Seasonal Variation of Triacylglycerol Profile of Bovine Milk
Abstract
:1. Introduction
2. Results
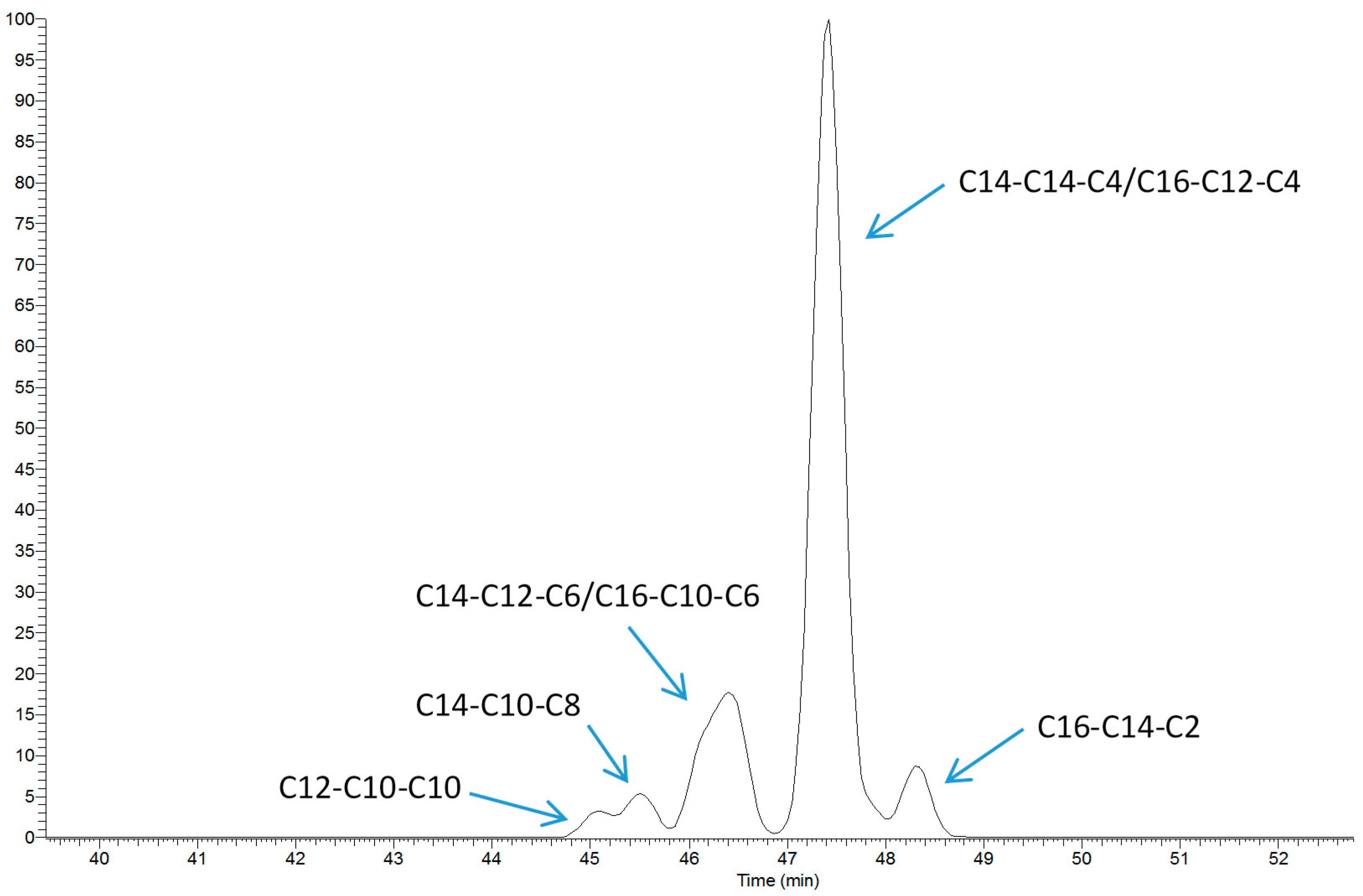
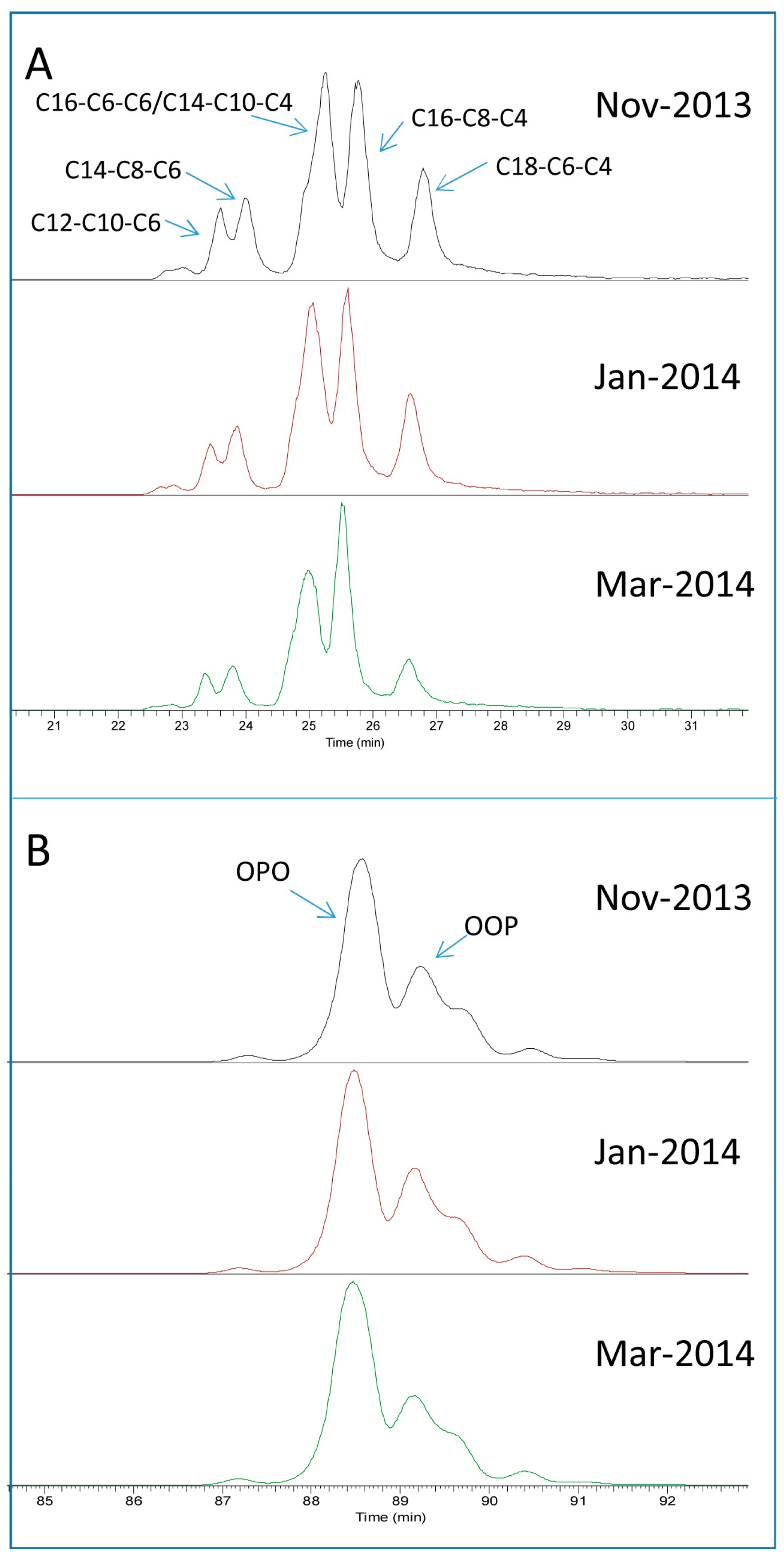
2.1. TAG Profile of Bovine Milk
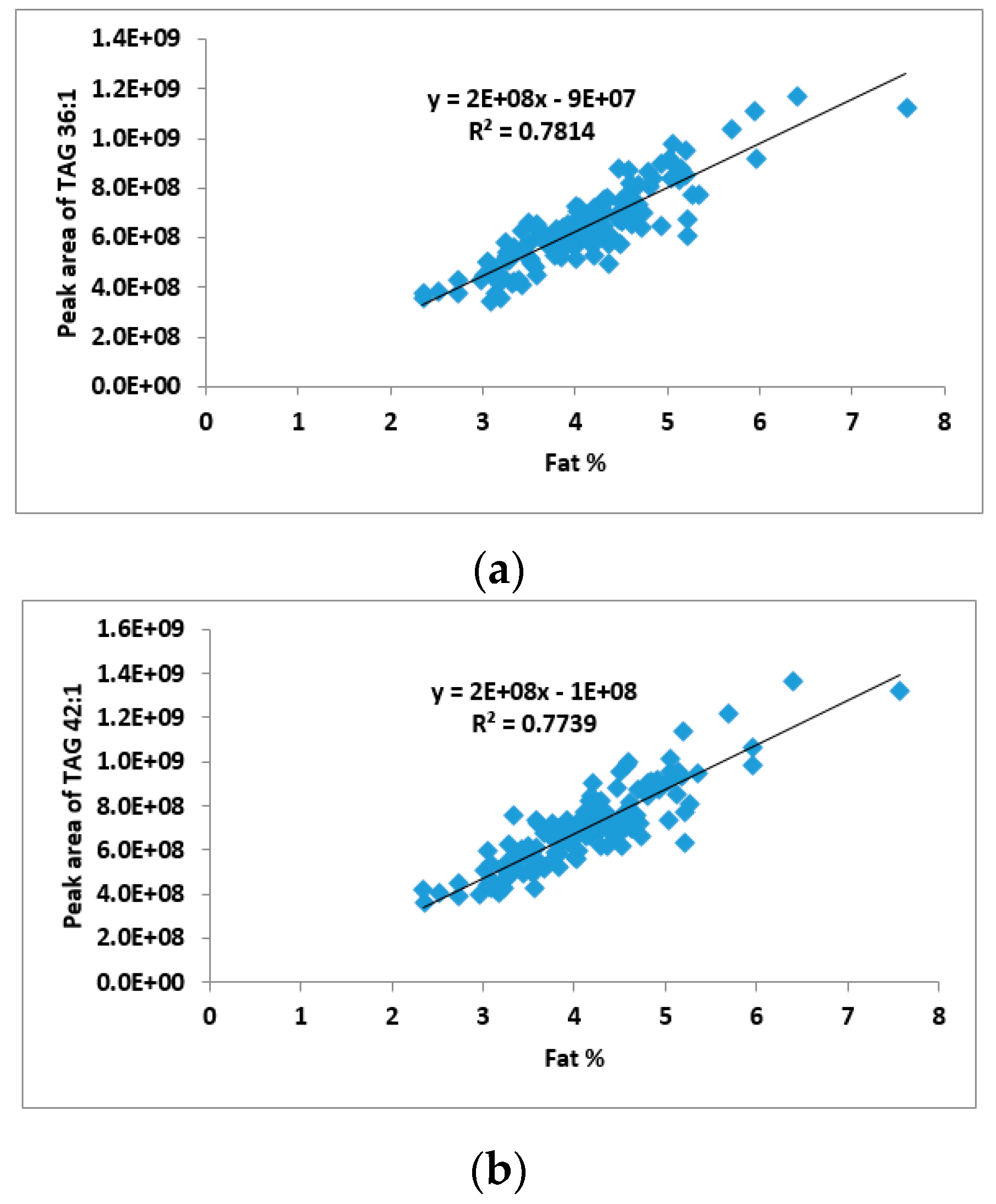
2.2. Correlation between TAG Groups and Total Fat Content
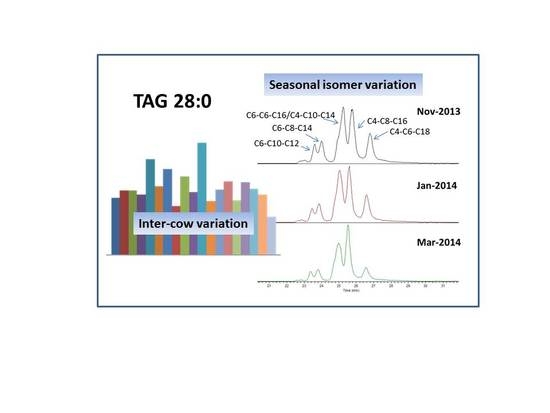
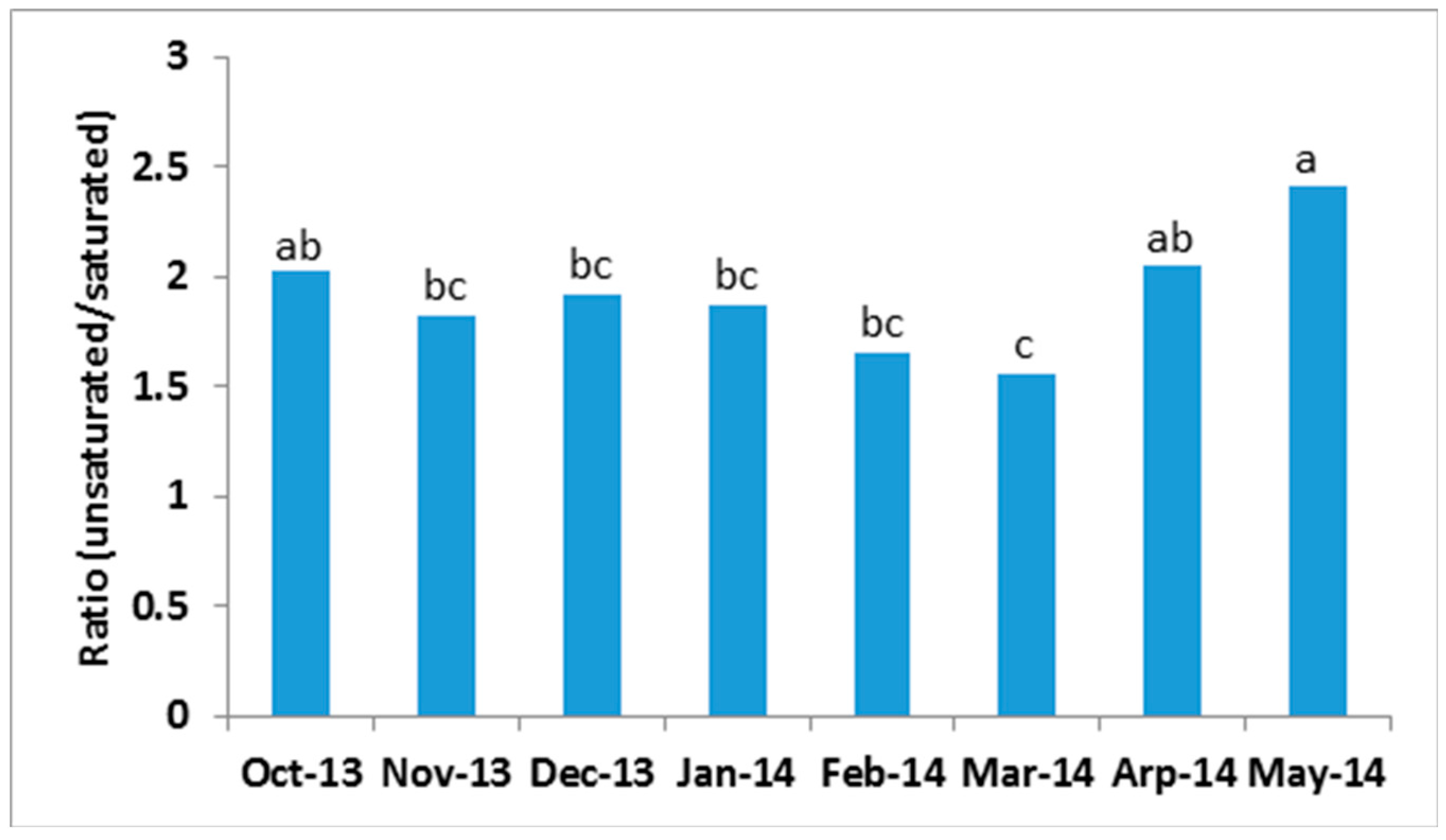
2.3. Seasonal Variation of Milk TAG Profile
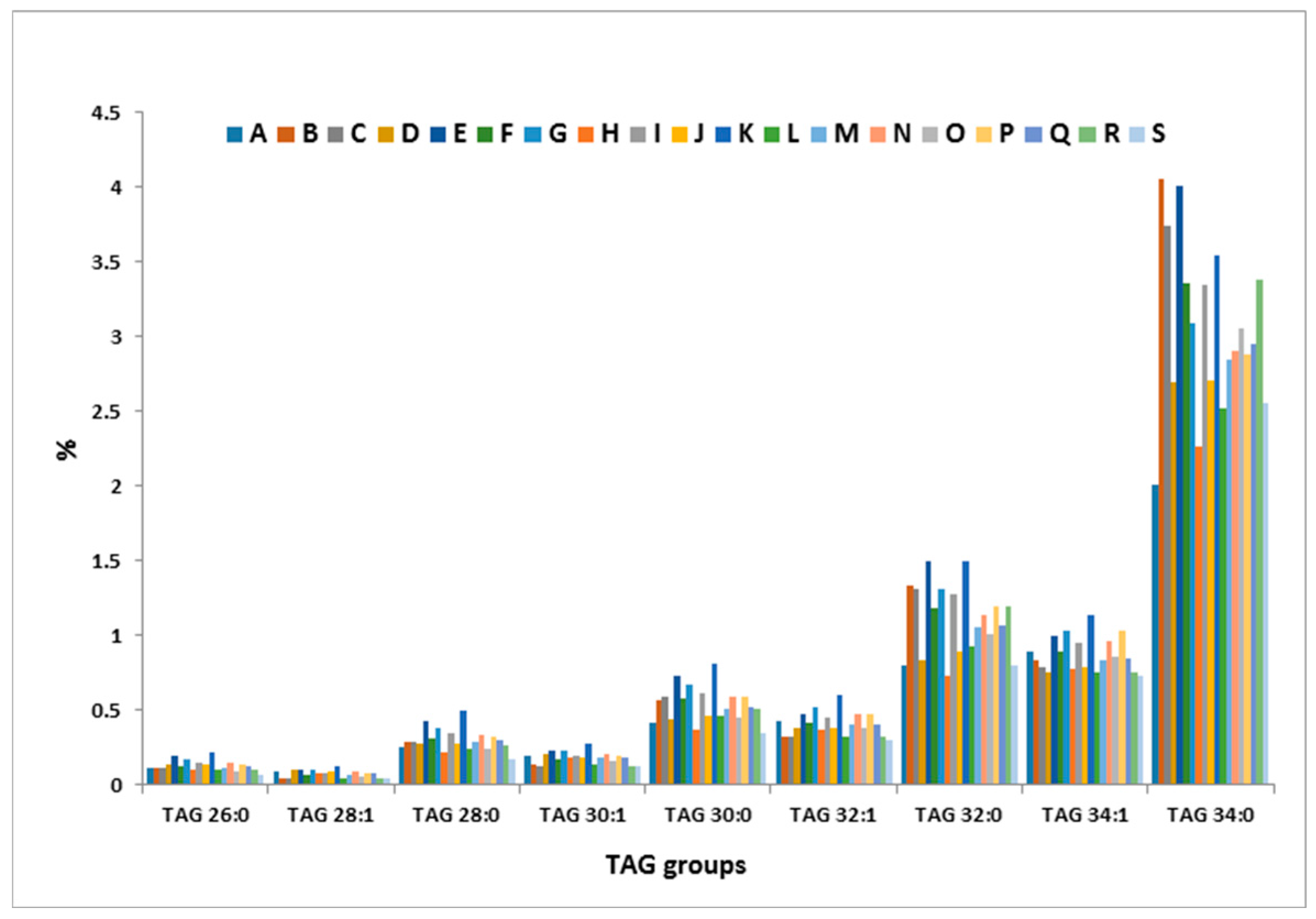
2.4. Inter-Cow Variation of TAG Profile
3. Discussion
4. Materials and Methods
4.1. Cows, Herd Management, and Milk Sample Collection
4.2. Chemicals
4.3. Lipid Extraction from Milk
4.4. TAG Analysis by LC-MS
4.5. Statistical Analysis of Data
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Mansson, H.L. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52. [Google Scholar] [CrossRef]
- Palmquist, D.L. Milk fat: Origin of fatty acids and influence of nutritional factors thereon. In Advanced Dairy Chemistry Volume 2: Lipids, 3rd ed.; Fox, P.F., McSweeney, P.L.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 43–92. [Google Scholar]
- Auldist, M.J.; Walsh, B.J.; Thomson, N.A. Seasonal and lactational influences on bovine milk composition in New Zealand. J. Dairy Res. 1998, 65, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.G. The composition of bovine milk lipids: January 1995 to December 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef]
