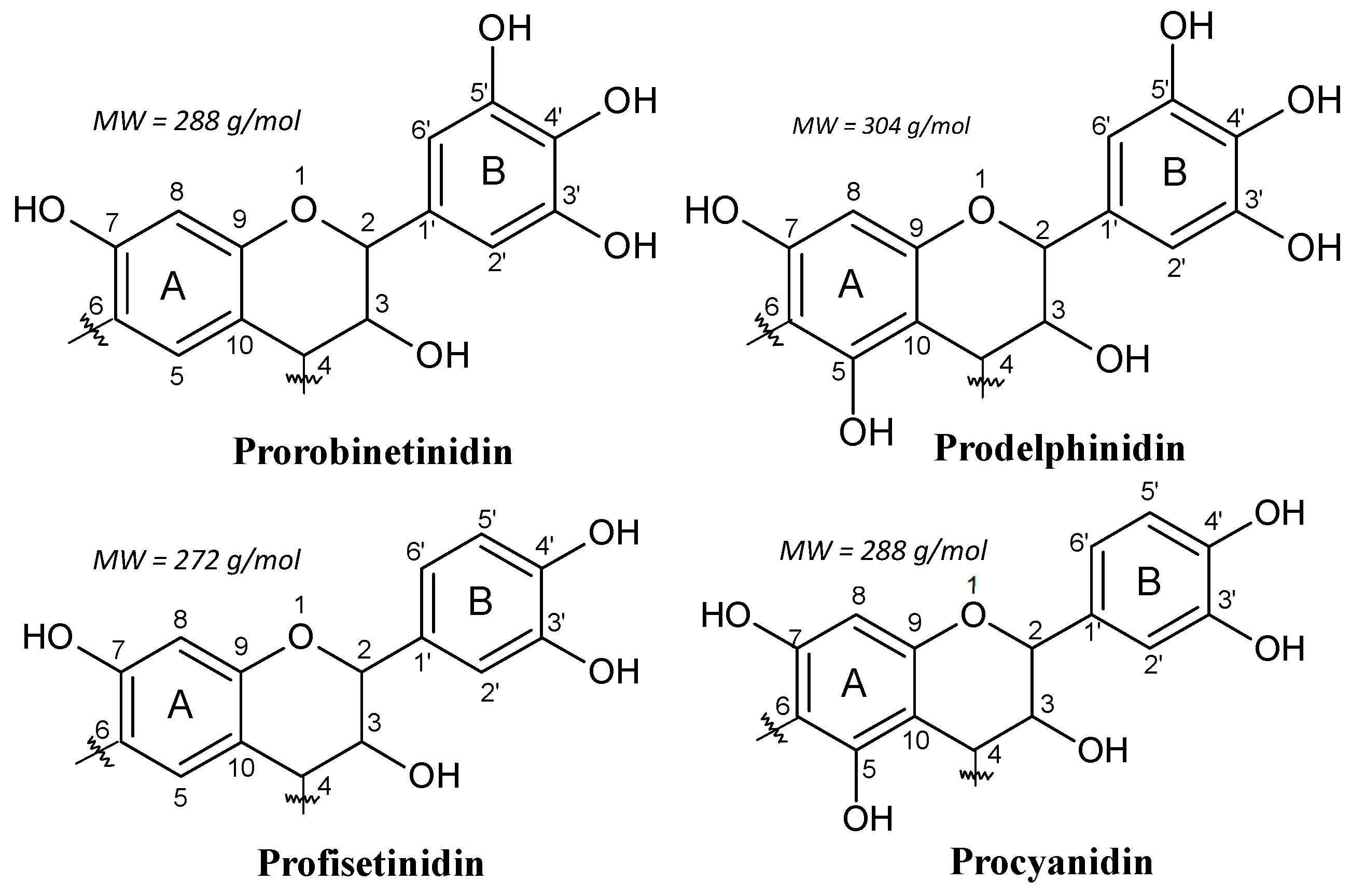
1.2. Condensed Tannins
1.3. Reactions of Condensed Flavonoid Tannins
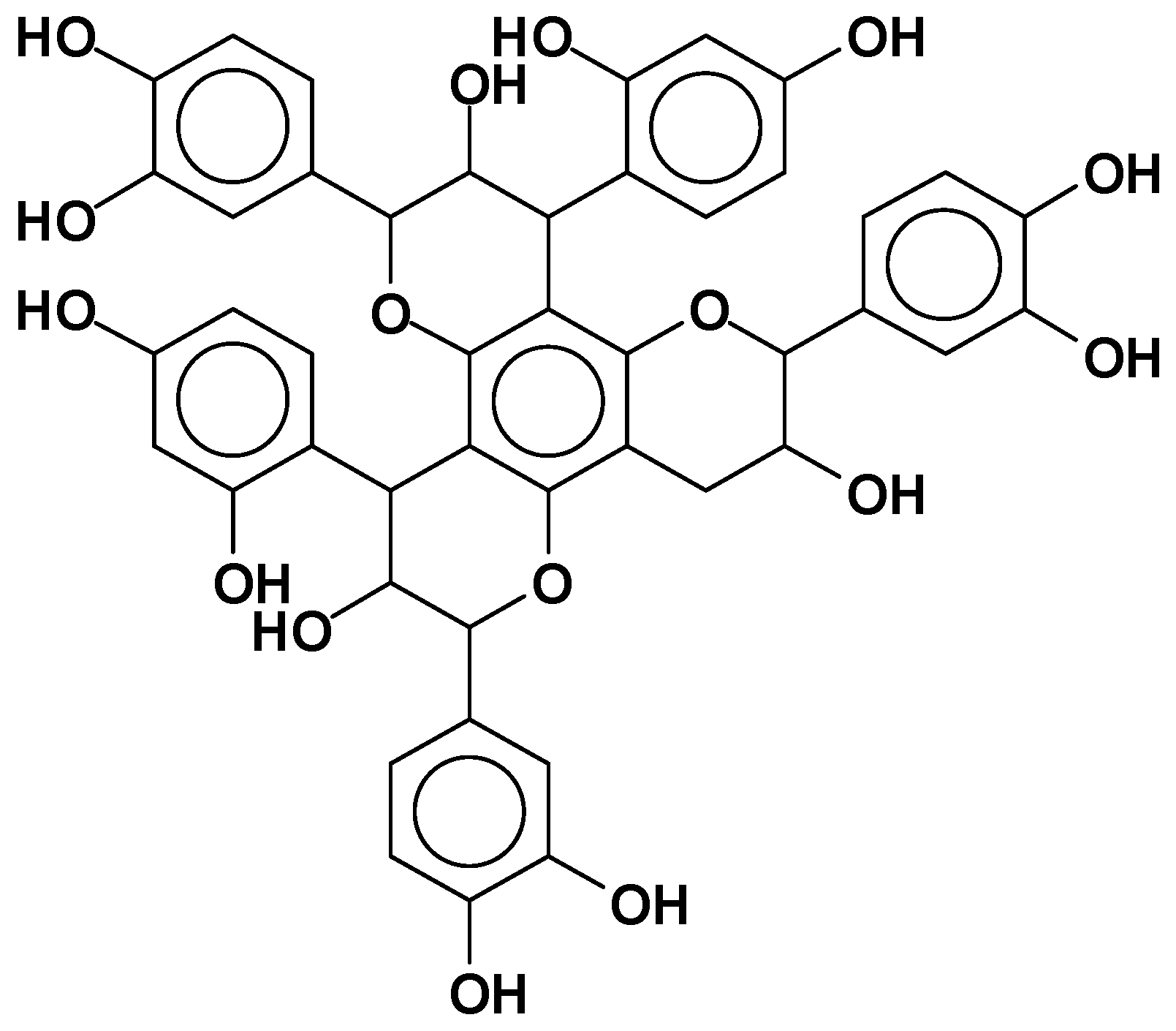
- Catechinic acid rearrangement: While this rearrangement is easily shown to occur in model compounds where the reaction is carried out in solution [13,21], it is much less evident and easily avoidable in tannin extracts where the colloidal nature of the extract markedly limits its occurrence. This is fortunate, as otherwise some fast-reacting tannins such as pine, pecan, cube gambier, etc. could not be used to produce resins, adhesives, and other thermosetting plastics [16,22,23,24,25,26].
- Catalytic tannin self-condensation: Polyflavonoid tannins have been found to self-condense and harden when in the presence of particular compounds acting as catalysts. Foremost, there is the catalytic effect of small amounts (2%−3%) of silica smoke, or nanosilica or silicates at high pH [27]. This reaction is fast and markedly exothermic, where a concentrated solution of tannin at 40%−50% in water gels and hardens at pH 12 and 25 °C in 20–30 min. The strong exothermic character of the reaction leads to this result, as the temperature increases by several tens of degrees in a short time [27]. Small amounts of boric acid and AlCl3 were found to have the same effect but are much less exothermic.
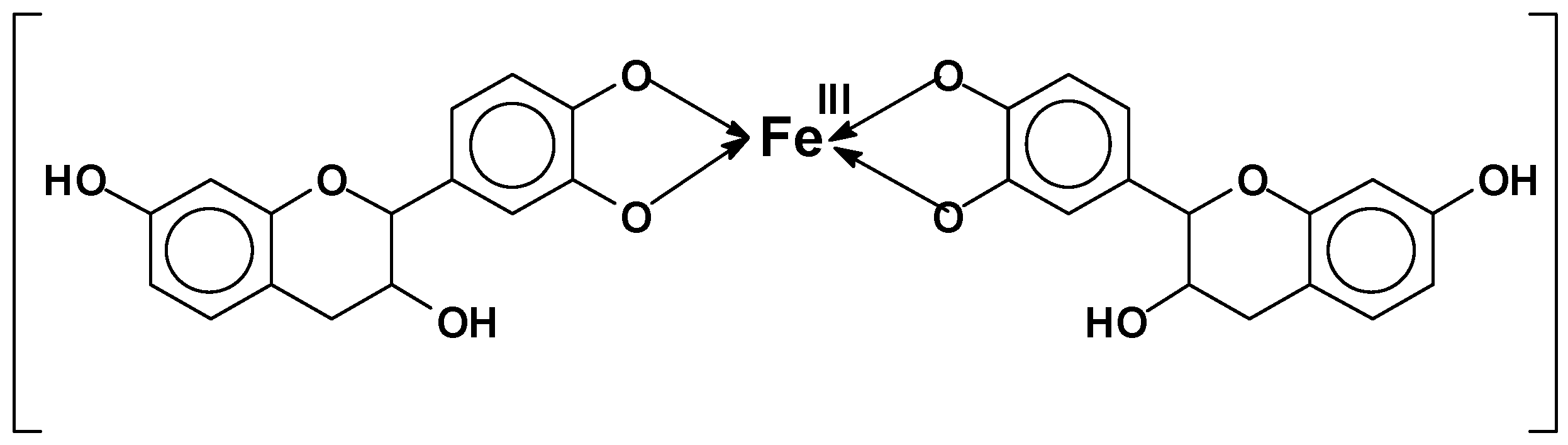
- Tannin complexation of metals: Tannins readily complex metal ions [28]. This characteristic is used to capture or precipitate toxic metals in water [29,30] and to isolate a rare metal such as germanium from its copper matrix, where it is mined for paint primers for metal application and several other applications. An old example is the formation of iron complexes, used to prepare intensely black/violet inks by the formation of ferric tannates (Figure 4). These coordination complexes are due to the ortho-diphenol hydroxyl groups on the tannin B-rings.
