Electrochemical Sensing Fabricated with Ta2O5 Nanoparticle-Electrochemically Reduced Graphene Oxide Nanocomposite for the Detection of Oxytetracycline
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Solutions
2.2. Equipment and Apparatus
2.3. Ta2O5 Nanoparticle Preparation
2.4. Preparation of Ta2O5-GO
2.5. Electrode Fabrication
2.6. Real Sample Pretreatment
2.7. Electrochemical Measurements
3. Results and Discussion
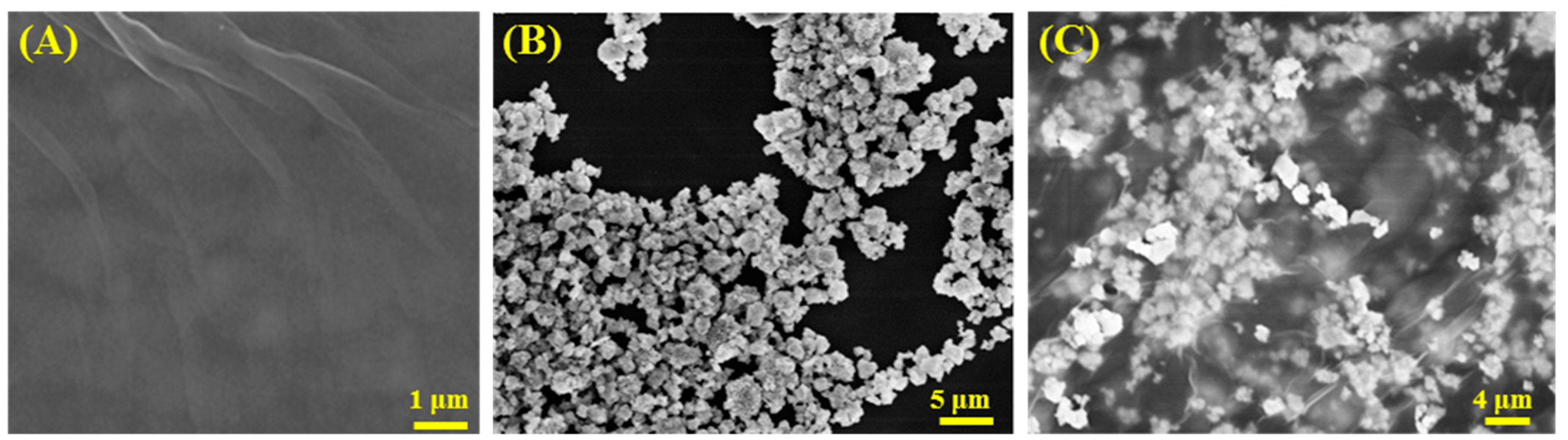
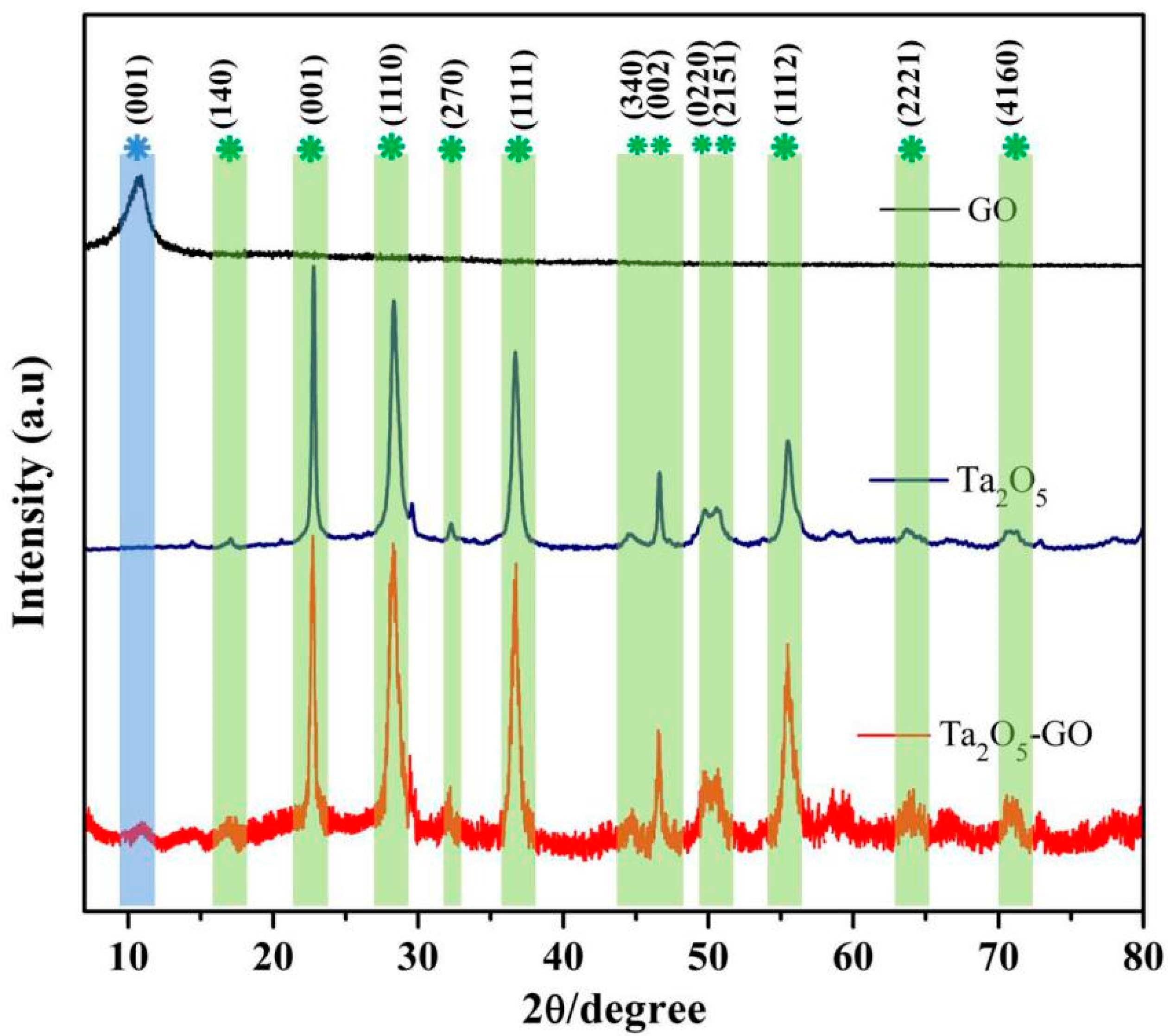
3.1. Materials Characterization
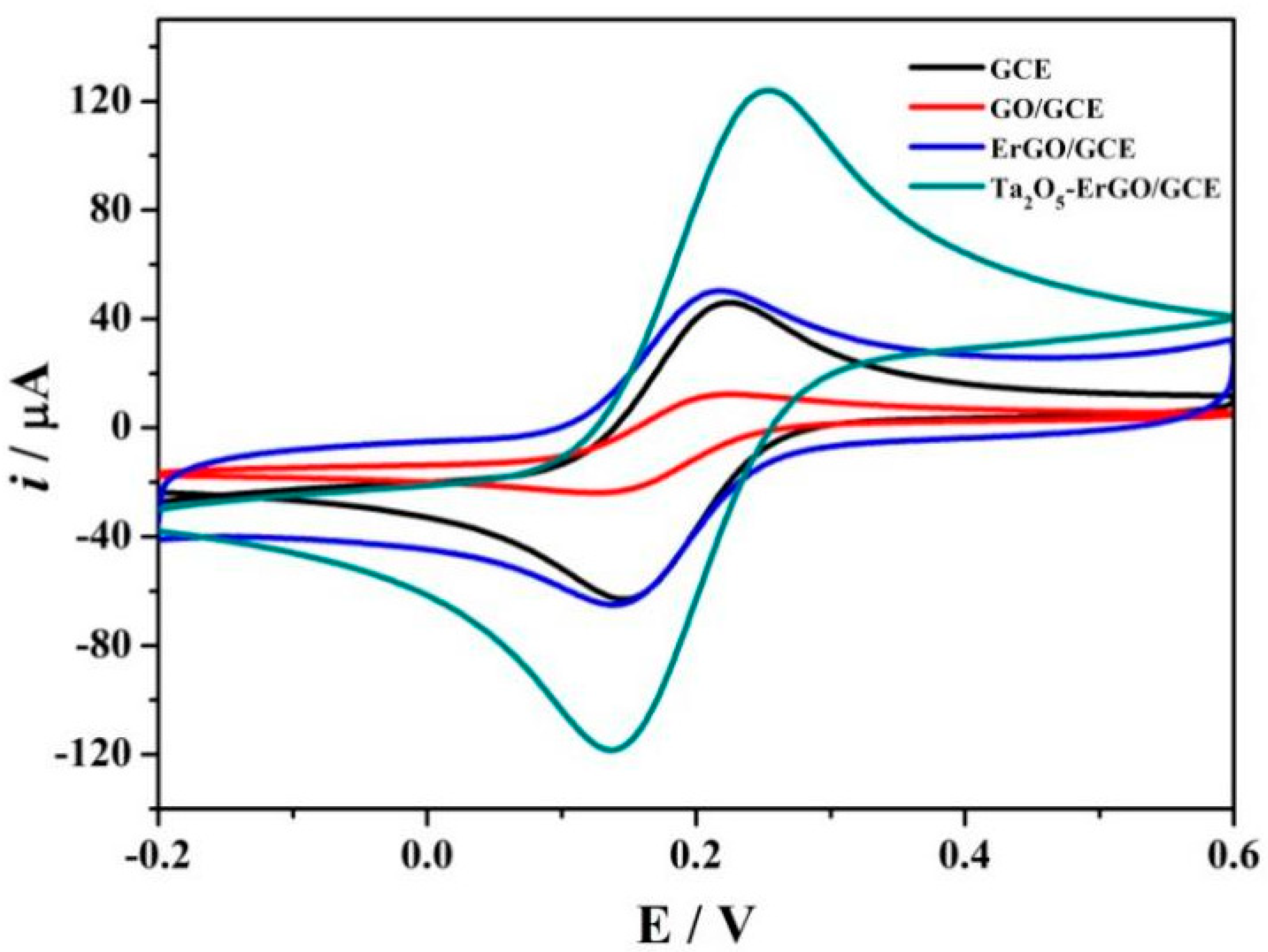
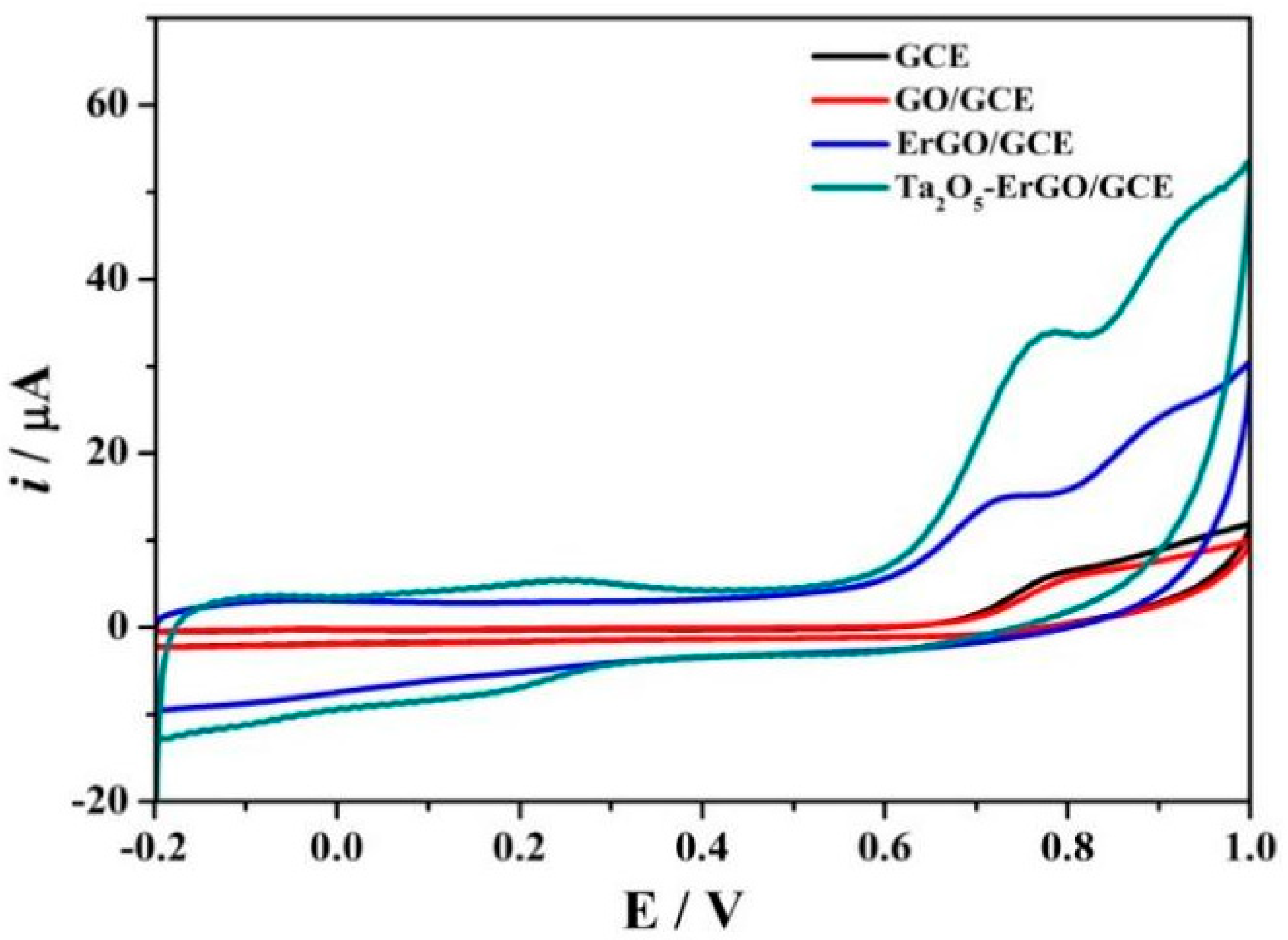
3.2. Electrochemical Characterization of the Modified Electrodes
3.3. Electrochemical Behavior of Oxytetracyclineon Various Electrodes
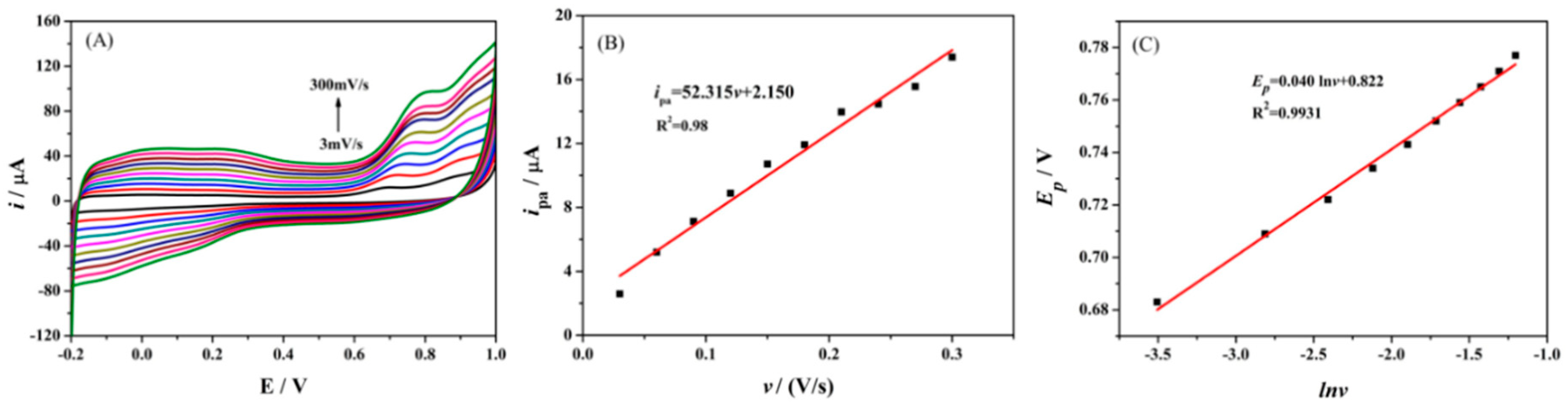
3.4. Electrochemical Kinetics of Oxytetracycline on Ta2O5-ErGO/GCE
3.5. Optimization of Determination Parameters
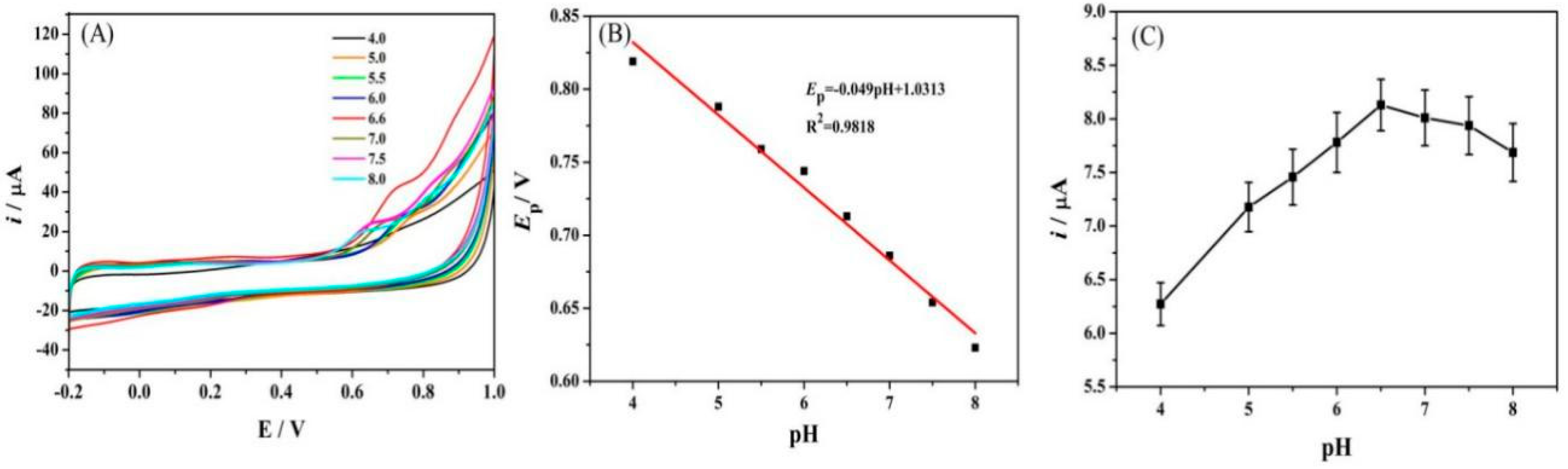
3.5.1. Effect of pH
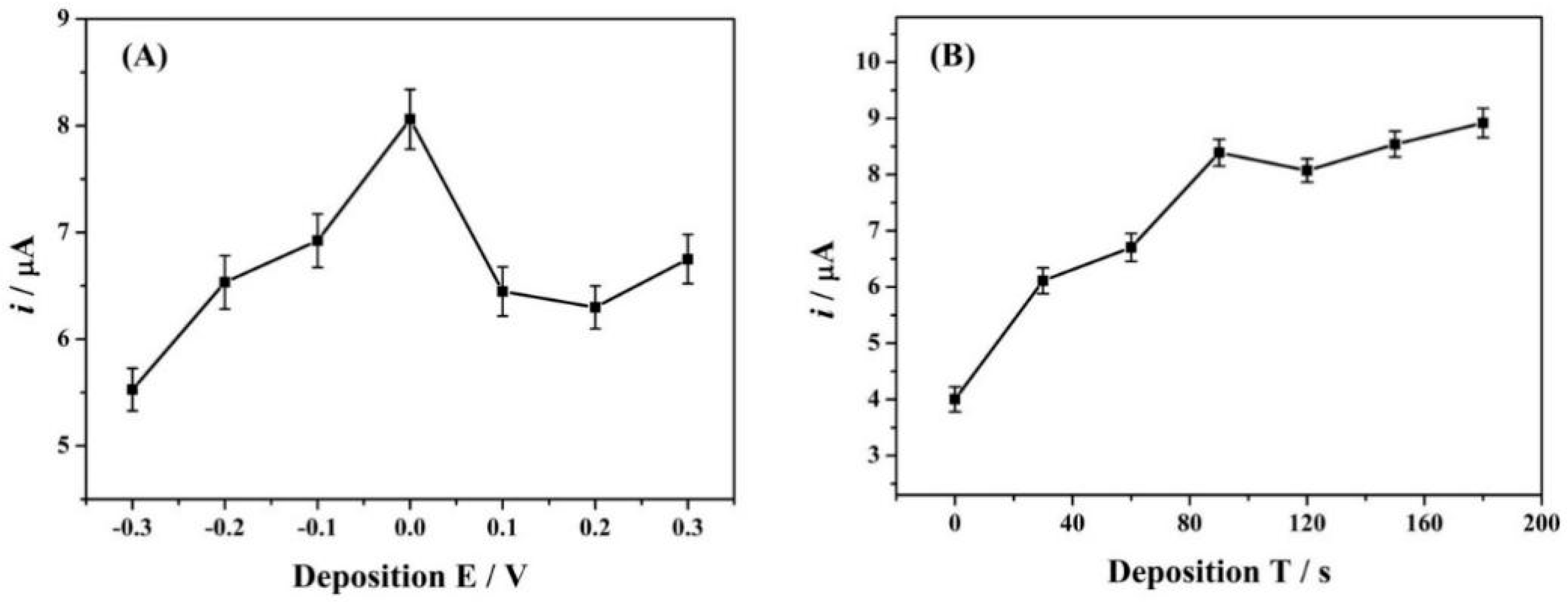
3.5.2. Effect of Deposition Parameters
3.6. Selectivity Reproducibility and Stability Investigation
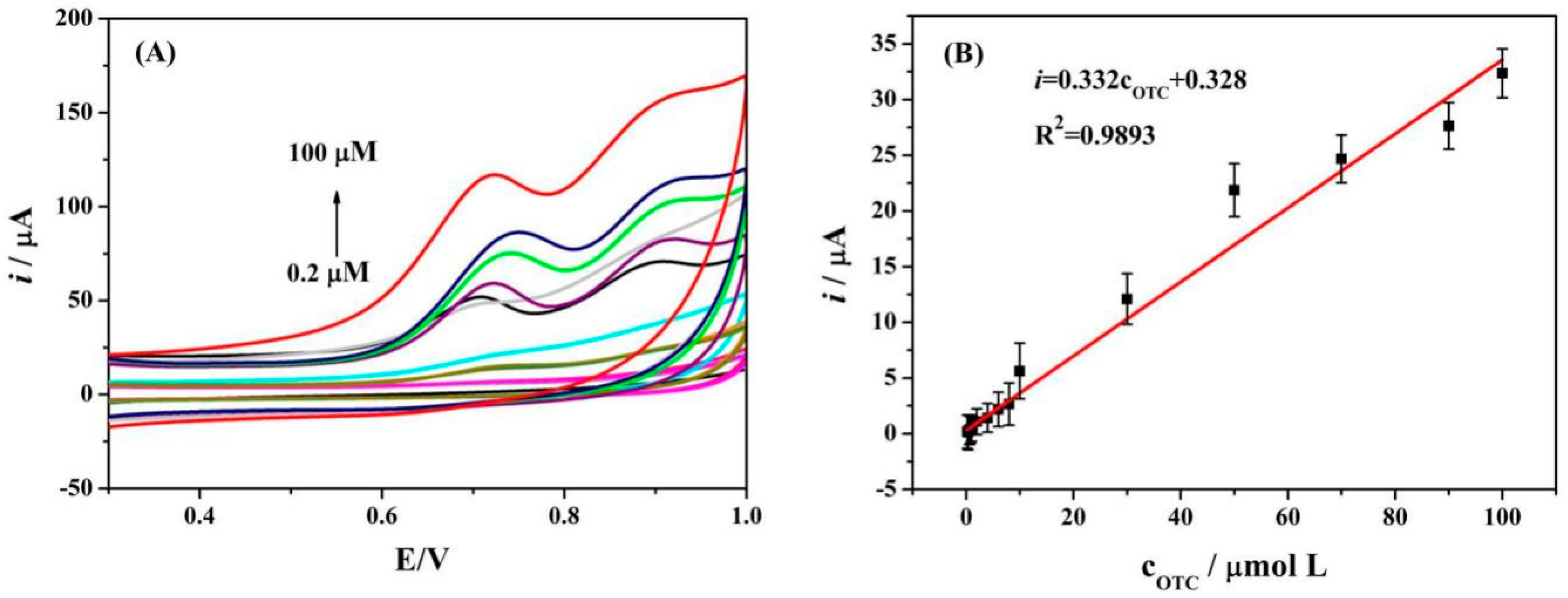
3.7. Calibration Curve of Oxytetracycline
3.8. Detection of Oxytetracycline in Real Sample
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lv, Y.; Wang, L.; Yang, L.; Zhao, C.; Sun, H. Synthesis and application of molecularly imprinted poly (methacrylic acid)–silica hybrid composite material for selective solid-phase extraction and high-performance liquid chromatography determination of oxytetracycline residues in milk. J. Chromatogr. A 2012, 1227, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, C.; Yan, X. Zeolite imidazolate framework-8 as sorbent for on-line solid-phase extraction coupled with high-performance liquid chromatography for the determination