- Heck, J.M.; van Valenberg, H.J.; Dijkstra, J.; van Hooijdonk, A.C. Seasonal variation in the Dutch bovine raw milk composition. J. Dairy Sci. 2009, 92, 4745–4755. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.P.; Wijesundera, C.; Dunshea, F.R.; Doyle, P.T. Seasonal and stage of lactation effects on milk fat composition in northern Victoria. Anim. Prod. Sci. 2013, 53, 560–572. [Google Scholar] [CrossRef]
- Moate, P.J.; Williams, S.R.O.; Torok, V.A.; Hannah, M.C.; Ribaux, B.E.; Tavendale, M.H.; Eckard, R.J.; Jacobs, J.L.; Auldist, M.J.; Wales, W.J. Grape marc reduces methane emissions when fed to dairy cows. J. Dairy Sci. 2014, 97, 5073–5087. [Google Scholar] [CrossRef] [PubMed]
- Tzompa-Sosa, D.A.; van Aken, G.A.; van Hooijdonk, A.C.; van Valenberg, H.J. Influence of C16:0 and long-chain saturated fatty acids on normal variation of bovine milk fat triacylglycerol structure. J. Dairy Sci. 2014, 97, 4542–4551. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Moate, P.; Ezernieks, V.; Cocks, B.G.; Rochfort, S. Identification and quantification of triacylglycerols containing n-3 long-chain polyunsaturated fatty acids in bovine milk. J. Dairy Sci. 2015, 98, 8473–8485. [Google Scholar] [CrossRef] [PubMed]