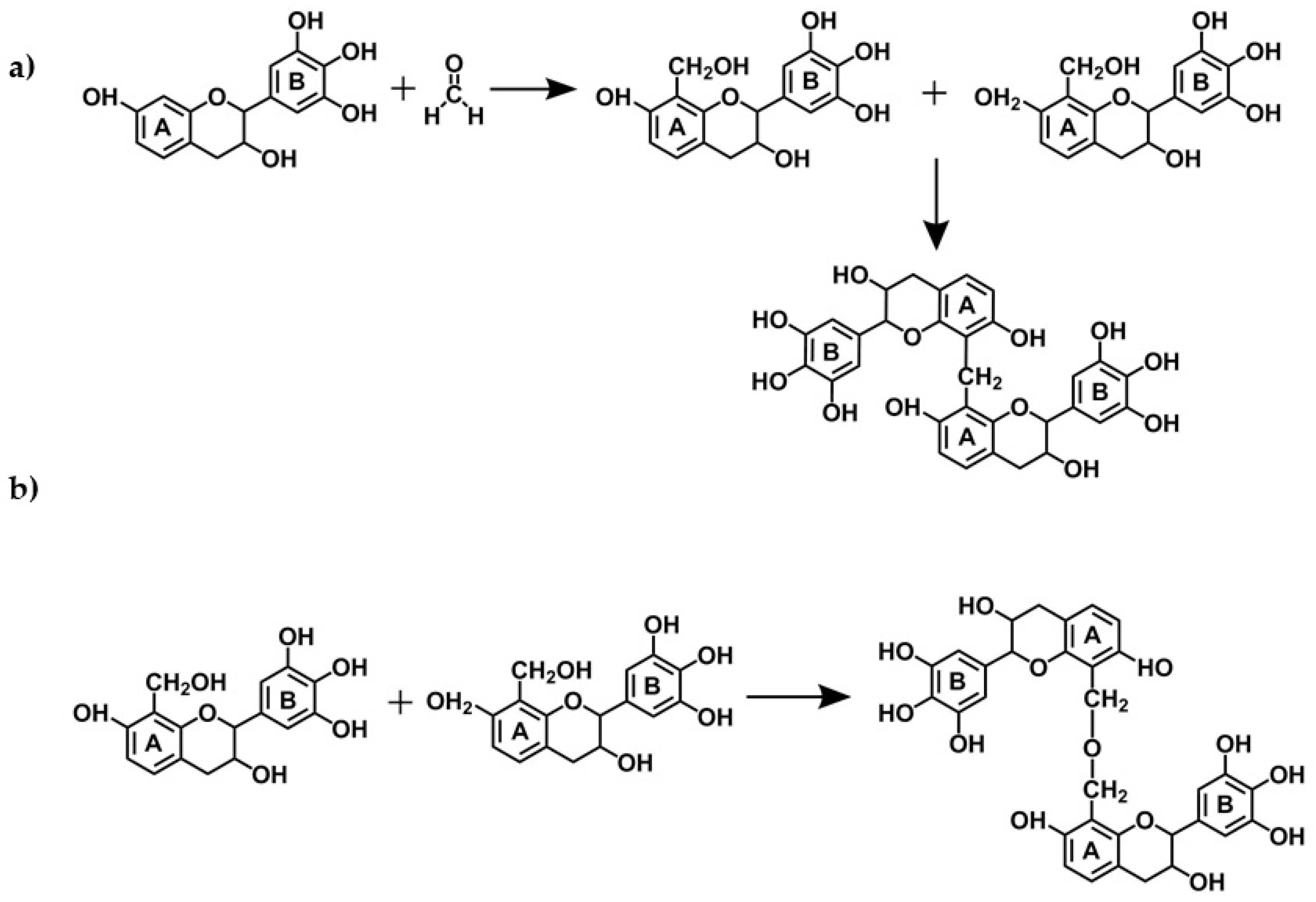
- Reactions of tannins with formaldehyde and other aldehydes: Due to their phenolic nature, tannins undergo the same alkali- or acid-catalyzed reaction with formaldehyde as phenols (see more details in Section 2.2.1.).
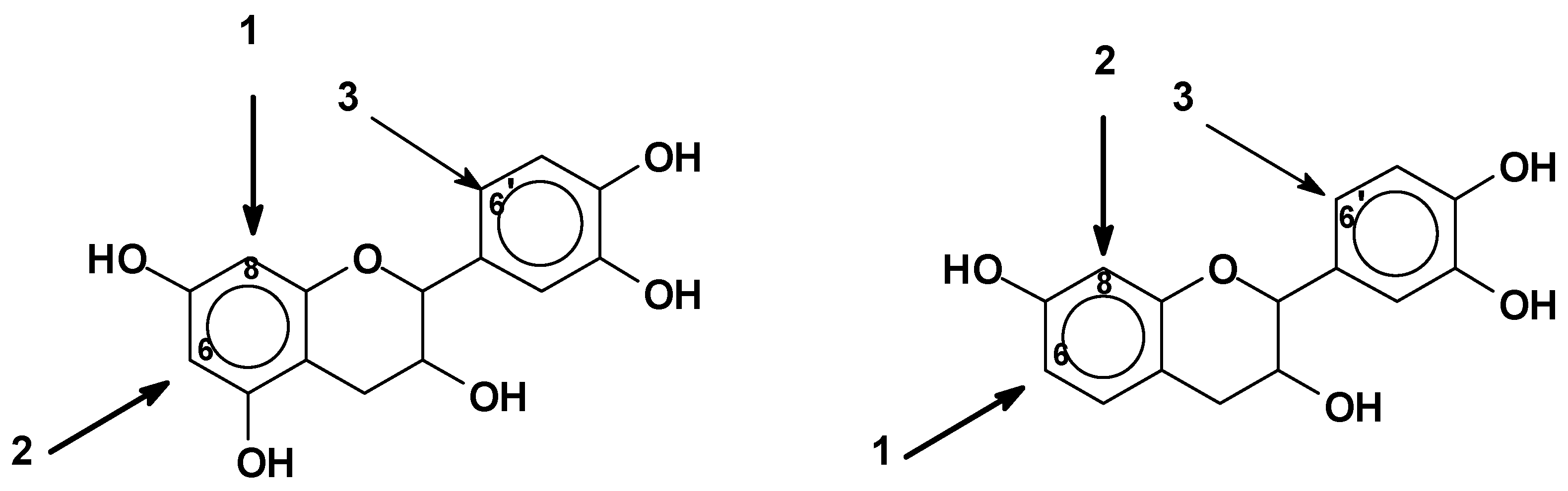
- Reactivity and orientation of electrophilic substitutions of flavonoids such as reaction with aldehydes: The relative accessibility and reactivity of flavonoid units is of interest for their use in resins, adhesives, and gels. A detailed description of reactions at different pHs is given in Section 2.2.2.
1.4. Industrial Extraction
1.5. Industrial Uses of Condensed Tannins
2. Tannin Gels
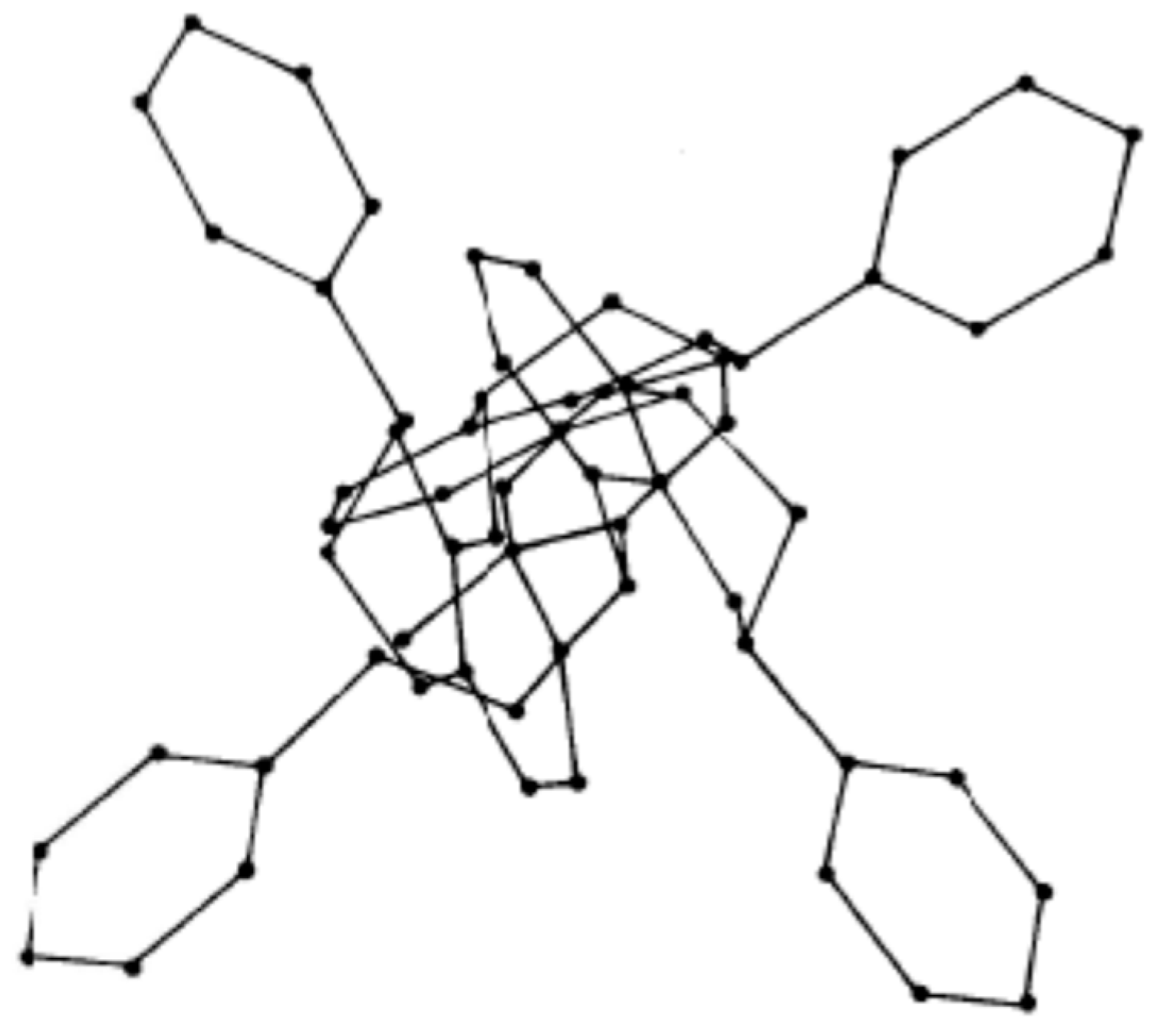
2.1. Tannin Gels Synthesis
2.2. Mechanisms
2.2.1. Tannin-Formaldehyde-Systems
2.2.2. Influence of pH and Mass Fraction
2.2.3. Tannin-Soy-Formaldehyde Gels
2.3. Preparation Methods and Conditions
2.3.1. Hydrogels Formulations at Normal Conditions
2.3.2. Solvent Exchange
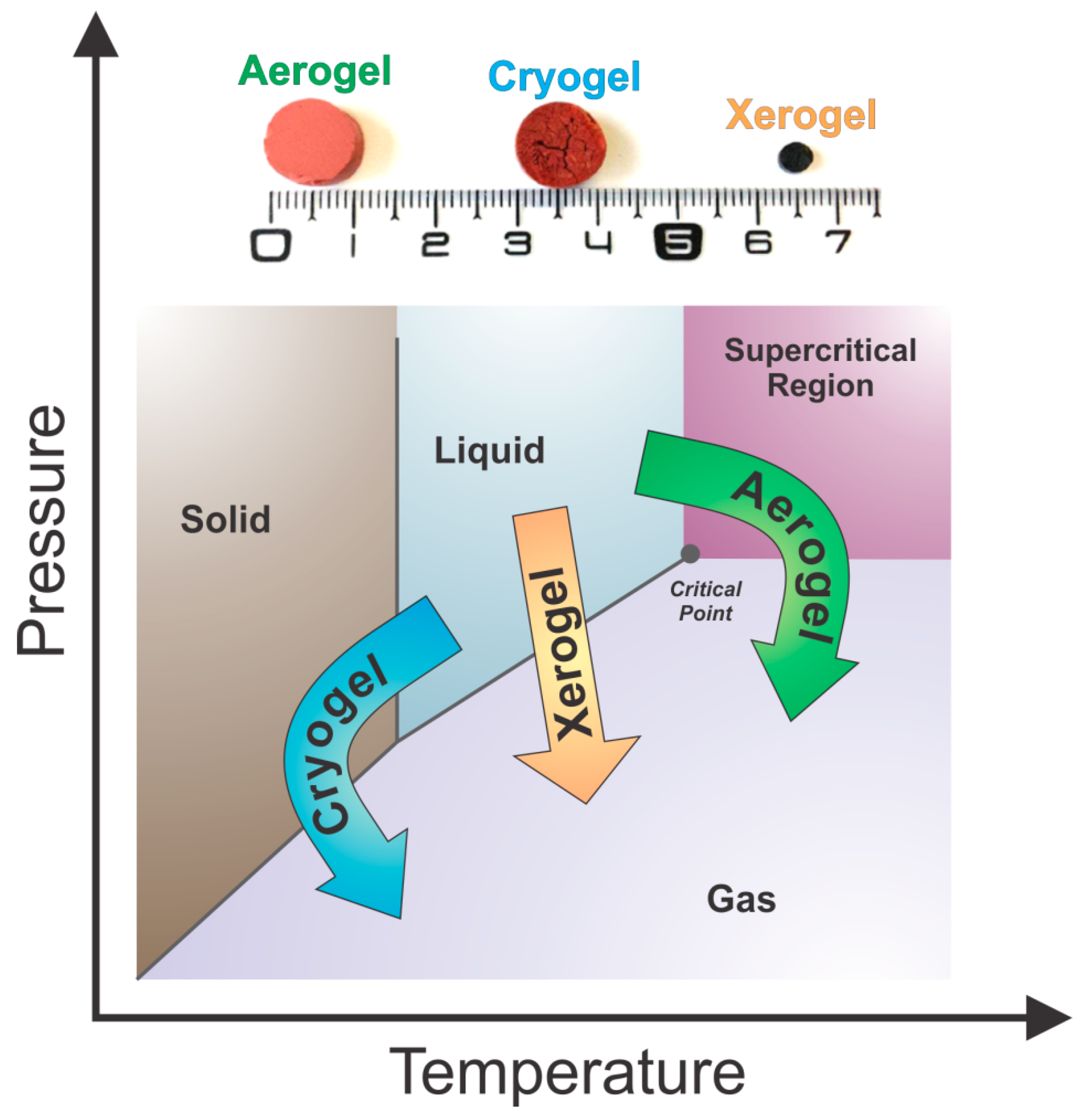
2.3.3. Drying Conditions and Final Physicochemical Properties of Tannin Gels
- Subcritical drying: Gels are dried under atmospheric conditions to form xerogels.