of tetracyclines in water and milk samples. J. Chromatogr. A 2013, 1304, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Guarddon, M.; Miranda, J.; Rodríguez, J.; Vázquez, B.; Cepeda, A.; Franco, C. Real-time polymerase chain reaction for the quantitative detection of teta and tetb bacterial tetracycline resistance genes in food. Int. J. Food Microbiol. 2011, 146, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Shaojun, J.; Zheng, S.; Daqiang, Y.; Lianhong, W.; Liangyan, C. Aqueous oxytetracycline degradation and the toxicity change of degradation compounds in photoirradiation process. J. Environ. Sci. 2008, 20, 806–813. [Google Scholar]
- Zhu, W.; Yang, J.; Wang, Z.; Wang, C.; Liu, Y.; Zhang, L. Rapid determination of 88 veterinary drug residues in milk using automated turborflow online clean-up mode coupled to liquid chromatography-tandem mass spectrometry. Talanta 2016, 148, 401–411. [Google Scholar] [CrossRef]
- Aga, D.S.; Goldfish, R.; Kulshrestha, P. Application of Elisa in determining the fate of tetracyclines in land-applied livestock wastes. Analyst 2003, 128, 658–662. [Google Scholar] [CrossRef]
- Li, Y.; Qu, L.; Li, D.; Song, Q.; Fathi, F.; Long, Y. Rapid and sensitive in-situ detection of polar antibiotics in water using a disposable ag–graphene sensor based on electrophoretic preconcentration and surface-enhanced Raman spectroscopy. Biosens. Bioelectron. 2013, 43, 94–100. [Google Scholar] [CrossRef]
- Sui, C.; Zhou, Y.; Wang, M.; Yin, H.; Wang, P.; Ai, S. Aptamer-based photoelectrochemical biosensor for antibiotic detection using ferrocene modified DNA as both aptamer and electron donor. Sens. Actuators B Chem. 2018, 266, 514–521. [Google Scholar] [CrossRef]
- Yan, K.; Liu, Y.; Yang, Y.; Zhang, J. A cathodic “signal-off” photoelectrochemical aptasensor for ultrasensitive and selective detection of oxytetracycline. Anal. Chem. 2015, 87, 12215–12220. [Google Scholar] [CrossRef]