- Dimick, P.S.; Reddy, S.Y.; Ziegler, G.R. Chemical and thermal characteristics of milk-fat fractions isolated by a melt crystallization. J. Am. Oil Chem. Soc. 1996, 73, 1647–1652. [Google Scholar] [CrossRef]
- Narine, S.S.; Marangoni, A.G. Relating structure of fat crystal networks to mechanical properties: A review. Food Res. Int. 1999, 32, 227–248. [Google Scholar] [CrossRef]
- Smiddy, M.; Huppertz, T.; van Ruth, S. Triacylglycerol and melting profiles of milk fat from several species. Int. Dairy J. 2012, 24, 64–69. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Ramel, P.R.; van Valenberg, H.J.; van Aken, G.A. Formation of β polymorphs in milk fats with large differences in triacylglycerol profiles. J. Agric. Food Chem. 2016, 64, 4152–4157. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.Q.; Huang, J.H.; Jin, Q.Z.; Guo, Z.; Liu, Y.F.; Cheong, L.Z.; Xu, X.B.; Wang, X.G. Model for human milk fat substitute evaluation based on triacylglycerol composition profile. J. Agric. Food Chem. 2013, 61, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Vyssotski, M.; Bloor, S.J.; Lagutin, K.; Wong, H.; Williams, D.B. Efficient separation and analysis of triacylglycerols: Quantitation of β-palmitate (OPO) in oils and infant formulas. J. Agric. Food Chem. 2015, 63, 5985–5992. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cocks, B.; Rochfort, S. Comparison of molecular species distribution of DHA-containing triacylglycerols in milk and different infant formulas by liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2016, 64, 2134–2144. [Google Scholar] [CrossRef] [PubMed]
- Lísa, M.; Netušilová, K.; Franěk, L.; Dvořáková, H.; Vrkoslav, V.; Holčapek, M. Characterization of fatty acid and triacylglycerol composition in animal fats using silver-ion and non-aqueous reversed-phase high-performance liquid chromatography/mass spectrometry and gas chromatography/flame ionization detection. J. Chromatogr. A 2011, 1218, 7499–7510. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.; Xu, M.; Day, L.; Singh, T.; Moore, S.C.; Mazzonetto, M.; Augustin, M.A. Milk fat globule size affects Cheddar cheese properties. Int. Dairy J. 2017, 70, 46–54. [Google Scholar] [CrossRef]
- Grisart, B.; Coppieters, W.; Farnir, F.; Karim, L.; Ford, C.; Berzi, P.; Cambisano, N.; Mni, M.; Reid, S.; Simon, P.; et al. Positional candidate cloning of a QTL in dairy cattle: Identification of a missense mutation in the bovine DGAT1 gene with major effect on milk yield and composition. Genome Res. 2002, 12, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, J.; Sun, D.; Ma, P.; Ding, X.; Yu, Y.; Zhang, Q. Genome wide association studies for milk production traits in Chinese Holstein population. PLoS ONE 2010, 5, e13661. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, A.C.; Visker, M.H.; van Arendonk, J.A.; Bovenhuis, H. Genomic regions associated with bovine milk fatty acids in both summer and winter milk samples. BMC Genet. 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wurmser, C.; Pausch, H.; Jung, S.; Reinhardt, F.; Tetens, J.; Thaller, G.; Fries, R. Identification and dissection of four major QTL affecting milk fat content in the German Holstein-Friesian population. PLoS ONE 2012, 7, e40711. [Google Scholar] [CrossRef] [PubMed]
- Gresti, J.; Bugaut, M.; Maniongui, C.; Bezard, J. Composition of molecular species of triacylglycerols in bovine milk fat. J. Dairy Sci. 1993, 76, 1850–1869. [Google Scholar] [CrossRef]
- Mottram, H.R.; Evershed, R.P. Elucidation of the composition of bovine milk fat triacylglycerols using high-performance liquid chromatography-atmospheric pressure chemical ionisation mass epectrometry. J. Chromatogr. A 2001, 926, 239–253. [Google Scholar] [CrossRef]