- Freeze-drying: Gels are dried at freezing conditions to produce cryogels.
- Supercritical drying: Gels are dried at a critical point of a working fluid to produce aerogels.
2.4. Hydrogels Formulations under Hydrothermal Conditions
2.5. Thermal Treatment of Organic Gels
2.6. Applications of Organic and Carbon Tannin Gels
3. Conclusions
- Tannins are the most abundant compounds from biomass after cellulose, hemicellulose, and lignin that can be used in a sustainable biorefinery plant. Among them, condensed polyflavonoid tannins exhibit a complex and heterogeneous structure with highly reactive sites in their structure that lead to condensation, polymerization or rearrangement under acid or alkaline conditions, in the presence of catalysts, metals, sulfite, and especially aldehydes (e.g., formaldehyde), which are efficient precursors for the preparation of solid gels.
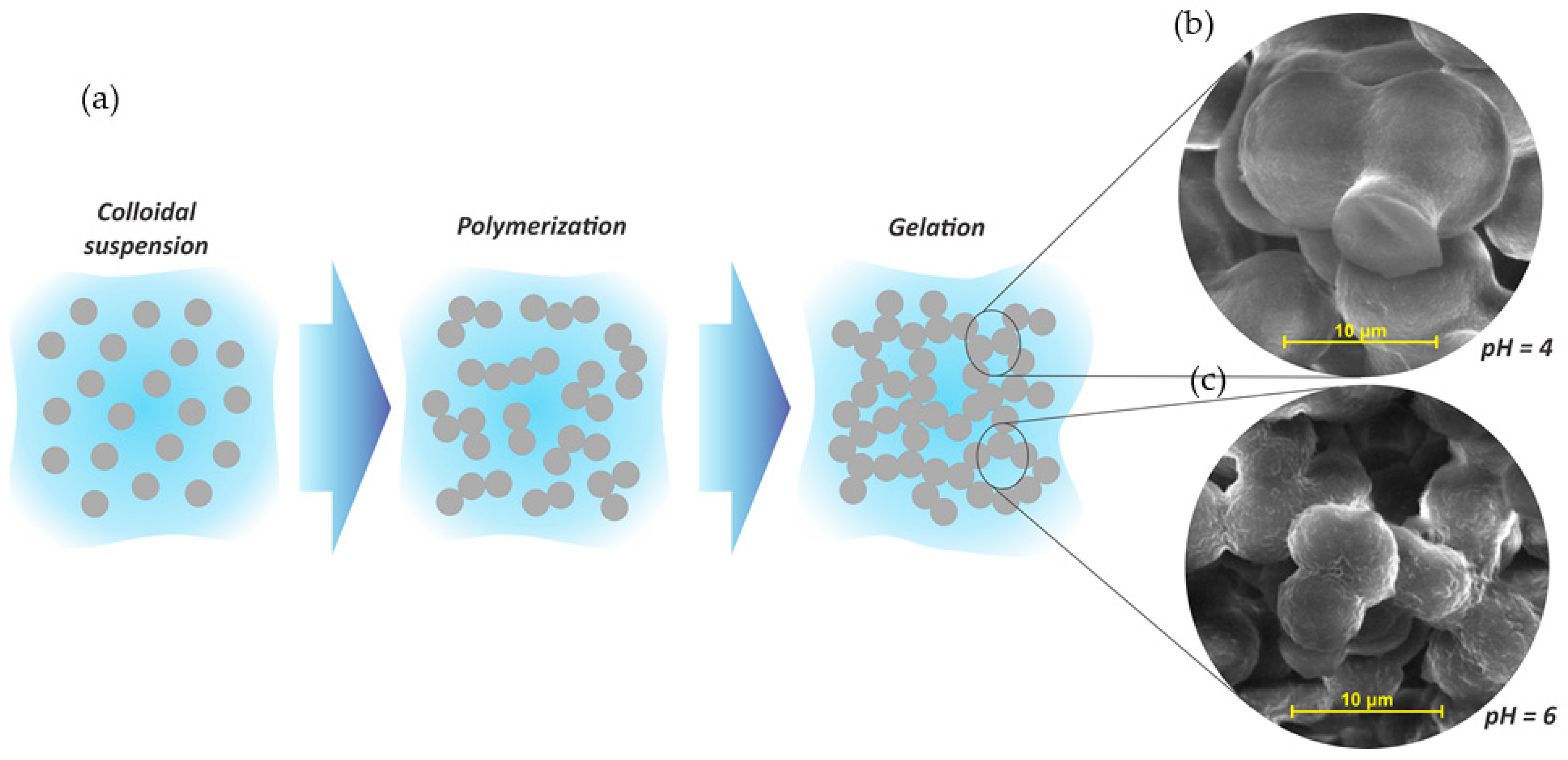
- The pH of the solution may also affect the final gels obtained under normal or hydrothermal conditions. Under normal conditions, gels prepared at low pH present high nodules, consequently high clusters, and high porosity, whereas at high pH, gels presented smaller nodules, reduced porosity, and narrow pores. In the case of hydrothermal gels, at low pH, gels attained low spherical nodules with higher porosity compared to bigger spherical particles of lower porosity at high pH. In the last case, smaller nodules favored the evolution of volatile matter during carbonization, and consequently improved the surface area and porosity development.
- The mass fraction of total solids from initial solutions has also an impact on final properties of tannin gels. Basically, very diluted systems lead to more porous gels, whereas high mass fractions tend to produce less porous materials. However, the most porous gels bear more capillary stresses during the drying step, which can be controlled by the addition of a surfactant in the medium.
- During tannin gel synthesis, the drying method defines the porous structure of the organic tannin gel, and consequently its application. Although aero- and cryo-gels are produced from costly techniques, their developed porous structure due to lower shrinkage is desired for several applications, which have not been tested or fully explored as yet (e.g., catalysis, column separation methods, chromatography, and thermal insulation).
- Carbonization and activation conditions play an important role in the porosity development of tannin gels. Optimal conditions of temperature, residence times, heating rate, and physical and chemical agents are suitable for the development of their porosity for specific applications. Thus, thermal treatment parameters conditions must be optimized during the production of tannin carbon gels.
- Tannin gels present interesting physicochemical properties for several applications. However, further studies should be performed to investigate their pore size distribution and the development of mesopores, which are crucial for most of the applications discussed: the removal of contaminants from wastewater, thermal insulation and energy storage. In addition, most studies on the application of tannin gels were based on small-scale tests.
- Thus, tannin organic and carbon gels are based on non-toxic, biosourced, and low-cost materials, which have textural properties (porosity or surface area) that are comparable to synthetic phenolic precursors such as phenol and resorcinol-formaldehyde gels.
Funding
Conflicts of Interest
References
- Arbenz, A.; Avérous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef]
- Haslam, E. Plant. Polyphenols: Vegetable Tannins Revisited; Chemistry and Pharmacology of Natural Products; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- De Hoyos-Martínez, P.L.; Merle, J.; Labidi, J.; Charrier-El Bouhtoury, F. Tannins extraction: A key point for their valorization and cleaner production. J. Clean. Prod. 2019, 206, 1138–1155. [Google Scholar] [CrossRef]
- Pizzi, A. Tannin-based adhesives. J. Macromol. Sci. Polymer Rev. 1980, 18, 247–315. [Google Scholar] [CrossRef]
- Pizzi, A. Recent developments in eco-efficient bio-based adhesives for wood bonding: Opportunities and issues. J. Adhes. Sci. Technol. 2006, 20, 829–846. [Google Scholar] [CrossRef]
- Roux, D.G.; Paulus, E. Condensed tannins. 8. The isolation and distribution of interrelated heartwood components of Schinopsis spp. Biochem. J. 1961, 78, 785–789. [Google Scholar] [CrossRef]
- Drewes, S.; Roux, D. Condensed tannins. 15. Interrelationships of flavonoid components in wattle-bark extract. Biochem. J. 1963, 87, 167–172. [Google Scholar] [CrossRef]
- Abdalla, S.; Pizzi, A.; Ayed, N.; Charrier, F.; Bahabri, F.; Ganash, A. MALDI-TOF and 13C NMR analysis of Tunisian Zizyphus jujuba root bark tannins. Ind. Crops Prod. 2014, 59, 277–281. [Google Scholar] [CrossRef]
- Drovou, S.; Pizzi, A.; Lacoste, C.; Zhang, J.; Abdulla, S.; El-Marzouki, F.M. Flavonoid tannins linked to long carbohydrate chains—MALDI-TOF analysis of the tannin extract of the African locust bean shells. Ind. Crops Prod. 2015, 67, 25–32. [Google Scholar] [CrossRef]
- Roux, D.G.; Ferreira, D.; Hundt, H.K.L.; Malan, E. Structure, stereochemistry, and reactivity of natural condensed tannins as basis for their extended industrial application. Appl. Polym. Symp. 1975, 28, 335–353. [Google Scholar]
- Roux, D.G. Recent advances in the chemistry and chemical utilization of the natural condensed tannins. Phytochemistry 1972, 11, 1219–1230. [Google Scholar] [CrossRef]
- Roux, D.G. Modern Applications of Mimosa Extract; Leather Industries Research Institute: Grahamstown, South Africa, 1965.
- Pizzi, A. Advanced Wood Adhesives Technology; Marcel Dekker: New York, NY, USA, 1994. [Google Scholar]
- Pizzi, A. Natural Phenolic Adhesives 1: Tannin. In Handbook of Adhesive Technology; Pizzi, A., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 2003; pp. 573–598. [Google Scholar]
- Christiansen, A.W.; Gillespie, R.H. Wood Adhesives in 1985: Status and Needs; Forest Products Laboratory, Ed.; Proceedings. Forest Products Research Society; FPRS: Madison, WI, USA, 1986. [Google Scholar]
- Pizzi, A.; Stephanou, A. Comparative and differential behaviour of pine vs. pecan nut tannin adhesives for particleboard. Holzforsch. Holzverwert. 1993, 45, 30–33. [Google Scholar]
- McGraw, G.W.; Rials, T.G.; Steynberg, J.P.; Hemingway, R.W. Chemistry of Pecan Tannins and Analysis of Cure of Pecan Tannin-Based Cold-Setting Adhesives with a DMA ‘Micro-Beam’ Test. In Plant Polyphenols. Basic Life Sciences; Hemingway, R.W., Laks, P.E., Eds.; Springer: Boston, MA, USA, 1992; Volume 59, pp. 979–990. [Google Scholar]
- Sealy-Fisher, V.J.; Pizzi, A. Increased pine tannins extraction and wood adhesives development by phlobaphenes minimization. Holz. Als. Roh. -Werkst. 1992, 50, 212–220. [Google Scholar] [CrossRef]
- Roux, D.G. Wattle Tannin and Mimosa Extract; Leather Industries Research Institute: Grahamstown, South Africa, 1965.