- Tan, B.; Zhao, H.; Du, L.; Gan, X.; Quan, X. A versatile fluorescent biosensor based on target-responsive graphene oxide hydrogel for antibiotic detection. Biosens. Bioelectron. 2016, 83, 267–273. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, S.; Liu, M.; Chang, Y.; Fan, X.; Quan, X. Fluorescent assay for oxytetracycline based on a long-chain aptamer assembled onto reduced graphene oxide. Microchim. Acta 2013, 180, 829–835. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards improvements for penetrating the blood–brain barrier—Recent progress from a material and pharmaceutical perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Tian, Y.; Wu, Y.; Magesa, F.; Deng, P.; Li, G. Facile preparation of Cu2O nanoparticles and reduced graphene oxide nanocomposite for electrochemical sensing of rhodamine b. Nanomaterials 2019, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Preparation of Cu2O-reduced graphene nanocomposite modified electrodes towards ultrasensitive dopamine detection. Sensors 2018, 18, 199. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; Deng, P.; Chen, D. Electrochemical sensor for rapid and sensitive detection of tryptophan by a cu2o nanoparticles-coated reduced graphene oxide nanocomposite. Biomolecules 2019, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; Deng, P.; Chen, D. Facile and Ultrasensitive Determination of 4-Nitrophenol Based on Acetylene Black Paste and Graphene Hybrid Electrode. Nanomaterials 2019, 9, 429. [Google Scholar] [CrossRef]