- Beccaria, M.; Sullini, G.; Cacciola, F.; Donato, P.; Dugo, P.; Mondello, L. High performance characterization of triacylglycerols in milk and milk-related samples by liquid chromatography and mass spectrometry. J. Chromatogr. A 2014, 1360, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.S.; Marur, V.R.; Sniatynski, M.J.; Greenberg, H.K.; Kirstal, B.S. Serum lipidomics profiling using LC-MS and high-energy collisional dissociation fragmentation: Focus on triglyceride detection and characterization. Anal. Chem. 2011, 83, 6648–6657. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Kamide, Y.; Hirai, M.Y.; Saito, K. Plant lipidomics based on hydrophilic interaction chromatography coupled to ion trap time-of-flight mass spectrometry. Metabolomics 2013, 9, S121–S131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.R.; Callahan, D.L.; Shrestha, P.; Liu, Q.; Petrie, J.R.; Singh, S.P. Lipidomics analysis of Arabidopsis seed genetically engineered to contain DHA. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Smet, K.; Coudijzer, K.; Fredrick, E.; De Campeneere, S.; De Block, J.; Wouters, J.; Raes, K.; Dewettinck, K. Crystallization behavior of milk fat obtained from linseed-fed cows. J. Dairy Sci. 2010, 93, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.K.; Andersen, K.K.; Kaufmann, N.; Wiking, L. Seasonal variation in the composition and melting behaviour of milk fat. J. Dairy Sci. 2014, 97, 4703–4712. [Google Scholar] [CrossRef] [PubMed]