- Pizzi, A. Sulfited tannins for exterior wood adhesives. Colloid Polym. Sci. 1979, 257, 37–40. [Google Scholar] [CrossRef]
- Ohara, S.; Hemingway, R.W. Condensed tannins: The formation of a diarylpropanol-catechinic acid dimer from base-catalyzed reactions of (+)-catechin. J. Wood Chem. Technol. 1991, 11, 195–208. [Google Scholar] [CrossRef]
- Pizzi, A. Pine tannin adhesives for particleboard. Holz Als Roh- Werkst. 1982, 40, 293–301. [Google Scholar] [CrossRef]
- Pizzi, A.; von Leyser, E.P.; Valenzuela, J.; Clark, J.G. The chemistry and development of pine tannin adhesives for exterior particleboard. Holzforschung 1993, 47, 168–174. [Google Scholar] [CrossRef]
- Valenzuela, J.; von Leyser, E.; Pizzi, A.; Westermeyer, C.; Gorrini, B. Industrial production of pine tannin-bonded particleboard and MDF. Eur. J. Wood Wood Prod. 2012, 70, 735–740. [Google Scholar] [CrossRef]
- Pizzi, A.; Valenezuela, J.; Westermeyer, C. Low formaldehyde emission, fast pressing, pine and pecan tannin adhesives for exterior particleboard. Holz Als Roh- Werkst. 1994, 52, 311–315. [Google Scholar] [CrossRef]
- Pizzi, A.; Stephanou, A. Fast vs. slow-reacting non-modified tannin extracts for exterior particleboard adhesives. Holz. Als. Roh. -Werkst. 1994, 52, 218–222. [Google Scholar] [CrossRef]
- Meikleham, N.; Pizzi, A.; Stephanou, A. Induced accelerated autocondensation of polyflavonoid tannins for phenolic polycondensates. I. 13C-NMR, 29Si-NMR, X-ray, and polarimetry studies and mechanism. J. Appl. Polym. Sci. 1994, 54, 1827–1845. [Google Scholar] [CrossRef]
- Slabbert, N. Complexation of condensed tannins with metal ions. In Plant Polyphenols: Biogenesis, Chemical Properties, and Significance; Hemingway, R.W., Laks, P.E., Eds.; Plenum Press: New York, NY, USA, 1992; pp. 421–436. [Google Scholar]
- Tondi, G.; Oo, C.W.; Pizzi, A.; Trosa, A.; Thevenon, M.F. Metal adsorption of tannin based rigid foams. Ind. Crops Prod. 2009, 29, 336–340. [Google Scholar] [CrossRef]
- Oo, C.W.; Kassim, M.J.; Pizzi, A. Characterization and performance of Rhizophora apiculata mangrove polyflavonoid tannins in the adsorption of copper (II) and lead (II). Ind. Crops Prod. 2009, 30, 152–161. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Prospectives and actual industrial applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A.; Cameron, F.A.; Eaton, N.J. The tridimensional structure of polyflavonoid tannins by conformational analysis. J. Macromol. Sci. Part. - Chem. 1985, 22, 515–540. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Essawy, H.A.; Barhoum, A.; Van Assche, G. Isocyanate free condensed tannin-based polyurethanes. Eur. Polym. J. 2015, 67, 513–526. [Google Scholar] [CrossRef]
- Pizzi, A. Tannin-based polyurethane adhesives. J. Appl. Polym. Sci. 1979, 23, 1889–1891. [Google Scholar] [CrossRef]
- Pizzi, A. Tannin-formaldehyde exterior wood adhesives through flavonoid B-ring cross linking. J. Appl. Polym. Sci. 1978, 22, 2397–2399. [Google Scholar] [CrossRef]
- Pizzi, A.; Stephanou, A. A 13C NMR study of polyflavonoid tannin adhesive intermediates. II. Colloidal state reactions. J. Appl. Polym. Sci. 1994, 51, 2125–2130. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crops Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Saayman, H.; Roux, D. The origins of tannins and flavonoids in black-wattle barks and heartwoods, and their associated ‘non-tannin’ components. Biochem. J. 1965, 97, 794–801. [Google Scholar] [CrossRef]
- Li, X.; Basso, M.C.; Braghiroli, F.L.; Fierro, V.; Pizzi, A.; Celzard, A. Tailoring the structure of cellular vitreous carbon foams. Carbon 2012, 50, 2026–2036. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.C.; Pizzi, A.; Laborie, M.-P.; Celzard, A.; Fierro, V. Pine tannin-based rigid foams: Mechanical and thermal properties. Ind. Crops Prod. 2013, 43, 245–250. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.-C.; Pizzi, A.; Celzard, A.; Ella Ebang, E.; Gallon, N.; Charrier, B. Pine (P. pinaster) and quebracho (S. lorentzii) tannin-based foams as green acoustic absorbers. Ind. Crops Prod. 2015, 67, 70–73. [Google Scholar] [CrossRef]
- Mitsunaga, T.; Doi, T.; Kondo, Y.; Abe, I. Color development of proanthocyanidins in vanillin-hydrochloric acid reaction. J. Wood Sci. 1998, 44, 125–130. [Google Scholar] [CrossRef]
- Clark-Lewis, J.W.; Roux, D.G. Natural occurrence of enantiomorphous leucoanthocyanidian: (+)-mollisacacidin (gleditsin) and quebracho(–)-leucofisetinidin. J. Chem. Soc. 1959, 1402–1406. [Google Scholar] [CrossRef]
- Navarrete, P.; Pizzi, A.; Pasch, H.; Rode, K.; Delmotte, L. MALDI-TOF and 13C NMR characterization of maritime pine industrial tannin extract. Ind. Crops Prod. 2010, 32, 105–110. [Google Scholar] [CrossRef]
- Abdalla, S.; Pizzi, A.; Ayed, N.; Charrier-El Bouthoury, F.; Charrier, B.; Bahabri, F.; Ganash, A. MALDI-TOF Analysis of Aleppo Pine (Pinus halepensis) bark tannin. BioResources 2014, 9, 3396–3406. [Google Scholar] [CrossRef]
- Ucar, M.B.; Ucar, G.; Pizzi, A.; Gonultas, O. Characterization of Pinus brutia bark tannin by MALDI-TOF MS and 13C NMR. Ind. Crops Prod. 2013, 49, 697–704. [Google Scholar] [CrossRef]
- Saad, H.; Charrier-El Bouhtoury, F.; Pizzi, A.; Rode, K.; Charrier, B.; Ayed, N. Characterization of pomegranate peels tannin extractives. Ind. Crops Prod. 2012, 40, 239–246. [Google Scholar] [CrossRef]
- Hundt, H.K.L.; Roux, D.G. Condensed tannins: Determination of the point of linkage in ‘terminal’(+)-catechin units and degradative bromination of 4-flavanylflavan-3,4-diols. J. Chem. Soc. Chem. Commun. 1978. [Google Scholar] [CrossRef]
- Botha, J.J.; Ferreira, D.; Roux, D.G. Condensed tannins. Circular dichroism method of assessing the absolute configuration at C-4 of 4-arylflavan-3-ols, and stereochemistry of their formation from flavan-3,4-diols. J. Chem. Soc. Chem. Commun. 1978. [Google Scholar] [CrossRef]
- Basso, M.C.; Lacoste, C.; Pizzi, A.; Fredon, E.; Delmotte, L. MALDI-TOF and 13C NMR analysis of flexible films and lacquers derived from tannin. Ind. Crops Prod. 2014, 61, 352–360. [Google Scholar] [CrossRef]
- Delgado-Sánchez, C.; Amaral-Labat, G.; Grishechko, L.I.; Sánchez–Sánchez, A.; Fierro, V.; Pizzi, A.; Celzard, A. Fire-resistant tannin–ethylene glycol gels working as rubber springs with tuneable elastic properties. J. Mater. Chem. A 2017, 5, 14720–14732. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol.—gel science. The physics and chemistry of Sol.—gel processing; Academic Press Inc.: San Diego, CA, USA, 1990. [Google Scholar]
- Szczurek, A.; Amaral-Labat, G.; Fierro, V.; Pizzi, A.; Celzard, A. The use of tannin to prepare carbon gels. Part II. Carbon cryogels. Carbon 2011, 49, 2785–2794. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Grishechko, L.; Szczurek, A.; Fierro, V.; Pizzi, A.; Kuznetsov, B.; Celzard, A. Highly mesoporous organic aerogels derived from soy and tannin. Green Chem. 2012, 14, 3099. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Pekala, R.W.; Schaefer, D.W. Structure of organic aerogels. 1. Morphology and scaling. Macromolecules 1993, 26, 5487–5493. [Google Scholar] [CrossRef]
- Job, N.; Pirard, R.; Marien, J.; Pirard, J.-P. Porous carbon xerogels with texture tailored by pH control during sol–gel process. Carbon 2004, 42, 619–628. [Google Scholar] [CrossRef]
- Wang, J.; Glora, M.; Petricevic, R.; Saliger, R.; Proebstle, H.; Fricke, J. Carbon cloth reinforced carbon aerogel films derived from resorcinol formaldehyde. J. Porous Mater. 2001, 8, 159–165. [Google Scholar] [CrossRef]
- Bock, V.; Emmerling, A.; Saliger, R.; Fricke, J. Structural investigation of resorcinol formaldehyde and carbon aerogels using SAXS and BET. J. Porous Mater. 1997, 4, 287–294. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Pizzi, A.; Celzard, A. Systematic studies of tannin–formaldehyde aerogels: Preparation and properties. Sci. Technol. Adv. Mater. 2013, 14, 015001. [Google Scholar] [CrossRef] [PubMed]
- Amaral-Labat, G.; Grishechko, L.I.; Fierro, V.; Kuznetsov, B.N.; Pizzi, A.; Celzard, A. Tannin-based xerogels with distinctive porous structures. Biomass Bioenergy 2013, 56, 437–445. [Google Scholar] [CrossRef]
- Job, N.; Panariello, F.; Marien, J.; Crine, M.; Pirard, J.-P.; Léonard, A. Synthesis optimization of organic xerogels produced from convective air-drying of resorcinol–formaldehyde gels. J. Non-Cryst. Solids 2006, 352, 24–34. [Google Scholar] [CrossRef]
- Job, N.; Théry, A.; Pirard, R.; Marien, J.; Kocon, L.; Rouzaud, J.-N.; Béguin, F.; Pirard, J.-P. Carbon aerogels, cryogels and xerogels: Influence of the drying method on the textural properties of porous carbon materials. Carbon 2005, 43, 2481–2494. [Google Scholar] [CrossRef]
- Castro, C.D.; Quintana, G.C. Mixture design approach on the physical properties of lignin-resorcinol-formaldehyde xerogels. Int. J. Polym. Sci. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. Aerogels Handbook. Advances in Sol-Gel Derived Materials and Technologies; Springer: New York, NY, USA, 2011. [Google Scholar]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Robb, S.A.; Lee, B.H.; McLemore, R.; Vernon, B.L. Simultaneously physically and chemically gelling polymer system utilizing a poly(NIPAAm-co-cysteamine)-based copolymer. Biomacromolecules 2007, 8, 2294–2300. [Google Scholar] [CrossRef]
- Pizzi, A.; Scharfetter, H.O. The chemistry and development of tannin-based adhesives for exterior plywood. J. Appl. Polym. Sci. 1978, 22, 1745–1761. [Google Scholar] [CrossRef]
- Ping, L.; Brosse, N.; Chrusciel, L.; Navarrete, P.; Pizzi, A. Extraction of condensed tannins from grape pomace for use as wood adhesives. Ind. Crops Prod. 2011, 33, 253–257. [Google Scholar] [CrossRef]
- Pizzi, A. Wood Adhesive Chemistry and Technology; Marcel Dekker: New York, NY, USA, 1983. [Google Scholar]
- Fraser, D.A.; Hall, R.W.; Raum, A.L.J. Preparation of ‘high-ortho’ novolak resins I. Metal ion catalysis and orientation effect. J. Appl. Chem. 2007, 7, 676–689. [Google Scholar] [CrossRef]
- Fraser, D.A.; Hall, R.W.; Jenkins, P.A.; Raum, A.L.J. Preparation of ‘high-ortho’ novolak resins. II. The course of the reaction. J. Appl. Chem. 2007, 7, 689–700. [Google Scholar] [CrossRef]
- Pizzi, A. Phenolic resins by reactions of coordinated metal ligands. J. Polym. Sci. Polym. Lett. Ed. 1979, 17, 489–492. [Google Scholar] [CrossRef]
- Pizzi, A. Phenolic and tannin-based adhesive resins by reactions of coordinated metal ligands. I. Phenolic chelates. J. Appl. Polym. Sci. 1979, 24, 1247–1255. [Google Scholar] [CrossRef]
- Pizzi, A. Phenolic and tannin-based adhesive resins by reactions of coordinated metal ligands. II. Tannin adhesive preparation, characteristics, and application. J. Appl. Polym. Sci. 1979, 24, 1257–1268. [Google Scholar] [CrossRef]
- Hillis, W.E.; Urbach, G. The reaction of (+)-catechin with formaldehyde. J. Appl. Chem. 2007, 9, 474–482. [Google Scholar] [CrossRef]
- Szczurek, A.; Amaral-Labat, G.; Fierro, V.; Pizzi, A.; Masson, E.; Celzard, A. The use of tannin to prepare carbon gels. Part I: Carbon aerogels. Carbon 2011, 49, 2773–2784. [Google Scholar] [CrossRef]
- Grishechko, L.I.; Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Kuznetsov, B.N.; Pizzi, A.; Celzard, A. New tannin–lignin aerogels. Ind. Crops Prod. 2013, 41, 347–355. [Google Scholar] [CrossRef]
- Amaral-Labat, G.A.; Pizzi, A.; Gonçalves, A.R.; Celzard, A.; Rigolet, S.; Rocha, G.J.M. Environment-friendly soy flour-based resins without formaldehyde. J. Appl. Polym. Sci. 2008, 108, 624–632. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.C.; Pizzi, A.; Celzard, A.; Laborie, M.-P. Natural albumin/tannin cellular foams. Ind. Crops Prod. 2015, 73, 41–48. [Google Scholar] [CrossRef]