- Li, G.; Xia, Y.; Tian, Y.; Wu, Y.; Liu, J.; He, Q.; Chen, D. Review—Recent Developments on Graphene-Based Electrochemical Sensors toward Nitrite. J. Electrochem. Soc. 2019, 166, B881. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Xia, Y.; Li, G.; Deng, P.; Chen, D. Novel electrochemical sensors based on cuprous oxide-electrochemically reduced graphene oxide nanocomposites modified electrode toward sensitive detection of sunset yellow. Molecules 2018, 23, 2130. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Ding, Z.; He, Q.; Ding, Z.; Liao, C.; Chen, D.; Ou, L. Fabrication and performance of ZnO doped tantalum oxide multilayer composite coatings on ti6al4v for orthopedic application. Nanomaterials 2019, 9, 685. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. A promising sensing platform toward dopamine using MnO2 nanowires/electro-reduced graphene oxide composites. Electrochim. Acta 2019, 296, 683–692. [Google Scholar] [CrossRef]
- Wan, X.; Yang, S.; Cai, Z.; He, Q.; Ye, Y.; Xia, Y.; Li, G.; Liu, J. Facile synthesis of MnO2 nanoflowers/n-doped reduced graphene oxide composite and its application for simultaneous determination of dopamine and uric acid. Nanomaterials 2019, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Manganese dioxide nanorods/electrochemically reduced graphene oxide nanocomposites modified electrodes for cost-effective and ultrasensitive detection of amaranth. Colloids Surf. B Biointerfaces 2018, 172, 565–572. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, G.; Liu, X.; Liu, J.; Deng, P.; Chen, D. Morphologically tunable MnO2 nanoparticles fabrication, modelling and their influences on electrochemical sensing performance toward dopamine. Catalysts 2018, 8, 323. [Google Scholar] [CrossRef]
- Ding, Z.; Deng, P.; Wu, Y.; Tian, Y.; Li, G.; Liu, J.; He, Q. A novel modified electrode for detection of the food colorant sunset yellow based on nanohybrid of MnO2 nanorods-decorated electrochemically reduced graphene oxide. Molecules 2019, 24, 1178. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Magesa, F.; Liu, J.; Li, G.; He, Q. Construction of effective electrochemical sensor for the determination of quinoline yellow based on different morphologies of manganese dioxide functionalized graphene. J. Food Compos. Anal. 2019, 84, 103280. [Google Scholar] [CrossRef]
- Lee, S.; Oh, J.; Kim, D.; Piao, Y. A sensitive electrochemical sensor using an iron oxide/graphene composite for the simultaneous detection of heavy metal ions. Talanta 2016, 160, 528–536. [Google Scholar] [CrossRef]
- Cai, Z.; Ye, Y.; Wan, X.; Liu, J.; Yang, S.; Xia, Y.; Li, G.; He, Q. Morphology–dependent electrochemical sensing properties of iron oxide–graphene oxide nanohybrids for dopamine and uric acid. Nanomaterials 2019, 9, 835. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Liu, S.; Wang, S. A comparative study of reduced graphene oxide modified TiO2, ZnO and Ta2O5 in visible light photocatalytic/photochemical oxidation of methylene blue. Appl. Catal. B Environ. 2014, 146, 162–168. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J.; Chen, D. Sensitive and selective detection of tartrazine based on TiO2-electrochemically reduced graphene oxide composite-modified electrodes. Sensors 2018, 18, 1911. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. Fabrication of amine-modified magnetite-electrochemically reduced graphene oxide nanocomposite modified glassy carbon electrode for sensitive dopamine determination. Nanomaterials 2018, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wu, Y.; Tian, Y.; Li, G.; Liu, J.; Deng, P.; Chen, D. Facile electrochemical sensor for nanomolarrutin detection based on magnetite nanoparticles and reduced graphene oxide decorated electrode. Nanomaterials 2019, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, P.; Tian, Y.; Ding, Z.; Li, G.; Liu, J.; Zuberi, Z.; He, Q. Rapid recognition and determination of tryptophan by carbon nanotubes and molecularly imprinted polymer-modified glassy carbon electrode. Bioelectrochemistry 2020, 131, 107393. [Google Scholar] [CrossRef] [PubMed]