- Dunshea, F.R.; Walker, G.P.; Ostrowska, E.; Doyle, P.T. Seasonal variation in the concentrations of conjugated linoleic and trans fatty acids in milk fat from commercial dairy farms is associated with pasture and grazing management and supplementary feeding practices. Aust. J. Exp. Agric. 2008, 48, 1062–1075. [Google Scholar] [CrossRef]
- Argov-Argaman, N.; Mesilati-Stahy, R.; Magen, Y.; Moallem, U. Elevated concentrate-to-forage ratio in dairy cow rations is associated with a shift in the diameter of milk fat globules and remodeling of their membranes. J. Dairy Sci. 2014, 97, 6285–6295. [Google Scholar] [CrossRef] [PubMed]
- Soyeurt, H.; Gillon, A.; Vanderick, S.; Mayeres, P.; Bertozzi, C.; Gengler, N. Estimation of heritability and genetic correlations for the major fatty acids in bovine milk. J. Dairy Sci. 2007, 90, 4435–4442. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, S.; Bovenhuis, H.; Stoop, W.M.; Bouwman, A.C.; van Arendonk, J.A.; Visker, M.H. Genetic correlation between composition of bovine milk fat in winter and summer, and DGAT1 and SCD1 by season interactions. J. Dairy Sci. 2013, 96, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. 2013, 8th edition. Available online: http://www.nhmrc.gov.au/publications/synopses/ea16syn.htm (accessed on 24 July 2013).
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tisssues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Liu, Z.; Moate, P.; Cocks, B.; Rochfort, S. Comprehensive polar lipid identification and quantification in milk by liquid chromatography–mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 978–979, 95–102. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, J.; Cocks, B.G.; Rochfort, S. Seasonal Variation of Triacylglycerol Profile of Bovine Milk. Metabolites 2017, 7, 24. https://doi.org/10.3390/metabo7020024
Liu Z, Wang J, Cocks BG, Rochfort S. Seasonal Variation of Triacylglycerol Profile of Bovine Milk. Metabolites. 2017; 7(2):24. https://doi.org/10.3390/metabo7020024
Chicago/Turabian StyleLiu, Zhiqian, Jianghui Wang, Benjamin G. Cocks, and Simone Rochfort. 2017. "Seasonal Variation of Triacylglycerol Profile of Bovine Milk" Metabolites 7, no. 2: 24. https://doi.org/10.3390/metabo7020024
APA StyleLiu, Z., Wang, J., Cocks, B. G., & Rochfort, S. (2017). Seasonal Variation of Triacylglycerol Profile of Bovine Milk. Metabolites, 7(2), 24. https://doi.org/10.3390/metabo7020024