- Yoshizawa, N.; Hatori, H.; Soneda, Y.; Hanzawa, Y.; Kaneko, K.; Dresselhaus, M.S. Structure and electrochemical properties of carbon aerogels polymerized in the presence of Cu2+. J. Non-Cryst. Solids 2003, 330, 99–105. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Pizzi, A.; Masson, E.; Celzard, A. “Blue glue”: A new precursor of carbon aerogels. Microporous Mesoporous Mater. 2012, 158, 272–280. [Google Scholar] [CrossRef]
- Nakano, Y.; Takeshita, K.; Tsutsumi, T. Adsorption mechanism of hexavalent chromium by redox within condensed-tannin gel. Water Res. 2001, 35, 496–500. [Google Scholar] [CrossRef]
- Zhan, X.-M.; Zhao, X. Mechanism of lead adsorption from aqueous solutions using an adsorbent synthesized from natural condensed tannin. Water Res. 2003, 37, 3905–3912. [Google Scholar] [CrossRef]
- Ogata, T.; Nakano, Y. Mechanisms of gold recovery from aqueous solutions using a novel tannin gel adsorbent synthesized from natural condensed tannin. Water Res. 2005, 39, 4281–4286. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Ogata, T.; Nakano, Y. Kinetic analysis of palladium(II) adsorption process on condensed-tannin gel based on redox reaction models. Water Res. 2007, 41, 3043–3050. [Google Scholar] [CrossRef]
- Şengil, İ.A.; Özacar, M. Biosorption of Cu(II) from aqueous solutions by mimosa tannin gel. J. Hazard. Mater. 2008, 157, 277–285. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Beltrán-Heredia, J.; Gibello-Pérez, P. Adsorbent biopolymers from tannin extracts for water treatment. Chem. Eng. J. 2011, 168, 1241–1247. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A.; Ritter, J.A. Preparation and properties of resorcinol-formaldehyde organic and carbon gels. Adv. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- García-González, C.A.; Camino-Rey, M.C.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- Fricke, J.; Tillotson, T. Aerogels: Production, characterization, and applications. Thin Solid Films 1997, 297, 212–223. [Google Scholar] [CrossRef]
- Amaral-Labat, G. Gels Poreux Biosourcés: Production, Caractérisation et Applications. Doctoral Dissertation, University of Lorraine, Épinal, France, 2013. [Google Scholar]
- Scherer, G.W.; Smith, D.M. Cavitation during drying of a gel. J. Non-Cryst. Solids 1995, 189, 197–211. [Google Scholar] [CrossRef]
- Daraoui, N.; Dufour, P.; Hammouri, H.; Hottot, A. Model predictive control during the primary drying stage of lyophilisation. Control. Eng. Pract. 2010, 18, 483–494. [Google Scholar] [CrossRef]
- Tamon, H.; Ishizaka, H.; Yamamoto, T.; Suzuki, T. Preparation of mesoporous carbon by freeze drying. Carbon 1999, 37, 2049–2055. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel insulation for building applications: A state-of-the-art review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Szczurek, A.; Fierro, V.; Celzard, A.; Menéndez, J.A.; Arenillas, A. Advances in tailoring the porosity of tannin-based carbon xerogels. Ind. Crops Prod. 2016, 82, 100–106. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Celzard, A. Unique bimodal carbon xerogels from soft templating of tannin. Mater. Chem. Phys. 2015, 149–150, 193–201. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Fierro, V.; Parmentier, J.; Pasc, A.; Celzard, A. Easy and eco-friendly synthesis of ordered mesoporous carbons by self-assembly of tannin with a block copolymer. Green Chem. 2016, 18, 3265–3271. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Brun, N.; García-González, C.A.; Smirnova, I.; Titirici, M.M. Hydrothermal synthesis of highly porous carbon monoliths from carbohydrates and phloroglucinol. RSC Adv. 2013, 3, 17088–17096. [Google Scholar] [CrossRef]
- Fellinger, T.-P.; White, R.J.; Titirici, M.-M.; Antonietti, M. Borax-mediated formation of carbon aerogels from glucose. Adv. Funct. Mater. 2012, 22, 3254–3260. [Google Scholar] [CrossRef]
- Wohlgemuth, S.-A.; White, R.J.; Willinger, M.-G.; Titirici, M.-M.; Antonietti, M. A one-pot hydrothermal synthesis of sulfur and nitrogen doped carbon aerogels with enhanced electrocatalytic activity in the oxygen reduction reaction. Green Chem. 2012, 14, 1515–1523. [Google Scholar] [CrossRef]
- White, R.J.; Yoshizawa, N.; Antonietti, M.; Titirici, M.-M. A sustainable synthesis of nitrogen-doped carbon aerogels. Green Chem. 2011, 13, 2428. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Fierro, V.; Izquierdo, M.T.; Parmentier, J.; Pizzi, A.; Celzard, A. Nitrogen-doped carbon materials produced from hydrothermally treated tannin. Carbon 2012, 50, 5411–5420. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Fierro, V.; Parmentier, J.; Vidal, L.; Gadonneix, P.; Celzard, A. Hydrothermal carbons produced from tannin by modification of the reaction medium: Addition of H+ and Ag+. Ind. Crops Prod. 2015, 77, 364–374. [Google Scholar] [CrossRef]
- Navarrete, P.; Pizzi, A.; Bertaud, F.; Rigolet, S. Condensed tannin reactivity inhibition by internal rearrangements: Detection by CP-MAS 13C NMR. Maderas Cienc. Tecnol. 2011, 13, 59–68. [Google Scholar] [CrossRef]
- Young, D.A.; Cronjé, A.; Botes, A.L.; Ferreira, D.; Roux, D.G. Synthesis of condensed tannins. Part 14. Biflavanoid profisetinidins as synthons. The Acid-induced ‘phlobaphene’ reaction. J. Chem. Soc. Perkin Trans. 1985, 1, 2521–2527. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Fierro, V.; Izquierdo, M.T.; Parmentier, J.; Pizzi, A.; Delmotte, L.; Fioux, P.; Celzard, A. High surface—highly N-doped carbons from hydrothermally treated tannin. Ind. Crops Prod. 2015, 66, 282–290. [Google Scholar] [CrossRef]
- Braghiroli, F.; Fierro, V.; Pizzi, A.; Rode, K.; Radke, W.; Delmotte, L.; Parmentier, J.; Celzard, A. Reaction of condensed tannins with ammonia. Ind. Crops Prod. 2013, 44, 330–335. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Fierro, V.; Szczurek, A.; Stein, N.; Parmentier, J.; Celzard, A. Hydrothermally treated aminated tannin as precursor of N-doped carbon gels for supercapacitors. Carbon 2015, 90, 63–74. [Google Scholar] [CrossRef]
- Deng, X.; Liu, X.; Duan, T.; Zhu, W.; Yi, Z.; Yao, W. Fabricating a graphene oxide—bayberry tannin sponge for effective radionuclide removal. Mater. Res. Express 2016, 3, 055002. [Google Scholar] [CrossRef]
- Braghiroli, F.; Fierro, V.; Szczurek, A.; Gadonneix, P.; Ghanbaja, J.; Parmentier, J.; Medjahdi, G.; Celzard, A. Hydrothermal treatment of tannin: A route to porous metal oxides and metal/carbon hybrid materials. Inorganics 2017, 5, 7. [Google Scholar] [CrossRef]
- Braghiroli, F.L. Polyphénols Végétaux Traités par Voie Humide: Synthèse de Carbones Biosourcés Hautement Poreux et Applications. Doctoral Dissertation, Université de Lorraine, Épinal, France, 2014. [Google Scholar]
- Pandey, A.; Bhaskar, T.; Stöcker, M.; Sukumaran, R. Recent Advances in Thermochemical Conversion of Biomass; Elsevier: Oxford, UK, 2015. [Google Scholar]
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Marsh, H.; Rodríguez-Reinoso, F. Chapter 5—Activation Processes (Thermal or Physical). In Activated Carbon; Elsevier Science Ltd.: Oxford, UK, 2006; pp. 243–321. [Google Scholar]
- Szczurek, A.; Amaral-Labat, G.; Fierro, V.; Pizzi, A.; Celzard, A. Chemical activation of tannin-based hydrogels by soaking in KOH and NaOH solutions. Microporous Mesoporous Mater. 2014, 196, 8–17. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Chapter 6—Activation Processes (Chemical). In Activated Carbon; Elsevier Science Ltd.: Oxford, UK, 2006; pp. 322–365. [Google Scholar]
- Reimerink, W.M.T.M. The use of activated carbon as catalyst and catalyst carrier in industrial applications. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1999; Volume 120, pp. 751–769. [Google Scholar]
- Chen, J.Y. Activated Carbon Fiber and Textiles; Elsevier: Duxford, UK, 2017; pp. 3–20. [Google Scholar]
- Wu, D.; Fu, R.; Sun, Z.; Yu, Z. Low-density organic and carbon aerogels from the sol–gel polymerization of phenol with formaldehyde. J. Non-Cryst. Solids 2005, 351, 915–921. [Google Scholar] [CrossRef]
- Szczurek, A.; Amaral-Labat, G.; Fierro, V.; Pizzi, A.; Masson, E.; Celzard, A. Porosity of resorcinol-formaldehyde organic and carbon aerogels exchanged and dried with supercritical organic solvents. Mater. Chem. Phys. 2011, 129, 1221–1232. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Szczurek, A.; Fierro, V.; Menéndez, J.A.; Arenillas, A.; Celzard, A. Towards a feasible and scalable production of bio-xerogels. J. Colloid Interface Sci. 2015, 456, 138–144. [Google Scholar] [CrossRef]
- Grishechko, L.I.; Amaral-Labat, G.; Fierro, V.; Szczurek, A.; Kuznetsov, B.N.; Celzard, A. Biosourced, highly porous, carbon xerogel microspheres. RSC Adv. 2016, 6, 65698–65708. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Stein, N.; Boulanger, C.; Pizzi, A.; Celzard, A. Pore structure and electrochemical performances of tannin-based carbon cryogels. Biomass Bioenergy 2012, 39, 274–282. [Google Scholar] [CrossRef]
- Szczurek, A.; Fierro, V.; Medjahdi, G.; Celzard, A. Carbon aerogels prepared by autocondensation of flavonoid tannin. Carbon Resour. Convers. 2019, 2, 72–84. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Izquierdo, M.T.; Mathieu, S.; González-Álvarez, J.; Celzard, A.; Fierro, V. Outstanding electrochemical performance of highly N- and O-doped carbons derived from pine tannin. Green Chem. 2017, 19, 2653–2665. [Google Scholar] [CrossRef]
- Fernández-Barbero, A.; Suárez, I.J.; Sierra-Martín, B.; Fernández-Nieves, A.; de las Nieves, F.J.; Marquez, M.; Rubio-Retama, J.; López-Cabarcos, E. Gels and microgels for nanotechnological applications. Adv. Colloid Interface Sci. 2009, 147–148, 88–108. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Bouafif, H.; Neculita, C.M.; Koubaa, A. Activated biochar as an effective sorbent for organic and inorganic contaminants in water. Water. Air. Soil Pollut. 2018, 229, 230. [Google Scholar] [CrossRef]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.-M. Biomass-derived porous carbon materials with different dimensions for supercapacitor electrodes: A review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Frackowiak, E.; Béguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Teng, H. Influence of oxygen treatment on electric double-layer capacitance of activated carbon fabrics. Carbon 2002, 40, 667–674. [Google Scholar] [CrossRef]
- Wei, L.; Sevilla, M.; Fuertes, A.B.; Mokaya, R.; Yushin, G. Hydrothermal carbonization of abundant renewable natural organic chemicals for high-performance supercapacitor electrodes. Adv. Energy Mater. 2011, 1, 356–361. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, L.-Z.; Zhou, M.-Q.; Guan, H.; Qiao, S.; Antonietti, M.; Titirici, M.-M. Nitrogen-containing hydrothermal carbons with superior performance in supercapacitors. Adv. Mater. 2010, 22, 5202–5206. [Google Scholar] [CrossRef]
- Si, W.; Zhou, J.; Zhang, S.; Li, S.; Xing, W.; Zhuo, S. Tunable N-doped or dual N, S-doped activated hydrothermal carbons derived from human hair and glucose for supercapacitor applications. Electrochim. Acta 2013, 107, 397–405. [Google Scholar] [CrossRef]
- Zapata-Benabithe, Z.; Diossa, G.; Castro, C.D.; Quintana, G. Activated carbon bio-xerogels as electrodes for supercapacitors applications. Procedia Eng. 2016, 148, 18–24. [Google Scholar] [CrossRef]
- Alvares Rodrigues, L.; Koibuchi Sakane, K.; Alves Nunes Simonetti, E.; Patrocínio Thim, G. Cr total removal in aqueous solution by PHENOTAN AP based tannin gel (TFC). J. Environ. Chem. Eng. 2015, 3, 725–733. [Google Scholar] [CrossRef]