- Anandan, S.; Pugazhenthiran, N.; Selvamani, T.; Hsieh, S.; Lee, G.; Wu, J. Investigation on photocatalytic potential of Au–Ta2O5 semiconductor nanoparticle by degrading methyl orange in aqueous solution by illuminating with visible light. Catal. Sci. Technol. 2012, 2, 2502–2507. [Google Scholar] [CrossRef]
- Krishnaprasanth, A.; Seetha, M. Solvent free synthesis of Ta2O5 nanoparticles and their photocatalytic properties. AIP Adv. 2018, 8, 055017. [Google Scholar] [CrossRef]
- Zhu, G.; Lin, T.; Cui, H.; Zhao, W.; Zhang, H.; Huang, F. Gray Ta2O5 nanowires with greatly enhanced photocatalytic performance. ACS Appl. Mater. Interfaces 2015, 8, 122–127. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, Z.; Wu, Z.; Xie, M.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; He, Q. Ta2O5/rgo nanocomposite modified electrodes for detection of tryptophan through electrochemical route. Nanomaterials 2019, 9, 811. [Google Scholar] [CrossRef]
- Xie, Z.; Li, G.; Fu, Y.; Sun, M.; Ye, B. Sensitive, simultaneous determination of chrysin and baicalein based on Ta2O5-chitosan composite modified carbon paste electrode. Talanta 2017, 165, 553–562. [Google Scholar] [CrossRef]
- Magesa, F.; Wu, Y.; Tian, Y.; Vianney, J.-M.; Buza, J.; He, Q.; Tan, Y. Graphene and Graphene Like 2D Graphitic Carbon Nitride: Electrochemical Detection of Food Colorants and Toxic Substances in Environment. Trends Environ. Anal. Chem. 2019, 23, e00064. [Google Scholar] [CrossRef]
- Yin, X.; Song, G.; Liu, Y. Vibration suppression of wind/traffic/bridge coupled system using multiple pounding tuned mass dampers (MPTMD). Sensors 2019, 19, 1133. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Yin, X.; Huang, Z.; Wang, L. Debonding detection of reinforced concrete (RC) beam with near-surface mounted (NSM) pre-stressed carbon fiber reinforced polymer (CFRP) plates using embedded piezoceramic smart aggregates (SAs). Appl. Sci. 2020, 10, 50. [Google Scholar] [CrossRef]
- Sun, J.; Gan, T.; Meng, W.; Shi, Z.; Zhang, Z.; Liu, Y. Determination of oxytetracycline in food using a disposable montmorillonite and acetylene black modified microelectrode. Anal. Lett. 2015, 48, 100–115. [Google Scholar] [CrossRef]
- Sun, J.; Gan, T.; Zhu, H.; Shi, Z.; Liu, Y. Direct electrochemical sensing for oxytetracycline in food using a zinc cation-exchanged montmorillonite. Appl. Clay Sci. 2014, 101, 598–603. [Google Scholar] [CrossRef]
- Du, J.; Song, Y.; Xie, S.; Feng, Y.; Jiang, J.; Xu, L. Electrochemical Biosensor Based on Hierarchical Nanoporous Composite Electrode for Detection of Oxytetracycline. Nanosci. Nanotechnol. Lett. 2018, 10, 1095–1100. [Google Scholar] [CrossRef]
- Lian, W.; Liu, S.; Yu, J.; Li, J.; Cui, M.; Xu, W.; Huang, J. Determination of oxytetracycline with a gold electrode modified by chitosan-multiwalled carbon nanotube multilayer films and gold nanoparticles. Anal. Lett. 2013, 46, 1117–1131. [Google Scholar] [CrossRef]
- Xie, S.; Xu, J.; Du, J.; Li, N.; Xu, L. Preparation of Ni–Co Alloy Electrodes by Pulsed Electrodeposition and Its Application in Detection of Oxytetracycline. Nanosci. Nanotechnol. Lett. 2016, 8, 527–531. [Google Scholar] [CrossRef]

| Electrode | Electrochemical Techniques | Linear Range (µM) | Limit of Detection (µM) | References |
|---|---|---|---|---|
| Montmorillonite/acetylene black modified carbon paste microelectrode | e DPV | 0.5–50 | 0.087 | [42] |