- Kunnambath, P.M.; Thirumalaisamy, S. Characterization and utilization of tannin extract for the selective adsorption of Ni(II) ions from water. J. Chem. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Nakajima, A. Electron spin resonance study on the vanadium adsorption by persimmon tannin gel. Talanta 2002, 57, 537–544. [Google Scholar] [CrossRef]
- Nakajima, A.; Ohe, K.; Baba, Y.; Kijima, T. Mechanism of gold adsorption by persimmon tannin gel. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2003, 19, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Alam, M.N.; Marutani, Y.; Ogata, T.; Morisada, S.; Nakano, Y. Improvement of Pd(II) adsorption performance of condensed-tannin gel by amine modification. Chem. Lett. 2009, 38, 956–957. [Google Scholar] [CrossRef]
- Morisada, S.; Kim, Y.-H.; Ogata, T.; Marutani, Y.; Nakano, Y. Improved adsorption behaviors of amine-modified tannin gel for palladium and platinum ions in acidic chloride solutions. Ind. Eng. Chem. Res. 2011, 50, 1875–1880. [Google Scholar] [CrossRef]
- Ogata, T.; Kim, Y.H.; Nakano, Y. Selective recovery process for gold utilizing a functional gel derived from natural condensed tannin. J. Chem. Eng. Jpn. 2007, 40, 270–274. [Google Scholar] [CrossRef]
- Gurung, M.; Adhikari, B.B.; Kawakita, H.; Ohto, K.; Inoue, K.; Alam, S. Selective recovery of precious metals from acidic leach liquor of circuit boards of spent mobile phones using chemically modified Persimmon tannin gel. Ind. Eng. Chem. Res. 2012, 51, 11901–11913. [Google Scholar] [CrossRef]
- Morisada, S.; Rin, T.; Ogata, T.; Kim, Y.-H.; Nakano, Y. Adsorption removal of boron in aqueous solutions by amine-modified tannin gel. Water Res. 2011, 45, 4028–4034. [Google Scholar] [CrossRef]
- Ogata, T.; Morisada, S.; Oinuma, Y.; Seida, Y.; Nakano, Y. Preparation of adsorbent for phosphate recovery from aqueous solutions based on condensed tannin gel. J. Hazard. Mater. 2011, 192, 698–703. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; González-Velasco, M.; Beltrán-Heredia, J.; Gragera-Carvajal, J.; Salguero-Fernández, J. Novel tannin-based adsorbent in removing cationic dye (Methylene Blue) from aqueous solution. Kinetics and equilibrium studies. J. Hazard. Mater. 2010, 174, 9–16. [Google Scholar] [CrossRef]
- Rahman, M.; Akter, N.; Karim, M.R.; Ahmad, N.; Rahman, M.M.; Siddiquey, I.A.; Bahadur, N.M.; Hasnat, M.A. Optimization, kinetic and thermodynamic studies for removal of Brilliant Red (X-3B) using Tannin gel. J. Environ. Chem. Eng. 2014, 2, 76–83. [Google Scholar] [CrossRef]
- Fayemiwo, O.M.; Daramola, M.O.; Moothi, K. Tannin-based adsorbents from green tea for removal of monoaromatic hydrocarbons in water: Preliminary investigations. Chem. Eng. Commun. 2018, 205, 549–556. [Google Scholar] [CrossRef]



| Precursors | Conditions | Main Findings | Ref. |
|---|---|---|---|
| Hydrogels Prepared Under Normal Conditions Organic Tannin Gels | |||
| Tannin + formaldehyde (TF) | Hydrogels prepared at 85 °C for 120 h; Mass fraction of total solids (MFTS) of 4–40 wt.%; NaOH and p-toluene sulfonic acid were used to change the initial pH of the solution (4.3); pH = 2−10; Supercritical drying in CO2 | Organic aerogels; SBET = 219–880 m2/g; Porosity = 40–97% The highest SBET (880 m2/g) and the highest micropore volume (0.28 cm3/g) were obtained for a gel prepared with MFTS of 10 wt.% and pH 6. The maximum mesopore volume (1.34 cm3/g) and the highest pore volume (21.5 cm3/g) were found for samples with a MFTS of 18 and 4 wt.% at pH 8 and 2, respectively. The transparency of gels was associated with the structure of the aquagel: larger nodules at low MFTS (˂ 18 wt.%) and low pH (especially 2) lead to larger clusters and pore volumes (21.5–4.9 cm3/g), reducing the transparency of the final gel. By increasing the pH (4–10) and MFTS (22–40 wt.%), the nodules were reduced, and the transparency tended to increase. Aerogels with MFTS greater than 22 wt.% with extremely high pH (10) resulted in a low porous material (0.48–1.01 cm3/g). The most diluted samples (4 and 6% of TF resin at pH 6 and 8, respectively) presented the highest shrinkage volumes (~ 87%) during drying step, which was caused by high capillary forces due to their low mechanical properties. These values are comparable to those of aerogels from natural biopolymer, such as cellulose (75 wt.%). The SBET values found for samples with MFTS lower than 20 wt.% were higher than those of any other bioresourced organic aerogel. | [60] |
| Tannin + formaldehyde + Pluronic (TFP) | Hydrogels prepared at 85 °C for 120 h with Pluronic® F-127, using a mass ratio of T/P of 2; NaOH and p-toluene sulfonic acid were used to change the initial pH of the solution (4.9); pH = 2−10; Subcritical drying in two steps: air and 80 °C | Organic xerogels; SBET = ˂ 1.5 m2/g; Porosity = 2–79% The surfactant P was employed as an additive to decrease the surface tension of the aqueous system inner porosity and consequently mitigate the shrinkage during drying step. The system TFP presented a correlation between porosity characteristics (pore volume and centered peak pore size measured through mercury porosimetry) and pH. Thus, pH played a crucial role in pluronic-tannin gels preparation. The xerogels prepared at pH 2–7 presented a bulk density comparable to those of aerogels (0.30–0.58 cm3/g). Such a system demonstrated an interesting and low-cost technique for preparing porous gels with tailored porosity on a large scale. The final xerogels presented unimodal (macroporosity) or bimodal (macro-mesoporosity) porosity. | [61] |
| Tannin + soy + formaldehyde (TSF) | Hydrogels prepared at 85 °C for 120 h; Proportions of T/S resin of 30 and 70 wt.% (on a dry basis), respectively; NaOH in pellets and H3PO4 (85%) were used to change the initial pH of the solution (8.2); pH 5.5−9; Mass ratio of total solids 14 wt.%; Supercritical drying in CO2 | Organic aerogels; SBET = 384–478 m2/g; Porosity = 84–88% The pH of the initial solution had a significant impact on gelation: the lowest gelation time, which happened at pH 6, produced the highest SBET (478 m2/g) and micropore volume (0.15 cm3/g). Curiously, this aerogel also presented the highest mesopore volume (2.29 cm3/g). A correlation between SBET and gelation time was noticed. The TSF aerogels were based on a filamentous structure, which is comparable to cellulosic and lignocellulosic aerogels. Organic aerogels are natural at 91%, and mesopore volumes (1.72–2.29 cm3/g) are among the greatest ever volumes reported for aerogels of natural origin, considering the same density (0.19–0.25 g/cm3). | [54] |
| Tannin + lignin + formaldehyde (TLF) | Hydrogels prepared at 85 °C for 120 h; Mass ratio of T/L of 0.11–1 (on a dry basis); Mass ratio of ((L + T)/F) of 0.83–2.5 (on a dry basis); Initial pH of 10; Supercritical drying in CO2 | Organic aerogels; SBET = 50–550 m2/g; Porosity = 72%–87% The SBET of TLF aerogels was mostly related to mesopores volumes (0.2–1.4 g/cm3), since micropores were extremely low (0.01–0.02 cm3/g). The highest SBET values were found for TLF gels at mass fractions of T/L=1 and (L + T)/F = 1.25, demonstrating that tannin could be replaced by lignin at 50 wt.%. Besides, there is an optimal quantity of formaldehyde that can be used to produce highly porous aerogels. Organic aerogels were synthesized using up to 71% of natural precursor. | [78] |
| Tannin + resorcinol + formaldehyde + sodium dodecyl sulfate (TRFSDS) | Preparation in oven: Hydrogels prepared at 85 °C for 72 h (gelation) and 48 h (curing); Preparation in a microwave: 85 °C for 3 h (gelation and curing) and 1–2 h (drying); NaOH and SDS were used as catalyst and surfactant, respectively; Initial pH of 5.5; SDS amount of 5 wt.%; Mass ratio of (T + R)/F of 1.2; MFTS of 25 wt.%; T was used in different proportions (0–100 wt.%) to replace R | Organic xerogels; Porosity = 20–85% The synthesis of tannin xerogels in a microwave oven allowed a reduction in time of 95%, including the time from sol-gel reaction until complete drying. Resorcinol could be replaced by tannin in the production of organic xerogels. However, the use of surfactant is always required because the structure collapses in the presence of large quantities of tannin (>25 wt.%). Materials with high proportion of tannin (75 wt.%) were produced with very small difference of porosity through conventional and microwave synthesis conditions (77% and 62%, respectively). | [124] |
| Carbon tannin gels | |||
| Tannin microspheres (TM) | Microspheres prepared at 80 °C for 1 h stirred at 200–1200 rpm; Mixture of surfactant (SPAN 80) (0–10%) and sunflower oil; Initial pH of 4.3; MFTS of 40 wt.%; Carbonization: 900 °C for 2 h; Heating rate of 2 °C/min | Carbon xerogels; SBET = 7–666 m2/g Microspheres were prepared by inverse emulsion polymerization in sunflower oil, followed by subcritical drying and carbonization. Higher stirring speed produced microspheres with low average size due to the shear rate that lead particles (microspheres) at smaller sizes. At the same time, smaller microspheres were produced by increasing the surfactant content and promoting greater contact between oil and the aqueous phase. This process formed small drops of resin that became microspheres after gelation. However, there was a limit amount of surfactant and speed stirring to produce small microspheres of 5 wt.% and 500 rpm, respectively. The mean deviation decreased with the increasing speed stirring (with or without surfactant) and the final microspheres presented narrow micropore size distribution centered at 0.4–0.5 nm. | [125] |
| Tannin + formaldehyde (TF) | Hydrogels prepared at 85 °C for 120 h; pH from 3.3 to 8.3; Supercritical drying in acetone; Carbonization: 900 °C for 2 h; Heating rate of 5 °C/min | Carbon aerogels; SBET = 580–720 m2/g; Porosity = 75–99% Carbon aerogels based on tannin-formaldehyde solutions were prepared in a broad pH range. The microstructure of gels presented the typical nodular shape of phenolic aerogels, where their size was determined by the initial pH of the solution. The polymerization reaction was performed with methylene or methylol bridges, as showed by 13C NMR analysis. The final material presented high mesopore fraction (57–78%). The synthesized TF aerogels were five times cheaper than resorcinol-formaldehyde aerogels. | [77] |
| Tannin + resorcinol + formaldehyde (TRF) | Hydrogels prepared at 50 °C for 10–160 min (depending on gelation time); Acetic acid or orthophosphoric acid were used to change the initial pH of the solution (8.15); pH = 2−8; Freeze-drying; Carbonization: 900 °C for 2 h; Heating rate of 5 °C/min | Carbon cryogels; SBET = 30–650 m2/g; Porosity = 32–80% Low cost cryogels were produced by replacing two third of resorcinol by tannin, which resulted in a reduction of the final cost of the gel by a factor of 2. Also, freeze-drying represents a low-cost drying method compared to supercritical drying. The SBET and porosity decreased by increasing pH. Furthermore, SBET values were comparable to resorcinol-formaldehyde cryogels and phenol-formaldehyde aerogels, which are gel materials based on more expensive precursors. | [53] |
| Tannin + formaldehyde (TF) | Hydrogels prepared at 85 °C for 120 h; Acetic acid or NaOH were used to change the initial pH of the solution (4.3); pH = 3.3−7.3; Freeze-drying; Carbonization: 900 °C for 2 h; Heating rate of 5 °C/min | Carbon cryogels; SBET = 399–1420 m2/g; Porosity = 94–96% Carbon cryogels presented high pore volumes, mostly distributed in macro- and micropores sizes. These materials also presented very low bulk densities (0.078–0.101 g/cm3), mostly composed by macropores (0.13–0.55 cm3/g). Materials prepared at pH higher than 6 presented higher SBET (1228–1420 m2/g) and higher micropore volumes. The electrochemical performances of those materials were tested, and the main results are presented in Table 3. | [126] |