| a Zn-Mt/GCE | DPV, f EIS | 0.80–40 | 0.12 | [43] |
| b Pd-HSiO1.5/Ni–Co | Electrodeposition | 400–0.01 | 10 | [44] |
| c CS-MWCNTs/ AuNPs | CV, amperometry | 0.03–80 | 0.027 | [45] |
| d Ni-Co Alloy electrode | CV | 10–500 | 10 | [46] |
| Ta2O5-ErGO/GCE | CV | 0.2–100 | 0.095 | Current study |
| Sample | Add (µM) | Total Found (µM) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| NO. 1 | 0.50 | 0.599 | 3.50 | 119.8 |
| NO. 2 | 5.00 | 6.045 | 3.27 | 120.9 |
| NO. 3 | 50.00 | 50.04 | 2.76 | 100.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magesa, F.; Wu, Y.; Dong, S.; Tian, Y.; Li, G.; Vianney, J.M.; Buza, J.; Liu, J.; He, Q. Electrochemical Sensing Fabricated with Ta2O5 Nanoparticle-Electrochemically Reduced Graphene Oxide Nanocomposite for the Detection of Oxytetracycline. Biomolecules 2020, 10, 110. https://doi.org/10.3390/biom10010110
Magesa F, Wu Y, Dong S, Tian Y, Li G, Vianney JM, Buza J, Liu J, He Q. Electrochemical Sensing Fabricated with Ta2O5 Nanoparticle-Electrochemically Reduced Graphene Oxide Nanocomposite for the Detection of Oxytetracycline. Biomolecules. 2020; 10(1):110. https://doi.org/10.3390/biom10010110
Chicago/Turabian StyleMagesa, Felista, Yiyong Wu, Shuai Dong, Yaling Tian, Guangli Li, John Mary Vianney, Joram Buza, Jun Liu, and Quanguo He. 2020. "Electrochemical Sensing Fabricated with Ta2O5 Nanoparticle-Electrochemically Reduced Graphene Oxide Nanocomposite for the Detection of Oxytetracycline" Biomolecules 10, no. 1: 110. https://doi.org/10.3390/biom10010110
APA StyleMagesa, F., Wu, Y., Dong, S., Tian, Y., Li, G., Vianney, J. M., Buza, J., Liu, J., & He, Q. (2020). Electrochemical Sensing Fabricated with Ta2O5 Nanoparticle-Electrochemically Reduced Graphene Oxide Nanocomposite for the Detection of Oxytetracycline. Biomolecules, 10(1), 110. https://doi.org/10.3390/biom10010110