| Tannin + pluronic + formaldehyde (TPF) | Hydrogels prepared at 85 °C for 120 h; pH from 2 to 10; Subcritical drying in two steps: air and 80 °C; Mass ratio of T/P of 0.5–2; Carbonization: 900 °C for 2 h; Heating rate of 5 °C/min | Carbon xerogels; SBET =5–877 m2/g; Porosity = 46%–86% The use of pluronic allowed the synthesis of the first carbon tannin based xerogels presenting density values in a range of 0.28–0.33 g/cm3, which are comparable to those of carbon aerogels derived from expensive precursors. Those densities are related to samples prepared at pH lower than 7 and low amounts of pluronic (Mass ratio of T/P of 2). Narrow bimodal macro-microporous or meso-microporous xerogels were produced as a function of pH and surfactant. For lower pluronic concentrations, the micropore size distribution remained centered at 0.5 nm, while the macroporosity was shifted as a function of both pluronic and pH. By increasing the amount of pluronic and keeping the same pH, the porosity changed from a macro-microporous structure to a meso-microporous structure. The final structure was highly porous with narrow non-ordered porosity. | [98] |
| Tannin (T) (autocondensation) | Tannin was added to solutions of Na2SiO3 (35 wt.%); Mass ratio of SiO2/T of 0.3–1.04; NaOH (2 mol/L) or HF (40 wt. %) were used as etching agents; Curing and ageing were performed at ambient temperature for 3 h; Supercritical drying in CO2; Carbonization: 900 °C for 2 h; Heating rate of 5 °C/min | Carbon aerogels; SBET NaOH = 451–709 m2/g; SBET HF= 542–783 m2/g; Porosity = 67%–82% Porous carbon gels were prepared with no formaldehyde addition. Na2SiO3 was used as catalyst for auto-condensation and as template for porosity formation. The final porous properties were controlled by silica/tannin ratio, while NaOH or HF were used as etching agents. XPS and Raman analyses showed the non-ordered aromatic structure of tannin-based carbon aerogels. Carbon aerogels washed with HF solutions presented better porosity (71%–82%) compared to NaOH (67%–75%). Samples prepared at low SiO2/T mass ratio (0.30–0.75) had no significant differences. However, the highest SiO2/T mass ratio (1.04) presented the lowest density, and consequently the highest porosity. By increasing the quantity of silica in the reactional medium, there was a decrease in the microporosity associated to SBET, whereas the highest values were found for SiO2/T = 0.45. Mesopores were better developed at SiO2/T mass fraction of 0.75 (1.71 cm3/g for samples etched with HF). | [127] |
| Tannin + sodium dodecyl sulfate + formaldehyde (TSDSF) | Hydrogels prepared at 85 °C for 72 h; NaOH and sodium dodecyl sulfate (SDS) were used as catalyst and surfactant, respectively; Initial pH of 5.5, SDS amount of 5–20 wt.% and T/F mass ratio of 0.6–2.6; MFTS of 25 wt.%; Supercritical drying in CO2; Carbonization: 900 °C for 2 h; Heating rate of 5 °C/min | Carbon xerogels; Porosity = 25%–78% There was an optimal amount of surfactant (10 wt.%) for producing highly porous carbon xerogels (density of 0.34 g/cm3 and porosity of 78%). However, when using 10 wt.% of surfactant and increasing the T/F mass ratio, the pore volumes increased, and the average pore size decreased. Also, the concentration of formaldehyde was important for attaining materials with greater porosity, since an ideal proportion of crosslinker promotes the creation of links between surrounding clusters. | [97] |
| Tannin + formaldehyde (TF) | Hydrogels prepared at 85 °C for 120 h; pH of 2, 5 and 8; Activation with NaOH or KOH; Mass ratio of NaOH/gel of 0.045, 0.181 and 0.724 (on a dry basis); Mass ratio of KOH/gel of 0.063, 0.253 and 1.013 (on a dry basis); Activation: 750 °C for 1 h; Heating rate of 5 °C/min | Activated carbon xerogels (ACX); SBET = 50–1810 m2/g The exchange step was performed with alkali solutions at different concentrations. Higher concentrations of alkali (NaOH or KOH) induced the development of surface area. In addition, the microstructure of ACX changed from nodular to microcellular carbon foam and, the effect of the pH was not meaningful in these different structures. A smaller dispersion of particle sizes and high surface areas related to micropores volumes were obtained using smaller mass ratio of alkalis (0.045–1.013), compared to usual values in the literature (>1). Probably, the soft structure of gels allowed the alkali solution to interpenetrate in its three-dimensional structure, causing greater alkali diffusion and favoring the formation of higher porosity. | [118] |
| Precursors | Conditions | Main Findings | Ref. |
|---|---|---|---|
| Hydrogels Prepared Under Hydrothermal Conditions Organic and Carbon Hydrothermal Tannin Gels | |||
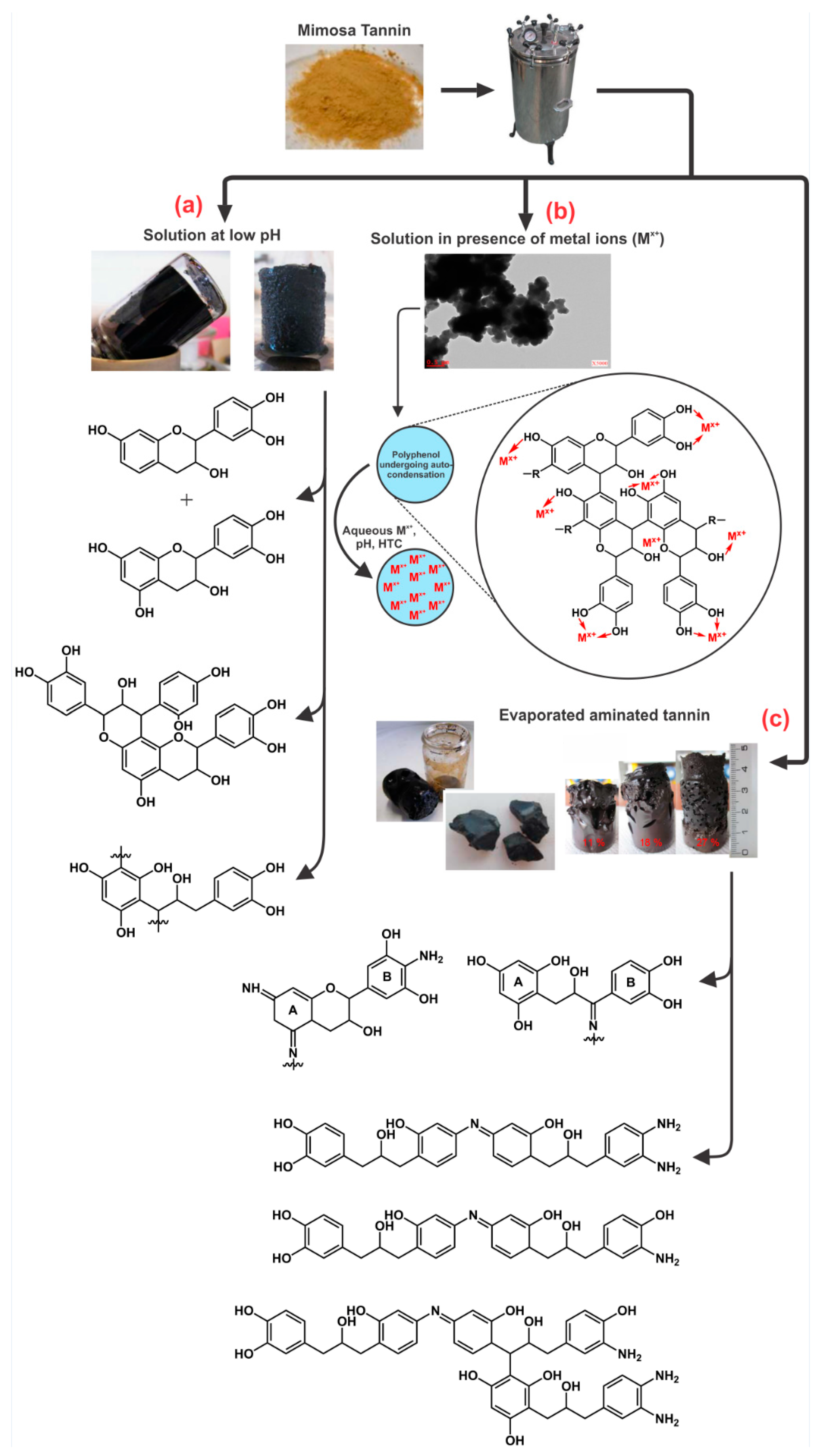
| Evaporated aminated tannin (EAT) | Hydrothermal gel: Amination at room temperature followed by evaporation and HTC of the solid material in water at 180 °C for 24 h; Subcritical drying in two steps: air and 80 °C; Carbonization: 900 °C for 3 h; Heating rate at 1 °C/min. | Xerogel: SBET = 32 m2/g This amination process under HTC conditions lead to a gel without any crosslinker agent. The mechanism was based on autocondensation and partly dehydrated tannin mainly through the heterocycle opening. Materials also had high percentage of nitrogen (up to 3.4%). Carbon xerogel: SBET = 500 m2/g The synthesized carbon gel had a porous structure comprised of a mixture of wider microporosity and mesoporosity (Isotherms Type I and IV). Materials had high percentage of nitrogen (up to 2.9%) and a micropore/total pore volume ratio of 72%. XPS analysis showed several forms of nitrogen such as pyridinic (N-6) and neutral amines in the hydrothermal xerogel, whereas pyridic (N-6), pyrrolic (N-5) and oxidized (N-X) (stable forms of nitrogen) were found in its carbon form. | [105] |
| Evaporated aminated tannin (EAT) | Hydrothermal gel: Amination at room temperature followed by evaporation and HTC of the solid material in water at 180–220 °C for 24 h; Subcritical drying in two steps: air and 80 °C; Carbonization: 900 °C for 3 h; Heating rate at 1 °C/min. | Xerogel: SBET = 64−102 m2/g A monolithic gel was obtained under HTC at temperatures from 180 to 220 °C. Not many differences in relation to the physicochemical properties were observed in this range of temperature. These gels were nitrogen rich materials (up to 4.3%). Carbon xerogel: SBET = 311−552 m2/g Higher HTC temperatures lead to lower surface areas (~ 300 m2/g) due to the formation of bigger and less porous spherical nodules. After carbonization, materials still had a high percentage of nitrogen (up to 2.9%). N-doped materials were successfully synthesized for electrochemical devices. These results were presented and discussed in Table 3. | [109] |
| Evaporated aminated tannin (EAT) | Hydrothermal gel: Amination at room temperature followed by evaporation and HTC of the solid material at mass fractions of 11, 18 and 27% in water at 180 °C for 24 h. The final gels were first soaked in ethanol for 3 days, and then subcritically (air and 80 °C), supercritically (CO2), and freeze-dried; Carbonization: 900 °C for 3 h; Heating rate at 1 °C/min. | Xerogel, aerogel and cryogel: SBET = 102−295 m2/g For the most diluted gels (11 wt.%), the porosity improved in this sequence: xerogel, cryogel and aerogel. For the least diluted gels, similar surface areas were obtained at any kind of drying method. The mechanism of gel formation was described in Section 2.4. Carbon xerogel, aerogel and cryogel: SBET = 496−860 m2/g Unlike organic gels, carbon gels prepared with the highest mass fraction (27 wt.%) had the highest surface area, up to 900 m2/g. It was then noticed that the most porous organic gels do not lead to carbon gels with the highest surface areas. | [111] |
| Tannin (T) | Hydrothermal gel: Tannin aqueous solution under HTC conditions at 180 °C for 24 h; pTSA (para-toluenosulfonic acid) was used to change the pH from 4.2 to 1; Subcritical drying in two steps: air and 80 °C; Carbonization: 900 °C for 3 h; Heating rate at 1 °C/min. | Xerogel: SBET = 1.25 m2/g Monolith material was obtained by reducing pH, resulting in a positive impact on the hydrochar yield. However, a very low surface area was obtained. Carbon xerogel: SBET = 796 m2/g Monolithic materials having spherical nodules at different diameters were obtained: 2.9 µm at pH 2; and 4.6 µm at pH 4.2. This trend was different from phenolic gels prepared with tannin-formaldehyde. The surface area of 796 m2/g was attained for the material prepared at lowest pH. Its higher microsphere nodule diameter might have promoted the gasification of low molecular weight gases, which enhanced the development of their surface area. | [106] |
| Evaporated aminated (pine) tannin (EAT) | Hydrothermal gel: Amination at room temperature followed by evaporation and HTC of the solid material in water at 180 °C for 24 h; Subcritical drying in two steps: air and 80 °C; Carbonization: 900 °C for 3 h; Heating rate at 1 °C/min. | Xerogel: Compacted monoliths having homogeneous spherical particles typical of gels. Carbon xerogel: SBET = 485 m2/g Micro-mesoporous carbon gels exhibited high surface area with high nitrogen and oxygen functionalized groups, which were applied as electrodes for electrochemical devices. These results were described in Table 3. | [128] |
| Bayberry tannin and graphene oxide (GO) | Hydrothermal gel: A mixture of graphene oxide and tannin at different mass ratios (1:0–1.5); Sonication for 30 min and HTC at 120 °C for 12 h.; Gels were soaked in HNO3 solution for 4 days; Freeze-drying. | Hydrothermal cryogel: SBET = 75 m2/g The monolith had a three-dimensional structure, consisting of crosslinked GO sheets, GO sheets, and tannin. The gels showed a type III isotherm characteristic of mesoporous materials, having surface area up to 75 m2/g. Its application as adsorbent to remove Sr2+ from water is described in Table 3 | [112] |
| Application | Type of Gel | Testing Conditions | Main Findings | References |
|---|---|---|---|---|
| Organic tannin gels | ||||
| Thermal insulator | Aerogel | Thermal conductivity of soy-tannin-formaldehyde in a cylindrical shape was performed at room temperature | Tannin gel conductivity is higher than that of air (0.033 W/mK compared to 0.026 W/mK). The presence of narrow mesopores and fewer macropores would be required to improve their performance as thermal insulators. | [54] |
| Thermal insulator | Aerogel | Thermal conductivity of tannin-formaldehyde in a cylindrical shape was performed at room temperature | Tannin gel conductivity is close to that of air (0.027 W/mK). Low thermal conductivity was reported for gels with low density and very narrow pores. | [60] |
| Adsorbent for water treatment | ||||
| Metal Pb2+ | Tannin-gel | Synthetic effluent 1000 mg/L, batch tests; ratio metal: adsorbent 0.1 g: 100 mL; Equilibrium time 5 h, pH = 1−7, T = 20 °C | Tannin-gel behavior as ionic exchanger: two Na+ ions exchanged by one Pb2+ ion. Pb removal efficiency increased from 58 to 115 mg Pb/g with increased pH from 3 to 4.2, respectively. | [84] |
| Metal Cr6+ | Tannin-gel | Synthetic effluent 1000 mg/L, batch tests; ratio metal: adsorbent 2.5 g:100 mL; Equilibrium time 30 min, pH = 0.85–5, T = 30 °C | Adsorption mechanism consists of four steps: 1) Esterification of chromate with tannin molecules; 2) Reduction of Cr6+ into Cr3+; 3) Formation of carboxyl groups by tannin oxidation; and 4) Ion exchange of Cr3+ with carboxyl and hydroxyl groups. Maximum adsorption capacity: 287 mgCr/g at pH 2. | [83] |
| Metal Cr6+ | Tannin-gel | Synthetic effluent 500–5000 mg/L, batch tests; ratio metal: adsorbent 0.2 g:100 mL; Equilibrium time 8 h, pH = 1–12, T = 25–45 °C | Cr adsorption reached the maximum value of 488 mg/g at pH 1 (25 °C). The mechanism of Cr adsorption is based on: 1) Cr(IV) adsorption by phenolic groups through chromate esterification with tannin-gel surface; 2) Cr(VI) reduction to Cr(III); 3) Carboxylate group formation due to tannin-gel oxidation; 4) Cr(III) retention on tannin-gel surface; and finally 5) Cr adsorption through hydroxyl and carboxyl groups. | [138] |
| Metal Cu2+ | Tannin-gel | Synthetic effluent 10−150 mg/L, batch tests; ratio metal: adsorbent 0.1 g:100 mL; Equilibrium time 3 h, pH = 2−5, T = 25 °C | Adsorption mechanism is a result of ion exchange or complexation between Cu2+ ions and phenolic groups present on tannin-gel surface. Adsorption decreases at lower pH due to ion exchange equilibrium. Maximum adsorption capacity: 44 mgCu/g at pH 5. | [87] |
| Metal Zn2+ | Tannin-gel | Synthetic effluent, batch tests; Equilibrium time 2 weeks, pH = 7, T = 20 °C | Tannin-gel made from lab-extracted Pine tannin presented the best performance for Zn removal. Maximum adsorption capacity: 65 mgZn/g | [88] |
| Metal Ni2+ | Tannin-gel | Synthetic and real effluent (synthetic and river water) 50−200 mg/L, batch tests; ratio metal: adsorbent 0.25−1 g: 100 mL; Equilibrium time 35 min, pH = 2–7, T = 23 °C | The adsorption of Ni ions took place in a homogeneous tannin gel surface (monolayer adsorption). Maximum adsorption capacity: 250 mgNi/g at pH 5 | [139] |
| Metal Sr2+ | Hydrothermal cryogel | Synthetic effluent 10−150 mg/L, batch tests; ratio metal: adsorbent 0.02 g:100 mL; Equilibrium time 10 h, pH = 9, T = 25 °C | Graphene oxide-tannin gel prepared under HTC conditions showed excellent adsorption performance for the removal of Sr2+ (68 mgSr/g). Surface chemical analysis showed that Sr2+ was largely dependent on oxygen functional groups, pH, salinity and ionic strength. | [112] |
| Rare metal V | Tannin-gel | Synthetic effluent 0.2 mM, batch tests; ratio metal: adsorbent 0.02 g:100 mL; Equilibrium time 1 h, pH = 1–8, T = 30 °C | V was efficiently adsorbed from different solutions: VOCl2 and NH4VO3. Stable compounds were formed between VO2+ (acid character) and catechol and pyrogallol (alkali behavior). V adsorption from NH4VO3 was based on adsorption of H3VO4 (pH = 3.75) and reduction of VO2+ to VO3- at pH 6. | [140] |
| Rare metal Au | Tannin-gel | Synthetic effluent 10 mg/L, batch tests; ratio metal: adsorbent 0.01 g:100 mL; pH = 2−3.8, T = 20 °C | Adsorption of Au took place through the reduction of trivalent Au ions into metallic Au as well as oxidation of hydroxyl groups present in tannin-gel to carbonyl groups. Maximum adsorption capacity: 8000 mgAu/g | [85] |
| Rare metal Au | Tannin-gel | Synthetic effluent 0.1 mM, column tests; Flow bed at 150 and 300 mL/h; pH = 2–6 | 98.5% of Au from HAuCl4 was adsorbed at pH 6. The mechanism of Au adsorption was based on: 1) Ligand exchange: AuCl4- with hydroxylphenyl groups present in the tannin-gel; 2) Reduction of Au(III) to Au(0); and 3) Adsorption of Au(0). | [141] |
| Rare metal Pd(II) | Tannin-gel | Synthetic effluent 10 mg/L, batch tests; ratio metal: adsorbent 0.04 g:100 mL; pH = 1.3−2.5, T = 25 °C | Adsorption of Pd(II) was based on the inner sphere redox reaction: Pd(II) ions were adsorbed as metallic Pd; hydroxyl groups were oxidized; and a ligand-substitute Pd(II) tannin inner sphere complex was formed. | [86] |
| Rare metal Pd(II) | Aminated tannin-gel freeze-dried | Synthetic effluent 100 mg/L, batch tests; ratio metal: adsorbent 0.1 g:100 mL; Acidic medium, T = 25 °C | Adsorption of Pd(II) was due to metal ions complexation and/or electrostatic interaction. Also, acidic metal ions had high affinity towards amine basic groups. Maximum adsorption capacity: 80 mgPd/g. | [142] |
| Rare metals Pd and Pt | Aminated tannin-gel freeze-dried | Synthetic effluent 0.001 M, batch tests, single and multiple metal solutions; ratio metal: adsorbent 0.1 g:100 mL; Equilibrium time 20 h, pH = 0–5, T = 25 °C | The adsorption of Pd and Pt on tannin-gel surface increased with increasing pH and temperature, and with decreasing chloride ion concentration. The amino groups presented in tannin-gel formed stable complex with metal ions but the adsorbability of Pd(II) was much higher than Pt(IV). Interesting that aminated tannin gel adsorbed mostly Pd(II) from mixed solutions even though it had good adsorbability for Pt(IV) from single metal solution | [143] |
| Rare metals Au, Pd, Pt and Rh | Tannin-gel | Synthetic effluent 1 mmol/L, batch tests, single and multiple solutions; ratio metal: adsorbent 0.1 g:100 mL; pH = 1, T = 25 °C | The predominant species of each metal were adsorbed by controlling the pH (equal to 1) as well as the redox potential differences between metals and tannin-gel. Au was selectively adsorbed and reduced because its redox potential was higher than that of tannin-gel. However, the other precious metals had much lower redox potential than that of tannin-gel. | [144] |
| Metals: Au(III), Pd(II), Pt(IV), Cu(II), Fe(III), Ni(II), Zn(II) | Tannin-gel | Synthetic and real effluent, batch and column tests, single and multiple metal solutions; ratio metal: adsorbent 0.1–2 g:100 mL; Flow bed at 5 mL/h; Equilibrium time 12 h, acidic pH, T = 30 °C | Rare metals were efficiently adsorbed through both batch and column tests. Tannin-gel selectively adsorbed Au(III), Pd(II) and Pt(IV) over other metals: Cu(II), Fe(III), Ni(II) and Zn(II). The mechanism of adsorption of precious metals was the combination of ion exchange, electrostatic interaction and coordination with thiocarbonyl group. Au(III) was reduced to elemental Au through abundant polyphenolic groups on tannin molecule. Tannin-gel was regenerated under acid solution up to five times. | [145] |
| Boron (B) | Aminated tannin-gel freeze-dried | Synthetic effluent 200 mg/L, batch tests; ratio contaminant: adsorbent 0.5 g:100 mL; Equilibrium time 20 h, pH = 8.8, T = 30 °C | Aminated and non-aminated gels efficiently adsorbed B at pH > 7. The adsorption of B took place through the chelate formation of tetrahydroxyborate ion and the hydroxyl and amino groups presented in tannin-gels. The adsorption capacity of the aminated cryogel was higher than that of non-aminated one due to the stable bonds between boron and nitrogen from amino groups. | [146] |
| Phosphate (P) | Tannin-gel freeze-dried | Synthetic effluent 100 mg/L, batch tests; ratio contaminant: adsorbent 0.5 g:100 mL; pH = 2−12, T = 25 °C | The gel impregnated with iron and oxidized with nitric acid showed adsorption selectivity for phosphate. The adsorption process was independent of the pH (from 3 to 12). Maximum adsorption capacity: 31 mgP/gFe | [147] |
| Organic MB | Tannin-gel | Synthetic effluent, batch tests; Equilibrium time 2 weeks, pH = 7, T = 20 °C | Tannin-gel made from lab-extracted Pine tannin presented the best performance for MB removal. Maximum adsorption capacity: 432 mgMB/g | [88] |
| Organic MB | Tannin-gel | Synthetic effluent 1000 mg/L, batch tests; Equilibrium time 15 days; pH = 4−10, T = 20 °C | Adsorption of MB was improved by increasing pH, probably because the dye appeared with a higher cationic degree and thus, it enhanced electrostatic interactions. Maximum adsorption capacity: 483 mgMB/g | [148] |
| Organic BR | Tannin-gel | Synthetic effluent 40 mg/L, batch tests; ratio contaminant: adsorbent 0.04 g:100 mL; Equilibrium time 1.5 h, pH = 2–8, T = 28 °C | Good adsorption of BR due to the presence of functional groups on tannin-gel structure: phenolic, carboxylic, alcoholic, ether and aromatic rings. Maximum adsorption capacity: 45 mgBR/g | [149] |
| Organic CTAB | Tannin-gel | Synthetic effluent, batch tests; Equilibrium time 2 weeks, pH = 7, T = 20 °C | Tannin-gel made from lab-extracted Pine tannin presented the best performance for CTAB removal. Maximum adsorption capacity: 773 mgCTAB/g | [88] |
| Benzene and toluene | Tannin-gel | Synthetic effluents 1% sol., batch tests; Equilibrium time 1 h; ratio contaminant: adsorbent 0.1 g:100 mL; pH = 2–8.6, T = 60 °C | The removal of toluene was more effective than benzene probably because of the interactions between the methyl groups on toluene and the OH groups on tannin gel. Results show up to 99% removal of toluene and benzene after 30 min batch tests. | [150] |
| Carbon tannin gels | ||||
| Thermal insulator | Carbon xerogel | Thermal conductivity of tannin-formaldehyde-surfactant in a cylindrical shape was performed at room temperature | Carbon tannin gel conductivity is higher than that of air (0.039 W/mK compared to 0.026 W/mK). The presence of narrow mesopores would be required to improve its performance as thermal insulator. | [97] |
| Electrochemistry | ||||
| Supercapacitor | Carbon cryogel | Supercapacitor device based on a three-electrode cell configuration with an aqueous acid electrolyte (4 M H2SO4) | Carbon cryogels prepared at pH higher than 6 had low density and high surface areas (up to 1200 m2/g). Thus, such materials as electrodes for supercapacitor reached capacitances as high as 100 F/g (scan rate 2 mV/s). In addition to mesopores, ultra- and supermicropores played an important role on their performance as electrodes for supercapacitor. | [126] |
| Supercapacitor | Hydrothermal carbon aero-, cryo- and xerogel | Supercapacitor device based on a three-electrode cell configuration with an aqueous acid electrolyte (4 M H2SO4) | Carbon aerogel, cryogel, and xerogel prepared from HTC of evaporated aminated tannin (MFTS of 27 wt.%) reached surfaces areas of 860, 754, and 585 m2/g and specific capacitances of 362, 387, and 330 F/g (scan rate 2 mV/s), respectively. The presence of nitrogen (2−3 wt.%) and oxygen (17−18 wt.%) functional groups played an important role on their performance for electrical energy storage, especially through pseudo-capacitance. However, mesostructuration within 3−13 nm should be created to improve the capacitance reduction at a higher scan rate. | [111] |
| Supercapacitor | Hydrothermal carbon xerogel | Supercapacitor device based on a three-electrode cell configuration with an aqueous acid electrolyte (1 M H2SO4) | Hydrothermal carbon xerogel made from evaporated aminated Pine tannin reached a surface area and a specific capacitance of 485 m2/g and 253 F/g (scan rate 0.5 mV/s), respectively. The material presented high concentration of nitrogen and oxygen functional groups (6 mmol/g) that played an important role on their performance as electrodes for a supercapacitor. | [128] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braghiroli, F.L.; Amaral-Labat, G.; Boss, A.F.N.; Lacoste, C.; Pizzi, A. Tannin Gels and Their Carbon Derivatives: A Review. Biomolecules 2019, 9, 587. https://doi.org/10.3390/biom9100587
Braghiroli FL, Amaral-Labat G, Boss AFN, Lacoste C, Pizzi A. Tannin Gels and Their Carbon Derivatives: A Review. Biomolecules. 2019; 9(10):587. https://doi.org/10.3390/biom9100587
Chicago/Turabian StyleBraghiroli, Flavia Lega, Gisele Amaral-Labat, Alan Fernando Ney Boss, Clément Lacoste, and Antonio Pizzi. 2019. "Tannin Gels and Their Carbon Derivatives: A Review" Biomolecules 9, no. 10: 587. https://doi.org/10.3390/biom9100587
APA StyleBraghiroli, F. L., Amaral-Labat, G., Boss, A. F. N., Lacoste, C., & Pizzi, A. (2019). Tannin Gels and Their Carbon Derivatives: A Review. Biomolecules, 9(10), 587. https://doi.org/10.3390/biom9100587







